Predictive Models of Odor Contribution and Thresholds for Volatiles in Identification of Novel Crop Aroma Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemical Reagents
2.3. Sample Preparation
2.4. GC–MS Conditions
2.5. Metabolome Data Analysis
2.6. Aroma Contribution and Odor Threshold Data Collection
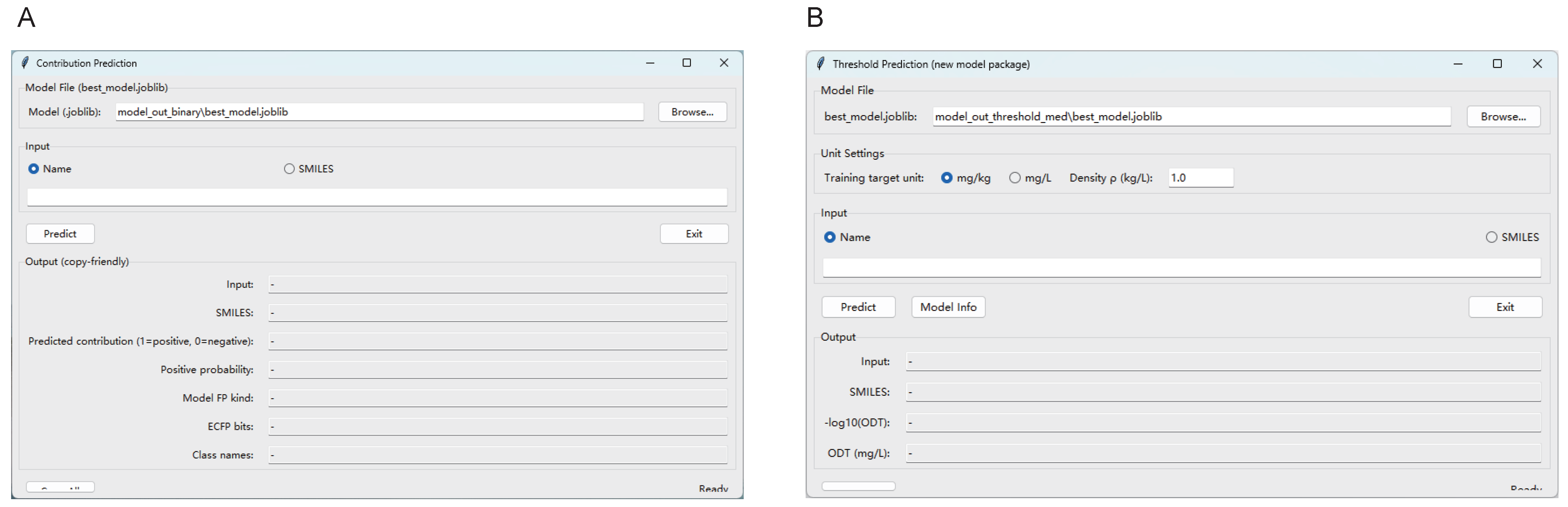
2.7. Model Development and Validation
| RF: |
| clf__n_estimators ∈ {200, 300, 400, 600} |
| clf__max_depth ∈ {10, 20, 30} |
| clf__min_samples_split ∈ {2, 5, 10} |
| clf__min_samples_leaf ∈ {1, 2, 4} |
| clf__max_features ∈ {“sqrt”, “log2”} |
| GBDT (gradient-boosted trees): |
| clf__n_estimators ∈ {300, 600, 900} |
| clf__max_depth ∈ {4, 6, 8} |
| clf__learning_rate ∈ {0.03, 0.05, 0.1} |
| clf__subsample ∈ {0.7, 0.9, 1.0} |
| clf__colsample_bytree ∈ {0.6, 0.8, 1.0} |
| clf__reg_lambda ∈ {0.0, 1.0, 3.0} |
| MLP: |
| clf__hidden_layer_sizes ∈ {(512,128), (256,128), (256,64)} |
| clf__alpha (L2) from 5 log-spaced values between 1 × 10−5 and 1 × 10−3 |
| clf__learning_rate_init from 5 log-spaced values between 1 × 10−4 and 1 × 10−3 |
| clf__batch_size ∈ {128, 256, 512} |
| GCN: |
| hidden dimension ∈ {64, 128, 256}; dropout ∈ {0.1, 0.3, 0.5}; |
| learning rate ∈ {1 × 10−3, 3 × 10−3, 5 × 10−4}; weight decay ∈ {0.0, 1 × 10−4, 5 × 10−4}; |
| batch size ∈ {64, 128, 256}. |
2.8. Serial Ddilution and Sensory Evaluation
3. Results
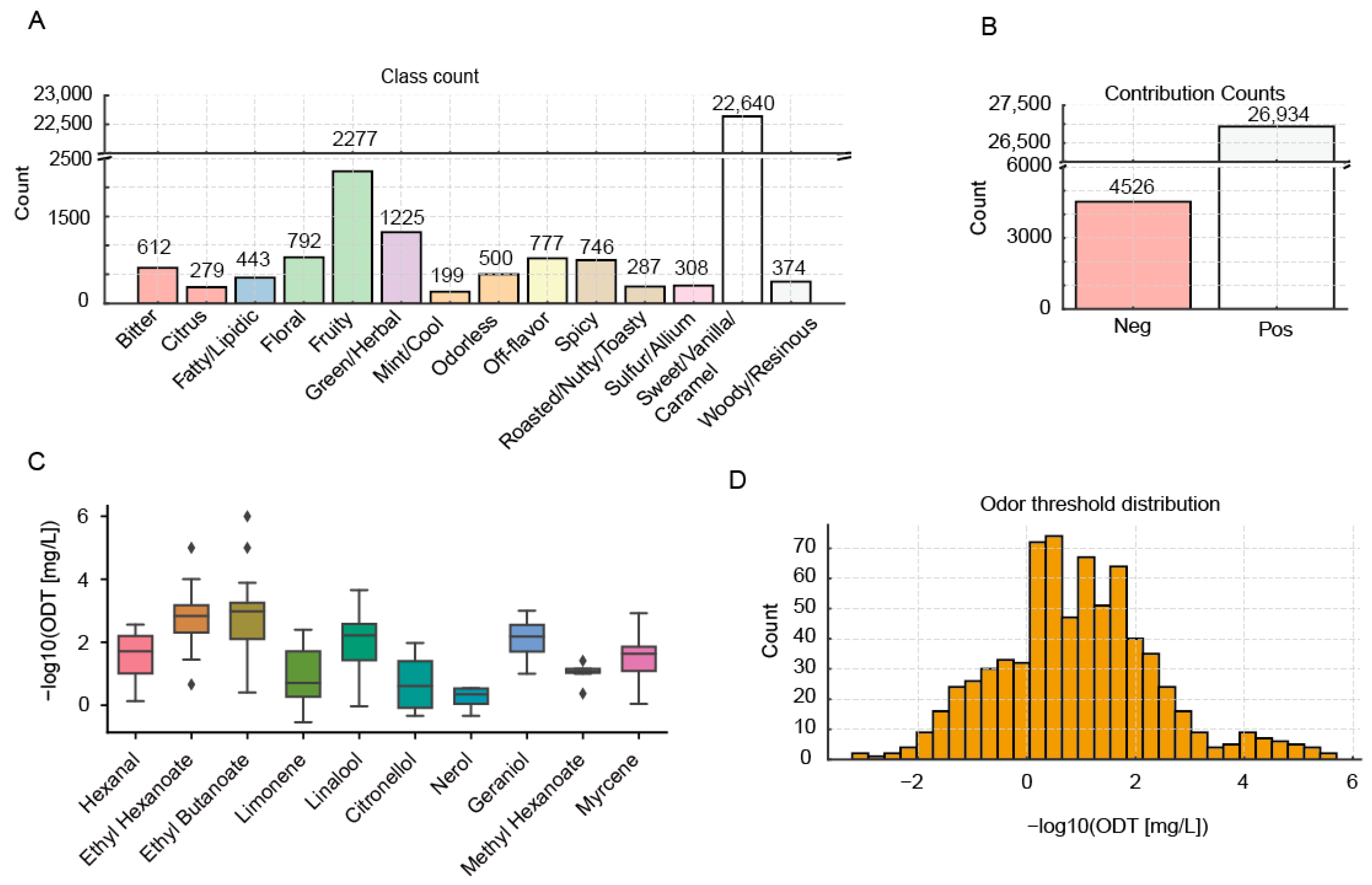
3.1. Data Collection
3.2. Development and Validation of Volatile Aroma Contribution and Odor Thresholds Predictive Model
3.3. Predictive Models Revealed Novel Aroma Compound in Passion Fruit
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, D.; Ma, X.; Xie, Q.; Yu, F. Understanding and engineering of aroma compounds in crops. Seed Biol. 2024, 3, e001. [Google Scholar] [CrossRef]
- Tieman, D.; Zhu, G.; Resende, M.F.R.; Lin, T.; Nguyen, C.; Bies, D.; Rambla, J.L.; Beltran, K.S.O.; Taylor, M.; Zhang, B.; et al. A chemical genetic roadmap to improved tomato flavor. Science 2017, 355, 391. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Hasing, T.; Johnson, T.S.; Garner, D.M.; Schwieterman, M.L.; Barbey, C.R.; Colquhoun, T.A.; Sims, C.A.; Resende, M.F.R.; Whitaker, V.M. Strawberry sweetness and consumer preference are enhanced by specific volatile compounds. Hortic. Res. 2021, 8, 66. [Google Scholar] [CrossRef]
