Serum Uric Acid in the PAMELA Study: Main Findings and Association with the Atherogenic Index of Plasma
Abstract
1. Introduction
2. The PAMELA Study: An Overview
2.1. Study Profile
2.2. Measured Variables
3. Main SUA Findings of the PAMELA Study
3.1. SUA and BP
3.2. SUA and High BP-Related Organ Damage
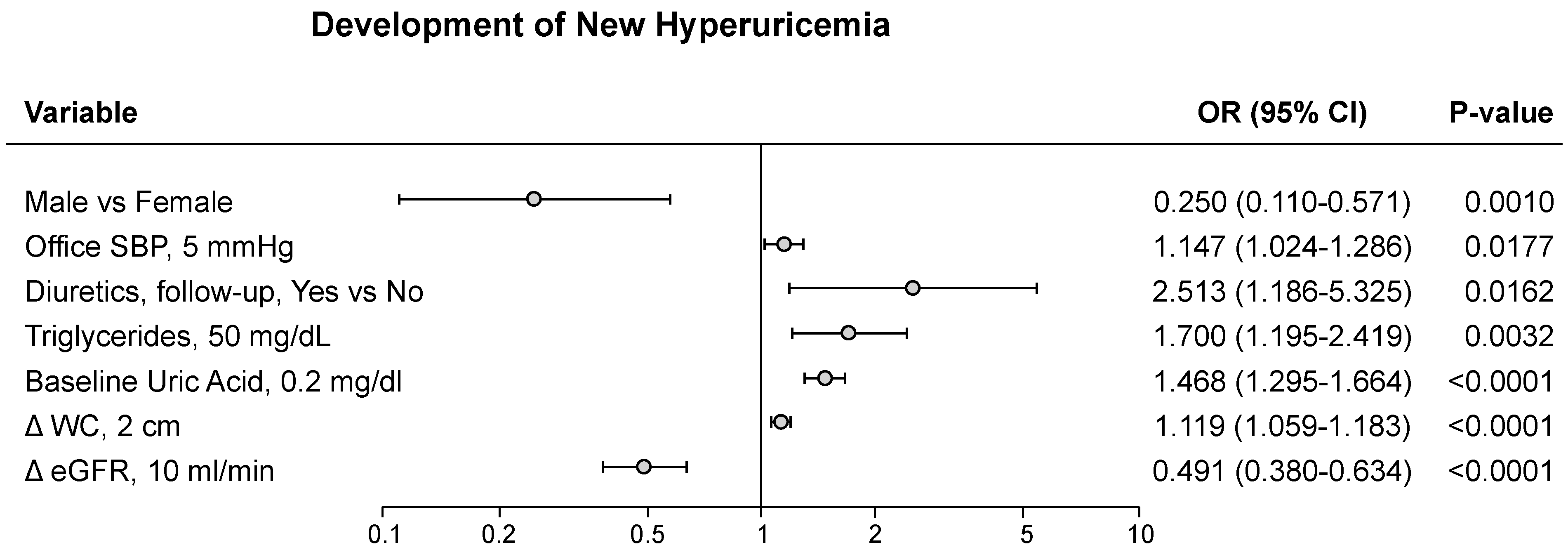
3.3. SUA and Metabolic Alterations
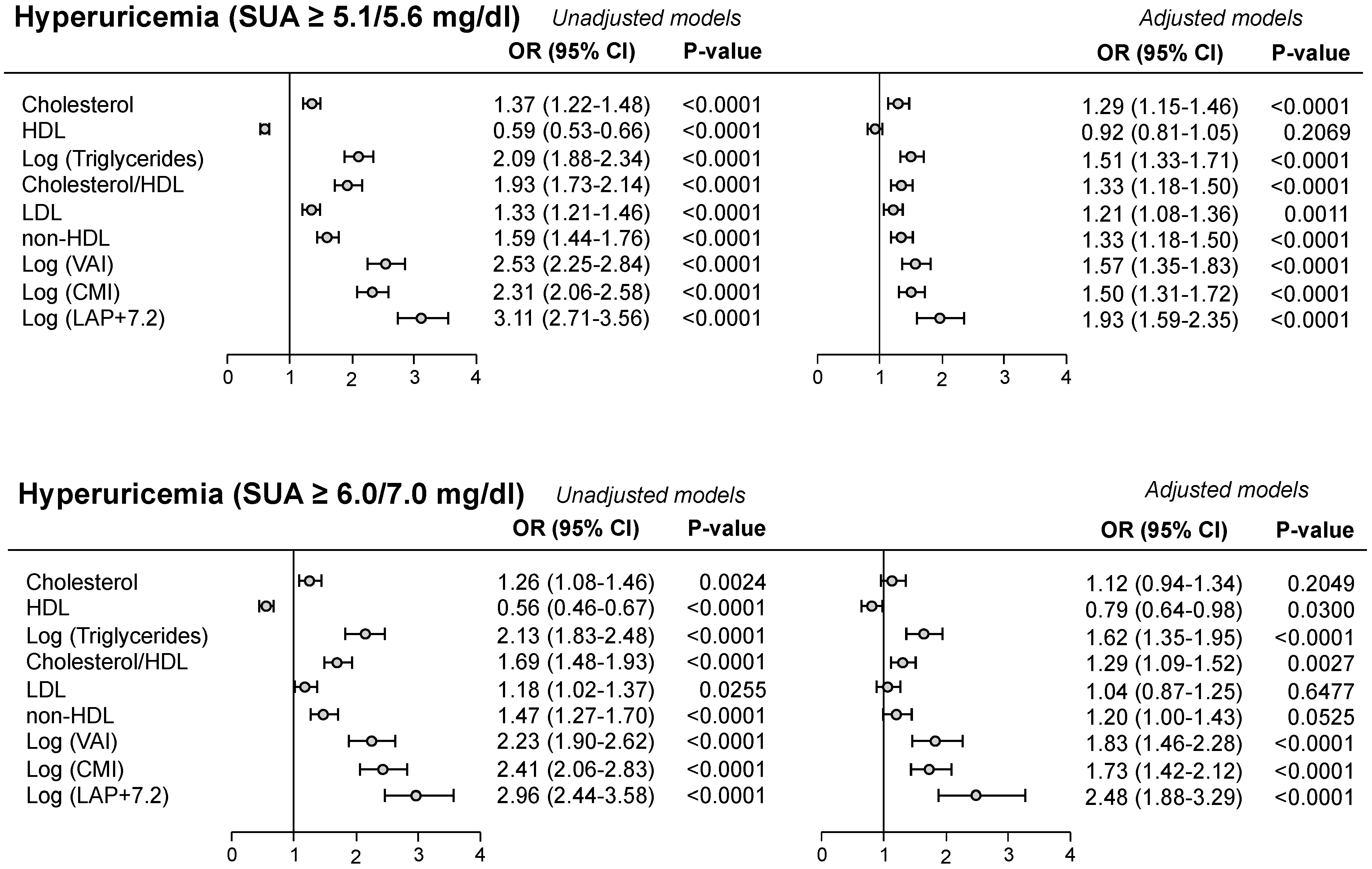
4. SUA, Lipid Profile, and Adiposity Indices
5. Relationships Between SUA and AIP
5.1. Results of Published Papers
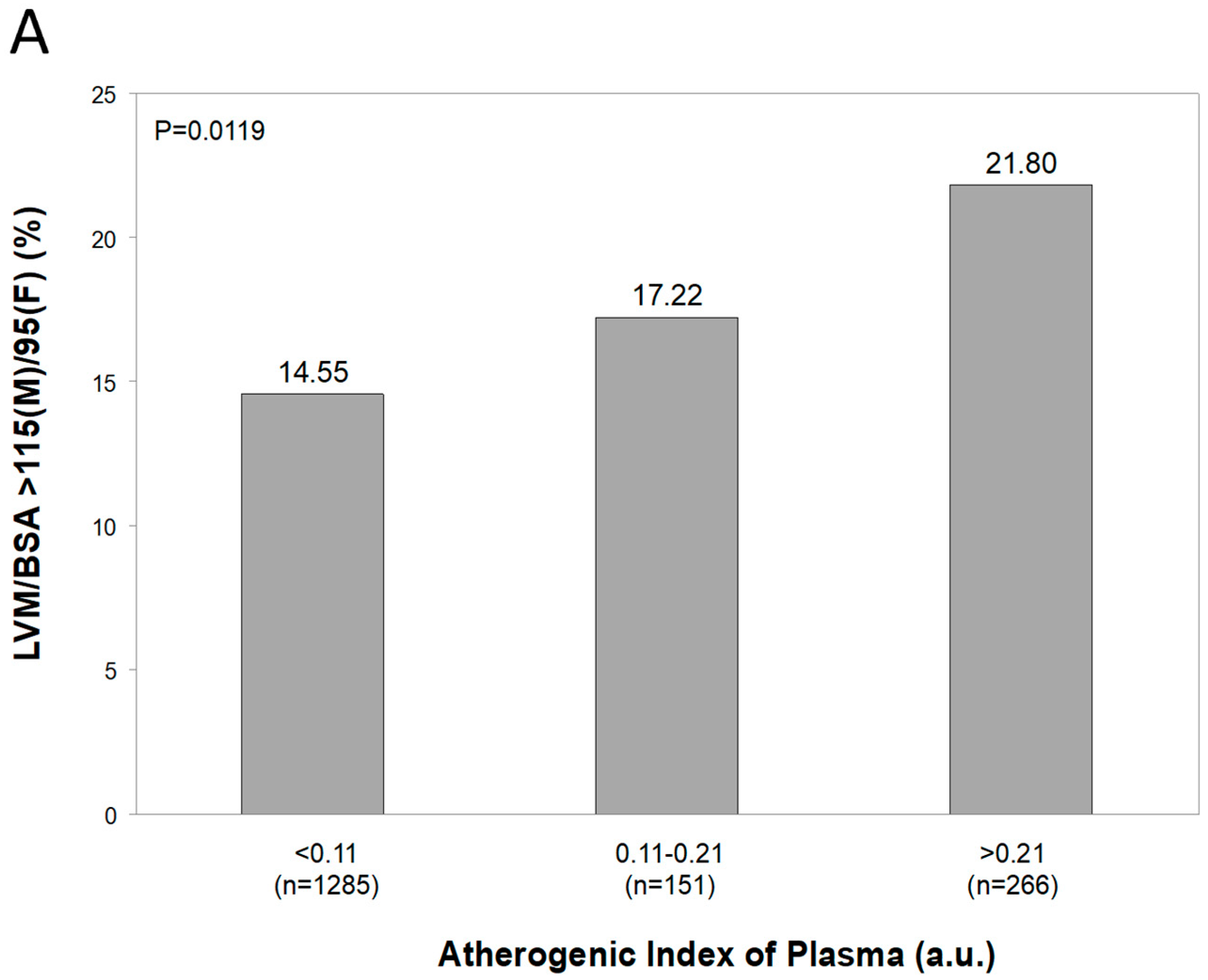
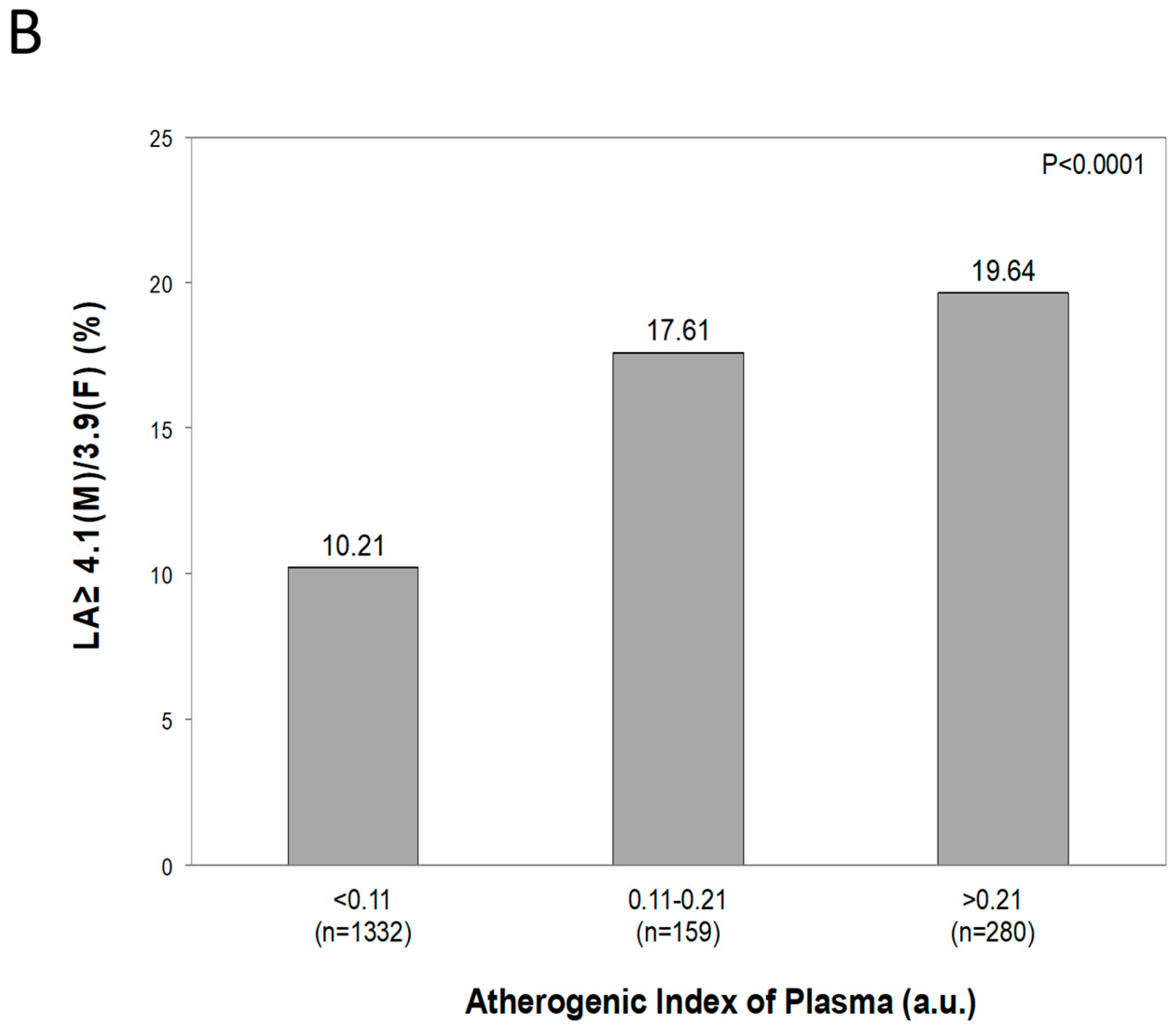
5.2. Original Data from the PAMELA Study
6. Comments and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaudhary, K.; Malhotra, K.; Sowers, J.; Aroor, A. Uric Acid—Key Ingredient in the Recipe for Cardiorenal Metabolic Syndrome. Cardiorenal Med. 2013, 3, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Maloberti, A.; Mengozzi, A.; Russo, E.; Cicero, A.F.G.; Angeli, F.; Rosei, E.A.; Barbagallo, C.M.; Bernardino, B.; Bombelli, M.; Cappelli, F.; et al. The Results of the URRAH (Uric Acid Right for Heart Health) Project: A Focus on Hyperuricemia in Relation to Cardiovascular and Kidney Disease and its Role in Metabolic Dysregulation. High Blood Press. Cardiovasc. Prev. 2023, 30, 411–425. [Google Scholar] [CrossRef]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension. J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [CrossRef]
