Adenosine Triphosphate and Adenylate Energy Charge in Ready-to-Eat Food
Abstract
1. Introduction
1.1. Nucleotide-Based Freshness Indices
1.2. Adenylate Energy Charge
1.3. Nucleotides in Thermally Processed and Canned Fish
2. Materials and Methods
2.1. Fast Protein and Metabolites Liquid Chromatography (FPMLC)
2.2. pH Measurements
2.3. ATP Biolomimscense
2.4. High-Performance Liquid Chromatography HPLC
2.5. Nuclear Magnetic Resonance (NMR) Spectroscopy
3. Results
3.1. A Comprehensive Analysis of the AEC as a Quality Factor in Absolutely Fresh Pork and Fish Based on Previously Published Experimental Data
3.2. The AEC Index of Thermally Processed Pork, Beef, Poultry, and Fish
3.3. Nucleotide-Based Freshness Indices of Canned Fish
3.4. Influence of High-Temperature Sterilization on Nucleotide-Based Freshness Indices at Different Stages of Cold Storage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, T.; Song, G.; Liu, X.; Xu, M.; Li, Y. Nucleotides as optimal candidates for essential nutrients in living organisms: A review. J. Funct. Foods 2021, 82, 104498. [Google Scholar] [CrossRef]
- Mouritsen, O.G. Umamification of food facilitates the green transition. Soil Ecol. Lett. 2023, 5, 1–3. [Google Scholar] [CrossRef]
- Hwang, Y.-H.; Ismail, I.; Joo, S.-T. Identification of Umami Taste in Sous-Vide Beef by Chemical Analyses, Equivalent Umami Concentration, and Electronic Tongue System. Foods 2020, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Ninomiya, K. Umami and food palatability. J. Nutr. 2000, 130, 921S–926S. [Google Scholar] [CrossRef] [PubMed]
- History–Khymos. Available online: https://khymos.org/molecular-gastronomy/history/ (accessed on 12 June 2024).
- Hong, H.; Regenstein, J.M.; Luo, Y. The importance of ATP-related compounds for the freshness and flavor of post-mortem fish and shellfish muscle: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Guzik, P.; Kulawik, P.; Zając, M.; Migdał, W. Microwave applications in the food industry: An overview of recent developments. Crit. Rev. Food Sci. Nutr. 2022, 62, 7989–8008. [Google Scholar] [CrossRef] [PubMed]
