Phenolic Compounds from Hypericum cerastoides (Spach) N. Robson: Dereplication via UHPLC-HRMS/MS, Isolation, Identification, and Preliminary Biological Evaluation Focusing on Radical-Scavenging, Anti-α-Glucosidase, and Pro-Lipase Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
2.3. Extraction and Isolation
2.3.1. Hypercerastoside A (HC4) (4,6-Dihydroxy Benzophenone-2-O-β-D-2″-Acetylglucopyranoside)
2.3.2. Hypercerastoside B (HC6) (4-O-{6-[(2E)-p-Hydroxycinnamoyl]-β-D-Glucopyranosyl}-6-isopropyl-tetrahydro-2H-pyran-2-one)
2.3.3. Hypercerastoside C (HC7) (Methyl 3-O-{6-[(2E)-p-hydroxycinnamoyl]-β-glucopyranosyl}-6-methyl-5-hydroxyheptanoate)
2.4. Acid Hydrolysis
2.5. Anti-α-Glucosidase Activity Assay
2.6. Assay for Modulation of Lipase Activity
2.7. DPPH Radical-Scavenging Activity Assay
2.8. ABTS Radical-Scavenging Activity Assay
2.9. Statistical Analysis
3. Results
3.1. Dereplication and Semi-Quantitative Determination of Polar Phenolic Compounds in Aerial Parts from Hypericum cerastoides by UHPLC-HRMS/MS Analysis
3.1.1. Flavan-3-ols and a Flavolignan
3.1.2. Hydroxycinnamic Acid Derivatives
3.1.3. Flavonol Aglycones and Their Glycosides
3.1.4. Benzophenones
3.2. Isolation and Identification of Some Polar Phenolic Compounds from the Ethylacetate Extract of the Aerial Parts of Hypericum cerastoides
3.3. Biological Activities of the Isolated Phenolic Compounds from the Ethyl Acetate Extract of the Aerial Parts of Hypericum cerastoides
3.3.1. Radical-Scavenging Activity of Compounds HC1–HC7
3.3.2. α-Glucosidase Inhibitory Activity of Compounds HC1–HC7
3.3.3. Lipase Activity of Compounds HC1–HC7
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crockett, S.; Robson, N. Taxonomy and Chemotaxonomy of the Genus Hypericum. Med. Aromat. Plant Sci. Biotechnol. 2011, 5, 1–13. [Google Scholar]
- Avato, P. A Survey on the Hypericum Genus: Secondary Metabolites and Bioactivity. Stud. Nat. Prod. Chem. 2005, 30, 603–634. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, W.; Wang, J.C. Recent Advances Regarding Constituents and Bioactivities of Plants from the Genus Hypericum. Chem. Biodivers. 2015, 12, 309–349. [Google Scholar] [CrossRef] [PubMed]