- Bombelli, M.; Ronchi, I.; Volpe, M.; Facchetti, R.; Carugo, S.; Dell’Oro, R.; Cuspidi, C.; Grassi, G.; Mancia, G. Prognostic value of serum uric acid: New-onset in and out-of-office hypertension and long-term mortality. J. Hypertens. 2014, 32, 1237–1244. [Google Scholar] [CrossRef]
- Maloberti, A.; Vanoli, J.; Finotto, A.; Bombelli, M.; Facchetti, R.; Redon, P.; Mancia, G.; Grassi, G. Uric acid relationships with lipid profile and adiposity indices: Impact of different hyperuricemic thresholds. J. Clin. Hypertens. 2022, 25, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Yeo, C.; Kaushal, S.; Lim, B.; Syn, N.; Oo, A.M.; Rao, J.; Koura, A.; Yeo, D. Impact of bariatric surgery on serum uric acid levels and the incidence of gout—A meta-analysis. Obes. Rev. 2019, 20, 1759–1770. [Google Scholar] [CrossRef] [PubMed]
- Bombelli, M.; Quarti-Trevano, F.; Tadic, M.; Facchetti, R.; Cuspidi, C.; Mancia, G.; Grassi, G. Uric acid and risk of new-onset metabolic syndrome, impaired fasting glucose and diabetes mellitus in a general Italian population: Data from the Pressioni Arteriose Monitorate E Loro Associazioni study. J. Hypertens. 2018, 36, 1492–1498. [Google Scholar] [CrossRef]
- Dobiášová, M.; Rašlová, K.; Rauchová, H.; Vohnout, B.; Ptáčková, K.; Frohlich, J. Atherogenic lipoprotein profile in families with and without history of early myocardial infarction. Physiol. Res. 2001, 50, 1–8. [Google Scholar] [CrossRef]
- Russo, E.; Viazzi, F.; Pontremoli, R.; Angeli, F.; Barbagallo, C.M.; Berardino, B.; Bombelli, M.; Cappelli, F.; Casiglia, E.; Cianci, R.; et al. Predictive value of TG/HDL-C and GFR-adjusted uric acid levels on cardiovascular mortality: The URRAH study. Lipids Health Dis. 2025, 24, 21. [Google Scholar] [CrossRef]
