Changes in Phenylacetylglutamine Levels Provide Add-On Value in Risk Stratification of Hypertensive Patients: A Longitudinal Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Plasma PAGln Measurements
2.3. Outcomes
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Association Between PAGln Concentrations and MACEs in Hypertension
3.3. Predictive Performance of PAGln in Hypertensive Patients
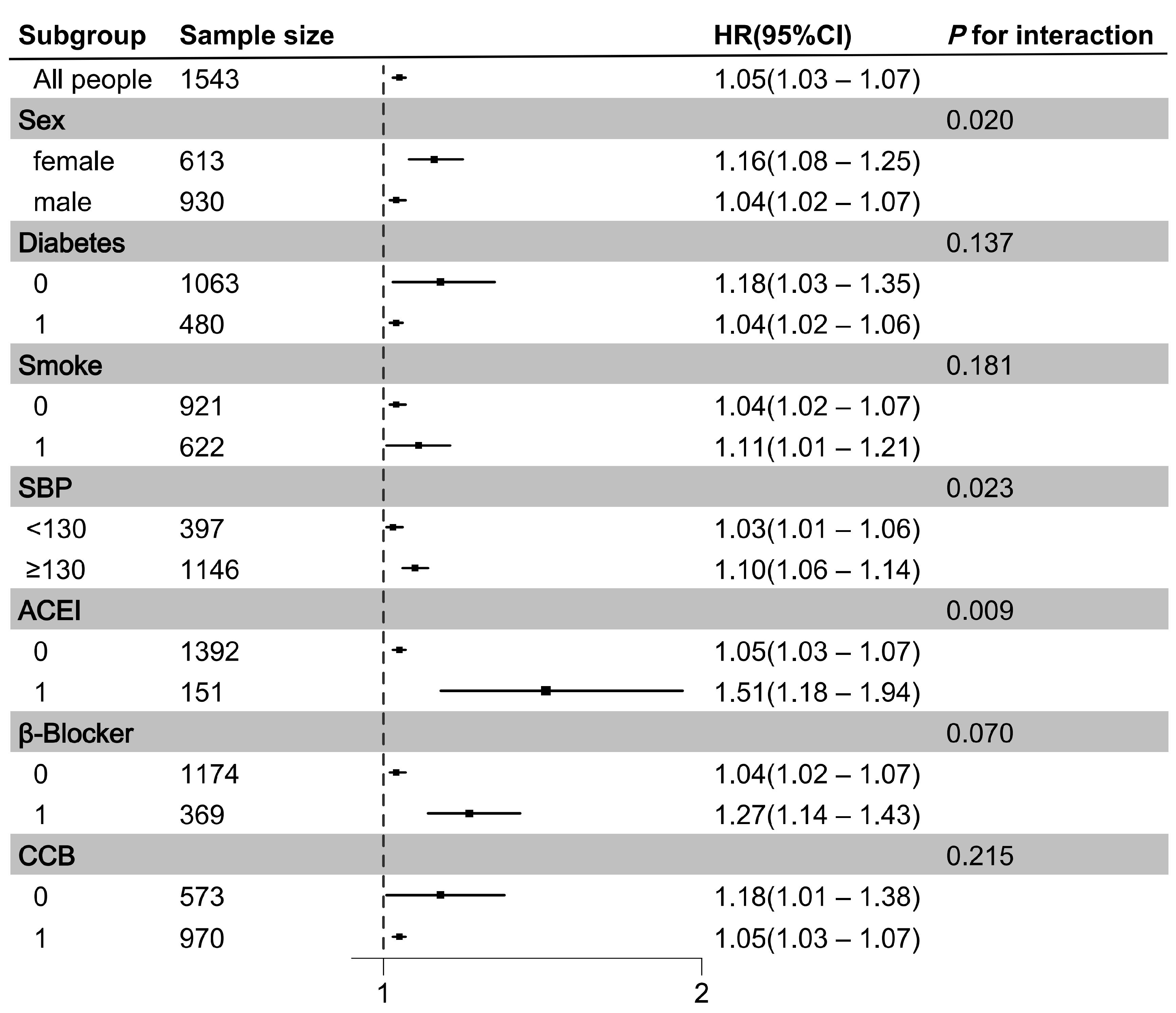
3.4. Associations Between PAGln and MACEs in Subgroups and Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cifkova, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Primers 2018, 4, 18014. [Google Scholar] [CrossRef] [PubMed]
- Al-Mashhadi, R.H.; Al-Mashhadi, A.L.; Nasr, Z.P.; Mortensen, M.B.; Lewis, E.A.; Camafeita, E.; Ravlo, K.; Al-Mashhadi, Z.; Kjaer, D.W.; Palmfeldt, J.; et al. Local Pressure Drives Low-Density Lipoprotein Accumulation and Coronary Atherosclerosis in Hypertensive Minipigs. J. Am. Coll. Cardiol. 2021, 77, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Bress, A.P.; Greene, T.; Derington, C.G.; Shen, J.; Xu, Y.; Zhang, Y.; Ying, J.; Bellows, B.K.; Cushman, W.C.; Whelton, P.K.; et al. Patient Selection for Intensive Blood Pressure Management Based on Benefit and Adverse Events. J. Am. Coll. Cardiol. 2021, 77, 1977–1990. [Google Scholar] [CrossRef]
- Rahimi, K.; Bidel, Z.; Nazarzadeh, M.; Copland, E.; Canoy, D.; Ramakrishnan, R.; Pinho-Gomes, A.C.; Woodward, M.; Adler, A.; Agodoa, L.; et al. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: An individual participant-level data meta-analysis. Lancet 2021, 397, 1625–1636. [Google Scholar] [CrossRef]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, 1269–1324. [Google Scholar] [CrossRef]
- Badoud, F.; Lam, K.P.; DiBattista, A.; Perreault, M.; Zulyniak, M.A.; Cattrysse, B.; Stephenson, S.; Britz-McKibbin, P.; Mutch, D.M. Serum and Adipose Tissue Amino Acid Homeostasis in the Metabolically Healthy Obese. J. Proteome Res. 2014, 13, 3455–3466. [Google Scholar] [CrossRef]
- Gao, A.W.; Sterken, M.G.; uit de Bos, J.; van Creij, J.; Kamble, R.; Snoek, B.L.; Kammenga, J.E.; Houtkooper, R.H. Natural genetic variation in C. elegans identified genomic loci controlling metabolite levels. Genome Res. 2018, 28, 1296–1308. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mandal, J.; Yang, T.; Cheng, X.; Yeo, J.Y.; McCarthy, C.G.; Wenceslau, C.F.; Koch, L.G.; Hill, J.W.; Vijay-Kumar, M.; et al. Metabolites and Hypertension: Insights into Hypertension as a Metabolic Disorder: 2019 Harriet Dustan Award. Hypertension 2020, 75, 1386–1396. [Google Scholar] [CrossRef]