- Robson, N.K.B. Studies in the Genus Hypericum L. (Hypericaceae) 5(1). Sections 10. Olympia to 15/16. Crossophyllum. Phytotaxa 2010, 4, 5–126. [Google Scholar] [CrossRef]
- Kizilarslan, Ç.; Özhatay, N. Wild Plants Used as Medicinal Purpose in the South Part of Izmit (Northwest Turkey). Turk. J. Pharm. Sci. 2012, 9, 199–218. [Google Scholar]
- Özkan, E.E.; Mat, A. An Overview on Hypericum Species of Turkey. J. Pharmacogn. Phytother. 2013, 5, 38–46. [Google Scholar] [CrossRef]
- Erken, S.; Malyer, H.; Demirci, F.; Demirci, B.; Baser, K.H.C. Chemical Investigations on Some Hypericum Species Growing in Turkey-I. Chem. Nat. Compd. 2001, 37, 434–438. [Google Scholar] [CrossRef]
- Semerdjieva, I.; Zheljazkov, V.D.; Dincheva, I.; Piperkova, N.; Maneva, V.; Cantrell, C.L.; Astatkie, T.; Stoyanova, A.; Ivanova, T. Essential Oil Composition of Seven Bulgarian Hypericum Species and Its Potential as a Biopesticide. Plants 2023, 12, 923. [Google Scholar] [CrossRef]
- Kitanov, G.M.; Nedialkov, P.T. Mangiferin and Isomangiferin in Some Hypericum Species. Biochem. Syst. Ecol. 1998, 26, 647–653. [Google Scholar] [CrossRef]
- Crockett, S.L.; Schaneberg, B.; Khan, I.A. Phytochemical Profiling of New and Old World Hypericum (St. John’s Wort) Species. Phytochem. Anal. 2005, 16, 479–485. [Google Scholar] [CrossRef]
- Konya-Konuk, R.; Aru, B.; Öztürk, C.; Kırmızıbekmez, H. A Novel Normonoterpene Glycoside and a New Benzophenone Derivative from Hypericum cerastioides and Their in Vitro Cytotoxic Activities. Fitoterapia 2024, 179, 106276. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile Discrimination of Aldose Enantiomers by Reversed-Phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef] [PubMed]
- Nedialkov, P.T.; Kokanova-Nedialkova, Z.; Buecherl, D.; Momekov, G.; Heilmann, J.; Nikolov, S. 30-Normedicagenic Acid Glycosides from Chenopodium Foliosum. Nat. Prod. Commun. 2012, 7, 1419–1422. [Google Scholar] [CrossRef]
- Kokanova-Nedialkova, Z.; Kondeva-Burdina, M.; Nedialkov, P.T. Neuroprotective, Anti-α-Glucosidase and Prolipase Active Flavonoids from Good King Henry (Chenopodium bonus-henricus L.). Nat. Prod. Res. 2021, 35, 5484–5488. [Google Scholar] [CrossRef]
