Hunting Metabolic Biomarkers for Exposure to Per- and Polyfluoroalkyl Substances: A Review
Abstract
1. Introduction
2. Introduction to PFAS
2.1. Classification
2.2. Sources
2.3. Human Exposure Pathway and Characteristics
2.3.1. Population Characteristics
2.3.2. Distribution Trend
2.3.3. Absorption and Distribution
3. Toxic Effects of PFAS
3.1. Liver Injury
3.2. Reproductive and Developmental Toxicity
3.3. Cardiovascular Toxicity
3.4. Glucose Homeostasis Disruption
3.5. Other Toxicological Impact
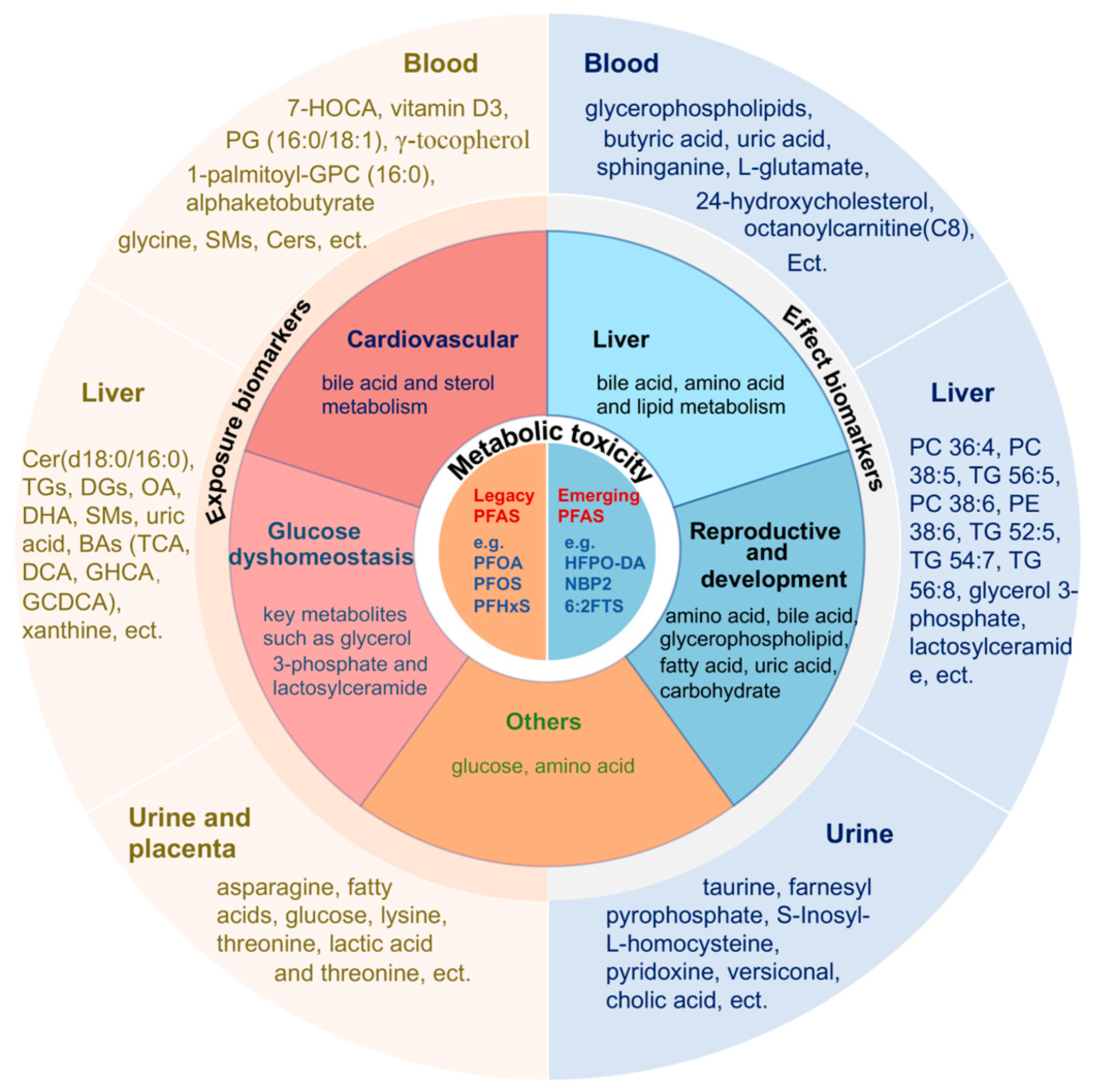
4. Potential Biomarkers
4.1. Exposure Biomarkers
4.1.1. Blood
| PFAS | Study Object | Sample Size | Sample Matrix | Main Finding(s) | Ref. |
|---|---|---|---|---|---|
| PFOA, PFNA, PFDA, PFUdA, PFTrDA, PFHxS, PFOS, 6:2 FTS, etc. | Non-occupationally exposed residents near industrial parks of Shandong Province | 91 | serum |
| Yang Y et al., 2024 [100] |
| PFOS, PFHxS, PFOA, PFNA | Pregnant African American Newborns in Atlanta, Georgia | 267 | serum |
| Taibl KR et al., 2023 [76] |
| 23PFSA, mainly PFHpA, PFOA, PFBS, PFPeS, PFHxS, PFHpS, PFOS, 6:2 FTS | Occupational workers and residents of fluorine chemical plants in Hubei, China | 225 | urine |
| He A et al., 2023 [96] |
| PFOS, PFHxS, PFHpS, PFOA, PFNA, PFDA | The Study of Latino Adolescents at Risk (SOLAR), Southern California Children’s Health Study (CHS) | 312, 137 | plasma |
| Goodrich JA et al., 2023 [104] |
| PFOA, PFOS, PFHXS, PFDA, PFUdA, PFNA | Cohort investigating pregnant women and children in the US VDAART cohort study | 459, 401 | plasma |
| Prince N et al., 2023 [101] |
| PFHxS, PFOA, PFHpS, PFNA, PFOS, 6:2 Cl-PFESA, PFDA, PFUdA, 8:2 Cl—PFESA | Male residents recruited from Guangzhou | 278 | serum |
| Chen Y et al., 2023 [90] |
| PFHxS, PFNA, PFOA, 2 PFOS isomers | Cohort with NAFLD who underwent laparoscopic bariatric surgery | 105 | Liver, serum |
| Sen P et al., 2022 [63] |
| PFOS, PFHxS, PFOA, PFDA, PFNA, PFUdA | A nested case-control study of HCC | 50 pairs | plasma |
| Goodrich JA et al., 2022 [97] |
| PFOA, PFNA, PFDA, PFUdA, PFHxS, PFHpS, 6:2 Cl-PFESA, 8:2 Cl-PFESA | Matched samples of pregnant women delivering in Beijing’s hospitals | 84 pairs | serum, cord blood |
| Li Y et al., 2021 [51] |
| PFOS, PFOA, PFHxS, PFNA, EtFOSAA, MeFOSAA, PFDA, PFOSA | A Diabetes Prevention Program project for a multicenter randomized clinical trial in people at risk for type 2 diabetes | 691 | plasma |
| Mitro SD et al., 2021 [105] |
| PFHxS, PFOS, PFOA, 6:2 Cl-PFESA, PFNA, PFDA, PFUdA, PFHpS | Cord blood samples stored in the Beijing Cord Blood Bank | 104 | cord blood |
| Sinisalu L et al., 2021 [102] |
| PFOA, PFOA, PFHxS, PFDA, PFNA, PFUdA | A case-control study on T2D | 187 pairs | plasma |
| Schillemans T et al., 2020 [91] |
| PFOS, PFOA, PFNA, PFHxS, PFUdA | The Human Early-Life Exposome project | 1105 pairs | serum |
| Stratakis N et al., 2020 [69] |
| PFOA, PFOS and PFHxS | NAFLD patients enrolled at Children’s Healthcare of Atlanta | 74 | plasma |
| Jin R et al., 2020 [62] |
| PFOA, PFOS, PFHxS | Overweight and Obese Hispanic Children in Downtown Los Angeles | 40 | plasma |
| Alderete TL et al., 2019 [93] |
| PFOA, PFOS, PFNA, PFHxS | The Health Outcomes and Measures of the Environment Study | 114 | serum |
| Kingsley SL et al., 2019 [103] |
| PFAS | Study Object | Dose | Sample Matrix | Main Finding(s) | Ref. |
|---|---|---|---|---|---|
| PFOA | Humanized PPARα mice | 8 μM/kg, 6–7 weeks | liver |
| Sen P et al., 2022 [63] |
| HFPO-DA or “GenX”, NBP2 | C57BL/6 mice | 0.5, 5, 100 mg/kg, 28 d | liver |
| Kirkwood-Donelson KI et al., 2024 [106] |
| FTEOs, PFOA | CD-1 mice | 5, 100 ng/L, 17.5 d | placenta |
| Adams H et al., 2024 [107] |
| PFOS | SD rats | 0.03, 0.3 mg/kg, 18 d | liver |
| Yu G et al., 2023 [94] |
| Mixture of PFOA, PFNA, PFDA, PFUdA, PFDoDA, PFTrDA, PFTeDA, PFOS | A/J mice | 3 g/week, 10 weeks | liver |
| Khan EA et al., 2023 [73] |
| PFOA | C57BL/6 mice | 1 ppm/kg, 40 ppb/kg, 4 weeks | Serum, liver |
| Gao B et al., 2022 [108] |
| Mixture of POPs with six PFAS: PFHxS, PFOS, PFOA, PFNA, PFDA, PFUdA | NOD/SHiLtJ mice | 0.14, 2.9 μg/kg, 12 weeks | serum |
| Sinioja T et al., 2022 [82] |
| PFAS mixture, PFOA, PFOS, PFNA, PFHxS, GenX | C57BL/6J mice | 10 mg/L, 12 weeks | plasma, liver |
| Roth K et al., 2021 [71] |
| PFHxA | ICR mice | 50, 200 mg/kg, 2 months | Serum, liver |
| Jiang L et al., 2021 [66] |
| PFOS | BALB/c mouse | 100, 1000 μg/kg, 2 months | liver |
| Li X et al., 2021 [72] |
4.1.2. Liver
4.1.3. Urine and Placenta
4.2. Effect Biomarkers
4.2.1. Blood
4.2.2. Liver
4.2.3. Urine
5. Summary and Outlook
5.1. Summary
5.2. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef] [PubMed]
- Gaines, L.G.T. Historical and current usage of per- and polyfluoroalkyl substances (PFAS): A literature review. Am. J. Ind. Med. 2023, 66, 353–378. [Google Scholar] [CrossRef] [PubMed]
