Gut Microbiota and Sinusoidal Vasoregulation in MASLD: A Portal Perspective
Abstract
1. Introduction
2. Gut Microbiota and Dysbiosis in MASLD
3. Vasoregulatory Effects of Microbiota-Derived Substances in MASLD
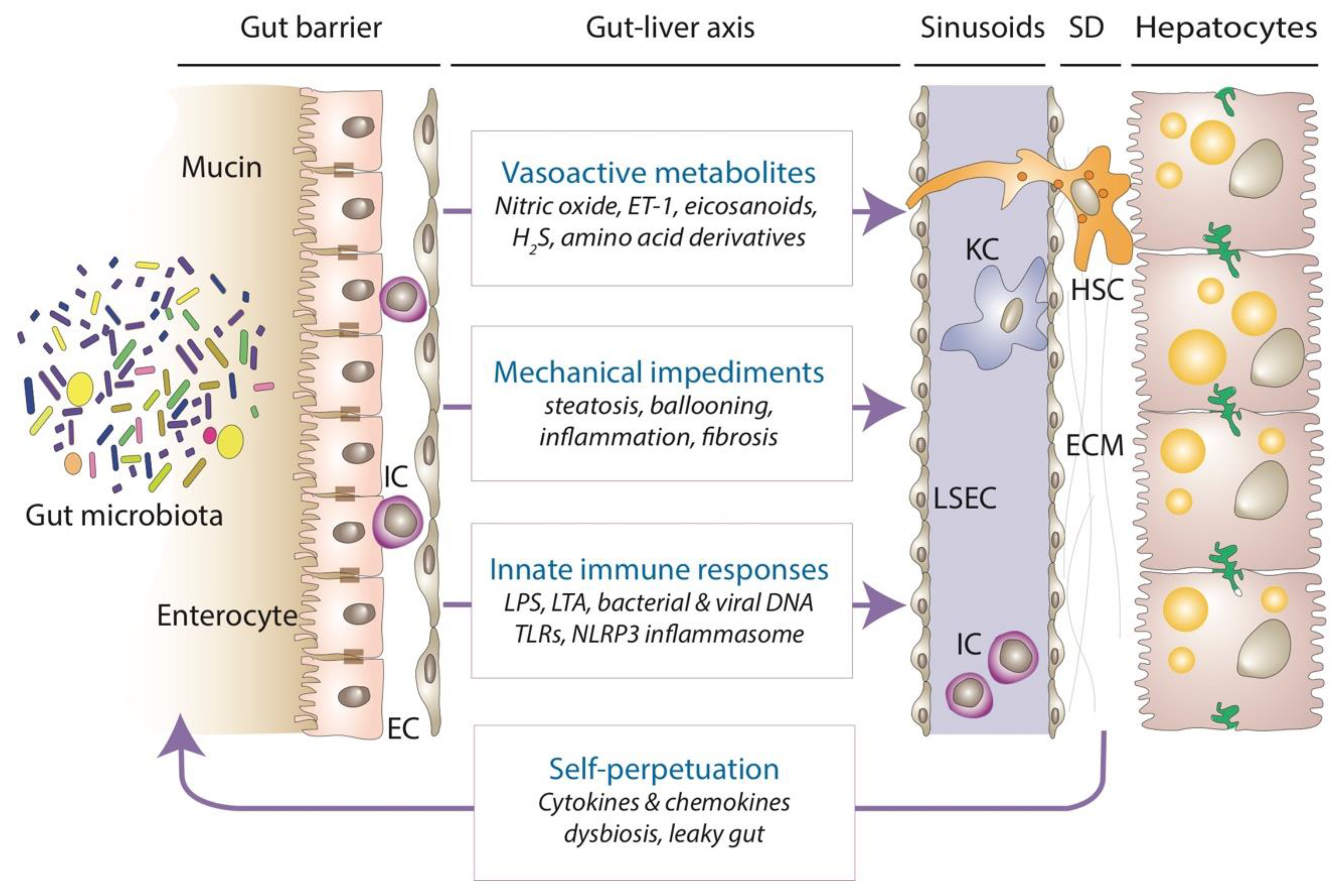
4. Indirect Impact of Gut Microbiota on Sinusoidal Hemodynamics in MASLD
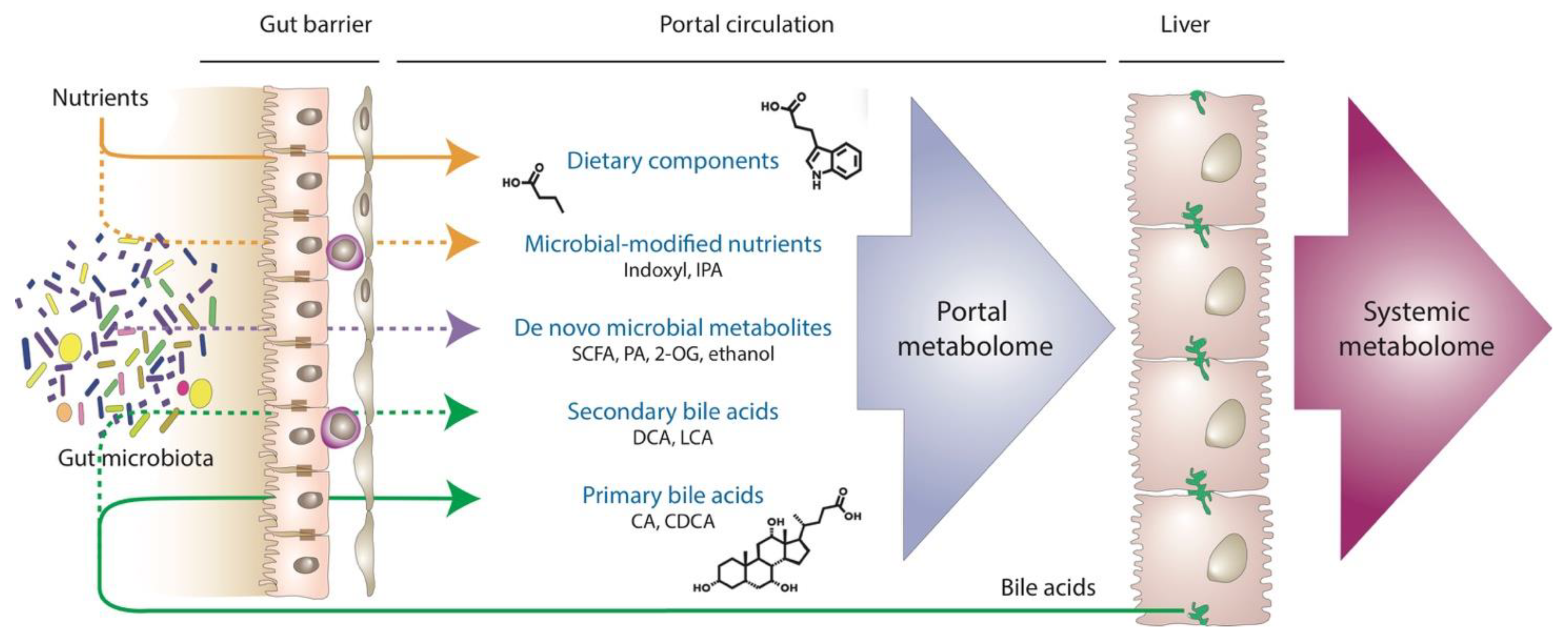
5. Portal Metabolomics: The Holy Grail to Understanding MASLD?
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Younossi, Z.; Golabi, P.; Paik, J.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Powell, E.E.; Wong, V.W.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Noureddin, N.; Huang, D.Q.; Bettencourt, R.; Siddiqi, H.; Majzoub, A.M.; Nayfeh, T.; Tamaki, N.; Izumi, N.; Nakajima, A.; Idilman, R.; et al. Natural history of clinical outcomes and hepatic decompensation in metabolic dysfunction-associated steatotic liver disease. Aliment. Pharmacol. Ther. 2024, 59, 1521–1526. [Google Scholar] [CrossRef]
- Song, R.; Li, Z.; Zhang, Y.; Tan, J.; Chen, Z. Comparison of NAFLD, MAFLD and MASLD characteristics and mortality outcomes in United States adults. Liver Int. 2024, 44, 1051–1060. [Google Scholar] [CrossRef]
- Brunt, E.M. Nonalcoholic steatohepatitis: Definition and pathology. Semin. Liver Dis. 2001, 21, 3–16. [Google Scholar] [CrossRef]
- Vollmar, B.; Menger, M.D. The hepatic microcirculation: Mechanistic contributions and therapeutic targets in liver injury and repair. Physiol. Rev. 2009, 89, 1269–1339. [Google Scholar] [CrossRef]
- Bosch, J.; Garcia-Pagan, J.C. Complications of cirrhosis. I. Portal hypertension. J. Hepatol. 2000, 32, 141–156. [Google Scholar] [CrossRef]
- Ripoll, C.; Groszmann, R.; Garcia-Tsao, G.; Grace, N.; Burroughs, A.; Planas, R.; Escorsell, A.; Garcia-Pagan, J.C.; Makuch, R.; Patch, D.; et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology 2007, 133, 481–488. [Google Scholar] [CrossRef]
- Berzigotti, A.; Seijo, S.; Reverter, E.; Bosch, J. Assessing portal hypertension in liver diseases. Expert. Rev. Gastroenterol. Hepatol. 2013, 7, 141–155. [Google Scholar] [CrossRef]
- Francque, S.; Verrijken, A.; Mertens, I.; Hubens, G.; Van Marck, E.; Pelckmans, P.; Van Gaal, L.; Michielsen, P. Noncirrhotic human nonalcoholic fatty liver disease induces portal hypertension in relation to the histological degree of steatosis. Eur. J. Gastroenterol. Hepatol. 2010, 22, 1449–1457. [Google Scholar] [CrossRef]
- Mendes, F.D.; Suzuki, A.; Sanderson, S.O.; Lindor, K.D.; Angulo, P. Prevalence and indicators of portal hypertension in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2012, 10, 1028–1033.e2. [Google Scholar] [CrossRef]
- Bassegoda, O.; Olivas, P.; Turco, L.; Mandorfer, M.; Serra-Burriel, M.; Tellez, L.; Kwanten, W.; Laroyenne, A.; Farcau, O.; Alvarado, E.; et al. Decompensation in Advanced Nonalcoholic Fatty Liver Disease May Occur at Lower Hepatic Venous Pressure Gradient Levels than in Patients With Viral Disease. Clin. Gastroenterol. Hepatol. 2022, 20, 2276–2286.e6. [Google Scholar] [CrossRef]
- Baffy, G.; Bosch, J. Overlooked subclinical portal hypertension in non-cirrhotic NAFLD: Is it real and how to measure it? J. Hepatol. 2022, 76, 458–463. [Google Scholar] [CrossRef]
