The Positive Regulatory Effect of DBT on Lipid Metabolism in Postpartum Dairy Cows
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of DBT
2.2. Animal Grouping, Management, and Sample Collection
2.3. Measurement of Serum Biochemical Indices
2.4. Faecal 16S rRNA Amplicon Sequencing and Data Processing
2.5. Targeted Analysis of Plasma Metabolites and Data Processing
2.6. Statistical Analysis
3. Results
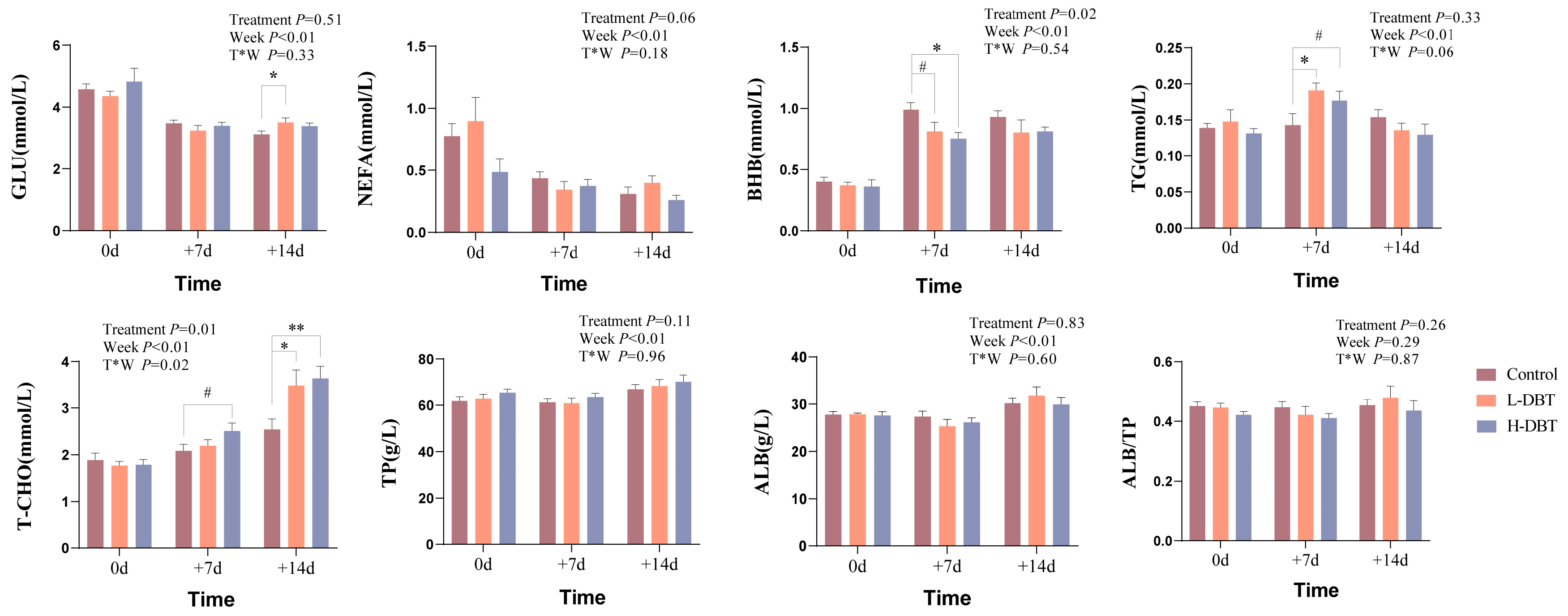
3.1. Effect of DBT on Serum Biochemical Indices in Postpartum Dairy Cows
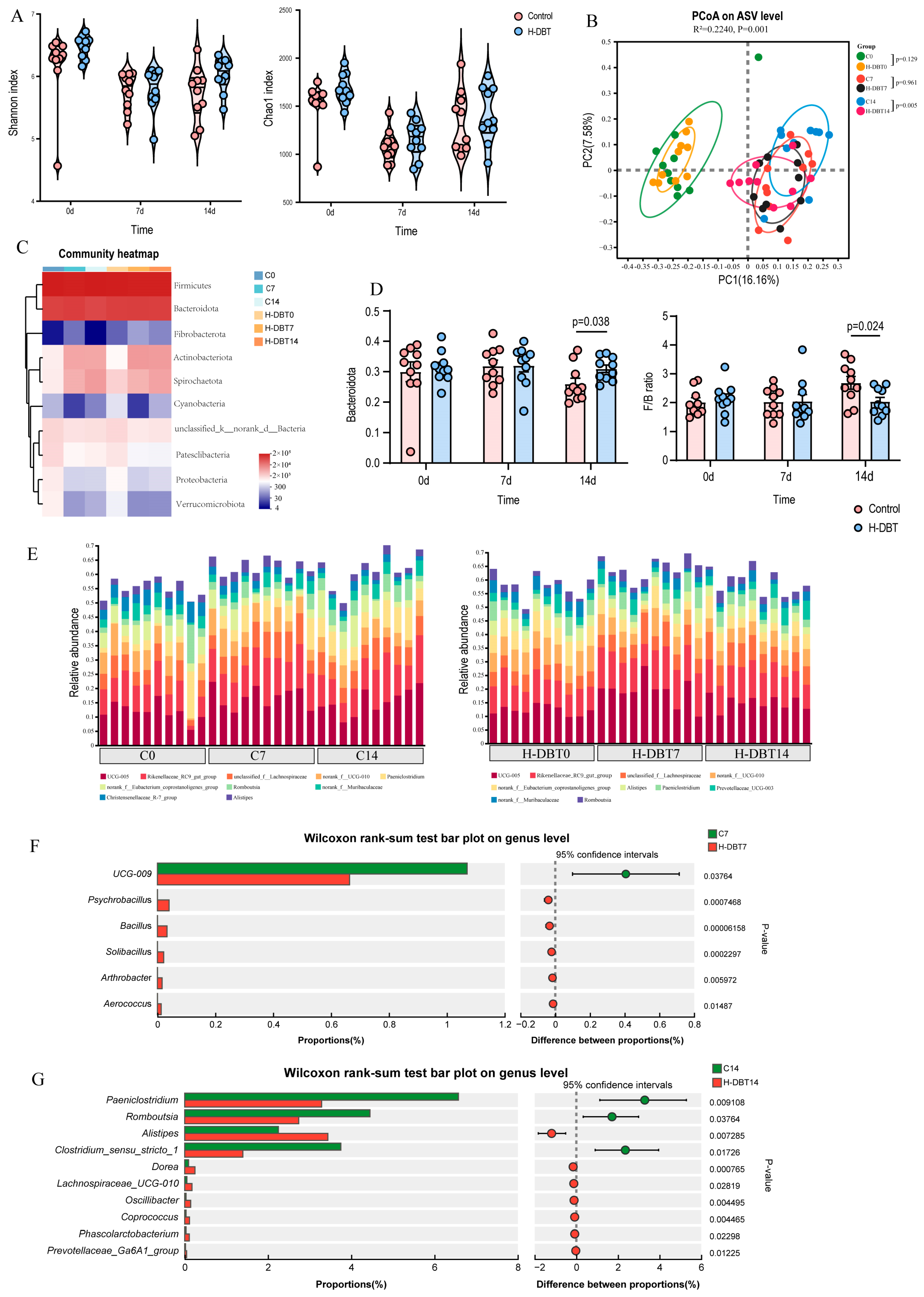
3.2. DBT-Modulated Intestinal Microbiota
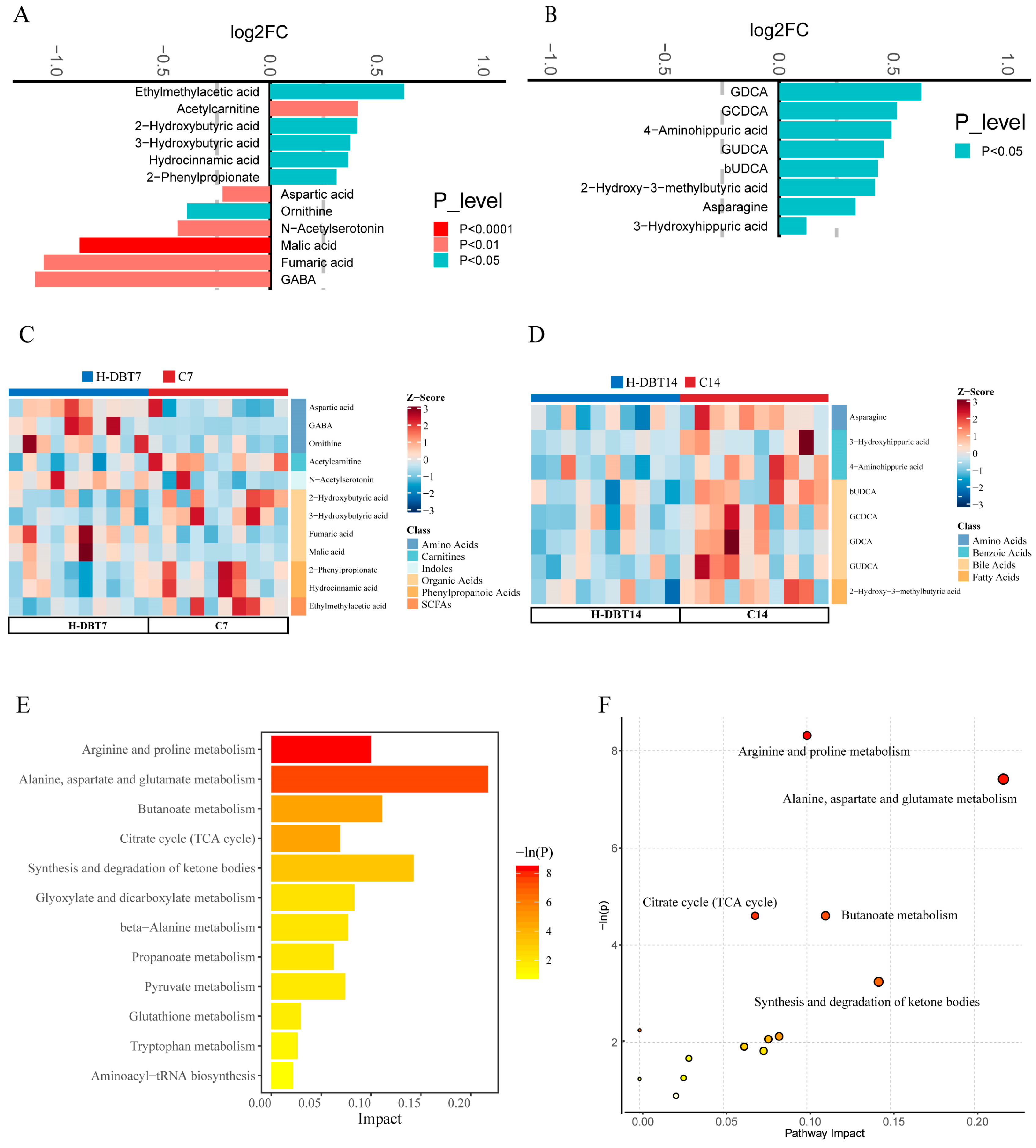
3.3. DBT Changed Plasma Metabolic Profiles
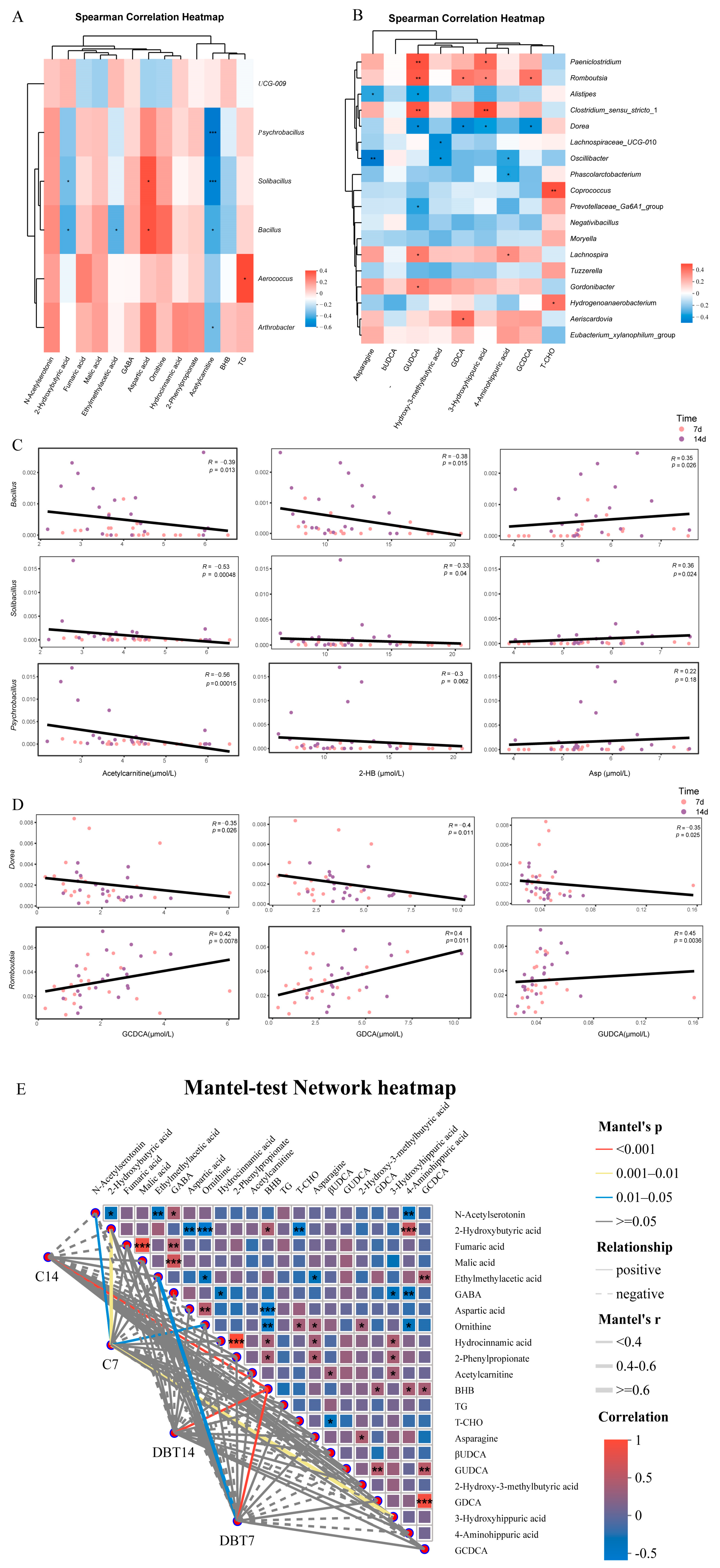
3.4. Correlation Among Phenotypes, Genera, and Plasma Metabolites
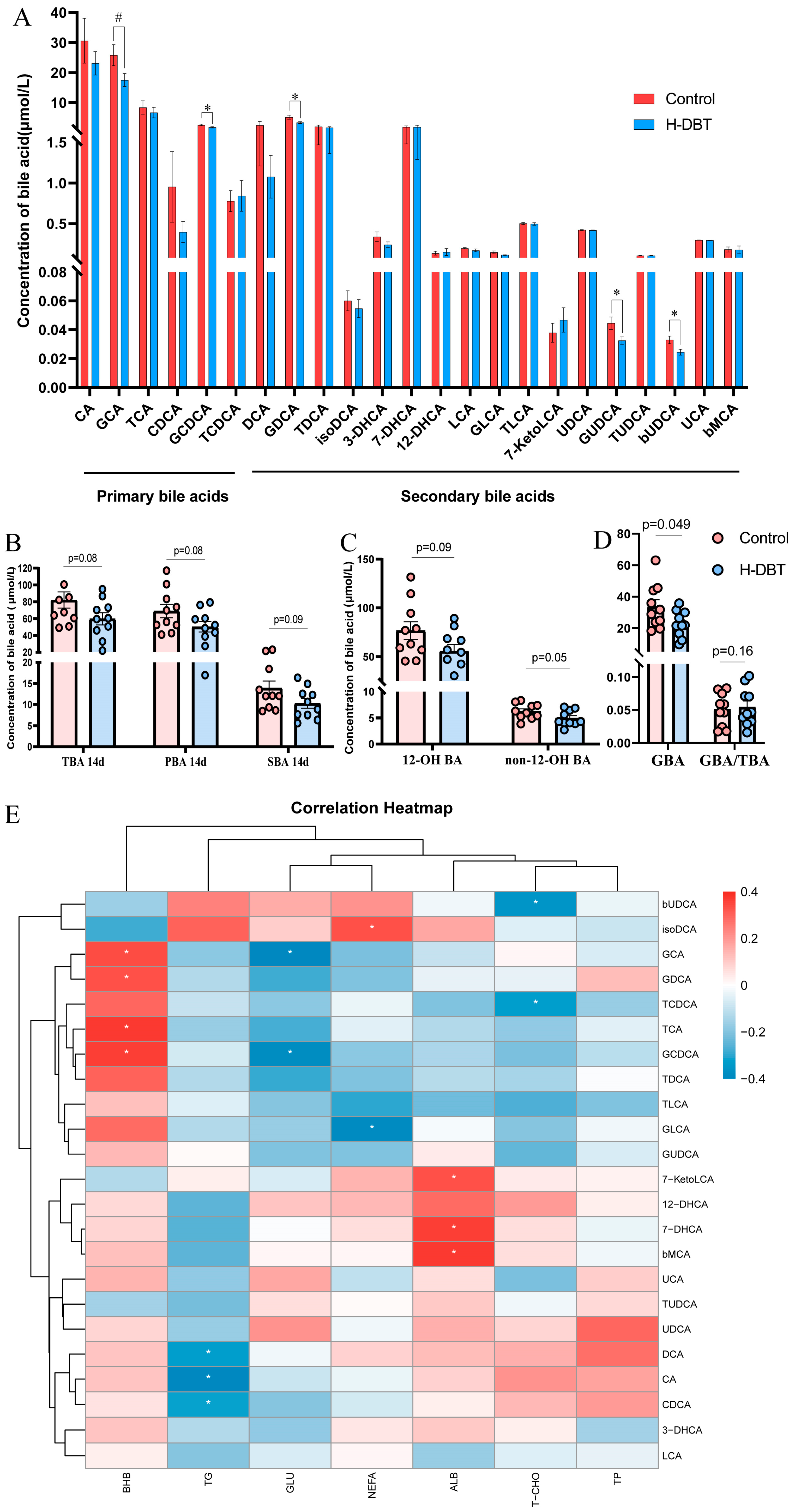
3.5. DBT Intervention Alters the Serum BA Profiles of Postpartum Dairy Cows
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drackley, J.K. ADSA Foundation Scholar Award. Biology of dairy cows during the transition period: The final frontier? J. Dairy Sci. 1999, 82, 2259–2273. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.Z.; Shen, L.H.; Jiang, J.; Huang, Y.X.; Bai, L.P.; Yu, S.M.; Yao, X.P.; Ren, Z.H.; Yang, Y.X.; Cao, S.Z. Plasma metabolite changes in dairy cows during parturition identified using untargeted metabolomics. J. Dairy Sci. 2019, 102, 4639–4650. [Google Scholar] [CrossRef]
- Ingvartsen, K.L.; Moyes, K. Nutrition, immune function and health of dairy cattle. Animal 2013, 7, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Bobe, G.; Young, J.W.; Beitz, D.C. Invited review: Pathology, etiology, prevention, and treatment of fatty liver in dairy cows. J. Dairy Sci. 2004, 87, 3105–3124. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.Y.; Tan, J.; Li, L.L.; Wang, Y.; Liu, M.; Jiang, L.S.; Zhao, Y.C. Longitudinal characterization of serum metabolome and lipidome reveals that the ceramide profile is associated with metabolic health in early postpartum cows experiencing different lipolysis. J. Dairy Sci. 2024, 107, 7446–7468. [Google Scholar] [CrossRef]
- Shapiro, H.; Kolodziejczyk, A.A.; Halstuch, D.; Elinav, E. Bile acids in glucose metabolism in health and disease. J. Exp. Med. 2018, 215, 383–396. [Google Scholar] [CrossRef]
- Du, X.; Liu, M.; Trevisi, E.; Ju, L.; Yang, Y.; Gao, W.; Song, Y.; Lei, L.; Zolzaya, M.; Li, X.; et al. Expression of hepatic genes involved in bile acid metabolism in dairy cows with fatty liver. J. Dairy Sci. 2024, 107, 8629–8641. [Google Scholar] [CrossRef]
- Dicks, L.; Schuh-von Graevenitz, K.; Prehn, C.; Sadri, H.; Murani, E.; Ghaffari, M.H.; Haussler, S. Bile acid profiles and mRNA abundance of bile acid-related genes in adipose tissue of dairy cows with high versus normal body condition. J. Dairy Sci. 2024, 107, 8688–8708. [Google Scholar] [CrossRef]
- Gaertner, T.; Gernand, E.; Gottschalk, J.; Donat, K. Relationships between body condition, body condition loss, and serum metabolites during the transition period in primiparous and multiparous cows. J. Dairy Sci. 2019, 102, 9187–9199. [Google Scholar] [CrossRef]