- Thijs, M.; Laletas, E.; Quinn, C.M.; Raguraman, S.V.; Carr, B.; Bierganns, P. Total and Class-Specific Determination of Fluorinated Compounds in Consumer and Food Packaging Samples Using Fluorine-19 Solid-State Nuclear Magnetic Resonance Spectroscopy. Anal. Chem. 2024, 96, 8282–8290. [Google Scholar] [CrossRef] [PubMed]
- Casal, P.; Zhang, Y.; Martin, J.W.; Pizarro, M.; Jiménez, B.; Dachs, J. Role of Snow Deposition of Perfluoroalkylated Substances at Coastal Livingston Island (Maritime Antarctica). Environ. Sci. Technol. 2017, 51, 8460–8470. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, J.; Ehlers, S.; Oberhausen, A.; Tischer, M.; Fürst, P.; Schafft, H.; Lahrssen-Wiederholt, M. Absorption, distribution, and milk secretion of the perfluoroalkyl acids PFBS, PFHxS, PFOS, and PFOA by dairy cows fed naturally contaminated feed. J. Agric. Food Chem. 2013, 61, 2903–2912. [Google Scholar] [CrossRef] [PubMed]
- Miner, K.R.; Clifford, H.; Taruscio, T.; Potocki, M.; Solomon, G.; Ritari, M.; Napper, I.E.; Gajurel, A.P.; Mayewski, P.A. Deposition of PFAS ‘forever chemicals’ on Mt. Everest. Sci. Total Environ. 2021, 759, 144421. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zheng, L.; Tian, L.; Wang, N.; Lei, L.; Wang, Y.; Dong, Q.; Huang, C.; Yang, D. Chronic PFOS Exposure Disrupts Thyroid Structure and Function in Zebrafish. Bull. Environ. Contam. Toxicol. 2018, 101, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Butenhoff, J.L.; Chang, S.C.; Olsen, G.W.; Thomford, P.J. Chronic dietary toxicity and carcinogenicity study with potassium perfluorooctanesulfonate in Sprague Dawley rats. Toxicology 2012, 293, 1–15. [Google Scholar] [CrossRef]
- Wang, Z.; DeWitt, J.C.; Higgins, C.P.; Cousins, I.T. A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)? Environ. Sci. Technol. 2017, 51, 2508–2518. [Google Scholar] [CrossRef]
- Lindstrom, A.B.; Strynar, M.J.; Libelo, E.L. Polyfluorinated compounds: Past, present, and future. Environ. Sci. Technol. 2011, 45, 7954–7961. [Google Scholar] [CrossRef]
- Xu, C.; Jiang, Z.Y.; Liu, Q.; Liu, H.; Gu, A. Estrogen receptor beta mediates hepatotoxicity induced by perfluorooctane sulfonate in mouse. Environ. Sci. Pollut. Res. Int. 2017, 24, 13414–13423. [Google Scholar] [CrossRef]
- Sun, P.; Nie, X.; Chen, X.; Yin, L.; Luo, J.; Sun, L.; Wan, C.; Jiang, S. Nrf2 Signaling Elicits a Neuroprotective Role against PFOS-mediated Oxidative Damage and Apoptosis. Neurochem. Res. 2018, 43, 2446–2459. [Google Scholar] [CrossRef]
- Soloff, A.C.; Wolf, B.J.; White, N.D.; Muir, D.; Courtney, S.; Hardiman, G.; Bossart, G.D.; Fair, P.A. Environmental perfluorooctane sulfonate exposure drives T cell activation in bottlenose dolphins. J. Appl. Toxicol. 2017, 37, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bai, Y.; Tang, C.; Cao, X.; Chang, F.; Chen, L. Impact of Perfluorooctane Sulfonate on Reproductive Ability of Female Mice through Suppression of Estrogen Receptor α-Activated Kisspeptin Neurons. Toxicol. Sci. 2018, 165, 475–486. [Google Scholar] [CrossRef]
- Liu, C.; Chang, V.W.; Gin, K.Y.; Nguyen, V.T. Genotoxicity of perfluorinated chemicals (PFCs) to the green mussel (Perna viridis). Sci. Total Environ. 2014, 487, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Toskos, T.; Panagiotakis, I.; Dermatas, D. Per- and polyfluoroalkyl substances—Challenges associated with a family of ubiquitous emergent contaminants. Waste Manag. Res. 2019, 37, 449–451. [Google Scholar] [CrossRef]
- Pezzatti, J.; Boccard, J.; Codesido, S.; Gagnebin, Y.; Joshi, A.; Picard, D.; González-Ruiz, V.; Rudaz, S. Implementation of liquid chromatography-high resolution mass spectrometry methods for untargeted metabolomic analyses of biological samples: A tutorial. Anal. Chim. Acta 2020, 1105, 28–44. [Google Scholar] [CrossRef]
- Gobelius, L.; Glimstedt, L.; Olsson, J.; Wiberg, K.; Ahrens, L. Mass flow of per- and polyfluoroalkyl substances (PFAS) in a Swedish municipal wastewater network and wastewater treatment plant. Chemosphere 2023, 336, 139182. [Google Scholar] [CrossRef] [PubMed]
- Abafe, O.A.; Macheka, L.R.; Abafe, O.T.; Chokwe, T.B. Concentrations and human exposure assessment of per and polyfluoroalkyl substances in farmed marine shellfish in South Africa. Chemosphere 2021, 281, 130985. [Google Scholar] [CrossRef]
- Gebbink, W.A.; van Leeuwen, S.P.J. Environmental contamination and human exposure to PFASs near a fluorochemical production plant: Review of historic and current PFOA and GenX contamination in the Netherlands. Environ. Int. 2020, 137, 105583. [Google Scholar] [CrossRef]
- Katz, D.R.; Sullivan, J.C.; Rosa, K.; Gardiner, C.L.; Robuck, A.R.; Lohmann, R.; Kincaid, C.; Cantwell, M.G. Transport and fate of aqueous film forming foam in an urban estuary. Environ. Pollut. 2022, 300, 118963. [Google Scholar] [CrossRef] [PubMed]
- Augustsson, A.; Lennqvist, T.; Osbeck, C.M.G.; Tibblin, P.; Glynn, A.; Nguyen, M.A.; Westberg, E.; Vestergren, R. Consumption of freshwater fish: A variable but significant risk factor for PFOS exposure. Environ. Res. 2021, 192, 110284. [Google Scholar] [CrossRef] [PubMed]
