Serum Metabolomics and NF-κB Pathway Analysis Revealed the Antipyretic Mechanism of Ellagic Acid on LPS-Induced Fever in Rabbits
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Experimental Design
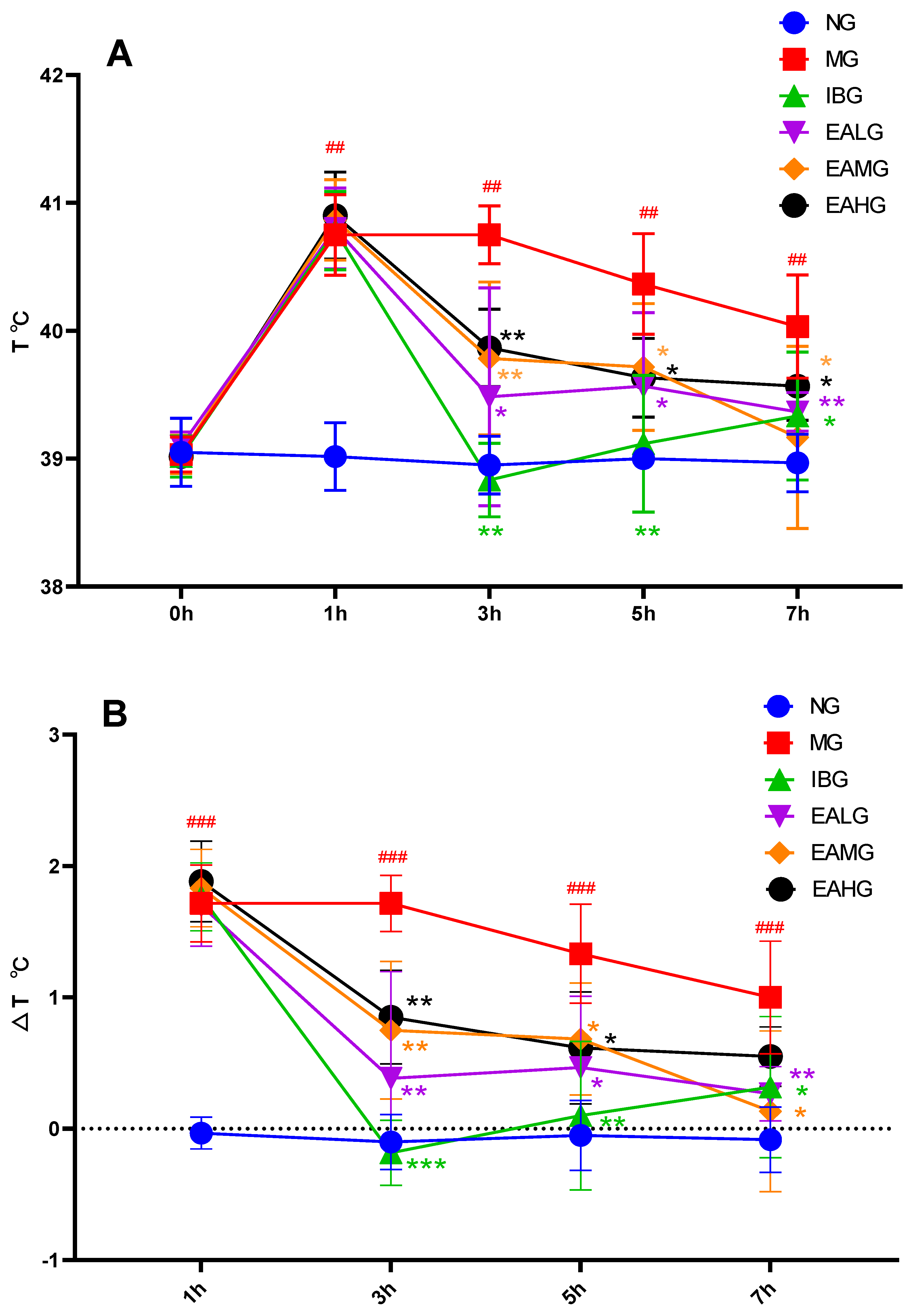
2.3. Measurement of Rectal Temperature
2.4. Sample Collection
2.5. Estimation of Oxidative Stress Biomarkers in Serum
2.6. Quantifcation of Cytokines in Serum
2.7. Quantifcation of cAMP and PGE2 in Cerebrospinal Fluid
2.8. Quantifcation of cAMP and 5-HT in Hypothalamus
2.9. Western Blotting
2.10. Detection of Serum Biomarker Levels with GC-MS
2.11. Statistical Analysis
3. Results
3.1. Effects of EA on LPS-Induced Fever in Rabbits
3.2. Effect of EA on MDA, SOD, GSH, TNF-α, IL-1β, and IL-6 in Serum
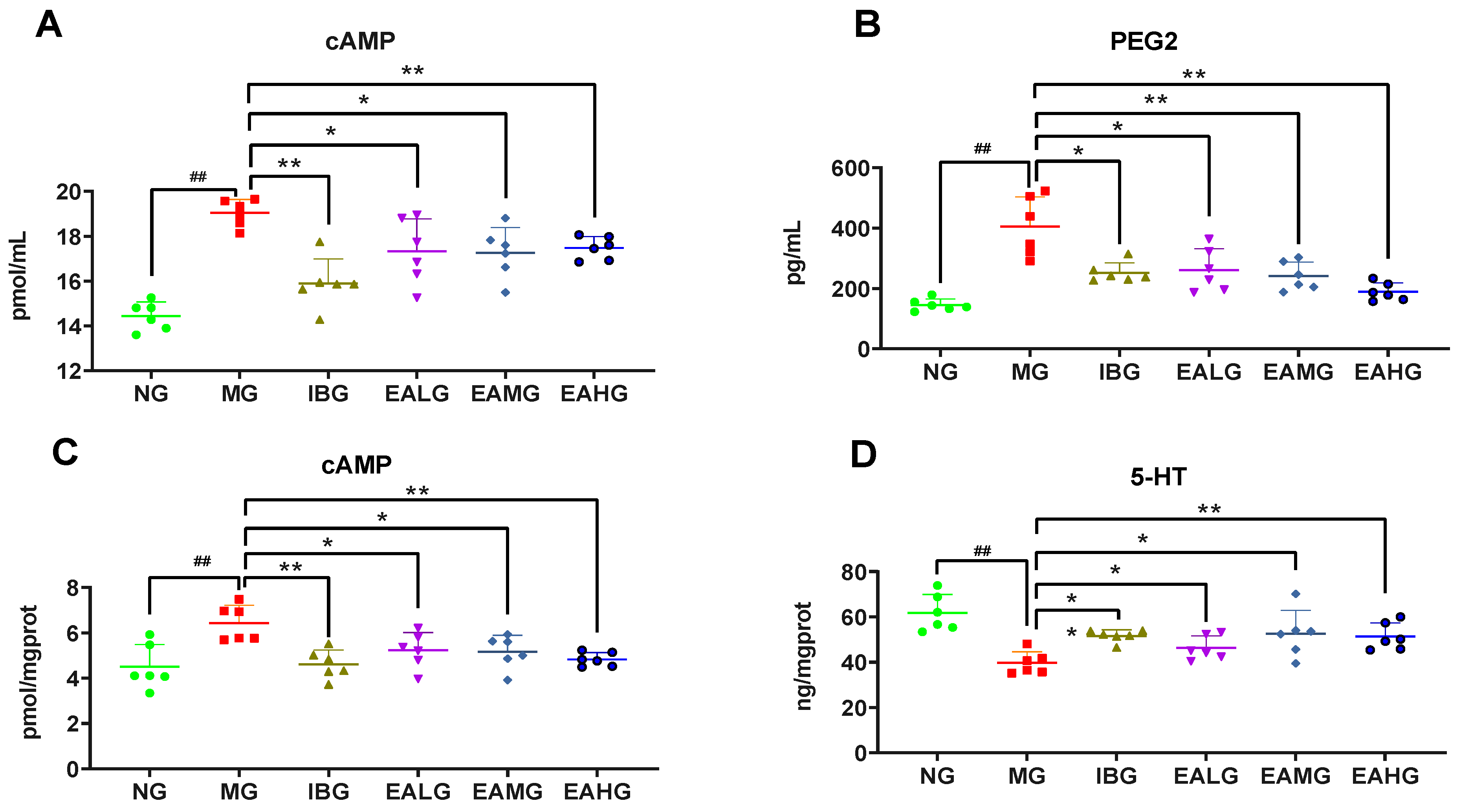
3.3. Effect of EA on cAMP and PGE2 in Cerebrospinal Fluid
3.4. Effect of EA on cAMP and 5-HT in Hypothalamus
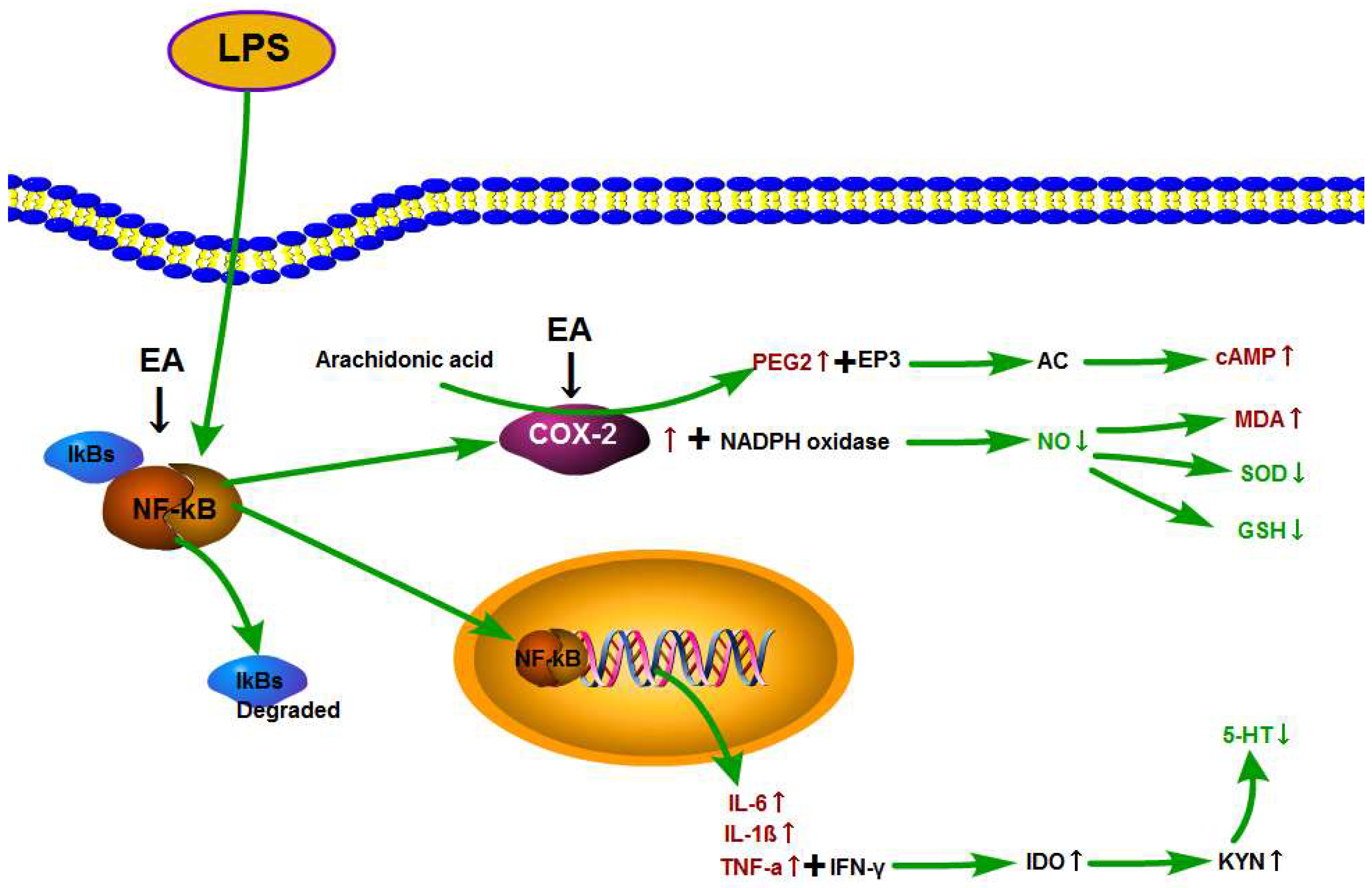
3.5. Effect of EA on p-NF-κB p65 and COX-2 Expression in Hypothalamus
3.6. Serum Metabolomics Profile and Multivariate Data Analysis
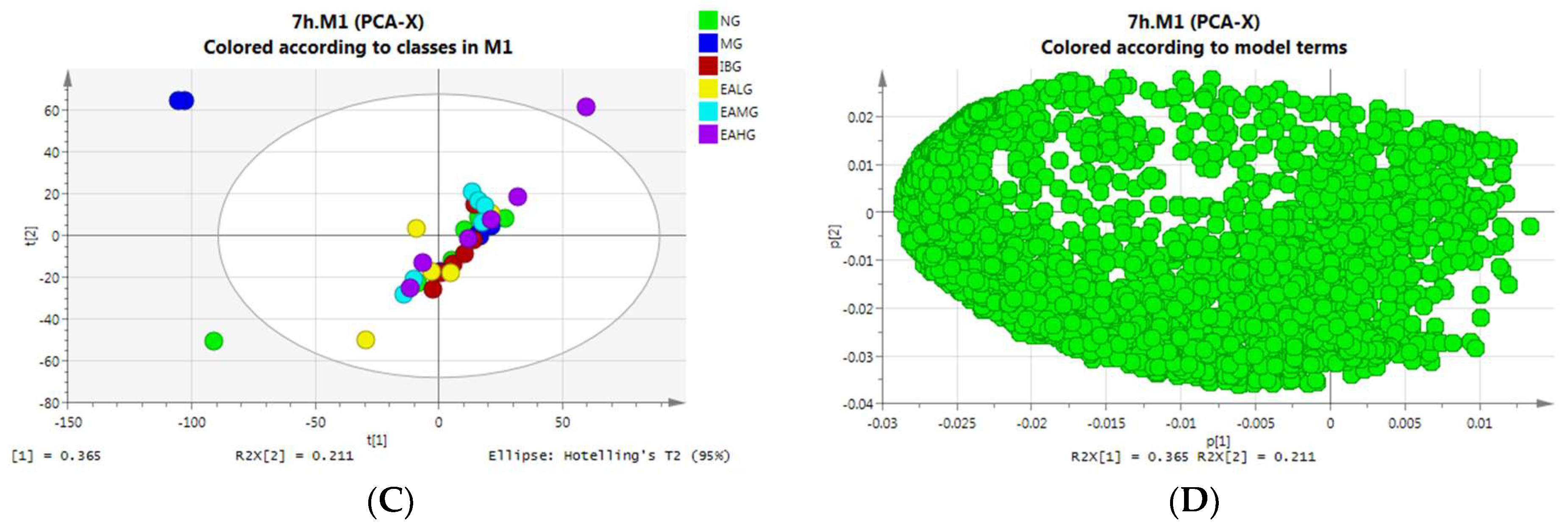
3.6.1. Principal Component Analysis (PCA)
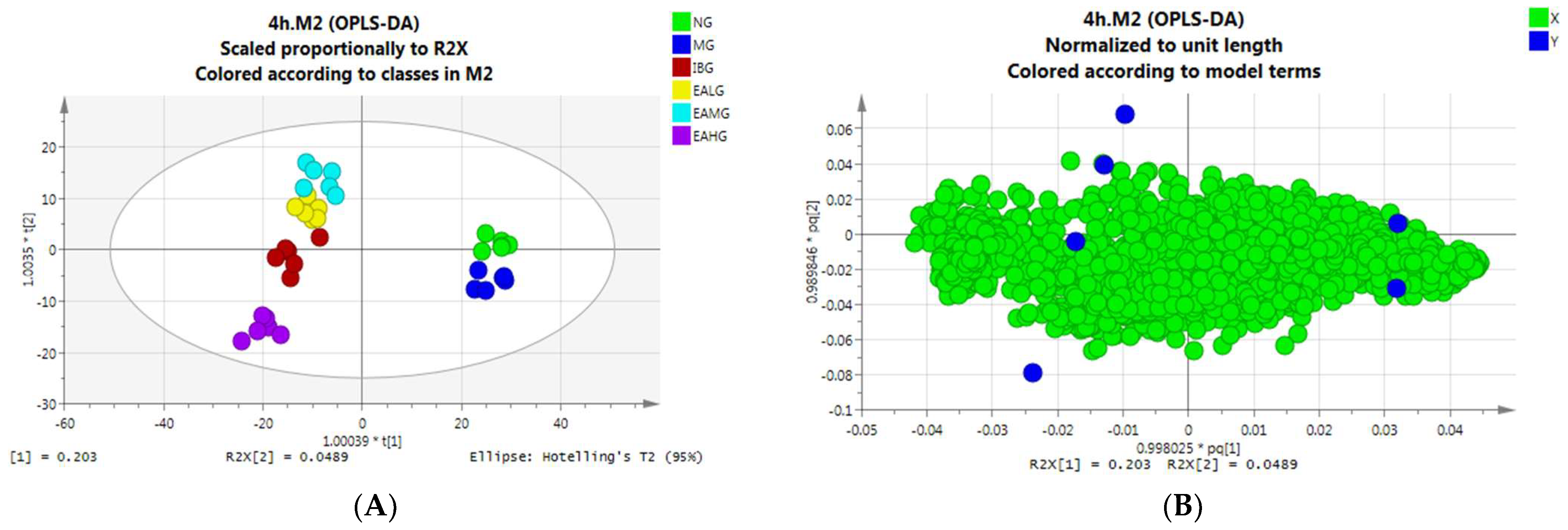
PCA Analysis of Serum Samples Collected at 4 h
PCA Analysis of Serum Samples Collected at 7 h
3.6.2. Orthogonal Partial Least Squares–Discriminant Analysis (OPLS-DA)
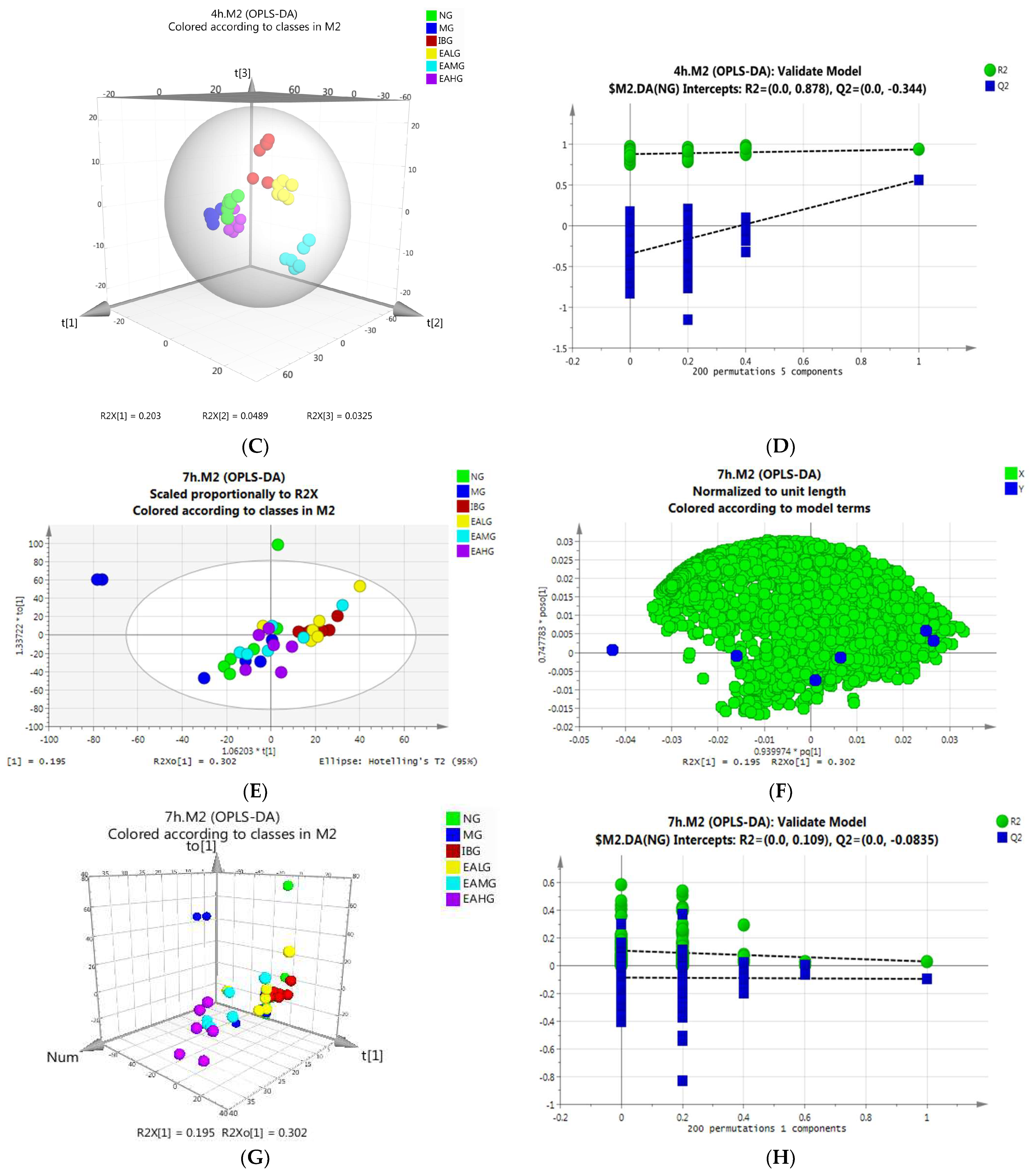
OPLS-DA Analysis of Serum Samples Collected at 4 h
OPLS-DA Analysis of Serum Samples Collected at 7 h
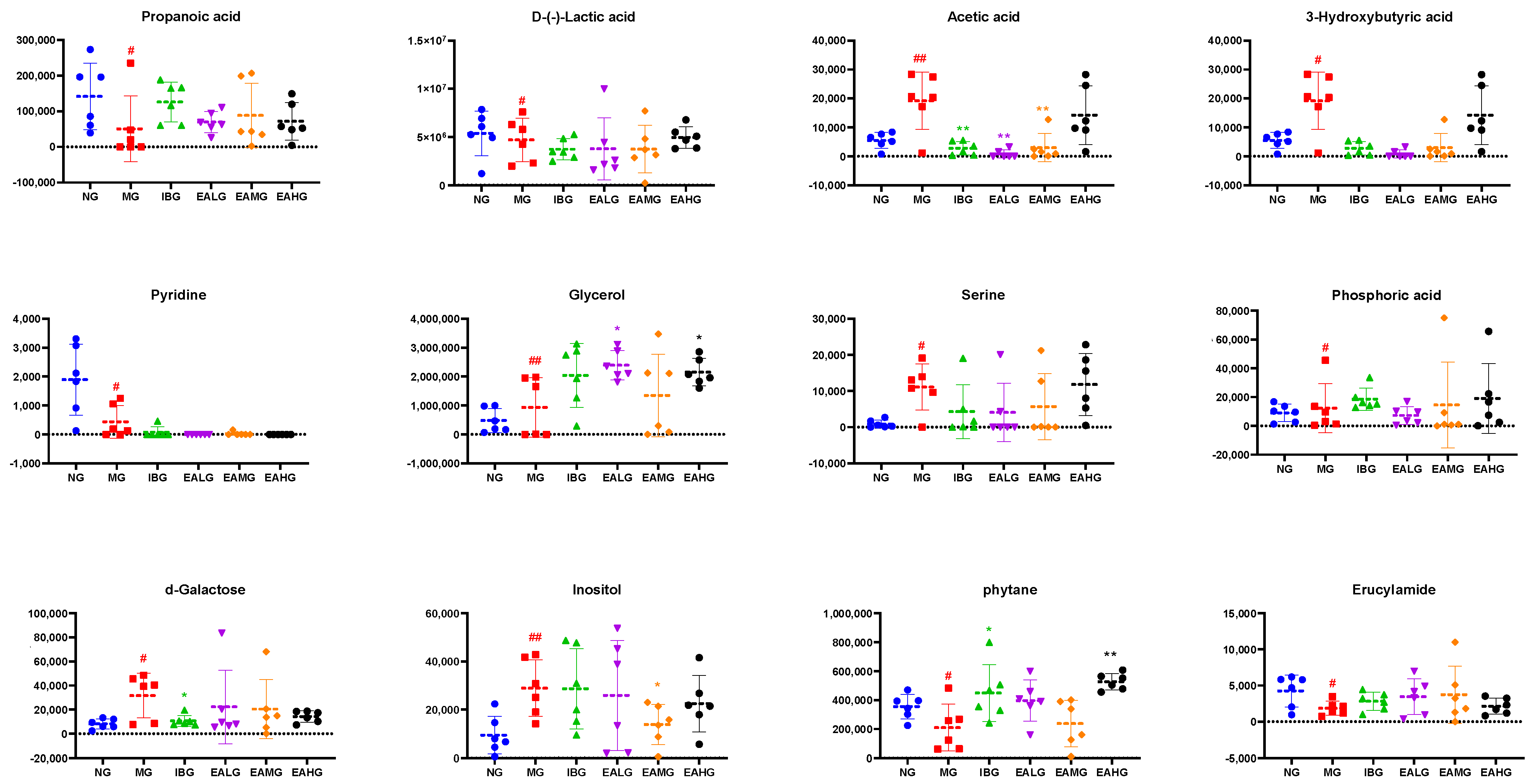
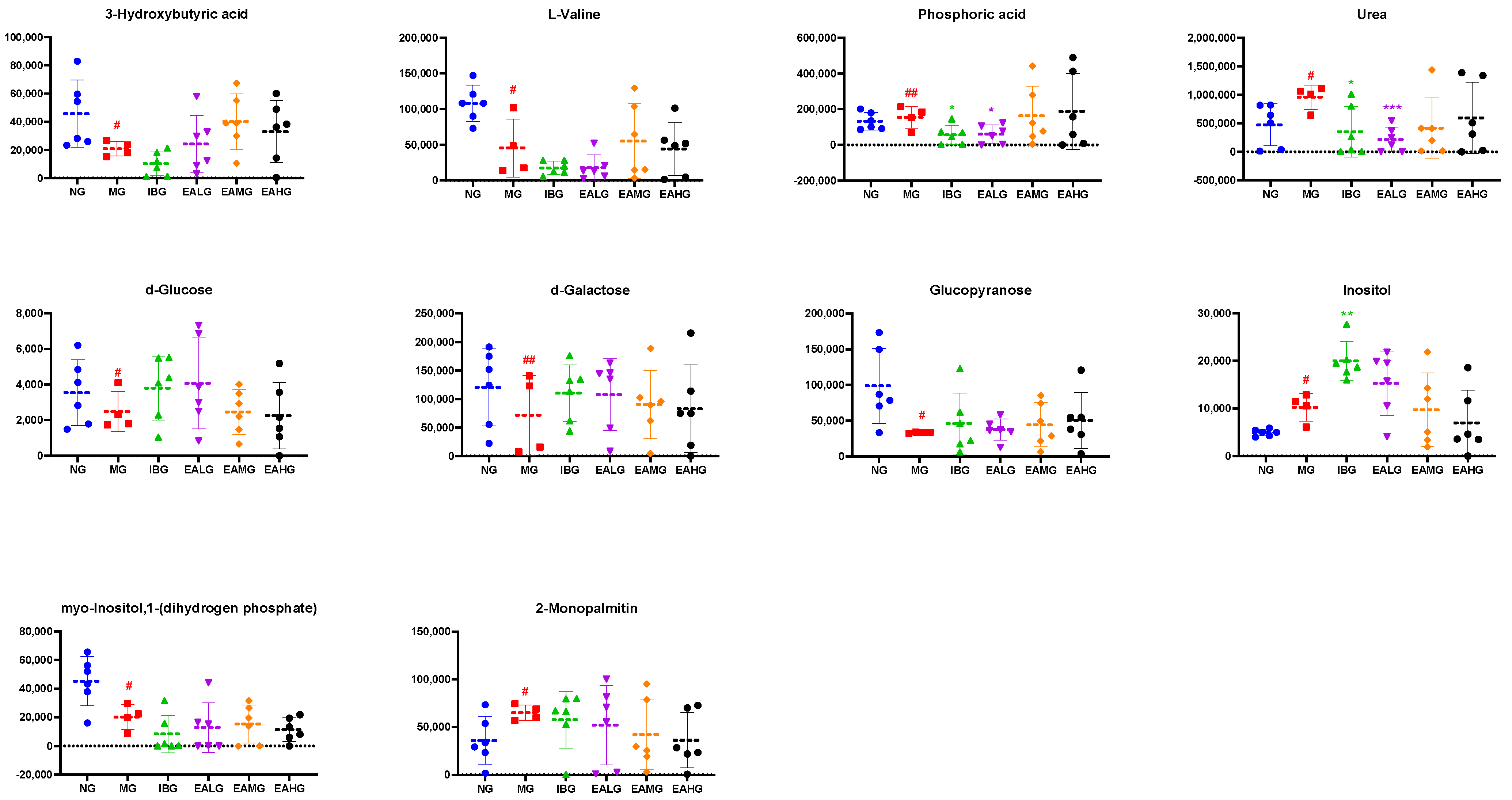
3.6.3. Identification of Potential Biomarkers
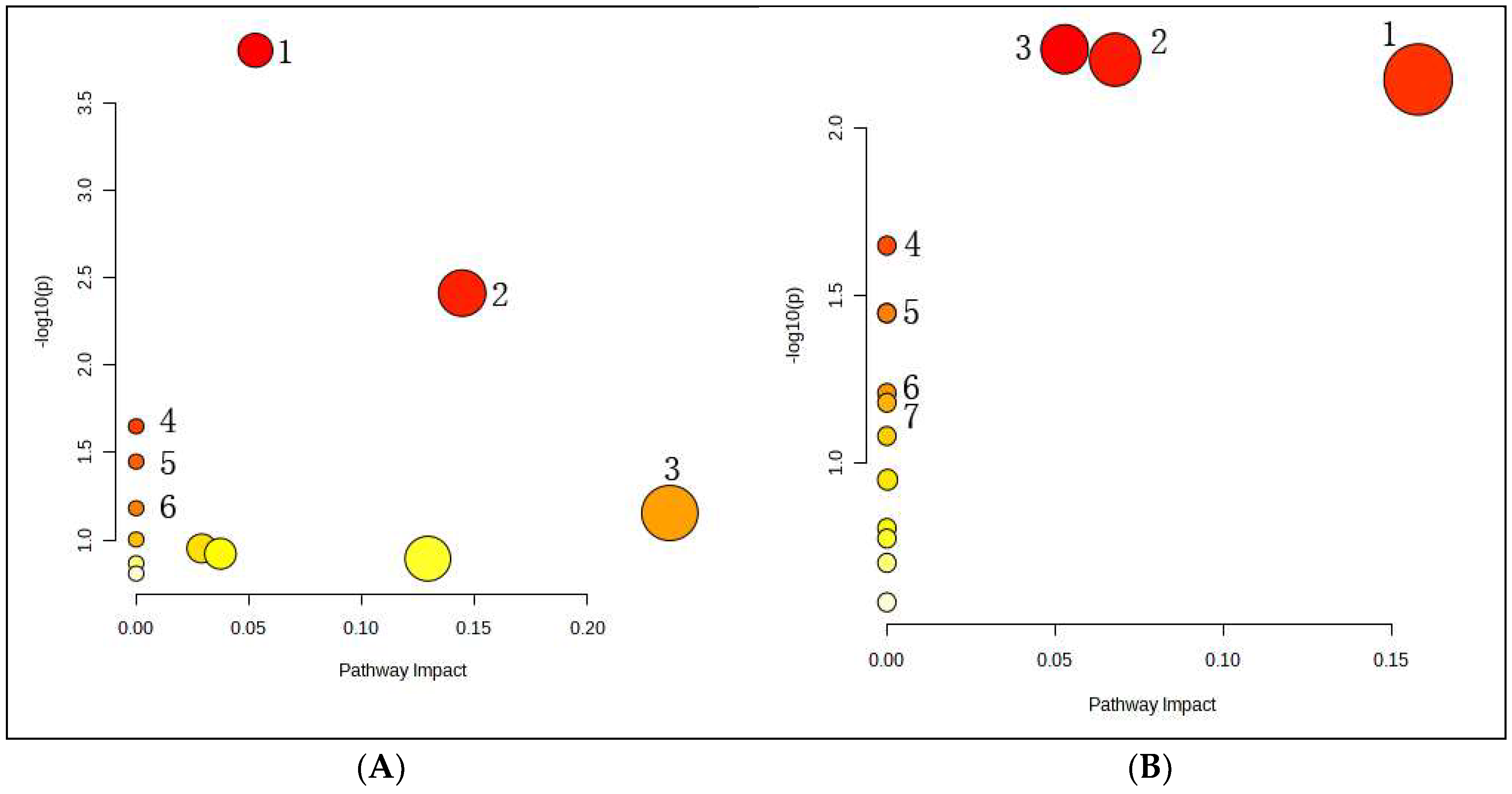
3.6.4. Metabolic Pathway Analysis of the Potential Biomarkers
3.6.5. Correlation Analysis between Metabolic and Inflammatory Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ogoina, D. Fever, fever patterns and diseases called ‘fever’—A review. J. Infect. Public. Health 2011, 4, 108–124. [Google Scholar] [CrossRef] [PubMed]
- Lammert, E. Metabolism of Human Diseases. In Organ Physiology and Pathophysiology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 313–317. [Google Scholar]
- Evans, S.S.; Repasky, E.A.; Fisher, D.T. Fever and the thermal regulation of immunity: The immune system feels the heat. Nat. Rev. Immunol. 2015, 15, 335–349. [Google Scholar] [CrossRef]