- Norvezh, F.; Jalali, M.R.; Tabandeh, M.R.; Hajikolaei, M.R.H.; Gooraninejad, S. Serum Apelin-36 alteration in late pregnancy and early lactation of dairy cows and its association with negative energy balance markers. Res. Vet. Sci. 2019, 125, 285–289. [Google Scholar] [CrossRef]
- Hassan, F.-u.; Nadeem, A.; Javed, M.; Saif-ur-Rehman, M.; Shahzad, M.A.; Azhar, J.; Shokrollahi, B. RETRACTED: Nutrigenomic Interventions to Address Metabolic Stress and Related Disorders in Transition Cows (Retracted Article). Biomed Res. Int. 2022, 2022, 2295017. [Google Scholar] [CrossRef]
- Lin, H.Q.; Gong, A.G.W.; Wang, H.Y.; Duan, R.; Dong, T.T.X.; Zhao, K.J.; Tsim, K.W.K. Danggui Buxue Tang (Astragali Radix and Angelicae Sinensis Radix) for menopausal symptoms: A review. J. Ethnopharmacol. 2017, 199, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Gong, A.G.W.; Duan, R.; Wang, H.Y.; Dong, T.T.X.; Tsim, K.W.K. Calycosin Orchestrates Osteogenesis of Danggui Buxue Tang in Cultured Osteoblasts: Evaluating the Mechanism of Action by Omics and Chemical Knock-out Methodologies. Front. Pharmacol. 2018, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.T.X.; Zhao, K.J.; Gao, Q.T.; Ji, Z.N.; Zhu, T.T.; Li, J.; Duan, R.; Cheung, A.W.H.; Tsim, K.W.K. Chemical and biological assessment of a chinese herbal decoction containing Radix Astragali and Radix Angelicae Sinensis: Determination of drug ratio in having optimized properties. J. Agric. Food Chem. 2006, 54, 2767–2774. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Li, J.; Cheung, J.K.H.; Duan, J.; Ding, A.; Cheung, A.W.H.; Zhao, K.; Li, W.Z.; Dong, T.T.; Tsim, K.W.K. Verification of the formulation and efficacy of Danggui Buxue Tang (a decoction of Radix Astragali and Radix Angelicae Sinensis): An exemplifying systematic approach to revealing the complexity of Chinese herbal medicine formulae. Chin. Med. 2007, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Qi, B.; Liang, Y.T.; Dong, T.T.X.; Wang, H.Y.; Tsim, K.W.K.; Zheng, Y. Danggui Buxue Tang, an ancient Chinese herbal decoction, protects -amyloid-induced cell death in cultured cortical neurons. Bmc Complement. Altern. Med. 2019, 19, 9. [Google Scholar] [CrossRef]
- Minh Nhat, T.; Kim, S.; Quynh Hoang Ngan, N.; Lee, S. Molecular Mechanisms Underlying Qi-Invigorating Effects in Traditional Medicine: Network Pharmacology-Based Study on the Unique Functions of Qi-Invigorating Herb Group. Plants 2022, 11, 2470. [Google Scholar] [CrossRef]
- Li, C.; Zhu, F.; Wang, S.; Wang, J.; Wu, B. Danggui Buxue Decoction Ameliorates Inflammatory Bowel Disease by Improving Inflammation and Rebuilding Intestinal Mucosal Barrier. Evid. -Based Complement. Altern. Med. 2021, 2021, 8853141. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Xiao, B.; Shui, S.; Yang, J.; Huang, R.; Dong, J. Metabolomics analysis of serum reveals the effect of Danggui Buxue Tang on fatigued mice induced by exhausting physical exercise. J. Pharm. Biomed. Anal. 2018, 151, 301–309. [Google Scholar] [CrossRef]