- Ragnarsdóttir, O.; Abdallah, M.A.; Harrad, S. Dermal uptake: An important pathway of human exposure to perfluoroalkyl substances? Environ. Pollut. 2022, 307, 119478. [Google Scholar] [CrossRef] [PubMed]
- Eun, H.; Yamazaki, E.; Taniyasu, S.; Miecznikowska, A.; Falandysz, J.; Yamashita, N. Evaluation of perfluoroalkyl substances in field-cultivated vegetables. Chemosphere 2020, 239, 124750. [Google Scholar] [CrossRef] [PubMed]
- Gazzotti, T.; Sirri, F.; Ghelli, E.; Zironi, E.; Zampiga, M.; Pagliuca, G. Perfluoroalkyl contaminants in eggs from backyard chickens reared in Italy. Food Chem. 2021, 362, 130178. [Google Scholar] [CrossRef] [PubMed]
- Sharp, S.; Sardiña, P.; Metzeling, L.; McKenzie, R.; Leahy, P.; Menkhorst, P.; Hinwood, A. Per- and Polyfluoroalkyl Substances in Ducks and the Relationship with Concentrations in Water, Sediment, and Soil. Environ. Toxicol. Chem. 2021, 40, 846–858. [Google Scholar] [CrossRef]
- Macheka, L.R.; Olowoyo, J.O.; Mugivhisa, L.L.; Abafe, O.A. Determination and assessment of human dietary intake of per and polyfluoroalkyl substances in retail dairy milk and infant formula from South Africa. Sci. Total Environ. 2021, 755, 142697. [Google Scholar] [CrossRef] [PubMed]
- Monge Brenes, A.L.; Curtzwiler, G.; Dixon, P.; Harrata, K.; Talbert, J.; Vorst, K. PFOA and PFOS levels in microwave paper packaging between 2005 and 2018. Food Addit. Contam. Part B Surveill. 2019, 12, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Toptancı, İ.; Ketenoglu, O.; Kıralan, M. Assessment of the migration of perfluorinated compounds and primary aromatic amines from PTFE-coated non-stick cookware marketed in Turkey. Environ. Sci. Pollut. Res. Int. 2022, 29, 38535–38549. [Google Scholar] [CrossRef]
- Cara, B.; Lies, T.; Thimo, G.; Robin, L.; Lieven, B. Bioaccumulation and trophic transfer of perfluorinated alkyl substances (PFAS) in marine biota from the Belgian North Sea: Distribution and human health risk implications. Environ. Pollut. 2022, 311, 119907. [Google Scholar] [CrossRef]
- Bolan, N.; Sarkar, B.; Vithanage, M.; Singh, G.; Tsang, D.C.W.; Mukhopadhyay, R.; Ramadass, K.; Vinu, A.; Sun, Y.; Ramanayaka, S.; et al. Distribution, behaviour, bioavailability and remediation of poly- and per-fluoroalkyl substances (PFAS) in solid biowastes and biowaste-treated soil. Environ. Int. 2021, 155, 106600. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Qiu, W.; Du, J.; Wan, Z.; Zhou, J.L.; Chen, H.; Liu, R.; Magnuson, J.T.; Zheng, C. Translocation, bioaccumulation, and distribution of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in plants. iScience 2022, 25, 104061. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, Z.; Lian, Y.; Sun, X.; Wu, Y.; Qiao, L.; Wang, M. Source, transportation, bioaccumulation, distribution and food risk assessment of perfluorinated alkyl substances in vegetables: A review. Food Chem. 2021, 349, 129137. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Zhang, J.; Pan, Y.; Dai, J.; Ji, C.; Tang, J. First Report on the Bioaccumulation and Trophic Transfer of Perfluoroalkyl Ether Carboxylic Acids in Estuarine Food Web. Environ. Sci. Technol. 2022, 56, 6046–6055. [Google Scholar] [CrossRef] [PubMed]
- Munoz, G.; Mercier, L.; Duy, S.V.; Liu, J.; Sauvé, S.; Houde, M. Bioaccumulation and trophic magnification of emerging and legacy per- and polyfluoroalkyl substances (PFAS) in a St. Lawrence River food web. Environ. Pollut. 2022, 309, 119739. [Google Scholar] [CrossRef]
- Göckener, B.; Eichhorn, M.; Lämmer, R.; Kotthoff, M.; Kowalczyk, J.; Numata, J.; Schafft, H.; Lahrssen-Wiederholt, M.; Bücking, M. Transfer of Per- and Polyfluoroalkyl Substances (PFAS) from Feed into the Eggs of Laying Hens. Part 1: Analytical Results Including a Modified Total Oxidizable Precursor Assay. J. Agric. Food Chem. 2020, 68, 12527–12538. [Google Scholar] [CrossRef]
- Lupton, S.J.; Smith, D.J.; Scholljegerdes, E.; Ivey, S.; Young, W.; Genualdi, S.; DeJager, L.; Snyder, A.; Esteban, E.; Johnston, J.J. Plasma and Skin Per- and Polyfluoroalkyl Substance (PFAS) Levels in Dairy Cattle with Lifetime Exposures to PFAS-Contaminated Drinking Water and Feed. J. Agric. Food Chem. 2022, 70, 15945–15954. [Google Scholar] [CrossRef] [PubMed]
- Aker, A.; Ayotte, P.; Caron-Beaudoin, E.; De Silva, A.; Ricard, S.; Gaudreau, É.; Lemire, M. Plasma concentrations of perfluoroalkyl acids and their determinants in youth and adults from Nunavik, Canada. Chemosphere 2023, 310, 136797. [Google Scholar] [CrossRef]
- Chen, F.; Yin, S.; Kelly, B.C.; Liu, W. Chlorinated Polyfluoroalkyl Ether Sulfonic Acids in Matched Maternal, Cord, and Placenta Samples: A Study of Transplacental Transfer. Environ. Sci. Technol. 2017, 51, 6387–6394. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, H.; Cui, Q.; Sheng, N.; Yeung, L.W.Y.; Guo, Y.; Sun, Y.; Dai, J. First Report on the Occurrence and Bioaccumulation of Hexafluoropropylene Oxide Trimer Acid: An Emerging Concern. Environ. Sci. Technol. 2017, 51, 9553–9560. [Google Scholar] [CrossRef]