- Dubey, A.; Tiwari, M.; Singh, Y.; Kumar, N.; Srivastava, K. Investigation of Anti-pyretic Activity of Vinpocetine in Wistar Rat. Int. J. Pharm. Res. 2020, 12, 1901–1906. [Google Scholar]
- Ahmad, S.; Rehman, T.; Abbasi, W.M. Invivo evaluation of antipyretic effects of some homeopathic ultra-high dilutions on Baker’s yeast-induced fever on Similia principle. J. Ayurveda Integr. Med. 2018, 9, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Cox, B.; Lee, T.F. Possible involvement of 5-hydroxytryptamine in dopamine-receptor-mediated hypothermia in the rat. J. Pharm. Pharmacol. 2011, 31, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.B.; Zuo, J.H.; Ma, C.; Lin, H.; Xu, Y.; Sun, J.M.; Ye, D.D.; Lv, G.F.; Lin, Z. Study on the antipyretic effect of gypsum and its compatibility on yeast-induced pyrexia rats based on NF-κ B signaling pathway. Mater. Express 2020, 10, 748–755. [Google Scholar] [CrossRef]
- Su, C.L.; Cheng, C.C.; Lin, M.T.; Yeh, H.C.; Lee, M.C.; Lee, J.C.; Won, S.J. Staphylococcal enterotoxin C1-induced pyrogenic cytokine production in human peripheral blood mononuclear cells is mediated by NADPH oxidase and nuclear factor-kappa B. FEBS J. 2007, 274, 3633–3645. [Google Scholar] [CrossRef] [PubMed]
- Pavliv, L.; Voss, B.; Rock, A. Pharmacokinetics, safety, and tolerability of a rapid infusion of i.v. ibuprofen in healthy adults. Am. J. Health-Syst. Pharm. 2011, 68, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Royston, G. Metabolic profiling: Pathways in discovery. Drug Discov. Today 2004, 9, 260–261. [Google Scholar]
- Eraslan, Z.; Cascante, M.; Günther, U.L. Metabolomics in Cell Biology. Handb. Exp. Pharmacol. 2023, 277, 181–207. [Google Scholar]
- Lippa, K.A.; Aristizabal-Henao, J.J.; Beger, R.D.; Bowden, J.A.; Broeckling, C.; Beecher, C.; Davis, W.C.; Dunn, W.B.; Flores, R.; Goodacre, R.; et al. Reference materials for MS-based untargeted metabolomics and lipidomics: A review by the metabolomics quality assurance and quality control consortium (mQACC). Metabolomics 2022, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S.; Naldrett, M.J. Mass spectrometry based untargeted metabolomics for plant systems biology. Emerg. Top. Life Sci. 2021, 5, 189–201. [Google Scholar] [PubMed]
