Serum Extracellular Vesicles as Pathogenetic Signals in Obese and Lean Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Samples
2.2. Laboratory Assessments
2.3. Chemicals, Reagents, and Antibodies
2.4. Serum EV Isolation via Iodixanol Density Gradient Ultracentrifugation
2.5. Nanoparticle Tracking Analysis (NTA)
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
3.1. Distinct Clinical Examination and Blood Test Profiling in Obese and Lean MASLD
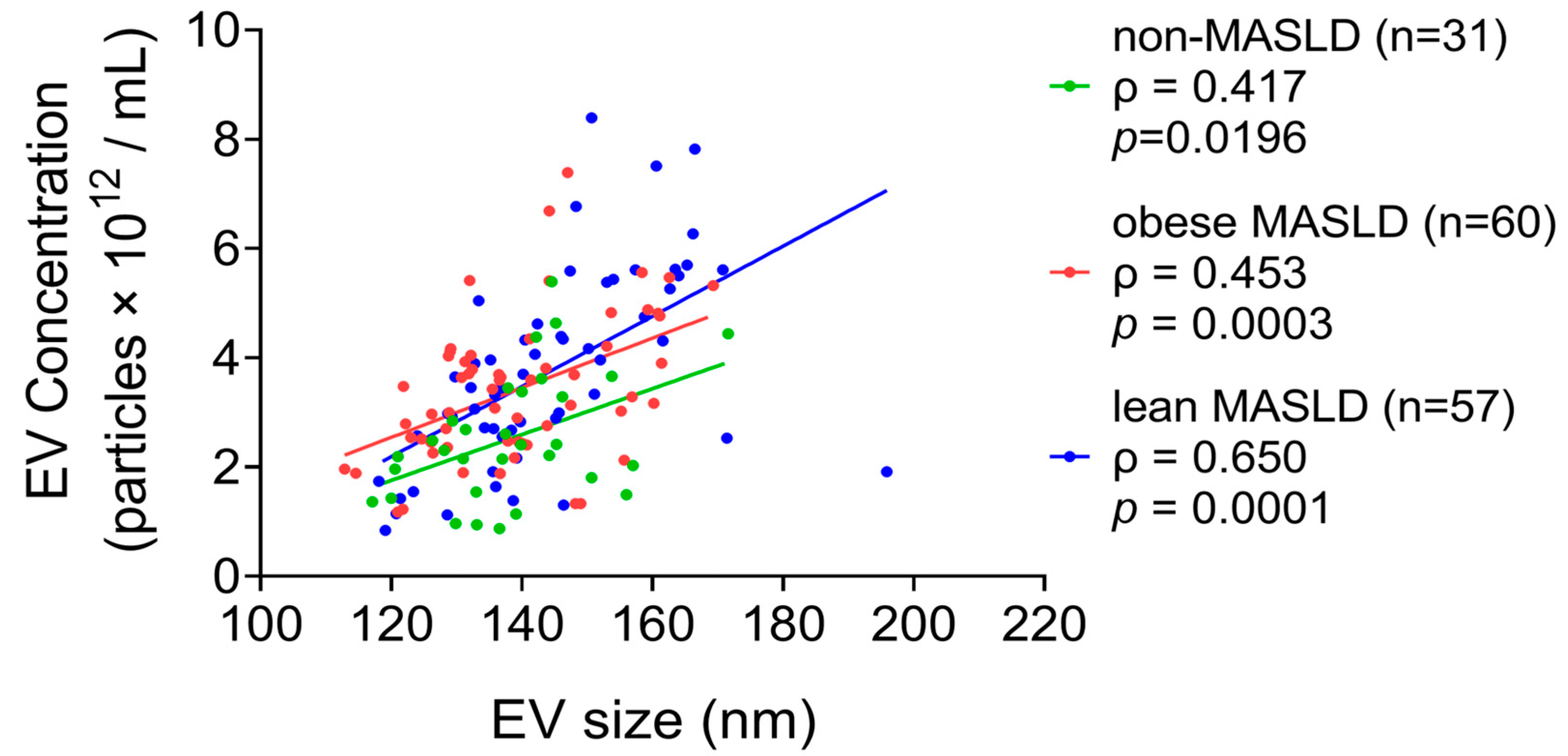
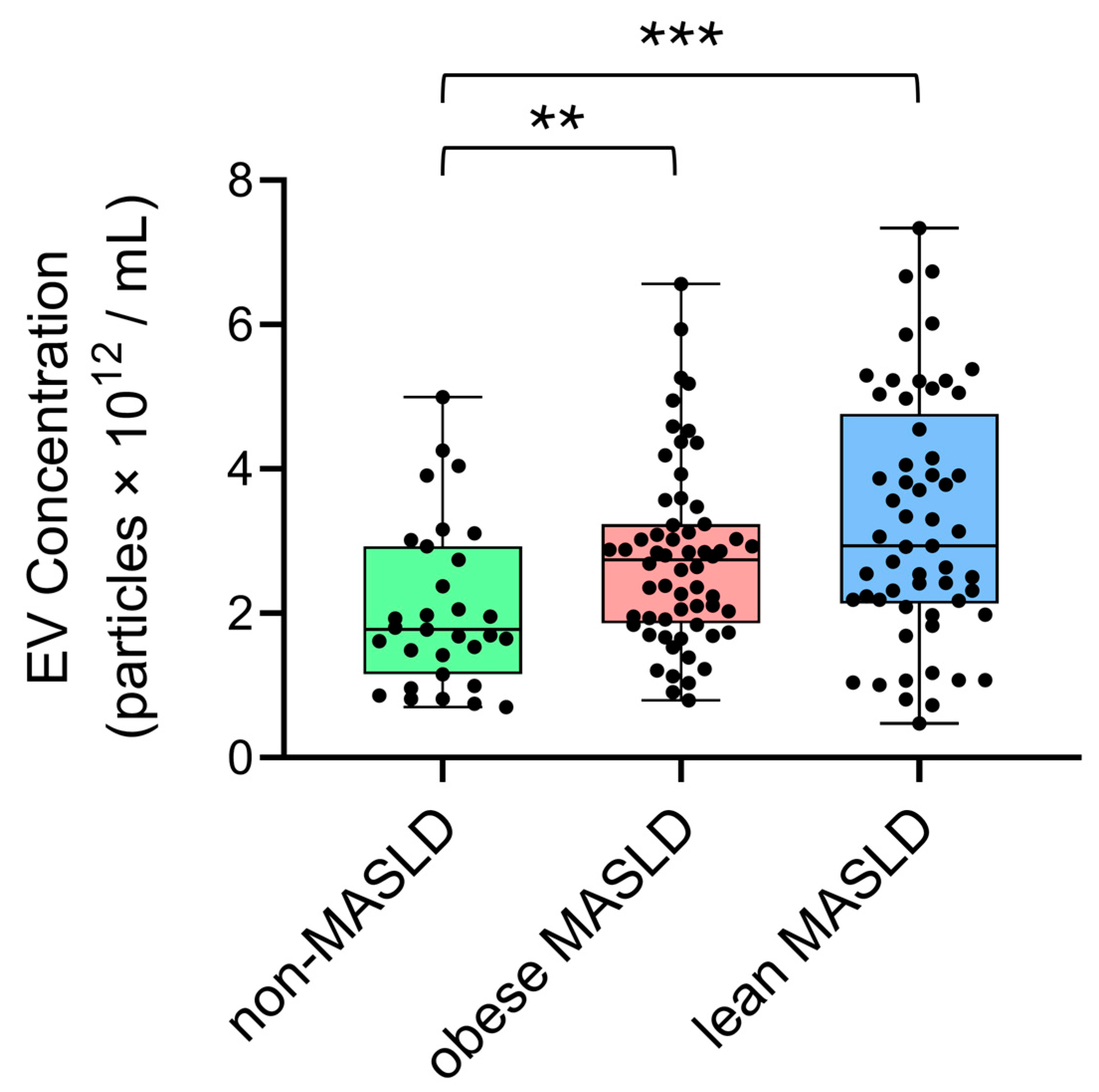
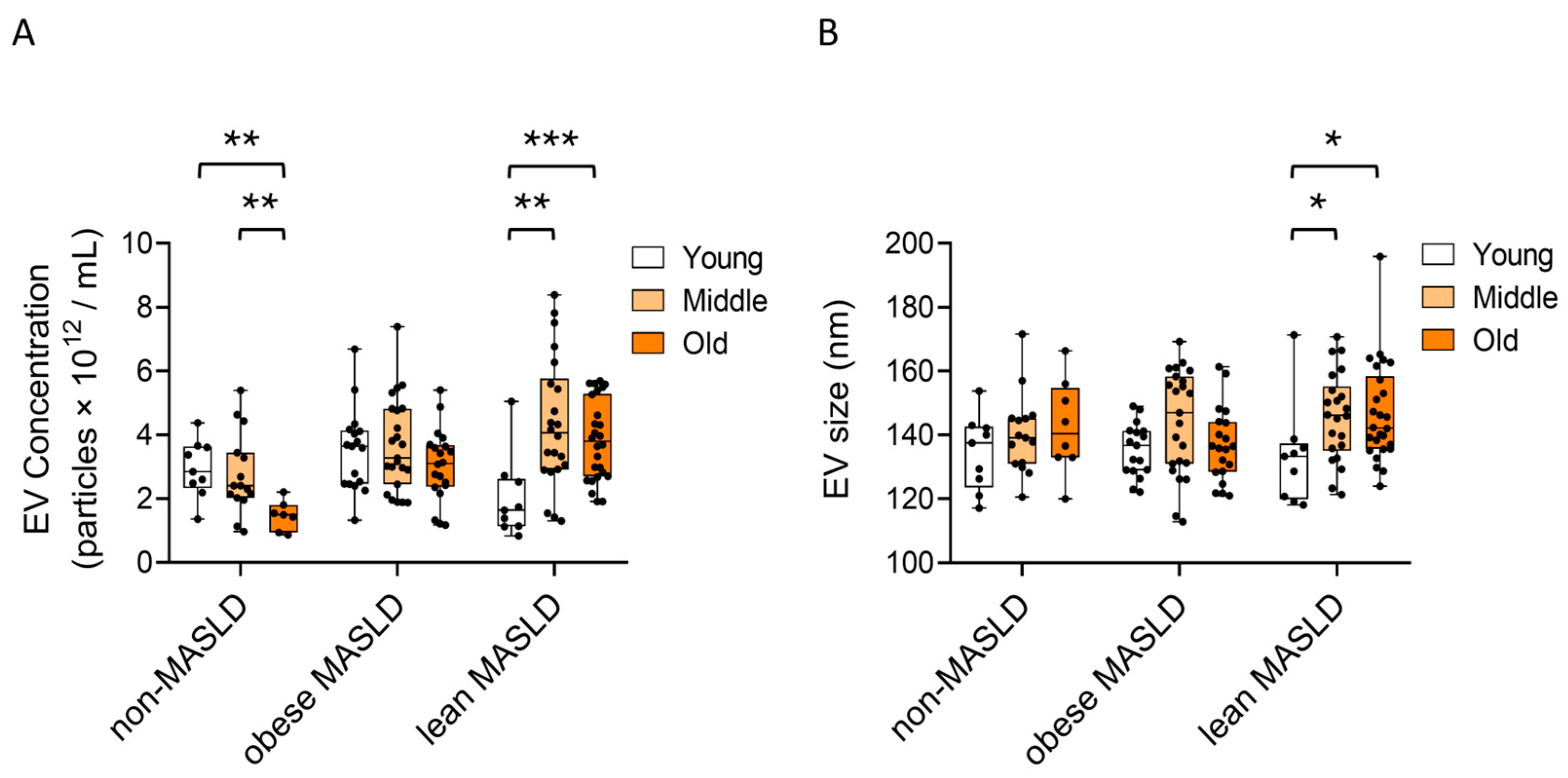
3.2. Elevated Circulating EV Concentrations in Both Lean and Obese MASLD
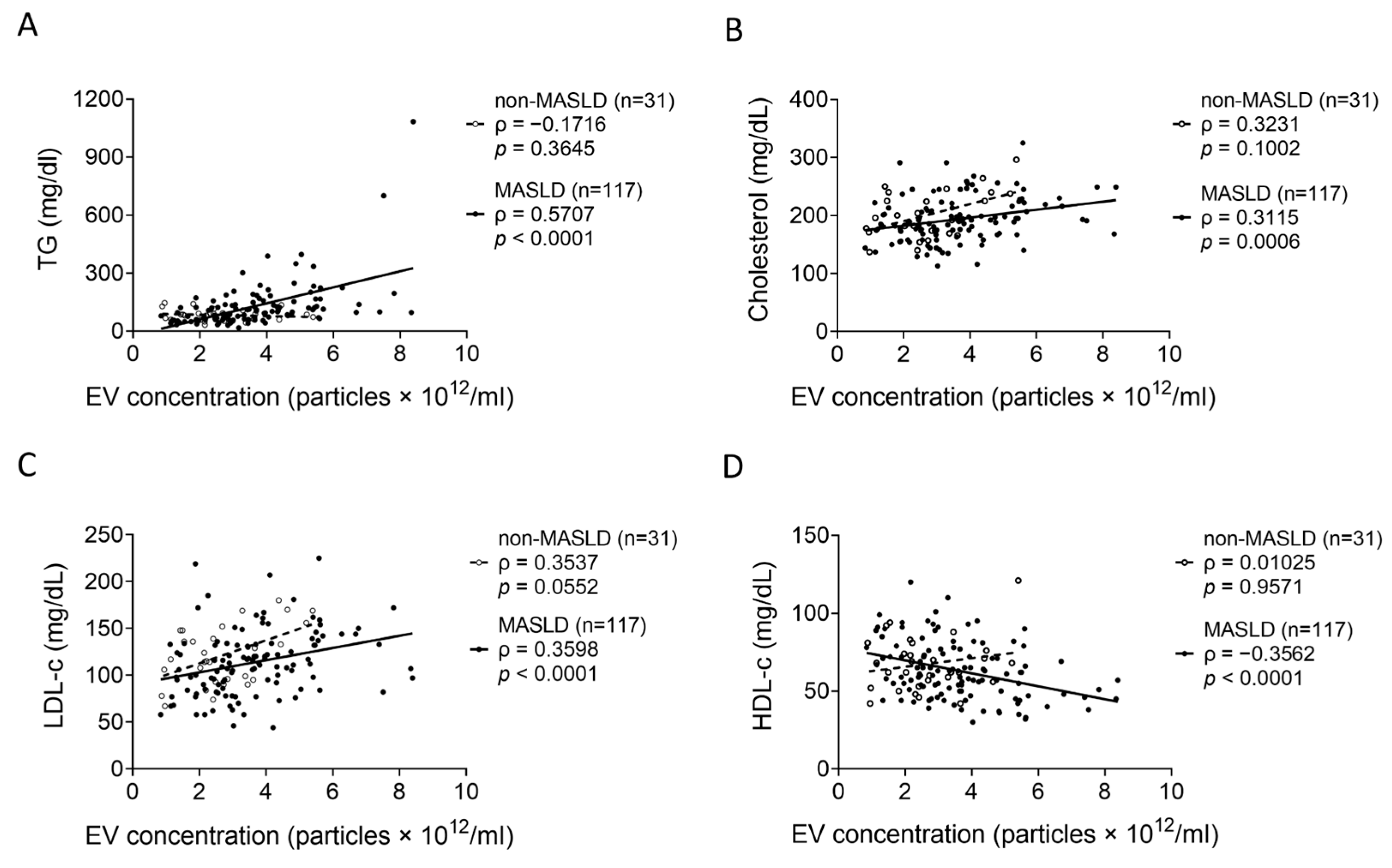
3.3. Correlations Between Serum EV Levels and Lipid Parameters in MASLD
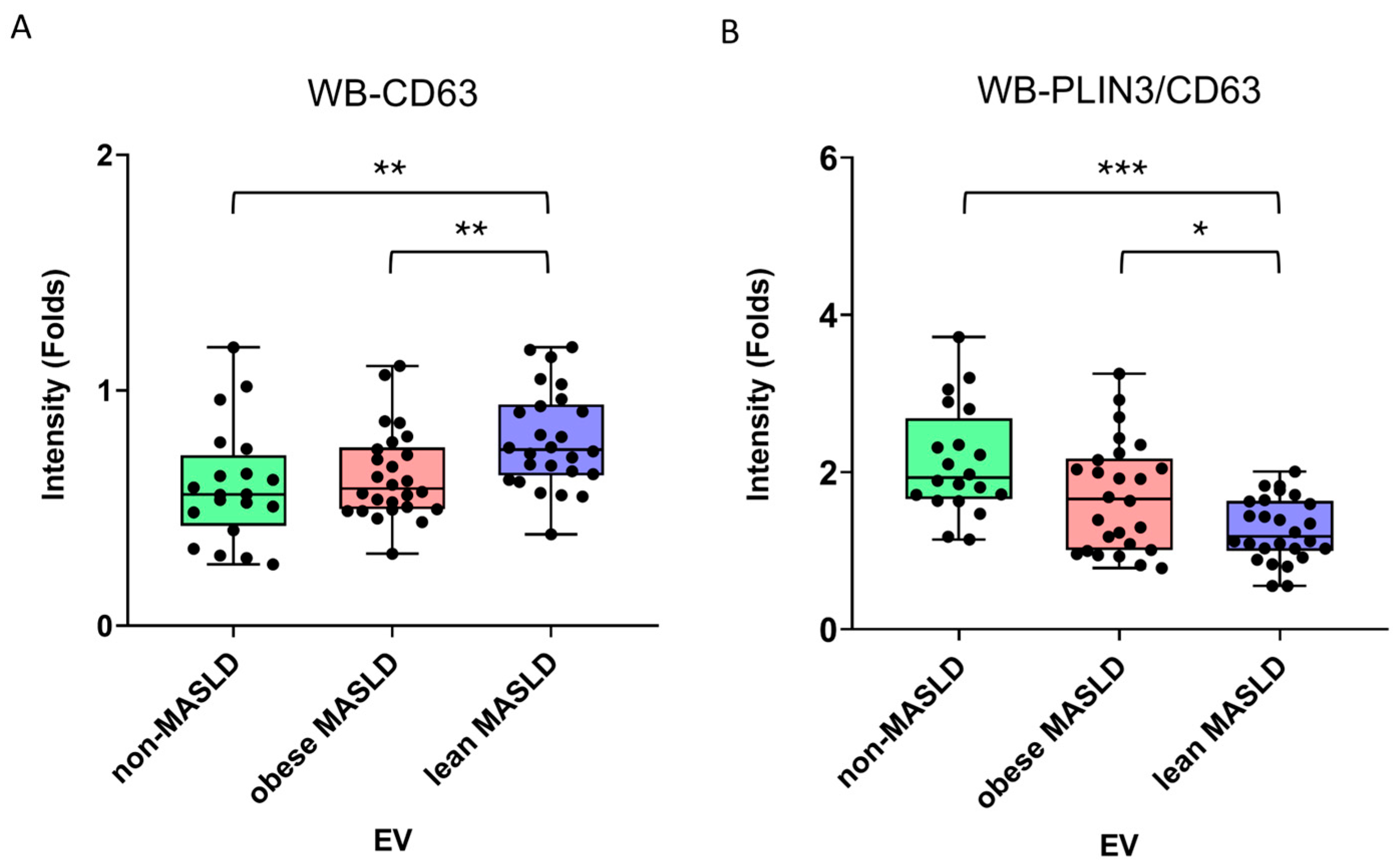
3.4. Decreasing PLIN3/CD63 Ratio in Serum EVs in Lean MASLD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| DBP | diastolic blood pressure |
| EVs | extracellular vesicles |
| GOT | glutamate oxaloacetate transaminase |
| GPT | glutamate pyruvate transaminase |
| HbA1c | hemoglobin A1c |
| HDL-c | high-density lipoprotein cholesterol |
| HOMA-IR | homeostasis model assessment of insulin resistance |
| HOMA-β | homeostasis model assessment of β-cell function |
| HRP | horseradish peroxidase |
| hsCRP | high-sensitivity C-reactive protein |
| LDL | low-density lipoprotein |
| LDs | lipid droplets |
| MASLD | metabolic dysfunction-associated steatotic liver disease |
| NTA | nanoparticle tracking analysis |
| PLIN | perilipin |
| PNPLA3 | patatin-like phospholipase domain-containing protein 3 |
| SBP | systolic blood pressure |
| T2DM | type 2 diabetes mellitus |
| TG | triglyceride |
| THR-β | thyroid hormone receptor beta |
| TM6SF2 | transmembrane 6 superfamily member 2 |
| VLDL | very-low-density lipoprotein |
| WBC | white blood cell |
References
- Gofton, C.; Upendran, Y.; Zheng, M.H.; George, J. MAFLD: How is it different from NAFLD? Clin. Mol. Hepatol. 2023, 29, S17–S31. [Google Scholar] [CrossRef]
- Sangro, P.; de la Torre Aláez, M.; Sangro, B.; D’Avola, D. Metabolic dysfunction-associated fatty liver disease (MAFLD): An update of the recent advances in pharmacological treatment. J. Physiol. Biochem. 2023, 79, 869–879. [Google Scholar] [CrossRef]
- Boccatonda, A.; Andreetto, L.; D’Ardes, D.; Cocco, G.; Rossi, I.; Vicari, S.; Schiavone, C.; Cipollone, F.; Guagnano, M.T. From NAFLD to MAFLD: Definition, Pathophysiological Basis and Cardiovascular Implications. Biomedicines 2023, 11, 883. [Google Scholar] [CrossRef]