- Suhong, Z.; Yanan, T.; Shanjie, W.; Fan, Y.; Junyan, X.; Zhifeng, Q.; Xinxin, L.; Mingming, C.; Peng, Z.; Guohua, Z.; et al. Prognostic value of gut microbiota-derived metabolites in patients with ST-segment elevation myocardial infarction. Am. J. Clin. Nutr. 2023, 117, 499–508. [Google Scholar] [CrossRef]
- Tan, Y.; Zhou, J.; Yang, S.; Li, J.; Zhao, H.; Song, L.; Yan, H. Addition of Plasma Myeloperoxidase and Trimethylamine N-Oxide to the GRACE Score Improves Prediction of Near-Term Major Adverse Cardiovascular Events in Patients with ST-Segment Elevation Myocardial Infarction. Front. Pharmacol. 2021, 12, 632075. [Google Scholar] [CrossRef]
- Nemet, I.; Li, X.S.; Haghikia, A.; Li, L.; Wilcox, J.; Romano, K.A.; Buffa, J.A.; Witkowski, M.; Demuth, I.; Konig, M.; et al. Atlas of gut microbe-derived products from aromatic amino acids and risk of cardiovascular morbidity and mortality. Eur. Heart J. 2023, 44, 3085–3096. [Google Scholar] [CrossRef] [PubMed]
- Marcin, C.; Beata, S.; Kamil, L.; Łukasz, Ł.; Mateusz, C.; Urszula, W.; Tomasz, R.; Jadwiga, R.; Magdalena, W. Co-toxicity of Endotoxin and Indoxyl Sulfate, Gut-Derived Bacterial Metabolites, to Vascular Endothelial Cells in Coronary Arterial Disease Accompanied by Gut Dysbiosis. Nutrients 2022, 14, 424. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Nemet, I.; Saha, P.P.; Gupta, N.; Zhu, W.; Romano, K.A.; Skye, S.M.; Cajka, T.; Mohan, M.L.; Li, L.; Wu, Y.; et al. A Cardiovascular Disease-Linked Gut Microbial Metabolite Acts via Adrenergic Receptors. Cell 2020, 180, 862–877.e22. [Google Scholar] [CrossRef]
- Wei, H.; Wu, J.; Wang, H.; Huang, J.; Li, C.; Zhang, Y.; Song, Y.; Zhou, Z.; Sun, Y.; Xiao, L.; et al. Increased circulating phenylacetylglutamine concentration elevates the predictive value of cardiovascular event risk in heart failure patients. J. Intern. Med. 2023, 294, 515–530. [Google Scholar] [CrossRef]
- Liu, L.-S.; Joint Committee for Guideline Revision. 2018 Chinese Guidelines for Prevention and Treatment of Hypertension—A report of the Revision Committee of Chinese Guidelines for Prevention and Treatment of Hypertension. J. Geriatr. Cardiol. 2019, 16, 182–241. [Google Scholar] [CrossRef]
