Abstract
There is a notable correlation between mitochondrial homeostasis and metabolic disruption. In this review, we report that obesity-induced disruption of mitochondrial homeostasis adversely affects lipid metabolism, adipocyte differentiation, oxidative capacity, inflammation, insulin sensitivity, and thermogenesis in thermogenic fat. Elevating mitochondrial homeostasis in thermogenic fat emerges as a promising avenue for developing treatments for metabolic diseases, including enhanced mitochondrial function, mitophagy, mitochondrial uncoupling, and mitochondrial biogenesis. The exerkines (e.g., myokines, adipokines, batokines) released during exercise have the potential to ameliorate mitochondrial homeostasis, improve glucose and lipid metabolism, and stimulate fat browning and thermogenesis as a defense against obesity-associated metabolic diseases. This comprehensive review focuses on the manifold benefits of exercise-induced exerkines, particularly emphasizing their influence on mitochondrial homeostasis and fat thermogenesis in the context of metabolic disorders associated with obesity.
1. Introduction
Obesity has evolved into a pervasive global epidemic, with its prevalence steadily increasing on a worldwide scale []. According to estimates from the World Health Organization (WHO), approximately 13% of adults globally grapple with obesity. Importantly, obesity serves as a precursor to metabolic syndrome, giving rise to a spectrum of complications, including but not limited to diabetes, hypertension, non-alcoholic fatty liver disease (NASH), cardiovascular disorders, neuropathic diseases, and cancer [,,,,]. In the COVID-19 pandemic, the increased mortality in patients with obesity is a noteworthy example. Hence, there is urgent need for effective treatment methods to prevent and mitigate obesity-associated metabolic disorders and their related complications.
Mitochondria play a pivotal role in preserving energy metabolism in adipose tissues. However, obesity leads to the pathological remodeling of mitochondrial morphology and dysfunction in adipocytes [,]. Dysfunction of mitochondria has adverse effects on glucose and lipid metabolism, oxidative capacity, insulin sensitivity, adipocyte differentiation, and thermogenesis in adipocytes, ultimately contributing to metabolic diseases [,,]. Enhancing mitochondrial function, achievable through various approaches such as mitochondria-targeted antioxidants, thiazolidinedione, dietary natural compounds, controlled caloric restriction, and regular exercise, plays a crucial role in maintaining metabolic homeostasis [,]. This contribution is evidenced by the promotion of thermogenesis in brown and beige adipocytes. As a secure, effective, and cost-efficient approach, regular exercise is widely embraced by individuals dealing with obesity and overweight [,]. Exerkines, induced by exercise, play pivotal roles in maintaining mitochondrial homeostasis, facilitating fat browning and thermogenesis as a defense against obesity-associated metabolic diseases [,]. In this review, our emphasis is on elucidating the advantages of regular exercise concerning fat thermogenesis and mitochondrial homeostasis in the context of metabolic diseases associated with obesity. We explore the role of exercise in stimulating the secretion of exerkines and its potential significance in preventing obesity-associated metabolic disorders.
2. Mitochondrial Homeostasis in Thermogenic Fat
Mitochondrial homeostasis encompasses the balance and regulation of various processes within mitochondria, including mitochondrial oxidative phosphorylation, mitochondrial dynamics, mitochondrial biogenesis, mitophagy, and mitochondrial uncoupling. This delicate equilibrium plays a pivotal role in sustaining energy metabolism, particularly in brown and beige adipocytes, distinguished by a heightened abundance of mitochondria. Disruption of mitochondrial homeostasis results in adverse effects on lipid metabolism, adipocyte differentiation, oxidative capacity, inflammation, insulin sensitivity, and thermogenesis, culminating in metabolic diseases [,,,]. Enhancing mitochondrial homeostasis in thermogenic fat emerges as a potential avenue for developing treatments for metabolic diseases (Figure 1).