- Yuan, Q.; Wang, H.; Gao, P.; Chen, W.; Lv, M.; Bai, S.; Wu, J. Prevalence and Risk Factors of Metabolic-Associated Fatty Liver Disease among 73,566 Individuals in Beijing, China. Int. J. Environ. Res. Public Health 2022, 19, 2096. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Yang, C.; Yang, S.; Xu, W.; Zhang, J.; Fu, W.; Feng, C. Association between the hyperuricemia and nonalcoholic fatty liver disease risk in a Chinese population: A retrospective cohort study. PLoS ONE 2017, 12, e0177249. [Google Scholar] [CrossRef]
- Ghazanfar, H.; Javed, N.; Qasim, A.; Zacharia, G.S.; Ghazanfar, A.; Jyala, A.; Shehi, E.; Patel, H. Metabolic Dysfunction-Associated Steatohepatitis and Progression to Hepatocellular Carcinoma: A Literature Review. Cancers 2024, 16, 1214. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Taub, R.; Neff, G.W.; Lucas, K.J.; Labriola, D.; Moussa, S.E.; Alkhouri, N.; Bashir, M.R. Resmetirom for nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled phase 3 trial. Nat. Med. 2023, 29, 2919–2928. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.K. Comparison between obese and non-obese nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2023, 29, S58–S67. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiang, L.; Qi, F.; Cao, Y.; Zhang, W.; Lv, T.; Zhou, X. The metabolic profiles and body composition of non-obese metabolic associated fatty liver disease. Front. Endocrinol. 2024, 15, 1322563. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xia, B.X.; Luo, T.; Wang, P. Association between physical activity and diet quality of obese and non-obese MAFLD. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 75–89. [Google Scholar] [CrossRef]
- WHO expert consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.; Shaw, J.; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Kim, D.; Kim, W.R. Nonobese fatty liver disease. Clin. Gastroenterol. Hepatol. 2017, 15, 474–485. [Google Scholar] [CrossRef]
- Eslam, M.; El-Serag, H.B.; Francque, S.; Sarin, S.K.; Wei, L.; Bugianesi, E.; George, J. Metabolic (dysfunction)-associated fatty liver disease in individuals of normal weight. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, Z.; Qin, H. Examining the pathogenesis of MAFLD and the medicinal properties of natural products from a metabolic perspective. Metabolites 2024, 14, 218. [Google Scholar] [CrossRef]
- Filipovic, B.; Marjanovic-Haljilji, M.; Mijac, D.; Lukic, S.; Kapor, S.; Kapor, S.; Starcevic, A.; Popovic, D.; Djokovic, A. Molecular Aspects of MAFLD-New Insights on Pathogenesis and Treatment. Curr. Issues Mol. Biol. 2023, 45, 9132–9148. [Google Scholar] [CrossRef]
- Zadoorian, A.; Du, X.; Yang, H. Lipid droplet biogenesis and functions in health and disease. Nat. Rev. Endocrinol. 2023, 19, 443–459. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Perilipins: A family of five fat-droplet storing proteins that play a significant role in fat homeostasis. J. Cell Biochem. 2024, 125, e30579. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, D.; Li, Y. Extracellular vesicles in pathogenesis and treatment of metabolic associated fatty liver disease. Front. Physiol. 2022, 13, 909518. [Google Scholar] [CrossRef]