- Goff, D.C.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’Donnell, C.J.; et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk. Circulation 2014, 129 (Suppl. 2), S49–S73. [Google Scholar] [CrossRef]
- Heianza, Y.; Tiwari, S.; Wang, X.; Watrous, J.D.; Rexrode, K.M.; Hu, F.B.; Alotaibi, M.; Jain, M.; Sun, Q.; Manson, J.E.; et al. Gut-Microbiota-Related Metabolite Phenylacetylglutamine and Risk of Incident Coronary Heart Disease Among Women. J. Clin. Endocrinol. Metab. 2024, dgae525. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Zhao, Z.; Song, X.; Qu, H.; Liu, H. Phenylacetylglutamine is associated with the degree of coronary atherosclerotic severity assessed by coronary computed tomographic angiography in patients with suspected coronary artery disease. Atherosclerosis 2021, 333, 75–82. [Google Scholar] [CrossRef]
- Ottosson, F.; Brunkwall, L.; Smith, E.; Orho-Melander, M.; Nilsson, P.M.; Fernandez, C.; Melander, O. The gut microbiota-related metabolite phenylacetylglutamine associates with increased risk of incident coronary artery disease. J. Hypertens. 2020, 38, 2427–2434. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension. J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Piechocki, M.; Przewłocki, T.; Pieniążek, P.; Trystuła, M.; Podolec, J.; Kabłak-Ziembicka, A. A Non-Coronary, Peripheral Arterial Atherosclerotic Disease (Carotid, Renal, Lower Limb) in Elderly Patients-A Review PART II-Pharmacological Approach for Management of Elderly Patients with Peripheral Atherosclerotic Lesions outside Coronary Territory. J. Clin. Med. 2024, 13, 1508. [Google Scholar] [CrossRef] [PubMed]
- Messerli, F.H.; Bangalore, S.; Mandrola, J.M. β blockers switched to first-line therapy in hypertension. Lancet 2023, 402, 1802–1804. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.S.; Hwang, S.Y.; Choi, J.H.; Lee, H.J.; Yoo, H.J.; Seo, J.-A.; Kim, S.G.; Kim, N.H.; Choi, D.S.; Baik, S.H.; et al. Effects of Low Muscle Mass on Albuminuria and Chronic Kidney Disease in Patients with Type 2 Diabetes: The Korean Sarcopenic Obesity Study (KSOS). J. Gerontol. Ser. A 2018, 73, 386–392. [Google Scholar] [CrossRef]
- Huang, L. Phenylacetylglutamine causes a pathologic inflammation state and enhances atherosclerosis through the b2-adrenergic receptor cAMP PKA NF-kappaB pathway in diabetes. Eur. Heart J. 2023, 44 (Suppl. 2), ehad655.3289. [Google Scholar] [CrossRef]
