Application of ATR-FTIR Spectroscopy for Analysis of Salt Stress in Brussels Sprouts
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Growth Environment
2.2. Experimental Design and Treatment
2.3. Growth and Chlorophyll Fluorescence Analysis
2.4. Analysis of Metabolite Changes Using ATR-FTIR Spectroscopy
2.5. Pearson Correlation Coefficient and Statistical Analysis
3. Results and Discussion
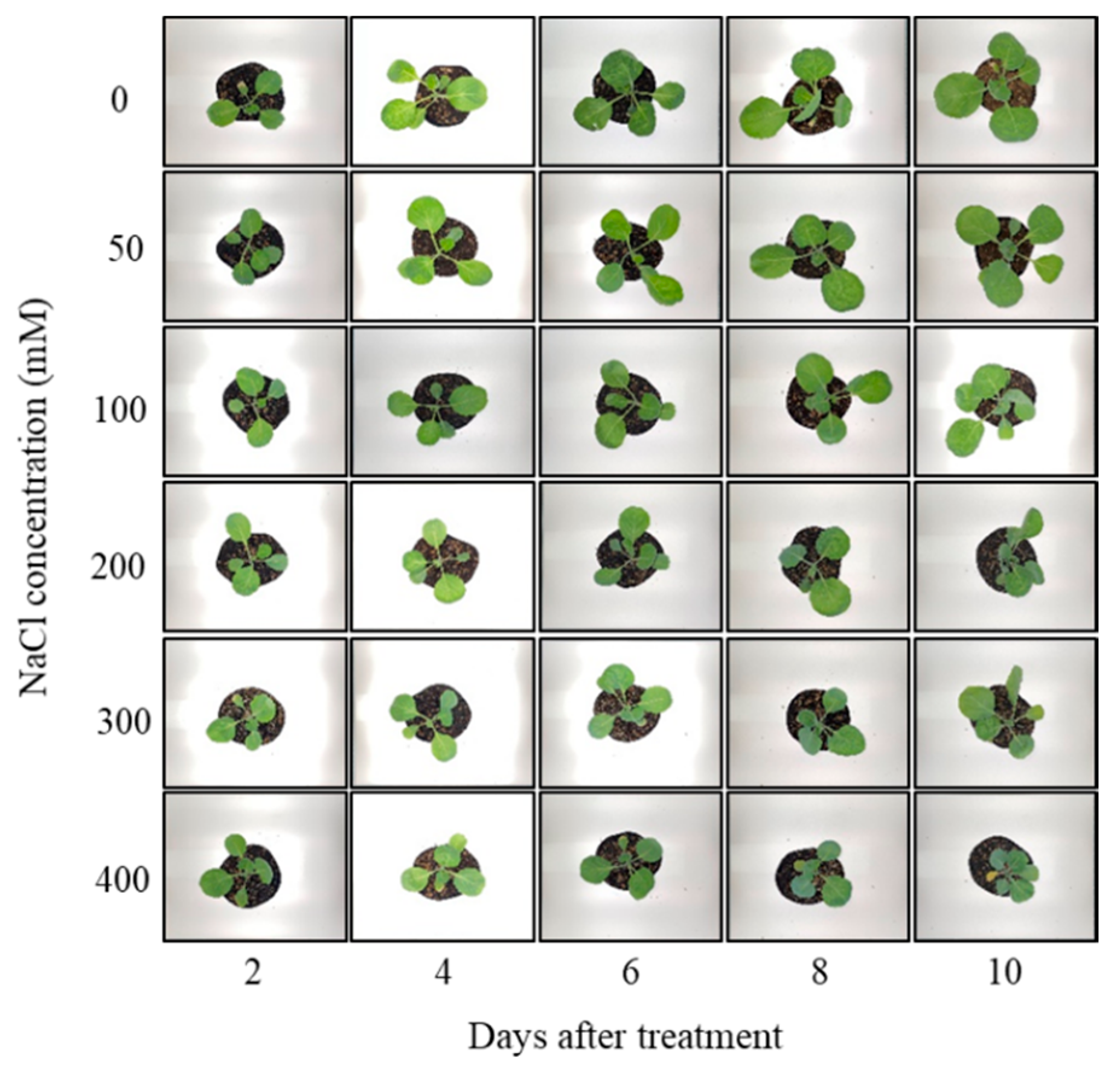
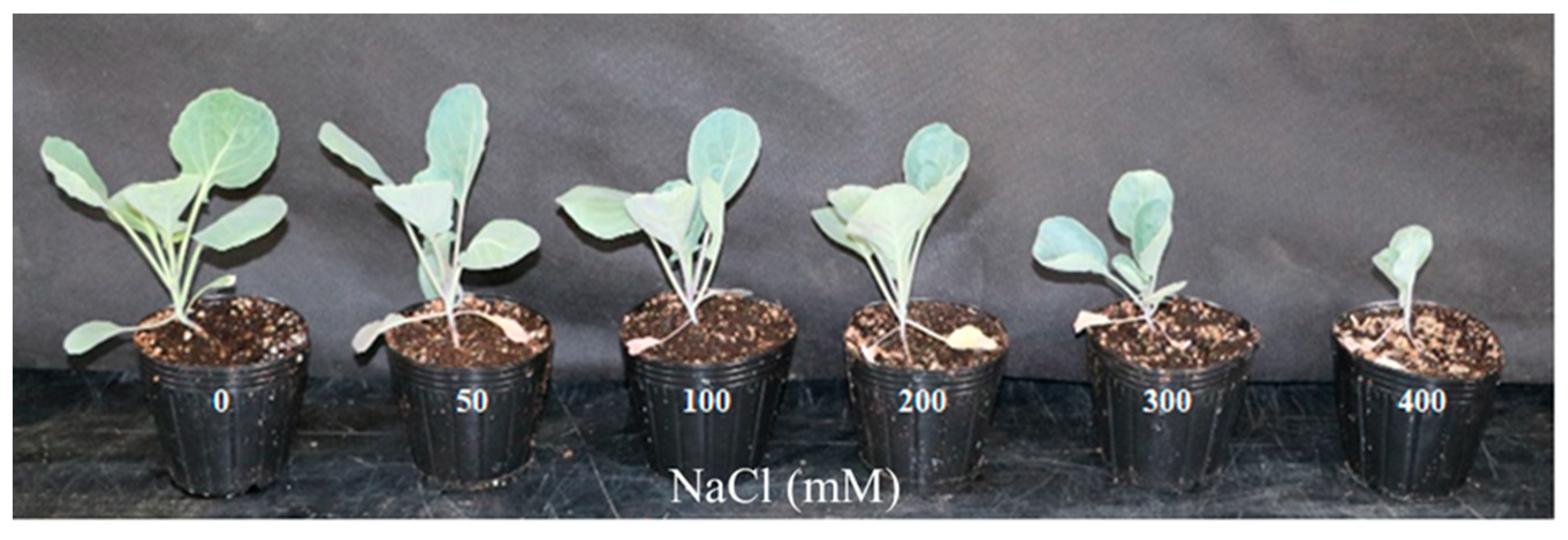
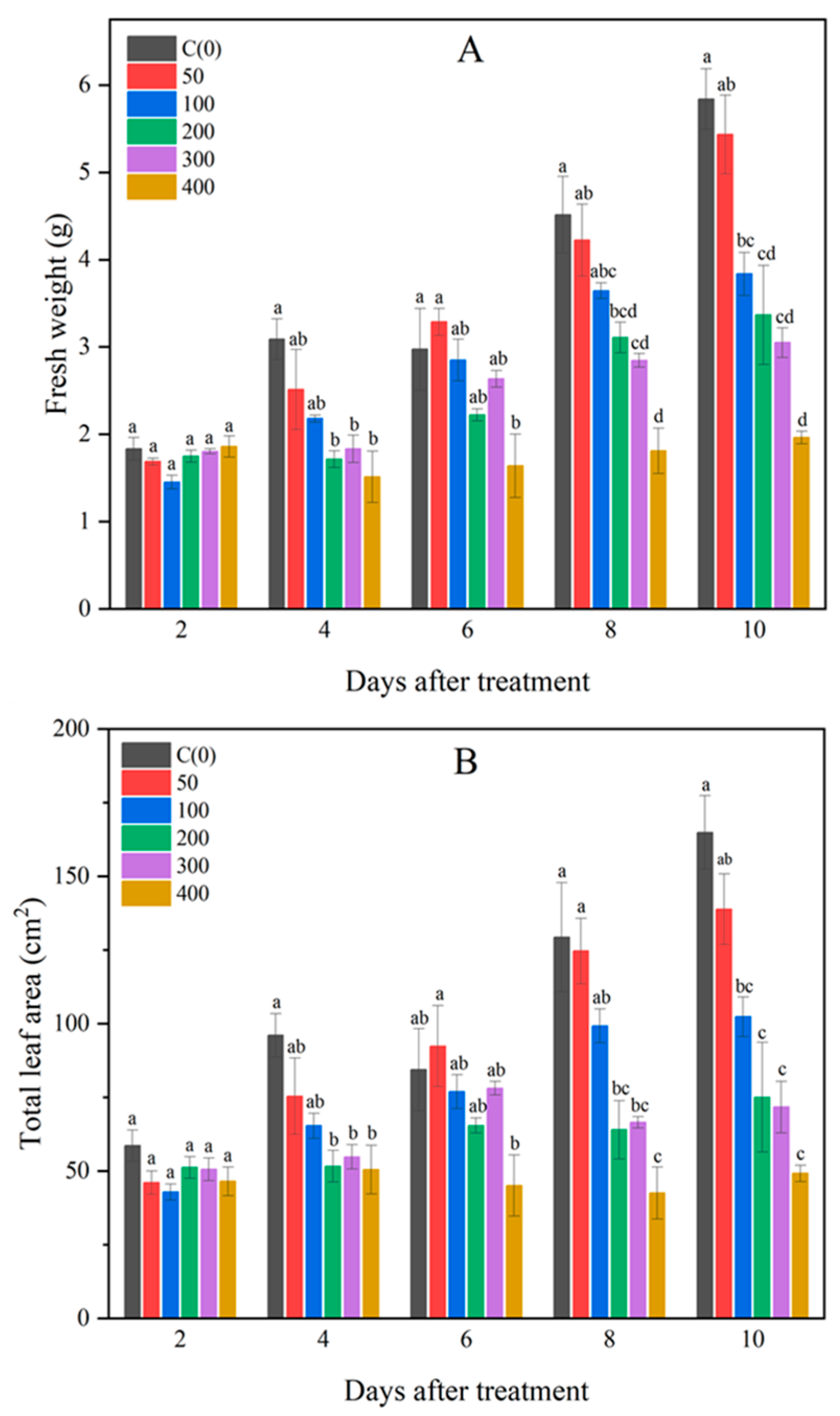
3.1. Changes in Fresh Weight and Leaf Area of Brussels Sprouts
3.2. Changes in Chlorophyll Fluorescence Parameters
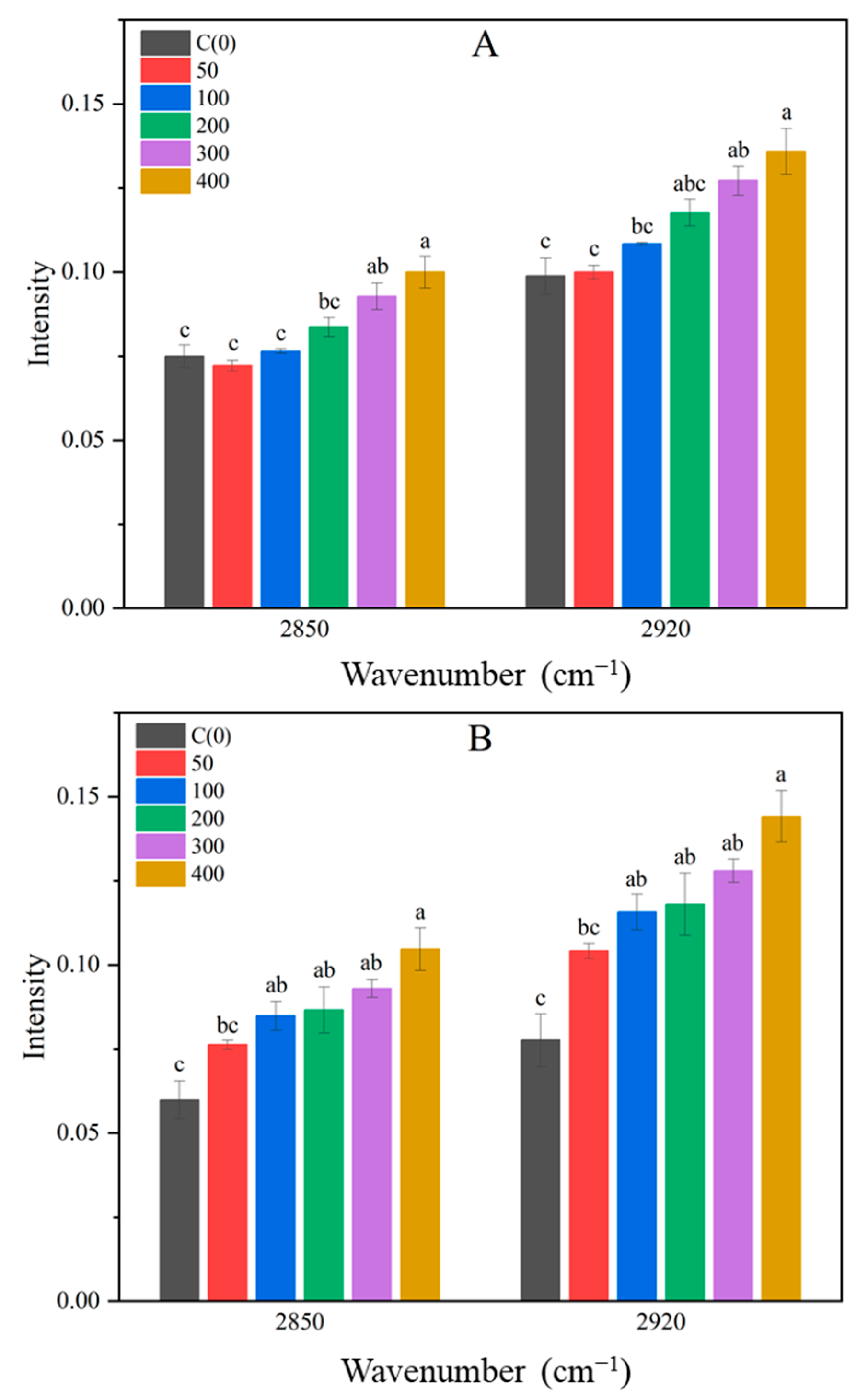
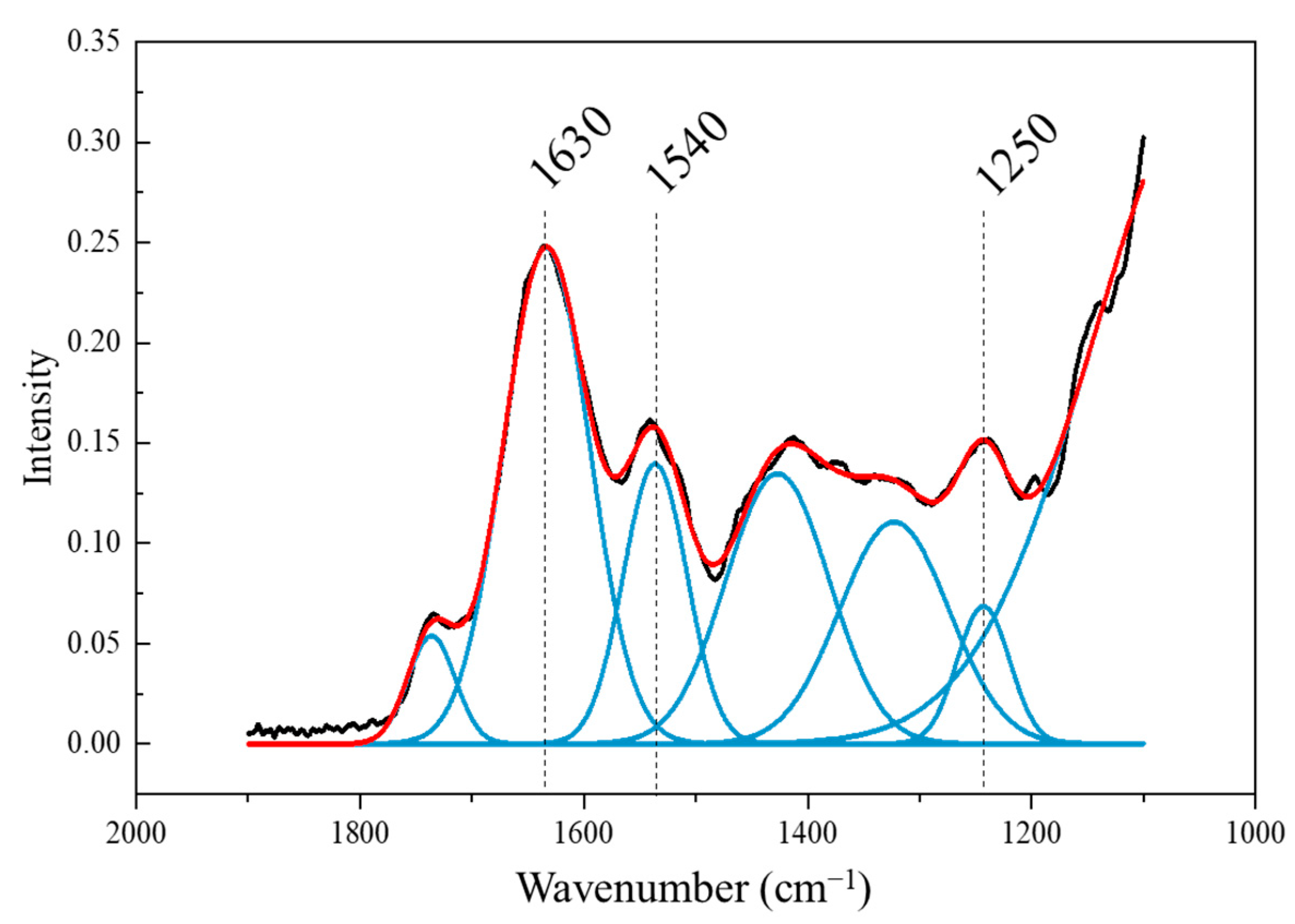
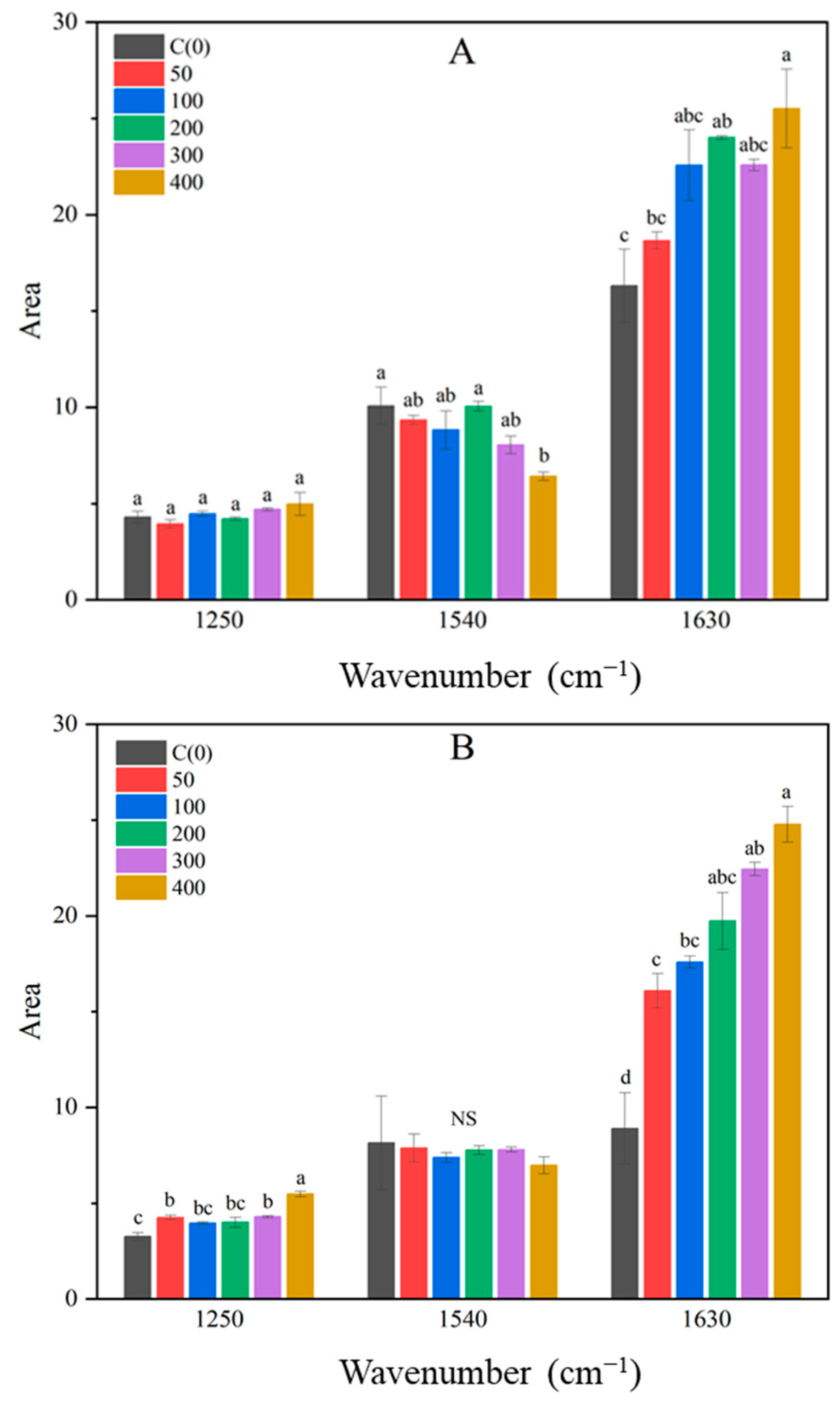
3.3. FTIR Spectrum Analysis of Brussels Sprout
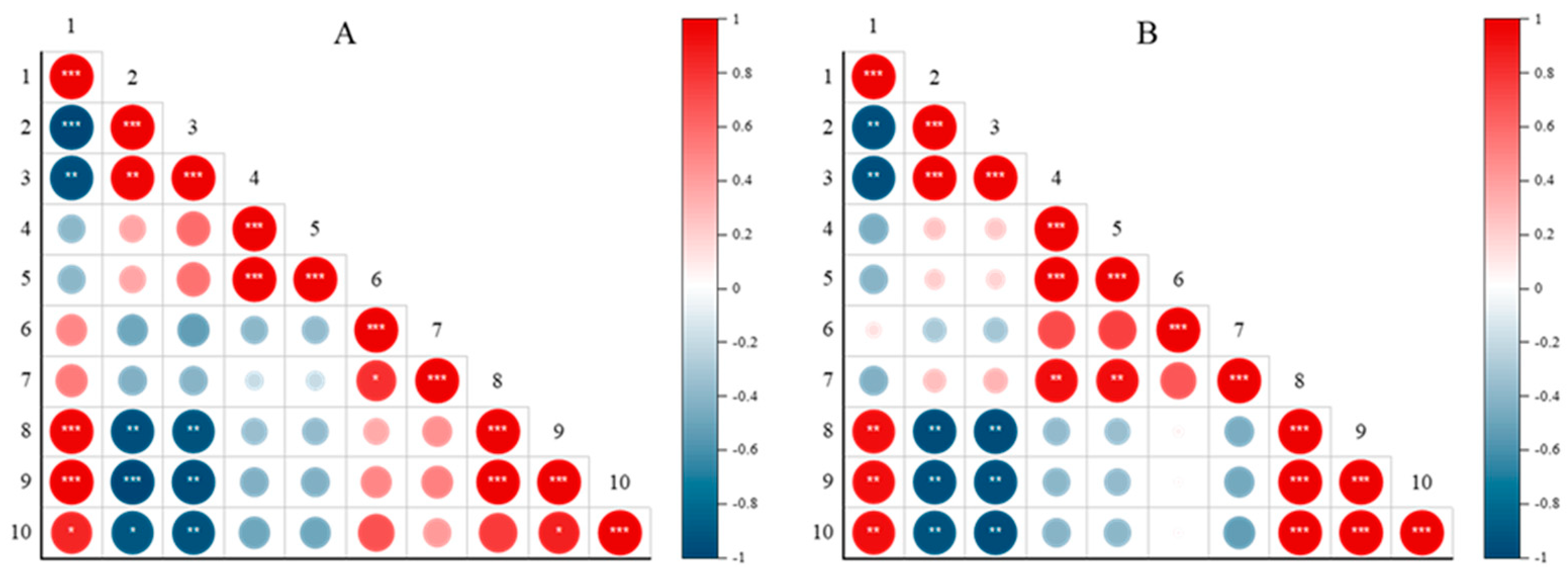
3.4. Comparison of Pearson Correlation Coefficients
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, J.; Upadhyay, A.K.; Prasad, K.; Bahadur, A.; Rai, M. Variability of carotenes, vitamin C, E and phenolics in brassica vegetables. J. Food Compos. Anal. 2007, 20, 106–112. [Google Scholar] [CrossRef]
