Maternal Inflammation During Pregnancy and Cord Blood Metabolomic Signatures in the Context of HIV Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Maternal Markers of Inflammation and Immune Activation
2.3. Cord Blood Markers of Inflammation
2.4. Cord Blood Metabolomics and Lipidomics
2.5. HIV Status, Demographics, and Clinical Information
2.6. Statistical Analysis
3. Results
3.1. Characteristics
3.2. Relationships Between Maternal Inflammatory Markers and Infant Cord Blood Inflammatory Markers
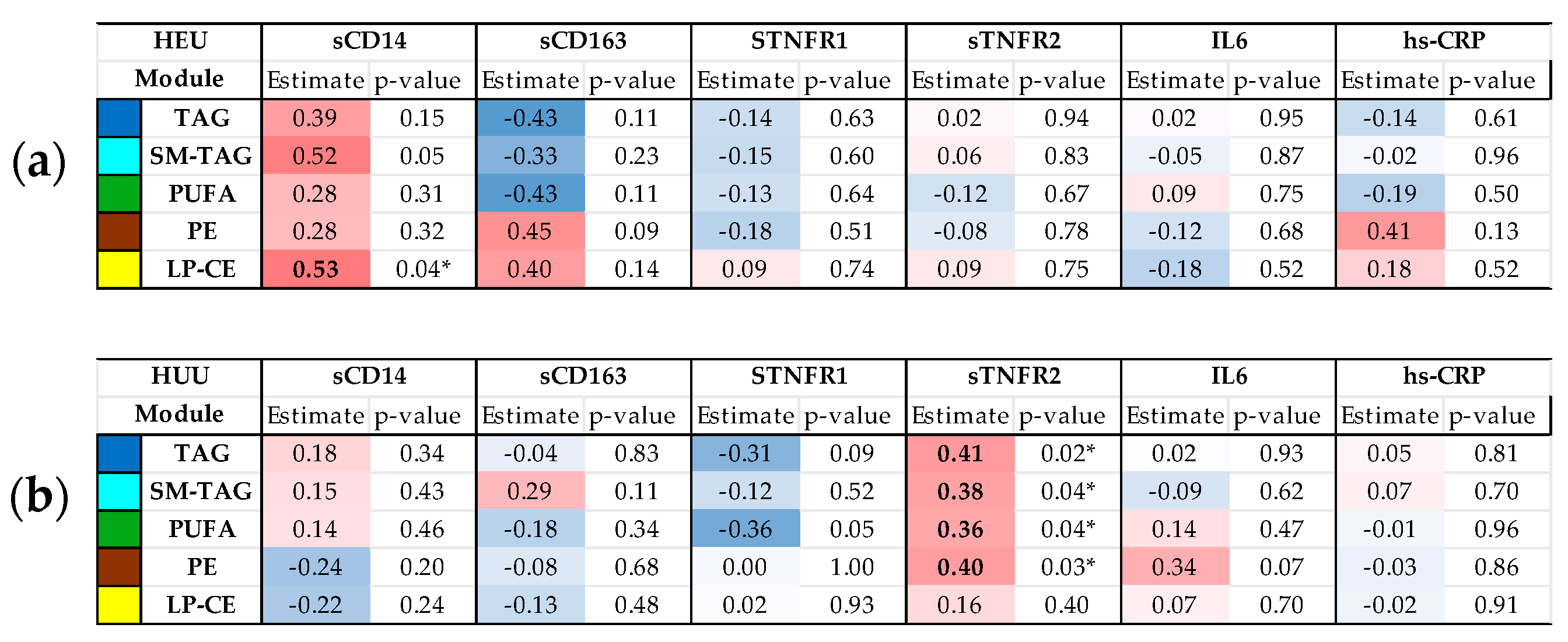
3.3. Relationships Between Maternal Inflammatory Markers and Cord Blood Metabolome/Lipidome by HIV Exposure Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ART | antiretroviral therapy |
| HEU | HIV-exposed uninfected |
| HUU | HIV-unexposed uninfected |
| hs-CRP | High-sensitivity C-reactive protein |
| IFN-γ | interferon gamma |
| TNFα | tumor necrosis factor alpha |
| sTNFR | soluble(s) TNF-α receptor |
| IL | interleukin |
| sCD14 | soluble CD14 |
| sCD163 | soluble CD163 |
| PWH | people living with HIV |
| PWoH | people without HIV |
References
- Shiau, S.; Jacobson, D.L.; Huo, Y.; Kacanek, D.; Yee, L.M.; Williams, D.B.; Haddad, L.B.; Serghides, L.; Powis, K.; Sperling, R.S.; et al. Unique Profile of Inflammation and Immune Activation in Pregnant People with HIV in the United States. J. Infect. Dis. 2023, 227, 720–730. [Google Scholar] [CrossRef]
- Vyas, P.; Mathad, J.S.; Leu, C.-S.; Naik, S.; Alexander, M.; Araújo-Pereira, M.; Kulkarni, V.; Deshpande, P.; Yadana, S.; Andrade, B.B.; et al. Impact of HIV Status on Systemic Inflammation During Pregnancy. AIDS 2021, 35, 2259–2268. [Google Scholar] [CrossRef]
- Hunt, P.W.; Lee, S.A.; Siedner, M.J. Immunologic Biomarkers, Morbidity, and Mortality in Treated HIV Infection. J. Infect. Dis. 2016, 214, S44–S50. [Google Scholar] [CrossRef]
- Sandler, N.G.; Wand, H.; Roque, A.; Law, M.; Nason, M.C.; Nixon, D.E.; Pedersen, C.; Ruxrungtham, K.; Lewin, S.R.; Emery, S.; et al. Plasma Levels of Soluble CD14 Independently Predict Mortality in HIV Infection. J. Infect. Dis. 2011, 203, 780–790. [Google Scholar] [CrossRef]
- Shafiq, M.; Mathad, J.S.; Naik, S.; Alexander, M.; Yadana, S.; Araújo-Pereira, M.; Kulkarni, V.; Deshpande, P.; Kumar, N.P.; Babu, S.; et al. Association of Maternal Inflammation During Pregnancy with Birth Outcomes and Infant Growth Among Women with or Without HIV in India. JAMA Netw. Open 2021, 4, e2140584. [Google Scholar] [CrossRef]