- Allemann, M.; Saeedi Saravi, S.S.; Lee, P.; Beer, J.H. Gut-microbiota-derived phenylacetylglutamine induces oxidative stress in endothelial cells via a NOX2-dependent pathway. Eur. Heart J. 2023, 44 (Suppl. 2), ehad655.2052. [Google Scholar] [CrossRef]
- Konstantinou, K.; Tsioufis, C.; Koumelli, A.; Mantzouranis, M.; Kasiakogias, A.; Doumas, M.; Tousoulis, D. Hypertension and patients with acute coronary syndrome: Putting blood pressure levels into perspective. J. Clin. Hypertens. 2019, 21, 1135–1143. [Google Scholar] [CrossRef]
- Wertheimer, A.; Collino, S.; Montoliu, I.; Martin, F.-P.J.; Scherer, M.; Mari, D.; Salvioli, S.; Bucci, L.; Ostan, R.; Monti, D.; et al. Metabolic Signatures of Extreme Longevity in Northern Italian Centenarians Reveal a Complex Remodeling of Lipids, Amino Acids, and Gut Microbiota Metabolism. PLoS ONE 2013, 8, e56564. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, N.; Arikawa, A.Y.; Chen, C. Inhibitory Effects of Green Tea Polyphenols on Microbial Metabolism of Aromatic Amino Acids in Humans Revealed by Metabolomic Analysis. Metabolites 2019, 9, 96. [Google Scholar] [CrossRef]
- Zhu, Y.; Dwidar, M.; Nemet, I.; Buffa, J.A.; Sangwan, N.; Li, X.S.; Anderson, J.T.; Romano, K.A.; Fu, X.; Funabashi, M.; et al. Two distinct gut microbial pathways contribute to meta-organismal production of phenylacetylglutamine with links to cardiovascular disease. Cell Host Microbe 2023, 31, 18–32.e9. [Google Scholar] [CrossRef]
- Mushtaq, N.; Hussain, S.; Yuan, L.; Zhang, S.; Li, H.; Ullah, S.; Xu, J. Gender Based Compositional Fluctuations in Gut Microbiota in Hypertension. Cardiovasc. Pharmacol. Open Access 2018, 7, 248. [Google Scholar] [CrossRef]
- Menni, C.; Mangino, M.; Cecelja, M.; Psatha, M.; Brosnan, M.J.; Trimmer, J.; Mohney, R.P.; Chowienczyk, P.; Padmanabhan, S.; Spector, T.D.; et al. Metabolomic study of carotid–femoral pulse-wave velocity in women. J. Hypertens. 2015, 33, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Avolio, A.; Seo, D.C.; Kim, B.S.; Kang, J.H.; Lee, M.Y.; Sung, K.C. Relationship Between Brachial-Ankle Pulse Wave Velocity and Incident Hypertension According to 2017 ACC/AHA High Blood Pressure Guidelines. J. Am. Heart Assoc. 2019, 8, e013019. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-H.; Chen, Y.-C.; Ho, C.-C.; Hsu, B.