- Sun, L.; Yang, Z.; Zhao, W.; Chen, Q.; Bai, H.; Wang, S.; Yang, L.; Bi, C.; Shi, Y.; Liu, Y. Integrated lipidomics, transcriptomics and network pharmacology analysis to reveal the mechanisms of Danggui Buxue Decoction in the treatment of diabetic nephropathy in type 2 diabetes mellitus. J. Ethnopharmacol. 2022, 283, 114699. [Google Scholar] [CrossRef]
- Xue, M.; Bian, Y.; Liu, Y.; Zhou, J.; Xu, J.; Zhang, L.; Huang, X. Danggui Buxue decoction ameliorates lipid metabolic defects involved in the initiation of diabetic atherosclerosis; identification of active compounds. J. Tradit. Chin. Med. 2020, 40, 414–421. [Google Scholar] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, M.; Chen, J.; Li, Y.; Kuang, Z.; Dende, C.; Raj, P.; Quinn, G.; Hu, Z.; Srinivasan, T.; et al. The gut microbiota reprograms intestinal lipid metabolism through long noncoding RNA Snhg9. Science 2023, 381, 851–856. [Google Scholar] [CrossRef]
- Ma, C.; Sun, Z.; Zeng, B.; Huang, S.; Zhao, J.; Zhang, Y.; Su, X.; Xu, J.; Wei, H.; Zhang, H. Cow-to-mouse fecal transplantations suggest intestinal microbiome as one cause of mastitis. Microbiome 2018, 6, 200. [Google Scholar] [CrossRef]
- Kim, H.S.; Whon, T.W.; Sung, H.; Jeong, Y.-S.; Jung, E.S.; Shin, N.-R.; Hyun, D.-W.; Kim, P.S.; Lee, J.-Y.; Lee, C.H.; et al. Longitudinal evaluation of fecal microbiota transplantation for ameliorating calf diarrhea and improving growth performance. Nat. Commun. 2021, 12, 161. [Google Scholar] [CrossRef]
- Luo, Z.; Yong, K.; Luo, Q.; Du, Z.; Ma, L.; Huang, Y.; Zhou, T.; Yao, X.; Shen, L.; Yu, S.; et al. Altered Fecal Microbiome and Correlations of the Metabolome with Plasma Metabolites in Dairy Cows with Left Displaced Abomasum. Microbiol. Spectr. 2022, 10, e01972-22. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Li, Y.J.; He, B.X.; Hu, J.J.; Mohsin, M.A.; Yu, H.R.; Wang, P.; Zhang, P.J.; Du, Y.L.; Huang, L.J.; et al. The Influence of Ketosis on the Rectal Microbiome of Chinese Holstein Cows. Pak. Vet. J. 2019, 39, 175–180. [Google Scholar] [CrossRef]
- Li, X.; Wu, D.; Niu, J.; Sun, Y.; Wang, Q.; Yang, B.; Kuang, H. Intestinal Flora: A Pivotal Role in Investigation of Traditional Chinese Medicine. Am. J. Chin. Med. 2021, 49, 237–268. [Google Scholar] [CrossRef]
- Wang, W.-k.; Fan, L.; Ge, F.; Li, Z.; Zhu, J.; Yin, K.; Xia, J.; Xue, M. Effects of Danggui Buxue decoction on host gut microbiota and metabolism in GK rats with type 2 diabetes. Front. Microbiol. 2022, 13, 1029409. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Bei, H.; Jia, L.; Huang, C.; Chen, Q.; Tao, C.; Chen, J.; Bo, H. Danggui Buxue Tang restores antibiotic-induced metabolic disorders by remodeling the gut microbiota. J. Ethnopharmacol. 2020, 259, 112953. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Luo, Z.; Huang, Y.; Zhou, T.; Ma, L.; Wu, D.; Yao, X.; Shen, L.; Yu, S.; Yong, K.; et al. Screening for potential warning biomarkers in cows with ketosis based on host-microbiota co-metabolism analysis. Front. Microbiol. 2024, 15, 1373402. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, L.; Liu, D.; Chen, H.; Tang, D.-D.; Zhao, Y.-Y. Metabolomics highlights pharmacological bioactivity and biochemical mechanism of traditional Chinese medicine. Chem. -Biol. Interact. 2017, 273, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Macrae, A.I.; Burrough, E.; Forrest, J.; Corbishley, A.; Russell, G.; Shaw, D.J. Risk factors associated with excessive negative energy balance in commercial United Kingdom dairy herds. Vet. J. 2019, 250, 15–23. [Google Scholar] [CrossRef]