- Koenen, M.T.; Brandt, E.F.; Kaczor, D.M.; Caspers, T.; Heinzmann, A.C.A.; Fischer, P.; Heinrichs, D.; Wirtz, T.H.; Trautwein, C.; Koenen, R.R.; et al. Extracellular Vesicles from Steatotic Hepatocytes Provoke Pro-Fibrotic Responses in Cultured Stellate Cells. Biomolecules 2022, 12, 698. [Google Scholar] [CrossRef]
- Urabe, F.; Kosaka, N.; Ito, K.; Kimura, T.; Egawa, S.; Ochiya, T. Extracellular vesicles as biomarkers and therapeutic targets for cancer. Am. J. Physiol. Cell Physiol. 2020, 318, C29–C39. [Google Scholar] [CrossRef]
- Marar, C.; Starich, B.; Wirtz, D. Extracellular vesicles in immunomodulation and tumor progression. Nat. Immunol. 2021, 22, 560–570. [Google Scholar] [CrossRef]
- Sheta, M.; Taha, E.A.; Lu, Y.; Eguchi, T. Extracellular Vesicles: New Classification and Tumor Immunosuppression. Biology 2023, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Yu, L.; Ma, T.; Xu, W.; Qian, H.; Sun, Y.; Shi, H. Small extracellular vesicles isolation and separation: Current techniques, pending questions and clinical applications. Theranostics 2022, 12, 6548–6575. [Google Scholar] [CrossRef]
- Fyfe, J.; Dye, D.; Razak, N.B.A.; Metharom, P.; Falasca, M. Immune evasion on the nanoscale: Small extracellular vesicles in pancreatic ductal adenocarcinoma immunity. Semin. Cancer Biol. 2023, 96, 36–47. [Google Scholar] [CrossRef]
- Pols, M.S.; Klumperman, J. Trafficking and function of the tetraspanin CD63. Exp. Cell Res. 2009, 315, 1584–1592. [Google Scholar] [CrossRef]
- Van Niel, G.; Charrin, S.; Simoes, S.; Romao, M.; Rochin, L.; Saftig, P.; Marks, M.S.; Rubinstein, E.; Raposo, G. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev. Cell 2011, 21, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Dixson, A.C.; Dawson, T.R.; Di Vizio, D.; Weaver, A.M. Context-specific regulation of extracellular vesicle biogenesis and cargo selection. Nat. Rev. Mol. Cell Biol. 2023, 24, 454–476. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, S.N.; Cheerathodi, M.R.; Nkosi, D.; York, S.B.; Meckes, D.G., Jr. Tetraspanin CD63 Bridges Autophagic and Endosomal Processes To Regulate Exosomal Secretion and Intracellular Signaling of Epstein-Barr Virus LMP1. J. Virol. 2018, 92, e01969-17. [Google Scholar] [CrossRef]
- Palmulli, R.; Couty, M.; Piontek, M.C.; Ponnaiah, M.; Dingli, F.; Verweij, F.J.; Charrin, S.; Tantucci, M.; Sasidharan, S.; Rubinstein, E.; et al. CD63 sorts cholesterol into endosomes for storage and distribution via exosomes. Nat. Cell Biol. 2024, 26, 1093–1109. [Google Scholar] [CrossRef]
- Yuana, Y.; Sturk, A.; Nieuwland, R. Extracellular vesicles in physiological and pathological conditions. Blood Rev. 2013, 27, 31–39. [Google Scholar] [CrossRef]
