Changes in Angiogenesis and Bone Turnover Markers in Patients with Gaucher Disease Developing Osteonecrosis
Abstract
1. Introduction
2. Materials and Methods
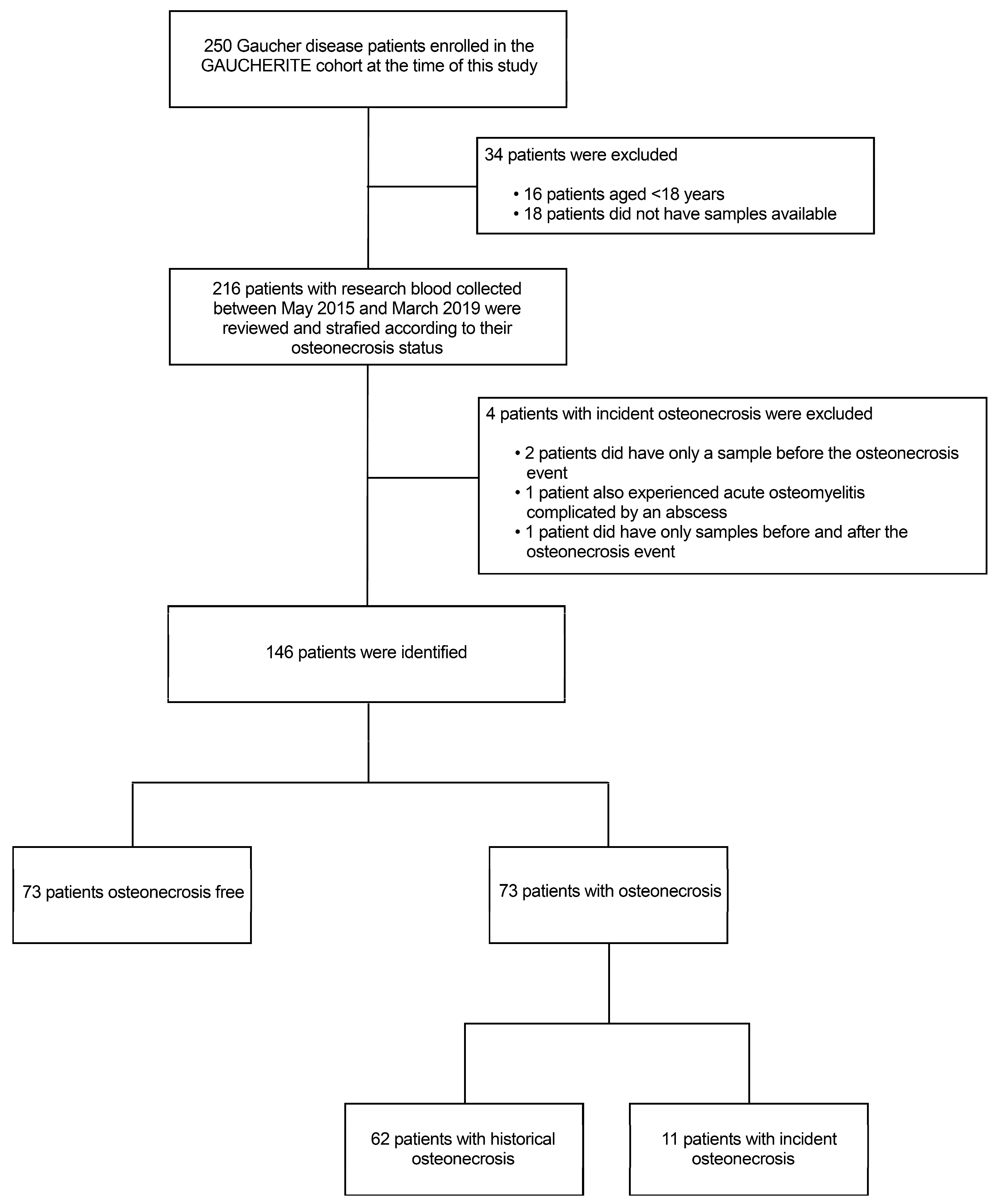
2.1. Patients
2.2. Blood Sampling and Serum Storage
2.3. Biochemistry
2.4. Biomarker Assays
2.5. Bone Disease Assessment
2.6. Gaucher Disease Severity Scoring System
2.7. Statistical Analysis
3. Results
3.1. Characteristics of the Subjects
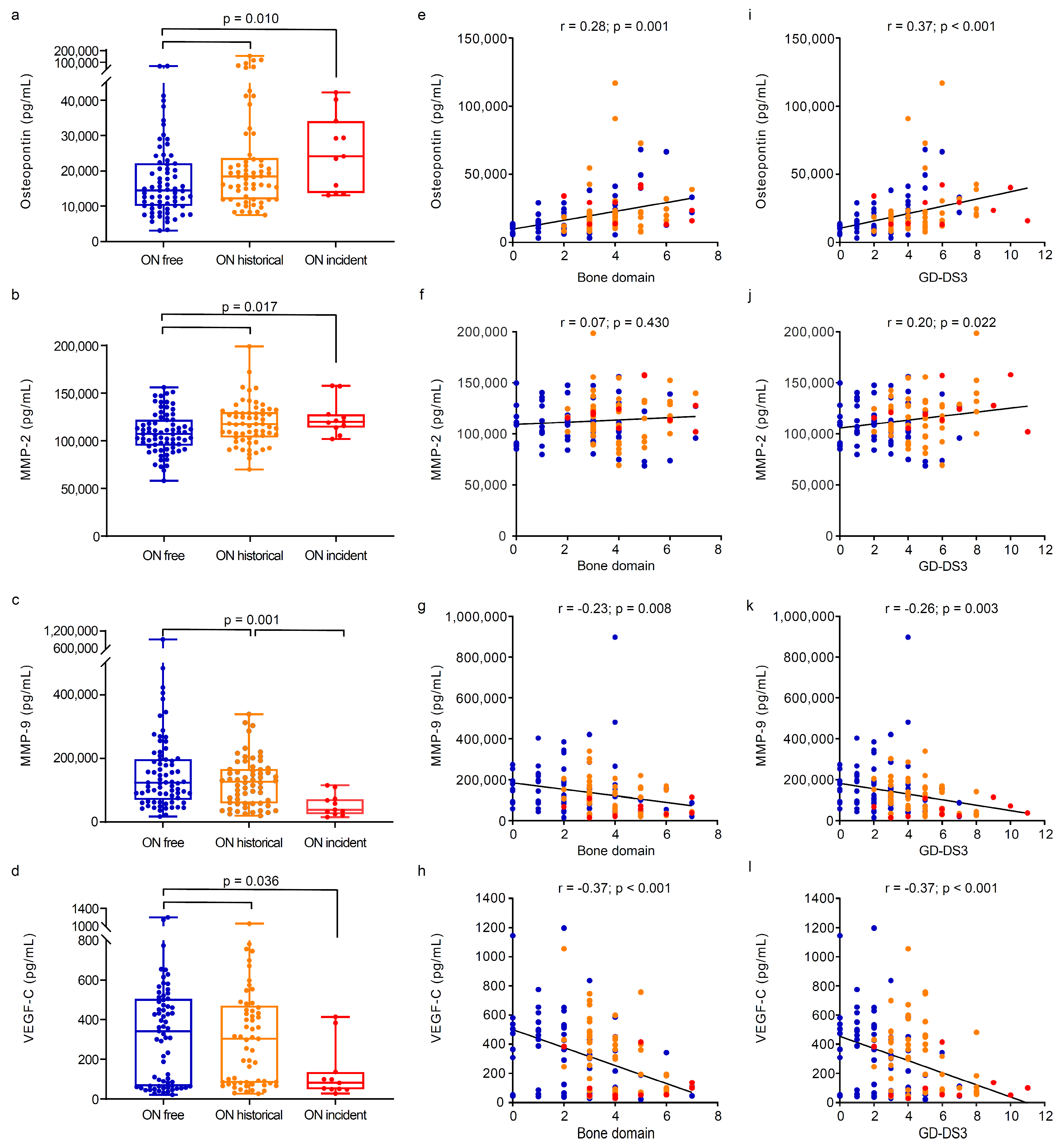
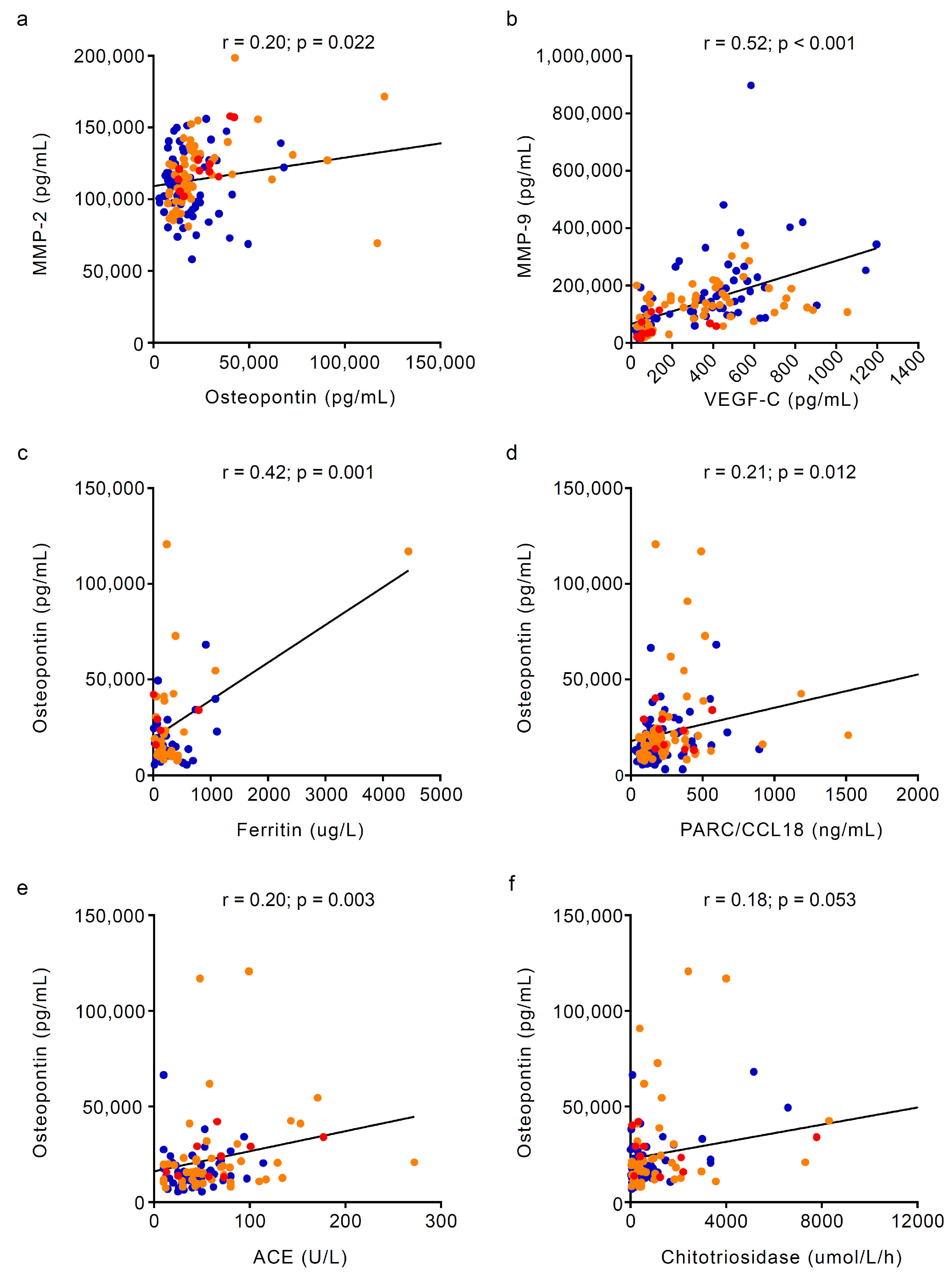
3.2. Circulating Markers of Angiogenesis and Bone Turnover Markers in Gaucher Disease Patients Stratified by Osteonecrosis Status
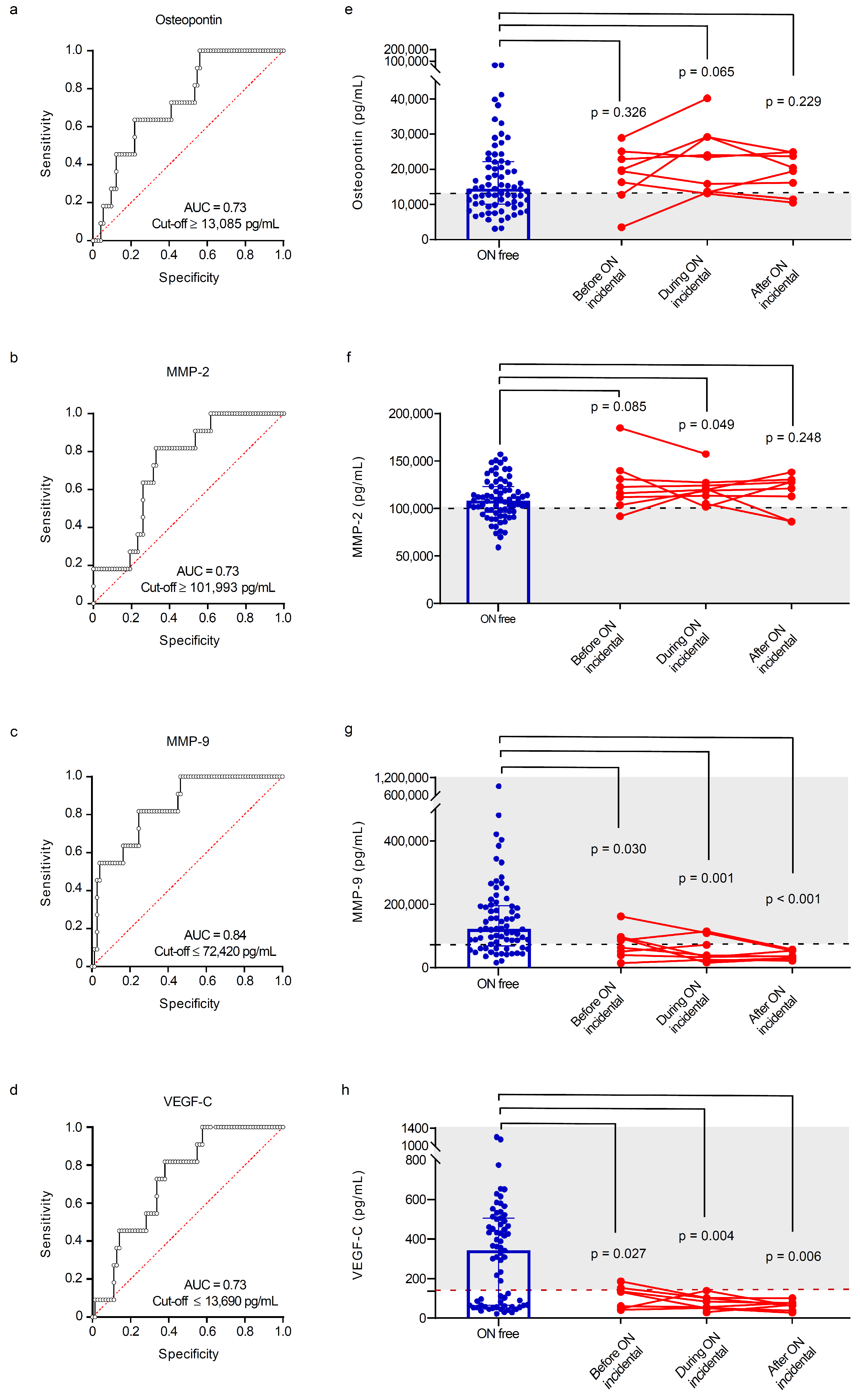
3.3. Sensitivity and Specificity of Angiogenesis and Bone Biomarkers for Detecting Osteonecrosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roh, J.; Subramanian, S.; Weinreb, N.J.; Kartha, R.V. Gaucher disease—More than just a rare lipid storage disease. J. Mol. Med. 2022, 100, 499–518. [Google Scholar] [CrossRef] [PubMed]
- Deegan, P.B.; Pavlova, E.; Tindall, J.; Stein, P.E.; Bearcroft, P.; Mehta, A.; Hughes, D.; Wraith, J.E.; Cox, T.M. Osseous manifestations of adult Gaucher disease in the era of enzyme replacement therapy. Medicine 2011, 90, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.; Mikosch, P.; Belmatoug, N.; Carubbi, F.; Cox, T.; Goker-Alpan, O.; Kindmark, A.; Mistry, P.; Poll, L.; Weinreb, N.; et al. Gaucher Disease in Bone: From Pathophysiology to Practice. J. Bone Miner. Res. 2019, 34, 996–1013. [Google Scholar] [CrossRef] [PubMed]
- de Fost, M.; van Noesel, C.J.; Aerts, J.M.; Maas, M.; Poll, R.G.; Hollak, C.E. Persistent bone disease in adult type 1 Gaucher disease despite increasing doses of enzyme replacement therapy. Haematologica 2008, 93, 1119–1120. [Google Scholar] [CrossRef]
- Baris, H.N.; Cohen, I.J.; Mistry, P.K. Gaucher disease: The metabolic defect, pathophysiology, phenotypes and natural history. Pediatr. Endocrinol. Rev. 2014, 12 (Suppl. S1), 72–81. [Google Scholar]
- Mucci, J.M.; Rozenfeld, P. Pathogenesis of Bone Alterations in Gaucher Disease: The Role of Immune System. J. Immunol. Res. 2015, 2015, 192761. [Google Scholar] [CrossRef]
- Goker-Alpan, O. Therapeutic approaches to bone pathology in Gaucher disease: Past, present and future. Mol. Genet. Metab. 2011, 104, 438–447. [Google Scholar] [CrossRef]
- Khan, A.; Hangartner, T.; Weinreb, N.J.; Taylor, J.S.; Mistry, P.K. Risk factors for fractures and avascular osteonecrosis in type 1 Gaucher disease: A study from the International Collaborative Gaucher Group (ICGG) Gaucher Registry. J. Bone Miner. Res. 2012, 27, 1839–1848. [Google Scholar] [CrossRef]