- Sevenoaks, T.; Wedderburn, C.J.; Donald, K.A.; Barnett, W.; Zar, H.J.; Stein, D.J.; Naudé, P.J.W. Association of Maternal and Infant Inflammation with Neurodevelopment in HIV-Exposed Uninfected Children in a South African Birth Cohort. Brain Behav. Immun. 2021, 91, 65–73. [Google Scholar] [CrossRef]
- Reikie, B.A.; Adams, R.C.M.; Leligdowicz, A.; Ho, K.; Naidoo, S.; Rusk, C.E.; de Beer, C.; Preiser, W.; Cotton, M.F.; Speert, D.P.; et al. Altered Innate Immune Development in HIV-Exposed Uninfected Infants. J. Acquir. Immune Defic. Syndr. 2014, 66, 245–255. [Google Scholar] [CrossRef]
- Jao, J.; Abrams, E.J. Metabolic Complications of in Utero Maternal HIV and Antiretroviral Exposure in HIV-Exposed Infants. Pediatr. Infect. Dis. J. 2014, 33, 734–740. [Google Scholar] [CrossRef]
- Jao, J.; Kirmse, B.; Yu, C.; Qiu, Y.; Powis, K.; Nshom, E.; Epie, F.; Tih, P.M.; Sperling, R.S.; Abrams, E.J.; et al. Lower Preprandial Insulin and Altered Fuel Use in HIV/Antiretroviral-Exposed Infants in Cameroon. J. Clin. Endocrinol. Metab. 2015, 100, 3260–3269. [Google Scholar] [CrossRef]
- Jao, J.; Bonner, L.B.; Dobinda, K.; Powis, K.M.; Sun, S.; Legbedze, J.; Mmasa, K.N.; Makhema, J.; Mmalane, M.; Kgole, S.; et al. Lower Insulin Sensitivity Through 36 Months of Life with in Utero HIV and Antiretroviral Exposure in Botswana: Results from the Tshilo Dikotla Study. Clin. Infect. Dis. 2024, 79, 727–733. [Google Scholar] [CrossRef]
- Hornburg, D.; Wu, S.; Moqri, M.; Zhou, X.; Contrepois, K.; Bararpour, N.; Traber, G.M.; Su, B.; Metwally, A.A.; Avina, M.; et al. Dynamic Lipidome Alterations Associated with Human Health, Disease and Ageing. Nat. Metab. 2023, 5, 1578–1594. [Google Scholar] [CrossRef]
- Schoeman, J.C.; Moutloatse, G.P.; Harms, A.C.; Vreeken, R.J.; Scherpbier, H.J.; Van Leeuwen, L.; Kuijpers, T.W.; Reinecke, C.J.; Berger, R.; Hankemeier, T.; et al. Fetal Metabolic Stress Disrupts Immune Homeostasis and Induces Proinflammatory Responses in Human Immunodeficiency Virus Type 1- and Combination Antiretroviral Therapy-Exposed Infants. J. Infect. Dis. 2017, 216, 436–446. [Google Scholar] [CrossRef]