- Long, M.T.; Noureddin, M.; Lim, J.K. AGA clinical practice update: Diagnosis and management of nonalcoholic fatty liver disease in lean individuals: Expert review. Gastroenterology 2022, 163, 764–774.e1. [Google Scholar] [CrossRef]
- Sun, H.Y.; Chen, S.F.; Lai, M.D.; Chang, T.T.; Chen, T.L.; Li, P.Y.; Shieh, D.B.; Young, K.C. Comparative proteomic profiling of plasma very-low-density and low-density lipoproteins. Clin. Chim. Acta 2010, 411, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Muthiah, M.; Ng, C.H.; Chan, K.E.; Fu, C.E.; Lim, W.H.; Tan, D.J.H.; Nah, B.; Kong, G.; Xiao, J.; Yong, J.N.; et al. Type 2 diabetes mellitus in metabolic-associated fatty liver disease vs. type 2 diabetes mellitus non-alcoholic fatty liver disease: A longitudinal cohort analysis. Ann. Hepatol. 2023, 28, 100762. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.D.; Cai, J.; Targher, G.; Byrne, C.D.; Shapiro, M.D.; Sung, K.C.; Somers, V.K.; Chahal, C.A.A.; George, J.; Chen, L.L.; et al. Metabolic dysfunction-associated fatty liver disease and implications for cardiovascular risk and disease prevention. Cardiovasc. Diabetol. 2022, 21, 270. [Google Scholar] [CrossRef]
- Ahadi, M.; Molooghi, K.; Masoudifar, N.; Namdar, A.B.; Vossoughinia, H.; Farzanehfar, M. A review of non-alcoholic fatty liver disease in non-obese and lean individuals. J. Gastroenterol. Hepatol. 2021, 36, 1497–1507. [Google Scholar] [CrossRef]
- Phipps, M.; Wattacheril, J. Non-alcoholic fatty liver disease (NAFLD) in non-obese individuals. Frontline Gastroenterol. 2020, 11, 478–483. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Albhaisi, S.; Chowdhury, A.; Sanyal, A.J. Non-alcoholic fatty liver disease in lean individuals. JHEP Rep. 2019, 1, 329–341. [Google Scholar] [CrossRef]
- Zou, B.; Yeo, Y.H.; Nguyen, V.H.; Cheung, R.; Ingelsson, E.; Nguyen, M.H. Prevalence, characteristics and mortality outcomes of obese, nonobese and lean NAFLD in the United States, 1999–2016. J. Intern. Med. 2020, 288, 139–151. [Google Scholar] [CrossRef]
- Hagström, H.; Nasr, P.; Ekstedt, M.; Hammar, U.; Stål, P.; Hultcrantz, R.; Kechagias, S. Risk for development of severe liver disease in lean patients with nonalcoholic fatty liver disease: A long-term follow-up study. Hepatol. Commun. 2017, 2, 48–57. [Google Scholar] [CrossRef]
- Wongtrakul, W.; Charatcharoenwitthaya, N.; Charatcharoenwitthaya, P. Lean non-alcoholic fatty liver disease and the risk of all-cause mortality: An updated meta-analysis. Ann. Hepatol. 2024, 29, 101288. [Google Scholar] [CrossRef]
