Prognostic Impact of Metabolic Syndrome and Steatotic Liver Disease in Hepatocellular Carcinoma Using Machine Learning Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Study Data
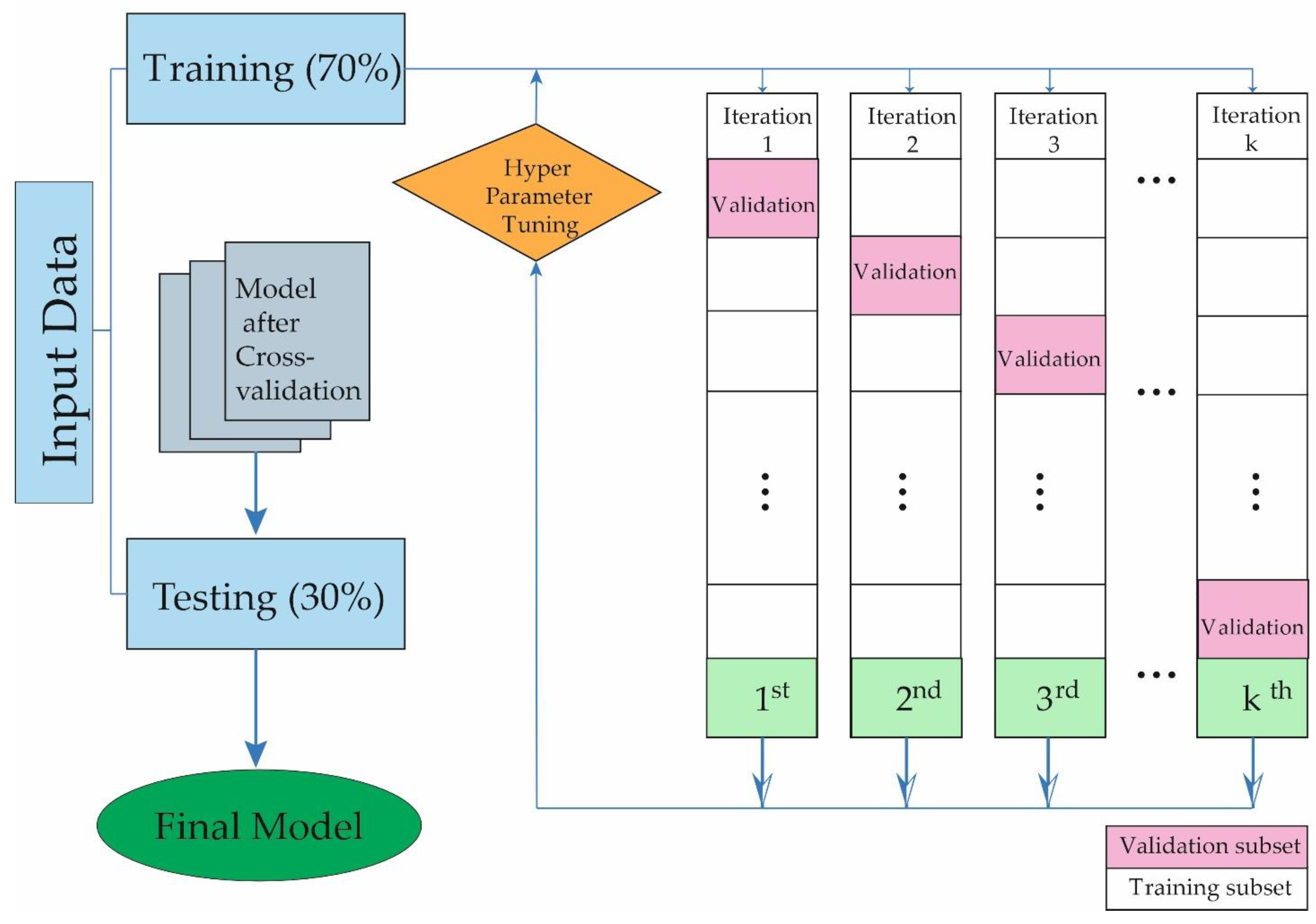
2.3. Development Model
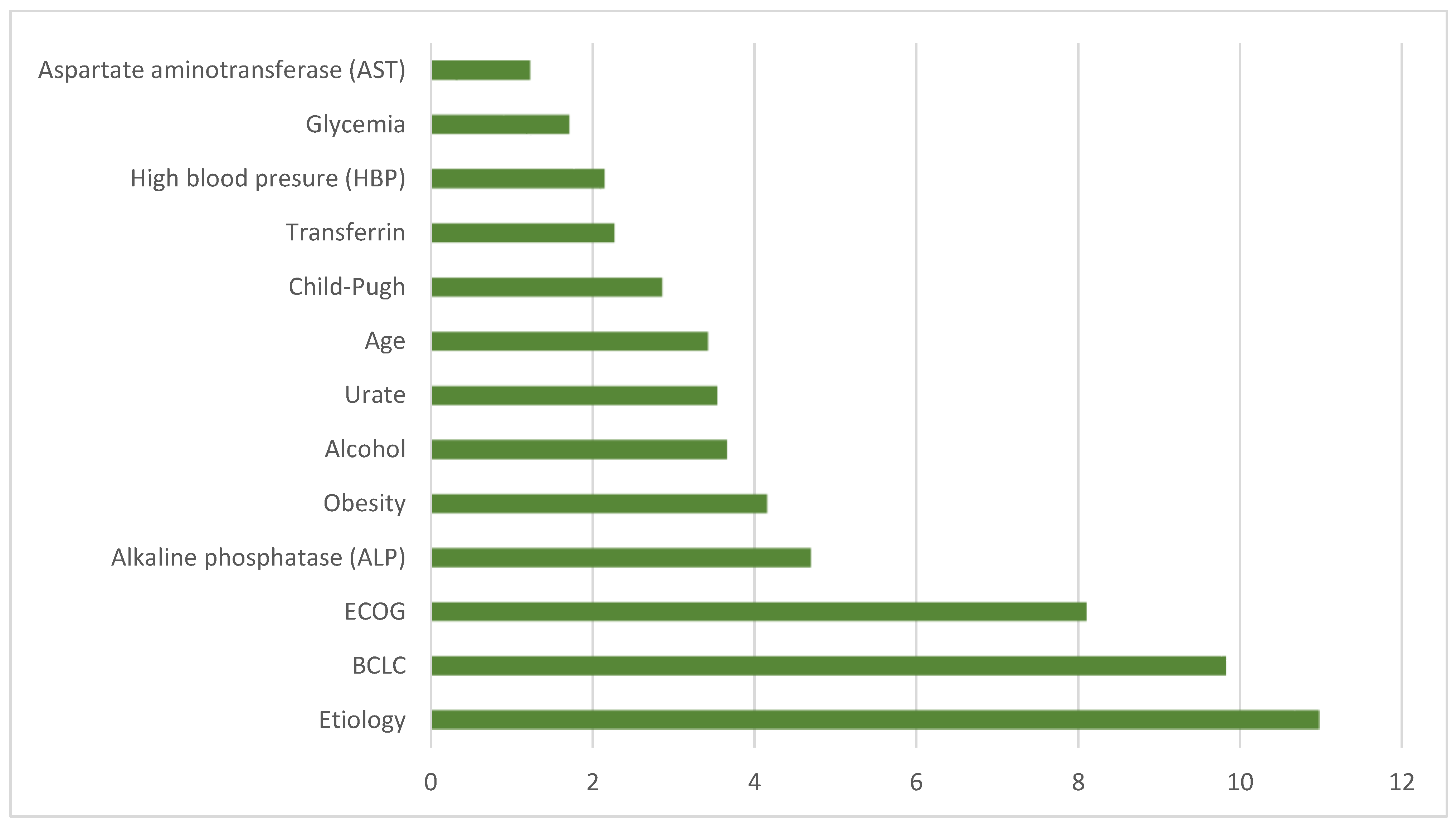
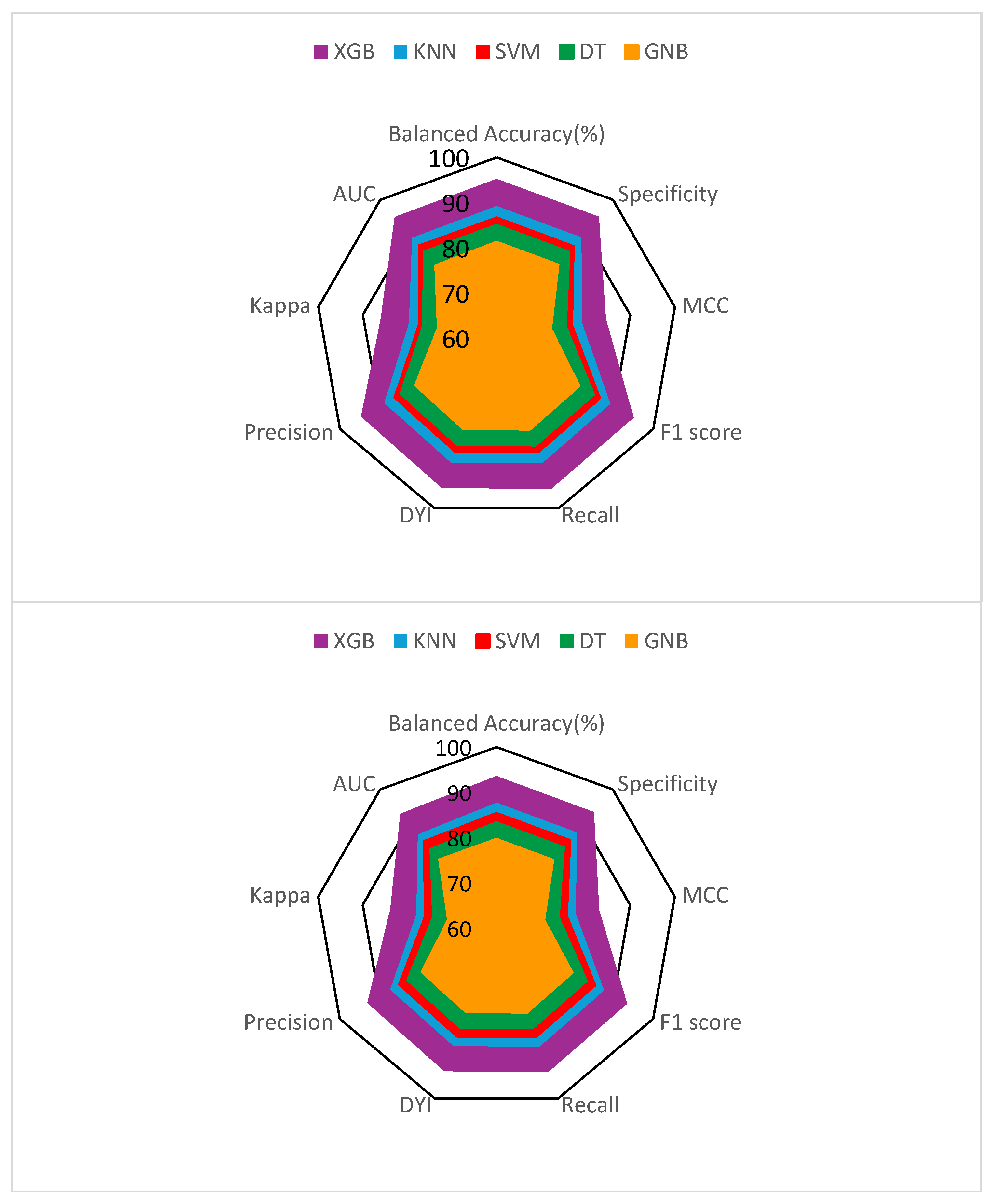
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhat, S.A.; Farooq, Z.; Ismail, H.; Corona-Avila, I.; Khan, W. Unraveling the Sweet Secrets of HCC: Glucometabolic Rewiring in Hepatocellular Carcinoma. Technol. Cancer Res. Treat. 2023, 22, 15330338231219434. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, X.; Liang, W.; Zhang, Z.; Zhang, S.; Xu, L.; Zhang, H.; Feng, Z.; Song, M.; Zhang, J.; et al. Deep learning-based accurate diagnosis and quantitative evaluation of microvascular invasion in hepatocellular carcinoma on whole-slide histopathology images. Cancer Med. 2024, 13, e7104. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Islami, F.; Ward, E.M.; Sung, H.; A Cronin, K.; Tangka, F.K.L.; Sherman, R.L.; Zhao, J.; Anderson, R.N.; Henley, S.J.; Yabroff, K.R.; et al. Annual Report to the Nation on the Status of Cancer, Part 1: National Cancer Statistics. JNCI J. Natl. Cancer Inst. 2021, 113, 1648–1669. [Google Scholar] [CrossRef] [PubMed]
- Phoolchund, A.G.S.; Khakoo, S.I. MASLD and the Development of HCC: Pathogenesis and Therapeutic Challenges. Cancers 2024, 16, 259. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Yang, F.; Zhu, X.; Qiao, G.; Zhang, C.; Tao, J.; Gao, X.; Xiao, M. Association between pro-inflammatory diet and liver cancer risk: A systematic review and meta-analysis. Public Health Nutr. 2023, 26, 2780–2789. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Ávila, M.A.; Ayuso, C.; Mínguez, B.; Varela, M.; Bilbao, I.; Bilbao, J.I.; Burrel, M.; Bustamante, J.; et al. Diagnosis and treatment of hepatocellular carcinoma. Update of the consensus document of the AEEH, AEC, SEOM, SERAM, SERVEI, and SETH. Med. Clínica 2021, 156, 463.e1–463.e30. [Google Scholar] [CrossRef]
- Batt, N.; Rodrigues, B.; Bloom, S.; Sawhney, R.; George, E.; Hodge, A.; Vootukuru, N.; McCrae, C.; Sood, S.; Roberts, S.; et al. Metabolic-associated fatty liver disease and hepatocellular carcinoma: A prospective study of characteristics and response to therapy. J. Gastroenterol. Hepatol. 2024. [Google Scholar] [CrossRef]
- Moon, J.H.; Jeong, S.; Jang, H.; Koo, B.K.; Kim, W. Metabolic dysfunction-associated steatotic liver disease increases the risk of incident cardiovascular disease: A nationwide cohort study. EClinicalMedicine 2023, 65, 102292. [Google Scholar] [CrossRef]