- Jao, J.; Balmert, L.C.; Sun, S.; Qiu, Y.; Kraus, T.A.; Kirmse, B.; Sperling, R.S.; Abrams, E.J.; Myer, L.; Arpadi, S.; et al. Distinct Cord Blood C-Peptide, Adipokine, and Lipidomic Signatures by in Utero HIV Exposure. Pediatr. Res. 2021, 92, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.E.; Groveman, S.A.; Lawson, M.L.; Clark, R.H.; Zemel, B.S. New Intrauterine Growth Curves Based on United States Data. Pediatrics 2010, 125, e214–e224. [Google Scholar] [CrossRef]
- Kuhn, M.; Jackson, S.; Cimentada, J. Corrr: Correlations in R 2025; The Comprehensive R Archive Network (CRAN). Available online: https://github.com/tidymodels/corrr (accessed on 1 September 2025).
- Francis, E.C.; Kechris, K.; Johnson, R.K.; Rawal, S.; Pathmasiri, W.; Rushing, B.R.; Du, X.; Jansson, T.; Dabelea, D.; Sumner, S.J.; et al. Maternal Serum Metabolomics in Mid-Pregnancy Identifies Lipid Pathways as a Key Link to Offspring Obesity in Early Childhood. Int. J. Mol. Sci. 2024, 25, 7620. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R Package for Weighted Correlation Network Analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Perez De Souza, L.; Alseekh, S.; Brotman, Y.; Fernie, A.R. Network-Based Strategies in Metabolomics Data Analysis and Interpretation: From Molecular Networking to Biological Interpretation. Expert. Rev. Proteom. 2020, 17, 243–255. [Google Scholar] [CrossRef]
- Akoto, C.; Norris, S.A.; Hemelaar, J. Maternal HIV Infection Is Associated with Distinct Systemic Cytokine Profiles Throughout Pregnancy in South African Women. Sci. Rep. 2021, 11, 10079. [Google Scholar] [CrossRef]
- Bebell, L.M.; Ngonzi, J.; Butler, A.; Kumbakumba, E.; Adong, J.; Loos, C.; Boatin, A.A.; Bassett, I.V.; Siedner, M.J.; Williams, P.L.; et al. Distinct Cytokine Profiles in Late Pregnancy in Ugandan People with HIV. Sci. Rep. 2024, 14, 10980. [Google Scholar] [CrossRef]
- Lohman-Payne, B.; Koster, J.; Gabriel, B.; Chilengi, R.; Forman, L.S.; Heeren, T.; Duffy, C.R.; Herlihy, J.; Crimaldi, S.; Gill, C.; et al. Persistent Immune Activation in Human Immunodeficiency Virus-Infected Pregnant Women Starting Combination Antiretroviral Therapy After Conception. J. Infect. Dis. 2022, 225, 1162–1167. [Google Scholar] [CrossRef]
- Prendergast, A.J.; Rukobo, S.; Chasekwa, B.; Mutasa, K.; Ntozini, R.; Mbuya, M.N.N.; Jones, A.; Moulton, L.H.; Stoltzfus, R.J.; Humphrey, J.H. Stunting Is Characterized by Chronic Inflammation in Zimbabwean Infants. PLoS ONE 2014, 9, e86928. [Google Scholar] [CrossRef]