- Renuka, N.; Barvkar, V.T.; Ansari, Z.; Zhao, C.; Wang, C.; Zhang, Y.; Nadaf, A.B. Co-functioning of 2AP precursor amino acids enhances 2-acetyl-1-pyrroline under salt stress in aromatic rice (Oryza sativa L.) cultivars. Sci. Rep. 2022, 12, 3911. [Google Scholar] [CrossRef] [PubMed]
- Pontes, M.; Marques, J.C.; Câmara, J.S. Headspace solid-phase microextraction-gas chromatography-quadrupole mass spectrometric methodology for the establishment of the volatile composition of Passiflora fruit species. Microchem. J. 2009, 93, 1–11. [Google Scholar] [CrossRef]
- Bryant, R.J.; McClung, A.M. Volatile profiles of aromatic and non-aromatic rice cultivars using SPME/GC–MS. Food Chem. 2011, 124, 501–513. [Google Scholar] [CrossRef]
- Bojko, B.; Reyes-Garcés, N.; Bessonneau, V.; Goryński, K.; Mousavi, F.; Souza Silva, E.A.; Pawliszyn, J. Solid-phase microextraction in metabolomics. TrAC Trends Anal. Chem. 2014, 61, 168–180. [Google Scholar] [CrossRef]
- Yuan, H.; Cao, G.; Hou, X.; Huang, M.; Du, P.; Tan, T.; Zhang, Y.; Zhou, H.; Liu, X.; Liu, L.; et al. Development of a widely targeted volatilomics method for profiling volatilomes in plants. Mol. Plant 2022, 15, 189–202. [Google Scholar] [CrossRef]
- Yuan, H.; Jiangfang, Y.; Liu, Z.; Su, R.; Li, Q.; Fang, C.; Huang, S.; Liu, X.; Fernie, A.R.; Luo, J. WTV2.0: A high-coverage plant volatilomics method with a comprehensive selective ion monitoring acquisition mode. Mol. Plant 2024, 17, 972–985. [Google Scholar] [CrossRef]
- Du, Z.; Jin, Y.; Wang, W.; Xia, K.; Chen, Z. Molecular and metabolic insights into floral scent biosynthesis during flowering in Dendrobium chrysotoxum. Front. Plant Sci. 2022, 13, 1030492. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, C.; Xu, K.; Tian, C.; Zhang, M.; Lu, L.; Zhu, C.; Lai, Z.; Guo, Y. A comprehensive investigation of macro-composition and volatile compounds in spring-picked and autumn-picked white tea. Foods 2022, 11, 3628. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Dang, J.; Shi, Z.; Shao, Y.; Sang, M.; Dai, S.; Yue, W.; Liu, C.; Wu, Q. Identification and characterization of a novel gene involved in glandular trichome development in Nepeta. tenuifolia. Front. Plant Sci. 2022, 13, 936244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Huang, S.; Wang, Q.; Shang, B.; Liu, J.; Xing, X.; Hong, Y.; Liu, H.; Duan, X.; Sun, H. Lipidomics and volatilomics reveal the changes in lipids and their volatile oxidative degradation products of brown rice during accelerated aging. Food Chem. 2023, 421, 136157. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Shang, S.; Tian, Y.; Gao, Y.; Song, Z.; Peng, L.; Li, Z.; Wang, B. Integrative analysis of sensory evaluation and non-targeted metabolomics to unravel tobacco leaf metabolites associated with sensory quality of heated tobacco. Front. Plant Sci. 2023, 14, 1123100. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, K.; Lu, Y.; Wu, W.; Wan, R.; Shi, X.; Liu, H.; Sun, Z.; Zhao, X. Analysis of non-volatile and volatile metabolites during Ziziphus jujube leaf black tea processing via widely targeted metabolomics. LWT 2024, 205, 116507. [Google Scholar] [CrossRef]
- Shen, C.; Yu, S.; Tan, X.; Luo, G.; Yu, Z.; Ju, J.; Yang, L.; Huang, Y.; Li, S.; Ji, R.; et al. Infestation of rice striped stem borer (Chilo suppressalis) larvae induces emission of volatile organic compounds in rice and repels female adult oviposition. Int. J. Mol. Sci. 2024, 25, 8827. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, J.; Liu, H.; Wang, Y. Rapid traceability of Gastrodia elata Blume origins and analysis of key volatile organic components using FTIR and HS-SPME-GC–MS combined with chemometrics. Food Chem. X. 2025, 29, 102770. [Google Scholar] [CrossRef]
- Van Gemert, L.J. Odour Thresholds: Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter: Zeist, The Netherlands, 2011. [Google Scholar]
- Nagata, Y.; Takeuchi, N. Measurement of odor threshold by triangle odor bag method. Odor Meas. Rev. 2003, 118, 118–127. [Google Scholar]
- Burlingame, G.A.; Doty, R.L.; Dietrich, A.M. Humans as sensors to evaluate drinking water taste and odor: A review. J.-Am. Water Works Assoc. 2017, 109, 13–24. [Google Scholar] [CrossRef]
- Huang, Y.; Bu, L.; Huang, K.; Zhang, H.; Zhou, S. Predicting odor sensory attributes of unidentified chemicals in water using fragmentation mass spectra with machine learning models. Environ. Sci. Technol. 2024, 58, 11504–11513. [Google Scholar] [CrossRef]
- Keller, A.; Gerkin, R.C.; Guan, Y.; Dhurandhar, A.; Turu, G.; Szalai, B.; Mainland, J.D.; Ihara, Y.; Yu, C.W.; Wolfinger, R.; et al. Predicting human olfactory perception from chemical features of odor molecules. Science 2017, 355, 820–826. [Google Scholar] [CrossRef]