- Gao, F.; Chen, G.; Byrne, C.D.; Targher, G.; Cheung, T.T.; Zheng, M.-H. Metabolic dysfunction-associated fatty liver disease and hepatocellular carcinoma: Present and future. HepatoBiliary Surg. Nutr. 2023, 12, 945–948. [Google Scholar] [CrossRef]
- Candita, G.; Rossi, S.; Cwiklinska, K.; Fanni, S.C.; Cioni, D.; Lencioni, R.; Neri, E. Imaging Diagnosis of Hepatocellular Carcinoma: A State-of-the-Art Review. Diagnostics 2023, 13, 625. [Google Scholar] [CrossRef]
- Elderkin, J.; Al Hallak, N.; Azmi, A.S.; Aoun, H.; Critchfield, J.; Tobon, M.; Beal, E.W. Hepatocellular Carcinoma: Surveillance, Diagnosis, Evaluation and Management. Cancers 2023, 15, 5118. [Google Scholar] [CrossRef] [PubMed]
- Sayiner, M.; Golabi, P.; Younossi, Z.M. Disease Burden of Hepatocellular Carcinoma: A Global Perspective. Dig. Dis. Sci. 2019, 64, 910–917. [Google Scholar] [CrossRef]
- Caines, A.; Selim, R.; Salgia, R. The Changing Global Epidemiology of Hepatocellular Carcinoma. Clin. Liver Dis. 2020, 24, 535–547. [Google Scholar] [CrossRef]
- Strazzabosco, M.; Cabibbo, G.; Colombo, M. Adjusting Barcelona Clinic Liver Cancer Staging System to the Evolving Landscape of Hepatocellular Carcinoma: A Look to the Future. Gastroenterology 2022, 162, 2106–2108. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Zhang, E.; Narasimman, M.; Rich, N.E.; Waljee, A.K.; Hoshida, Y.; Yang, J.D.; Reig, M.; Cabibbo, G.; Nahon, P.; et al. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: A meta-analysis. J. Hepatol. 2022, 77, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Kinsey, E.; Lee, H.M. Management of Hepatocellular Carcinoma in 2024: The Multidisciplinary Paradigm in an Evolving Treatment Landscape. Cancers 2024, 16, 666. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Gil-Rojas, S.; Suárez, M.; Martínez-Blanco, P.; Torres, A.M.; Martínez-García, N.; Blasco, P.; Torralba, M.; Mateo, J. Application of Machine Learning Techniques to Assess Alpha-Fetoprotein at Diagnosis of Hepatocellular Carcinoma. Int. J. Mol. Sci. 2024, 25, 1996. [Google Scholar] [CrossRef]
- Chen, V.L.; Xu, D.; Wicha, M.S.; Lok, A.S.; Parikh, N.D. Utility of Liquid Biopsy Analysis in Detection of Hepatocellular Carcinoma, Determination of Prognosis, and Disease Monitoring: A Systematic Review. Clin. Gastroenterol. Hepatol. 2020, 18, 2879–2902.e9. [Google Scholar] [CrossRef]