- Kang, W.-Y.; Song, Y.-L.; Zhang, L. α-Glucosidase Inhibitory and Antioxidant Properties and Antidiabetic Activity of Hypericum ascyron L. Med. Chem. Res. 2011, 20, 809–816. [Google Scholar] [CrossRef]
- McDougall, G.J.; Kulkarni, N.N.; Stewart, D. Berry Polyphenols Inhibit Pancreatic Lipase Activity in Vitro. Food Chem. 2009, 115, 193–199. [Google Scholar] [CrossRef]
- Kokanova-Nedialkova, Z.; Nedialkov, P. Antioxidant Properties of 6-Methoxyflavonol Glycosides from the Aerial Parts of Chenopodium bonus-henricus L. Bulg. Chem. Commun. 2017, 49, 253–258. [Google Scholar]
- Marinov, T.; Kokanova-Nedialkova, Z.; Nedialkov, P. UHPLC-HRMS-Based Profiling and Simultaneous Quantification of the Hydrophilic Phenolic Compounds from the Aerial Parts of Hypericum aucheri Jaub. & Spach (Hypericaceae). Pharmacia 2024, 71, 1–11. [Google Scholar] [CrossRef]
- Liu, X.; Yan, X.; Bi, J.; Wu, X.; Liu, J.; Zhou, M. Identification of Phenolic Compounds and Antioxidant Activity of Guava Dehydrated by Different Drying Methods. Dry. Technol. 2020, 38, 987–1000. [Google Scholar] [CrossRef]
- Beltrame, F.L.; Filho, E.R.; Barros, F.A.P.; Cortez, D.A.; Cass, Q.B. A Validated Higher-Performance Liquid Chromatography Method for Quantification of Cinchonain Ib in Bark and Phytopharmaceuticals of Trichilia catigua Used as Catuaba. J. Chromatogr. A 2006, 1119, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tu, Z.; Xie, X.; Lu, Y.; Wang, Z.; Wang, H.; Sha, X. Antihyperglycemic, Antioxidant Activities of Two Acer palmatum Cultivars, and Identification of Phenolics Profile by UPLC-QTOF-MS/MS: New Natural Sources of Functional Constituents. Ind. Crops Prod. 2016, 89, 522–532. [Google Scholar] [CrossRef]
- Mohd Jusoh, N.H.; Subki, A.; Yeap, S.K.; Yap, K.C.; Jaganath, I.B. Pressurized Hot Water Extraction of Hydrosable Tannins from Phyllanthus tenellus Roxb. BMC Chem. 2019, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- March, R.E.; Miao, X.-S. A Fragmentation Study of Kaempferol Using Electrospray Quadrupole Time-of-Flight Mass Spectrometry at High Mass Resolution. Int. J. Mass Spectrom. 2004, 231, 157–167. [Google Scholar] [CrossRef]