- Zhang, T.; Zhang, A.; Qiu, S.; Yang, S.; Wang, X. Current Trends and Innovations in Bioanalytical Techniques of Metabolomics. Crit. Rev. Anal. Chem. 2015, 46, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, M.; Sun, H.; Yang, J.C.; Huang, Y.X.; Huang, J.Q.; Lei, X.; Sun, L.H. Selenium deficiency-induced multiple tissue damage with dysregulation of immune and redox homeostasis in broiler chicks under heat stress. Sci. China-Life Sci. 2023, 66, 2056–2069. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, H.; Gao, J.; Zhu, M.; Wang, Y.; Jiang, C.; Xu, M. Metabolic disorder in the progression of heart failure. Sci. China-Life Sci. 2019, 62, 1153–1167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Mao, H.; Zhu, J.; Zhou, R.; Zhang, L.; Jiang, H. Comparison of differential metabolites in brain tissue of aged marmosets and serum of elderly patients after prolonged anesthesia. Front. Mol. Neurosci. 2023, 16, 1134239. [Google Scholar] [CrossRef]
- Wang, X.; Han, W.; Yang, J.; Westaway, D.; Li, L. Development of chemical isotope labeling LC-MS for tissue metabolomics and its application for brain and liver metabolome profiling in Alzheimer’s disease mouse model. Anal. Chim. Acta 2019, 1050, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Bruzzone, C.; Gil-Redondo, R.; Seco, M.; Barragán, R.; de la Cruz, L.; Cannet, C.; Schäfer, H.; Fang, F.; Diercks, T.; Bizkarguenaga, M.; et al. A molecular signature for the metabolic syndrome by urine metabolomics. Cardiovasc. Diabetol. 2021, 20, 155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Sun, H.; Wang, P.; Han, Y.; Wang, X. Modern analytical techniques in metabolomics analysis. Analyst 2011, 137, 293–300. [Google Scholar] [CrossRef]
- Amathieu, R.; Triba, M.N.; Goossens, C.; Bouchemal, N.; Nahon, P.; Savarin, P.; Le Moyec, L. Nuclear magnetic resonance based metabolomics and liver diseases: Recent advances and future clinical applications. World J. Gastroenterol. 2016, 22, 417–426. [Google Scholar] [CrossRef]
- Ramautar, R.; Somsen, G.W.; de Jong, G.J. CE-MS in metabolomics. Electrophoresis 2009, 30, 276–291. [Google Scholar] [CrossRef] [PubMed]
- Romanovsky, A.A.; Ivanov, A.I.; Shimansky, Y.P. Selected contribution: Ambient temperature for experiments in rats: A new method for determining the zone of thermal neutrality. J. Appl. Physiol. 2002, 92, 2667–2679. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Shen, M.; Huang, L.; Wu, T.; Xie, J. Mesona chinensis Benth polysaccharides alleviates liver injury by beneficial regulation of gut microbiota in cyclophosphamide-induced mice. Food Sci. Hum. Wellness 2022, 11, 74–84. [Google Scholar] [CrossRef]