- Wang, Y.; Fleishman, J.S.; Li, T.; Li, Y.; Ren, Z.; Chen, J.; Ding, M. Pharmacological therapy of metabolic dysfunction-associated steatotic liver disease-driven hepatocellular carcinoma. Front. Pharmacol. 2024, 14, 1336216. [Google Scholar] [CrossRef] [PubMed]
- Rajula, H.S.R.; Verlato, G.; Manchia, M.; Antonucci, N.; Fanos, V. Comparison of Conventional Statistical Methods with Machine Learning in Medicine: Diagnosis, Drug Development, and Treatment. Medicina 2020, 56, 455. [Google Scholar] [CrossRef] [PubMed]
- Ngiam, K.Y.; Khor, I.W. Big data and machine learning algorithms for health-care delivery. Lancet Oncol. 2019, 20, e262–e273. [Google Scholar] [CrossRef] [PubMed]
- Suárez, M.; Martínez, R.; Torres, A.M.; Torres, B.; Mateo, J. A Machine Learning Method to Identify the Risk Factors for Liver Fibrosis Progression in Nonalcoholic Steatohepatitis. Dig. Dis. Sci. 2023, 68, 3801–3809. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Wang, Z.; Liu, F.-Y.; Cheng, Z.-G.; Yu, X.; Han, Z.; Zhong, H.; Yu, J.; Liang, P. A Hybrid Machine Learning Model Based on Semantic Information Can Optimize Treatment Decision for Naïve Single 3–5-cm HCC Patients. Liver Cancer 2022, 11, 256–267. [Google Scholar] [CrossRef]
- Chan, W.-K.; Chuah, K.-H.; Rajaram, R.B.; Lim, L.-L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J. Obes. Metab. Syndr. 2023, 32, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Vallejo, L.; Guatibonza-García, V.; Mantzoros, C.S. Recent guidelines for Non-Alcoholic Fatty Liver disease (NAFLD)/Fatty Liver Disease (FLD): Are they already outdated and in need of supplementation? Metabolism 2022, 136, 155248. [Google Scholar] [CrossRef]
- Schutte, A.E.; Kollias, A.; Stergiou, G.S. Blood pressure and its variability: Classic and novel measurement techniques. Nat. Rev. Cardiol. 2022, 19, 643–654. [Google Scholar] [CrossRef]
- Khanna, D.; Peltzer, C.; Kahar, P.; Parmar, M.S. Body Mass Index (BMI): A Screening Tool Analysis. Cureus 2022, 14, e22119. [Google Scholar] [CrossRef]
- Tapper, E.B.; Parikh, N.D. Diagnosis and Management of Cirrhosis and Its Complications A Review. JAMA—J. Am. Med. Assoc. 2023, 329, 1589–1602. [Google Scholar] [CrossRef]
- Bochnakova, T. Hepatic Venous Pressure Gradient. Clin. Liver Dis. 2021, 17, 144. [Google Scholar] [CrossRef] [PubMed]