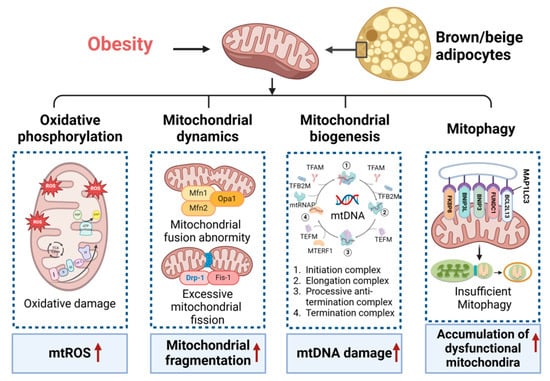
Figure 1.
Mitochondrial homeostasis in thermogenesis fat. Obesity results in the disruption of mitochondrial homeostasis in thermogenesis fat, affecting mitochondrial oxidative phosphorylation, dynamics, biogenesis, and mitophagy. This is evidenced by the increased mitochondrial reactive oxygen species (mtROS), mitochondrial fragmentation, mitochondrial DNA (mtDNA) damage, and the accumulation of dysfunctional mitochondria. Mfn1/2, mitofusin 1 and 2; Opa1, optic atrophy 1; Drp-1, dynamin-related protein 1; Fis-1, mitochondrial fission protein 1; TFAM, mitochondrial transcription factor A; TFB2M, mitochondrial transcription factors B2; mtRNAP, mitochondrial RNA polymerase; TEFM, transcription elongation factor of mitochondria; MTERF1, mitochondrial transcription termination factor 1; MAPP1LC3, microtubule-associated protein 1 light chain 3; BCL2L13, Bcl-2-like protein 13; FUNDC1, FUN14 domain-containing protein 1; BNIP3, BCL2 interacting protein 3; FKBP8, FK506 binding protein 8. Red arrows indicate an increase (up arrow).
2.1. Mitochondrial Function in Thermogenic Fat
Mitochondria play a crucial role in metabolism of adipose tissue, as evidenced by their involvement in crucial metabolic pathways, such as lipolysis and lipogenesis. These functions of mitochondrial are essential for supporting energy metabolism in thermogenic adipocytes. Dysfunction of mitochondrial function in brown and beige adipocytes is associated with disrupted thermogenesis and energy balance in obesity and aging. Given that the metabolism of thermogenic fat is primarily oxidative, the effective regulation of thermogenesis in these cells involved manipulating the rate-limiting steps in mitochondrial respiration and oxidative phosphorylation []. Furthermore, recent studies have revealed that mitochondria can be exchanged between cells, such as adipocytes and macrophages, to regulate metabolism, homeostasis, and thermogenesis in brown and beige adipocytes. This exchange is facilitated through the release of extracellular vesicles (EVs) carrying oxidatively damaged mitochondrial components, thereby preventing the breakdown of the thermogenic program [,,]. Additionally, mitochondria-derived EVs have been shown to decrease the expression of peroxisome proliferator-activated receptor-γ and uncoupling protein 1 (UCP1). Phospholipid cardiolipin (CL) and phosphatidic acid (PA) play pivotal roles in regulating mitochondrial morphology and mitochondrial function []. Lipocalin 2, a protein that binds to PA, assumes a crucial role in the remodeling of acyl-chain remodeling in phospholipids and in regulating mitochondrial function within thermogenic fat. This process is particularly significant during inflammation induced by obesity and metabolic stimulation resulting from cellular aging [].
A recent study has indicated that there is a sex-based difference in the impact on adipose mitochondrial function and the development of metabolic syndrome. In females, adipose mitochondrial function shows heightened activity, encompassing elevated mitochondrial oxidative phosphorylation, mitochondrial DNA content, and augmented production of mitochondrial reactive oxygen species. These factors are closely linked to adiposity, insulin resistance, and plasma lipid levels []. Several studies have underscored the pivotal role of mitochondrial metabolism in the pro-inflammatory activation of adipose tissue macrophages in response to obesity [,]. The application of a near-infrared fluorophore with a preferential accumulation in the mitochondria of adipose tissue macrophages has been shown to mitigate pro-inflammatory activation by enhancing the levels of mitochondrial complex and oxidative phosphorylation []. Additionally, the respiratory chains of mitochondria in interscapular brown adipose tissue depend on UCP1 [].