- Podsędek, A. Natural antioxidants and antioxidant capacity of brassica vegetables: A review. LWT-Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Kushad, M.M.; Brown, A.F.; Kurilich, A.C.; Juvik, J.A.; Klein, B.P.; Wallig, M.A.; Jeffery, E.H. Variation of glucosinolates in vegetable crops of Brassica oleracea. J. Agric. Food Chem. 1999, 47, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Jeon, K.W.; Kim, M.G.; Heo, S.H.; Boo, K.H.; Kim, J.H.; Kim, C.S. Comparative analysis of active components and antioxidant activities of Brussels sprouts (Brassica oleracea var. gemmifera) and cabbage (Brassica oleracea var. capitata). J. Appl. Biol. Chem. 2022, 65, 413–419. [Google Scholar] [CrossRef]
- Heaney, R.K.; Fenwick, G.R. Glucosinolates in brassica vegetables. Analysis of 22 varieties of Brussels sprout (Brassica oleracea var. gemmifera). J. Sci. Food Agric. 1980, 31, 785–793. [Google Scholar] [CrossRef]
- KATI (Korea Agricultural Trade Information). 2014 Agriculture, Forestry and Fisheries Import & Export & Statistics. 2015. Available online: https://www.kati.net/board/pubilshedMaterialsView.do?board_seq=37727&menu_dept2=48 (accessed on 20 November 2023).
- KATI (Korea Agricultural Trade Information). 2022 Agriculture and Forestry Export & Import Statistics. 2023. Available online: https://www.kati.net/board/pubilshedMaterialsList.do?dateSearch=&srchFr=&srchTo=&srchTp=&srchWord=&page=1 (accessed on 20 November 2023).
