Skeletal Muscle Metabolism Is Dynamic during Porcine Postnatal Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Harvest and Sample Collection
2.2. Backfat Measurement
2.3. Histochemistry
2.4. Mitochondria Isolation
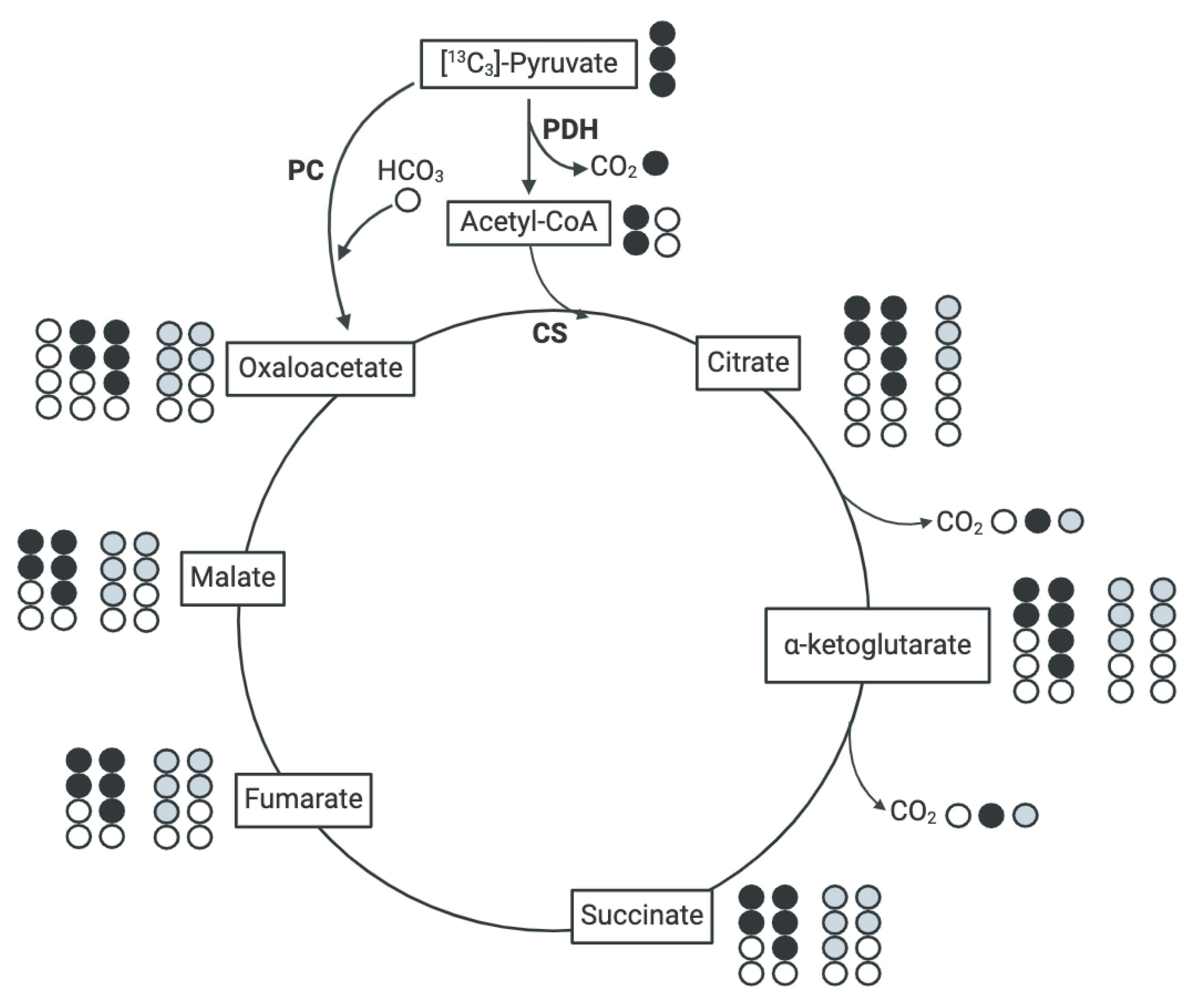
2.5. In Vitro [13C3]-Pyruvate and [13C5]-Glutamate Tracing in Isolated Mitochondria
2.6. Mass Spectrometry of TCA Metabolites
2.7. Western Blotting
2.8. Statistics Section
3. Results
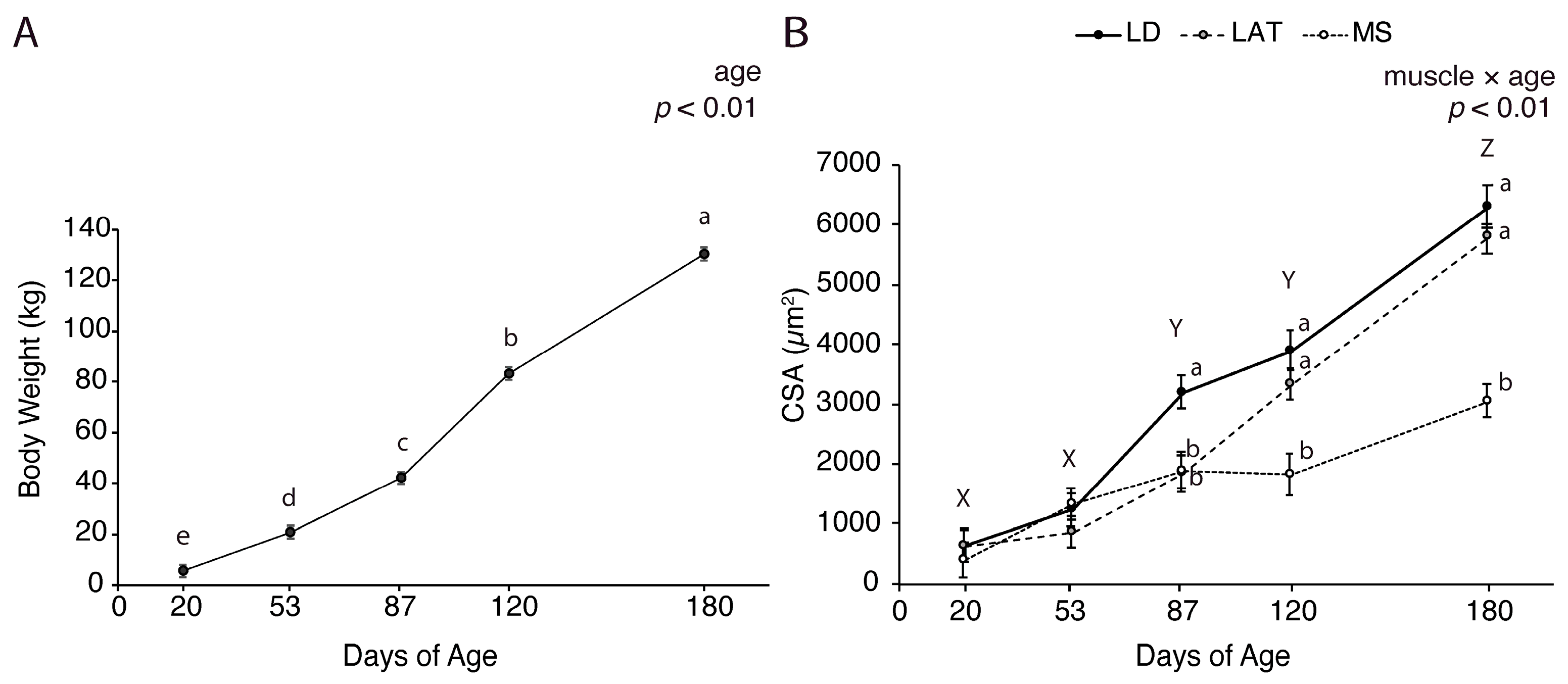
3.1. Muscle and Fat Growth Characterization
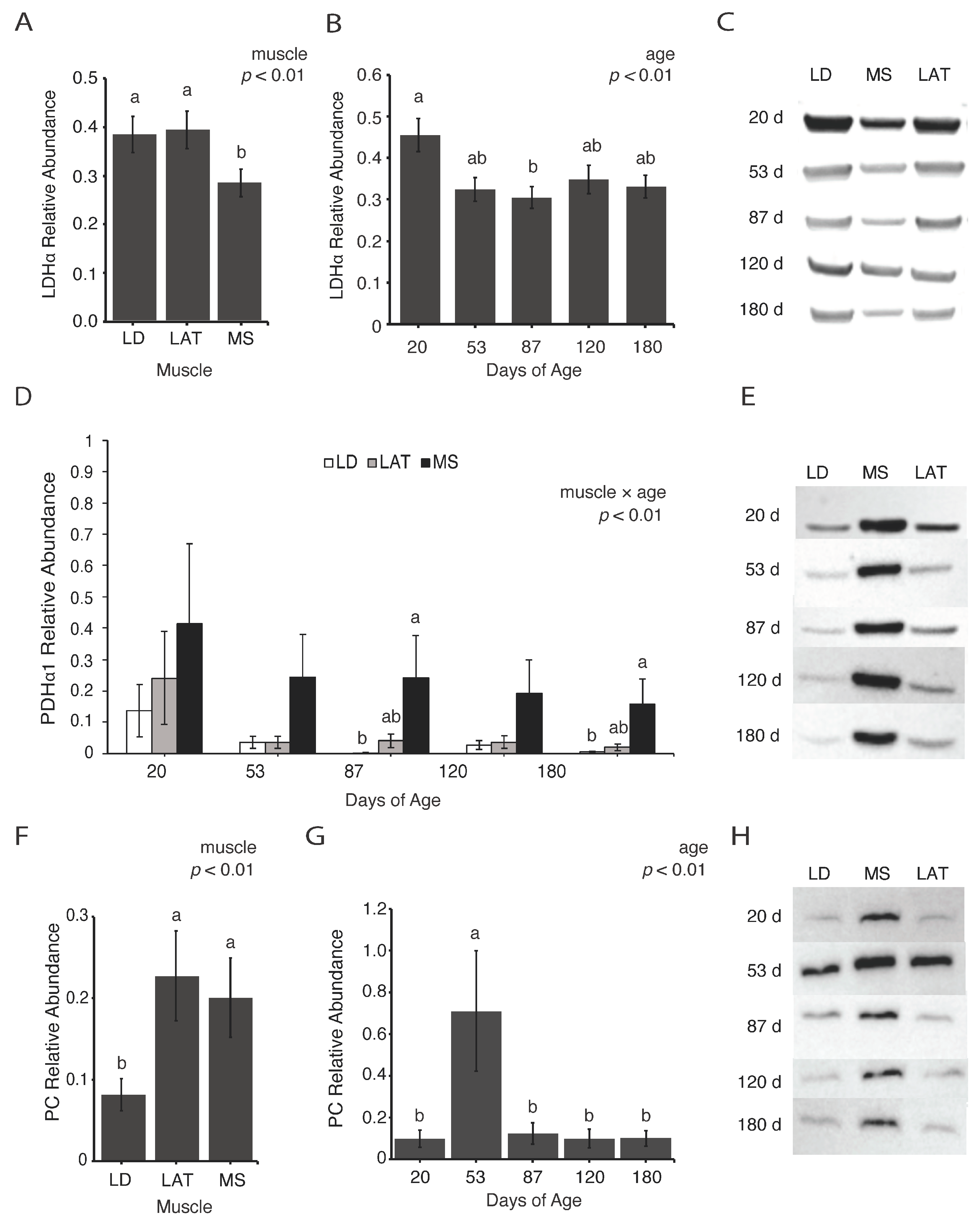
3.2. Enzymes Involved in Pyruvate Metabolism Are Variable with Age
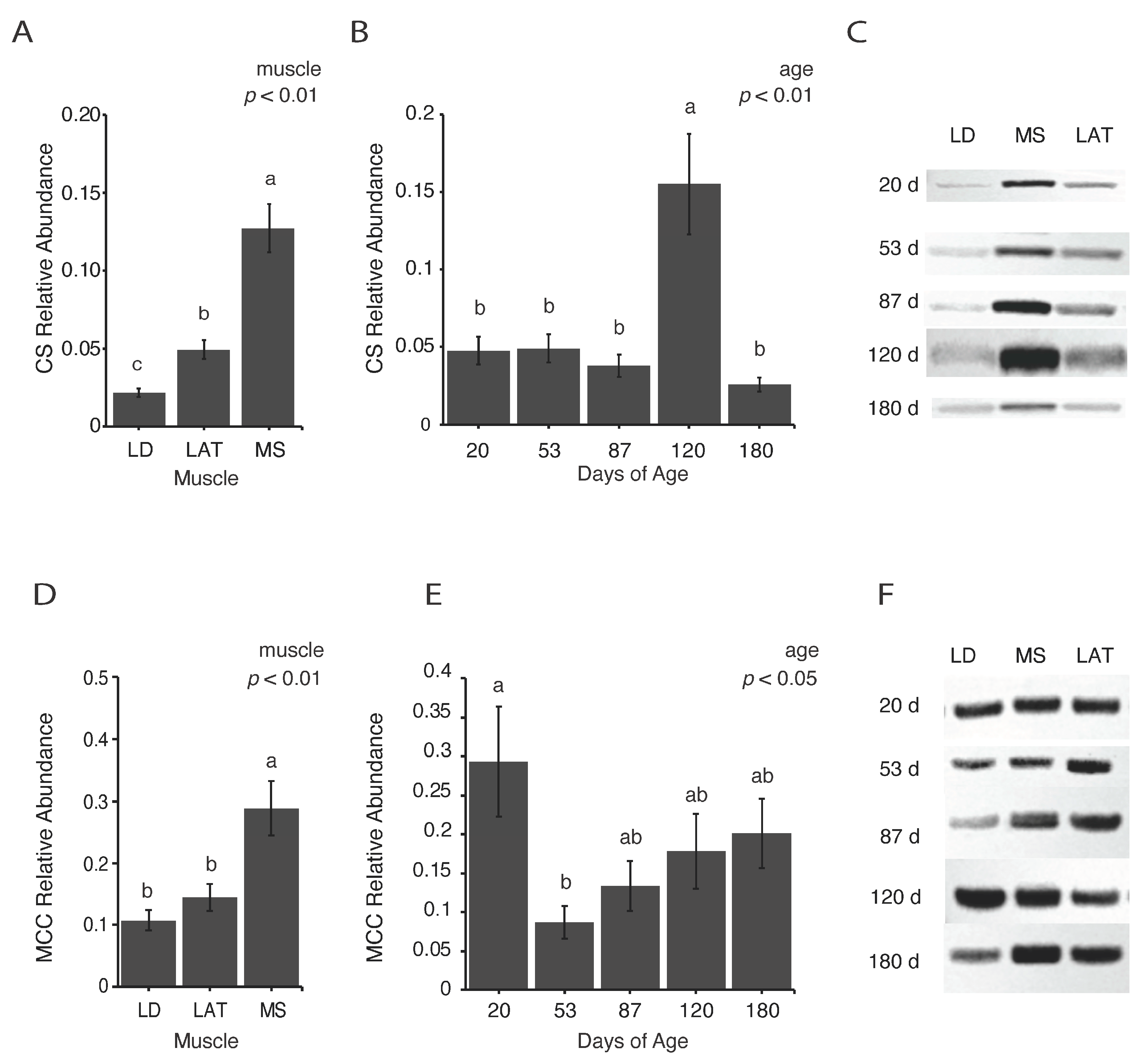