- Prajitha, N.; Athira, S.S.; Mohanan, P.V. Pyrogens, a polypeptide produces fever by metabolic changes in hypothalamus: Mechanisms and detections. Immunol. Lett. 2018, 204, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, T.H. Pathogen Recognition and Inflammatory Signaling in Innate Immune Defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef]
- Ivanov, A.I.; Romanovsky, A.A. Prostaglandin E2 as a mediator of fever: Synthesis and catabolism. Front. Biosci. 2004, 9, 1977–1993. [Google Scholar] [CrossRef] [PubMed]
- Joachim, R.; Blatteis, C.M. Mechanisms of fever production and lysis: Lessons from experimental LPS fever. Compr. Physiol. 2014, 4, 1563–1604. [Google Scholar]
- Baradaran Rahimi, V.; Ghadiri, M.; Ramezani, M.; Askari, V.R. Antiinflammatory and anti-cancer activities of pomegranate and its constituent, ellagic acid: Evidence from cellular, animal, and clinical studies. Phytother. Res. 2020, 34, 685–720. [Google Scholar] [CrossRef] [PubMed]
- El-Shitany, N.A.; El-Bastawissy, E.A.; El-Desoky, K. Ellagic acid protects against carrageenan-induced acute inflammation through inhibition of nuclear factor kappa B, inducible cyclooxygenase and proinflammatory cytokines and enhancement of interleukin-10 via an antioxidant mechanism. Int. Immunopharmacol. 2014, 19, 290–299. [Google Scholar] [CrossRef]
- Zhao, L.; Mehmood, A.; Soliman, M.M.; Iftikhar, A.; Iftikhar, M.; Aboelenin, S.M.; Wang, C. Protective Effects of Ellagic Acid Against Alcoholic Liver Disease in Mice. Front. Nutr. 2012, 8, 744520. [Google Scholar] [CrossRef]
- Maruthamuthu, V.; Henry, L.J.K.; Ramar, M.K.; Kandasamy, R. Myxopyrum serratulum ameliorates airway inflammation in LPS-stimulated RAW 264.7 macrophages and OVA-induced murine model of allergic asthma. J. Ethnopharmacol. 2020, 255, 112369. [Google Scholar] [CrossRef]
- Marín, M.; Giner, R.M.; Ríos, J.L.; Recio, M.C. Intestinal anti-inflammatory activity of ellagic acid in the acute and chronic dextrane sulfate sodium models of mice colitis. J. Ethnopharmacol. 2013, 150, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Mady, F.M.; Shaker, M.A. Enhanced anticancer activity and oral bioavailability of ellagic acid through encapsulation in biodegradable polymeric nanoparticles. Int. J. Nanomed. 2017, 12, 7405–7417. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.Z.; Lee, J.J.; Huang, W.T.; Liao, J.F.; Lin, M.T. Inhibition of nuclear factor-kappa B prevents staphylococcal enterotoxin A-induced fever. Mol. Cell. Biochem. 2004, 262, 177–185. [Google Scholar] [CrossRef]