- Wood, W.A.; Deal, A.M.; Stover, A.M.; Basch, E. Comparing Clinician-Assessed and Patient-Reported Performance Status for Predicting Morbidity and Mortality in Patients with Advanced Cancer Receiving Chemotherapy. JCO Oncol. Pract. 2021, 17, e111–e118. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; She, X.; Liu, Z.; Gao, X.; Lu, L.; Huang, J.; Lu, C.; Lin, Y.; Liang, R.; Ye, J. Advances in post-operative prognostic models for hepatocellular carcinoma. J. Zhejiang Univ. B 2023, 24, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhong, J.; Sun, C.; Zhang, J. Effects of aerobic exercise on TC, HDL-C, LDL-C and TG in patients with hyperlipidemia. Medicine 2021, 100, e25103. [Google Scholar] [CrossRef] [PubMed]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive Assessment of Liver Disease in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1264–1281.e4. [Google Scholar] [CrossRef] [PubMed]
- Ergul Aydin, Z.; Kamisli Ozturk, Z. Performance Analysis of XGBoost Classifier with Missing Data. Manch. J. Artif. Intell. Appl. Sci. 2021, 2, 166–170. [Google Scholar]
- Ma, B.; Meng, F.; Yan, G.; Yan, H.; Chai, B.; Song, F. Diagnostic classification of cancers using extreme gradient boosting algorithm and multi-omics data. Comput. Biol. Med. 2020, 121, 103761. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Liu, Y.; Hou, C.; Fan, J.; Zheng, B.; Yin, J. Expediting the Accuracy-Improving Process of SVMs for Class Imbalance Learning. IEEE Trans. Knowl. Data Eng. 2021, 33, 3550–3567. [Google Scholar] [CrossRef]
- Kaul, S.; Fayaz, S.A.; Zaman, M.; Butt, M.A. Is Decision Tree Obsolete in Its Original Form? A Burning Debate. Rev. D’intelligence Artif. 2022, 36, 105–113. [Google Scholar] [CrossRef]
- Di Stefano, M.; Galati, S.; Ortore, G.; Caligiuri, I.; Rizzolio, F.; Ceni, C.; Bertini, S.; Bononi, G.; Granchi, C.; Macchia, M.; et al. Machine Learning-Based Virtual Screening for the Identification of Cdk5 Inhibitors. Int. J. Mol. Sci. 2022, 23, 10653. [Google Scholar] [CrossRef]
- Gou, J.; Sun, L.; Du, L.; Ma, H.; Xiong, T.; Ou, W.; Zhan, Y. A representation coefficient-based k-nearest centroid neighbor classifier. Expert Syst. Appl. 2022, 194, 116529. [Google Scholar] [CrossRef]
- Stefanowitsch, A. Corpus Linguistics: A Guide to the Methodology; Language Science Press: Berlin, Germany, 2020. [Google Scholar] [CrossRef]
- Luengo, J.; García-Gil, D.; Ramírez-Gallego, S.; García, S.; Herrera, F. Big Data Preprocessing: Enabling Smart Data; Springer International Publishing: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-A.; Moon, J.H.; Kim, W. Critical appraisal of metabolic dysfunction-associated steatotic liver disease: Implication of Janus-faced modernity. Clin. Mol. Hepatol. 