3.3. Enzymes Involved in Citrate Metabolism Vary with Age
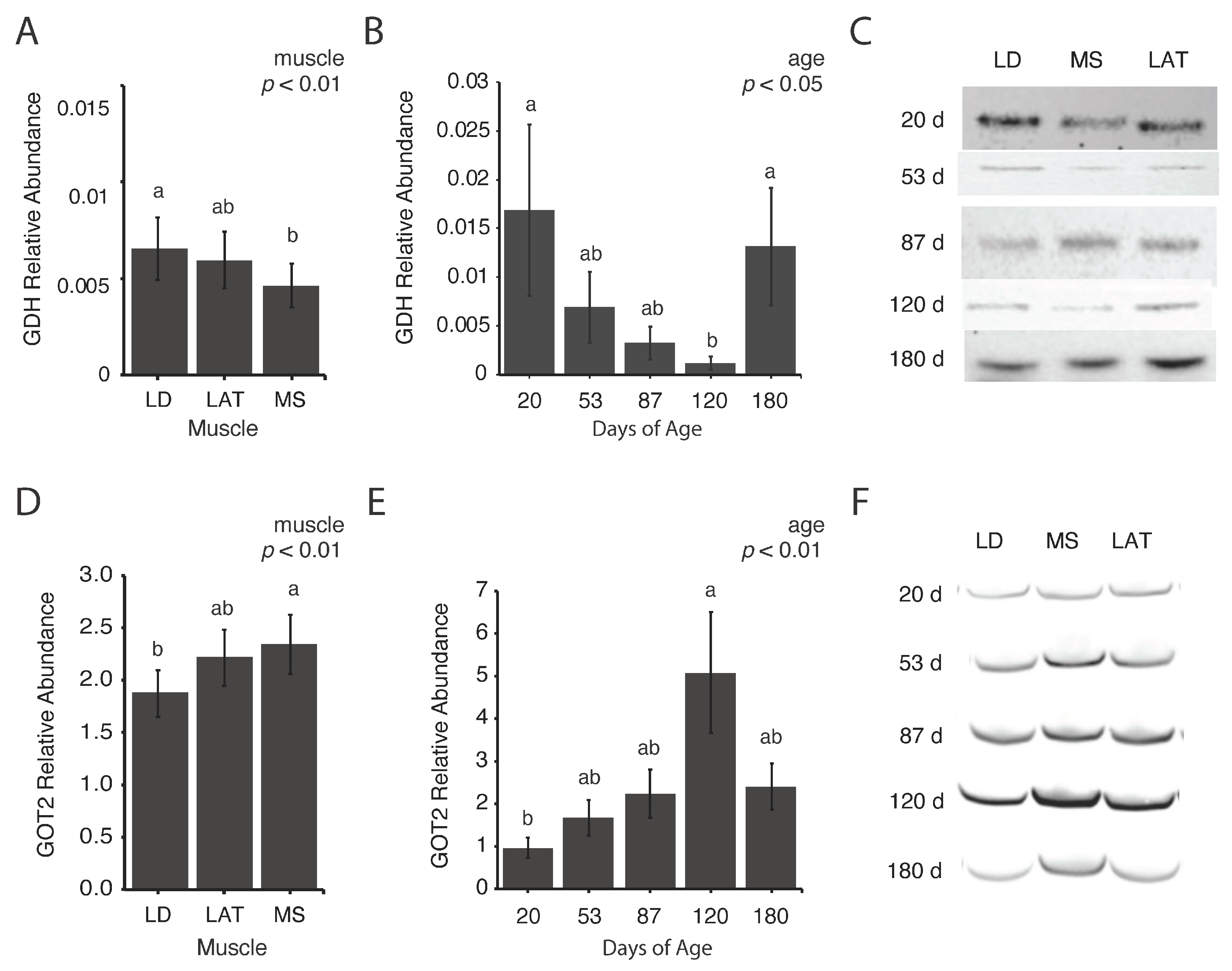
3.4. Abundance of Mitochondrial Enzymes Involved in Amino Acid Metabolism Differs Based on Inherent Muscle Metabolism
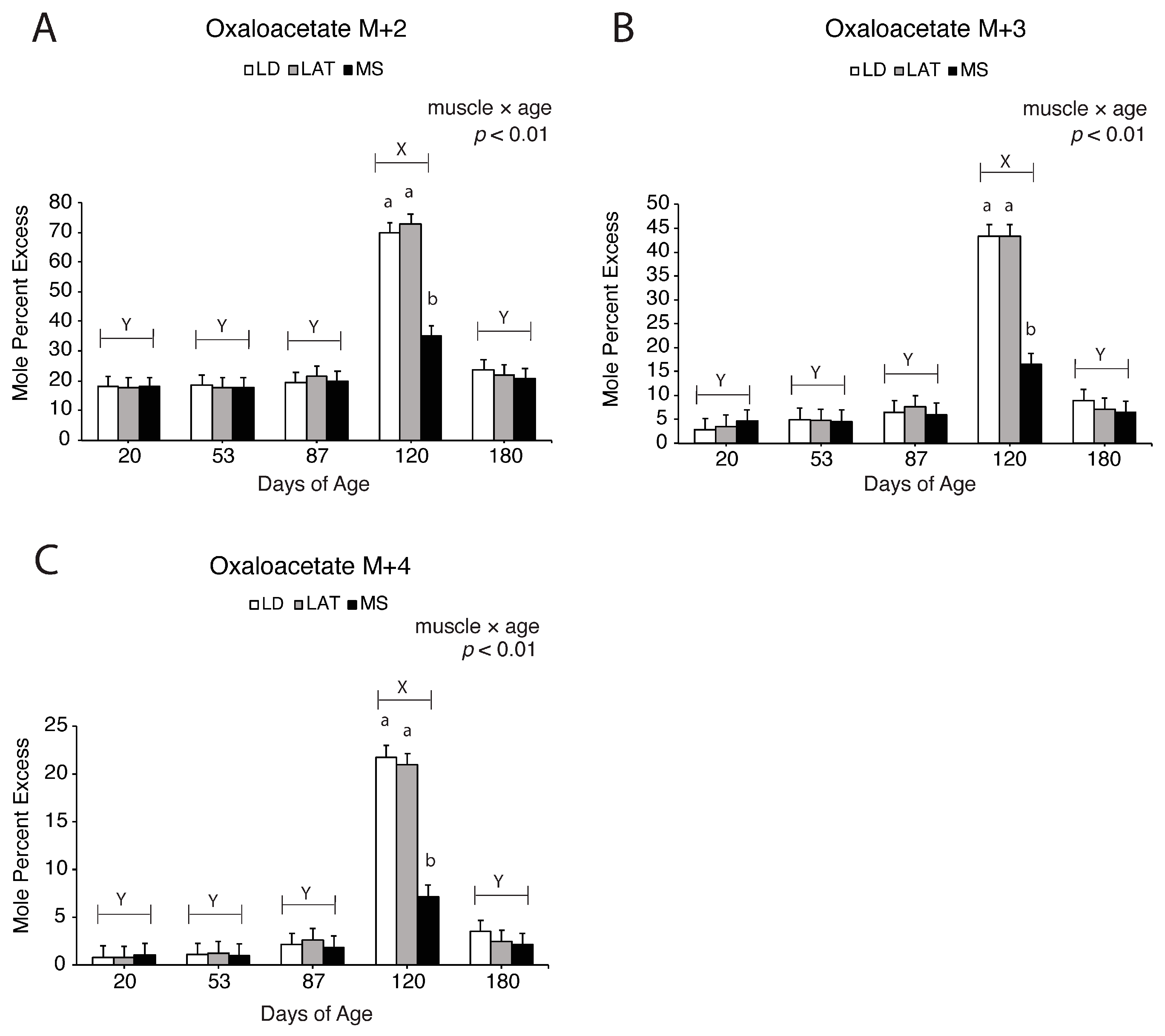
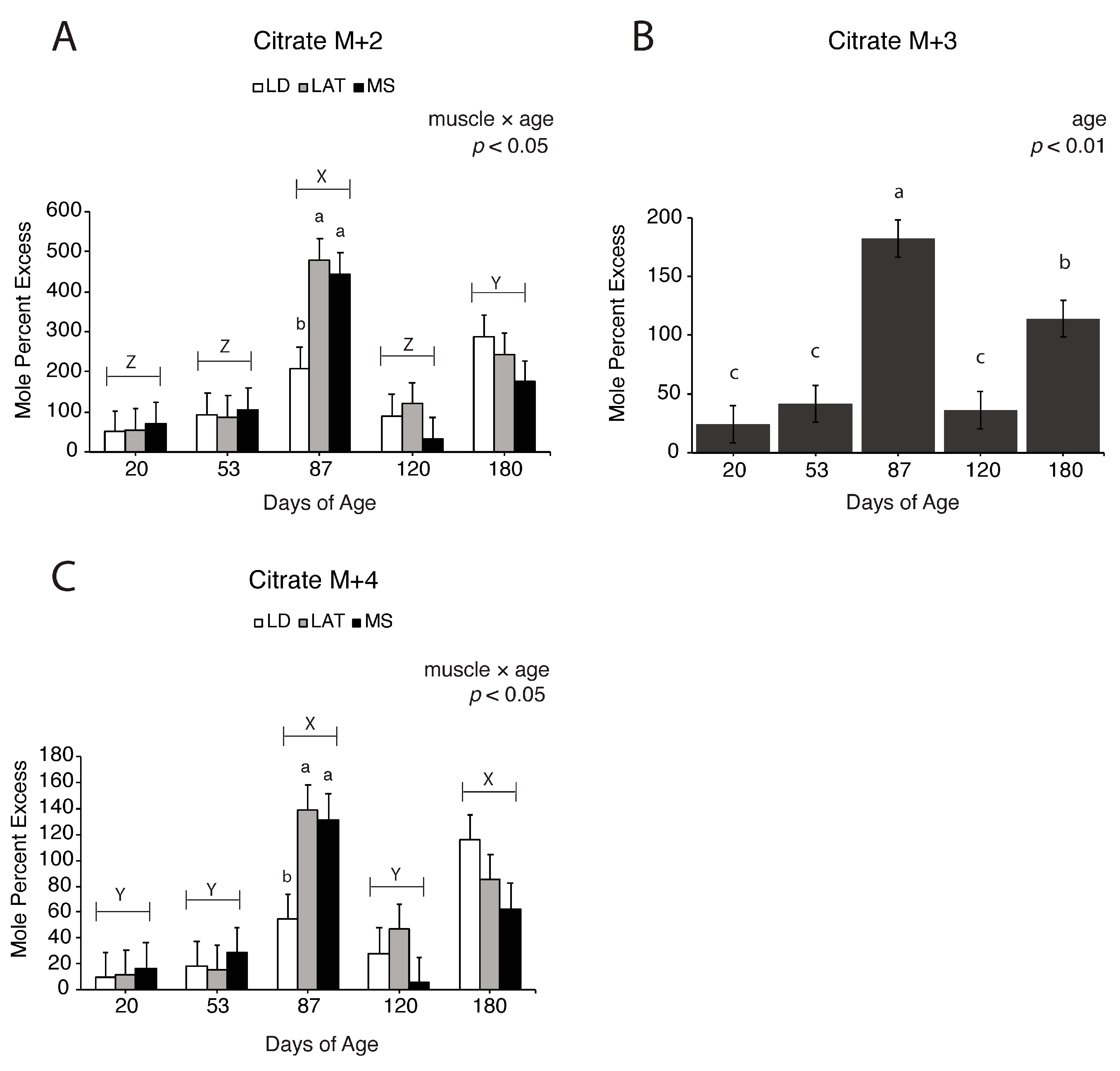
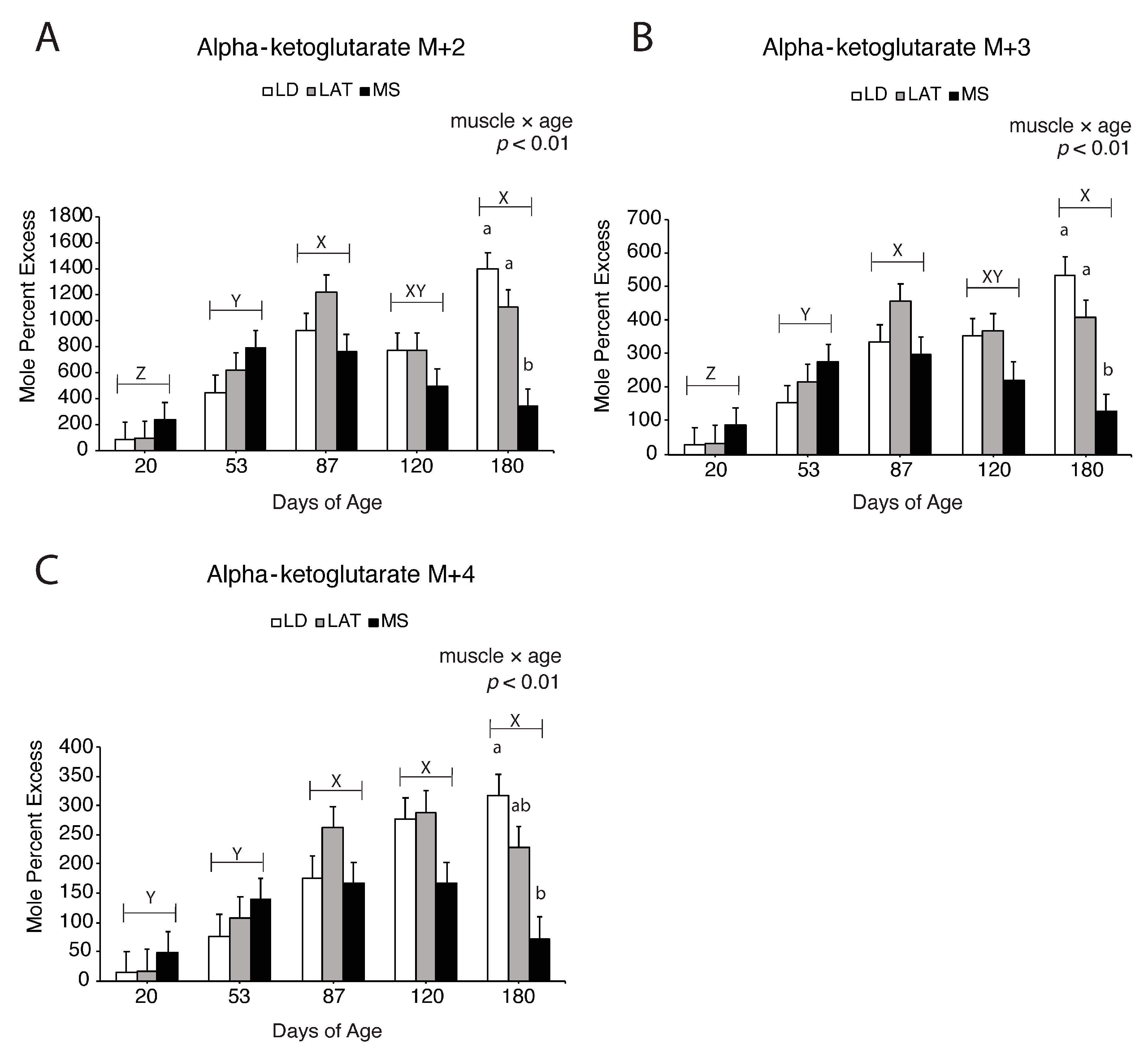
3.5. Assessment of Pyruvate-Derived TCA Intermediate Metabolism in the TCA Cycle
3.6. Assessment of Glutamate Entry into the TCA Cycle
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- United States Department of Agriculture Foreign Agricultural Service. USDA Production, Supply, and Distribution Database; USDA: Washington, DC, USA, 2024. Available online: https://apps.fas.usda.gov/psdonline/app/index.html#/app/home (accessed on 5 May 2024).
- Dohlman; Hansen, J.; Boussios, D. USDA Agricultural Projections to 2031; World Agricultural Outlook Board, Office of Chief Economist: Washington, DC, USA, 2022.
- Tokach, M.D.; Goodband, B.D.; O’Quinn, T.G. Performance-enhancing technologies in swine production. Anim. Front. 2016, 6, 15–21. [Google Scholar] [CrossRef][Green Version]
- McBride, W.D.; Key, N. U.S. Hog Production from 1992 to 2009: Technology, Restructuring, and Productivity Growth; USDA Economic Research Service: Washington, DC, USA, 2013.
- Brewer, M.S.; Zhu, L.G.; McKeith, F.K. Marbling effects on quality characteristics of pork loin chops: Consumer purchase intent, visual and sensory characteristics. Meat Sci. 2001, 59, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Ngapo, T.; Fortin, J.; Martin, J.-F. Do pig farmers preferences bias consumer choice for pork? Response to critique of the pork preference studies. Meat Sci. 2010, 85, 788–791. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, W.; Li, M.; Gao, P.; He, Z.; Wu, Y.; Cai, C.; Li, B.; Cao, G. Transcriptome profile of skeletal muscle at different developmental stages in Large White and Mashen pigs. Can. J. Anim. Sci. 2019, 99, 867–880. [Google Scholar] [CrossRef]