- Ospina, P.A.; Nydam, D.V.; Stokol, T.; Overton, T.R. Associations of elevated nonesterified fatty acids and β-hydroxybutyrate concentrations with early lactation reproductive performance and milk production in transition dairy cattle in the northeastern United States. J. Dairy Sci. 2010, 93, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Ospina, P.A.; Nydam, D.V.; Stokol, T.; Overton, T.R. Evaluation of nonesterified fatty acids and β-hydroxybutyrate in transition dairy cattle in the northeastern United States: Critical thresholds for prediction of clinical diseases. J. Dairy Sci. 2010, 93, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Macrae, A.I.; Burrough, E.; Forrest, J.; Corbishley, A.; Russell, G.; Shaw, D.J. Prevalence of excessive negative energy balance in commercial United Kingdom dairy herds. Vet. J. 2019, 248, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Ospina, P.A.; McArt, J.A.; Overton, T.R.; Stokol, T.; Nydam, D.V. Using Nonesterified Fatty Acids and β-Hydroxybutyrate Concentrations During the Transition Period for Herd-Level Monitoring of Increased Risk of Disease and Decreased Reproductive and Milking Performance. Vet. Clin. North Am. -Food Anim. Pract. 2013, 29, 387–412. [Google Scholar] [CrossRef] [PubMed]
- McArt, J.A.A.; Nydam, D.V.; Oetzel, G.R. Epidemiology of subclinical ketosis in early lactation dairy cattle. J. Dairy Sci. 2012, 95, 5056–5066. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, Z.; Shepley, E.; Endres, M.I.; Cramer, G.; Caixeta, L.S. Assessment of milk yield and composition, early reproductive performance, and herd removal in multiparous dairy cattle based on the week of diagnosis of hyperketonemia in early lactation. J. Dairy Sci. 2022, 105, 4410–4420. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.; McArt, J.A.A. Hyperketonemia A Marker of Disease, a Sign of a High-Producing Dairy Cow, or Both? Vet. Clin. North Am. -Food Anim. Pract. 2023, 39, 307–324. [Google Scholar] [CrossRef]
- Gross, J.J.; Schwinn, A.-C.; Mueller, E.; Munger, A.; Dohme-Meier, F.; Bruckmaier, R.M. Plasma cholesterol levels and short-term adaptations of metabolism and milk production during feed restriction in early lactating dairy cows on pasture. J. Anim. Physiol. Anim. Nutr. 2021, 105, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Qiao, Q.; Gao, Y.; Hou, J.; Hu, M.; Du, Y.; Zhao, K.; Li, X. Gut Microbiota and Their Role in Health and Metabolic Disease of Dairy Cow. Front. Nutr. 2021, 8, 701511. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, P.; Guo, A.; Yang, Y.; Chen, F.; Zhang, Q. Research progress on the regulation of production traits by gastrointestinal microbiota in dairy cows. Front. Vet. Sci. 2023, 10, 1206346. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Du, Z.; Huang, Y.; Zhou, T.; Wu, D.; Yao, X.; Shen, L.; Yu, S.; Yong, K.; Wang, B.; et al. Alterations in the gut microbiota and its metabolites contribute to metabolic maladaptation in dairy cows during the development of hyperketonemia. Msystems 2024, 9, e00023-24. [Google Scholar] [CrossRef]