2.2. Mitochondrial Biogenesis in Obesity
Previous studies have suggested that obesity leads to a decline in mitochondrial biogenesis, a reduction in the expression of genes responsible for mitochondrial respiratory complex components, and a decrease in respiration/mitochondrial oxidative phosphorylation (OXPHOS) in adipose tissue [,]. Specifically, the cardiotrophin-like cytokine factor 1 (CLCF1) has been identified as a key player in inducing the whitening of brown adipose tissue and impeding thermogenesis. This effect is achieved by suppressing mitochondrial biogenesis through the activation of the STAT3/PGC1α signaling pathway in response to obesity []. It is important to observe that enhancing mitochondrial biogenesis is critical for addressing metabolic diseases associated with obesity.
A recent study has identified that the thyroid hormone triiodothyronine (T3) triggers thermogenesis by uncoupling electron transport from ATP synthesis in BAT mitochondria. T3 enhances fatty acid oxidation, autophagic flux, mitophagy, mitochondrial respiration, and mitochondrial biogenesis []. Additionally, Parkin plays a role in maintaining mitochondrial homeostasis in white adipocytes by orchestrating a balance between mitophagy and Pgc1alpha-mediated mitochondrial biogenesis. This suggests a promising therapeutic target within adipocytes to address obesity and obesity-associated disorders. CL, a phospholipid located in the inner membrane of mitochondria, exerts a major role in maintaining mitochondrial metabolism and structural integrity. It is essential for the well-being of various organs, including fat, liver, heart, skeletal muscle, brain, and kidney [,,,,]. Furthermore, CL acts as a pivotal regulator in the thermogenic programs, connecting with mitochondrial biogenesis and function in response to regular exercise []. Lifelong exercise has been identified as a beneficial factor, mitigating age-related changes in mitochondrial biogenesis, inflammation, and lipolysis in perirenal fat and liver tissues. This positive impact may involve the inhibition of inflammation through activating the c-Jun N-terminal kinase (JNK), p38 mitogen-activated protein kinase (MAPK), and AKT pathways in adipose tissue [].
2.3. Mitophagy in Thermogenic Fat
Autophagy is necessary for the efficient turnover of damaged organelles, including mitochondria (mitophagy). Mitophagy refers to a selective process in which damaged mitochondria are isolated and subsequently eliminated through autophagic degradation []. The adipose-specific deletion of autophagy-related 7 results in a large volume of cytosol and contained more mitochondria in mutant white adipocytes []. Furthermore, exposure to cold induces adaptive thermogenesis by enhancing the autophagy of lipids and mitophagy in brown and beige adipocytes [,]. Recent studies have revealed that T3 not only regulates mitochondrial homeostasis by inducing lipophagy and mitophagy in the liver and skeletal muscle [,], but also triggers thermogenesis. This involved inducing the expression of mitochondrial UCP1, promoting autophagy-dependent fatty acid oxidation, and regulating autophagy, activity, and turnover of mitochondria in BAT and aging skeletal muscle [,]. In contrast, BAT primarily relies on upregulated mitophagy and mitochondrial biogenesis to ensure mitochondrial quality control. Consequently, promoting autophagy to induce mitochondrial turnover in BAT could hold therapeutic potential for enhancing thermogenesis and addressing obesity and associated metabolic conditions.
Nevertheless, diminished mitophagy may also prove essential in the browning of white adipose, allowing for a substantial increase in mitochondrial mass during this remodeling process. Furthermore, even with the suppression of p62 and optineurin, rosiglitazone continued to promote UCP1 expression, endorsing the idea that a reduction in mitophagy machinery facilitates beige remodeling []. Deficiency in FUNDC1, a mediator of mitophagy, triggered a retrograde response in muscle, leading to the upregulation of fibroblast growth factor 21 (FGF21) expression. This, in turn, facilitated the thermogenic remodeling of adipose tissue in response to obesity []. The tumor suppressor p53 enhances insulin sensitivity in aged adipose tissue by triggering mitophagy [].