- Cuyckens, F.; Claeys, M. Mass Spectrometry in the Structural Analysis of Flavonoids. J. Mass Spectrom. 2004, 39, 1–15. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, A.; Zhang, Y.; Sun, H.; Meng, X.; Yan, G.; Wang, X. Application of Ultra-Performance Liquid Chromatography with Time-of-Flight Mass Spectrometry for the Rapid Analysis of Constituents and Metabolites from the Extracts of Acanthopanax senticosus Harms Leaf. Pharmacogn. Mag. 2016, 12, 145–152. [Google Scholar] [CrossRef]
- An, H.; Wang, H.; Lan, Y.; Hashi, Y.; Chen, S. Simultaneous Qualitative and Quantitative Analysis of Phenolic Acids and Flavonoids for the Quality Control of Apocynum venetum L. Leaves by HPLC–DAD–ESI–IT–TOF–MS and HPLC–DAD. J. Pharm. Biomed. Anal. 2013, 85, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.C.; Liu, L.T.; Bian, J.J.; Yan, C.Q.; Ye, L.; Zhao, M.X.; Huang, Q.S.; Wang, W.; Liang, K.; Shi, Z.F.; et al. Identification of Multiple Constituents in Shuganjieyu Capsule and Rat Plasma after Oral Administration by Ultra-Performance Liquid Chromatography Coupled with Electrospray Ionization and Ion Trap Mass Spectrometry. Acta Chromatogr. 2018, 30, 95–102. [Google Scholar] [CrossRef]
- Cuyckens, F.; Claeys, M. Determination of the Glycosylation Site in Flavonoid Mono-O-Glycosides by Collision-Induced Dissociation of Electrospray-Generated Deprotonated and Sodiated Molecules. J. Mass Spectrom. 2005, 40, 364–372. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, X.; Jiang, Y.; Yu, L.; Huang, X.; Dong, Z.; Yang, S.; He, W.; Zeng, J.; Qing, Z. Systematic Identification Metabolites of Hemerocallis citrina Borani by High-Performance Liquid Chromatography/Quadrupole-Time-of-Flight Mass Spectrometry Combined with a Screening Method. J. Pharm. Biomed. Anal. 2020, 186, 113314. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhou, Z.; Yao, S.; Li, S.; Yang, W.; Jiang, B.; Liu, X.; Wu, W.; Qv, H.; Guo, D. Neutral Loss Ion Mapping Experiment Combined with Precursor Mass List and Dynamic Exclusion for Screening Unstable Malonyl Glucoside Conjugates. J. Am. Soc. Mass Spectrom. 2016, 27, 99–107. [Google Scholar] [CrossRef]