- Orellana, L.H.; Ben Francis, T.; Ferraro, M.; Hehemann, J.-H.; Fuchs, B.M.; Amann, R.I. Verrucomicrobiota are specialist consumers of sulfated methyl pentoses during diatom blooms. ISME J. 2022, 16, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Fujio-Vejar, S.; Vasquez, Y.; Morales, P.; Magne, F.; Vera-Wolf, P.; Ugalde, J.A.; Navarrete, P.; Gotteland, M. The Gut Microbiota of Healthy Chilean Subjects Reveals a High Abundance of the Phylum Verrucomicrobia. Front. Microbiol. 2017, 8, 1221. [Google Scholar] [CrossRef]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A relevant minority for the maintenance of gut homeostasis. Dig. Liver Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef]
- Lamontagne, J.; Rico, D.E.; Perdomo, C.M.; Ronholm, J.; Gervais, R.; Chouinard, P.Y. Effects of direct-fed Bacillus subtilis and Bacillus licheniformis on production performance and milk fatty acid profile in dairy cows. J. Dairy Sci. 2023, 106, 1815–1825. [Google Scholar] [CrossRef]
- Jia, P.; Dong, L.-F.; Tu, Y.; Diao, Q.-Y. Bacillus subtilis and Macleaya cordata extract regulate the rumen microbiota associated with enteric methane emission in dairy cows. Microbiome 2023, 11, 229. [Google Scholar] [CrossRef]
- Jia, P.; Tu, Y.; Liu, Z.; Li, F.; Yan, T.; Ma, S.; Dong, L.; Diao, Q. Diets supplementation with Bacillus subtilis and Macleaya cordata extract improve production performance and the metabolism of energy and nitrogen, while reduce enteric methane emissions in dairy cows. Anim. Feed Sci. Technol. 2022, 294, 115481. [Google Scholar] [CrossRef]
- Couch, C.E.; Stagaman, K.; Spaan, R.S.; Combrink, H.J.; Sharpton, T.J.; Beechler, B.R.; Jolles, A.E. Diet and gut microbiome enterotype are associated at the population level in African buffalo. Nat. Commun. 2021, 12, 2267. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Zhou, X.; Tao, G.; Hao, W.; Wang, L.; Lan, Z.; Song, Y.; Wu, M.; Huang, J.-Q. Ferulic acid and feruloylated oligosaccharides alleviate anxiety and depression symptom via regulating gut microbiome and microbial metabolism. Food Res. Int. 2022, 162, 111887. [Google Scholar] [CrossRef]
- Pinnell, L.J.; Reyes, A.A.; Wolfe, C.A.; Weinroth, M.D.; Metcalf, J.L.; Delmore, R.J.; Belk, K.E.; Morley, P.S.; Engle, T.E. Bacteroidetes and Firmicutes Drive Differing Microbial Diversity and Community Composition Among Micro-Environments in the Bovine Rumen. Front. Vet. Sci. 2022, 9, 897996. [Google Scholar] [CrossRef]
- Fukiya, S.; Arata, M.; Kawashima, H.; Yoshida, D.; Kaneko, M.; Minamida, K.; Watanabe, J.; Ogura, Y.; Uchida, K.; Itoh, K.; et al. Conversion of cholic acid and chenodeoxycholic acid into their 7-oxo derivatives by Bacteroides intestinalis AM-1 isolated from human feces. Fems Microbiol. Lett. 2009, 293, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yu, S.; Li, L.; Zhao, H.; Li, Y.; Jiang, L.; Liu, M. Feeding citrus flavonoid extracts decreases bacterial endotoxin and systemic inflammation and improves immunometabolic status by modulating hindgut microbiome and metabolome in lactating dairy cows. Anim. Nutr. 2023, 13, 386–400. [Google Scholar] [CrossRef] [PubMed]