2023, 29, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Wong, V.W. Implications of the new nomenclature of steatotic liver disease and definition of metabolic dysfunction-associated steatotic liver disease. Aliment. Pharmacol. Ther. 2023, 59, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.M.; Pose, E.; Reddy, K.R.; Russo, M.W.; Kamath, P.S. Nonalcoholic Fatty Liver Disease Gets Renamed as Metabolic Dysfunction–Associated Steatotic Liver Disease: Progress But with Challenges. Gastroenterology 2023, 166, 229–234. [Google Scholar] [CrossRef]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef] [PubMed]
- Nasr, P.; Wester, A.; Ekstedt, M.; Strandberg, R.; Kechagias, S.; Shang, Y.; Widman, L.; Hagström, H. Misclassified Alcohol-related Liver Disease is Common in Presumed Metabolic Dysfunction-associated Steatotic Liver Disease and Highly Increases Risk for Future Cirrhosis. Clin. Gastroenterol. Hepatol. 2024, 22, 1048–1057.e2. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.; Peng, X.; Li, X.; An, K.; He, H.; Fu, X.; Li, S.; An, Z. Unmasking the enigma of lipid metabolism in metabolic dysfunction-associated steatotic liver disease: From mechanism to the clinic. Front. Med. 2023, 10, 1294267. [Google Scholar] [CrossRef]
- Syed-Abdul, M.M. Lipid Metabolism in Metabolic-Associated Steatotic Liver Disease (MASLD). Metabolites 2024, 14, 12. [Google Scholar] [CrossRef]
- Gensluckner, S.; Wernly, B.; Datz, C.; Aigner, E. Iron, Oxidative Stress, and Metabolic Dysfunction—Associated Steatotic Liver Disease. Antioxidants 2024, 13, 208. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tsung, A.; Mishra, L.; Huang, H. Regulatory T cell: A double-edged sword from metabolic-dysfunction-associated steatohepatitis to hepatocellular carcinoma. EBioMedicine 2024, 101, 105031. [Google Scholar] [CrossRef] [PubMed]
- Schilcher, K.; Dayoub, R.; Kubitza, M.; Riepl, J.; Klein, K.; Buechler, C.; Melter, M.; Weiss, T.S. Saturated Fat-Mediated Upregulation of IL-32 and CCL20 in Hepatocytes Contributes to Higher Expression of These Fibrosis-Driving Molecules in MASLD. Int. J. Mol. Sci. 2023, 24, 13222. [Google Scholar] [CrossRef] [PubMed]
- Ghandian, S.; Thapa, R.; Garikipati, A.; Barnes, G.; Green-Saxena, A.; Calvert, J.; Mao, Q.; Das, R. Machine learning to predict progression of non-alcoholic fatty liver to non-alcoholic steatohepatitis or fibrosis. JGH Open 2022, 6, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Etienne, Q.; Lebrun, V.; Komuta, M.; Navez, B.; Thissen, J.-P.; Leclercq, I.A.; Lanthier, N. Fetuin-A in Activated Liver Macrophages Is a Key Feature of Non-Alcoholic Steatohepatitis. Metabolites 2022, 12, 625. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Saha, P.; Bose, D.; Trivedi, A.; More, M.; Xiao, S.; Diehl, A.M.; Chatterjee, S. Hepatic NLRP3-Derived Hsp70 Binding to TLR4 Mediates MASLD to MASH Progression upon Inhibition of PP2A by Harmful Algal Bloom Toxin Microcystin, a Second Hit. Int. J. Mol. Sci. 2023, 24, 16354. [Google Scholar] [CrossRef] [PubMed]
- Syamprasad, N.; Jain, S.; Rajdev, B.; Panda, S.R.; Kumar, G.J.; Shaik, K.M.; Shantanu, P.; Challa, V.S.; Jorvekar, S.B.; Borkar, R.M.; et al. AKR1B1 drives hyperglycemia-induced metabolic reprogramming in MASLD-associated hepatocellular carcinoma. JHEP Rep. 2024, 6, 100974. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, S.; Kobori, T.; Yoneda, M.; Ogawa, Y.; Honda, Y.; Kessoku, T.; Imajo, K.; Saito, S.; Nakajima, A.; Hotta, K. Identification of differentially methylated regions associated with both liver fibrosis and hepatocellular carcinoma. BMC Gastroenterol. 2024, 24, 57. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Dungubat, E.; Kusano, H.; Fukusato, T. Pathology and Pathogenesis of Metabolic Dysfunction-Associated Steatotic Liver Disease-Associated Hepatic Tumors. Biomedicines 2023, 11, 2761. [Google Scholar] [CrossRef]
- Kakehashi, A.; Suzuki, S.; Wanibuchi, H. Recent Insights into the Biomarkers, Molecular Targets and Mechanisms of Non-Alcoholic Steatohepatitis-Driven Hepatocarcinogenesis. Cancers 2023, 15, 4566. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Niu, K.; Zhang, W.; Lv, Q.; Zhang, Y. How CLSPN could demystify its prognostic value and potential molecular mechanism for hepatocellular carcinoma: A crosstalk study. Comput. Biol. Med. 2024, 172, 108260. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.H.; Lim, W.H.; Lim, G.E.H.; Tan, D.J.H.; Syn, N.; Muthiah, M.D.; Huang, D.Q.; Loomba, R. Mortality Outcomes by Fibrosis Stage in Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2023, 21, 931–939.e5. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn, D.J.; Holleboom, A.G.; Takkenberg, R.B.; Verheij, J.; Lantinga, M.A. Can liver stiffness measurement accurately predict disease progression and clinical outcome in patients with metabolic dysfunction-associated steatotic liver disease and bridging fibrosis or cirrhosis? HepatoBiliary Surg. Nutr. 2023, 12, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Pelusi, S.; Ronzoni, L.; Rondena, J.; Rosso, C.; Pennisi, G.; Dongiovanni, P.; Margarita, S.; Carpani, R.; Soardo, G.; Prati, D.; et