-G. Serum Phenylacetylglutamine among Potential Risk Factors for Arterial Stiffness Measuring by Carotid–Femoral Pulse Wave Velocity in Patients with Kidney Transplantation. Toxins 2024, 16, 111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rojas, S.; Singh, S.; Musich, P.R.; Gutierrez, M.; Yao, Z.; Thewke, D.; Jiang, Y. Wnt2 Contributes to the Development of Atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 751720. [Google Scholar] [CrossRef]
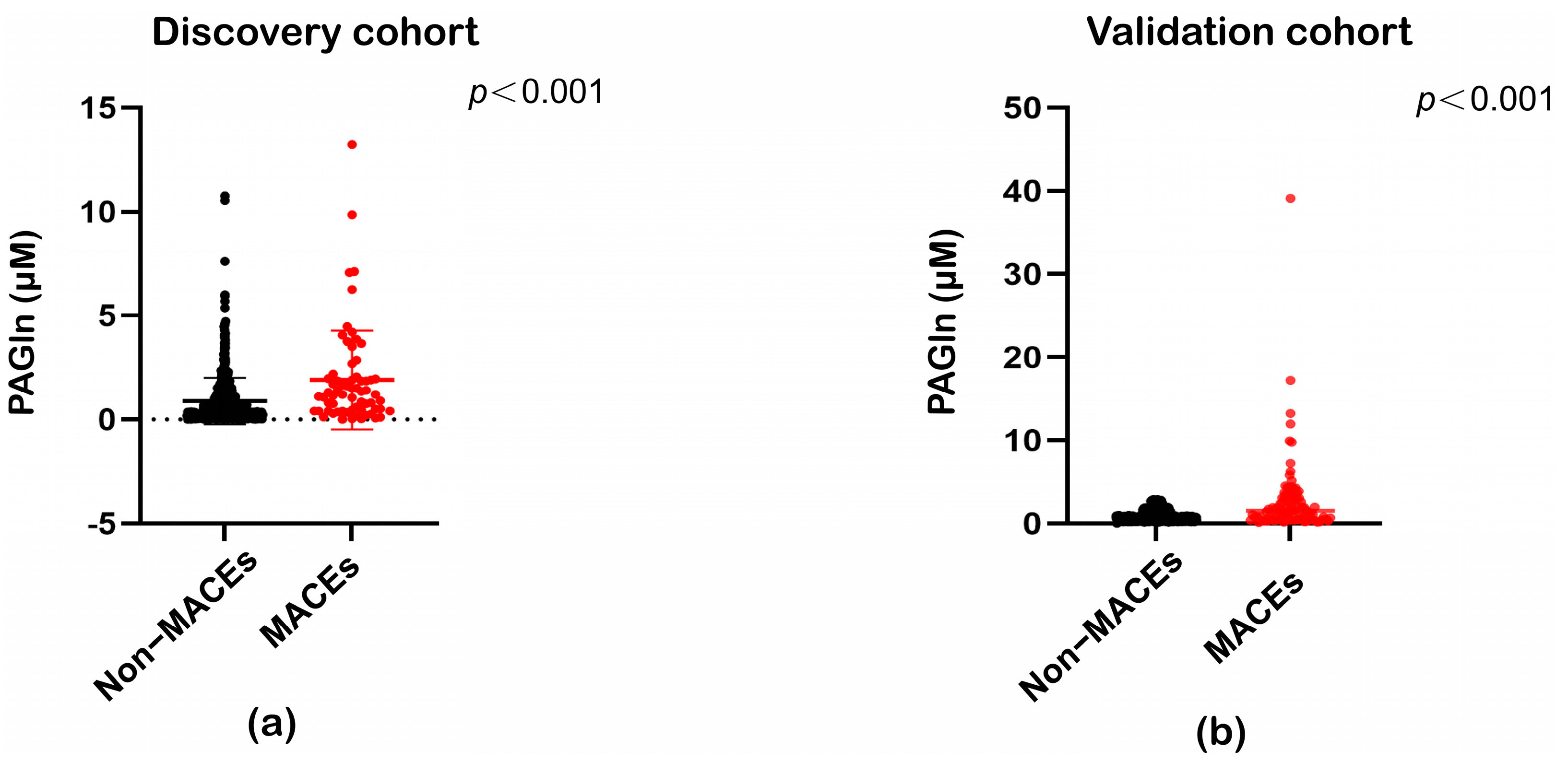

| Discovery Cohort | Validation Cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| All (n = 792) | Low 2 (n = 548) | High 2 (n = 244) | p Value | All (n = 751) | Low 2 (n = 328) | High 2 (n = 423) | p Value | |
| Demographic characteristics | ||||||||
| Age (years) | 50 [39, 61] | 47 [37, 59] | 57 [45, 67] | <0.001 | 57 [47, 66] | 51 [40, 60] | 62 [52, 69] | <0.001 |
| Sex (male) | 480 (60.61%) | 347 (63.32%) | 133 (54.51%) | 0.019 | 450 (59.92%) | 199 (60.67%) | 251 (59.34%) | 0.712 |
| Smoking | 330 (41.67%) | 226 (41.24%) | 104 (42.62%) | 0.716 | 292 (38.88%) | 129 (39.33%) | 163 (38.53%) | 0.825 |
| Systolic pressure (mmHg) | 140 [130, 154] | 140 [130, 154] | 140 [129, 153] | 0.488 | 139 [128, 151] | 141 [130, 154] | 138 [127, 150] | 0.022 |
| Heart rate (bpm) | 71 [64, 80] | 71 [64, 80] | 69 [62, 79] | 0.012 | 75 [68, 82] | 75 [68, 82] | 75 [68, 82] | 0.926 |
| Medical history | ||||||||
| History of diabetes | 240 (30.30%) | 149 (27.19%) | 91 (37.30%) | 0.004 | 240 (31.96%) | 83 (25.30%) | 157 (37.12%) | 0.001 |
| History of CHD | 70 (8.84%) | 32 (7.16%) | 38 (27.27%) | <0.001 | 181 (24.10%) | 54 (16.46%) | 127 (30.02%) | <0.001 |
| History of TIA | 104 (13.13%) | 63 (11.50%) | 41 (16.80%) | 0.041 | 102 (13.58%) | 26 (7.93%) | 76 (17.97%) | <0.001 |
| Laboratory measurements | ||||||||
| TC (mmol/L) | 4.73 [4.07, 5.43] | 4.83 [4.20, 5.47] | 4.52 [3.75, 5.34] | <0.001 | 4.57 [3.80, 5.34] | 4.79 [3.96, 5.48] | 4.37 [3.59, 5.23] | <0.001 |
| TG (mmol/L) | 1.51 [1.04, 2.20] | 1.57 [1.10, 2.25] | 1.36 [0.94, 2.07] | 0.007 | 1.84 [1.25, 3.11] | 1.95 [1.32, 3.39] | 1.78 [1.15, 2.76] | 0.004 |
| HDL cholesterol (mmol/L) | 1.16 [1.01, 1.36] | 1.15 [1.02, 1.36] | 1.16 [0.98, 1.36] | 0.781 | 1.13 [0.95, 1.32] | 1.13 [0.96, 1.29] | 1.12 [0.94, 1.34] | 0.857 |
| LDL cholesterol (mmol/L) | 2.91 [2.25, 3.47] | 2.96 [2.36, 3.49] | 2.66 [2.05, 3.43] | 0.005 | 2.76 [2.13, 3.38] | 2.93 [2.32, 3.55] | 2.62 [2.00, 3.22] | <0.001 |