- Van Boekel, M.; Fogliano, V.; Pellegrini, N.; Stanton, C.; Scholz, G.; Lalljie, S.; Somoza, V.; Knorr, D.; Jasti, P.R.; Eisenbrand, G. A review on the beneficial aspects of food processing. Mol. Nutr. Food Res. 2010, 54, 1215–1247. [Google Scholar] [CrossRef] [PubMed]
- Nerín, C.; Aznar, M.; Carrizo, D. Food contamination during food process. Trends Food Sci. Technol. 2016, 48, 63–68. [Google Scholar] [CrossRef]
- Saito, T.; Arai, K.-I.; Matsuyoshi, M. A New Method for Estimating the Freshness of Fish. Nippon Suisan Gakkaishi 1959, 24, 749–750. [Google Scholar] [CrossRef]
- Karube, I.; Matsuoka, H.; Suzuki, S.; Watanabe, E.; Toyama, K. Determination of Fish Freshness with an Enzyme Sensor System. J. Agric. Food Chem. 1984, 32, 314–319. [Google Scholar] [CrossRef]
- Freezing at Sea. Available online: https://www.fao.org/4/v3630e/v3630e14.htm (accessed on 12 June 2024).
- Yokoyama, Y.; Sakaguchi, M.; Kawai, F.; Kanamori, M. Changes in Concentration of ATP-related Compounds in Various Tissues of Oyster during Ice Storage. Nippon Suisan Gakkaishi 1992, 58, 2125–2136. [Google Scholar] [CrossRef]
- Wang, Q.; Xue, C.; Li, Z.; Fu, X.; Xu, J.; Xue, Y. Changes in the contents of ATP and its related breakdown compounds in various tissues of oyster during frozen storage. J. Ocean Univ. China 2007, 6, 407–412. [Google Scholar] [CrossRef]
- Dong, S.; Niu, Y.; Wei, H.; Lin, Y.; Lu, X.; Yamashita, T.; Yu, K.; Takaki, K.; Yuan, C. Effect of super-chilling storage on maintenance of quality and freshness of the Pacific oyster (Crassostrea gigas). Food Qual. Saf. 2023, 7, fyad008. [Google Scholar] [CrossRef]
- Camacho, C.; Correia, T.; Teixeira, B.; Mendes, R.; Valente, L.M.P.; Pessoa, M.F.; Nunes, M.L.; Gonçalves, A. Nucleotides and free amino acids in sea urchin Paracentrotus lividus gonads: Contributions for freshness and overall taste. Food Chem. 2023, 404 Pt A, 134505. [Google Scholar] [CrossRef]