- Yang, S.-H.; Du, Y.; Li, X.-L.; Zhang, Y.; Li, S.; Xu, R.-X.; Zhu, C.-G.; Guo, Y.-L.; Wu, N.-Q.; Qing, P.; et al. Triglyceride to High-Density Lipoprotein Cholesterol Ratio and Cardiovascular Events in Diabetics With Coronary Artery Disease. Am. J. Med. Sci. 2017, 354, 117–124. [Google Scholar] [CrossRef]
- Andraschko, L.M.; Gazi, G.; Leucuta, D.-C.; Popa, S.-L.; Chis, B.A.; Ismaiel, A. Atherogenic Index of Plasma in Metabolic Syndrome—A Systematic Review and Meta-Analysis. Medicina 2025, 61, 611. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Quarti-Trevano, F.; Dell’oro, R.; Cuspidi, C.; Mancia, G. The PAMELA research project: A 25-year long journey. Panminerva Medica 2021, 63, 430–435. [Google Scholar] [CrossRef]
- Grassi, G.; Vanoli, J.; Facchetti, R.; Mancia, G. Uric Acid, Hypertensive Phenotypes, and Organ Damage: Data from the Pamela Study. Curr. Hypertens. Rep. 2022, 24, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Bombelli, M.; Facchetti, R.; Madotto, F.; Corrao, G.; Trevano, F.Q.; Grassi, G.; Sega, R. Long-Term Prognostic Value of Blood Pressure Variability in the General Population. Hypertension 2007, 49, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Cuspidi, C.; Facchetti, R.; Bombelli, M.; Sala, C.; Tadic, M.; Grassi, G.; Mancia, G. Uric Acid and New Onset Left Ventricular Hypertrophy: Findings from the PAMELA Population. Am. J. Hypertens. 2017, 30, 279–285. [Google Scholar] [CrossRef]
- Seravalle, G.; Grassi, G. Obesity and hypertension. Pharmacol. Res. 2017, 122, 1–7. [Google Scholar] [CrossRef]