- Wilkinson, A.L.; Pedersen, S.H.; Urassa, M.; Michael, D.; Andreasen, A.; Todd, J.; Kinung’hi, S.M.; Changalucha, J.; McDermid, J.M. Maternal Systemic or Cord Blood Inflammation Is Associated with Birth Anthropometry in a Tanzanian Prospective Cohort. Trop. Med. Int. Health 2017, 22, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Grebenciucova, E.; VanHaerents, S. Interleukin 6: At the Interface of Human Health and Disease. Front. Immunol. 2023, 14, 1255533. [Google Scholar] [CrossRef]
- Dahlgren, J.; Samuelsson, A.-M.; Jansson, T.; Holmäng, A. Interleukin-6 in the Maternal Circulation Reaches the Rat Fetus in Mid-Gestation. Pediatr. Res. 2006, 60, 147–151. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, W.; Chen, C.; Yan, B.; Zhu, L.; Chen, X.; Peng, C. The Mechanisms of Lysophosphatidylcholine in the Development of Diseases. Life Sci. 2020, 247, 117443. [Google Scholar] [CrossRef]
- Rhee, E.P.; Cheng, S.; Larson, M.G.; Walford, G.A.; Lewis, G.D.; McCabe, E.; Yang, E.; Farrell, L.; Fox, C.S.; O’Donnell, C.J.; et al. Lipid Profiling Identifies a Triacylglycerol Signature of Insulin Resistance and Improves Diabetes Prediction in Humans. J. Clin. Investig. 2011, 121, 1402–1411. [Google Scholar] [CrossRef]
- Fessler, M.B.; Rudel, L.L.; Brown, J.M. Toll-like Receptor Signaling Links Dietary Fatty Acids to the Metabolic Syndrome. Curr. Opin. Lipidol. 2009, 20, 379–385. [Google Scholar] [CrossRef]
- Sengottuvel, V.; Hota, M.; Oh, J.; Galam, D.L.; Wong, B.H.; Wenk, M.R.; Ghosh, S.; Torta, F.; Silver, D.L. Deficiency in the Omega-3 Lysolipid Transporter Mfsd2a Leads to Aberrant Oligodendrocyte Lineage Development and Hypomyelination. J. Clin. Investig. 2023, 133, e164118. [Google Scholar] [CrossRef]
- Wajant, H.; Siegmund, D. TNFR1 and TNFR2 in the Control of the Life and Death Balance of Macrophages. Front. Cell Dev. Biol. 2019, 7, 91. [Google Scholar] [CrossRef]
- Fiedler, T.; Fairless, R.; Pichi, K.; Fischer, R.; Richter, F.; Kontermann, R.E.; Pfizenmaier, K.; Diem, R.; Williams, S.K. Co-Modulation of TNFR1 and TNFR2 in an Animal Model of Multiple Sclerosis. J. Neuroinflamm. 2023, 20, 100. [Google Scholar] [CrossRef]
- Fischer, R.; Kontermann, R.E.; Pfizenmaier, K. Selective Targeting of TNF Receptors as a Novel Therapeutic Approach. Front. Cell Dev. Biol. 2020, 8, 401. [Google Scholar] [CrossRef]