- Cambero, M.I.; Jaramillo, C.J.; Ordoñez, J.A.; Cobos, A.; Pereira-Lima, C.I.; García de Fernando, G.D. Effect of cooking conditions on the flavour compounds and composition of shrimp (Parapenaeus longirostris) broth. Z. Lebensm. Forsch. A 1998, 206, 311–322. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Sakaguchi, M.; Kawai, F.; Kanamori, M. Chemical Indices for Assessing Freshness of Shellfish during Storage. Fish. Sci. 1994, 60, 329–333. [Google Scholar] [CrossRef]
- Fijisawa, K.; Yoshino, M. Activities of adenylate-degrading enzymes in muscles from vertebrates and invertebrates. Comp. Biochem. Physiol. B 1987, 86, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Shibata, M.; ElMasry, G.; Nakazawa, N.; Nakauchi, S.; Hagiwara, T.; Osako, K.; Okazaki, E. Expeditious prediction of post-mortem changes in frozen fish meat using three-dimensional fluorescence fingerprints. Biosci. Biotechnol. Biochem. 2019, 83, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, D.E.; Walton, G.M. Adenosine triphosphate conservation in metabolic regulation. Rat liver citrate cleavage enzyme. J. Biol. Chem. 1967, 242, 3239–3241. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, D.E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry 1968, 7, 4030–4034. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.G.; Fall, L.; Atkinson, D.E. Adenylate energy charge in Escherichia coli during growth and starvation. J. Bacteriol. 1971, 108, 1072–1086. [Google Scholar] [CrossRef]
- Walesby, N.J.; Johnston, I.A. Temperature acclimation in brook trout muscle: Adenine nucleotide concentrations, phosphorylation state and adenylate energy charge. J. Comp. Physiol. B 1980, 139, 127–133. [Google Scholar] [CrossRef]
- Erikson, U.; Beyer, A.; Sigholt, T. Muscle High-Energy Phosphates and Stress Affect K-Values during Ice Storage of Atlantic Salmon (Salmo salar). J. Food Sci. 1997, 62, 43–47. [Google Scholar] [CrossRef]
- Fukushima, H.; Yamada, K.; Wada, R.; Maeda, T.; Matsumiya, M. Thermal stabilities of inosine monophosphate-degrading enzymes in several fish muscles. Int. J. Food Prop. 2020, 23, 1158–1167. [Google Scholar] [CrossRef]
- Hattula, T.; Kiesvaara, M. Breakdown products of adenosine triphosphate in heated fishery products as an indicator of raw material freshness and of storage quality. LWT Food Sci. Technol. 1996, 29, 135–139. [Google Scholar] [CrossRef]