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Hao, S.; Wang, Y.; Yan, Y.; Liu, Y.; Wang, J.; Chen, S. A review on plant responses to salt stress and their mechanisms of salt resistance. Horticulturae 2021, 7, 132. [Google Scholar] [CrossRef]
- Dehghan, S.; Sadeghi, M.; Pöppel, A.; Fischer, R.; Lakes-Harlan, R.; Kavousi, H.R.; Vilcinskasm, A.; Rahnamaeian, M. Differential inductions of phenylalanine ammonia-lyase and chalcone synthase during wounding, salicylic acid treatment, and salinity stress in safflower, Carthamus tinctorius. Biosci. Rep. 2014, 34, e00114. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a multifaceted signalling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Cho, M.C.; Yang, E.Y.; Lee, J.G. Response to salt stress in lettuce: Changes in chlorophyll fluorescence parameters, phytochemical contents, and antioxidant activities. Agronomy 2020, 10, 1627. [Google Scholar] [CrossRef]
- Strauss, A.J.; Krüger, G.H.J.; Strasser, R.J.; Van Heerden, P.D.R. Ranking of dark chilling tolerance in soybean genotypes probed by the chlorophyll a fluorescence transient OJIP. Environ. Exp. Bot. 2006, 56, 147–157. [Google Scholar] [CrossRef]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A.; et al. Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [CrossRef]
- Fadlelmoula, A.; Pinho, D.; Carvalho, V.H.; Catarino, S.O.; Minas, G. Fourier Transform Infrared (FTIR) Spectroscopy to Analyse Human Blood over the Last 20 Years: A Review towards Lab-on-a-Chip Devices. Micromachines 2022, 13, 187. [Google Scholar] [CrossRef]