- Pérez, F.; Nadal, M.; Navarro-Ortega, A.; Fàbrega, F.; Domingo, J.L.; Barceló, D.; Farré, M. Accumulation of perfluoroalkyl substances in human tissues. Environ. Int. 2013, 59, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.M.; Zhang, S.; Hoffman, K.; Miranda, M.L.; Stapleton, H.M. Concentrations of per- and polyfluoroalkyl substances (PFAS) in human placental tissues and associations with birth outcomes. Chemosphere 2022, 295, 133873. [Google Scholar] [CrossRef] [PubMed]
- Onteeru, M.; Barnes, L.E.; O’Connell, K.; Bhimani, J.; Du, M.; Romano, M.E.; Kantor, E.D. Association between fish oil supplements use and serum per- and polyfluoroalkyl substances (PFAS): Results from the National Health and Nutrition Examination Survey. Environ. Res. 2022, 215, 114205. [Google Scholar] [CrossRef]
- Yu, C.H.; Riker, C.D.; Lu, S.E.; Fan, Z.T. Biomonitoring of emerging contaminants, perfluoroalkyl and polyfluoroalkyl substances (PFAS), in New Jersey adults in 2016–2018. Int. J. Hyg. Environ. Health 2020, 223, 34–44. [Google Scholar] [CrossRef]
- Richterová, D.; Govarts, E.; Fábelová, L.; Rausová, K.; Rodriguez Martin, L.; Gilles, L.; Remy, S.; Colles, A.; Rambaud, L.; Riou, M.; et al. PFAS levels and determinants of variability in exposure in European teenagers—Results from the HBM4EU aligned studies (2014–2021). Int. J. Hyg. Environ. Health 2023, 247, 114057. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, M.; Gallego-Picó, A.; Cutanda, F.; Huetos, O.; Esteban, M.; Pérez-Gómez, B.; Castaño, A. Perfluorinated alkyl substances in Spanish adults: Geographical distribution and determinants of exposure. Sci. Total Environ. 2017, 603–604, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cai, D.; Chu, C.; Li, Q.; Zhou, Y.; Hu, L.; Yang, B.; Dong, G.; Zeng, X.; Chen, D. Transplacental Transfer of Per- and Polyfluoroalkyl Substances (PFASs): Differences between Preterm and Full-Term Deliveries and Associations with Placental Transporter mRNA Expression. Environ. Sci. Technol. 2020, 54, 5062–5070. [Google Scholar] [CrossRef] [PubMed]
- Mamsen, L.S.; Jönsson, B.A.G.; Lindh, C.H.; Olesen, R.H.; Larsen, A.; Ernst, E.; Kelsey, T.W.; Andersen, C.Y. Concentration of perfluorinated compounds and cotinine in human foetal organs, placenta, and maternal plasma. Sci. Total Environ. 2017, 596–597, 97–105. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Li, Y.; Hao, Y.; Li, J.; Zhang, L.; Wang, P.; Yin, Y.; Zhang, S.; Li, T.; et al. Occurrence of per- and polyfluoroalkyl substances (PFASs) in raw milk and feed from nine Chinese provinces and human exposure risk assessment. Chemosphere 2022, 300, 134521. [Google Scholar] [CrossRef]
- Norén, E.; Lindh, C.; Glynn, A.; Rylander, L.; Pineda, D.; Nielsen, C. Temporal trends, 2000–2017, of perfluoroalkyl acid (PFAA) concentrations in serum of Swedish adolescents. Environ. Int. 2021, 155, 106716. [Google Scholar] [CrossRef]
- Li, Y.; Lu, X.; Yu, N.; Li, A.; Zhuang, T.; Du, L.; Tang, S.; Shi, W.; Yu, H.; Song, M.; et al. Exposure to legacy and novel perfluoroalkyl substance disturbs the metabolic homeostasis in pregnant women and fetuses: A metabolome-wide association study. Environ. Int. 2021, 156, 106627. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Zhou, Y.; Li, Q.Q.; Bloom, M.S.; Lin, S.; Yu, Y.J.; Chen, D.; Yu, H.Y.; Hu, L.W.; Yang, B.Y.; et al. Are perfluorooctane sulfonate alternatives safer? New insights from a birth cohort study. Environ. Int. 2020, 135, 105365. [Google Scholar] [CrossRef]
- Choi, G.W.; Choi, E.J.; Kim, J.H.; Kang, D.W.; Lee, Y.B.; Cho, H.Y. Gender differences in pharmacokinetics of perfluoropentanoic acid using non-linear mixed-effect modeling in rats. Arch. Toxicol. 2020, 94, 1601–1612. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, L.; Zhang, X.; Liu, P.; Deji, Z.; Xing, Y.; Zhou, Y.; Lin, X.; Huang, Z. Per- and polyfluoroalkyl substances exposure and its influence on the intestinal barrier: An overview on the advances. Sci. Total Environ. 2022, 852, 158362. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Teng, M.; Zhao, X.; Li, Y.; Sun, J.; Zhao, W.; Ruan, Y.; Leung, K.M.Y.; Wu, F. Insight into the binding model of per- and polyfluoroalkyl substances to proteins and membranes. Environ. Int. 2023, 175, 107951. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zhou, Z.; Hu, Z.; Wei, C.; Li, J.; Wang, L.; Liu, G.; Zhang, J.; Wang, Y.; Wang, T.; et al. Effect of Enterohepatic Circulation on the Accumulation of Per- and Polyfluoroalkyl Substances: Evidence from Experimental and Computational Studies. Environ. Sci. Technol. 2022, 56, 3214–3224. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Choi, E.J.; Choi, G.W.; Lee, Y.B.; Cho, H.Y. Exploring sex differences in human health risk assessment for PFNA and PFDA using a PBPK model. Arch. Toxicol. 