- Mu, H.; Li, L.; Yang, C.; Chen, S.; Yan, B.; Gao, H.; Wei, C. Effects of dietary fishmeal levels on adenosine triphosphate-related compounds and freshness of raw and cooked muscle in large yellow croaker Larimichthys crocea. Aquac. Rep. 2022, 26, 101304. [Google Scholar] [CrossRef]
- Kuda, T.; Fujita, M.; Goto, H.; Yano, T. Effects of freshness on ATP-related compounds in retorted chub mackerel Scomber japonicus. LWT Food Sci. Technol. 2007, 40, 1186–1190. [Google Scholar] [CrossRef]
- Kuda, T.; Fujita, M.; Goto, H.; Yano, T. Effects of retort conditions on ATP-related compounds in pouched fish muscle. LWT Food Sci. Technol. 2008, 41, 469–473. [Google Scholar] [CrossRef]
- Vázquez-Ortiz, F.A.; Pacheco-Aguilar, R.; Lugo-Sanchez, M.E.; Villegas-Ozuna, R.E. Application of the Freshness Quality Index (K Value) for Fresh Fish to Canned Sardines from Northwestern Mexico. J. Food Compos. Anal. 1997, 10, 158–165. [Google Scholar] [CrossRef]
- Sünter, A.; Kuznetsov, A.; Raudsepp, P.; Püssa, T.; Toom, L.; Konoplev, G.; Stepanova, O.S.; Stepanova, O.V.; Lyalin, D.; Frorip, A.; et al. Manifestation of Heat-Induced Valuable Dietary Nucleotide Salvage in Food Prepared from Aged Fish in Fast Protein and Metabolites Liquid Chromatography, ATP-Bioluminescence Assay, and NMR Spectra. AppliedChem 2023, 3, 334–349. [Google Scholar] [CrossRef]
- Kuznetsov, A.; Frorip, A.; Sünter, A.; Kasvand, N.; Korsakov, V.; Konoplev, G.; Stepanova, O.; Rusalepp, L.; Anton, D.; Püssa, T.; et al. Fast Protein and Metabolites (Nucleotides and Nucleosides) Liquid Chromatography Technique and Chemical Sensor for the Assessment of Fish and Meat Freshness. Chemosensors 2023, 11, 69. [Google Scholar] [CrossRef]
- Rundlöf, T.; Mathiasson, M.; Bekiroglu, S.; Hakkarainen, B.; Bowden, T.; Arvidsson, T. Survey and qualification of internal standards for quantification by ¹H NMR spectroscopy. J. Pharmaceut. Biomed. 2010, 52, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Honikel, K.O.; Fischer, C. A Rapid method for the detection of PSE and DFD porcine muscles. J. Food Sci. 1977, 42, 1633–1636. [Google Scholar] [CrossRef]