- Karanga, Y.; Ilboudo, O.; Tapsoba, I.; Colson, E.; Gerbaux, P.; Bonzi-Coulibaly, Y.L. Characterization of Flavonoids in the Ethyl Acetate Extract from Euphorbia hirta L. by Liquid Chromatography and Tandem Mass Spectrometry. Sci. Struct. Matičre 2022, 6, 123–136. [Google Scholar]
- Li, X.; Chen, H.; Jia, Y.; Peng, J.; Li, C. Inhibitory Effects against Alpha-Amylase of an Enriched Polyphenol Extract from Pericarp of Mangosteen (Garcinia mangostana). Foods 2022, 11, 1001. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, W.-X.; Zheng, M.-F.; Xu, Q.-L.; Wan, F.-H.; Wang, J.; Lei, T.; Zhou, Z.-Y.; Tan, J.-W. Bioactive Quinic Acid Derivatives from Ageratina adenophora. Molecules 2013, 18, 14096–14104. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.K. Carbon-13 NMR of Flavonoids; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 1-4832-9074-3. [Google Scholar]
- Mabry, T.; Markham, K.R.; Thomas, M.B. The Systematic Identification of Flavonoids; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1970; ISBN 3-642-88458-X. [Google Scholar]
- Pauli, G.F.; Poetsch, F.; Nahrstedt, A. Structure Assignment of Natural Quinic Acid Derivatives Using Proton Nuclear Magnetic Resonance Techniques. Phytochem. Anal. 1998, 9, 177–185. [Google Scholar] [CrossRef]
- Ilieva, Y. Acylphloroglucinols from Species of the Genus Hypericum L.: Isolation, Structural Characterization and Testing for Cytotoxic and Antineoplastic Activity. Ph.D. Thesis, Medical University of Sofia, Sofia, Bulgaria, 2019. [Google Scholar]
- Liu, H.; Orjala, J.; Sticher, O.; Rali, T. Acylated Flavonol Glycosides from Leaves of Stenochlaena palustris. J. Nat. Prod. 1999, 62, 70–75. [Google Scholar] [CrossRef]
- Heck, A.M.; Yanovski, J.A.; Calis, K.A. Orlistat, a New Lipase Inhibitor for the Management of Obesity. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2000, 20, 270–279. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-Antioxidant Activity Relationships of Flavonoids and Phenolic Acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Kusunoki, M.; Tsutsumi, K.; Tana, C.; Sato, D.; Nakamura, T. Lipoprotein Lipase Activation Improves the Cachexia and Obesity. J. Obes. Weight Loss Ther. 2013, 3, 1–6. [Google Scholar] [CrossRef]