2019, 93, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Pizzurro, D.M.; Seeley, M.; Kerper, L.E.; Beck, B.D. Interspecies differences in perfluoroalkyl substances (PFAS) toxicokinetics and application to health-based criteria. Regul. Toxicol. Pharmacol. 2019, 106, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Dzierlenga, A.L.; Robinson, V.G.; Waidyanatha, S.; DeVito, M.J.; Eifrid, M.A.; Gibbs, S.T.; Granville, C.A.; Blystone, C.R. Toxicokinetics of perfluorohexanoic acid (PFHxA), perfluorooctanoic acid (PFOA) and perfluorodecanoic acid (PFDA) in male and female Hsd:Sprague dawley SD rats following intravenous or gavage administration. Xenobiotica 2020, 50, 722–732. [Google Scholar] [CrossRef]
- Chang, S.C.; Das, K.; Ehresman, D.J.; Ellefson, M.E.; Gorman, G.S.; Hart, J.A.; Noker, P.E.; Tan, Y.M.; Lieder, P.H.; Lau, C.; et al. Comparative pharmacokinetics of perfluorobutyrate in rats, mice, monkeys, and humans and relevance to human exposure via drinking water. Toxicol. Sci. 2008, 104, 40–53. [Google Scholar] [CrossRef]
- Attanasio, R. Sex differences in the association between perfluoroalkyl acids and liver function in US adolescents: Analyses of NHANES 2013–2016. Environ. Pollut. 2019, 254, 113061. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; McConnell, R.; Catherine, C.; Xu, S.; Walker, D.I.; Stratakis, N.; Jones, D.P.; Miller, G.W.; Peng, C.; Conti, D.V.; et al. Perfluoroalkyl substances and severity of nonalcoholic fatty liver in Children: An untargeted metabolomics approach. Environ. Int. 2020, 134, 105220. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Qadri, S.; Luukkonen, P.K.; Ragnarsdottir, O.; McGlinchey, A.; Jäntti, S.; Juuti, A.; Arola, J.; Schlezinger, J.J.; Webster, T.F.; et al. Exposure to environmental contaminants is associated with altered hepatic lipid metabolism in non-alcoholic fatty liver disease. J. Hepatol. 2022, 76, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ouyang, T.; Liu, H.; Cao, L.; Chen, W. Perfluoroalkyl substance (PFAS) exposure and risk of nonalcoholic fatty liver disease in the elderly: Results from NHANES 2003–2014. Environ. Sci. Pollut. Res. Int. 2023, 30, 64342–64351. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhu, L.; Wang, Q.; Shan, G. Tissue distribution and bioaccumulation of legacy and emerging per-and polyfluoroalkyl substances (PFASs) in edible fishes from Taihu Lake, China. Environ. Pollut. 2021, 268, 115887. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Hong, Y.; Xie, G.; Zhang, J.; Zhang, H.; Cai, Z. Comprehensive multi-omics approaches reveal the hepatotoxic mechanism of perfluorohexanoic acid (PFHxA) in mice. Sci. Total Environ. 2021, 790, 148160. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Pan, R.; Liang, X.; Wu, X.; Wu, Y.; Zhang, H.; Zhao, J.; Chen, W. Perfluorooctanoic acid-induced liver injury is potentially associated with gut microbiota dysbiosis. Chemosphere 2021, 266, 129004. [Google Scholar] [CrossRef]
- Jiang, L.; Hong, Y.; Xiao, P.; Wang, X.; Zhang, J.; Liu, E.; Li, H.; Cai, Z. The Role of Fecal Microbiota in Liver Toxicity Induced by Perfluorooctane Sulfonate in Male and Female Mice. Environ. Health Perspect. 2022, 130, 67009. [Google Scholar] [CrossRef] [PubMed]
- Stratakis, N.; Conti, D.V.; Jin, R.; Margetaki, K.; Valvi, D.; Siskos, A.P.; Maitre, L.; Garcia, E.; Varo, N.; Zhao, Y.; et al. Prenatal Exposure to Perfluoroalkyl Substances Associated with Increased Susceptibility to Liver Injury in Children. Hepatology 2020, 72, 1758–1770. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Lv, J.; Zhou, S.; Lin, S.; Huang, S.; Zheng, L.; Deng, G.; Feng, Y.; Zhang, G.; et al. Overall and individual associations between per- and polyfluoroalkyl substances and liver function indices and the metabolic mechanism. Environ. Int. 2024, 183, 108405. [Google Scholar] [CrossRef]
- Roth, K.; Yang, Z.; Agarwal, M.; Liu, W.; Peng, Z.; Long, Z.; Birbeck, J.; Westrick, J.; Liu, W.; Petriello, M.C. Exposure to a mixture of legacy, alternative, and replacement per- and polyfluoroalkyl substances (PFAS) results in sex-dependent modulation of cholesterol metabolism and liver injury. Environ. Int. 2021, 157, 106843. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, T.; Wang, Z.; Wei, J.; Liu, J.; Zhang, Y.; Zhao, Z. Distribution of perfluorooctane sulfonate in mice and its effect on liver lipidomic. Talanta 2021, 226, 122150. [Google Scholar] [CrossRef] [PubMed]
- Khan, E.A.; Grønnestad, R.; Krøkje, Å.; Bartosov, Z.; Johanson, S.M.; Müller, M.H.B.; Arukwe, A. Alteration of hepato-lipidomic homeostasis in A/J mice fed an environmentally relevant PFAS mixture. Environ. Int. 2023, 173, 107838. [Google Scholar] [CrossRef] [PubMed]