2.4. Mitochondrial Uncoupling in Obesity
Mitochondrial uncoupling is characterized by a dissociation between the generation of mitochondrial membrane potential and its utilization for ATP synthesis, a process vital for mitochondria-dependent energy production. Recent research has revealed that mitochondrial uncoupling extends beyond its association with mitochondrial dysfunction and is also implicated in various biological processes, including the production of ROS, autophagy, cell death, protein secretion, and metabolic adaptation in brown and beige adipocytes []. The subtle uncoupling of oxidative phosphorylation achieved through numerous mitochondria-targeted penetrating cations plays a role in the reported therapeutic benefits by inducing autophagy and mitophagy []. The initiation of mitochondrial uncoupling, whether through synthetic or natural uncoupling agents or by activating UCPs, sets in motion multiple cellular mechanisms [,].
Mitochondria uncoupling can serve a dual role, offering protection against cell death and apoptosis in certain instances while potentially promoting them, contingent upon factors such as cell type, the specific mitochondrial uncoupler employed, and the intensity of mitochondrial uncoupling [,]. The mitochondrial uncouplers like carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) or 2,4-dinitrophenol (DNP) may disturb the equilibrium of various, including Ca2+, Na+, and K+ at cytosolic, mitochondrial, or lysosomal levels [,,]. A recently identified mitochondrial uncoupler, BAM15, improves body fat mass, inflammation, and insulin resistance in obese mice []. The recognition of UCP1′s involvement is pivotal in understanding the thermogenic processes within brown and beige adipocytes []. More recently, thermogenic processes that are independent of UCP1 have been observed in thermogenic fat [,].
3. The Impact of Exercise on Thermogenic Fat
3.1. Exercise-Induced Browning of White Adipocytes
Consistent exercise training (e.g., 11 days of wheel cage running, swim training performed for 90 min daily, 5 days/week) substantially reduces adipocyte size, enhances mitochondrial biogenesis and glucose uptake, stimulates adipokine secretion, and improves overall metabolic health in WAT [,]. Four-week wheel-running exercise training dramatically curtails body weight gain, promotes energy expenditure, and increases UCP1-dependent thermogenesis []. Prolonged treadmill-running exercise training (3 m/min for 5 min, increased to 4.8–5 m/min for 5 min, and then reaching a maximum of 7.2–8 m/min for 20 min; 0% slope) induces adaptability in white adipose depots, as demonstrated by increasing free fatty acid (FFA) oxidation, reduced inflammation, and diminished macrophage infiltration in aged obese female mice []. Prolonged exercise (e.g., treadmill with 10 m/min for the first 60 min, followed by 1 m/min increment increases at 15-min intervals or treadmill with a fixed 10% slope at a constant 18 m/min speed for 60 min daily for 5 d/wk (8 wk) before test) leads to mitochondrial biogenesis and beiging in WAT by regulating myokines, including IL-6, FGF21, apelin, meteorin-like protein (Metrnl), lactate, beta-aminoisobutyric acid (BAIBA), brain-derived neurotrophic factor (BDNF), musclin, myostatin, and irisin [,,,]. The activation of the Wnt/β-catenin signaling pathway and PGC-1α-related pathways drives the adipocyte population necessary for beiging, which is involved in mitochondrial biogenesis and function [] (Figure 2).
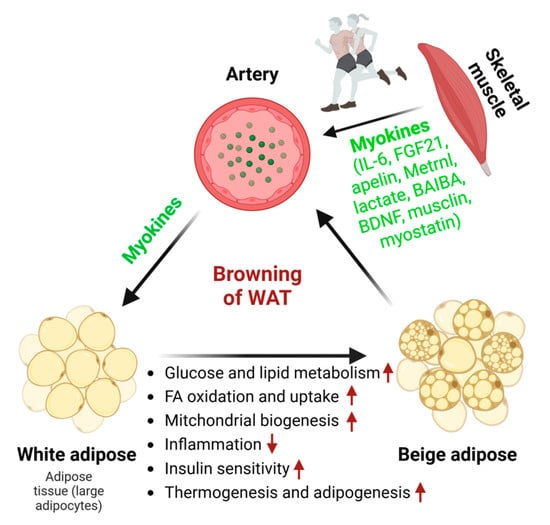
Figure 2.