- Basiri, M.; Ghaffari, M.E.; Ruan, J.; Murugesan, V.; Kleytman, N.; Belinsky, G.; Akhavan, A.; Lischuk, A.; Guo, L.; Klinger, K.; et al. Osteonecrosis in Gaucher disease in the era of multiple therapies: Biomarker set for risk stratification from a tertiary referral center. eLife 2023, 12, e87537. [Google Scholar] [CrossRef]
- Hulkova, H.; Poupetova, H.; Harzer, K.; Mistry, P.; Aerts, J.M.; Elleder, M. Abnormal nonstoring capillary endothelium: A novel feature of Gaucher disease. Ultrastructural study of dermal capillaries. J. Inherit. Metab. Dis. 2010, 33, 69–78. [Google Scholar] [CrossRef]
- Klimkowska, M.; Machaczka, M.; Palmblad, J. Aberrant bone marrow vascularization patterns in untreated patients with Gaucher disease type 1. Blood Cells Mol. Dis. 2018, 68, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.J.; Fuller, M.; Saville, J.T.; Cox, T.M. Reduced cerebral vascularization in experimental neuronopathic Gaucher disease. J. Pathol. 2018, 244, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Assouline-Dayan, Y.; Chang, C.; Greenspan, A.; Shoenfeld, Y.; Gershwin, M.E. Pathogenesis and natural history of osteonecrosis. Semin. Arthritis Rheum. 2002, 32, 94–124. [Google Scholar] [CrossRef] [PubMed]
- D’Amore, S.; Page, K.; Donald, A.; Taiyari, K.; Tom, B.; Deegan, P.; Tan, C.Y.; Poole, K.; Jones, S.A.; Mehta, A.; et al. In-depth phenotyping for clinical stratification of Gaucher disease. Orphanet J. Rare Dis. 2021, 16, 431. [Google Scholar] [CrossRef]
- Malamitsi-Puchner, A.; Tziotis, J.; Tsonou, A.; Protonotariou, E.; Sarandakou, A.; Creatsas, G. Changes in serum levels of vascular endothelial growth factor in males and females throughout life. J. Soc. Gynecol. Investig. 2000, 7, 309–312. [Google Scholar] [CrossRef]
- Thrailkill, K.M.; Moreau, C.S.; Cockrell, G.; Simpson, P.; Goel, R.; North, P.; Fowlkes, J.L.; Bunn, R.C. Physiological matrix metalloproteinase concentrations in serum during childhood and adolescence, using Luminex Multiplex technology. Clin. Chem. Lab. Med. 2005, 43, 1392–1399. [Google Scholar] [CrossRef]
- Nourkami-Tutdibi, N.; Graf, N.; Beier, R.; Zemlin, M.; Tutdibi, E. Plasma levels of osteopontin from birth to adulthood. Pediatr. Blood Cancer 2020, 67, e28272. [Google Scholar] [CrossRef]
- Khoswanto, C. Role of matrix metalloproteinases in bone regeneration: Narrative review. J. Oral Biol. Craniofac. Res. 2023, 13, 539–543. [Google Scholar] [CrossRef]
- Human Tissue Act 2004. Available online: https://www.legislation.gov.uk/ukpga/2004/30/contents (accessed on 22 September 2024).
- Human Tissue (Scotland) Act 2006. Available online: https://www.legislation.gov.uk/asp/2006/4/contents (accessed on 22 September 2024).
- Giraldo, P.; Lopez de Frutos, L.; Cebolla, J.J. Biomarker combination is necessary for the assessment of Gaucher disease? Ann. Transl. Med. 2018, 6, S81. [Google Scholar] [CrossRef]