- Nababan, S.H.H.; Lesmana, C.R.A. Portal Hypertension in Nonalcoholic Fatty Liver Disease: From Pathogenesis to Clinical Practice. J. Clin. Transl. Hepatol. 2022, 10, 979–985. [Google Scholar] [CrossRef]
- Vonghia, L.; Magrone, T.; Verrijken, A.; Michielsen, P.; Van Gaal, L.; Jirillo, E.; Francque, S. Peripheral and hepatic vein cytokine levels in correlation with non-alcoholic fatty liver disease (NAFLD)-related metabolic, histological, and haemodynamic features. PLoS ONE 2015, 10, e0143380. [Google Scholar] [CrossRef]
- Van der Graaff, D.; Kwanten, W.J.; Couturier, F.J.; Govaerts, J.S.; Verlinden, W.; Brosius, I.; D’Hondt, M.; Driessen, A.; De Winter, B.Y.; De Man, J.G.; et al. Severe steatosis induces portal hypertension by systemic arterial hyporeactivity and hepatic vasoconstrictor hyperreactivity in rats. Lab. Investig. 2018, 98, 1263–1275. [Google Scholar] [CrossRef]
- DeLeve, L.D. Liver sinusoidal endothelial cells in hepatic fibrosis. Hepatology 2015, 61, 1740–1746. [Google Scholar] [CrossRef]
- Baffy, G. Origins of portal hypertension in nonalcoholic fatty liver disease. Dig. Dis. Sci. 2018, 63, 563–576. [Google Scholar] [CrossRef]
- Arab, J.P.; Martin-Mateos, R.M.; Shah, V.H. Gut-liver axis, cirrhosis and portal hypertension: The chicken and the egg. Hepatol. Int. 2018, 12, 24–33. [Google Scholar] [CrossRef]
- Baffy, G. Potential mechanisms linking gut microbiota and portal hypertension. Liver Int. 2019, 39, 598–609. [Google Scholar] [CrossRef]
- Miele, L.; Valenza, V.; La Torre, G.; Montalto, M.; Cammarota, G.; Ricci, R.; Masciana, R.; Forgione, A.; Gabrieli, M.L.; Perotti, G.; et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009, 49, 1877–1887. [Google Scholar] [CrossRef]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef]
- Portincasa, P.; Khalil, M.; Graziani, A.; Fruhbeck, G.; Baffy, G.; Garruti, G.; Di Ciaula, A.; Bonfrate, L. Gut microbes in metabolic disturbances. Promising role for therapeutic manipulations? Eur. J. Intern. Med. 2024, 119, 13–30. [Google Scholar] [CrossRef]
- Wang, R.; Tang, R.; Li, B.; Ma, X.; Schnabl, B.; Tilg, H. Gut microbiome, liver immunology, and liver diseases. Cell. Mol. Immunol. 2021, 18, 4–17. [Google Scholar] [CrossRef]
- Mouries, J.; Brescia, P.; Silvestri, A.; Spadoni, I.; Sorribas, M.; Wiest, R.; Mileti, E.; Galbiati, M.; Invernizzi, P.; Adorini, L.; et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J. Hepatol. 2019, 71, 1216–1228. [Google Scholar] [CrossRef]
- Forlano, R.; Martinez-Gili, L.; Takis, P.; Miguens-Blanco, J.; Liu, T.; Triantafyllou, E.; Skinner, C.; Loomba, R.; Thursz, M.; Marchesi, J.R.; et al. Disruption of gut barrier integrity and host-microbiome interactions underlie MASLD severity in patients with type-2 diabetes mellitus. Gut Microbes 2024, 16, 2304157. [Google Scholar] [CrossRef]
- Loomba, R.; Seguritan, V.; Li, W.; Long, T.; Klitgord, N.; Bhatt, A.; Dulai, P.S.; Caussy, C.; Bettencourt, R.; Highlander, S.K.; et al. Gut Microbiome-Based Metagenomic Signature for Non-invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell Metab. 2017, 25, 1054–1062.e5. [Google Scholar] [CrossRef]
- Lee, G.; You, H.J.; Bajaj, J.S.; Joo, S.K.; Yu, J.; Park, S.; Kang, H.; Park, J.H.; Kim, J.H.; Lee, D.H.; et al. Distinct signatures of gut microbiome and metabolites associated with significant fibrosis in non-obese NAFLD. Nat. Commun. 2020, 11, 4982. [Google Scholar] [CrossRef]