- Batlle, N.; Aristoy, M.-C.; Toldrá, F. Early Postmortem Detection of Exudative Pork Meat Based on Nucleotide Content. J. Food Sci. 2000, 65, 413–416. [Google Scholar] [CrossRef]
- Batlle, N.; Aristoy, M.-C.; Toldrá, F. ATP Metabolites During Aging of Exudative and Nonexudative Pork Meats. J. Food Sci. 2001, 66, 68–71. [Google Scholar] [CrossRef]
- Moesgaard, B.; Quistorff, B.; Christensen, V.G.; Therkelsen, I.; Jørgensen, P.F. Differences of post-mortem ATP turnover in skeletal muscle of normal and heterozygote malignant-hyperthermia pigs: Comparison of (31)P-NMR and analytical biochemical measurements. Meat Sci. 1995, 39, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Matarneh, S.K. Defining the Role of Mitochondria in Fresh Meat Quality Development. Ph.D. Thesis, Virginia Polytechnic Institute, Blacksburg, VA, USA, 12 July 2017. [Google Scholar]
- Kato, N.; Uchiyama, H.; Uda, F. A rapid method for determination of inosine, hypoxanthine uric acid, and nucleotides in fish muscle by continuous gradient column chromatography. Bull. Jpn. Soc. Sci. Fish. 1973, 39, 1039–1044. (In Japanese) [Google Scholar] [CrossRef][Green Version]
- Ando, S.; Hirohito Kodama, H.; Kawai, Y.; Hatano, M. Analysis of Purine Nucleotides by High Performance Liquid Chromatography: Its application for anadromous salmonid fishes. Bull. Fac. Fish. Hokkaido Univ. 1988, 39, 142–150. [Google Scholar]
- Hu, W.; Haraguchi, H.; Takeuchi, T. Analysis of nucleotides by high-performance liquid chromatography with phosphorus-selective detection. J. Chromatogr. A 1991, 557, 441–449. [Google Scholar] [CrossRef]
- Vetter, R.D.; Hodson, R.E. Use of Adenylate Concentrations and Adenylate Energy Charge as Indicators of Hypoxic Stress in Estuarine Fish. Can. J. Fish. Aquat. Sci. 1982, 39, 535–541. [Google Scholar] [CrossRef]
- Heath, A.G. Changes in tissue adenylates and water content of bluegill, Lepomis macrochirus, exposed to copper. J. Fish Biol. 1984, 24, 299–309. [Google Scholar] [CrossRef]