- Bonjour, J.P.; Ammann, P.; Rizzoli, R. Importance of preclinical studies in the development of drugs for treatment of osteoporosis: A review related to the 1998 WHO guidelines. Osteoporos. Int. 1999, 9, 379–393. [Google Scholar] [CrossRef]
- D’Amore, S.; Sano, H.; Chappell, D.D.G.; Chiarugi, D.; Baker, O.; Page, K.; Ramaswami, U.; Johannesdottir, F.; Cox, T.M.; Deegan, P.; et al. Radiographic Cortical Thickness Index Predicts Fragility Fracture in Gaucher Disease. Radiology 2023, 307, e212779. [Google Scholar] [CrossRef] [PubMed]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.; Rajan, P.S.; Deegan, P.; Cox, T.M.; Bearcroft, P. Quantifying the Erlenmeyer flask deformity. Br. J. Radiol. 2012, 85, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, N.J.; Cappellini, M.D.; Cox, T.M.; Giannini, E.H.; Grabowski, G.A.; Hwu, W.L.; Mankin, H.; Martins, A.M.; Sawyer, C.; vom Dahl, S.; et al. A validated disease severity scoring system for adults with type 1 Gaucher disease. Genet. Med. 2010, 12, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.N.; Racine, J.; Jones, L.C.; Aaron, R.K. Pathophysiology and risk factors for osteonecrosis. Curr. Rev. Musculoskelet. Med. 2015, 8, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, E.V.; Deegan, P.B.; Tindall, J.; McFarlane, I.; Mehta, A.; Hughes, D.; Wraith, J.E.; Cox, T.M. Potential biomarkers of osteonecrosis in Gaucher disease. Blood Cells Mol. Dis. 2011, 46, 27–33. [Google Scholar] [CrossRef]
- Paiva, K.B.S.; Granjeiro, J.M. Matrix Metalloproteinases in Bone Resorption, Remodeling, and Repair. Prog. Mol. Biol. Transl. Sci. 2017, 148, 203–303. [Google Scholar] [CrossRef]
- Ivanova, M.M.; Dao, J.; Loynab, N.; Noor, S.; Kasaci, N.; Friedman, A.; Goker-Alpan, O. The Expression and Secretion Profile of TRAP5 Isoforms in Gaucher Disease. Cells 2024, 13, 716. [Google Scholar] [CrossRef]
- Luukkonen, J.; Pascual, L.M.; Patlaka, C.; Lang, P.; Turunen, S.; Halleen, J.; Nousiainen, T.; Valkealahti, M.; Tuukkanen, J.; Andersson, G.; et al. Increased amount of phosphorylated proinflammatory osteopontin in rheumatoid arthritis synovia is associated to decreased tartrate-resistant acid phosphatase 5B/5A ratio. PLoS ONE 2017, 12, e0182904. [Google Scholar] [CrossRef]
- Bai, R.J.; Li, Y.S.; Zhang, F.J. Osteopontin, a bridge links osteoarthritis and osteoporosis. Front. Endocrinol. 2022, 13, 1012508. [Google Scholar] [CrossRef]