The effect of myokines on white adipose tissue browning. Exercise training promotes the browning of WAT by triggering the secretion of various myokines from skeletal muscle. This is substantiated by reductions in adipocyte size, heightened lipid and glucose metabolism, increased FFA oxidation and uptake, enhanced mitochondrial biogenesis, improved insulin sensitivity, brown adipogenesis, and thermogenesis. Myokines are secreted from skeletal muscle in response to exercise. Red arrows indicate an increase (up arrow) or a decrease (down arrow).
3.2. Exercise Modulates Brown Adipose Tissue
The efficacy of BAT has been reported to be blunted by metabolic diseases [], cardiovascular diseases [], and aging [,,,]. While traditionally recognized as a thermogenic tissue, BAT communicates with distant organs, such as the heart, through its endocrine function. Remarkably, four weeks of swimming exercise (90 min twice per day) induce the release of the small extracellular vesicles (sEVs) from BAT, conferring cardioprotection by delivering the cardioprotective miRNAs to the heart during myocardial ischemia/reperfusion (MI/R) injury []. Meanwhile, the augmented exercise capacity induced by BAT is mediated through mitochondrial biogenesis, antioxidant defense, and enhanced hindlimb perfusion. Therefore, BAT serves as a mediator for heightened exercise capacity, a mechanism further potentiated by the disruption of the regulator of G protein signaling 14 (RGS14) []. Mitochondrial homeostasis in BAT plays a pivotal role in the thermoregulatory and metabolic processes. Voluntary physical exercise promotes thermogenesis, insulin sensitivity, mitochondrial activity, and biogenesis in BAT [,,]. CL is a key effector of brown/beige adipocytes’ thermogenic programs and is linked to mitochondrial biogenesis and function in response to regular exercise [].
3.3. Exercise-Induced UCP1-Dependent Thermogenesis
Situated in the inner membrane of mitochondria, UCP1 induces a proton leak across this membrane, facilitating the conversion of electrochemical energy into heat. Notably, mice lacking UCP1 exhibit impaired thermogenesis, underscoring the pivotal role of UCP1 in nonshivering heat production. Given that UCP1 is a key regulator in the thermogenesis of brown adipocytes and a subset of white adipocytes, various other functional thermogenic elements exert their impacts in a UCP1-dependent manner. Beige adipocytes expressing UCP1 can be activated through exposure to cold, administration of β-adrenergic agonists, or engagement in exercise training to stimulate thermogenesis [,,].
Exercise training is said to confer benefits, at least in part, by enhancing mitochondrial uncoupling-driven thermogenesis. The global stimulation of mitochondrial uncoupling by exercise training contributes to the restructuring of skeletal muscle cell physiology. Furthermore, acute physical exercise training results in an upregulation of BAT UCP1 protein expression in individuals with obesity []. The immediate impact of a single exercise session on thermogenesis can be elucidated through the increase in leptin-induced hypothalamic ERK1/2 phosphorylation. Indeed, a single exercise session elevates hypothalamic sphingine-1-phosphate (S1P) levels and STAT3 phosphorylation events that ultimately enhance UCP1-dependent BAT thermogenesis [,]. In WAT, the impact of exercise training appears to be contrary. Specifically, exercise training (17 m/min, 45 min/day, 5 days, 8 weeks) is observed to diminish the protein expression of UCP1 and PGC-1α in the subcutaneous WAT of mice subjected to a high-fat diet []. Additionally, exercise training triggers the release of myokines by skeletal muscle. Among these myokines, irisin holds particular significance. Irisin’s primary and extensively studied role is to instigate the browning of WAT, consequently promoting UCP-1-dependent mitochondrial uncoupling [].
3.4. Exercise-Induced UCP1-Independent Thermogenesis
UCP1 has significantly advanced our comprehension of how these cells participate in thermogenesis []. However, cold-acclimated Ucp1 knockout mice still display tolerance to cold exposure, suggesting the presence of compensatory thermogenic mechanisms []. Subsequently, thermogenic processes independent of UCP1 have been elucidated, both within thermogenic fat and in other tissues. A recent study has identified several UCP1-independent thermogenic effectors using Ucp1 knockout mice, including creatine, Dio2, calcium-ATPase, glycerol-3-phosphate shuttle, PGC-1α, and Cox II [].