- Ledoux, A.C.; Perkins, N.D. NF-kappaB and the cell cycle. Biochem. Soc. Trans. 2014, 42, 76–81. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Kulkarni, V.H.; Chakraborty, M.; Habbu, P.V.; Ray, A. Ellagic acid restored lead-induced nephrotoxicity by anti-inflammatory, anti-apoptotic and free radical scavenging activities. Heliyon 2021, 7, e05921. [Google Scholar] [CrossRef]
- Mitchell, J.P.; Carmody, R.J. NF-κB and the Transcriptional Control of Inflammation. Int. Rev. Cell Mol. Biol. 2018, 335, 41–84. [Google Scholar] [PubMed]
- Ootsuka, Y.; Blessing, W.W.; Steiner, A.A.; Romanovsky, A.A. Fever response to intravenous prostaglandin E2 is mediated by the brain but does not require afferent vagal signaling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1294. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smale, S.T. Hierarchies of NF-κB target-gene regulation. Nat. Immunol. 2011, 12, 689. [Google Scholar] [CrossRef]
- Dixit, V.; Mak, T.W. NF-κB Signaling: Many Roads Lead To Madrid. Cell 2002, 111, 615–619. [Google Scholar] [CrossRef]
- Tapia, M.; Bosch, M.; Rolfe, M.; Ross, J.S.; Gascón, P.; Perona, R.; Rovira, A.; Albanell, J. Pharmacological inhibition and silencing of classical IKK-NF-κB pathway by siRNA sensitizes cancer cells to doxorubicin. J. Clin. Oncol. 2006, 24 (Suppl. 18), 2059. [Google Scholar] [CrossRef]
- Appleby, S.B.; Ristimäki, A.; Neilson, K.; Narko, K.; Hla, T. Structure of the human cyclooxygenase-2 gene. Biochem. J. 1994, 302 Pt 3, 723. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, I.N.; Chang AS, Y.; Teng, C.M.; Chen, C.C.; Yang, C.R. Aciculatin inhibits lipopolysaccharide-mediated inducible nitric oxide synthase and cyclooxygenase-2 expression via suppressing NF-κB and JNK/p38 MAPK activation pathways. J. Biomed. Sci. 2011, 18, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sheng, L.; Yang, T.; Qiu, Z.P.; Zheng, G.H.; Wang, G.H. Effects of ellagic acid on inflammation and oxidative stress induced by AKT gene transfection in mice with fatty liver disease. China J. Chin. Mater. Medica 2019, 44, 1869. [Google Scholar]