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Comp. Physiol. 2021, 10, 785–809. [Google Scholar]
- Gerrard, D.; Grant, A. Principles of Animal Growth and Development; Kendall/Hunt Publishing Company: Dubuque, IA, USA, 2003. [Google Scholar]
- Julien, I.B.; Sephton, C.F.; Dutchak, P.A. Metabolic Networks Influencing Skeletal Muscle Fiber Composition. Front. Cell Dev. Biol. 2018, 6, 125. [Google Scholar]
- Fu, L.; Xu, Y.; Hou, Y.; Qi, X.; Zhou, L.; Liu, H.; Luan, Y.; Jing, L.; Miao, Y.; Zhao, S.; et al. Proteomic analysis indicates that mitochondrial energy metabolism in skeletal muscle tissue is negatively correlated with feed efficiency in pigs. Sci. Rep. 2017, 7, 45291. [Google Scholar] [CrossRef] [PubMed]
- Bottje, W.; Iqbal, M.; Tang, Z.X.; Cawthon, D.; Okimoto, R.; Wing, T.; Cooper, M. Association of mitochondrial function with feed efficiency within a single genetic line of male broilers. Poult. Sci. 2002, 81, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Kolath, W.H.; Kerley, M.S.; Golden, J.W.; Keisler, D.H. The relationship between mitochondrial function and residual feed intake in Angus steers. J. Anim. Sci. 2006, 84, 861–865. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirments of Swine, Eleventh Revised ed.; The National Academies Press: Washington, DC, USA, 2012.