- Kang, B.E.; Park, A.; Yang, H.; Jo, Y.; Oh, T.G.; Jeong, S.M.; Ji, Y.; Kim, H.L.; Kim, H.N.; Auwerx, J.; et al. Machine learning-derived gut microbiome signature predicts fatty liver disease in the presence of insulin resistance. Sci. Rep. 2022, 12, 21842. [Google Scholar] [CrossRef]
- Donia, M.S.; Fischbach, M.A. Small molecules from the human microbiota. Science 2015, 349, 1254766. [Google Scholar] [CrossRef]
- Ryou, M.; Stylopoulos, N.; Baffy, G. Nonalcoholic fatty liver disease and portal hypertension. Explor. Med. 2020, 1, 149–169. [Google Scholar] [CrossRef]
- Laleman, W.; Vanderschueren, E.; Van der Merwe, S.; Chang, K.J. The use of endoscopic ultrasound in the diagnosis and management of portal hypertension. Best. Pract. Res. Clin. Gastroenterol. 2022, 60–61, 101811. [Google Scholar] [CrossRef]
- Baliss, M.; Patel, D.; Madi, M.Y.; Bazarbashi, A.N. EUS-Guided Vascular Interventions. J. Clin. Med. 2023, 12, 2165. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Bonfrate, L.; Portincasa, P. The role of microbiota in nonalcoholic fatty liver disease. Eur. J. Clin. Investig. 2022, 52, e13768. [Google Scholar] [CrossRef]
- Cai, W.; Qiu, T.; Hu, W.; Fang, T. Changes in the intestinal microbiota of individuals with non-alcoholic fatty liver disease based on sequencing: An updated systematic review and meta-analysis. PLoS ONE 2024, 19, e0299946. [Google Scholar] [CrossRef]
- Alferink, L.J.M.; Radjabzadeh, D.; Erler, N.S.; Vojinovic, D.; Medina-Gomez, C.; Uitterlinden, A.G.; de Knegt, R.J.; Amin, N.; Ikram, M.A.; Janssen, H.L.A.; et al. Microbiomics, Metabolomics, Predicted Metagenomics, and Hepatic Steatosis in a Population-Based Study of 1,355 Adults. Hepatology 2021, 73, 968–982. [Google Scholar] [CrossRef]
- Mascardi, M.F.; Mazzini, F.N.; Suarez, B.; Ruda, V.M.; Marciano, S.; Casciato, P.; Narvaez, A.; Haddad, L.; Anders, M.; Orozco, F.; et al. Integrated analysis of the transcriptome and its interaction with the metabolome in metabolic associated fatty liver disease: Gut microbiome signatures, correlation networks, and effect of PNPLA3 genotype. Proteomics 2023, 23, e2200414. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Khalil, M.; Angelis, M.; Calabrese, F.M.; D’Amato, M.; Wang, D.Q.; Di Ciaula, A. Intestinal Barrier and Permeability in Health, Obesity and NAFLD. Biomedicines 2021, 10, 83. [Google Scholar] [CrossRef]
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology 2013, 57, 601–609. [Google Scholar] [CrossRef]
- Ralli, T.; Saifi, Z.; Tyagi, N.; Vidyadhari, A.; Aeri, V.; Kohli, K. Deciphering the role of gut metabolites in non-alcoholic fatty liver disease. Crit. Rev. Microbiol. 2023, 49, 815–833. [Google Scholar] [CrossRef]
- Yang, M.; Qi, X.; Li, N.; Kaifi, J.T.; Chen, S.; Wheeler, A.A.; Kimchi, E.T.; Ericsson, A.C.; Rector, R.S.; Staveley-O’Carroll, K.F.; et al. Western diet contributes to the pathogenesis of non-alcoholic steatohepatitis in male mice via remodeling gut microbiota and increasing production of 2-oleoylglycerol. Nat. Commun. 2023, 14, 228. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, S.E.; Lee, N.Y.; Kim, J.H.; Jung, J.H.; Jang, M.K.; Park, S.H.; Lee, M.S.; Kim, D.J.; Kim, H.S.; et al. Role of Microbiota-Derived Metabolites in Alcoholic and Non-Alcoholic Fatty Liver Diseases. Int. J. Mol. Sci. 2021, 23, 426. [Google Scholar] [CrossRef]