al. Prevalence and determinants of liver disease in relatives of italian patients with advanced MASLD. Clin. Gastroenterol. Hepatol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Mosca, A.; Manco, M.; Braghini, M.R.; Cianfarani, S.; Maggiore, G.; Alisi, A.; Vania, A. Environment, Endocrine Disruptors, and Fatty Liver Disease Associated with Metabolic Dysfunction (MASLD). Metabolites 2024, 14, 71. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Murugesan, S.; Singh, S.; Uy, K.N.; Kaur, J.; Mann, N.; Sekhon, R.K. The Influence of Coffee on Reducing Metabolic Dysfunction-Associated Steatotic Liver Disease in Patients with Type 2 Diabetes: A Review. Cureus 2023, 15, e50118. [Google Scholar] [CrossRef]
- Suárez, M.; Gil-Rojas, S.; Martínez-Blanco, P.; Torres, A.M.; Ramón, A.; Blasco-Segura, P.; Torralba, M.; Mateo, J. Machine Learning-Based Assessment of Survival and Risk Factors in Non-Alcoholic Fatty Liver Disease-Related Hepatocellular Carcinoma for Optimized Patient Management. Cancers 2024, 16, 1114. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L. Hepatic Fibrosis and Cancer: The Silent Threats of Metabolic Syndrome. Diabetes Metab. J. 2024, 48, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Chicco, D.; Oneto, L. Computational intelligence identifies alkaline phosphatase (ALP), alpha-fetoprotein (AFP), and hemoglobin levels as most predictive survival factors for hepatocellular carcinoma. Health Inform. J. 2021, 27, 1460458220984205. [Google Scholar] [CrossRef]
- Rathmell, J.C. Obesity, Immunity, and Cancer. New Engl. J. Med. 2021, 384, 1160–1162. [Google Scholar] [CrossRef]
- Nagai, K.; Nagai, K.; Iwaki, M.; Kobayashi, T.; Nogami, A.; Oka, M.; Saito, S.; Yoneda, M. Frontiers of Collaboration between Primary Care and Specialists in the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease: A Review. Life 2023, 13, 2144. [Google Scholar] [CrossRef] [PubMed]
- Anderson, O.B.; Berdzuli, N.; Ilbawi, A.; Kestel, D.; Kluge, H.P.; Krech, R.; Mikkelsen, B.; Neufeld, M.; Poznyak, V.; Rekve, D.; et al. Health and cancer risks associated with low levels of alcohol consumption. Lancet Public Health 2023, 8, e6–e7. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cheng, Y.; Mao, C.; Liu, S.; Xiao, D.; Huang, J.; Tao, Y. Emerging mechanisms and targeted therapy of ferroptosis in cancer. Mol. Ther. 2021, 29, 2185–2208. [Google Scholar] [CrossRef] [PubMed]