- Shang, L.; Liu, C.; Tomiura, Y.; Hayashi, K. Machine-learning-based olfactometer: Prediction of odor perception from physicochemical features of odorant molecules. Anal. Chem. 2017, 89, 11999–12005. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, R.; Ranjta, S.; Varadwaj, P.K. SMILES to Smell: Decoding the structure–odor relationship of chemical compounds using the deep neural network approach. J. Chem. Inf. Model 2021, 61, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.M.C.; Santana, V.V.; Rodrigues, A.E.; Ribeiro, A.M.; Idelfonso, B.R.N. A framework for predicting odor threshold values of perfumes by scientific machine learning and transfer learning. Heliyon 2023, 9, e20813. [Google Scholar] [CrossRef]
- Huang, Y.; Bu, L.; Zhu, S.; Zhou, S. Integration of nontarget analysis with machine learning modeling for prioritization of odorous volatile organic compounds in surface water. J. Hazard. Mater. 2024, 471, 134367. [Google Scholar] [CrossRef]
- Ji, H.; Pu, D.; Yan, W.; Zhang, Q.; Zuo, M.; Zhang, Y. Recent advances and application of machine learning in food flavor prediction and regulation. Trends Food Sci. Tech. 2023, 138, 738–751. [Google Scholar] [CrossRef]
- Zeng, X.; Cao, R.; Xi, Y.; Li, X.; Yu, M.; Zhao, J.; Cheng, J.; Li, J. Food flavor analysis 4.0: A cross-domain application of machine learning. Trends Food Sci. Tech. 2023, 138, 116–125. [Google Scholar] [CrossRef]
- Arn, H.; Acree, T. Flavornet: A database of aroma compounds based on odor potency in natural products. Dev. Food Sci. 1998, 40, 27–28. [Google Scholar]
- Lee, B.K.; Mayhew, E.J.; Sanchez-Lengeling, B.; Wei, J.N.; Qian, W.W.; Little, K.A.; Andres, M.; Nguyen, B.B.; Moloy, T.; Yasonik, J.; et al. A principal odor map unifies diverse tasks in olfactory perception. Science 2023, 381, 999–1006. [Google Scholar] [CrossRef]
- Hamel, E.A.; Castro, J.B.; Gould, T.J.; Pellegrino, R.; Liang, Z.; Coleman, L.A.; Patel, F.; Wallace, D.S.; Bhatnagar, T.; Mainland, J.D.; et al. Pyrfume: A window to the world’s olfactory data. Sci. Data 2024, 11, 1220. [Google Scholar] [CrossRef]
- Ollitrault, G.; Achebouche, R.; Dreux, A.; Murail, S.; Audouze, K.; Tromelin, A.; Taboureau, O. Pred-O3, a web server to predict molecules, olfactory receptors and odor relationships. Nucleic Acids Res. 2024, 52, W507–W512. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef]