- Hoyles, L.; Fernandez-Real, J.M.; Federici, M.; Serino, M.; Abbott, J.; Charpentier, J.; Heymes, C.; Luque, J.L.; Anthony, E.; Barton, R.H.; et al. Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat. Med. 2018, 24, 1070–1080. [Google Scholar] [CrossRef]
- Wang, Q.; Mackay, C.R. High metabolite concentrations in portal venous blood as a possible mechanism for microbiota effects on the immune system and Western diseases. J. Allergy Clin. Immunol. 2024, 153, 980–982. [Google Scholar] [CrossRef]
- Chiang, J.Y.L.; Ferrell, J.M. Bile Acid Biology, Pathophysiology, and Therapeutics. Clin. Liver Dis. 2020, 15, 91–94. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Garruti, G.; Lunardi Baccetto, R.; Molina-Molina, E.; Bonfrate, L.; Wang, D.Q.; Portincasa, P. Bile Acid Physiology. Ann. Hepatol. 2017, 16, S4–S14. [Google Scholar] [CrossRef]
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef]
- Ji, Y.; Gao, Y.; Chen, H.; Yin, Y.; Zhang, W. Indole-3-Acetic Acid Alleviates Nonalcoholic Fatty Liver Disease in Mice via Attenuation of Hepatic Lipogenesis, and Oxidative and Inflammatory Stress. Nutrients 2019, 11, 2062. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, Q.; Shao, K.; Liu, L.; Ma, X.; Zhang, F.; Zhang, X.; Meng, L.; Yan, C.; Zhao, X. Indole-3-acetic acid improves the hepatic mitochondrial respiration defects by PGC1a up-regulation. Cell. Signal. 2022, 99, 110442. [Google Scholar] [CrossRef]
- Poeta, M.; Pierri, L.; Vajro, P. Gut-Liver Axis Derangement in Non-Alcoholic Fatty Liver Disease. Children 2017, 4, 66. [Google Scholar] [CrossRef]
- Giannelli, V.; Di Gregorio, V.; Iebba, V.; Giusto, M.; Schippa, S.; Merli, M.; Thalheimer, U. Microbiota and the gut-liver axis: Bacterial translocation, inflammation and infection in cirrhosis. World J. Gastroenterol. 2014, 20, 16795–16810. [Google Scholar] [CrossRef]
- Luo, L.; Chang, Y.; Sheng, L. Gut-liver axis in the progression of nonalcoholic fatty liver disease: From the microbial derivatives-centered perspective. Life Sci. 2023, 321, 121614. [Google Scholar] [CrossRef]
- Garcia-Lezana, T.; Raurell, I.; Bravo, M.; Torres-Arauz, M.; Salcedo, M.T.; Santiago, A.; Schoenenberger, A.; Manichanh, C.; Genesca, J.; Martell, M.; et al. Restoration of a healthy intestinal microbiota normalizes portal hypertension in a rat model of nonalcoholic steatohepatitis. Hepatology 2018, 67, 1485–1498. [Google Scholar] [CrossRef]
- Rabot, S.; Membrez, M.; Bruneau, A.; Gerard, P.; Harach, T.; Moser, M.; Raymond, F.; Mansourian, R.; Chou, C.J. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010, 24, 4948–4959. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Zhang, W.; Mackay, C.R.; Gershwin, M.E. Immunomodulatory Effects of Microbiota-Derived Short-Chain Fatty Acids in Autoimmune Liver Diseases. J. Immunol. 2023, 210, 1629–1639. [Google Scholar] [CrossRef]
- Juanola, O.; Ferrusquia-Acosta, J.; Garcia-Villalba, R.; Zapater, P.; Magaz, M.; Marin, A.; Olivas, P.; Baiges, A.; Bellot, P.; Turon, F.; et al. Circulating levels of butyrate are inversely related to portal hypertension, endotoxemia, and systemic inflammation in patients with cirrhosis. FASEB J. 2019, 33, 11595–11605. [Google Scholar] [CrossRef]