- Cann-Moisan, C.; Caroff, J.; Sebert, P.; Barthelemy, L. Determination of nucleotide concentrations with high performance liquid chromatography (HPLC): Application to fish. Aquaculture 1989, 76, 135–143. [Google Scholar] [CrossRef]
- Cann-Moisan, C.; Caroff, J.; Sebert, P.; Barthelemy, L. Comparative study of energetic nucleotides in young and adult trout (Salmo gairdneri R.). Aquaculture 1989, 81, 91–96. [Google Scholar] [CrossRef]
- Van Waarde, A. Towards a valid preparation for the in vitro study of energy metabolism in fish muscles. Comp. Biochem. Physiol. Part B Comp. Biochem. 1983, 75, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Nishiguchi, S.; Fukuda, T.; Ando, M.; Tsukamasa, Y. Effects of temperature treatment before thawing on NAD+ and ATP concentrations in frozen fish meats prepared by instant killing and quick freezing and on pH after thawing. Nippon Suisan Gakkaishi 2020, 86, 494–501. [Google Scholar] [CrossRef]
- Li, D.; Qin, N.; Zhang, L.; Li, Q.; Prinyawiwatkul, W.; Luo, Y. Degradation of adenosine triphosphate, water loss and textural changes in frozen common carp (Cyprinus carpio) fillets during storage at different temperatures. Int. J. Refrig. 2019, 98, 294–301. [Google Scholar] [CrossRef]
- Tikk, M.; Tikk, K.; Tørngren, M.A.; Meinert, L.; Aaslyng, M.D.; Karlsson, A.H.; Andersen, H.J. Development of Inosine Monophosphate and Its Degradation Products during Aging of Pork of Different Qualities in Relation to Basic Taste and Retronasal Flavor Perception of the Meat. J. Agric. Food Chem. 2006, 54, 7769–7777. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.V.; Olsen, K.; Mouritsen, O.G. Umami synergy as the scientific principle behind taste-pairing champagne and oysters. Sci. Rep. 2020, 10, 20077. [Google Scholar] [CrossRef] [PubMed]
- Cambero, M.I.; Pereira-Lima, C.I.; Ordoñez, J.A.; García de Fernando, G.D. Beef broth flavour: Relation of components with the flavour developed at different cooking temperatures. J. Sci. Food Agric. 2000, 80, 1519–1528. [Google Scholar] [CrossRef]
- Abramova, L.S.; Kozin, A.V.; Guseva, E.S.; Lavrikova, K.A. Assessment of the palatability of Atlantic salmon by NMR spectroscopy. Food Syst. 2023, 6, 350–357. (In Russian) [Google Scholar] [CrossRef]