- Liu, N.; Karunakaran, C.; Lahlali, R.; Warkentin, T.; Bueckert, R.A. Genotypic and heat stress effects on leaf cuticles of field pea using ATR-FTIR spectroscopy. Planta 2019, 249, 601–613. [Google Scholar] [CrossRef]
- Barreto, M.S.C.; Dos Reis, J.V.; Muraoka, T.; Jemo, M.; Vergutz, L.; Alleoni, L.R.F. Diffuse reflectance infrared Fourier transform spectroscopy for a qualitative evaluation of plant leaf pigment extraction. Analyst 2021, 146, 3440–3448. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yen, H.E. Early salt stress effects on the changes in chemical composition in leaves of ice plant and Arabidopsis. A Fourier transform infrared spectroscopy study. Plant Physiol. 2002, 130, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Wolkers, W.F.; Hoekstra, F.A. Aging of dry desiccation-tolerant pollen does not affect protein secondary structure. Plant Physiol. 1995, 109, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Sanada, A.; Agehara, S. Characterizing Root Morphological Responses to Exogenous Tryptophan in Soybean (Glycine max) Seedlings Using a Scanner-Based Rhizotron System. Plants 2023, 12, 186. [Google Scholar] [CrossRef]
- Cessna, S.; Demmig-Adams, B.; Adams III, W.W. Exploring photosynthesis and plant stress using inexpensive chlorophyll fluorometers. J. Nat. Resour. Life Sci. Educ. 2010, 39, 22–30. [Google Scholar] [CrossRef]
- Giordano, M.; Kansiz, M.; Heraud, P.; Beardall, J.; Wood, B.; McNaughton, D. Fourier transform infrared spectroscopy as a novel tool to investigate changes in intracellular macromolecular pools in the marine microalga Chaetoceros muellerii (Bacillariophyceae). J. Phycol. 2001, 37, 271–279. [Google Scholar] [CrossRef]
- Pimentel, L.G.; Barreto, M.S.C.; da Silva Oliveira, D.M.; Cherubin, M.R.; Demattê, J.A.M.; Cerri, C.E.P.; Cerri, C.C. Diffuse Reflectance Infrared Fourier Transform (DRIFT) spectroscopy to assess decomposition dynamics of sugarcane straw. BioEnergy Res. 2019, 12, 909–919. [Google Scholar] [CrossRef]
- Deb, M.K.; Verma, D. Fourier transform infrared spectroscopic determination of ammonium at sub-microgram level in waters and biological fluids following removal of nitrate from sample matrix by zerovalent iron nanoparticles. Microchim. Acta 2010, 169, 23–31. [Google Scholar] [CrossRef]
- Zeroual, W.; Choisy, C.; Doglia, S.M.; Bobichon, H.; Angiboust, J.F.; Manfait, M. Monitoring of bacterial growth and structural analysis as probed by FT-IR spectroscopy. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 1994, 1222, 171–178. [Google Scholar] [CrossRef]