| Concentration (mg/L) | Detected (Yes/Total n) | Descriptors (Term: Count) | Intensity (Mean ± SD) |
|---|---|---|---|
| acetic acid, 2-phenylethyl ester | |||
| 0 | 0/20 | None: 0 | 0.00 ± 0.00 |
| 0.1 | 6/20 | sweet: 3; floral: 2; alcoholic: 1; fruity: 1; rose: 1; woody: 1 | 0.47 ± 0.77 |
| 1 | 18/20 | floral: 10; cooling: 3; sweet: 3; minty: 2; rose: 2; alcoholic: 1; fermented: 1; fruity: 1; honey: 1; leafy: 1; sour: 1; woody: 1 | 1.82 ± 0.88 |
| 10 | 20/20 | floral: 14; sweet: 5; alcoholic: 1; cooling: 1; fruity: 1; honey: 1; minty: 1; rose: 1; woody: 1 | 2.49 ± 0.81 |
| 100 | 20/20 | floral: 15; sweet: 7; fruity: 4; rose: 4; alcoholic: 1; honey: 1; minty: 1 | 3.77 ± 0.53 |
| 1000 | 20/20 | floral: 15; sweet: 7; fruity: 4; rose: 3; minty: 2; fermented: 1; honey: 1; pungent: 1 | 4.55 ± 0.60 |
| menthyl acetate | |||
| 0 | 0/20 | None: 0 | 0.00 ± 0.00 |
| 0.01 | 2/20 | grassy: 1; sweet: 1 | 0.10 ± 0.31 |
| 0.1 | 5/20 | grassy: 3; cooling: 1; honey: 1; minty: 1; other: 1 | 0.30 ± 0.55 |
| 1 | 10/20 | grassy: 5; leaf: 2; other: 2; cooling: 1; honey: 1; leafy: 1; minty: 1 | 0.72 ± 0.87 |
| 10 | 18/20 | grassy: 9; leaf: 8; fruity: 5; cooling: 2; honey: 1 | 2.06 ± 0.96 |
| 100 | 20/20 | grassy: 17; leaf: 13; cooling: 9; other: 4; floral: 1; honey: 1; sour: 1 | 3.25 ± 0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Li, S.; Luo, J.; Yuan, H. Predictive Models of Odor Contribution and Thresholds for Volatiles in Identification of Novel Crop Aroma Compounds. Metabolites 2025, 15, 747. https://doi.org/10.3390/metabo15110747
Li Q, Li S, Luo J, Yuan H. Predictive Models of Odor Contribution and Thresholds for Volatiles in Identification of Novel Crop Aroma Compounds. Metabolites. 2025; 15(11):747. https://doi.org/10.3390/metabo15110747
Chicago/Turabian StyleLi, Qiao, Shaofang Li, Jie Luo, and Honglun Yuan. 2025. "Predictive Models of Odor Contribution and Thresholds for Volatiles in Identification of Novel Crop Aroma Compounds" Metabolites 15, no. 11: 747. https://doi.org/10.3390/metabo15110747
APA StyleLi, Q., Li, S., Luo, J., & Yuan, H. (2025). Predictive Models of Odor Contribution and Thresholds for Volatiles in Identification of Novel Crop Aroma Compounds. Metabolites, 15(11), 747. https://doi.org/10.3390/metabo15110747