The voluntary wheel running exercise can mitigate cold-induced weight loss, and in this process, UCP-1 does not appear to play a role []. Muscle, functioning as a thermogenic organ, actively contributes to maintaining body temperature in cold conditions. Sarcolipin (SLN), which uncouples calcium transport from adenosine triphosphate hydrolysis by sarco/endoplasmic reticulum Ca2+-ATPase (SERCA), has been proposed as a potential mechanism for oxidative metabolism and nonshivering thermogenesis in skeletal muscle [,,,]. A recent study has provided additional evidence that Ca2+ cycling plays a regulatory role in thermogenesis within beige adipocytes and contributes to overall energy homeostasis in the body []. In Ucp1 knockout mice, alterations in Ca2+ cycling were observed, as evidenced by the increased fatty acid oxidation. These findings suggest that Ca2+ cycling plays a role in Ucp1-independent thermogenesis in WAT. Furthermore, the study indicates that myokine (leptin) triggers thermogenesis through a UCP1-independent mechanism involving futile substrate cycling [,,] (Figure 3).
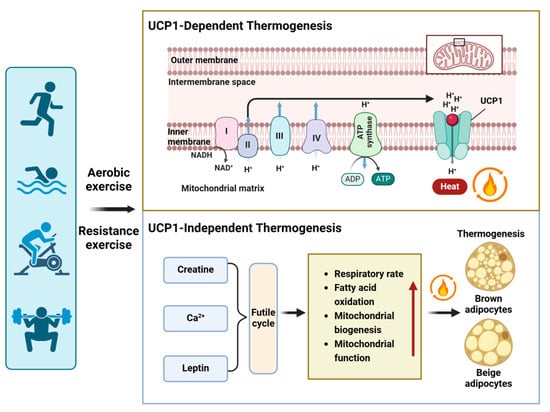
Figure 3.
Exercise-induced thermogenesis in adipocytes. Exercise not only activates UCP1-dependent thermogenesis but also induces UCP1-independent thermogenesis by increasing various futile cycles, such as creatine futile cycling, Ca2+ futile cycling, and leptin-induced TAG-fatty acid cycling. The mechanisms of UCP1-independent thermogenesis involve respiratory rate, fatty acid oxidation, mitochondrial biogenesis, and function. Red arrows indicate an increase (up arrow).
4. Potential Impact of Exerkines on Mitochondrial Homeostasis in Thermogenic Fat
Emerging evidence suggests that regular exercise is a widely recognized therapeutic tool and highly effective intervention for mitigating obesity-associated metabolic syndrome. It plays a crucial role in mitochondrial homeostasis as well as contributing significantly to individual thermogenic activity [,,,,,]. Exercise-induced circulating factors, referred to as exerkines, are implicated in the activation and metabolism of BAT and promote the browning of WAT [,,,]. Exercise induces the secretion of exerkines from various tissues, including skeletal muscle (myokines), white adipose tissue (adipokines), and brown adipose tissue (batokines).
4.1. The Impact of Myokines on Mitochondrial Homeostasis
Exercise has been recognized as a therapeutic approach for managing obesity-related metabolic diseases by mitigating abdominal adiposity and metabolic syndrome. Despite this acknowledgement, the underlying mechanisms of how regular exercise training serves as a therapeutic modality for abdominal fat remain rudimentary. Exercise-induced myokines have the potential to stimulate the browning of WAT by modulating lipid metabolism in response to obesity-associated metabolic diseases. Notably, the myokines involved include IL-6 [], FGF21 [], apelin [], Meteorin-like (Metrnl) [,,], lactate [], β-aminoisobutyric acid (BAIBA) [,], BDNF [], musclin [], myostatin [], and irisin-an exercise-induced myokine dependence PGC-1α [] (Table 1).

Table 1.
The benefits of exerkines in obesity-associated metabolic diseases.