- Lin, E.Y.; Xi, W.; Aggarwal, N.; Shinohara, M.L. Osteopontin (OPN)/SPP1: From its biochemistry to biological functions in the innate immune system and the central nervous system (CNS). Int. Immunol. 2023, 35, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Nyman, J.S.; Lynch, C.C.; Perrien, D.S.; Thiolloy, S.; O’Quinn, E.C.; Patil, C.A.; Bi, X.; Pharr, G.M.; Mahadevan-Jansen, A.; Mundy, G.R. Differential effects between the loss of MMP-2 and MMP-9 on structural and tissue-level properties of bone. J. Bone Miner. Res. 2011, 26, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Dubey, S.; Varney, M.L.; Dave, B.J.; Singh, R.K. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J. Immunol. 2003, 170, 3369–3376. [Google Scholar] [CrossRef] [PubMed]
- Mott, J.D.; Werb, Z. Regulation of matrix biology by matrix metalloproteinases. Curr. Opin. Cell Biol. 2004, 16, 558–564. [Google Scholar] [CrossRef]
- Saran, U.; Gemini Piperni, S.; Chatterjee, S. Role of angiogenesis in bone repair. Arch. Biochem. Biophys. 2014, 561, 109–117. [Google Scholar] [CrossRef]
- Johnson, C.; Sung, H.J.; Lessner, S.M.; Fini, M.E.; Galis, Z.S. Matrix metalloproteinase-9 is required for adequate angiogenic revascularization of ischemic tissues: Potential role in capillary branching. Circ. Res. 2004, 94, 262–268. [Google Scholar] [CrossRef]
- He, M.; Wang, J.; Wang, G.; Tian, Y.; Jiang, L.; Ren, Z.; Qiu, C.; Fu, Q. Effect of glucocorticoids on osteoclast function in a mouse model of bone necrosis. Mol. Med. Rep. 2016, 14, 1054–1060. [Google Scholar] [CrossRef][Green Version]
- Fang, S.; Li, Y.; Chen, P. Osteogenic effect of bone marrow mesenchymal stem cell-derived exosomes on steroid-induced osteonecrosis of the femoral head. Drug Des. Dev. Ther. 2019, 13, 45–55. [Google Scholar] [CrossRef]
- Li, G.; Ji, F.; Guo, W.; Wei, B. Decreased serum MMP-9 levels in patients with nontraumatic osteonecrosis of the femoral head. BMC Musculoskelet. Disord. 2023, 24, 240. [Google Scholar] [CrossRef]
- Di Natale, P.; Villani, G.R.; Parini, R.; Scarpa, M.; Parenti, G.; Pontarelli, G.; Grosso, M.; Sersale, G.; Tomanin, R.; Sibilio, M.; et al. Molecular markers for the follow-up of enzyme-replacement therapy in mucopolysaccharidosis type VI disease. Biotechnol. Appl. Biochem. 2008, 49, 219–223. [Google Scholar] [CrossRef]
- Batzios, S.P.; Zafeiriou, D.I.; Vargiami, E.; Karakiulakis, G.; Papakonstantinou, E. Differential expression of matrix metalloproteinases in the serum of patients with mucopolysaccharidoses. JIMD Rep. 2012, 3, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, S.; Zheng, L.P.; Brogi, E.; Kearney, M.; Pu, L.Q.; Bunting, S.; Ferrara, N.; Symes, J.F.; Isner, J.M. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J. Clin. Investig. 1994, 93, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Banai, S.; Jaklitsch, M.T.; Shou, M.; Lazarous, D.F.; Scheinowitz, M.; Biro, S.; Epstein, S.E.; Unger, E.F. Angiogenic-induced enhancement of collateral blood flow to ischemic myocardium by vascular endothelial growth factor in dogs. Circulation 1994, 89, 2183–2189. [Google Scholar] [CrossRef] [PubMed]