- Hærvig, K.K.; Petersen, K.U.; Hougaard, K.S.; Lindh, C.; Ramlau-Hansen, C.H.; Toft, G.; Giwercman, A.; Høyer, B.B.; Flachs, E.M.; Bonde, J.P.; et al. Maternal Exposure to Per- and Polyfluoroalkyl Substances (PFAS) and Male Reproductive Function in Young Adulthood: Combined Exposure to Seven PFAS. Environ. Health Perspect. 2022, 130, 107001. [Google Scholar] [CrossRef]
- Rickard, B.P.; Rizvi, I.; Fenton, S.E. Per- and poly-fluoroalkyl substances (PFAS) and female reproductive outcomes: PFAS elimination, endocrine-mediated effects, and disease. Toxicology 2022, 465, 153031. [Google Scholar] [CrossRef]
- Taibl, K.R.; Dunlop, A.L.; Barr, D.B.; Li, Y.Y.; Eick, S.M.; Kannan, K.; Ryan, P.B.; Schroder, M.; Rushing, B.; Fennell, T.; et al. Newborn metabolomic signatures of maternal per- and polyfluoroalkyl substance exposure and reduced length of gestation. Nat. Commun. 2023, 14, 3120. [Google Scholar] [CrossRef]
- Ouidir, M.; Buck Louis, G.M.; Kanner, J.; Grantz, K.L.; Zhang, C.; Sundaram, R.; Rahman, M.L.; Lee, S.; Kannan, K.; Tekola-Ayele, F.; et al. Association of Maternal Exposure to Persistent Organic Pollutants in Early Pregnancy with Fetal Growth. JAMA Pediatr. 2020, 174, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Kelsey, K.; Zhu, C.; Pennell, K.D.; Yao, Q.; Manz, K.E.; Zheng, Y.F.; Braun, J.M.; Liu, Y.; Papandonatos, G.; et al. Adverse birth outcomes related to concentrations of per- and polyfluoroalkyl substances (PFAS) in maternal blood collected from pregnant women in 1960–1966. Environ. Res. 2024, 241, 117010. [Google Scholar] [CrossRef]
- Zeng, X.; Chen, T.; Cui, Y.; Zhao, J.; Chen, Q.; Yu, Z.; Zhang, Y.; Han, L.; Chen, Y.; Zhang, J. In utero exposure to perfluoroalkyl substances and early childhood BMI trajectories: A mediation analysis with neonatal metabolic profiles. Sci. Total Environ. 2023, 867, 161504. [Google Scholar] [CrossRef]
- Zhuchen, H.Y.; Wang, J.Y.; Liu, X.S.; Shi, Y.W. Research Progress on Neurodevelopmental Toxicity in Offspring after Indirect Exposure to PFASs in Early Life. Toxics 2023, 11, 571. [Google Scholar] [CrossRef]
- Liu, D.; Yan, S.; Liu, Y.; Chen, Q.; Ren, S. Association of prenatal exposure to perfluorinated and polyfluoroalkyl substances with childhood neurodevelopment: A systematic review and meta-analysis. Ecotoxicol. Environ. Saf. 2024, 271, 115939. [Google Scholar] [CrossRef] [PubMed]
- Sinioja, T.; Bodin, J.; Duberg, D.; Dirven, H.; Berntsen, H.F.; Zimmer, K.; Nygaard, U.C.; Orešič, M.; Hyötyläinen, T. Exposure to persistent organic pollutants alters the serum metabolome in non-obese diabetic mice. Metabolomics 2022, 18, 87. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Liu, X.; Nian, M.; Wang, Y.; Qiu, J.; Yu, H.; Chen, X.; Zhang, J. Environmental exposure to per- and polyfluoroalkyl substances mixture and male reproductive hormones. Environ. Int. 2021, 152, 106496. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Barr, D.B.; Ryan, P.B.; Panuwet, P.; Smarr, M.M.; Liu, K.; Kannan, K.; Yakimavets, V.; Tan, Y.; Ly, V.; et al. Per- and polyfluoroalkyl substance (PFAS) exposure, maternal metabolomic perturbation, and fetal growth in African American women: A meet-in-the-middle approach. Environ. Int. 2022, 158, 106964. [Google Scholar] [CrossRef]
- Conley, J.M.; Lambright, C.S.; Evans, N.; Medlock-Kakaley, E.; Hill, D.; McCord, J.; Strynar, M.J.; Wehmas, L.C.; Hester, S.; MacMillan, D.K.; et al. Developmental toxicity of Nafion byproduct 2 (NBP2) in the Sprague-Dawley rat with comparisons to hexafluoropropylene oxide-dimer acid (HFPO-DA or GenX) and perfluorooctane sulfonate (PFOS). Environ. Int. 2022, 160, 107056. [Google Scholar] [CrossRef]
- Schillemans, T.; Yan, Y.; Ribbenstedt, A.; Donat-Vargas, C.; Lindh, C.H.; Kiviranta, H.; Rantakokko, P.; Wolk, A.; Landberg, R.; Åkesson, A.; et al. OMICs Signatures Linking Persistent Organic Pollutants to Cardiovascular Disease in the Swedish Mammography Cohort. Environ. Sci. Technol. 2024, 58, 1036–1047. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.; Wu, L.Y.; Liu, J.J.; Fang, Q.L.; Qian, Z.M.; Chu, C.; Li, Q.Q.; Su, F.; Zhang, Y.T.; Zhou, P.; et al. The effects of Cl-PFESAs exposure on blood lipids—A community-based large population study in Guangzhou. Sci. Total Environ. 2022, 806, 150634. [Google Scholar] [CrossRef] [PubMed]
- Schillemans, T.; Bergdahl, I.A.; Hanhineva, K.; Shi, L.; Donat-Vargas, C.; Koponen, J.; Kiviranta, H.; Landberg, R.; Åkesson, A.; Brunius, C. Associations of PFAS-related plasma metabolites with cholesterol and triglyceride concentrations. Environ. Res. 2023, 216, 114570. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, T.; Walker, D.I.; Thomas, D.C.; Qiu, C.; Chatzi, L.; Alderete, T.L.; Kim, J.S.; Conti, D.V.; Breton, C.V.; et al. Dysregulated lipid and fatty acid metabolism link perfluoroalkyl substances exposure and impaired glucose metabolism in young adults. Environ. Int. 2020, 145, 106091. [Google Scholar] [CrossRef]