| HCY (μmol/L) | 11.40 [9.00, 15.70] | 11.10 [9.15, 16.35] | 12.15 [9.15, 16.35] | 0.307 | 13.20 [11.00, 16.50] | 13.15 [10.90, 15.85] | 13.20 [11.00, 17.00] | 0.588 |
| Cr (μmol/L) | 68.90 [57.45, 80.30] | 68.10 [56.85, 78.70] | 71.30 [59.30, 85.05] | 0.005 | 70.90 [60.50, 83.90] | 70.45 [60.90, 84.80] | 71.20 [59.70, 82.80] | 0.663 |
| Fasting blood glucose (mmol/L) | 5.60 [5.13, 6.52] | 5.58 [5.13, 6.41] | 5.62 [5.12, 6.86] | 0.206 | 5.67 [5.04, 6.99] | 5.63 [5.04, 6.63] | 5.72 [5.05, 7.12] | 0.160 |
| Echocardiography | ||||||||
| EF (%) | 65 [62, 68] | 65 [62, 68] | 65 [62, 68] | 0.927 | 65 [62, 68] | 65 [62, 68] | 65 [62, 68] | 0.944 |
| PWV (m/s) | 15.19 [13.92, 17.25] | 15.02 [13.82, 16.99] | 15.76 [14.20, 17.89] | 0.003 | 14.94 [13.65, 17.11] | 14.93 [13.67, 16.90] | 15.00 [13.58, 17.21] | 0.685 |
| ABI | 1.16 [1.11, 1.20] | 1.16 [1.11, 1.20] | 1.16 [1.10, 1.20] | 0.448 | 1.18 [1.12, 1.24] | 1.18 [1.12, 1.25] | 1.18 [1.12, 1.24] | 0.369 |
| Carotid plaque 3 | 577 (72.85%) | 375 (68.43%) | 202 (82.79%) | <0.001 | 477 (63.52%) | 175 (53.35%) | 302 (71.39%) | <0.001 |
| Medication | ||||||||
| β-blocker | 254 (32.07%) | 170 (31.02%) | 84 (34.43%) | 0.343 | 115 (15.31%) | 48 (14.63%) | 67 (15.84%) | 0.649 |
| ACEI | 82 (10.35%) | 53 (9.67%) | 29 (11.89%) | 0.041 | 69 (9.19%) | 34 (10.37%) | 35 (8.27%) | 0.325 |
| ARB | 365 (46.09%) | 260 (47.45%) | 105 (43.03%) | 0.250 | 257 (34.22%) | 105 (32.01%) | 152 (35.93%) | 0.261 |
| CCB | 543 (68.56%) | 363 (66.24%) | 180 (73.77%) | 0.035 | 427 (56.86%) | 194 (59.15%) | 233 (55.08%) | 0.265 |
| Diuretic | 155 (19.57%) | 102 (18.61%) | 53 (21.72%) | 0.482 | 80 (10.65%) | 33 (10.06%) | 47 (11.11%) | 0.644 |
| Plasma PAGln (μmol/L) | Number of Events (%) | Crude Model | Multivariable 1 1 | Multivariable 2 2 | Multivariable 3 3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| HR [95% CI] | p Value | HR [95% CI] | p Value | HR [95% CI] | p Value | HR [95% CI] | p Value | ||
| Discovery cohort | |||||||||
| PAGln < 1.047 | 69.19% | 1.0 [referent] | 1.0 [referent] | 1.0 [referent] | 1.0 [referent] | ||||
| PAGln ≥ 1.047 | 30.81% | 3.02 [1.85–4.93] | <0.001 | 2.20 [1.33–3.65] | 0.002 | 2.19 [1.31–3.66] | 0.003 | 2.32 [1.38–3.89] | 0.001 |
| PAGln 4 (continuous variable) | 9.09% | 1.29 [1.18–1.41] | <0.001 | 1.17 [1.06–1.28] | 0.030 | 1.13 [1.01–1.25] | 0.033 | 1.12 [1.00–1.25] | 0.043 |