- Anjani, K.; Lhomme, M.; Sokolovska, N.; Poitou, C.; Aron-Wisnewsky, J.; Bouillot, J.L.; Lesnik, P.; Bedossa, P.; Kontush, A.; Clement, K.; et al. Circulating phospholipid profiling identifies portal contribution to NASH signature in obesity. J. Hepatol. 2015, 62, 905–912. [Google Scholar] [CrossRef]
- Nimer, N.; Choucair, I.; Wang, Z.; Nemet, I.; Li, L.; Gukasyan, J.; Weeks, T.L.; Alkhouri, N.; Zein, N.; Tang, W.H.W.; et al. Bile acids profile, histopathological indices and genetic variants for non-alcoholic fatty liver disease progression. Metabolism 2021, 116, 154457. [Google Scholar] [CrossRef]
- Marchiano, S.; Biagioli, M.; Bordoni, M.; Morretta, E.; Di Giorgio, C.; Vellecco, V.; Roselli, R.; Bellini, R.; Massa, C.; Cari, L.; et al. Defective Bile Acid Signaling Promotes Vascular Dysfunction, Supporting a Role for G-Protein Bile Acid Receptor 1/Farnesoid X Receptor Agonism and Statins in the Treatment of Nonalcoholic Fatty Liver Disease. J. Am. Heart Assoc. 2023, 12, e031241. [Google Scholar] [CrossRef]
- Guan, H.; Zhang, X.; Kuang, M.; Yu, J. The gut-liver axis in immune remodeling of hepatic cirrhosis. Front. Immunol. 2022, 13, 946628. [Google Scholar] [CrossRef]
- Chen, D.; Le, T.H.; Shahidipour, H.; Read, S.A.; Ahlenstiel, G. The Role of Gut-Derived Microbial Antigens on Liver Fibrosis Initiation and Progression. Cells 2019, 8, 1324. [Google Scholar] [CrossRef]
- van der Graaff, D.; Chotkoe, S.; De Winter, B.; De Man, J.; Casteleyn, C.; Timmermans, J.P.; Pintelon, I.; Vonghia, L.; Kwanten, W.J.; Francque, S. Vasoconstrictor antagonism improves functional and structural vascular alterations and liver damage in rats with early NAFLD. JHEP Rep. 2022, 4, 100412. [Google Scholar] [CrossRef]
- Francque, S.; Laleman, W.; Verbeke, L.; Van Steenkiste, C.; Casteleyn, C.; Kwanten, W.; Van Dyck, C.; D’Hondt, M.; Ramon, A.; Vermeulen, W.; et al. Increased intrahepatic resistance in severe steatosis: Endothelial dysfunction, vasoconstrictor overproduction and altered microvascular architecture. Lab. Investig. 2012, 92, 1428–1439. [Google Scholar] [CrossRef]
- Gonzalez-Paredes, F.J.; Hernandez Mesa, G.; Morales Arraez, D.; Marcelino Reyes, R.; Abrante, B.; Diaz-Flores, F.; Salido, E.; Quintero, E.; Hernandez-Guerra, M. Contribution of Cyclooxygenase End Products and Oxidative Stress to Intrahepatic Endothelial Dysfunction in Early Non-Alcoholic Fatty Liver Disease. PLoS ONE 2016, 11, e0156650. [Google Scholar] [CrossRef]
- Renga, B.; Mencarelli, A.; Migliorati, M.; Distrutti, E.; Fiorucci, S. Bile-acid-activated farnesoid X receptor regulates hydrogen sulfide production and hepatic microcirculation. World J. Gastroenterol. 2009, 15, 2097–2108. [Google Scholar] [CrossRef]
- Fiorucci, S.; Distrutti, E. Linking liver metabolic and vascular disease via bile acid signaling. Trends Mol. Med. 2022, 28, 51–66. [Google Scholar] [CrossRef]
- Krishnan, S.; Ding, Y.; Saedi, N.; Choi, M.; Sridharan, G.V.; Sherr, D.H.; Yarmush, M.L.; Alaniz, R.C.; Jayaraman, A.; Lee, K. Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Rep. 2018, 23, 1099–1111. [Google Scholar] [CrossRef]