- Pufe, T.; Wildemann, B.; Petersen, W.; Mentlein, R.; Raschke, M.; Schmidmaier, G. Quantitative measurement of the splice variants 120 and 164 of the angiogenic peptide vascular endothelial growth factor in the time flow of fracture healing: A study in the rat. Cell Tissue Res. 2002, 309, 387–392. [Google Scholar] [CrossRef]
- Suzuki, O.; Bishop, A.T.; Sunagawa, T.; Katsube, K.; Friedrich, P.F. VEGF-promoted surgical angiogenesis in necrotic bone. Microsurgery 2004, 24, 85–91. [Google Scholar] [CrossRef]
- Dudley, A.C.; Griffioen, A.W. Pathological angiogenesis: Mechanisms and therapeutic strategies. Angiogenesis 2023, 26, 313–347. [Google Scholar] [CrossRef]
- Kim, T.; Hong, J.M.; Lee, J.; Oh, B.; Park, E.K.; Lee, C.; Bae, S.; Kim, S. Promoter polymorphisms of the vascular endothelial growth factor gene is associated with an osteonecrosis of the femoral head in the Korean population. Osteoarthr. Cartil. 2008, 16, 287–291. [Google Scholar] [CrossRef]
- Hong, J.M.; Kim, T.H.; Kim, H.J.; Park, E.K.; Yang, E.K.; Kim, S.Y. Genetic association of angiogenesis- and hypoxia-related gene polymorphisms with osteonecrosis of the femoral head. Exp. Mol. Med. 2010, 42, 376–385. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, J.S.; Kang, E.H.; Lee, Y.K.; Kim, S.Y.; Song, Y.W.; Koo, K.H. Vascular endothelial growth factor polymorphisms in patients with steroid-induced femoral head osteonecrosis. J. Orthop. Res. 2012, 30, 21–27. [Google Scholar] [CrossRef]
- Vincenzi, B.; Napolitano, A.; Zoccoli, A.; Iuliani, M.; Pantano, F.; Papapietro, N.; Denaro, V.; Santini, D.; Tonini, G. Serum VEGF levels as predictive marker of bisphosphonate-related osteonecrosis of the jaw. J. Hematol. Oncol. 2012, 5, 56. [Google Scholar] [CrossRef]
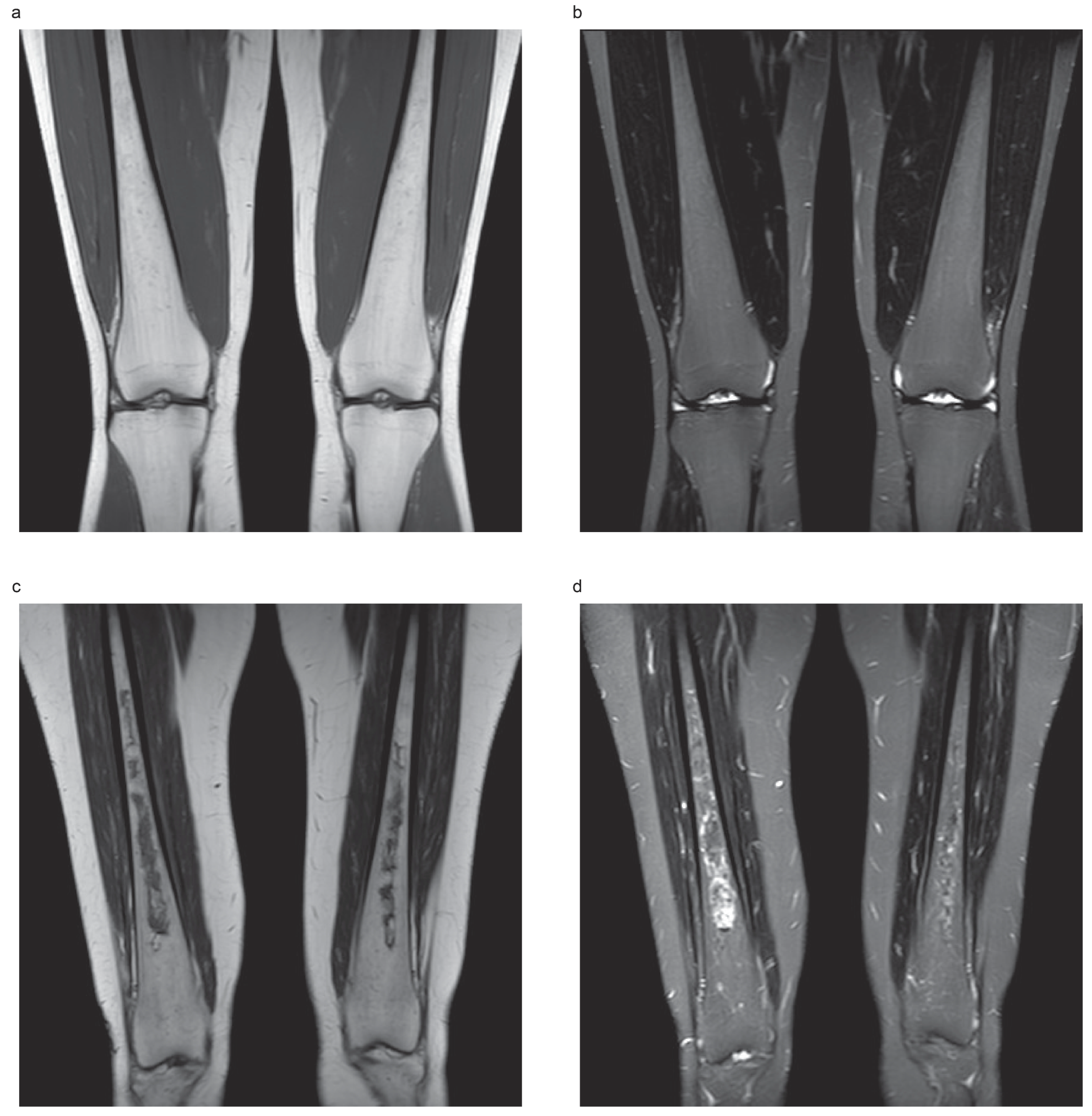
| Variable | Osteonecrosis—Free | Osteonecrosis—Historical | Osteonecrosis—Incident | p-Value |
|---|---|---|---|---|
| N | 73 (36M:37F) | 62 (28M:34F) | 11 (6M:5F) | |
| Age, years, median (IQR) | 47 (34.4–60.3) | 47.7 (35.8–60.9) | 49.5 (39.2–66.8) | 0.446 |
| GENOTYPE, N % | ||||
| Homozygous N370S/N370S | 18 (25%) | 4 (6.5%) | 1 (9%) | 0.036 + |
| Homozygous L444P/L444P | 3 (4%) | 6 (10%) | - | |
| Homozygous other/other a | 1 (1%) | - | - | |
| Heterozygous N370S/L444P | 14 (19%) | 11 (18%) | 2 (18%) | |
| Heterozygous N370S/other a | 29 (39.7%) | 32 (52%) | 4 (36.5%) | |
| Heterozygous L444P/other a | 1 (1%) | 4 (6.5%) | - | |
| Heterozygous other/other a | 7 (10%) | 5 (8%) | 4 (36.5%) | |
| TYPE OF GAUCHER DISEASE, N % | ||||
| Type 1 | 68 (93%) | 55 (89%) | 10 (91%) | 0.665 + |