- Peixoto da Silva, S.; Santos, J.M.O.; Costa e Silva, M.P.; Gil da Costa, R.M.; Medeiros, R. Cancer Cachexia and Its Pathophysiology: Links with Sarcopenia, Anorexia and Asthenia. J. Cachexia Sarcopenia Muscle 2020, 11, 619–635. [Google Scholar] [CrossRef] [PubMed]



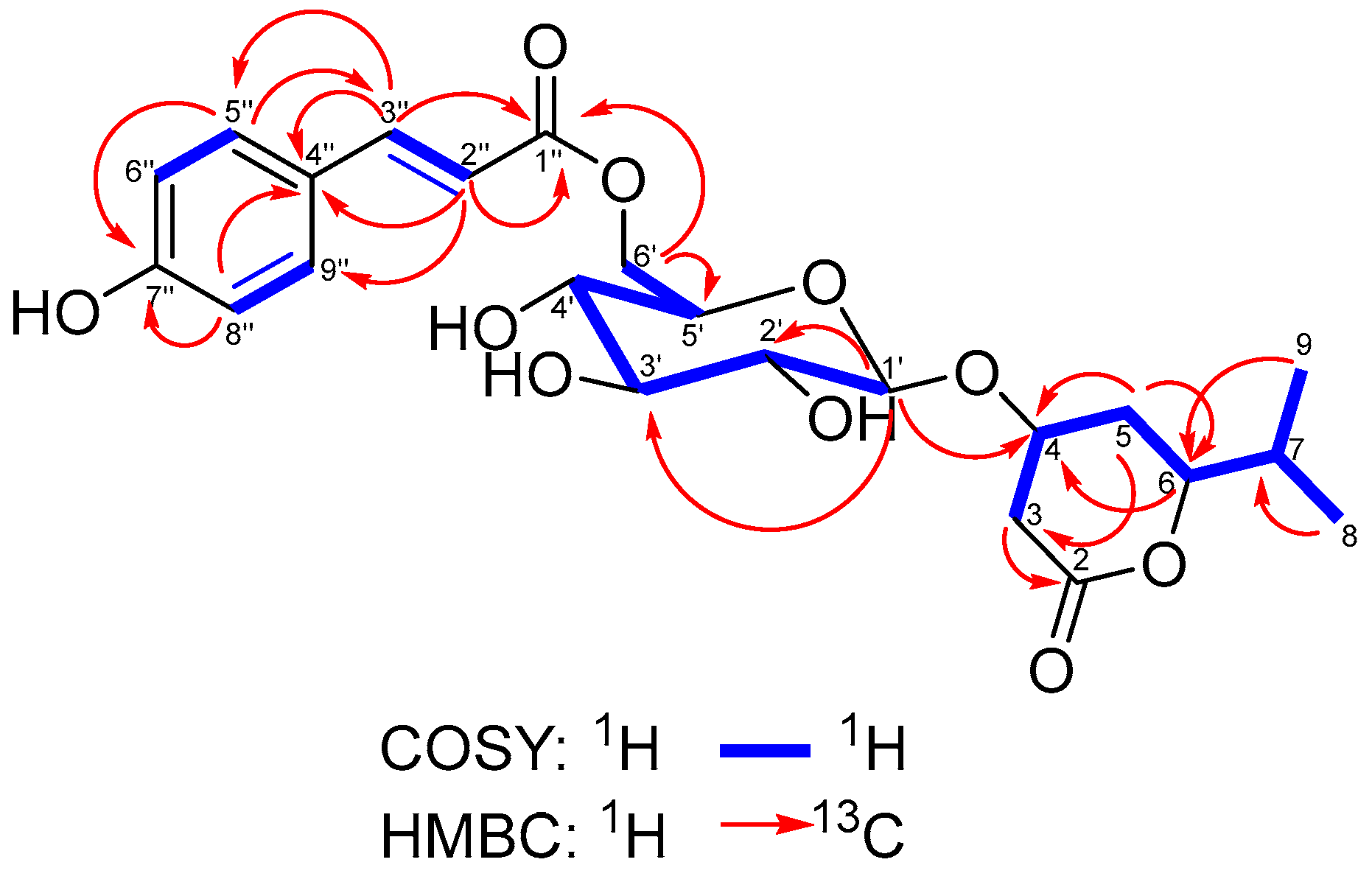
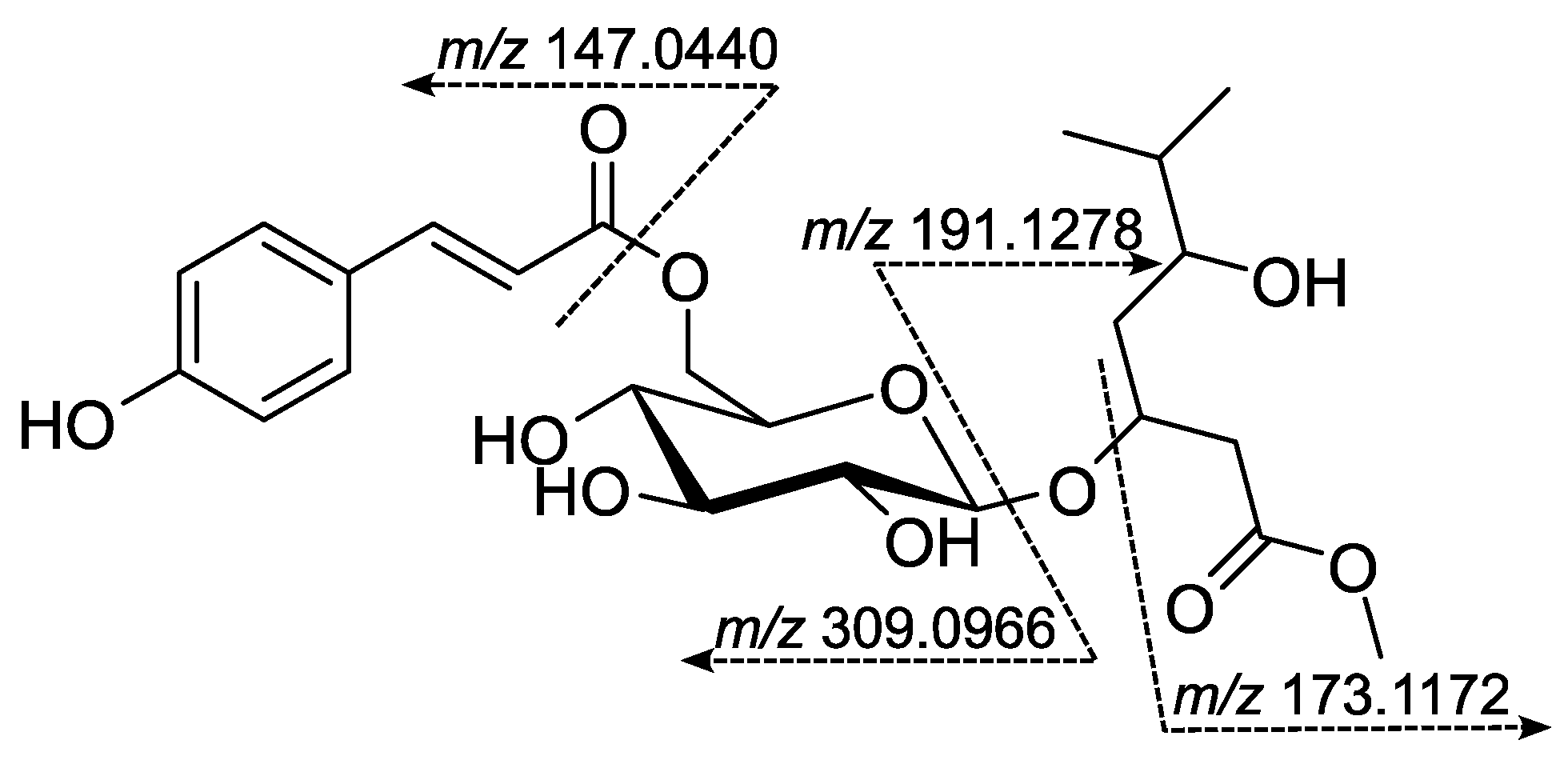

| Position | δH, Int., Mult. (J in Hz) | δC (ppm), Mult. 1 |
|---|---|---|
| 1 | - | 109.4, C |
| 2 | - | 158.6, C |
| 3 | 6.32, 1H, d (2.0) | 95.8, CH |
| 4 | - | 162.4, C |
| 5 | 6.18, 1H, d (2.0) | 98.0, CH |
| 6 | - | 160.0, C |
| 1′ | - | 140.3, C |
| 2′ | 7.72, 1H, m | 129.9, CH |
| 3′ | 7.44, 1H, m | 128.9, CH |
| 4′ | 7.55, 1H, m | 133.1, CH |
| 5′ | 7.44, 1H, m | 128.9, CH |
| 6′ | 7.72, 1H, m | 129.9, CH |
| C=O | - | 196.5, C |
| 1″ | 5.01, 1H, d (8.0) | 99.6, CH |
| 2″ | 4.46, 1H, dd (8.0, 9.6) | 73.7, CH |
| 3″ | 3.55, 1H, dd (9.6, 9.0) | 75.9, CH |
| 4″ | 3.41, 1H, dd (8.9, 9.6) | 71.3, CH |
| 5″ | 3.50, 1H, m | 77.9, CH |
| 6″ | 3.90, 1H, dd (2.6, 11.9) 3.71, 1H, dd (5.6, 11.9) | 62.4, CH2 |
| 1‴ | - | 169.3, C |
| 2‴ | 1.78, 3H, s | 20.7, CH3 |
| Position | δH, Int., Mult. (J in Hz) | δC (ppm), Mult. 1 |
|---|---|---|
| 2 | - | 169.8, C |
| 3 | 2.67, 2H, d (3.8) | 37.0, CH2 |
| 4 | 4.35, 1H, m | 72.9, CH |
| 5 | 2.27, 1H, m; 1.74, 1H, m | 31.7, CH2 |
| 6 | 4.45, 1H, m | 80.9, CH |
| 7 | 1.80, 1H, m | 33.1, CH |
| 8 | 0.91, 2H, d (6.8) | 18.4, CH3 |
| 9 | 0.91, 2H, d (6.8) | 17.7, CH3 |
| 1′ | 4.50, 1H, d (7.8) | 104.2, CH |
| 2′ | 3.24, 1H, dd (9.0, 7.8) | 74.7, CH |
| 3′ | 3.45, 1H, dd (9.0, 8.8) | 77.6, CH |
| 4′ | 3.40, 1H, dd (9.2, 8.8) | 71.2, CH |
| 5′ | 3.61, 1H, m | 75.0, CH |
| 6′ | 4.52, 1H, dd (11.8, 2.0); 4.29, 1H, dd (11.8, 6.4) | 64.3, CH2 |
| 1″ | - | 167.5, C |
| 2″ | 6.39, 1H, d (16.0) | 115.3, CH |
| 3″ | 7.64, 1H, d (16.0) | 145.6, CH |
| 4″ | 126.9, C | |
| 5″ | 7.57, 2H, d (8.6) | 131.0, CH |
| 6″ | 6.91, 2H, d (8.6) | 116.7, CH |