- Chen, Y.; Lv, J.; Fu, L.; Wu, Y.; Zhou, S.; Liu, S.; Zheng, L.; Feng, W.; Zhang, L. Metabolome-wide association study of four groups of persistent organic pollutants and abnormal blood lipids. Environ. Int. 2023, 173, 107817. [Google Scholar] [CrossRef]
- Schillemans, T.; Shi, L.; Donat-Vargas, C.; Hanhineva, K.; Tornevi, A.; Johansson, I.; Koponen, J.; Kiviranta, H.; Rolandsson, O.; Bergdahl, I.A.; et al. Plasma metabolites associated with exposure to perfluoroalkyl substances and risk of type 2 diabetes—A nested case-control study. Environ. Int. 2021, 146, 106180. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Tam, C.H.T.; Wong, K.K.; Ozaki, R.; Lowe, W.L., Jr.; Metzger, B.E.; Chow, E.; Tam, W.H.; Wong, C.K.C.; Ma, R.C.W. Epidemic-specific association of maternal exposure to per- and polyfluoroalkyl substances (PFAS) and their components with maternal glucose metabolism: A cross-sectional analysis in a birth cohort from Hong Kong. Sci. Total Environ. 2024, 917, 170220. [Google Scholar] [CrossRef] [PubMed]
- Alderete, T.L.; Jin, R.; Walker, D.I.; Valvi, D.; Chen, Z.; Jones, D.P.; Peng, C.; Gilliland, F.D.; Berhane, K.; Conti, D.V.; et al. Perfluoroalkyl substances, metabolomic profiling, and alterations in glucose homeostasis among overweight and obese Hispanic children: A proof-of-concept analysis. Environ. Int. 2019, 126, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, J.; Liu, Y.; Luo, T.; Meng, X.; Zhang, R.; Huang, B.; Sun, Y.; Zhang, J. Metabolic perturbations in pregnant rats exposed to low-dose perfluorooctanesulfonic acid: An integrated multi-omics analysis. Environ. Int. 2023, 173, 107851. [Google Scholar] [CrossRef] [PubMed]
- Stanifer, J.W.; Stapleton, H.M.; Souma, T.; Wittmer, A.; Zhao, X.; Boulware, L.E. Perfluorinated Chemicals as Emerging Environmental Threats to Kidney Health: A Scoping Review. Clin. J. Am. Soc. Nephrol. 2018, 13, 1479–1492. [Google Scholar] [CrossRef] [PubMed]
- He, A.; Li, J.; Li, Z.; Lu, Y.; Liang, Y.; Zhou, Z.; Man, Z.; Lv, J.; Wang, Y.; Jiang, G. Novel Insights into the Adverse Health Effects of per- and Polyfluoroalkyl Substances on the Kidney via Human Urine Metabolomics. Environ. Sci. Technol. 2023, 57, 16244–16254. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, J.A.; Walker, D.; Lin, X.; Wang, H.; Lim, T.; McConnell, R.; Conti, D.V.; Chatzi, L.; Setiawan, V.W. Exposure to perfluoroalkyl substances and risk of hepatocellular carcinoma in a multiethnic cohort. JHEP Rep. 2022, 4, 100550. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Teitelbaum, S.L.; Dolios, G.; Dang, L.T.; Tu, P.; Wolff, M.S.; Petrick, L.M. Molecular Gatekeeper Discovery: Workflow for Linking Multiple Exposure Biomarkers to Metabolomics. Environ. Sci. Technol. 2022, 56, 6162–6171. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Furnary, T.; Vasiliou, V.; Yan, Q.; Nyhan, K.; Jones, D.P.; Johnson, C.H.; Liew, Z. Non-targeted metabolomics and associations with per- and polyfluoroalkyl substances (PFAS) exposure in humans: A scoping review. Environ. Int. 2022, 162, 107159. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Yang, M.; Wei, S.; Li, Y. Integrated Analysis of Per- and Polyfluoroalkyl Substance Exposure and Metabolic Profiling of Elderly Residents Living near Industrial Plants. Environ. Sci. Technol. 2024, 58, 4104–4114. [Google Scholar] [CrossRef]
- Prince, N.; Begum, S.; Mínguez-Alarcón, L.; Génard-Walton, M.; Huang, M.; Soeteman, D.I.; Wheelock, C.; Litonjua, A.A.; Weiss, S.T.; Kelly, R.S.; et al. Plasma concentrations of per- and polyfluoroalkyl substances are associated with perturbations in lipid and amino acid metabolism. Chemosphere 2023, 324, 138228. [Google Scholar] [CrossRef] [PubMed]
- Sinisalu, L.; Yeung, L.W.Y.; Wang, J.; Pan, Y.; Dai, J.; Hyötyläinen, T. Prenatal exposure to poly-/per-fluoroalkyl substances is associated with alteration of lipid profiles in cord-blood. Metabolomics 2021, 17, 103. [Google Scholar] [CrossRef]
- Kingsley, S.L.; Walker, D.I.; Calafat, A.M.; Chen, A.; Papandonatos, G.D.; Xu, Y.; Jones, D.P.; Lanphear, B.P.; Pennell, K.D.; Braun, J.M. Metabolomics of childhood exposure to perfluoroalkyl substances: A cross-sectional study. Metabolomics 2019, 15, 95. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, J.A.; Walker, D.I.; He, J.; Lin, X.; Baumert, B.O.; Hu, X.; Alderete, T.L.; Chen, Z.; Valvi, D.; Fuentes, Z.C.; et al. Metabolic Signatures of Youth Exposure to Mixtures of Per- and Polyfluoroalkyl Substances: A Multi-Cohort Study. Environ. Health Perspect. 2023, 131, 27005. [Google Scholar] [CrossRef]
- Mitro, S.D.; Liu, J.; Jaacks, L.M.; Fleisch, A.F.; Williams, P.L.; Knowler, W.C.; Laferrère, B.; Perng, W.; Bray, G.A.; Wallia, A.; et al. Per- and polyfluoroalkyl substance plasma concentrations and metabolomic markers of type 2 diabetes in the Diabetes Prevention Program trial. Int. J. Hyg. Environ. Health 2021, 232, 113680. [Google Scholar] [CrossRef]