| Type 3 | 5 (7%) | 7 (11%) | 1 (9%) | |
| Age at Gaucher disease presentation, years, median (IQR) | 26.5 (13.8–37.8) | 8.2 (4.2–20.3) | 6.5 (4.4–25.3) | <0.001 1, 2 ≠ 0 ‡ |
| OSTEONECROSIS, N % | ||||
| Symptomatic osteonecrosis events | - | 36 | 9 | 0.001 + |
| Asymptomatic osteonecrosis events | - | 30 | 11 | |
| Age at first osteonecrosis, years, median (IQR) | - | 25.07 (11.88–37.43) | 21.95 (12.6–37.8) | 0.845 # |
| FRACTURE, N % | ||||
| No | 69 (94.5%) | 46 (74%) | 7 (64%) | 0.001 + |
| Yes | 4 (5.5%) | 16 (26%) | 4 (36%) | |
| Age at first fracture, years, median (IQR) | 57.65 (16.85–61.59) | 45.39 (25.15–52.95) | 23.9 (13.7–34.8) | 0.176 |
| OSTEOARTHRITIS, N % | ||||
| No | 42 (58%) | 27 (43.5%) | 1 (9%) | 0.007 + |
| Yes | 31 (42%) | 35 (56.5%) | 10 (91%) | |
| Age at osteoarthritis diagnosis, years, median (IQR) | 43 (34.3–53.7) | 41.8 (33.7–52.4) | 40.4 (32.9–53.9) | 0.885 |
| ORTHOPEDIC PROCEDURE, N % | ||||
| No | 66 (90%) | 34 (55%) | 4 (36%) | <0.001 + |
| Yes | 7 (10%) | 28 (45%) | 7 (64%) | |
| Age at first orthopaedic procedure, years, median (IQR) | 46.1 (13–55.2) | 33.3 (14.83–47.65) | 46.9 (19.1–54.3) | 0.281 |
| Variable | Osteonecrosis—Free | Osteonecrosis—Historical | Osteonecrosis—Incident | p-Value |
| ERLENMEYER FLASK DEFORMITY (EFD), N % | ||||
| No | 49 (67%) | 22 (35.5%) | 4 (36%) | 0.001 + |
| Yes | 24 (33%) | 40 (64.5%) | 7 (64%) | |
| Age at EFD diagnosis, years, median (IQR) | 37.2 (25.5–48) | 36.02 (22.8–49.35) | 45.5 (34.3–49.4) | 0.567 |
| LYTIC LESION, N % | ||||
| No | 71 (97%) | 58 (94%) | 10 (91%) | 0.474 + |
| Yes | 2 (3%) | 4 (6%) | 1 (9%) | |
| Age at first lytic lesion, years, median (IQR) | 48.0 (27.3–68.8) | 30.6 (9.0–42.4) | 47.2 (47.2–47.2) | 0.343 |
| GAUCHER DISEASE TYPE 1 SEVERITY SCORING SYSTEM (GD-DS3) | ||||
| Bone domain, median (IQR) | 2 (0.3–3) | 4 (3–5) | 4.5 (3–6.3) | <0.001 1, 2 ≠ 0 |
| Haematologic domain, median (IQR) | 0 (0–0.75) | 0 (0–0) | 0 (0–1) | 0.435 |
| Visceral domain, median (IQR) | 0 (0–1) | 0 (0–2) | 1 (0–3.3) | 0.057 |
| Total Gaucher DS3 score, median (IQR) | 2 (1–4) | 4 (3–6) | 6 (4–9) | <0.001 1, 2 ≠ 0 ‡ |
| BONE MARROW TRANSPLANT, N % | ||||
| No | 73 (100%) | 60 (97%) | 11 (100%) | 0.253 + |
| Yes | - | 2 (3%) | - | |
| Age at bone marrow transplant, years, median (IQR) | - | 6.3 (1.7–10.8) | - | - |
| SPLENECTOMY, N % | ||||
| No | 66 (90%) | 37 (60%) | 6 (55%) | <0.001 + |
| Yes | 7 (10%) | 25 (40%) | 5 (45%) | |
| Age at splenectomy, years, median (IQR) | 27 (24.09–54.05) | 11.72 (5.02–20.76) | 15.4 (7.2–21.7) | 0.011 1 ≠ 0 ‡ |
| GAUCHER SPECIFIC TREATMENT, N % | ||||
| No | 7 (10%) | 2 (3%) | - | 0.209 + |
| Yes | 66 (90%) | 60 (97%) | 11 (100%) | |