- Sowah, S.A.; Milanese, A.; Schuebel, R.; Wirbel, J.; Kartal, E.; Johnson, T.S.; Hirche, F.; Grafetstaetter, M.; Nonnenmacher, T.; Kirsten, R.; et al. Calorie restriction improves metabolic state independently of gut microbiome composition: A randomized dietary intervention trial. Genome Med. 2022, 14, 30. [Google Scholar] [CrossRef]
- Chen, D.; Yang, Z.; Chen, X.; Huang, Y.; Yin, B.; Guo, F.; Zhao, H.; Zhao, T.; Qu, H.; Huang, J.; et al. The effect of Lactobacillus rhamnosus hsryfm 1301 on the intestinal microbiota of a hyperlipidemic rat model. Bmc Complement. Altern. Med. 2014, 14, 386. [Google Scholar] [CrossRef]
- Bai, Y.; Zhao, T.; Gao, M.; Zou, Y.; Lei, X. A Novel Gene Alignment in Dorea sp. AM58-8 Produces 7-Dehydroxy-3β Bile Acids from Primary Bile Acids. Biochemistry 2022, 61, 2870–2878. [Google Scholar] [CrossRef]
- Wei, Y.; Liang, J.; Su, Y.; Wang, J.; Amakye, W.K.; Pan, J.; Chu, X.; Ma, B.; Song, Y.; Li, Y.; et al. The associations of the gut microbiome composition and short-chain fatty acid concentrations with body fat distribution in children. Clin. Nutr. 2021, 40, 3379–3390. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, T.; Wang, H.; Chen, L.; Zhou, Z. Studies on nutritional intervention of rice starch- oleic acid complex (resistant starch type V) in rats fed by high-fat diet. Carbohydr. Polym. 2020, 246, 116637. [Google Scholar] [CrossRef] [PubMed]
- Therdtatha, P.; Song, Y.; Tanaka, M.; Mariyatun, M.; Almunifah, M.; Manurung, N.E.P.; Indriarsih, S.; Lu, Y.; Nagata, K.; Fukami, K.; et al. Gut Microbiome of Indonesian Adults Associated with Obesity and Type 2 Diabetes: A Cross-Sectional Study in an Asian City, Yogyakarta. Microorganisms 2021, 9, 897. [Google Scholar] [CrossRef]
- Yin, H.; Huang, J.; Guo, X.; Xia, J.; Hu, M. Romboutsia lituseburensis JCM1404 supplementation ameliorated endothelial function via gut microbiota modulation and lipid metabolisms alterations in obese rats. FEMS Microbiol. Lett. 2023, 370, fnad016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, X.; Liu, H.; Wang, P.; Li, L.; Bionaz, M.; Lin, P.; Yao, J. Altered bile acid and correlations with gut microbiome in transition dairy cows with different glucose and lipid metabolism status. J. Dairy Sci. 2024, 107, 9915–9933. [Google Scholar] [CrossRef]
- Jones, L.L.; McDonald, D.A.; Borum, P.R. Acylcarnitines: Role in brain. Prog. Lipid Res. 2010, 49, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Knottnerus, S.J.G.; Bleeker, J.C.; Wust, R.C.I.; Ferdinandusse, S.; Ijlst, L.; Wijburg, F.A.; Wanders, R.J.A.; Visser, G.; Houtkooper, R.H. Disorders of mitochondrial long-chain fatty acid oxidation and the carnitine shuttle. Rev. Endocr. Metab. Disord. 2018, 19, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Pietrocola, F.; Galluzzi, L.; Bravo-San Pedro, J.M.; Madeo, F.; Kroemer, G. Acetyl Coenzyme A: A Central Metabolite and Second Messenger. Cell Metab. 2015, 21, 805–821. [Google Scholar] [CrossRef] [PubMed]