- Maloberti, A.; Dell’oRo, R.; Bombelli, M.; Quarti-Trevano, F.; Facchetti, R.; Mancia, G.; Grassi, G. Long-term increase in serum uric acid and its predictors over a 25 year follow-up: Results of the PAMELA study. Nutr. Metab. Cardiovasc. Dis. 2023, 34, 223–229. [Google Scholar] [CrossRef]
- Palatini, P.; Virdis, A.; Masi, S.; Mengozzi, A.; Casiglia, E.; Tikhonoff, V.; Cicero, A.F.; Ungar, A.; Parati, G.; Rivasi, G.; et al. Hyperuricemia increases the risk of cardiovascular mortality associated with very high HdL-cholesterol level. Nutr. Metab. Cardiovasc. Dis. 2022, 33, 323–330. [Google Scholar] [CrossRef]
- Agabiti Rosei, C.; Paini, A.; Buso, G.; Maloberti, A.; Giannattasio, C.; Salvetti, M.; Casiglia, E.; Tikhonoff, V.; Angeli, F.; Barbagallo, C.M.; et al. Serum uric acid, hypertriglyceridemia and carotid plaques: A subanalysis of the URic Acid Right for Heart health (URRAH) study. Metabolites 2024, 14, 323. [Google Scholar] [CrossRef]
- D’eLia, L.; Masulli, M.; Virdis, A.; Casiglia, E.; Tikhonoff, V.; Angeli, F.; Barbagallo, C.M.; Bombelli, M.; Cappelli, F.; Cianci, R.; et al. Triglyceride-glucose Index and Mortality in a Large Regional-based Italian Database (URRAH Project). J. Clin. Endocrinol. Metab. 2024, 110, e470–e477. [Google Scholar] [CrossRef]
- Baliarsingh, S.; Sharma, N.; Mukherjee, R. Serum uric acid: Marker for atherosclerosis as it is positively associated with “atherogenic index of plasma”. Arch. Physiol. Biochem. 