| Age at Gaucher specific treatment initiation, years, median (IQR) | 34.5 (25.4–45.01) | 29.25 (20.15–42.4) | 35.4 (20.4–45.9) | 0.555 |
| Time from Gaucher disease presentation and treatment initiation, years, median (IQR) | 3 (0.9–10.3) | 15.4 (4.4–24.3) | 16.9 (1–30.4) | <0.001 1, 2 ≠ 0 ‡ |
| BONE TREATMENT, N % | ||||
| No | 28 (38%) | 20 (32%) | 3 (27%) | 0.652 + |
| Yes | 45 (62%) | 42 (68%) | 8 (73%) | |
| Variable | Osteonecrosis— Free | Osteonecrosis—Historical | Osteonecrosis—Incident | p-Value |
| STEROID TREATMENT, N % | ||||
| No | 59 (81%) | 52 (84%) | 11 (100%) | 0.277 + |
| Yes | 14 (19%) | 10 (16%) | - | |
| Variable | Osteonecrosis—Free | Osteonecrosis—Historical | Osteonecrosis—Incident | p-Value |
|---|---|---|---|---|
| Haemoglobin, g/L, median (IQR) | 139 (129–146.5) | 136 (131–150) | 137 (128–152) | 0.873 |
| White cell count, ×109/L, median (IQR) | 5.7 (4.7–7.5) | 6.4 (5.0–8.4) | 6.6 (3.7–9) | 0.319 |
| Platelets, ×109/L, median (IQR) | 178 (145–219) | 194 (160–252) | 237 (152–325) | 0.043 2 ≠ 0 ‡ |
| Alkaline phosphatase, U/L, median (IQR) | 67.5 (55.8–84) | 67 (56–83) | 73 (55.5–95.3) | 0.864 |
| Calcium, mmol/L, median (IQR) | 2.3 (2.3–2.4) | 2.4 (2.3–2.4) | 2.3 (2.3–2.4) | 0.229 |
| Corrected calcium, mmol/L, median (IQR) | 2.4 (2.3–2.5) | 2.4 (2.3–2.5) | 2.4 (2.4–2.4) | 0.300 |
| Vitamin D, ug/L, median (IQR) | 61.1 (43–84.5) | 58.3 (39.8–82.0) | 56.8 (40.1–67.7) | 0.749 |
| Vitamin B12, ng/L, median (IQR) | 405 (310–556) | 399 (317.5–507) | 425 (356–538) | 0.861 |
| Ferritin, ug/L, median (IQR) | 153 (76.3–535.3) | 190 (119.2–371.1) | 73.8 (27.8–460.5) | 0.332 |
| ACE, U/L, median (IQR) | 40 (25–58) | 51 (38–80) | 66 (35–87) | 0.040 2 ≠ 0 ‡ |
| Chitotriosidase, umol/L/h, median (IQR) | 303 (125.5–878) | 519 (254.5–1301.5) | 479.5 (199.5–2138.3) | 0.128 |
| PARC/CCL18, ng/mL, median (IQR) | 149.5 (104.3–251.5) | 209 (117.3–378.8) | 224 (171–391.8) | 0.055 |
| Fibrinogen, g/L, median (IQR) | 2.67 (2.02–3.17) | 2.59 (2.16–2.83) | 2.92 (2.66–3.1) | 0.141 |
| Variable | Osteonecrosis—Free | Osteonecrosis—Historical | Osteonecrosis—Incident | p-Value |
|---|---|---|---|---|
| Osteonectin, pg/mL, median (IQR) | 769,231 (508,562.5–971,934) | 785,403.5 (585,508–1,003,565) | 322,027 (124,400.5–857,722.5) | 0.306 |
| Osteopontin, pg/mL, median (IQR) | 14,462 (10,057–22,202.5) | 18,474 (11,951.25–23,575.25) | 24,113 (13,754–34,091) | 0.010 1, 2 ≠ 0 ‡ |
| Osteocalcin, pg/mL, median (IQR) | 28,629 (19,786–34,474.5) | 26,751 (19,038–35,658.5) | 28,341 (17,304–45,209) | 0.576 |
| MMP-1, pg/mL, median (IQR) | 4945 (1699–11,427) | 5378.5 (2889.75–8872) | 3058 (980–6332) | 0.253 |
| MMP-2, pg/mL, median (IQR) | 10,7193 (95,016.5–122,201) | 116,699.5 (102,860.8–129,362.3) | 119,970 (113,784–127,694) | 0.017 1, 2 ≠ 0 ‡ |