- Huc, T.; Nowinski, A.; Drapala, A.; Konopelski, P.; Ufnal, M. Indole and indoxyl sulfate, gut bacteria metabolites of tryptophan, change arterial blood pressure via peripheral and central mechanisms in rats. Pharmacol. Res. 2018, 130, 172–179. [Google Scholar] [CrossRef]
- Yuan, X.; Yang, J.; Huang, Y.; Li, J.; Li, Y. Gut Microbiota Metabolite 3-Indolepropionic Acid Directly Activates Hepatic Stellate Cells by ROS/JNK/p38 Signaling Pathways. Biomolecules 2023, 13, 1464. [Google Scholar] [CrossRef]
- Pasarin, M.; La Mura, V.; Gracia-Sancho, J.; Garcia-Caldero, H.; Rodriguez-Vilarrupla, A.; Garcia-Pagan, J.C.; Bosch, J.; Abraldes, J.G. Sinusoidal endothelial dysfunction precedes inflammation and fibrosis in a model of NAFLD. PLoS ONE 2012, 7, e32785. [Google Scholar] [CrossRef]
- Carpino, G.; Del Ben, M.; Pastori, D.; Carnevale, R.; Baratta, F.; Overi, D.; Francis, H.; Cardinale, V.; Onori, P.; Safarikia, S.; et al. Increased Liver Localization of Lipopolysaccharides in Human and Experimental NAFLD. Hepatology 2020, 72, 470–485. [Google Scholar] [CrossRef]
- Mridha, A.R.; Haczeyni, F.; Yeh, M.M.; Haigh, W.G.; Ioannou, G.N.; Barn, V.; Ajamieh, H.; Adams, L.; Hamdorf, J.M.; Teoh, N.C.; et al. TLR9 is up-regulated in human and murine NASH: Pivotal role in inflammatory recruitment and cell survival. Clin. Sci. 2017, 131, 2145–2159. [Google Scholar] [CrossRef]
- Sookoian, S.; Salatino, A.; Castano, G.O.; Landa, M.S.; Fijalkowky, C.; Garaycoechea, M.; Pirola, C.J. Intrahepatic bacterial metataxonomic signature in non-alcoholic fatty liver disease. Gut 2020, 69, 1483–1491. [Google Scholar] [CrossRef]
- Csak, T.; Pillai, A.; Ganz, M.; Lippai, D.; Petrasek, J.; Park, J.K.; Kodys, K.; Dolganiuc, A.; Kurt-Jones, E.A.; Szabo, G. Both bone marrow-derived and non-bone marrow-derived cells contribute to AIM2 and NLRP3 inflammasome activation in a MyD88-dependent manner in dietary steatohepatitis. Liver Int. 2014, 34, 1402–1413. [Google Scholar] [CrossRef]
- Gracia-Sancho, J.; Maeso-Diaz, R.; Fernandez-Iglesias, A.; Navarro-Zornoza, M.; Bosch, J. New cellular and molecular targets for the treatment of portal hypertension. Hepatol. Int. 2015, 9, 183–191. [Google Scholar] [CrossRef]
- Shah, V.; Haddad, F.G.; Garcia-Cardena, G.; Frangos, J.A.; Mennone, A.; Groszmann, R.J.; Sessa, W.C. Liver sinusoidal endothelial cells are responsible for nitric oxide modulation of resistance in the hepatic sinusoids. J. Clin. Investig. 1997, 100, 2923–2930. [Google Scholar] [CrossRef]
- Hammoutene, A.; Rautou, P.E. Role of liver sinusoidal endothelial cells in non-alcoholic fatty liver disease. J. Hepatol. 2019, 70, 1278–1291. [Google Scholar] [CrossRef]
- Miyao, M.; Kotani, H.; Ishida, T.; Kawai, C.; Manabe, S.; Abiru, H.; Tamaki, K. Pivotal role of liver sinusoidal endothelial cells in NAFLD/NASH progression. Lab. Investig. 2015, 95, 1130–1144. [Google Scholar] [CrossRef]
- Poisson, J.; Lemoinne, S.; Boulanger, C.; Durand, F.; Moreau, R.; Valla, D.; Rautou, P.E. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J. Hepatol. 2017, 66, 212–227. [Google Scholar] [CrossRef]
- Fernandez, M. Molecular pathophysiology of portal hypertension. Hepatology 2015, 61, 1406–1415. [Google Scholar] [CrossRef]