- Zhang, K.; Lei, N.; Li, M.; Li, J.; Li, C.; Shen, Y.; Guo, P.; Xiong, L.; Xie, Y. Cang-Ai Volatile Oil Ameliorates Depressive Behavior Induced by Chronic Stress Through IDO-Mediated Tryptophan Degradation Pathway. Front. Psychiatry 2021, 12, 91991. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Xu, L.; Tang, L.; Liu, F.; Chen, Z.; Zhang, J.; Chen, L.; Pang, C.; Yu, X. Role of the indoleamine-2,3-dioxygenase/kynurenine pathway of tryptophan metabolism in behavioral alterations in a hepatic encephalopathy rat model. J. Neuroinflammation 2018, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Isobe, K.; Jung, H.J.; Yang, C.R.; Claxton, J.N.; Sandoval, P.; Burg, M.B.; Raghuram, V.; Knepper, M.A. Systems-level identification of PKA-dependent signaling in epithelial cells. Proc. Natl. Acad. Sci. USA 2017, 114, E8875–E8884. [Google Scholar] [CrossRef] [PubMed]
- Oka, T. Prostaglandin E2 as a mediator of fever: The role of prostaglandin E (EP) receptors. Front. Bioence 2004, 9, 3046–3057. [Google Scholar] [CrossRef]
- Martinez, C.S.; Piagette, J.T.; Escobar, A.G.; Martín, Á.; Palacios, R.; Peçanha, F.M.; Vassallo, D.V.; Exley, C.; Alonso, M.J.; Miguel, M.; et al. Aluminum exposure at human dietary levels promotes vascular dysfunction and increases blood pressure in rats: A concerted action of NAD(P)H oxidase and COX-2. Toxicology 2017, 390, 10–21. [Google Scholar] [CrossRef]

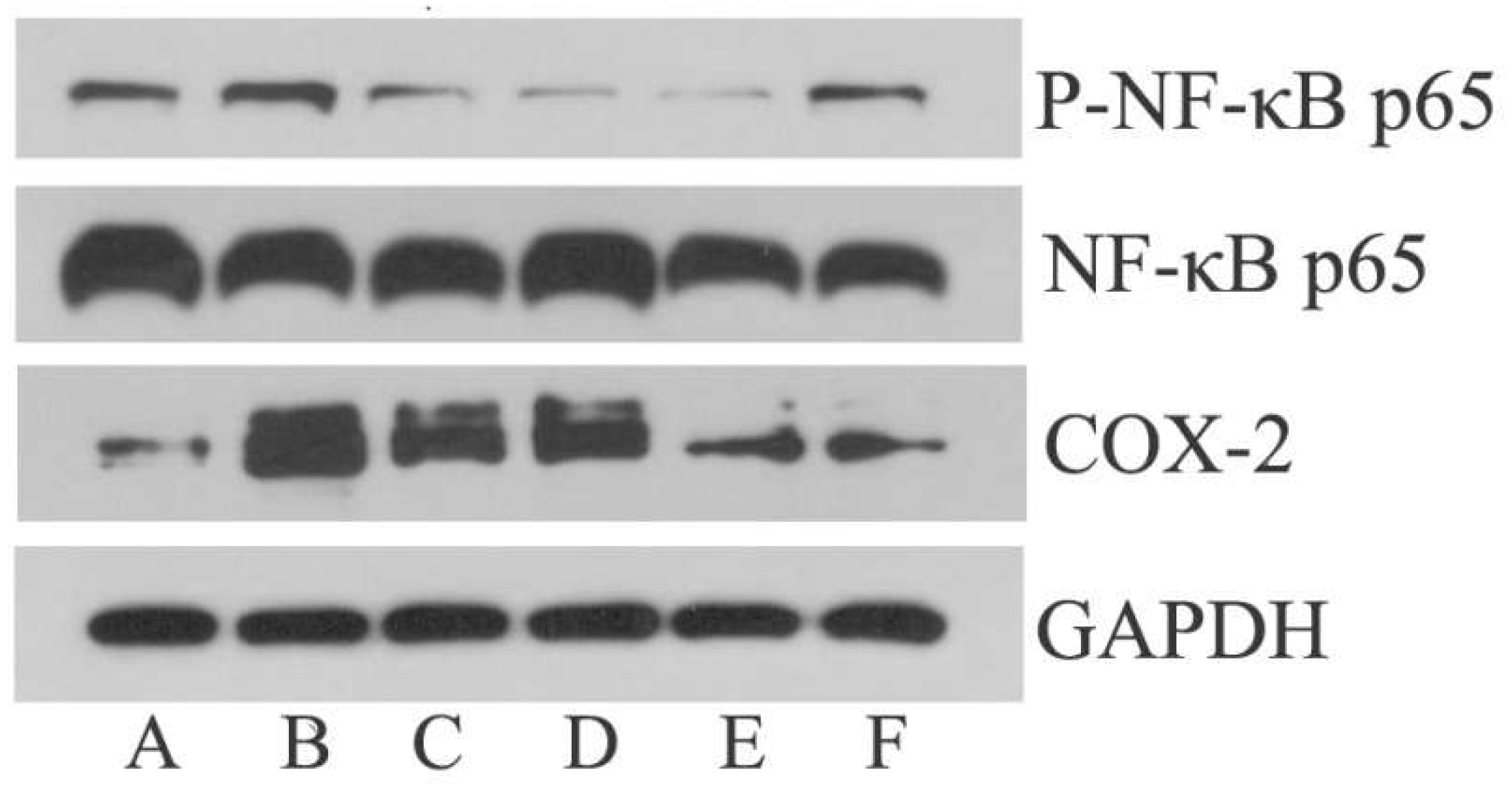


| Groups (Dose) | p-NF-κB p65/GAPDH | COX-2/GAPDH |
|---|---|---|
| NG | 0.29 ± 0.06 | 0.39 ± 0.1 |
| MG | 0.54 ± 0.06 ## | 1.44 ± 0.37 # |
| IBG (20 mg/kg) | 0.28 ± 0.1 ▲ | 0.74 ± 0.36 |
| EALG (26 mg/kg) | 0.24 ± 0.14 ▲ | 1.11 ± 0.23 |
| EAMG(52 mg/kg) | 0.18 ± 0.11 ▲ | 0.53 ± 0.15 ▲ |
| EAHG(104 mg/kg) | 0.29 ± 0.06 ▲▲ | 0.39 ± 0.16 ▲ |
| HMDB | tR/min | Metabolite | Formula | m/z | KEGG | p | Match Score % | NG vs. MG | MG vs. IBG | MG vs. EALG | MG vs. EAMG | MG vs. EAHG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HMDB0000237 | 5.60 | Propanoic acid | C3H6O2 | 28,29,74 | C00163 | 0.028 | 80.01 | ↓ | ↑ | ↑ | ↑ | ↑ |
| HMDB0001311 | 5.92 | D-(-)-Lactic acid | C3H6O3 | 147,117,191 | C00256 | 0.043 | 94.4 | ↑ | ↑ | ↑ | ↑ | ↑ |
| HMDB0000042 | 6.30 | Acetic acid | C2H4O2 | 43,45,60 | C00033 | 0.024 | 86.59 | ↑ | ↓ | ↑ | ↑ | ↑ |
| HMDB0000357 | 7.41 | 3-Hydroxybutyric acid | C4H8O3 | 147,117,191 | C01089 | 0.043 | 94.87 | ↑ | ↓ | ↓ | ↓ | ↑ |
| HMDB0000926 | 7.71 | Pyridine | C5H5N | 79,52,40 | C00747 | 0.038 | 82.1 | ↓ | ↑ | ↑ | ↑ | ↑ |
| HMDB0000131 | 8.88 | Glycerol | C3H8O3 | 205,117,103 | C00116 | 0.006 | 96.25 | ↑ | ↑ | ↓ | ↓ | ↓ |
| HMDB0062263 | 9.74 | Serine | C3H7NO3 | 204,218,100 | C00716 | 0.037 | 89.75 | ↑ | ↓ | ↑ | ↑ | ↑ |