- Åberg, F.; Danford, C.J.; Thiele, M.; Talbäck, M.; Rasmussen, D.N.; Jiang, Z.G.; Hammar, N.; Nasr, P.; Ekstedt, M.; But, A.; et al. A Dynamic Aspartate-to-Alanine Aminotransferase Ratio Provides Valid Predictions of Incident Severe Liver Disease. Hepatol. Commun. 2021, 5, 1021–1035. [Google Scholar] [CrossRef]
- Lee, K.H.; Choi, G.H.; Yun, J.; Choi, J.; Goh, M.J.; Sinn, D.H.; Jin, Y.J.; Kim, M.A.; Yu, S.J.; Jang, S.; et al. Machine learning-based clinical decision support system for treatment recommendation and overall survival prediction of hepatocellular carcinoma: A multi-center study. NPJ Digit. Med. 2024, 7, 2. [Google Scholar] [CrossRef]

| Methods | Accuracy | Specificity | Recall | Precision | AUC |
|---|---|---|---|---|---|
| XGB | 93.59 | 93.48 | 93.70 | 92.93 | 0.93 |
| SVM | 85.42 | 85.32 | 85.52 | 84.81 | 0.85 |
| DT | 83.60 | 83.50 | 83.70 | 83.00 | 0.83 |
| GNB | 79.93 | 79.84 | 80.02 | 79.36 | 0.80 |
| KNN | 87.69 | 87.59 | 87.80 | 87.07 | 0.87 |
| Methods | DYI | MCC | F1 Score | Kappa |
|---|---|---|---|---|
| XGB | 93.52 | 83.05 | 93.31 | 83.85 |
| SVM | 85.41 | 75.79 | 85.16 | 75.94 |
| DT | 83.60 | 74.18 | 83.35 | 74.43 |
| GNB | 79.93 | 70.92 | 79.69 | 71.06 |
| KNN | 87.69 | 77.81 | 87.43 | 77.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Rojas, S.; Suárez, M.; Martínez-Blanco, P.; Torres, A.M.; Martínez-García, N.; Blasco, P.; Torralba, M.; Mateo, J. Prognostic Impact of Metabolic Syndrome and Steatotic Liver Disease in Hepatocellular Carcinoma Using Machine Learning Techniques. Metabolites 2024, 14, 305. https://doi.org/10.3390/metabo14060305
Gil-Rojas S, Suárez M, Martínez-Blanco P, Torres AM, Martínez-García N, Blasco P, Torralba M, Mateo J. Prognostic Impact of Metabolic Syndrome and Steatotic Liver Disease in Hepatocellular Carcinoma Using Machine Learning Techniques. Metabolites. 2024; 14(6):305. https://doi.org/10.3390/metabo14060305
Chicago/Turabian StyleGil-Rojas, Sergio, Miguel Suárez, Pablo Martínez-Blanco, Ana M. Torres, Natalia Martínez-García, Pilar Blasco, Miguel Torralba, and Jorge Mateo. 2024. "Prognostic Impact of Metabolic Syndrome and Steatotic Liver Disease in Hepatocellular Carcinoma Using Machine Learning Techniques" Metabolites 14, no. 6: 305. https://doi.org/10.3390/metabo14060305
APA StyleGil-Rojas, S., Suárez, M., Martínez-Blanco, P., Torres, A. M., Martínez-García, N., Blasco, P., Torralba, M., & Mateo, J. (2024). Prognostic Impact of Metabolic Syndrome and Steatotic Liver Disease in Hepatocellular Carcinoma Using Machine Learning Techniques. Metabolites, 14(6), 305. https://doi.org/10.3390/metabo14060305