- Marrone, G.; Shah, V.H.; Gracia-Sancho, J. Sinusoidal communication in liver fibrosis and regeneration. J. Hepatol. 2016, 65, 608–617. [Google Scholar] [CrossRef]
- Friedman, S.L. Hepatic stellate cells: Protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 2008, 88, 125–172. [Google Scholar] [CrossRef]
- Iwakiri, Y.; Groszmann, R.J. Vascular endothelial dysfunction in cirrhosis. J. Hepatol. 2007, 46, 927–934. [Google Scholar] [CrossRef]
- Distrutti, E.; Mencarelli, A.; Santucci, L.; Renga, B.; Orlandi, S.; Donini, A.; Shah, V.; Fiorucci, S. The methionine connection: Homocysteine and hydrogen sulfide exert opposite effects on hepatic microcirculation in rats. Hepatology 2008, 47, 659–667. [Google Scholar] [CrossRef]
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef]
- Singh, S.B.; Lin, H.C. Hydrogen Sulfide in Physiology and Diseases of the Digestive Tract. Microorganisms 2015, 3, 866–889. [Google Scholar] [CrossRef]
- Yuan, S.; Zhang, H.M.; Li, J.X.; Li, Y.; Wang, Q.; Kong, G.Y.; Li, A.H.; Nan, J.X.; Chen, Y.Q.; Zhang, Q.G. Gasotransmitters in non-alcoholic fatty liver disease: Just the tip of the iceberg. Eur. J. Pharmacol. 2023, 954, 175834. [Google Scholar] [CrossRef]
- Lambooy, S.; Heida, A.; Joschko, C.; Nakladal, D.; van Buiten, A.; Kloosterhuis, N.; Huijkman, N.; Gerding, A.; van de Sluis, B.; Henning, R.; et al. Selective Hepatic Cbs Knockout Aggravates Liver Damage, Endothelial Dysfunction and ROS Stress in Mice Fed a Western Diet. Int. J. Mol. Sci. 2023, 24, 7019. [Google Scholar] [CrossRef]
- Konopelski, P.; Ufnal, M. Indoles—Gut Bacteria Metabolites of Tryptophan with Pharmacotherapeutic Potential. Curr. Drug Metab. 2018, 19, 883–890. [Google Scholar] [CrossRef]
- Ijaz, S.; Yang, W.; Winslet, M.C.; Seifalian, A.M. Impairment of hepatic microcirculation in fatty liver. Microcirculation 2003, 10, 447–456. [Google Scholar] [CrossRef]
- Parker, K.J.; Ormachea, J.; Drage, M.G.; Kim, H.; Hah, Z. The biomechanics of simple steatosis and steatohepatitis. Phys. Med. Biol. 2018, 63, 105013. [Google Scholar] [CrossRef]
- Ogawa, S.; Moriyasu, F.; Yoshida, K.; Oshiro, H.; Kojima, M.; Sano, T.; Furuichi, Y.; Kobayashi, Y.; Nakamura, I.; Sugimoto, K. Relationship between liver tissue stiffness and histopathological findings analyzed by shear wave elastography and compression testing in rats with non-alcoholic steatohepatitis. J. Med. Ultrason. 2016, 43, 355–360. [Google Scholar] [CrossRef]
- Pandey, E.; Nour, A.S.; Harris, E.N. Prominent Receptors of Liver Sinusoidal Endothelial Cells in Liver Homeostasis and Disease. Front. Physiol. 2020, 11, 873. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Mehta, G.; Gustot, T.; Mookerjee, R.P.; Garcia-Pagan, J.C.; Fallon, M.B.; Shah, V.H.; Moreau, R.; Jalan, R. Inflammation and portal hypertension—The undiscovered country. J. Hepatol. 2014, 61, 155–163. [Google Scholar] [CrossRef]
- Miyake, Y.; Yamamoto, K. Role of gut microbiota in liver diseases. Hepatol. Res. 2013, 43, 139–146. [Google Scholar] [CrossRef]
- Xu, T.; Du, Y.; Fang, X.B.; Chen, H.; Zhou, D.D.; Wang, Y.; Zhang, L. New insights into Nod-like receptors (NLRs) in liver diseases. Int. J. Physiol. Pathophysiol. Pharmacol. 2018, 10, 1–16. [Google Scholar]