| Per 1-SD 5 | 1.59 [1.29–1.97] | <0.001 | 1.34 [1.09–1.65] | 0.005 | 1.30 [1.06–1.60] | 0.012 | 1.33 [1.08–1.63] | 0.006 | |
| Validation cohort | |||||||||
| PAGln < 1.047 | 43.68% | 1.0 [referent] | 1.0 [referent] | 1.0 [referent] | 1.0 [referent] | ||||
| PAGln ≥ 1.047 | 56.32% | 4.15 [2.33–7.38] | <0.001 | 2.21 [1.21–4.05] | 0.010 | 2.08 [1.13–3.82] | 0.018 | 2.01 [1.12–3.78] | 0.020 |
| PAGln 4 (continuous variable) | 12.26% | 1.06 [1.05–1.08] | <0.001 | 1.05 [1.03–1.08] | <0.001 | 1.05 [1.02–1.07] | <0.001 | 1.05 [1.02–1.07] | <0.001 |
| Per 1-SD 5 | 2.39 [1.90–3.00] | <0.001 | 1.85 [1.43–2.40] | <0.001 | 1.79 [1.33–2.23] | <0.001 | 1.69 [1.31–2.18] | <0.001 | |
| C-Statistic (95% CI) | p Value | NRI (95% CI) | NRI p Value | IDI (95% CI) | IDI p Value | |
|---|---|---|---|---|---|---|
| Discovery cohort | ||||||
| ASCVD 1 | 0.725 [0.665–0.784] | reference | reference | |||
| ASCVD + PAGln | 0.736 [0.674–0.797] | 0.001 | 0.069 [0.005–0.133] | 0.036 | 0.025 [0.002–0.048] | 0.031 |
| Validation cohort | ||||||
| ASCVD | 0.775 [0.726–0.824] | reference | reference | |||
| ASCVD + PAGln | 0.779 [0.730–0.828] | 0.010 | 0.045 [0.010–0.080] | 0.012 | 0.099 [0.011–0.187] | 0.028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Jia, L.; Qiao, B.; Gong, Y.; Gao, S.; Wang, Y.; Du, J. Changes in Phenylacetylglutamine Levels Provide Add-On Value in Risk Stratification of Hypertensive Patients: A Longitudinal Cohort Study. Metabolites 2025, 15, 64. https://doi.org/10.3390/metabo15010064
Xu X, Jia L, Qiao B, Gong Y, Gao S, Wang Y, Du J. Changes in Phenylacetylglutamine Levels Provide Add-On Value in Risk Stratification of Hypertensive Patients: A Longitudinal Cohort Study. Metabolites. 2025; 15(1):64. https://doi.org/10.3390/metabo15010064
Chicago/Turabian StyleXu, Xuan, Lixin Jia, Bokang Qiao, Yanyan Gong, Shan Gao, Yuan Wang, and Jie Du. 2025. "Changes in Phenylacetylglutamine Levels Provide Add-On Value in Risk Stratification of Hypertensive Patients: A Longitudinal Cohort Study" Metabolites 15, no. 1: 64. https://doi.org/10.3390/metabo15010064
APA StyleXu, X., Jia, L., Qiao, B., Gong, Y., Gao, S., Wang, Y., & Du, J. (2025). Changes in Phenylacetylglutamine Levels Provide Add-On Value in Risk Stratification of Hypertensive Patients: A Longitudinal Cohort Study. Metabolites, 15(1), 64. https://doi.org/10.3390/metabo15010064