- Francis, E.C.; Dumolt, J.H.; Zemski-Berry, K.; Jansson, T.; Powell, T.L. Maternal Plasma Choline Levels Are Positively Correlated with Maternal and Placental Phospholipid-DHA Content in Females with Obesity Who Receive DHA Supplementation. J. Nutr. 2025, 155, 880–889. [Google Scholar] [CrossRef]
- Yamamoto, N.; Hashimoto, A.; Takemoto, Y.; Okuyama, H.; Nomura, M.; Kitajima, R.; Togashi, T.; Tamai, Y. Effect of the Dietary Alpha-Linolenate/Linoleate Balance on Lipid Compositions and Learning Ability of Rats. II. Discrimination Process, Extinction Process, and Glycolipid Compositions. J. Lipid Res. 1988, 29, 1013–1021. [Google Scholar] [CrossRef]
- Jao, J.; Kacanek, D.; Yu, W.; Williams, P.L.; Patel, K.; Burchett, S.; Scott, G.; Abrams, E.J.; Sperling, R.S.; Van Dyke, R.B.; et al. Neurodevelopment of HIV-Exposed Uninfected Infants Born to Women with Perinatally Acquired HIV in the United States. J. Acquir. Immune Defic. Syndr. 2020, 84, 213–219. [Google Scholar] [CrossRef]

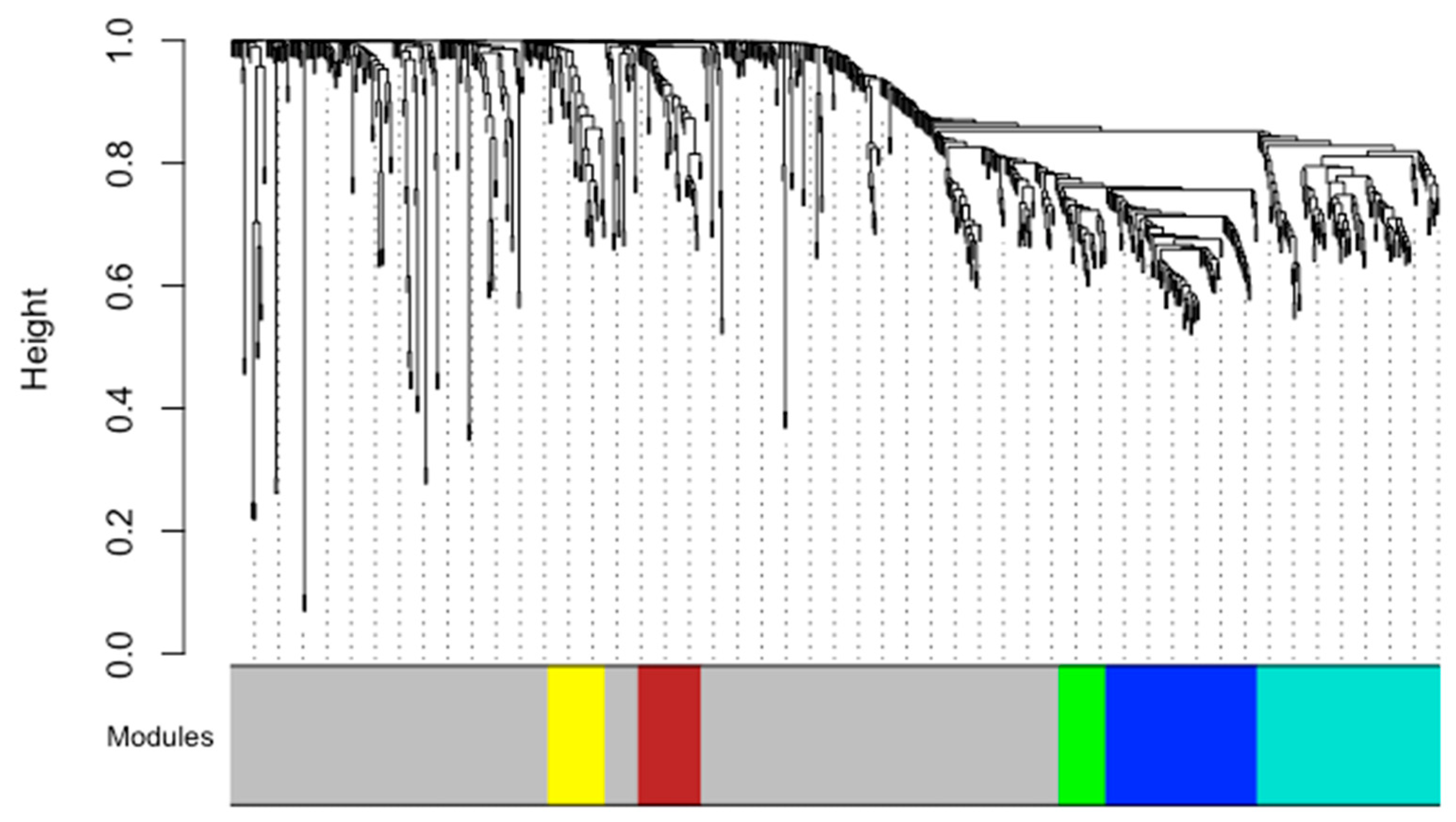
| Maternal Characteristics | PWoH (N = 47) | PWH (N = 22) | p-Value | |
|---|---|---|---|---|
| Age (years) | Mean (SD) | 24.2 (4.71) | 29.0 (5.84) | <0.001 |
| Race/ethnicity | N (%) | |||
| White | 1 (2.13) | 1 (4.55) | 0.75 | |
| Black/African American | 19 (40.4) | 9 (40.9) | ||
| Hispanic | 23 (48.9) | 9 (40.9) | ||
| Other | 4 (8.52) | 3 (13.6) | ||
| Highest education level | N (%) | |||
| Some high school or less | 7 (14.9) | 2 (9.09) | 0.80 | |
| High school diploma or equivalent | 16 (34.0) | 8 (36.4) | ||
| Some college or higher | 24 (51.1) | 12 (54.6) | ||
| Employed | N (%) | 16 (34.0) | 7 (31.8) | 0.86 |
| Family history of diabetes | N (%) | 12 (57.1) | 5 (38.5) | 0.29 |
| Missing | 26 | 9 | ||
| Illicit substance or alcohol use in pregnancy | N (%) | 0 (0.0) | 1 (4.76) | 0.31 |
| Missing | 0 | 1 | ||
| Tobacco use in pregnancy | N (%) | 0 (0.0) | 1 (5.0) | 0.30 |
| Missing | 0 | 2 | ||
| Pre-pregnancy BMI (kg/m2) | Mean (SD) | 26.4 (5.49) | 27.5 (7.63) | 0.56 |
| CD4 cell count at enrollment > 350 cells/mm3 | N (%) | 11 (50) | -- | |
| HIV RNA level < 100 copies/mL at delivery | N (%) | -- | 20 (90.9) | -- |
| Antiretroviral therapy during pregnancy | N (%) | -- | ||
| No ART | -- | 2 (9.09) | ||
| NNRTI-based 1 | 6 (27.3) | |||
| PI-based 2 | 11 (50.0) | |||