- Kirkwood-Donelson, K.I.; Chappel, J.; Tobin, E.; Dodds, J.N.; Reif, D.M.; DeWitt, J.C.; Baker, E.S. Investigating mouse hepatic lipidome dysregulation following exposure to emerging per- and polyfluoroalkyl substances (PFAS). Chemosphere 2024, 354, 141654. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.; Hanrahan, J.; Kiefte, S.; O’Brien, T.; Mercer, G.V.; Steeves, K.L.; Schneider, C.M.; Jobst, K.J.; Cahill, L.S. Differential impact of perfluorooctanoic acid and fluorotelomer ethoxylates on placental metabolism in mice. Chemosphere 2024, 356, 141923. [Google Scholar] [CrossRef]
- Gao, B.; Tu, P.; Chi, L.; Shen, W.; Gao, N. Perfluorooctanoic Acid-Disturbed Serum and Liver Lipidome in C57BL/6 Mice. Chem. Res. Toxicol. 2022, 35, 2252–2259. [Google Scholar] [CrossRef]
| Legacy PFAS | ||
|---|---|---|
| Name | Structural Formula | CASRN |
| Long-chain PFCA | CnF2n+1COOH, n ≥ 7 | - |
| Long-chain PFSA | CnF2n+1SO3H, n ≥ 6 | - |
| Emerging PFAS | ||
| Name | Structural Formula | CASRN |
| Short-chain PFCA | CnF2n+1COOH, n = 2–5 | - |
| Short-chain PFSA | CnF2n+1SO3H, n = 2–5 | - |
| HFPO-DA or “GenX” | C3F7O(CF3)CFCOOH | 13252-13-6 |
| NBP2 | CF3CHFOC3F6OC2F4SO3H | 749836-20-2 |
| 6:2 FTS | C8H4F13SO3H | 27619-97-2 |
| 6:2 Cl-PFESA | Cl(CF2)6O(CF2)2SO3− | 73606-19-6 |
| 8:2 Cl-PFESA | Cl(CF2)8O(CF2)2SO3− | 83329-89-9 |
| PFOSA | C8H17SO2NH2 | 754-91-6 |
| EtFOSAA | C8F17SO2N(C3H7)COOH | 2991-50-6 |
| MeFOSAA | C8F17SO2N(C2H5)COOH | 2355-31-9 |
| 6:2 FTEOs | C6F13(C2H4O)nH, n = 4–12 | 1640092-35-8 a |
| PFAS | Study Object | Sample Size | Toxic Effect | Sample Matrix | Main Finding(s) | Ref. |
| PFHxS, PFHpS, PFOA, PFNA, PFDA, PFOS, PFUdA, 6:2Cl-PFESA, 8:2Cl-PFESA | Residents of Guangzhou, China | 278 | Liver function indicators (APOB, GGT, DBIL) | serum |
| Chen Y et al., 2024 [90] |
| PFOS, PFHxS, PFOA, PFNA | Pregnant African American Newborns in Atlanta, Georgia | 267 | Premature labor and gestational age at delivery | serum |
| Taibl KR et al., 2023 [76] |
| PFHxS, PFOA, PFHpS, PFNA, PFOS, 6:2 Cl-PFESA, PFDA, PFUdA | Male residents of Guangzhou | 278 | Dyslipidemia (TC and LDL) | plasma |
| Chen Y et al., 2023 [90] |
| PFOA, PFOS, PFNA, PFUdA, PFDA, PFHxS, PFBS, PFDoA, PFHpA, PFOSA | A birth cohort study in Shanghai, China | 1671 | Obesity trajectory in children by age 4 | plasma |
| Zeng X et al., 2023 [79] |
| 23 PFASs, mainly PFHpA, PFOA, PFBS, PFPeS, PFHxS, PFHpS, PFOS, 6:2FTS | Workers and residents in Hubei, China | 225 | Renal function indicators (Urea, Creatine, Uric acid) | urine |
| He A et al., 2023 [96] |
| PFHxS, PFOS, PFOA, PFNA | A birth cohort of African American pregnant women | 313 | Decreased fetal growth | serum |
| Chang CJ et al., 2022 [84] |
| PFOS, PFHxS, PFOA, PFDA, PFNA, PFUdA | A nested case-control study | 50 pairs | Non-viral HCC risk | plasma |
| Goodrich JA et al., 2022 [97] |
| PFDA, PFNA, PFUdA | A nested case-control study | 187 | Cholesterol and triglycerides | plasma |
| Schillemans T et al., 2023 [88] |
| PFOS, PFOA, PFNA, PFHxS, PFUdA | The Human Early-Life Exposome project | 1105 | Risk of liver injury in children | serum |
| Stratakis N et al., 2020 [69] |
| PFOA, PFOS and PFHxS | Cross-sectional cohort of NAFLD | 74 | Histologic severity of NAFLD | plasma |
| Jin R et al., 2020 [62] |
| PFOA, PFOA, PFHxS, PFDA, PFNA, PFUdA | a case-control study on T2D nested | 187 pairs | Type 2 diabetes risk | plasma |
| Schillemans T et al., 2021 [91] |
| PFOA, PFOS, PFHxS | Hispanic children in Los Angeles | 40 | glucose homeostasis | plasma |
| Alderete TL et al., 2019 [93] |
| PFAS | Study Object | Dose | Toxic Effect | Sample Matrix | Main Finding(s) | Ref. |
| Eight PFAS mixtures (PFOA, PFNA, PFDA, PFUdA, PFDoDA, PFTrDA, PFTeDA, PFOS), | A/J mice | 3 g/mouse/week, 10 weeks | Hepatosomatic index (HSI) | liver |
| Khan EA et al., 2023 [73] |
| PFOS | SD rats | 0.03 and 0.3 mg/kg, GD1-18d | fasting glucose homeostasis | liver |
| Yu G et al., 2023 [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Cai, D.; Chen, Q.; Zhu, Z.; Zhang, S.; Wang, Z.; Hu, Z.; Shen, H.; Meng, Z. Hunting Metabolic Biomarkers for Exposure to Per- and Polyfluoroalkyl Substances: A Review. Metabolites 2024, 14, 392. https://doi.org/10.3390/metabo14070392
Ma X, Cai D, Chen Q, Zhu Z, Zhang S, Wang Z, Hu Z, Shen H, Meng Z. Hunting Metabolic Biomarkers for Exposure to Per- and Polyfluoroalkyl Substances: A Review. Metabolites. 2024; 14(7):392. https://doi.org/10.3390/metabo14070392
Chicago/Turabian StyleMa, Xue, Delei Cai, Qing Chen, Zhoujing Zhu, Shixin Zhang, Ziyu Wang, Zhengyan Hu, Haitao Shen, and Zhen Meng. 2024. "Hunting Metabolic Biomarkers for Exposure to Per- and Polyfluoroalkyl Substances: A Review" Metabolites 14, no. 7: 392. https://doi.org/10.3390/metabo14070392
APA StyleMa, X., Cai, D., Chen, Q., Zhu, Z., Zhang, S., Wang, Z., Hu, Z., Shen, H., & Meng, Z. (2024). Hunting Metabolic Biomarkers for Exposure to Per- and Polyfluoroalkyl Substances: A Review. Metabolites, 14(7), 392. https://doi.org/10.3390/metabo14070392