- Eitan, E.; Green, J.; Bodogai, M.; Mode, N.A.; Bæk, R.; Jørgensen, M.M.; Freeman, D.W.; Witwer, K.W.; Zonderman, A.B.; Biragyn, A.; et al. Age-Related Changes in Plasma Extracellular Vesicle Characteristics and Internalization by Leukocytes. Sci. Rep. 2017, 7, 1342. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Eguchi, A.; Tempaku, M.; Honda, T.; Togashi, K.; Iwasa, M.; Hasegawa, H.; Takei, Y.; Sumida, Y.; Taguchi, O. Circulating extracellular vesicles are associated with lipid and insulin metabolism. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E574–E582. [Google Scholar] [CrossRef]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in extracellular vesicle formation and function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Jankovičová, J.; Sečová, P.; Michalková, K.; Antalíková, J. Tetraspanins, More than Markers of Extracellular Vesicles in Reproduction. Int. J. Mol. Sci. 2020, 21, 7568. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, W.; Fu, R.; Ma, Z.; Hu, Y.; Liu, Y.; Ding, Z. Endoplasmic reticulum stress increases exosome biogenesis and packaging relevant to sperm maturation in response to oxidative stress in obese mice. Reprod. Biol. Endocrinol. 2022, 20, 161. [Google Scholar] [CrossRef]
- Chettimada, S.; Lorenz, D.R.; Misra, V.; Dillon, S.T.; Reeves, R.K.; Manickam, C.; Morgello, S.; Kirk, G.D.; Mehta, S.H.; Gabuzda, D. Exosome markers associated with immune activation and oxidative stress in HIV patients on antiretroviral therapy. Sci. Rep. 2018, 8, 7227. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef]
- Palumbos, S.D.; Popolow, J.; Goldsmith, J.; Holzbaur, E.L.F. Autophagic stress activates distinct compensatory secretory pathways in neurons. Proc. Natl. Acad. Sci. USA 2025, 122, e2421886122. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.K.; Ngo, J.M.; Lehman, I.M.; Schekman, R. Annexin A6 mediates calcium-dependent exosome secretion during plasma membrane repair. eLife 2023, 12, e86556. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.H.; Chen, E.; Li, L.; Saha, P.; Lee, H.J.; Huang, L.S.; Shelness, G.S.; Chan, L.; Chang, B.H.J. The constitutive lipid droplet protein PLIN2 regulates autophagy in liver. Autophagy 2017, 13, 1130–1144. [Google Scholar] [CrossRef] [PubMed]
- McManaman, J.L.; Bales, E.S.; Orlicky, D.J.; Jackman, M.; MacLean, P.S.; Cain, S.; Crunk, A.E.; Mansur, A.; Graham, C.E.; Bowman, T.A.; et al. Perilipin-2-null mice are protected against diet-induced obesity, adipose inflammation, and fatty liver disease. J. Lipid Res. 2013, 54, 1346–1359. [Google Scholar] [CrossRef]
- Najt, C.P.; Lwande, J.S.; McIntosh, A.L.; Senthivinayagam, S.; Gupta, S.; Kuhn, L.A.; Atshaves, B.P. Structural and functional assessment of perilipin 2 lipid binding domain(s). Biochemistry 2014, 53, 7051–7066. [Google Scholar] [CrossRef] [PubMed]
- Sztalryd, C.; Brasaemle, D.L. The perilipin family of lipid droplet proteins: Gatekeepers of intracellular lipolysis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1221–1232. [Google Scholar] [CrossRef]