| INSTI-based 3 | 2 (9.09) | |||
| >3 classes of antiretrovirals | 1 (4.6) | |||
| Maternal markers of inflammation and immune activation | ||||
| IL-6 (pg/mL) | Median (IQR) | 1.51 (0.84, 6.75) | 0.73 (0.49, 1.82) | 0.017 |
| hs-CRP (ng/mL) | Median (IQR) | 2922 (1212, 28,283) | 6003 (2724, 12,582) | 0.26 |
| sTNFR1 (ng/mL) | Median (IQR) | 2.08 (1.42, 2.54) | 1.52 (1.19, 1.90) | 0.03 |
| sTNFR2 (ng/mL) | Median (IQR) | 5.05 (2.36, 17.99) | 16.78 (4.71, 43.82) | 0.07 |
| sCD14 (ng/mL) | Median (IQR) | 1808 (1604, 1939) | 1525 (1353, 1703) | 0.08 |
| sCD163 (ng/mL) | Median (IQR) | 528 (428, 765) | 459 (343, 584) | 0.02 |
| Infant characteristics | HUU (N = 47) | HEU (N = 22) | ||
| Preterm (<37 weeks gestational age) | N (%) | 2 (4.26) | 2 (9.09) | 0.59 |
| Low birthweight (<1500 g) | N (%) | 3 (6.38) | 4 (18.2) | 0.20 |
| Small-for-gestational age | N (%) | 3 (6.38) | 4 (18.18) | 0.20 |
| C-section delivery | N (%) | 4 (8.51) | 16 (72.73) | <0.001 |
| Birth weight-for-age Z-score | Median (IQR) | −0.19 (−0.82, 0.40) | −0.57 (−1.12, −0.16) | 0.054 |
| Birth length-for-age Z-score | Median (IQR) | 0.089 (−0.46, 0.70) | −0.251 (−0.82, 0.46) | 0.16 |
| Infant cord blood markers of inflammation | ||||
| IL-6 (pg/mL) | Median (IQR) | 13.74 (13.74, 35.87) | 14.59 (13.74, 44.70) | 0.77 |
| TNFα (pg/mL) | Median (IQR) | 10.91 (8.67,14.73) | 14.01 (10.18,16.78) | 0.54 |
| IFN-γ (pg/mL) | Median (IQR) | 0.60 (0.60, 0.61) | 0.60 (0.60, 0.61) | 0.07 |
| Missing | 1 | 2 | ||
| IL-10 (pg/mL) | Median (IQR) | 1.67 (0.36, 3.03) | 1.84 (0.82, 3.97) | 0.95 |
| Missing | 1 | 3 |
| HEU | HUU | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal sCD14 | Maternal sTNFR2 | |||||||||||||
| Cord Blood LP-CE Module | β | p-Value | Cord Blood PE Module | β | p-Value | Cord Blood PUFA Module | β | p-Value | Cord Blood SM-TAG Module | β | p-Value | Cord Blood TAG Module | β | p-Value |
| LPC (16:0) | 2.70 | 0.012 * | PE(18:1/18:1) | 0.30 | 0.010 * | TAG54:5-FA16:0 | 0.26 | 0.034 * | TAG50:0-FA18:0 | 0.32 | 0.010 * | TAG54:5-FA16:0 | 0.30 | 0.009 ** |
| LPC (18:1) | 2.60 | 0.017 * | PE(P-18:1/20:4) | 0.23 | 0.046 * | TAG54:6-FA20:4 | 0.25 | 0.043 * | TAG50:1-FA18:1 | 0.31 | 0.013 * | TAG54:6-FA20:4 | 0.28 | 0.012 * |
| LPC (20:4) | 2.47 | 0.011 * | PE(P-18:0/20:4) | 0.24 | 0.16 | TAG56:6-FA22:4 | 0.25 | 0.047 * | TAG48:1-FA18:1 | 0.30 | 0.016 * | TAG56:7-FA22:5 | 0.28 | 0.016 * |