| MMP-3, pg/mL, median (IQR) | 12,663 (7838.5–18,749.5) | 12,721.5 (9052.5–17,771.25) | 12,314 (10,295–18,204) | 0.627 |
| MMP-9, pg/mL, median (IQR) | 121,223 (68,135.5–195,583.5) | 126,312 (58,997–165,988.3) | 38,711 (24,822–72,420) | 0.001 2 ≠ 1,0 ‡ |
| MMP-10, pg/mL, median (IQR) | 1113 (745.5–1408) | 1095 (892.75–1425.25) | 919 (571–974) | 0.058 |
| TIMP-1, pg/mL, median (IQR) | 299,558 (215,000.5–34,8074) | 306,410.5 (190,432.5–360,033.5) | 190,183 (161,362–285,583) | 0.147 |
| Osteoprotegerin, pg/mL, median (IQR) | 297 (229–381) | 314 (251.75–407) | 284 (239–406) | 0.589 |
| FGF, pg/mL, median (IQR) | 3 (1.85–5.1) | 3.8 (2.4–6.125) | 3 (2–3.7) | 0.156 |
| FLT-1, pg/mL, median (IQR) | 92.7 (70.75–113.55) | 92.65 (72.1–116.8) | 83.6 (67.1–127.8) | 0.834 |
| PLGF, pg/mL, median (IQR) | 6.8 (5.5–8.2) | 6.1 (4.875–7.5) | 5.8 (5.1–7.3) | 0.098 |
| TIE-2, pg/mL, median (IQR) | 5162 (4314.5–5693.5) | 4786.5 (3964.75–5752.25) | 4820 (4348–5088) | 0.634 |
| VEGF-A, pg/mL, median (IQR) | 185.7 (84.55–298.25) | 182.35 (69.225–504.68) | 73.1 (37–127) | 0.056 |
| VEGF-C, pg/mL, median (IQR) | 342.4 (64.6–505.4) | 304.3 (81.05–471.4) | 80.9 (49.1–136.9) | 0.036 2 ≠ 1, 0 ‡ |
| VEGF-D, pg/mL, median (IQR) | 1246 (1001.5–1880.5) | 1260 (966–1735) | 854 (696–1318) | 0.072 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amore, S.; Poole, K.E.; Ramaswami, U.; Hughes, D.; Page, K.; Solimando, A.G.; Vacca, A.; Cox, T.M.; Deegan, P., on behalf of the MRC GAUCHERITE Consortium. Changes in Angiogenesis and Bone Turnover Markers in Patients with Gaucher Disease Developing Osteonecrosis. Metabolites 2024, 14, 601. https://doi.org/10.3390/metabo14110601
D’Amore S, Poole KE, Ramaswami U, Hughes D, Page K, Solimando AG, Vacca A, Cox TM, Deegan P on behalf of the MRC GAUCHERITE Consortium. Changes in Angiogenesis and Bone Turnover Markers in Patients with Gaucher Disease Developing Osteonecrosis. Metabolites. 2024; 14(11):601. https://doi.org/10.3390/metabo14110601
Chicago/Turabian StyleD’Amore, Simona, Kenneth Eric Poole, Uma Ramaswami, Derralynn Hughes, Kathleen Page, Antonio Giovanni Solimando, Angelo Vacca, Timothy Martin Cox, and Patrick Deegan on behalf of the MRC GAUCHERITE Consortium. 2024. "Changes in Angiogenesis and Bone Turnover Markers in Patients with Gaucher Disease Developing Osteonecrosis" Metabolites 14, no. 11: 601. https://doi.org/10.3390/metabo14110601
APA StyleD’Amore, S., Poole, K. E., Ramaswami, U., Hughes, D., Page, K., Solimando, A. G., Vacca, A., Cox, T. M., & Deegan, P., on behalf of the MRC GAUCHERITE Consortium. (2024). Changes in Angiogenesis and Bone Turnover Markers in Patients with Gaucher Disease Developing Osteonecrosis. Metabolites, 14(11), 601. https://doi.org/10.3390/metabo14110601