- Menegat, M.B.; Dritz, S.S.; Tokach, M.D.; Woodworth, J.C.; DeRouchey, J.M.; Goodband, R.D. Phase-feeding strategies based on lysine specifications for grow-finish pigs. J. Anim. Sci. 2020, 98, skz366. [Google Scholar]
- Scheffler, T.L.; Scheffler, J.M.; Park, S.; Kasten, S.C.; Wu, Y.; McMillan, R.P.; Hulver, M.W.; Frisard, M.I.; Gerrard, D.E. Fiber hypertrophy and increased oxidative capacity can occur simultaneously in pig glycolytic skeletal muscle. Am. J. Physiol. Cell Physiol. 2014, 306, C354–C363. [Google Scholar] [CrossRef] [PubMed]
- Matarneh, S.K.; Yen, C.-N.; Bodmer, J.; El-Kadi, S.W.; Gerrard, D.E. Mitochondria influence glycolytic and tricarboxylic acid cycle metabolism under postmortem simulating conditions. Meat Sci. 2021, 172, 108316. [Google Scholar] [CrossRef] [PubMed]
- Bergmeyer, H.U. Methods of Enzymatic Analysis. In Methods of Enzymatic Analysis; Verlag Chemi: Weinheim, Germany, 1984; pp. 883–889. [Google Scholar]
- El-Kadi, S.W.; Baldwin, R.L.; McLeod, K.R.; Sunny, N.E.; Bequette, B.J. Glutamate Is the Major Anaplerotic Substrate in the Tricarboxylic Acid Cycle of Isolated Rumen Epithelial and Duodenal Mucosal Cells from Beef Cattle. J. Nutr. 2009, 139, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Inigo, M.; Deja, S.; Burgess, S.C. Ins and Outs of the TCA Cycle: The Central Role of Anaplerosis. Annu. Rev. Nutr. 2021, 41, 19–47. [Google Scholar] [CrossRef] [PubMed]
- Menegat, M.B.; Goodband, R.D.; DeRouchey, J.M.; Tokach, M.D.; Woodworth, J.C.; Dritz, S.S. Kansas State University Swine Nutrition Guide: Nursery Phase Feeding Program; Kansas State University: Manhattan, KS, USA, 2019. [Google Scholar]
- England, E.M.; Matarneh, S.K.; Scheffler, T.L.; Wachet, C.; Gerrard, D.E. pH inactivation of phosphofructokinase arrests postmortem glycolysis. Meat Sci. 2014, 98, 850–857. [Google Scholar] [CrossRef]
- Gravel, S.-P.; Andrzejewski, S.; Avizonis, D.; St-Pierre, J. Stable Isotope Tracer Analysis in Isolated Mitochondria from Mammalian Systems. Metabolites 2014, 4, 166–183. [Google Scholar] [CrossRef] [PubMed]
- King, N. Amino Acids and the Mitochondria, in Mitochondria: The Dynamic Organelle; Schaffer, S.W., Suleiman, M.S., Eds.; Springer: New York, NY, USA, 2007; pp. 151–166. [Google Scholar]
- Huo, W.; Weng, K.; Li, Y.; Zhang, Y.; Zhang, Y.; Xu, Q.; Chen, G. Comparison of muscle fiber characteristics and glycolytic potential between slow- and fast-growing broilers. Poult. Sci. 2022, 101, 101649. [Google Scholar] [CrossRef]
- Lefaucheur, L.; Ecolan, P.; Barzic, Y.-M.; Marion, J.; Le Dividich, J. Early Postnatal Food Intake Alters Myofiber Maturation in Pig Skeletal Muscle. J. Nutr. 2003, 133, 140–147. [Google Scholar] [CrossRef]
- Becker, L.L.; Scholtz, E.E.; DeRouchey, J.M.; Tokach, M.D.; Woodworth, J.C.; Goodband, R.D.; A De Jong, J.; Wu, F.; Berg, K.M.; Ward, J.P.; et al. Effects of standardized ileal digestible lysine on growth performance and economic return in duroc-sired finishing pigs. Transl. Anim. Sci. 2022, 6, txac069. [Google Scholar] [CrossRef]
- James, B.; Goodband, R.; Unruh, J.; Tokach, M.D.; Nelssen, J.; Dritz, S.; O’quinn, P.; Andrews, B. Effect of creatine monohydrate on finishing pig growth performance, carcass characteristics and meat quality. Anim. Feed. Sci. Technol. 2002, 96, 135–145. [Google Scholar] [CrossRef]
- Kerkaert, H.R.; Cemin, H.S.; Woodworth, J.C.; DeRouchey, J.M.; Dritz, S.S.; Tokach, M.D.; Goodband, R.D.; Haydon, K.D.; Hastad, C.W.; Post, Z.B. Improving performance of finishing pigs with added valine, isoleucine, and tryptophan: Validating a meta-analysis model. J. Anim. Sci. 2021, 99, skab006. [Google Scholar] [CrossRef] [PubMed]