- Stani, C.; Vaccari, L.; Mitri, E.; Birarda, G. FTIR investigation of the secondary structure of type I collagen: New insight into the amide III band. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 118006. [Google Scholar] [CrossRef]
- Gao, W.G.; Liu, X.C.; Chen, M.F. In situ ATR-FTIR investigation and theoretical calculation of the interactions of chromate and citrate on the surface of haematite (α-Fe2O3). RSC Adv. 2017, 7, 41011–41016. [Google Scholar] [CrossRef]
- Martínez, E.J.; Gil, M.V.; Fernandez, C.; Rosas, J.G.; Gómez, X. Anaerobic codigestion of sludge: Addition of butcher’s fat waste as a cosubstrate for increasing biogas production. PLoS ONE 2016, 11, e0153139. [Google Scholar] [CrossRef] [PubMed]
- Tunçtürk, M.; Tunçtürk, R.; Yildirim, B.; Çiftçi, V. Effect of salinity stress on plant fresh weight and nutrient composition of some Canola (Brassica napus L.) cultivars. Afr. J. Biotechnol. 2011, 10, 1827–1832. [Google Scholar]
- Beinsan, C.; Camen, D.; Sumalan, R.; Babau, M. Study concerning salt stress effect on leaf area dynamics and chlorophyll content in four bean local landraces from Banat area. In Proceedings of the 44th Croatian & 4th International Symposium on Agriculture, Opatija, Bosnia and Herzegovina, Opatija, Croatia, 16–20 February 2009; Volume 4, pp. 416–419. Available online: https://api.semanticscholar.org/CorpusID:92595911 (accessed on 22 November 2023).