2012, 119, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Bortolasci, C.C.; Vargas, H.O.; Nunes, S.O.V.; de Melo, L.G.P.; de Castro, M.R.P.; Moreira, E.G.; Dodd, S.; Barbosa, D.S.; Berk, M.; Maes, M. Factors influencing insulin resistance in relation to atherogenicity in mood disorders, the metabolic syndrome and tobacco use disorder. J. Affect. Disord. 2015, 179, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Nansseu, J.R.; Moor, V.J.; Nouaga, M.E.; Zing-Awona, B.; Tchanana, G.; Ketcha, A. Atherogenic index of plasma and risk of car-diovascular disease among Cameroonian postmenopausal women. Lipids Health Dis. 2016, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Biyik, Z.; Guney, I. Relationship Between Uric Acid, Proteinuria, and Atherogenic Index of Plasma in Renal Transplant Patients. Transplant. Proc. 2018, 50, 3376–3380. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, C.; Yang, J.; Seery, S.; Qi, Y.; Wang, W.; Zhang, K.; Shao, C.; Tang, Y.-D. Atherogenic index of plasma for non-diabetic, coronary artery disease patients after percutaneous coronary intervention: A prospective study of the long-term outcomes in China. Cardiovasc. Diabetol. 2022, 21, 29. [Google Scholar] [CrossRef]
- Akbas, E.M.; Timuroglu, A.; Ozcicek, A.; Ozcicek, F.; Demirtas, L.; Gungor, A.; Akbas, N. Association of uric acid, atherogenic index of plasma and albuminuria in diabetes mellitus. Int. J. Clin. Exp. Med. 2014, 7, 5737–5743. [Google Scholar]