| HMDB0002142 | 10.42 | Phosphoric acid | H3PO4 | 299,300,133 | C00009 | 0.031 | 93.28 | ↑ | ↓ | ↑ | ↓ | ↓ |
| HMDB0000143 | 17.93 | d-Galactose | C6H12O6 | 147,205,103 | C00984 | 0.014 | 88.64 | ↑ | ↓ | ↑ | ↑ | ↑ |
| HMDB0000211 | 20.14 | Inositol | C6H12O6 | 147,217,305 | C00137 | 0.008 | 91.71 | ↑ | ↓ | ↑ | ↑ | ↑ |
| NA | 22.23 | phytane | C20 H42 | 57,71,43 | NA | 0.018 | 82.72 | ↓ | ↑ | ↑ | ↑ | ↓ |
| NA | 29.71 | Erucylamide | C22H43NO | 59,72,55 | NA | 0.042 | 84.56 | ↓ | ↑ | ↓ | ↑ | ↓ |
| HMDB | tR/min | Metabolite | Formula | m/z | KEGG | p | Match Score % | NG vs. MG | MG vs. IBG | MG vs. EALG | MG vs. EAMG | MG vs. EAHG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HMDB0000357 | 7.57 | 3-Hydroxybutyric acid | C4H8O3 | 147,117,191 | C01089 | 0.029 | 94.19 | ↓ | ↓ | ↑ | ↑ | ↑ |
| HMDB0000883 | 8.41 | L-Valine | C5H11NO2 | 146,156,130 | C00183 | 0.045 | 93.42 | ↓ | ↓ | ↓ | ↑ | ↓ |
| HMDB0002142 | 10.43 | Phosphoric acid | H3PO4 | 299,300,133 | C00009 | 0.001 | 92.77 | ↑ | ↓ | ↓ | ↑ | ↑ |
| HMDB0000294 | 11.59 | Urea | CH4N2O | 60,44,17 | C00086 | 0.030 | 92.61 | ↑ | ↓ | ↓ | ↓ | ↓ |
| HMDB0000122 | 18.00 | d-Glucose | C6H12O6 | 217,218,133 | C00221 | 0.035 | 90.2 | ↓ | ↑ | ↑ | ↓ | ↓ |
| HMDB0000143 | 18.26 | d-Galactose | C6H12O6 | 147,205,103 | C00984 | 0.005 | 92.88 | ↓ | ↑ | ↑ | ↑ | ↑ |
| NA | 19.07 | Glucopyranose | C6H12O6 | 217,218,133 | NA | 0.020 | 95.95 | ↓ | ↑ | ↑ | ↑ | ↑ |
| HMDB0000211 | 20.29 | Inositol | C6H12O6 | 147,217,305 | C00137 | 0.035 | 94.12 | ↑ | ↑ | ↑ | ↓ | ↓ |
| HMDB0000213 | 24.13 | myo-Inositol,1-(dihydrogen phosphate) | C6H13O9P | 73,147,271 | C01177 | 0.017 | 85.35 | ↓ | ↓ | ↓ | ↓ | ↓ |
| HMDB0011533 | 25.25 | 2-Monopalmitin | C19H38O4 | 129,103,147 | NA | 0.026 | 80.76 | ↑ | ↓ | ↓ | ↓ | ↓ |
| Metabolite | The Correlation Coefficient: r | |||||
|---|---|---|---|---|---|---|
| MDA | SOD | GSH | IL-1β | IL-6 | TNF-α | |
| Propanoic acid | −0.391 * | +0.153 | +0.422 * | −0.319 | −0.280 | −0.330 * |
| D-(-)-Lactic acid | −0.101 | +0.102 | +0.013 | −0.106 | +0.058 | +0.090 |
| Acetic acid | +0.247 | −0.304 | −0.332 * | +0.318 | +0.253 | +0.168 |
| 3-Hydroxybutyric acid | −0.128 | +0.095 | +0.088 | +0.068 | −0.054 | +0.134 |
| Pyridine | −0.266 | +0.225 | +0.237 | −0.502 ** | −0.388 | −0.359 |
| glycerol | +0.119 | +0.005 | +0.074 | +0.235 | +0.187 | +0.250 |
| Serine | +0.014 | −0.138 | −0.136 | +0.394 * | +0.290 | +0.304 |