- Itabe, H.; Yamaguchi, T.; Nimura, S.; Sasabe, N. Perilipins: A diversity of intracellular lipid droplet proteins. Lipids Health Dis. 2017, 16, 83. [Google Scholar] [CrossRef]
- Bulankina, A.V.; Deggerich, A.; Wenzel, D.; Mutenda, K.; Wittmann, J.G.; Rudolph, M.G.; Burger, K.N.J.; Höning, S. TIP47 functions in the biogenesis of lipid droplets. J. Cell Biol. 2009, 185, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Nose, F.; Yamaguchi, T.; Kato, R.; Aiuchi, T.; Obama, T.; Hara, S.; Yamamoto, M.; Itabe, H. Crucial role of perilipin-3 (TIP47) in formation of lipid droplets and PGE2 production in HL-60-derived neutrophils. PLoS ONE 2013, 8, e71542. [Google Scholar] [CrossRef]
- Choi, Y.M.; Ajjaji, D.; Fleming, K.D.; Borbat, P.P.; Jenkins, M.L.; Moeller, B.E.; Fernando, S.; Bhatia, S.R.; Freed, J.H.; Burke, J.E.; et al. Structural insights into perilipin 3 membrane association in response to diacylglycerol accumulation. Nat. Commun. 2023, 14, 3204. [Google Scholar] [CrossRef]
- Khaddaj, R.; Stribny, J.; Cottier, S.; Schneiter, R. Perilipin 3 promotes the formation of membrane domains enriched in diacylglycerol and lipid droplet biogenesis proteins. Front. Cell Dev. Biol. 2023, 11, 1116491. [Google Scholar] [CrossRef]
- Gurjar, S.; Bhat, A.R.; Upadhya, R.; Shenoy, R.P. Extracellular vesicle-mediated approaches for the diagnosis and therapy of MASLD: Current advances and future prospective. Lipids Health Dis. 2025, 24, 5. [Google Scholar] [CrossRef]
- Boonkaew, B.; Charoenthanakitkul, D.; Suntornnont, N.; Ariyachet, C.; Tangkijvanich, P. Extracellular vesicles in metabolic dysfunction-associated steatotic liver disease: From intercellular signaling to clinical translation. World J. Hepatol. 2025, 17, 108259. [Google Scholar] [CrossRef] [PubMed]
- Keingeski, M.B.; Longo, L.; Brum da Silva Nunes, V.; Figueiró, F.; Dallemole, D.R.; Pohlmann, A.R.; Vier Schmitz, T.M.; da Costa Lopez, P.L.; Álvares-da-Silva, M.R.; Uribe-Cruz, C. Extracellular Vesicles and Their Correlation with Inflammatory Factors in an Experimental Model of Steatotic Liver Disease Associated with Metabolic Dysfunction. Metab. Syndr. Relat. Disord. 2024, 22, 394–401. [Google Scholar] [CrossRef] [PubMed]

| Non-MASLD (n = 57) | Obese MASLD (n = 83) | Lean MASLD (n = 87) | p-Value * | |
|---|---|---|---|---|
| Gender (F/M) | 42/15 | 40/43 | 55/32 | |
| Age (yr) | 44.00 (32.00–62.00) | 50.00 (38.00–65.00) | 49.50 (36.00–65.00) | |
| BMI (kg/m2) | 22.17 (20.41–23.87) | 26.04 (24.57–28.57) | 21.78 (20.22–22.59) | AC |
| Waist circumference (cm) | 79.00 (74.00–85.00) | 92.00 (84.00–98.50) | 83.00 (80.25–91.25) | ABC |
| SBP (mmHg) | 125.00 (112.00–135.00) | 137.50 (126.00–145.00) | 140.00 (134.50–144.50) | AB |
| DBP (mmHg) | 75.00 (68.00–80.00) | 83.00 (75.00–88.00) | 86.00 (76.50–88.00) | AB |