- Orabi, D.; Osborn, L.J.; Fung, K.; Massey, W.; Horak, A.J., 3rd; Aucejo, F.; Choucair, I.; DeLucia, B.; Wang, Z.; Claesen, J.; et al. A surgical method for continuous intraportal infusion of gut microbial metabolites in mice. JCI Insight 2021, 6, e145607. [Google Scholar] [CrossRef]
- Osborn, L.J.; Orabi, D.; Goudzari, M.; Sangwan, N.; Banerjee, R.; Brown, A.L.; Kadam, A.; Gromovsky, A.D.; Linga, P.; Cresci, G.A.M.; et al. A Single Human-Relevant Fast Food Meal Rapidly Reorganizes Metabolomic and Transcriptomic Signatures in a Gut Microbiota-Dependent Manner. Immunometabolism 2021, 3, e210029. [Google Scholar] [CrossRef]
- Schierwagen, R.; Alvarez-Silva, C.; Madsen, M.S.A.; Kolbe, C.C.; Meyer, C.; Thomas, D.; Uschner, F.E.; Magdaleno, F.; Jansen, C.; Pohlmann, A.; et al. Circulating microbiome in blood of different circulatory compartments. Gut 2019, 68, 578–580. [Google Scholar] [CrossRef]
- Stefater, M.A.; Pacheco, J.A.; Bullock, K.; Pierce, K.; Deik, A.; Liu, E.; Clish, C.; Stylopoulos, N. Portal Venous Metabolite Profiling After RYGB in Male Rats Highlights Changes in Gut-Liver Axis. J. Endocr. Soc. 2020, 4, bvaa003. [Google Scholar] [CrossRef]
- Tietz-Bogert, P.S.; Kim, M.; Cheung, A.; Tabibian, J.H.; Heimbach, J.K.; Rosen, C.B.; Nandakumar, M.; Lazaridis, K.N.; LaRusso, N.F.; Sung, J.; et al. Metabolomic Profiling of Portal Blood and Bile Reveals Metabolic Signatures of Primary Sclerosing Cholangitis. Int. J. Mol. Sci. 2018, 19, 3188. [Google Scholar] [CrossRef]
- Meijnikman, A.S.; Davids, M.; Herrema, H.; Aydin, O.; Tremaroli, V.; Rios-Morales, M.; Levels, H.; Bruin, S.; de Brauw, M.; Verheij, J.; et al. Microbiome-derived ethanol in nonalcoholic fatty liver disease. Nat. Med. 2022, 28, 2100–2106. [Google Scholar] [CrossRef]
- Laleman, W.; Vanderschueren, E.; Mehdi, Z.S.; Wiest, R.; Cardenas, A.; Trebicka, J. Endoscopic procedures in hepatology: Current trends and new developments. J. Hepatol. 2024, 80, 124–139. [Google Scholar] [CrossRef]
- Hajifathalian, K.; Westerveld, D.; Kaplan, A.; Dawod, E.; Herr, A.; Ianelli, M.; Saggese, A.; Kumar, S.; Fortune, B.E.; Sharaiha, R.Z. Simultaneous EUS-guided portosystemic pressure measurement and liver biopsy sampling correlate with clinically meaningful outcomes. Gastrointest. Endosc. 2022, 95, 703–710. [Google Scholar] [CrossRef]
- Ryou, M.; Stylopoulos, N. Endoscopic ultrasound-guided sampling and profiling of portal circulation in human patients for metabolic research studies and biomarker assessment. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G584–G588. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baffy, G.; Portincasa, P. Gut Microbiota and Sinusoidal Vasoregulation in MASLD: A Portal Perspective. Metabolites 2024, 14, 324. https://doi.org/10.3390/metabo14060324
Baffy G, Portincasa P. Gut Microbiota and Sinusoidal Vasoregulation in MASLD: A Portal Perspective. Metabolites. 2024; 14(6):324. https://doi.org/10.3390/metabo14060324
Chicago/Turabian StyleBaffy, Gyorgy, and Piero Portincasa. 2024. "Gut Microbiota and Sinusoidal Vasoregulation in MASLD: A Portal Perspective" Metabolites 14, no. 6: 324. https://doi.org/10.3390/metabo14060324
APA StyleBaffy, G., & Portincasa, P. (2024). Gut Microbiota and Sinusoidal Vasoregulation in MASLD: A Portal Perspective. Metabolites, 14(6), 324. https://doi.org/10.3390/metabo14060324