- Fang, M.; Ivanisevic, J.; Benton, H.P.; Johnson, C.H.; Patti, G.J.; Hoang, L.T.; Uritboonthai, W.; Kurczy, M.E.; Siuzdak, G. Thermal degradation of small molecules: A global metabolomic investigation. Anal. Chem. 2015, 87, 10935–10941. [Google Scholar] [CrossRef] [PubMed]
- Bakke, M.; Suzuki, S. Development of a novel hygiene monitoring system based on the detection of total adenylate (ATP+ADP+AMP). J. Food Prot. 2018, 81, 729–737. [Google Scholar] [CrossRef] [PubMed]
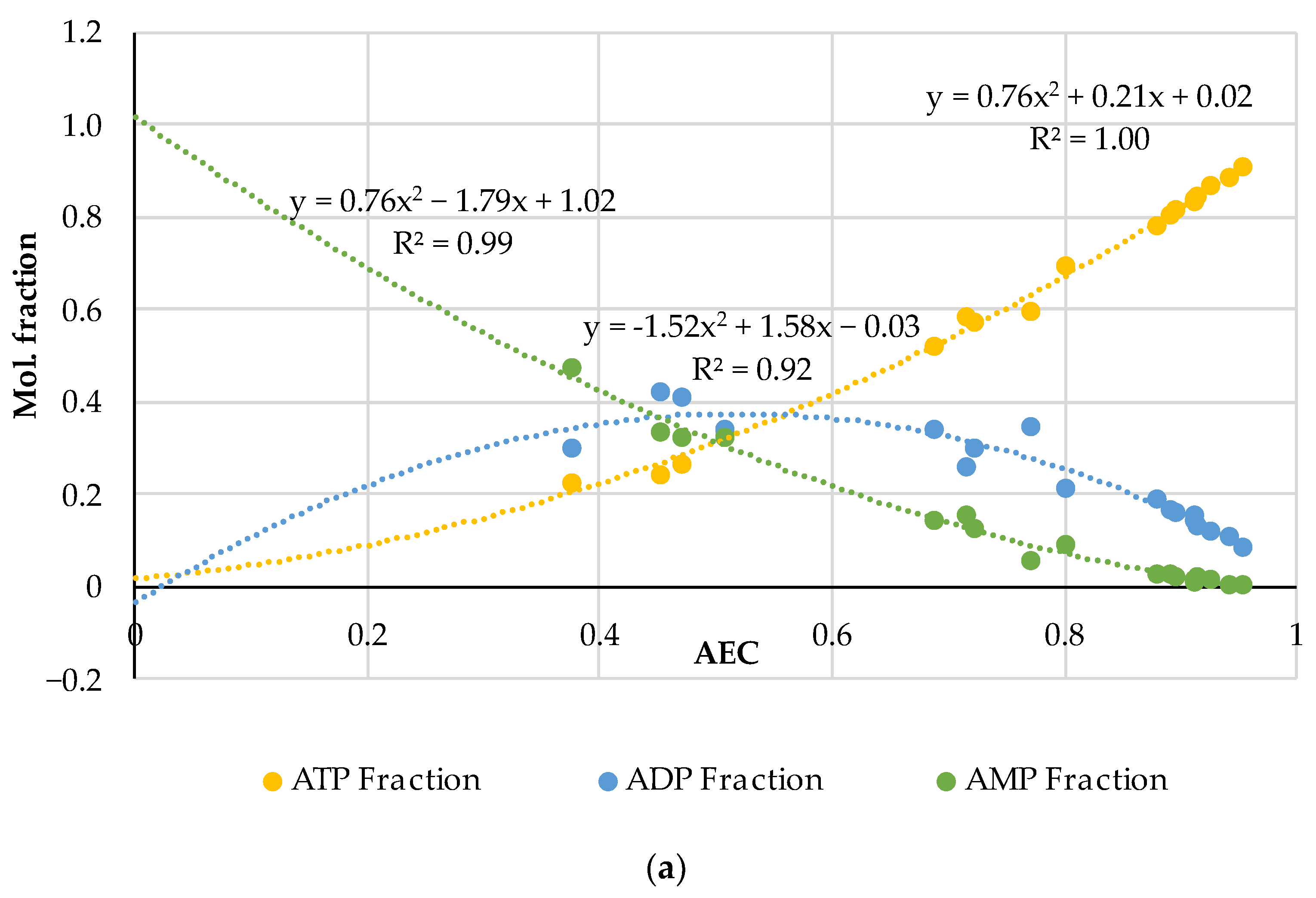
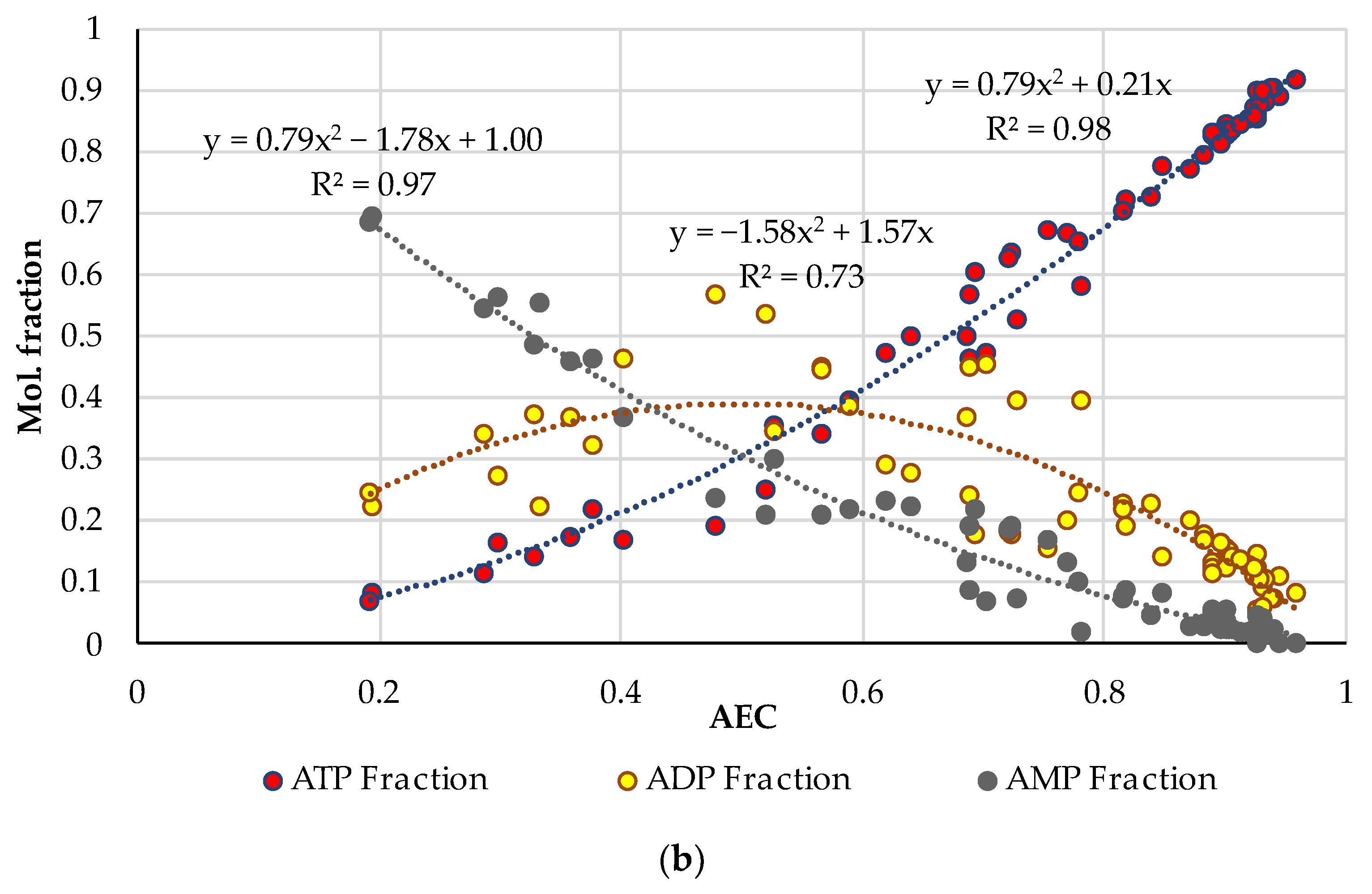
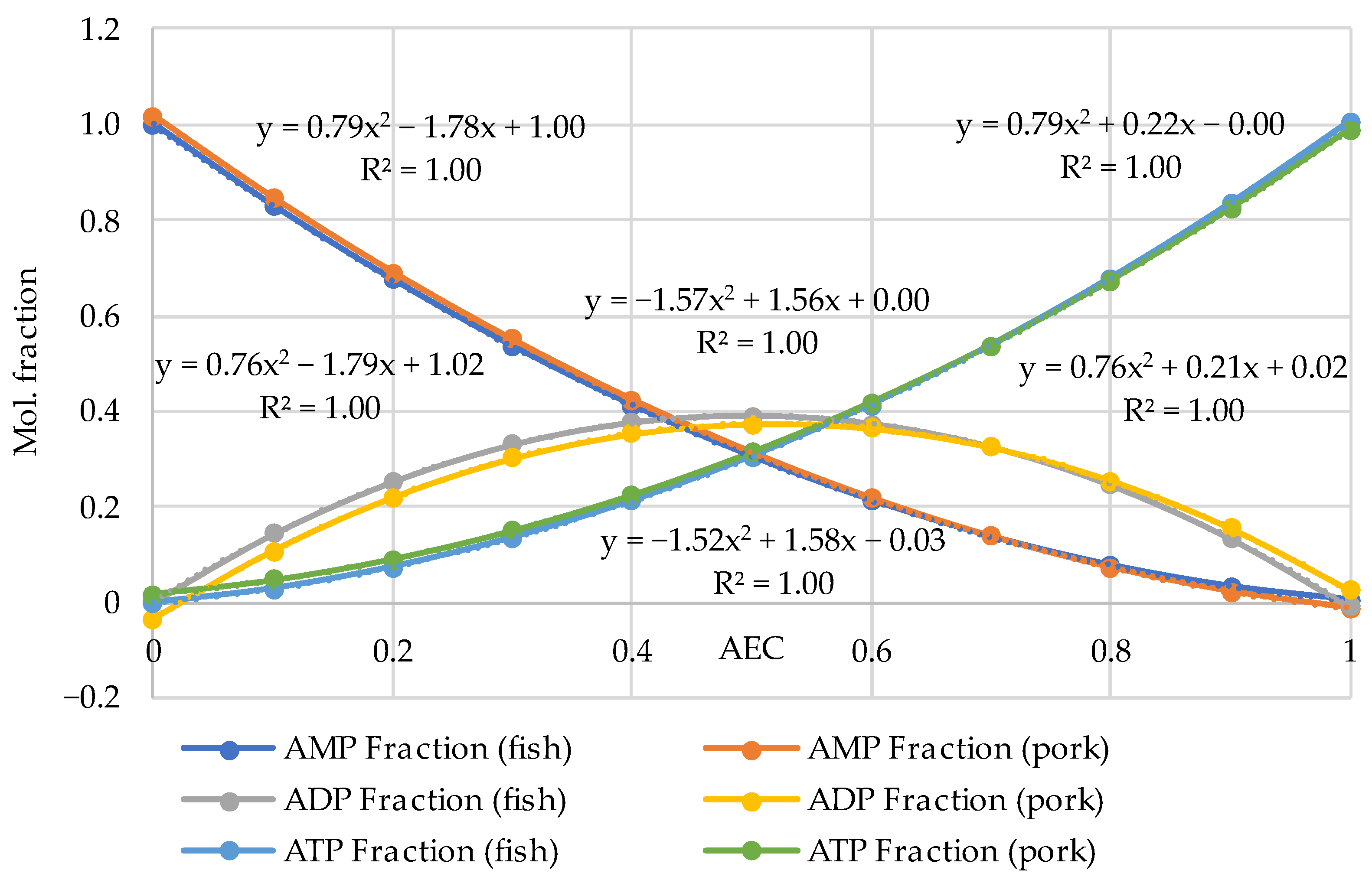

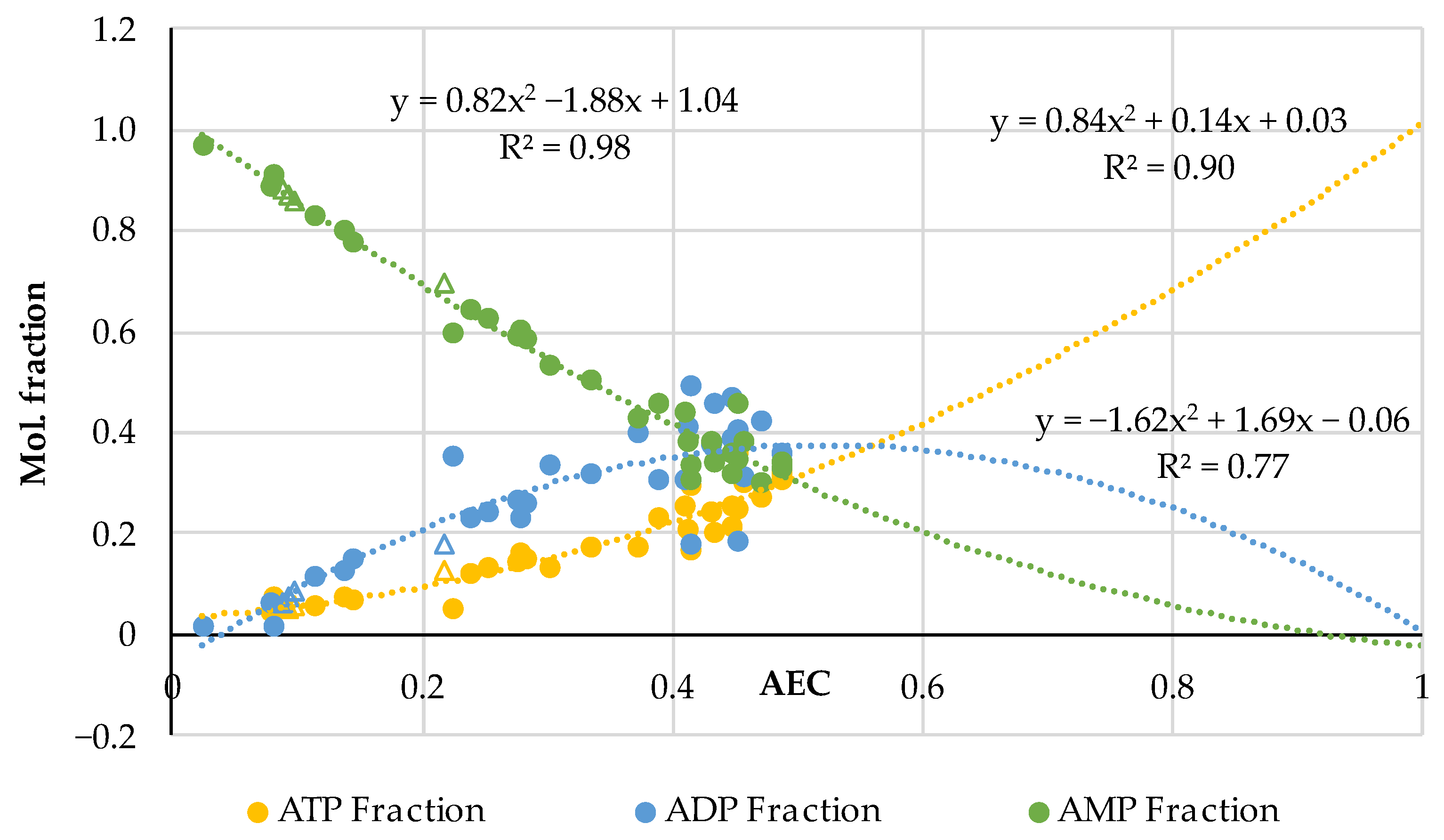
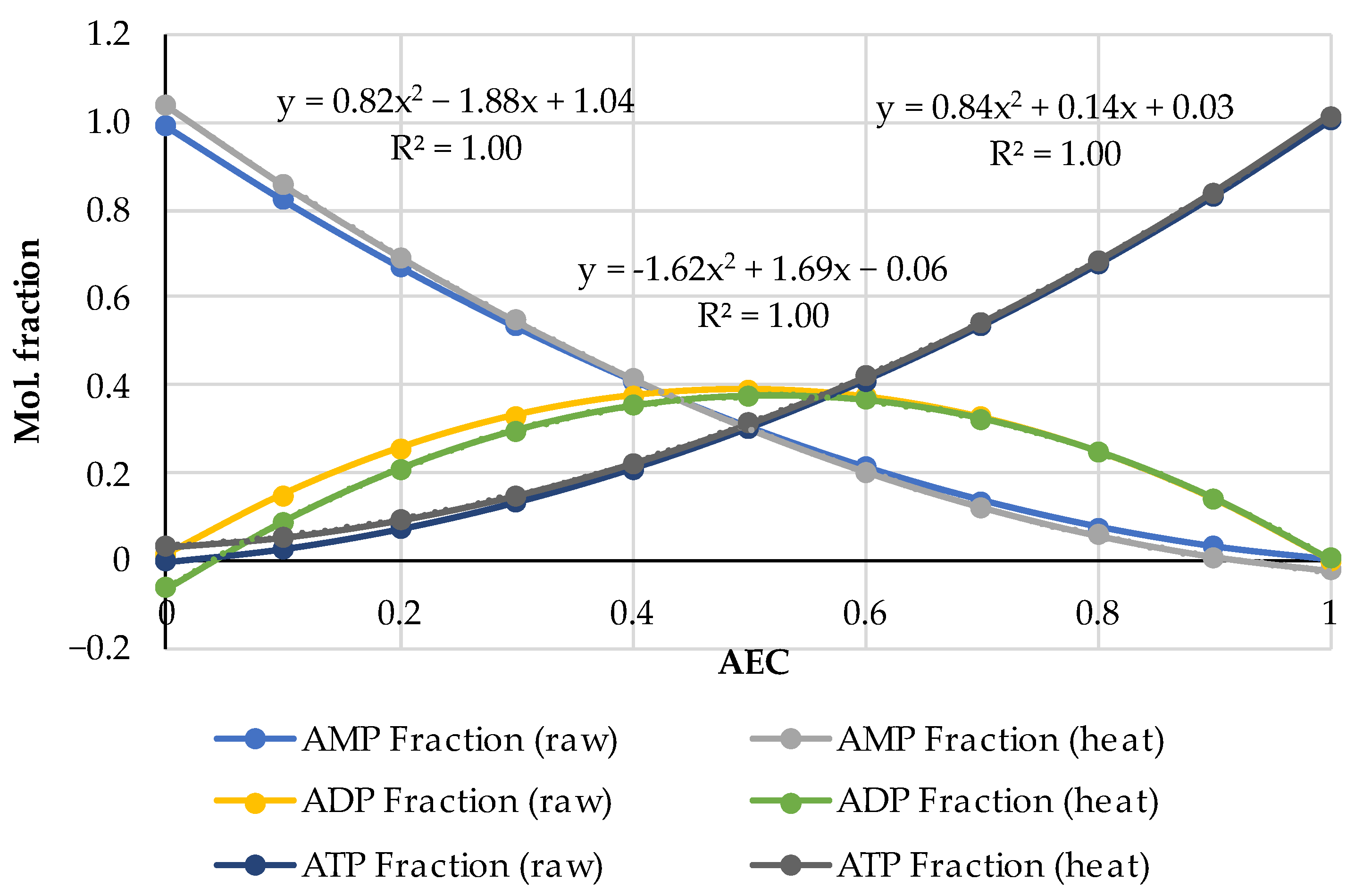
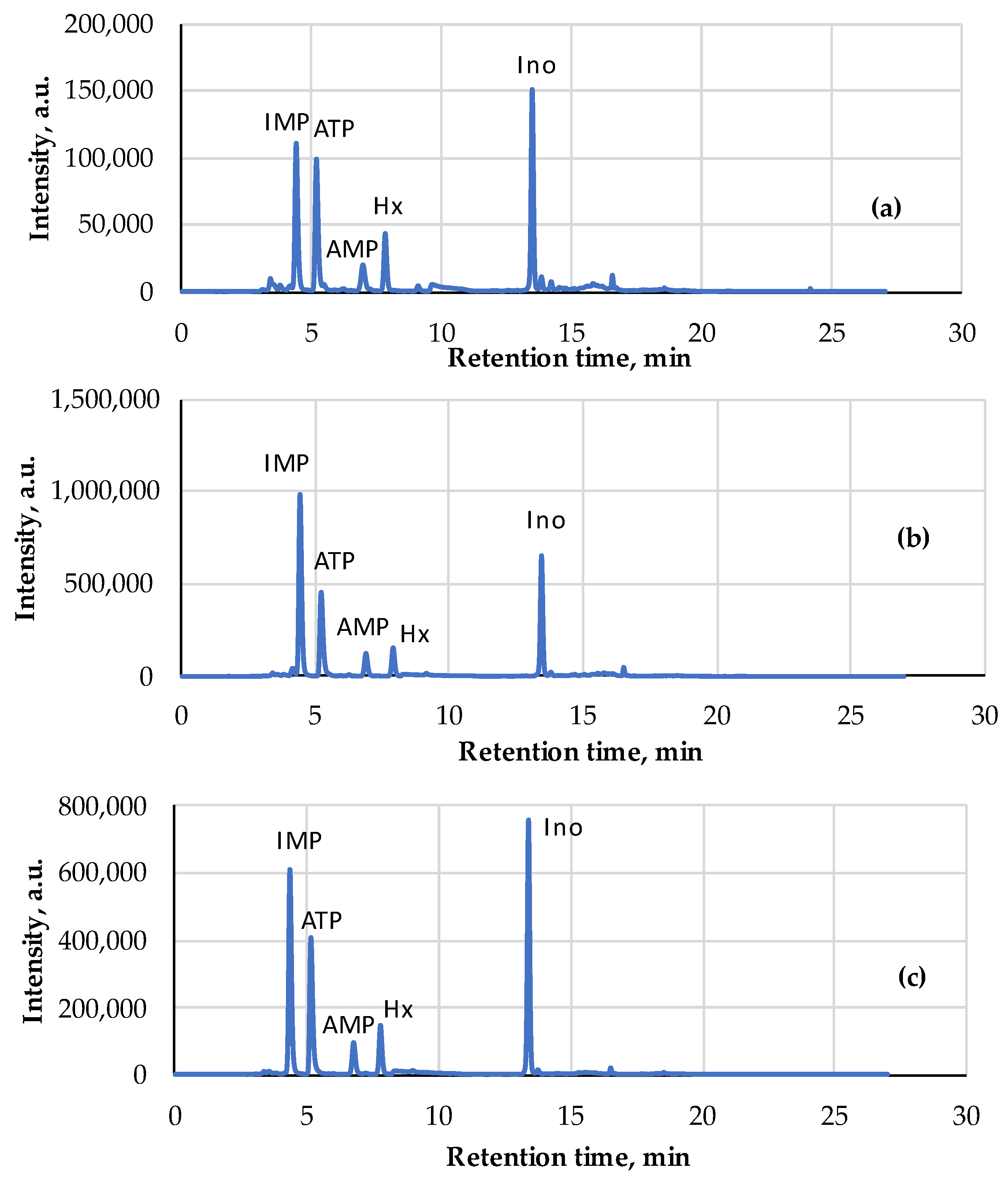
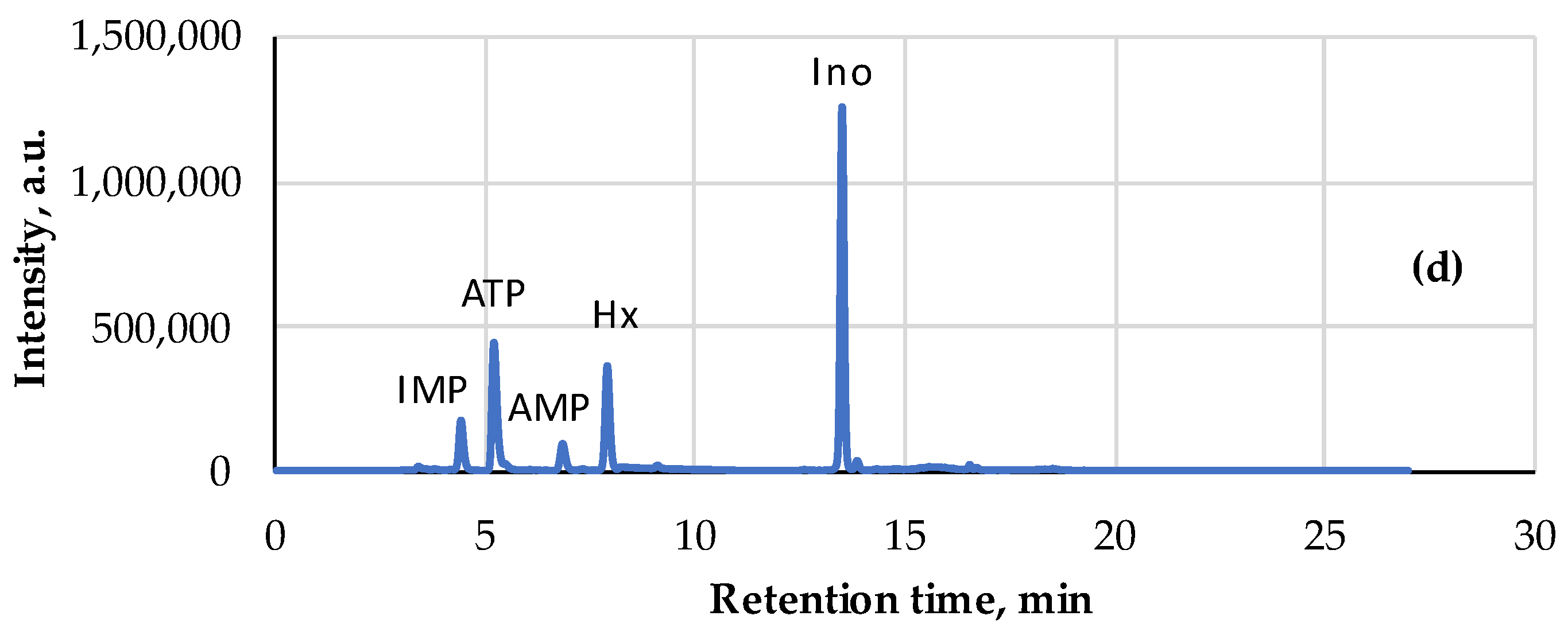
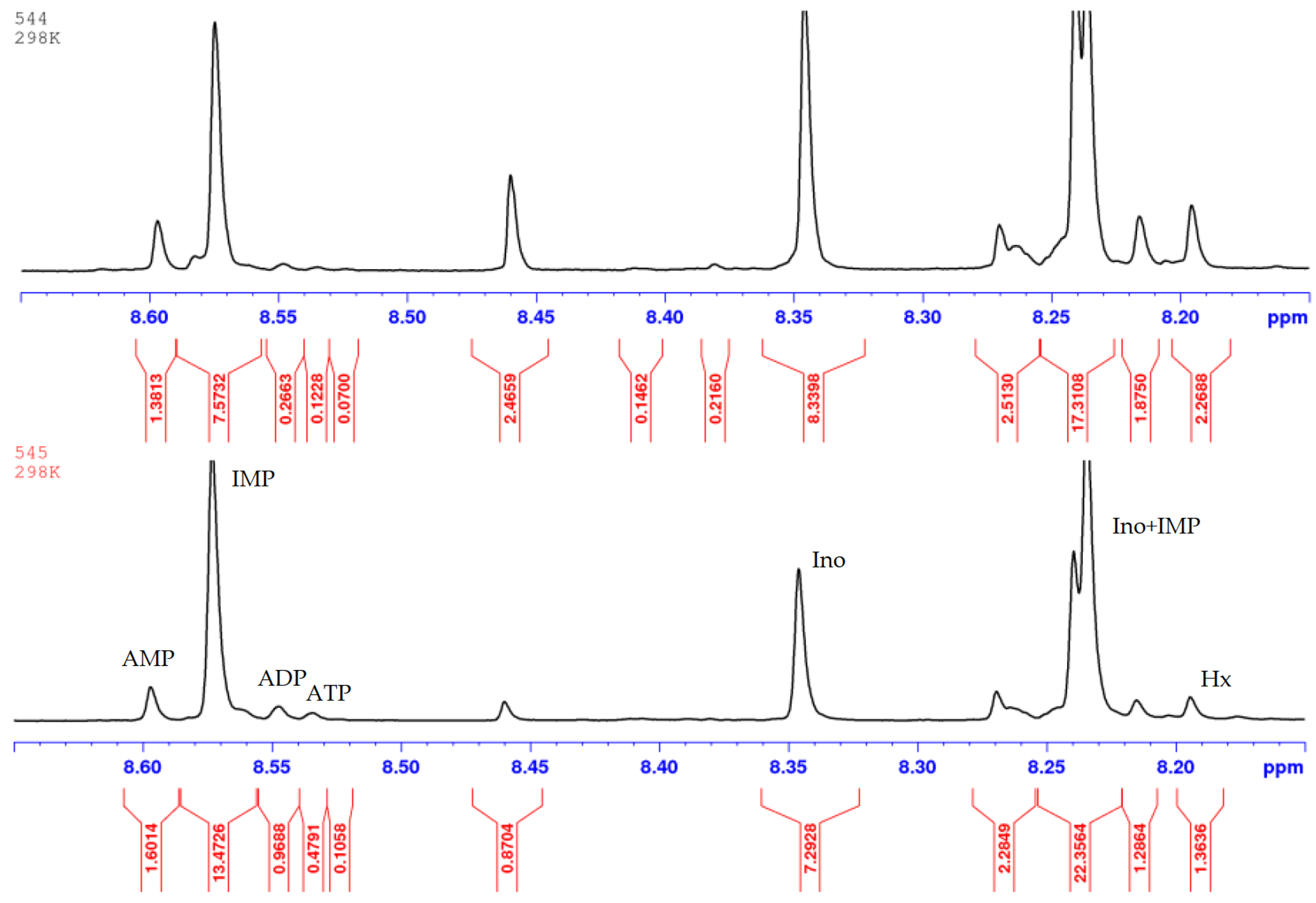


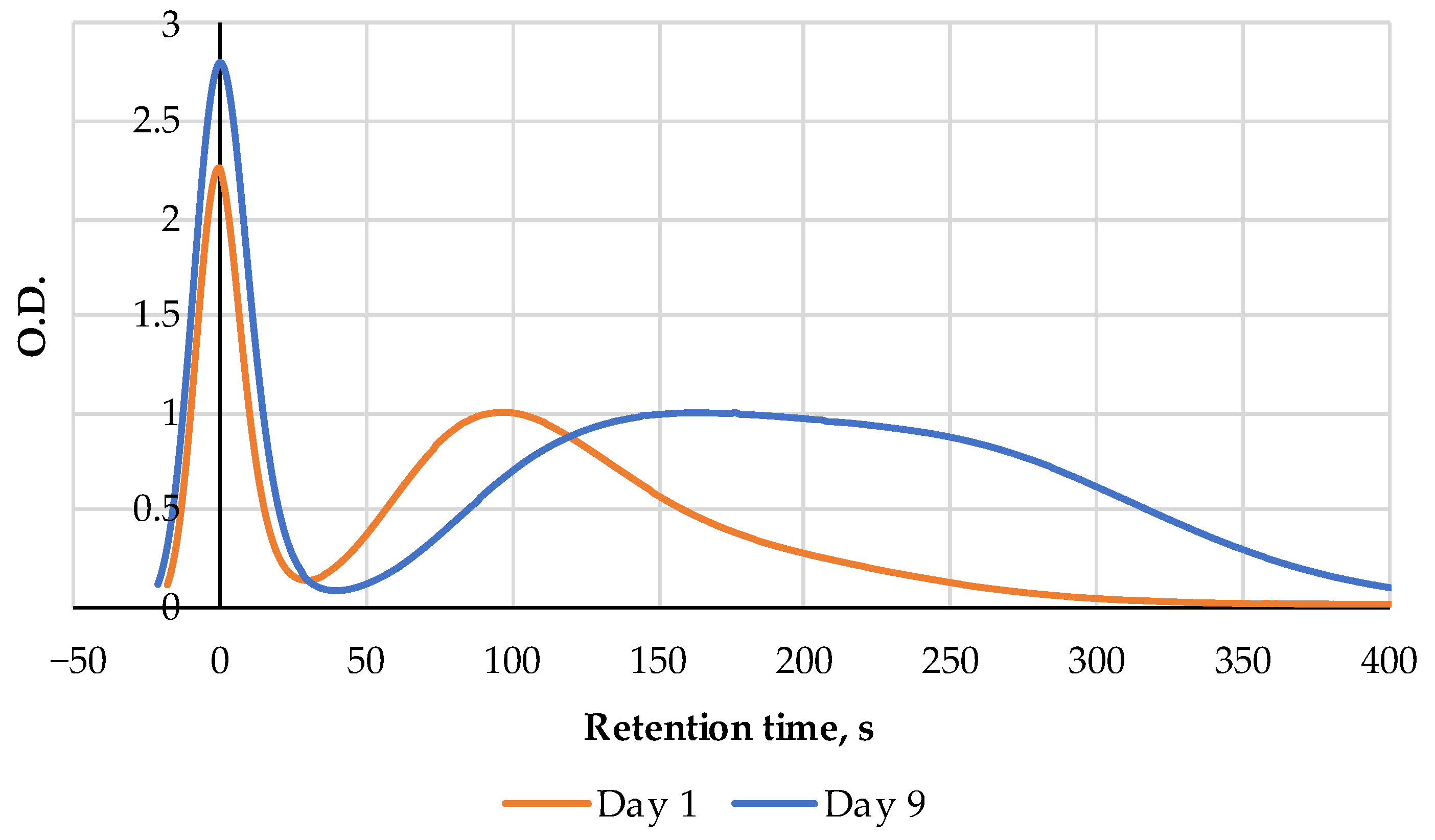


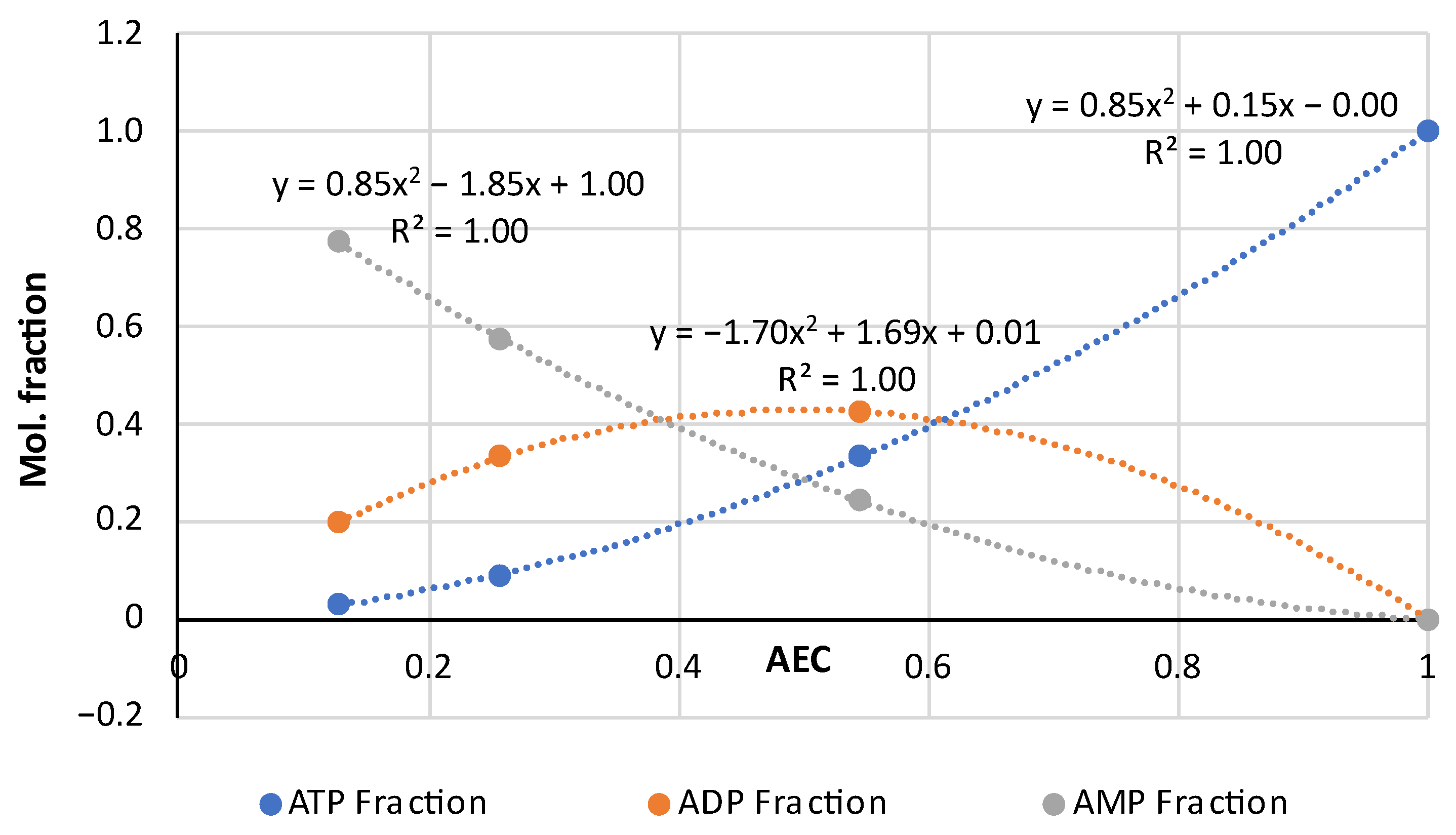
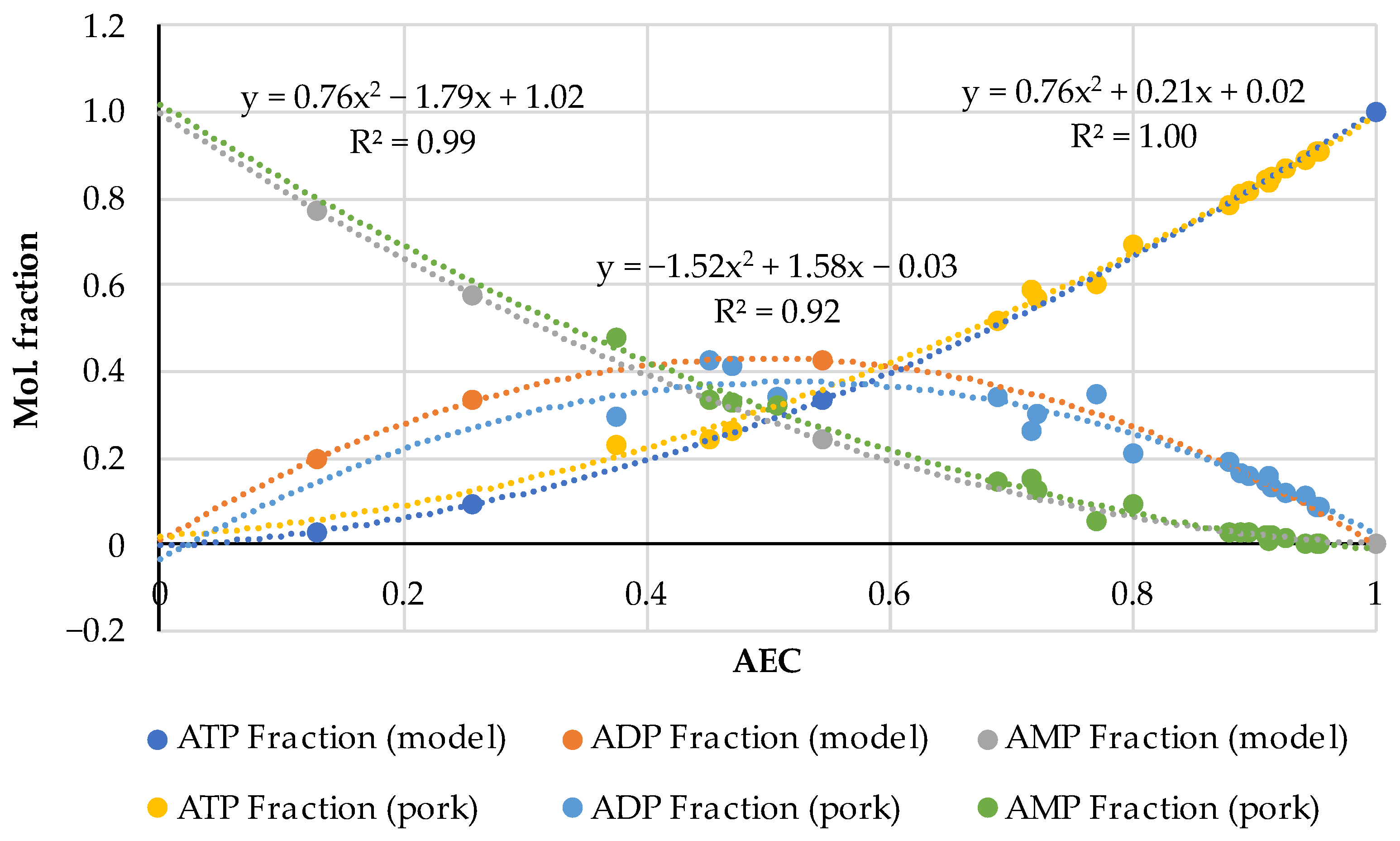
| Pork | AEC | Storage, Hours | Ref. |
|---|---|---|---|
| Normal (averaged) | 0.85 | 1–2 | [35,36,37] |
| Low quality | 0.715 | 2 | [37] |
| PSE | 0.65 | 1 | [35] |
| DFD | 0.6 | 1 | [35] |
| Normal | 0.91 | 2 | [38] |
| Heterozygote | 0.77 | 2 | [38] |
| Wild-type | 0.90 | 0 | [39] |
| AMPK | 0.93 | 0 | [39] |
| Meat | ATP | ADP | AMP | IMP | Ino | Hx | AEC | Condition |
|---|---|---|---|---|---|---|---|---|
| Broiler file | 0.005 | 0.036 | 0.061 | 0.67 | 0.65 | 0.32 | 0.225 | Boiled 75 °C 20 min |
| Chicken schnitzel | 0.009 | 0.017 | 0.047 | 0.41 | 0.77 | 0.2 | 0.24 | Bought as ready-to-eat food |
| Chicken schnitzel | 0.007 | 0.012 | 0.075 | 0.45 | 1.0 | 0.24 | 0.138 | After MW at 400 W 1 min |
| Minced pork balls | 0.001 | 0.001 | 0.06 | 0.22 | 0.36 | 0.24 | 0.024 | Bought as ready-to-eat food |
| Minced pork balls MW irradiated | 0.003 | 0.004 | 0.057 | 0.21 | 0.39 | 0.24 | 0.078 | After MW at 400 W 1 min |
| Sample # | Fish Specie | Time, s | HPLC/NMR Sample Code |
|---|---|---|---|
| 1 | Tuna | 176 | |
| 2 | Tuna | 188 | |
| 3 | Pink Salmon | 172.5 | |
| 4 | Pink Salmon | 187.5 | |