| GOT (IU/L) | 20.00 (17.00–24.00) | 23.00 (19.00–28.00) | 21.00 (18.00–26.00) | A |
| GPT (IU/L) | 18.00 (13.50–23.50) | 22.00 (17.00–34.50) | 19.50 (14.50–24.50) | AC |
| ALP (IU/L) | 166.50 (148.50–200.50) | 190.00 (158.00–237.00) | 186.00 (158.50–233.00) | |
| T-Bil (mg/dL) | 0.595 (0.455–0.780) | 0.640 (0.440–0.790) | 0.605 (0.425–0.805) | |
| D-Bil (mg/dL) | 0.160 (0.105–0.210) | 0.165 (0.120–0.215) | 0.160 (0.110–0.205) | |
| Albumin (g/dL) | 4.70 (4.30–4.85) | 4.60 (4.50–4.80) | 4.70 (4.40–4.80) | |
| Creatinine (mg/dL) | 0.650 (0.600–0.825) | 0.780 (0.670–0.920) | 0.695 (0.610–0.885) | AC |
| Uric acid (mg/dL) | 5.00 (4.30–5.95) | 5.80 (4.75–6.70) | 5.10 (4.40–5.80) | C |
| Cholesterol (mg/dL) | 190.00 (169.00–216.00) | 184.00 (167.00–207.50) | 198.00 (174.00–217.00) | |
| TG (mg/dL) | 79.50 (58.00–103.00) | 108.00 (71.50–167.50) | 77.00 (50.50–113.50) | AC |
| HDL-c (mg/dL) | 62.00 (57.00–74.00) | 56.00 (45.00–65.00) | 66.00 (57.00–82.00) | AC |
| LDL-c (mg/dL) | 113.00 (94.00–136.00) | 113.00 (95.00–132.00) | 115.00 (90.50–134.00) | |
| HbA1c (%) | 5.50 (530–5.80) | 5.60 (5.40–5.85) | 5.50 (5.30–5.80) | AC |
| Glucose (mg/dL) | 89.00 (81.50–96.50) | 91.00 (84.50–97.50) | 89.00 (85.00–100.00) | |
| Insulin (μIU/mL) | 4.85 (3.20–7.90) | 5.95 (4.60–8.55) | 3.80 (2.65–5.70) | C |
| HOMA-IR | 1.00 (0.67–1.74) | 1.36 (0.99–1.78) | 0.84 (0.57–1.37) | C |
| HOMA-beta | 72.00 (50.40–105.33) | 84.96 (61.09–117.14) | 51.97 (32.77–77.02) | C |
| WBC (μL) | 6500 (5400–8230) | 6800 (5860–8130) | 6050 (5240–7250) | C |
| Lymphocytes (%) | 32.60 (24.90–36.70) | 33.50 (27.70–39.00) | 31.40 (26.65–36.10) | |
| Neutrophils (%) | 57.60 (54.60–66.70) | 58.00 (53.10–62.50) | 60.90 (53.75–65.50) | |
| PLT (×1000/μL) | 251.00 (224.50–287.00) | 243.00 (207.00–289.00) | 239.50 (200.00–286.00) |
| Young (20–39 yrs) | Middle Age (40–59 yrs) | Old Age (≥60 yrs) | p-Value * | |
|---|---|---|---|---|
| Non-MASLD | ||||
| Gender (F/M) | 13/7 | 18/2 | 11/6 | |
| Age (yr) | 28.00 (25.50–32.00) | 44.50 (41.50–50.50) | 66.00 (62.50–70.00) | <0.001 |
| Waist circumference (cm) | 75.50 (73.00–81.50) | 80.00 (70.50–84.50) | 83.00 (77.75–86.50) | 0.033 |
| SBP (mmHg) | 119.00 (109.00–122.00) | 124.00 (112.00–132.50) | 135.00 (130.00–140.00) | <0.001 |
| DBP (mmHg) | 70.00 (68.50–75.50) | 74.50 (67.50–82.00) | 82.50 (76.00–85.00) | 0.026 |
| GOT (IU/L) | 17.50 (16.00–19.50) | 21.50 (16.00–24.00) | 23.00 (18.50–26.50) | 0.033 |
| TG (mg/dL) | 59.00 (43.50–73.00) | 77.50 (59.50–98.50) | 101.50 (83.00–124.00) | 0.001 |
| HbA1c (%) | 5.25 (5.10–5.40) | 5.50 (5.30–5.65) | 5.85 (5.80–6.20) | <0.001 |
| Glucose (mg/dL) | 82.50 (79.00–91.00) | 86.00 (82.00–88.00) | 104.50 (98.50–120.00) | 0.002 |
| Obese MASLD | ||||
| Gender (F/M) | 13/17 | 14/14 | 13/12 | |
| Age (yr) | 36.00 (28.00–38.00) | 51.00 (46.50–56.00) | 69.00 (66.00–73.00) | <0.001 |