- Huang, J.; Chen, C.; Jie, C.; Li, R.; Chen, C. L-shaped relationship between atherogenic index of plasma with uric acid levels and hyperuricemia risk. Front. Endocrinol. 2024, 15, 1461599. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, T.; Zhou, W.; Zhu, L.; Yu, C.; Bao, H.; Li, J.; Cheng, X. Threshold effect of atherogenic index of plasma on type 2 diabetes mellitus and modification by uric acid in normal-weight adults with hypertension. Front. Endocrinol. 2024, 15, 1495340. [Google Scholar] [CrossRef]
- Chang, Y.; Li, Y.; Guo, X.; Guo, L.; Sun, Y. Atherogenic Index of Plasma Predicts Hyperuricemia in Rural Population: A Cross-Sectional Study from Northeast China. Int. J. Environ. Res. Public Health 2016, 13, 879. [Google Scholar] [CrossRef]


| Variable | r | p-Value |
|---|---|---|
| Age | 0.24578 | <0.0001 |
| Sex (males) | 0.34147 | <0.0001 |
| BMI | 0.34527 | <0.0001 |
| SBP Office | 0.25679 | <0.0001 |
| DBP Office | 0.25837 | <0.0001 |
| Heart Rate Office | −0.02744 | 0.2172 |
| Antihypertensive drug | 0.18966 | <0.0001 |
| Glycemia * | 0.29839 | <0.0001 |
| Total cholesterol | 0.24833 | <0.0001 |
| HDL cholesterol | −0.73456 | <0.0001 |
| Triglycerides * | 0.9382 | <0.0001 |
| SUA | 0.43008 | <0.0001 |
| Creatinine | 0.30255 | <0.0001 |
| GFR | −0.14117 | <0.0001 |
| Variable | Number | r | p-Value |
|---|---|---|---|
| Female | 1005 | 0.39015 | <0.0001 |
| Male | 1030 | 0.25628 | <0.0001 |
| Age < 65 years | 1634 | 0.43106 | <0.0001 |
| Age ≥ 65 years | 401 | 0.35186 | <0.0001 |
| No antihypertensive drug | 1637 | 0.42725 | <0.0001 |
| Antihypertensive drug | 393 | 0.31914 | <0.0001 |
| Office BP < 140/90 mmhg | 1174 | 0.43525 | <0.0001 |
| Office BP ≥ 140/90 mmhg | 855 | 0.35746 | <0.0001 |