| Phosphoric acid | −0.178 | +0.123 | −0.052 | +0.033 | +0.120 | +0.022 |
| d-Galactose | +0.147 | −0.314 | −0.325 | +0.367 * | +0.361 | +0.331 |
| Inositol | −0.029 | −0.179 | −0.105 | +0.360 * | +0.523 | +0.247 |
| phytane | −0.307 | +0.255 | +0.215 | −0.154 | −0.160 | −0.084 |
| Erucylamide | +0.007 | +0.079 | +0.089 | −0.150 | −0.316 | −0.128 |
| Metabolite | The Correlation Coefficient: r | |||||
|---|---|---|---|---|---|---|
| MDA | SOD | GSH | IL-1β | IL-6 | TNF-α | |
| 3-Hydroxybutyric acid | −0.046 | +0.316 | +0.247 | −0.263 | −0.369 | −0.471 |
| L-Valine | −0.339 * | +0.284 | +0.513 ** | −0.501 ** | −0.515 | −0.463 * |
| Phosphoric acid | −0.242 | +0.015 | +0.012 | +0.03 | −0.098 | −0.13 |
| Urea | −0.032 | −0.354 * | −0.208 | +0.081 | +0.156 | +0.211 |
| d-Glucose | −0.039 | +0.027 | −0.059 | −0.037 | +0.144 | −0.091 |
| d-Galactose | −0.14 | +0.061 | +0.249 | −0.181 | −0.034 | −0.009 |
| Glucopyranose | −0.414 | +0.38 | +0.297 | −0.577 | −0.313 | −0.428 |
| Inositol | +0.188 | −0.249 * | −0.336 ** | +0.293 ** | 0.387 | +0.278 |
| myo-Inositol,1-(dihydrogen phosphate) | −0.388 * | +0.153 | +0.395 ** | −0.531 ** | −0.347 | −0.445 * |
| 2-Monopalmitin | +0.232 ** | −0.291 ** | −0.397 | +0.353 ** | +0.29 | +0.261 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, F.-F.; Xu, L.-B.; Zhu, H.; Yu, X.-Q.; Deng, L.-Y.; Qin, H.-Z.; Lin, S. Serum Metabolomics and NF-κB Pathway Analysis Revealed the Antipyretic Mechanism of Ellagic Acid on LPS-Induced Fever in Rabbits. Metabolites 2024, 14, 407. https://doi.org/10.3390/metabo14080407
Xie F-F, Xu L-B, Zhu H, Yu X-Q, Deng L-Y, Qin H-Z, Lin S. Serum Metabolomics and NF-κB Pathway Analysis Revealed the Antipyretic Mechanism of Ellagic Acid on LPS-Induced Fever in Rabbits. Metabolites. 2024; 14(8):407. https://doi.org/10.3390/metabo14080407
Chicago/Turabian StyleXie, Feng-Feng, Li-Ba Xu, Hua Zhu, Xiu-Qi Yu, Lin-Yu Deng, Hui-Zhen Qin, and Si Lin. 2024. "Serum Metabolomics and NF-κB Pathway Analysis Revealed the Antipyretic Mechanism of Ellagic Acid on LPS-Induced Fever in Rabbits" Metabolites 14, no. 8: 407. https://doi.org/10.3390/metabo14080407
APA StyleXie, F.-F., Xu, L.-B., Zhu, H., Yu, X.-Q., Deng, L.-Y., Qin, H.-Z., & Lin, S. (2024). Serum Metabolomics and NF-κB Pathway Analysis Revealed the Antipyretic Mechanism of Ellagic Acid on LPS-Induced Fever in Rabbits. Metabolites, 14(8), 407. https://doi.org/10.3390/metabo14080407