- Humer, E.; Khol-Parisini, A.; Metzler-Zebeli, B.U.; Gruber, L.; Zebeli, Q. Alterations of the Lipid Metabolome in Dairy Cows Experiencing Excessive Lipolysis Early Postpartum. PLoS ONE 2016, 11, e0158633. [Google Scholar] [CrossRef]
- Swartz, T.H.; Bradford, B.J.; Mamedova, L.K.; Estes, K.A. Effects of dietary rumen-protected choline supplementation to periparturient dairy cattle on inflammation, metabolism, and performance during an intramammary lipopolysaccharide challenge. J. Dairy Sci. 2023, 106, 8561–8582. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, M.-L.; Huang, B.; Zhao, F.-R.; Li, Y.; Cui, X.-T.; Lin, R. Acetylcarnitine Is Associated With Cardiovascular Disease Risk in Type 2 Diabetes Mellitus. Front. Endocrinol. 2021, 12, 806819. [Google Scholar] [CrossRef]
- Nie, Q.; Xing, M.; Chen, H.; Hu, J.; Nie, S. Metabolomics and Lipidomics Profiling Reveals Hypocholesterolemic and Hypolipidemic Effects of Arabinoxylan on Type 2 Diabetic Rats. J. Agric. Food Chem. 2019, 67, 10614–10623. [Google Scholar] [CrossRef]
- Qin, F.; Li, J.; Mao, T.; Feng, S.; Li, J.; Lai, M. 2 Hydroxybutyric Acid-Producing Bacteria in Gut Microbiome and Fusobacterium nucleatum Regulates 2 Hydroxybutyric Acid Level In Vivo. Metabolites 2023, 13, 451. [Google Scholar] [CrossRef] [PubMed]
- Ikmal, S.I.Q.S.; Huri, H.Z.; Vethakkan, S.R.; Ahmad, W.A.W. Potential Biomarkers of Insulin Resistance and Atherosclerosis in Type 2 Diabetes Mellitus Patients with Coronary Artery Disease. Int. J. Endocrinol. 2013, 2013, 698567. [Google Scholar] [CrossRef]
- Sousa, A.P.; Cunha, D.M.; Franco, C.; Teixeira, C.; Gojon, F.; Baylina, P.; Fernandes, R. Which Role Plays 2-Hydroxybutyric Acid on Insulin Resistance? Metabolites 2021, 11, 835. [Google Scholar] [CrossRef] [PubMed]
- Shantavasinkul, P.C.; Muehlbauer, M.J.; Bain, J.R.; Ilkayeva, O.R.; Craig, D.M.; Newgard, C.B.; Svetkey, L.P.; Shah, S.H.; Torquati, A. Improvement in insulin resistance after gastric bypass surgery is correlated with a decline in plasma 2-hydroxybutyric acid. Surg. Obes. Relat. Dis. 2018, 14, 1126–1132. [Google Scholar] [CrossRef]
- Li, B.; Hong, Y.; Gu, Y.; Ye, S.; Hu, K.; Yao, J.; Ding, K.; Zhao, A.; Jia, W.; Li, H. Functional Metabolomics Reveals that Astragalus Polysaccharides Improve Lipids Metabolism through Microbial Metabolite 2-Hydroxybutyric Acid in Obese Mice. Engineering 2022, 9, 111–122. [Google Scholar] [CrossRef]
- Rico, J.E.; Barrientos-Blanco, M.A. Invited review: Ketone biology-The shifting paradigm of ketones and ketosis in the dairy cow. J. Dairy Sci. 2024, 107, 3367–3388. [Google Scholar] [CrossRef] [PubMed]
- Suthar, V.S.; Canelas-Raposo, J.; Deniz, A.; Heuwieser, W. Prevalence of subclinical ketosis and relationships with postpartum diseases in European dairy cows. J. Dairy Sci. 2013, 96, 2925–2938. [Google Scholar] [CrossRef]
- Naviaux, R.K.; Naviaux, J.C.; Li, K.; Bright, A.T.; Alaynick, W.A.; Wang, L.; Baxter, A.; Nathan, N.; Anderson, W.; Gordon, E. REPLY TO VOGT ET AL.: Metabolomics and chronic fatigue syndrome. Proc. Natl. Acad. Sci. USA 2016, 113, E7142–E7143. [Google Scholar] [CrossRef]