| 5 | Mediterranean Sardine | 138 | 545 |
| 6 | Rainbow Trout | 189.5 | |
| 7 | Sockeye | 165 | |
| 8 | Mackerel | 164 | |
| 9 | Salmon | 192.5 | |
| 10 | Chum Salmon | 188.5 | |
| 11 | Rainbow Trout | 180 | |
| 12 | Pink Salmon | 168.5 | |
| 13 | Tuna | 164.5 | |
| 14 | Atlantic salmon | 171 | |
| 15 | Coho Salmon | 156.5 | |
| 16 | Trout | 164 | 546 |
| 17 | Iwashi | 154 | 544 |
| 18 | Pink Salmon | 178.5 | 547 |
| Canned Fish | Index Time, s FPMLC | KI, HPLC | KI, NMR | K, HPLC | K, NMR | AEC, NMR | Estimate Storage Period before Canning at +2…+4 °C, Days |
|---|---|---|---|---|---|---|---|
| Iwashi (Moreslav) | 154 | 61 | 57 | 42 | 53 | 0.11 | 3–7 |
| Mediterranean sardine (Connétable) | 138 | 43 | 37 | 31 | 34 | 0.28 | 0–2 |
| Rainbow trout (Ecofood) | 164 | 57 | 54 | 41 | 3–7 | ||
| Pink salmon (Moreslav) | 179 | 89 | 91 | 64 | >8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konoplev, G.; Sünter, A.; Kuznetsov, A.I.; Raudsepp, P.; Püssa, T.; Toom, L.; Rusalepp, L.; Anton, D.; Stepanova, O.V.; Lyalin, D.; et al. Adenosine Triphosphate and Adenylate Energy Charge in Ready-to-Eat Food. Metabolites 2024, 14, 440. https://doi.org/10.3390/metabo14080440
Konoplev G, Sünter A, Kuznetsov AI, Raudsepp P, Püssa T, Toom L, Rusalepp L, Anton D, Stepanova OV, Lyalin D, et al. Adenosine Triphosphate and Adenylate Energy Charge in Ready-to-Eat Food. Metabolites. 2024; 14(8):440. https://doi.org/10.3390/metabo14080440
Chicago/Turabian StyleKonoplev, Georgii, Alar Sünter, Artur I. Kuznetsov, Piret Raudsepp, Tõnu Püssa, Lauri Toom, Linda Rusalepp, Dea Anton, Oksana V. Stepanova, Daniil Lyalin, and et al. 2024. "Adenosine Triphosphate and Adenylate Energy Charge in Ready-to-Eat Food" Metabolites 14, no. 8: 440. https://doi.org/10.3390/metabo14080440
APA StyleKonoplev, G., Sünter, A., Kuznetsov, A. I., Raudsepp, P., Püssa, T., Toom, L., Rusalepp, L., Anton, D., Stepanova, O. V., Lyalin, D., Abramova, L., Kozin, A., Stepanova, O. S., Frorip, A., & Roasto, M. (2024). Adenosine Triphosphate and Adenylate Energy Charge in Ready-to-Eat Food. Metabolites, 14(8), 440. https://doi.org/10.3390/metabo14080440