| LPC (18:2) | 2.36 | 0.029 * | PE(O-18:0/20:4) | 0.22 | 0.06 | TAG56:6-FA18:1 | 0.24 | 0.047 * | TAG48:1-FA14:0 | 0.30 | 0.018 * | TAG56:6-FA18:2 | 0.27 | 0.019 * |
| LPE (18:1) | 2.34 | 0.022 * | PE(P-16:0/20:4) | 0.19 | 0.08 | TAG56:7-FA18:2 | 0.24 | 0.045 * | TAG54:3-FA16:0 | 0.28 | 0.022 * | TAG56:7-FA20:4 | 0.26 | 0.021 * |
| LPC (20:3) | 2.19 | 0.07 | PE(O-16:0/22:4) | 0.18 | 0.13 | TAG56:7-FA20:4 | 0.24 | 0.06 | TAG50:2-FA16:0 | 0.27 | 0.031 * | TAG56:6-FA18:1 | 0.25 | 0.034 * |
| LPE (18:2) | 2.09 | 0.05 | PE(P-16:0/22:5) | 0.16 | 0.11 | TAG56:6-FA18:2 | 0.24 | 0.05 | TAG54:3-FA20:2 | 0.27 | 0.030 * | TAG56:6-FA16:0 | 0.25 | 0.032 * |
| LPC (20:2) | 2.05 | 0.05 | PE(P-18:0/18:1) | 0.14 | 0.25 | TAG56:7-FA22:5 | 0.23 | 0.06 | TAG53:2-FA16:0 | 0.24 | 0.048 * | TAG56:7-FA22:4 | 0.23 | 0.050 * |
| LPE (20:3) | 1.73 | 0.16 | PE(P-16:0/18:1) | 0.14 | 0.27 | TAG56:6-FA16:0 | 0.23 | 0.07 | TAG52:2-FA16:0 | 0.24 | 0.05 | TAG56:7-FA18:2 | 0.22 | 0.06 |
| LPE (20:4) | 1.65 | 0.13 | PE(P-18:0/22:5) | 0.10 | 0.40 | TAG56:7-FA22:4 | 0.21 | 0.10 | TAG50:2-FA18:0 | 0.20 | 0.11 | TAG56:6-FA22:4 | 0.00 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, T.; Francis, E.C.; Kinkade, C.; Sperling, R.S.; Qiu, Y.; Kurland, I.J.; Jao, J.; Shiau, S. Maternal Inflammation During Pregnancy and Cord Blood Metabolomic Signatures in the Context of HIV Exposure. Metabolites 2025, 15, 765. https://doi.org/10.3390/metabo15120765
Fu T, Francis EC, Kinkade C, Sperling RS, Qiu Y, Kurland IJ, Jao J, Shiau S. Maternal Inflammation During Pregnancy and Cord Blood Metabolomic Signatures in the Context of HIV Exposure. Metabolites. 2025; 15(12):765. https://doi.org/10.3390/metabo15120765
Chicago/Turabian StyleFu, Tianyue, Ellen C. Francis, Carolyn Kinkade, Rhoda S. Sperling, Yunping Qiu, Irwin J. Kurland, Jennifer Jao, and Stephanie Shiau. 2025. "Maternal Inflammation During Pregnancy and Cord Blood Metabolomic Signatures in the Context of HIV Exposure" Metabolites 15, no. 12: 765. https://doi.org/10.3390/metabo15120765
APA StyleFu, T., Francis, E. C., Kinkade, C., Sperling, R. S., Qiu, Y., Kurland, I. J., Jao, J., & Shiau, S. (2025). Maternal Inflammation During Pregnancy and Cord Blood Metabolomic Signatures in the Context of HIV Exposure. Metabolites, 15(12), 765. https://doi.org/10.3390/metabo15120765