- Ferrebee, C.B.; Dawson, P.A. Metabolic effects of intestinal absorption and enterohepatic cycling of bile acids. Acta Pharm. Sin. B 2015, 5, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Zhu, S.; Tang, Y.; Liu, X.; Jia, M.; Malmuthuge, N.; Valencak, T.G.; McFadden, J.W.; Liu, J.-X.; Sun, H.-Z. Gut microbiome is linked to functions of peripheral immune cells in transition cows during excessive lipolysis. Microbiome 2023, 11, 40. [Google Scholar] [CrossRef]
- Woolbright, B.L.; Jaeschke, H. Novel insight into mechanisms of cholestatic liver injury. World J. Gastroenterol. 2012, 18, 4985–4993. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.-J.; Mai, C.-T.; Zhu, Y.-Z.; Liu, X.-C.; Xie, Y. Bile acids as regulatory molecules and potential targets in metabolic diseases. Life Sci. 2021, 287, 120152. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yun, C.; Sun, L.; Xia, J.; Wu, Q.; Wang, Y.; Wang, L.; Zhang, Y.; Liang, X.; Wang, L.; et al. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat. Med. 2019, 25, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Bai, Y.; Tong, F.; Yan, J.; Zhang, R.; Zhong, Y.; Tan, H.; Ma, X. Glycoursodeoxycholic acid regulates bile acids level and alters gut microbiota and glycolipid metabolism to attenuate diabetes. Gut Microbes 2023, 15, 2192155. [Google Scholar] [CrossRef] [PubMed]


| Comparison | Number of Cows with BHB ≥ 1 mmol/L | Total | χ2 | p |
|---|---|---|---|---|
| Control | 7 | 10 | 5.051 | 0.025 > 0.0167 |
| L-DBT | 2 | 10 | ||
| Control | 7 | 10 | 7.500 | 0.006 < 0.0167 |
| H-DBT | 1 | 10 | ||
| L-DBT | 2 | 10 | 0.392 | 0.531 > 0.0167 |
| H-DBT | 1 | 10 |
| Comparison | Number of Cows with BHB ≥ 1 mmol/L | Total | χ2 | p |
|---|---|---|---|---|
| Control | 5 | 10 | 0.833 | 0.361 > 0.0167 |
| L-DBT | 3 | 10 | ||
| Control | 5 | 10 | 3.810 | 0.051 > 0.0167 |
| H-DBT | 1 | 10 | ||
| L-DBT | 3 | 10 | 1.250 | 1.250 > 0.0167 |
| H-DBT | 1 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Yong, K.; Luo, Z.; Du, Z.; Zhou, T.; Li, X.; Yao, X.; Shen, L.; Yu, S.; Huang, Y.; et al. The Positive Regulatory Effect of DBT on Lipid Metabolism in Postpartum Dairy Cows. Metabolites 2025, 15, 58. https://doi.org/10.3390/metabo15010058
Zhou Z, Yong K, Luo Z, Du Z, Zhou T, Li X, Yao X, Shen L, Yu S, Huang Y, et al. The Positive Regulatory Effect of DBT on Lipid Metabolism in Postpartum Dairy Cows. Metabolites. 2025; 15(1):58. https://doi.org/10.3390/metabo15010058
Chicago/Turabian StyleZhou, Zheng, Kang Yong, Zhengzhong Luo, Zhenlong Du, Tao Zhou, Xiaoping Li, Xueping Yao, Liuhong Shen, Shumin Yu, Yixin Huang, and et al. 2025. "The Positive Regulatory Effect of DBT on Lipid Metabolism in Postpartum Dairy Cows" Metabolites 15, no. 1: 58. https://doi.org/10.3390/metabo15010058
APA StyleZhou, Z., Yong, K., Luo, Z., Du, Z., Zhou, T., Li, X., Yao, X., Shen, L., Yu, S., Huang, Y., & Cao, S. (2025). The Positive Regulatory Effect of DBT on Lipid Metabolism in Postpartum Dairy Cows. Metabolites, 15(1), 58. https://doi.org/10.3390/metabo15010058