- Royall, R.Q.; Goodband, R.D.; Tokach, M.D.; DeRouchey, J.M.; Woodworth, J.C.; Gebhardt, J.T. Effects of adding potassium bicarbonate to diets with high or low crystalline lysine to influence dietary cation-anion difference on finishing pig growth performance. Transl. Anim. Sci. 2022, 6, txac107. [Google Scholar] [CrossRef]
- Khattri, R.B.; Puglise, J.; Ryan, T.E.; Walter, G.A.; Merritt, M.E.; Barton, E.R. Isolated murine skeletal muscles utilize pyruvate over glucose for oxidation. Metabolomics 2022, 18, 105. [Google Scholar] [CrossRef]
- Panchal, A.R.; Comte, B.; Huang, H.; Kerwin, T.; Darvish, A.; Rosiers, C.D.; Brunengraber, H.; Stanley, W.C.; Kajimoto, M.; Ledee, D.R.; et al. Partitioning of pyruvate between oxidation and anaplerosis in swine hearts. Am. J. Physiol. Circ. Physiol. 2000, 279, H2390–H2398. [Google Scholar] [CrossRef]
- Gopal, K.; Abdualkader, A.M.; Li, X.; Greenwell, A.A.; Karwi, Q.G.; Altamimi, T.R.; Saed, C.; Uddin, G.M.; Darwesh, A.M.; Jamieson, K.L.; et al. Loss of muscle PDH induces lactic acidosis and adaptive anaplerotic compensation via pyruvate-alanine cycling and glutaminolysis. J. Biol. Chem. 2023, 299, 105375. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Cooper, D.E.; Scheidemantle, G.; Locasale, J.W.; Kirsch, D.G.; Liu, X. 13C tracer analysis suggests extensive recycling of endogenous CO2 in vivo. Cancer Metab. 2022, 10, 11. [Google Scholar] [CrossRef]
- Owen, O.E.; Kalhan, S.C.; Hanson, R.W. The Key Role of Anaplerosis and Cataplerosis for Citric Acid Cycle Function. J. Biol. Chem. 2002, 277, 30409–30412. [Google Scholar] [CrossRef] [PubMed]
- Wellen, K.E.; Hatzivassiliou, G.; Sachdeva, U.M.; Bui, T.V.; Cross, J.R.; Thompson, C.B. ATP-Citrate Lyase Links Cellular Metabolism to Histone Acetylation. Science 2009, 324, 1076–1080. [Google Scholar] [CrossRef]
- Das, S.; Morvan, F.; Jourde, B.; Meier, V.; Kahle, P.; Brebbia, P.; Toussaint, G.; Glass, D.J.; Fornaro, M. ATP Citrate Lyase Improves Mitochondrial Function in Skeletal Muscle. Cell Metab. 2015, 21, 868–876. [Google Scholar] [CrossRef]
- Holecek, M.; Sispera, L.; Skalska, H. Enhanced Glutamine Availability Exerts Different Effects on Protein and Amino Acid Metabolism in Skeletal Muscle from Healthy and Septic Rats. J. Parenter. Enter. Nutr. 2015, 39, 847–854. [Google Scholar] [CrossRef]
- Rezaei, R.; Knabe, D.A.; Tekwe, C.D.; Dahanayaka, S.; Ficken, M.D.; Fielder, S.E.; Eide, S.J.; Lovering, S.L.; Wu, G. Dietary supplementation with monosodium glutamate is safe and improves growth performance in postweaning pigs. Amino Acids 2013, 44, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Albrecht, E.; Stange, K.; Li, Z.; Schregel, J.; Sciascia, Q.L.; Metges, C.C.; Maak, S. Glutamine supplementation stimulates cell proliferation in skeletal muscle and cultivated myogenic cells of low birth weight piglets. Sci. Rep. 2021, 11, 13432. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rimmer, L.A.; Geisbrecht, E.R.; Chao, M.D.; O’Quinn, T.G.; Woodworth, J.C.; Zumbaugh, M.D. Skeletal Muscle Metabolism Is Dynamic during Porcine Postnatal Growth. Metabolites 2024, 14, 357. https://doi.org/10.3390/metabo14070357
Rimmer LA, Geisbrecht ER, Chao MD, O’Quinn TG, Woodworth JC, Zumbaugh MD. Skeletal Muscle Metabolism Is Dynamic during Porcine Postnatal Growth. Metabolites. 2024; 14(7):357. https://doi.org/10.3390/metabo14070357
Chicago/Turabian StyleRimmer, Linnea A., Erika R. Geisbrecht, Michael D. Chao, Travis G. O’Quinn, Jason C. Woodworth, and Morgan D. Zumbaugh. 2024. "Skeletal Muscle Metabolism Is Dynamic during Porcine Postnatal Growth" Metabolites 14, no. 7: 357. https://doi.org/10.3390/metabo14070357
APA StyleRimmer, L. A., Geisbrecht, E. R., Chao, M. D., O’Quinn, T. G., Woodworth, J. C., & Zumbaugh, M. D. (2024). Skeletal Muscle Metabolism Is Dynamic during Porcine Postnatal Growth. Metabolites, 14(7), 357. https://doi.org/10.3390/metabo14070357