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef]
- Panda, D.; Dash, P.K.; Dhal, N.K.; Rout, N.C. Chlorophyll fluorescence parameters and chlorophyll content in mangrove species grown in different salinity. Gen. Appl. Plant Physiol. 2006, 32, 175–180. [Google Scholar]
- Zlatev, Z.S.; Yordanov, I.T. Effects of soil drought on photosynthesis and chlorophyll fluorescence in bean plants. Bulg. J. Plant Physiol. 2004, 30, 3–18. [Google Scholar]
- Pak, V.A.; Nabipour, M.; Meskarbashee, M. Effect of salt stress on chlorophyll content, fluorescence, Na and K ions content in rape plants (Brassica napus L.). Asian J. Agric. Res. 2009, 3, 28–37. [Google Scholar] [CrossRef]
- Saddiq, M.S.; Iqbal, S.; Hafeez, M.B.; Ibrahim, A.M.; Raza, A.; Fatima, E.M.; Baloch, H.; Jahanzaib; Woodrow, P.; Ciarmiello, L.F. Effect of salinity stress on physiological changes in winter and spring wheat. Agronomy 2021, 11, 1193. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Kim, Y.H.; Lee, J.G. Detection of temperature stress using chlorophyll fluorescence parameters and stress-related chlorophyll and proline content in paprika (Capsicum annuum L.) seedlings. Hortic. Sci. Technol. 2018, 36, 619–629. [Google Scholar] [CrossRef]
- Zribi, L.; Fatma, G.; Fatma, R.; Salwa, R.; Hassan, N.; Néjib, R.M. Application of chlorophyll fluorescence for the diagnosis of salt stress in tomato “Solanum lycopersicum (variety Rio Grande)”. Sci. Hortic. 2009, 120, 367–372. [Google Scholar] [CrossRef]
- Bacarin, M.A.; Deuner, S.; Silva, F.S.P.D.; Cassol, D.; Silva, D.M. Chlorophyll a fluorescence as indicative of the salt stress on Brassica napus L. Braz. J. Plant Physiol. 2011, 23, 245–253. [Google Scholar] [CrossRef]
- Kaouther, Z.; Mariem, B.F.; Fardaous, M.; Cherif, H. Impact of salt stress (NaCl) on growth, chlorophyll content and fluorescence of Tunisian cultivars of chili pepper (Capsicum frutescens L.). J. Stress Physiol. Biochem. 2012, 8, 236–252. [Google Scholar]
- Jouyban, Z. The effects of salt stress on plant growth. Tech. J. Eng. Appl. Sci. 2012, 2, 7–10. [Google Scholar]
- Akyol, T.Y.; Yilmaz, O.; Uzılday, B.; Uzılday, R.Ö.; Türkan, İ. Plant response to salinity: An analysis of ROS formation, signaling, and antioxidant defense. Turk. J. Bot. 2020, 44, 1–13. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef]
- Chen, S.; Pan, X.; Li, Y.; Cui, L.; Zhang, Y.; Zhang, Z.; Pan, G.; Yang, J.; Cao, P.; Yang, A. Identification and characterization of chalcone synthase gene family members in Nicotiana tabacum. J. Plant Growth Regul. 2017, 36, 374–384. [Google Scholar] [CrossRef]
- Chen, S.; Wu, F.; Li, Y.; Qian, Y.; Pan, X.; Li, F.; Wang, Y.; Wu, Z.; Fu, C.; Lin, H.; et al. NtMYB4 and NtCHS1 are critical factors in the regulation of flavonoid biosynthesis and are involved in salinity responsiveness. Front. Plant Sci. 2019, 10, 178. [Google Scholar] [CrossRef]
- Eryılmaz, F. The relationships between salt stress and anthocyanin content in higher plants. Biotechnol. Biotechnol. Equip. 2006, 20, 47–52. [Google Scholar] [CrossRef]
- Naing, A.H.; Kim, C.K. Abiotic stress-induced anthocyanins in plants: Their role in tolerance to abiotic stresses. Physiol. Plant. 2021, 172, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Singh, S.P.; Shekhawat, K.; Rathore, S.S.; Pandey, A.; Kumar, S.; Singh, D.K.; Choudhry, S.B.; Kumar, S.; Singh, D. Comparative analysis for understanding salinity tolerance mechanism in Indian mustard (Brassica juncea L.). Acta Physiol. Plant. 2019, 41, 1–14. [Google Scholar] [CrossRef]
- Javed, M.; Zafar, Z.U.; Ashraf, M. Leaf proteome analysis signified that photosynthesis and antioxidants are key indicators of salinity tolerance in canola (Brassica napus L.). Pak. J. Bot. 2019, 51, 1955–1968. [Google Scholar] [CrossRef]