| 7″ | 160.7, CH | |
| 8″ | 6.91, 2H, d (8.6) | 116.7, CH |
| 9″ | 7.57, 2H, d (8.6) | 131.0, CH |
| Position | δH, Int., Mult. (J in Hz) | δC (ppm), Mult. 1 |
|---|---|---|
| 1 | - | 172.8, C |
| 2 | 2.65, 2H, m | 40.2, CH2 |
| 3 | 4.30, 1H, m | 76.8, CH |
| 4 | 1.78, 1H, m; 1.69, 1H, m | 40.3, CH2 |
| 5 | 3.54, 1H, m | 73.8, CH |
| 6 | 1.64, 1H, m | 34.4, CH |
| 7 | 0.85, 3H, d (6.8) | 19.3, CH3 |
| 8 | 0.83, 3H, d (6.8) | 17.4, CH3 |
| 9 | 3.64, 3H, s | 51.8, CH3 |
| 1′ | 4.49, 1H, d (7.8) | 103.9, CH |
| 2′ | 3.18, 1H, dd (9.0, 8.8) | 74.9, CH |
| 3’ | 3.45, 1H, dd (9.0, 8.8) | 77.7, CH |
| 4’ | 3.37, 1H, dd (9.0, 8.8) | 71.4, CH |
| 5’ | 3.61, 1H, m | 75.0, CH |
| 6’ | 4.52, 1H, dd (11.8, 2.2) 4.27, 1H, dd (11.8, 6.6) | 64.5, CH2 |
| 1’’ | - | 167.5, C |
| 2’’ | 6.40, 1H, d (16.0) | 116.7, CH |
| 3’’ | 7.63, 1H, d (16.0) | 145.5, CH |
| 4″ | - | 127.0, C |
| 5″ | 7.57, 2H, d (8.6) | 131.0, CH |
| 6″ | 6.91, 2H, d (8.6) | 115.5, CH |
| 7″ | - | 160.6, C |
| 8″ | 6.91, 2H, d (8.6) | 116.7, CH |
| 9″ | 7.57, 2H, d (8.6) | 131.0, CH |
| Compounds | DPPH % 1 | ABTS % 2 |
|---|---|---|
| coumaroylquinic acid (HC1) | 18.95 ± 0.36 | 22.12 ± 0.63 |
| myricetin-3-O-glucoside (HC2) | 84.32 ± 0.23 | 97.23 ± 0.45 |
| myricetin-3-O-galactoside (HC3) | 82.70 ± 0.34 | 96.04 ± 0.37 |
| hypercerastoside A (HC4) | 14.63 ± 0.50 | 70.20 ± 0.23 |
| methyl ester of chlorogenic acid (HC5) | 84.57 ± 0.27 | 71.27 ± 0.08 |
| hypercerastoside B (HC6) | 15.93 ± 0.33 | 25.50 ± 0.12 |
| hypercerastoside C (HC7) | 11.48 ± 0.21 | 26.88 ± 0.33 |
| Vit C | 59.44 ± 0.42 | 66.21 ± 0.45 |
| Trolox | 88.33 ± 0.33 | 94.16 ± 0.32 |
| Compounds | α-Glucosidase Inhibitory Activity (IC50 ± SD) 1 |
|---|---|
| coumaroylquinic acid (HC1) | 44 ± 5 µM |
| myricetin-3-O-glucoside (HC2) | NA 2 |
| myricetin-3-O-galactoside (HC3) | 206 ± 13 µM |
| hypercerastoside A (HC4) | NA 2 |
| methyl ester of chlorogenic acid (HC5) | NA 2 |
| hypercerastoside B (HC6) | 371 ± 9 µM |
| hypercerastoside C (HC7) | NA 2 |
| Acarbose | 206 ± 10 µM |
| Compounds | Pro-Lipase Activity (%) 1 at | ||||
|---|---|---|---|---|---|
| 200 µM | 100 µM | 50 µM | 25 µM | 12.5 µM | |