| BMI < 25 kg/m2 | 961 | 0.34454 | <0.0001 |
| 25 ≤ BMI < 30 kg/m2 | 757 | 0.40381 | <0.0001 |
| BMI ≥ 30 kg/m2 | 271 | 0.30076 | <0.0001 |
| No diabetes mellitus | 1964 | 0.44639 | <0.0001 |
| diabetes mellitus | 71 | 0.06196 | 0.6078 |
| Variable | Beta (Std Error) | p-Value |
|---|---|---|
| Age | 0.00048 (0.70585) | 0.0009 |
| Sex (Male) | 0.01369 (6.21816) | <0.0001 |
| BMI | 0.00141 (6.10144) | <0.0001 |
| Antihypertensive drugs | 0.01664 (0.27906) | 0.0373 |
| Total plasma cholesterol | 0.00014 (4.77346) | <0.0001 |
| SUA | 0.00545 (4.85739) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maloberti, A.; Facchetti, R.; Cuspidi, C.; Grassi, G. Serum Uric Acid in the PAMELA Study: Main Findings and Association with the Atherogenic Index of Plasma. Metabolites 2025, 15, 671. https://doi.org/10.3390/metabo15100671
Maloberti A, Facchetti R, Cuspidi C, Grassi G. Serum Uric Acid in the PAMELA Study: Main Findings and Association with the Atherogenic Index of Plasma. Metabolites. 2025; 15(10):671. https://doi.org/10.3390/metabo15100671
Chicago/Turabian StyleMaloberti, Alessandro, Rita Facchetti, Cesare Cuspidi, and Guido Grassi. 2025. "Serum Uric Acid in the PAMELA Study: Main Findings and Association with the Atherogenic Index of Plasma" Metabolites 15, no. 10: 671. https://doi.org/10.3390/metabo15100671
APA StyleMaloberti, A., Facchetti, R., Cuspidi, C., & Grassi, G. (2025). Serum Uric Acid in the PAMELA Study: Main Findings and Association with the Atherogenic Index of Plasma. Metabolites, 15(10), 671. https://doi.org/10.3390/metabo15100671