- Mittal, S.; Kumari, N.; Sharma, V. Differential response of salt stress on Brassica juncea: Photosynthetic performance, pigment, proline, D1 and antioxidant enzymes. Plant Physiol. Biochem. 2012, 54, 17–26. [Google Scholar] [CrossRef]
- Pavlović, I.; Pěnčík, A.; Novák, O.; Vujčić, V.; Brkanac, S.R.; Lepeduš, H.; Strnad, M.; Salopek-Sondi, B. Short-term salt stress in Brassica rapa seedlings causes alterations in auxin metabolism. Plant Physiol. Biochem. 2018, 125, 74–84. [Google Scholar] [CrossRef]
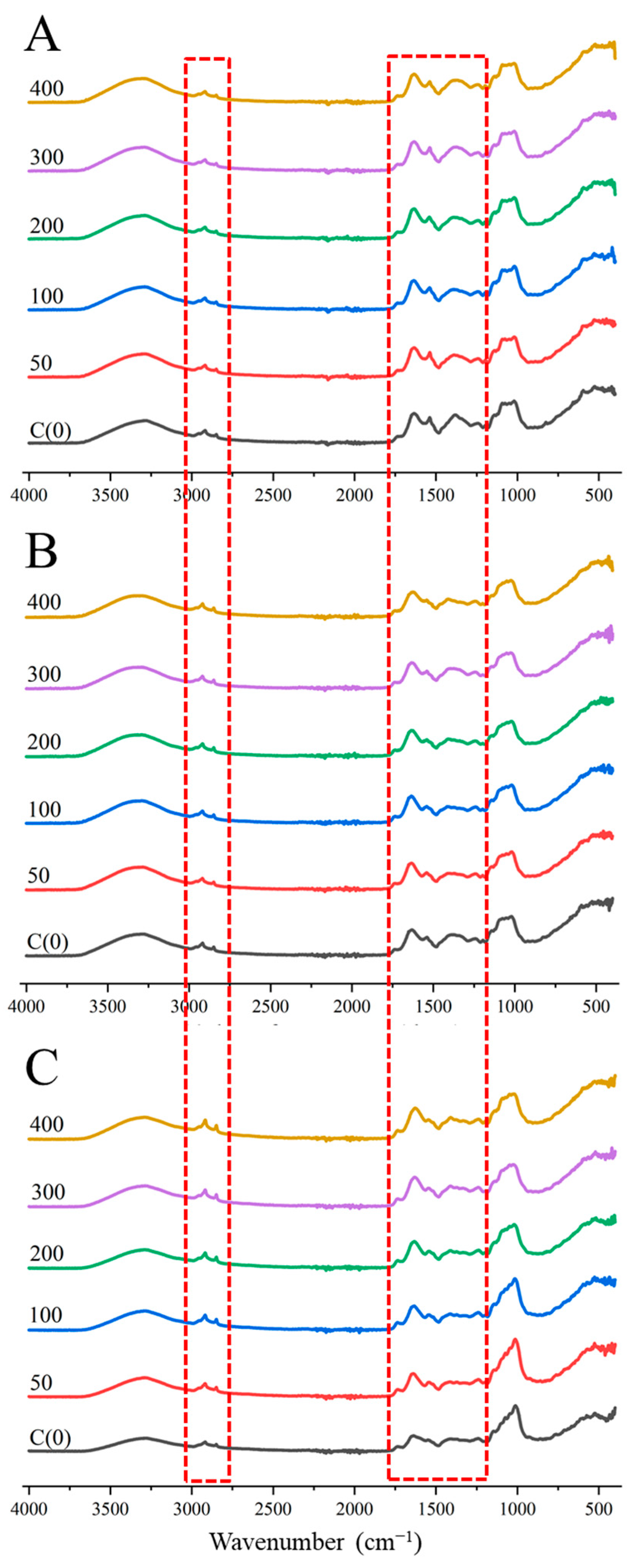
| Wavenumber (cm−1) | Band Assignments | References |
|---|---|---|
| 900~1200 | Fingerprint region of carbohydrate | [18,23,24] |
| 1250 | C-N band (amide III) | [18] |
| 1400 | Asymmetric N-H band of NH4+ | [25] |
| 1455 | Asymmetric deformation band of CH3 and CH2 (protein) | [26] |
| 1540 | N-H band (amide II) | [18,23] |
| 1600~1700 | C=O band (amide I) | [18,23,27] |
| 1740 | C=O band of COOH (lipids and fatty acids) C-OH band of COOH | [23,28] |
| 2850, 2920 | C-H band (lipid and plastid structure) | [17,29] |
| around 3400 | O-H and N-H band (protein and carbohydrate) | [18] |
| Parameter | Treatment | Days after Treatment | ||||
|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 10 | ||
| Fv/Fm | C(0) | 0.740 | 0.769 | 0.783 | 0.797 a* | 0.781 |
| 50 | 0.753 | 0.778 | 0.791 | 0.801 a | 0.773 | |
| 100 | 0.753 | 0.773 | 0.784 | 0.797 a | 0.782 | |
| 200 | 0.729 | 0.767 | 0.789 | 0.767 b | 0.789 | |
| 300 | 0.749 | 0.786 | 0.787 | 0.781 ab | 0.764 | |
| 400 | 0.738 | 0.792 | 0.790 | 0.794 ab | 0.772 | |
| p-value | 0.5593 | 0.0802 | 0.9791 | 0.0095 | 0.3321 | |
| 2 | 4 | 6 | 8 | 10 | ||
| Fv/F0 | C(0) | 2.859 | 3.339 | 3.621 | 3.946 a | 3.584 |
| 50 | 3.105 | 3.525 | 3.803 | 4.027 a | 3.419 | |
| 100 | 3.061 | 3.425 | 3.640 | 3.956 a | 3.611 | |
| 200 | 2.693 | 3.317 | 3.744 | 3.297 b | 3.752 | |
| 300 | 3.002 | 3.700 | 3.750 | 3.579 ab | 3.279 | |
| 400 | 2.842 | 3.819 | 3.804 | 3.883 ab | 3.408 | |
| p-value | 0.5170 | 0.0805 | 0.9785 | 0.0139 | 0.3229 | |
| 2 | 4 | 6 | 8 | 10 | ||
| ABS/RC | C(0) | 1.781 | 1.903 | 1.850 | 1.678 | 1.854 |
| 50 | 2.050 | 1.895 | 1.851 | 1.781 | 1.701 | |
| 100 | 2.158 | 1.595 | 1.910 | 1.832 | 1.856 | |
| 200 | 1.779 | 1.722 | 1.875 | 1.802 | 1.988 | |
| 300 | 2.208 | 1.688 | 1.869 | 1.848 | 1.816 | |
| 400 | 1.760 | 1.659 | 1.898 | 1.780 | 1.818 | |
| p-value | 0.1644 | 0.3388 | 0.9963 | 0.9876 | 0.7585 | |
| 2 | 4 | 6 | 8 | 10 | ||
| TRo/RC | C(0) | 0.232 | 0.239 | 0.247 | 0.225 | 0.246 |
| 50 | 0.265 | 0.235 | 0.240 | 0.237 | 0.226 | |
| 100 | 0.283 | 0.198 | 0.248 | 0.239 | 0.239 | |
| 200 | 0.242 | 0.220 | 0.243 | 0.232 | 0.246 | |
| 300 | 0.298 | 0.204 | 0.231 | 0.254 | 0.217 | |
| 400 | 0.238 | 0.201 | 0.247 | 0.237 | 0.232 | |
| p-value | 0.3852 | 0.5411 | 0.9604 | 0.9792 | 0.8188 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, S.-M.; Kim, C.-S.; Lee, J.-J.; Chung, J.-S. Application of ATR-FTIR Spectroscopy for Analysis of Salt Stress in Brussels Sprouts. Metabolites 2024, 14, 470. https://doi.org/10.3390/metabo14090470
Yun S-M, Kim C-S, Lee J-J, Chung J-S. Application of ATR-FTIR Spectroscopy for Analysis of Salt Stress in Brussels Sprouts. Metabolites. 2024; 14(9):470. https://doi.org/10.3390/metabo14090470
Chicago/Turabian StyleYun, Su-Min, Cheol-Soo Kim, Jeung-Joo Lee, and Jung-Sung Chung. 2024. "Application of ATR-FTIR Spectroscopy for Analysis of Salt Stress in Brussels Sprouts" Metabolites 14, no. 9: 470. https://doi.org/10.3390/metabo14090470
APA StyleYun, S.-M., Kim, C.-S., Lee, J.-J., & Chung, J.-S. (2024). Application of ATR-FTIR Spectroscopy for Analysis of Salt Stress in Brussels Sprouts. Metabolites, 14(9), 470. https://doi.org/10.3390/metabo14090470