| HC1 | 31.24 ± 0.70 | 24.44 ± 0.46 | 15.05 ± 0.27 | 10.86 ± 0.03 | 7.15 ± 0.17 |
| HC2 | 479.69 ± 7.63 | 257.38 ± 3.68 | 137.99 ± 2.69 | 72.56 ± 1.25 | 61.94 ± 0.44 |
| HC3 | 492.57 ± 10.25 | 263.68 ± 4.96 | 153.47 ± 3.56 | 85.85 ± 1.19 | 46.98 ± 0.48 |
| HC4 | NA 2 | NA 2 | NA 2 | NA 2 | NA 2 |
| HC5 | 169.79 ± 4.23 | 90.09 ± 2.19 | 46.87 ± 1.12 | 28.24 ± 0.61 | 21.39 ± 0.21 |
| HC6 | 36.55 ± 0.57 | 27 ± 0.50 | 15.96 ± 0.23 | 13.75 ± 0.31 | 8.28 ± 0.17 |
| HC7 | NA 1 | NA 1 | NA 1 | NA 1 | NA 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokanova-Nedialkova, Z.; Ilieva, Y.; Marinov, T.; Nedialkov, P.T. Phenolic Compounds from Hypericum cerastoides (Spach) N. Robson: Dereplication via UHPLC-HRMS/MS, Isolation, Identification, and Preliminary Biological Evaluation Focusing on Radical-Scavenging, Anti-α-Glucosidase, and Pro-Lipase Activities. Metabolites 2025, 15, 643. https://doi.org/10.3390/metabo15100643
Kokanova-Nedialkova Z, Ilieva Y, Marinov T, Nedialkov PT. Phenolic Compounds from Hypericum cerastoides (Spach) N. Robson: Dereplication via UHPLC-HRMS/MS, Isolation, Identification, and Preliminary Biological Evaluation Focusing on Radical-Scavenging, Anti-α-Glucosidase, and Pro-Lipase Activities. Metabolites. 2025; 15(10):643. https://doi.org/10.3390/metabo15100643
Chicago/Turabian StyleKokanova-Nedialkova, Zlatina, Yana Ilieva, Teodor Marinov, and Paraskev T. Nedialkov. 2025. "Phenolic Compounds from Hypericum cerastoides (Spach) N. Robson: Dereplication via UHPLC-HRMS/MS, Isolation, Identification, and Preliminary Biological Evaluation Focusing on Radical-Scavenging, Anti-α-Glucosidase, and Pro-Lipase Activities" Metabolites 15, no. 10: 643. https://doi.org/10.3390/metabo15100643
APA StyleKokanova-Nedialkova, Z., Ilieva, Y., Marinov, T., & Nedialkov, P. T. (2025). Phenolic Compounds from Hypericum cerastoides (Spach) N. Robson: Dereplication via UHPLC-HRMS/MS, Isolation, Identification, and Preliminary Biological Evaluation Focusing on Radical-Scavenging, Anti-α-Glucosidase, and Pro-Lipase Activities. Metabolites, 15(10), 643. https://doi.org/10.3390/metabo15100643