| ALP (IU/L) | 167.00 (142.00–198.00) | 210.00 (176.50–254.00) | 192.00 (158.00–237.00) | 0.009 |
| HbA1c (%) | 5.50 (5.40–5.70) | 5.65 (5.40–5.85) | 5.80 (5.50–6.30) | 0.003 |
| Lean MASLD | ||||
| Gender (F/M) | 20/9 | 20/9 | 15/14 | |
| Age (yr) | 31.00 (25.00–36.00) | 50.00 (46.00–54.00) | 69.00 (65.00–75.00) | <0.001 |
| SBP (mmHg) | 135.50 (133.00–140.00) | 140.00 (134.00–142.00) | 143.00 (138.00–154.00) | 0.018 |
| GOT (IU/L) | 18.50 (17.00–23.00) | 23.00 (19.00–27.00) | 23.00 (19.00–27.00) | 0.011 |
| Albumin (g/dL) | 4.80 (4.55–5.05) | 4.70 (4.55–4.80) | 4.60 (4.35–4.70) | 0.012 |
| Cholesterol (mg/dL) | 195.50 (176.00–208.00) | 213.00 (191.00–227.00) | 191.00 (158.00–212.00) | 0.024 |
| HbA1c (%) | 5.30 (5.10–5.50) | 5.50 (5.30–5.80) | 5.70 (5.50–5.80) | 0.001 |
| Glucose (mg/dL) | 86.00 (84.00–90.00) | 88.00 (84.50–90.00) | 99.00 (87.00–105.00) | 0.036 |
| Non-MASLD (n = 20) | Obese MASLD (n = 26) | Lean MASLD (n = 26) | p-Value * | |
|---|---|---|---|---|
| CD9 | 0.65 (0.46–0.98) | 0.46 (0.34–0.80) | 0.52 (0.39–0.70) | 0.231 |
| CD63 | 0.56 (0.44–0.70) | 0.58 (0.50–0.75) | 0.75 (0.64–0.93) | 0.003 |
| PLIN2/CD9 | 1.02 (0.75–1.85) | 0.81 (0.62–2.48) | 1.70 (1.29–1.99) | 0.166 |
| PLIN3/CD9 | 2.73 (0.78–3.47) | 1.66 (0.96–3.42) | 1.69 (1.32–2.29) | 0.993 |
| PLIN2/CD63 | 1.43 (1.05–2.04) | 1.16 (0.55–1.49) | 1.15 (0.87–1.32) | 0.075 |
| PLIN3/CD63 | 1.93 (1.67–2.57) | 1.66 (1.01–2.15) | 1.18 (1.03–1.63) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-Y.; Hsu, C.-Y.; Chung, W.-P.; Sun, H.-Y.; Kao, T.-C.; Chen, T.-Y.; Li, X.-M.; Huang, W.-L.; Young, K.-C. Serum Extracellular Vesicles as Pathogenetic Signals in Obese and Lean Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease. Metabolites 2025, 15, 746. https://doi.org/10.3390/metabo15110746
Chen C-Y, Hsu C-Y, Chung W-P, Sun H-Y, Kao T-C, Chen T-Y, Li X-M, Huang W-L, Young K-C. Serum Extracellular Vesicles as Pathogenetic Signals in Obese and Lean Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease. Metabolites. 2025; 15(11):746. https://doi.org/10.3390/metabo15110746
Chicago/Turabian StyleChen, Chi-Yi, Che-Yu Hsu, Wei-Pang Chung, Hung-Yu Sun, Tzu-Ching Kao, Tzu-Yi Chen, Xing-Min Li, Wei-Lung Huang, and Kung-Chia Young. 2025. "Serum Extracellular Vesicles as Pathogenetic Signals in Obese and Lean Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease" Metabolites 15, no. 11: 746. https://doi.org/10.3390/metabo15110746
APA StyleChen, C.-Y., Hsu, C.-Y., Chung, W.-P., Sun, H.-Y., Kao, T.-C., Chen, T.-Y., Li, X.-M., Huang, W.-L., & Young, K.-C. (2025). Serum Extracellular Vesicles as Pathogenetic Signals in Obese and Lean Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease. Metabolites, 15(11), 746. https://doi.org/10.3390/metabo15110746