4.2. The Impact of Adipokines on Mitochondrial Homeostasis
Adipokines secreted by exercise-trained white adipose tissue: their endocrine effects on enhanced glucose tolerance, fatty acid metabolism, and insulin sensitivity. Noteworthy, adipokines include adiponectin, leptin, SFRP4, FGF21, TGF-β2, follistatin-like 1, omentin, and vaspin [,,,,,,,,,]. These contribute to the reduction in inflammation through adipokines like SFRP5, TNF-α, IL-6, Wnt family member 5A, and MCP-1 [,,,]. Moreover, exercise-trained WAT promotes the emergence of thermogenic brown-like adipocytes with adipokines such as apelin, TGF-β, follistatin, and myostatin [,,]. This collective action not only supports mitochondrial homeostasis but also enhances overall metabolic homeostasis in response to exercise [,,,,,,] (Figure 4, Table 1).
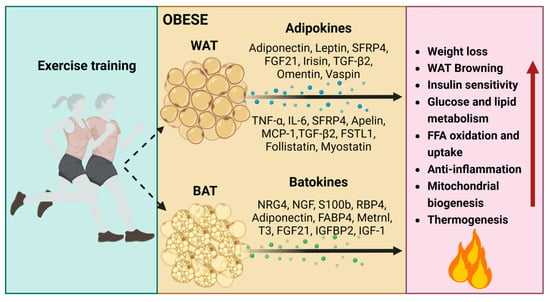
Figure 4.
The impacts of regular exercise-induced adipokines and batokines on obesity. Adipokines and batokines induced by regular exercise play crucial roles in influencing glucose tolerance, fatty acid metabolism, insulin sensitivity, inflammation, mitochondrial homeostasis, the sympathetic neural network, as well as thermogenesis in both BAT and WAT. T3, thyroid hormone; IGFBP2, insulin-like growth factor binding protein 2; IGF-1, insulin-like growth factor 1. Red arrows indicate an increase (up arrow).
A comprehensive meta-analysis study revealed that exercise, coupled with dietary intervention, significantly modulates adiponectin and leptin levels in individuals who are overweight or obese []. Adiponectin regulates mitochondrial biogenesis and insulin resistance in individuals with obesity subjected to treadmill running exercise []. The exercise-responsive transcript, SFRP4, emerges as a key mediator in facilitating long-term exercise-induced enhancements in insulin resistance []. Additionally, TGF-β2, an exercise-induced adipokine, mediates glucose homeostasis, improves insulin sensitivity, increases FFA uptake and oxidation, and promotes mitochondrial function in response to diabetes [,]. FSTL1 plays a significant role in inflammation, glucose metabolism, and insulin sensitivity, particularly in the context of obesity and exercise []. A 12-week aerobic exercise training increases the expression of adipokine omentin in visceral AT, leading to the regulation of insulin sensitivity, glucose homeostasis, and anti-inflammatory effects against type 2 diabetes mellitus []. Omentin also serves as a positive regulator of mitochondrial biogenesis by activating AMPK-PGC1alpha pathway []. Prolonged exercise treatment dramatically upregulates apelin, a key contributor to the presence of thermogenic brown-like adipocytes. This elevation is associated with increased glucose uptake, mitochondrial biogenesis, and decreased insulin resistance in individuals with diabetes [,,]. Additionally, aerobic exercise training induces the activation of the fat browning-related pathway (AMPK/Sirt1/PGC-1α), improves free fatty acid oxidation, reduces inflammation, promotes mitochondrial biogenesis, and facilitates the synthesis and secretion of exerkines, including irisin, TNF-α, IL-10, and MCP-1 [,,] (Table 1)
4.3. The Impact of Batokines on Mitochondrial Homeostasis
Regular exercise-induced sympathetic nervous system (SNS) plays a significant role in the thermogenesis of brown/beige adipocytes and the maintenance of mitochondrial homeostasis by regulating the release of neurotrophic batokines [,,]. These batokines contribute to the remodeling of the sympathetic neural network and the promotion of thermogenesis, including neuregulin-4 (NRG4) [,,], nerve growth factor (NGF) [,], and calcium-binding protein B (S100b) [,,] (Figure 4). The neurotrophic batokines further stimulate the formation of brown/beige adipocytes and enhance mitochondrial biogenesis by uncoupling oxidative phosphorylation from ATP production.
NRG4 mitigates the onset of obesity and fosters metabolic well-being by increasing BAT thermogenic activity in response to exercise (e.g., high-intensity interval training, circuit resistance training). This is substantiated by an upregulation in the expression of thermogenic markers (UCP1 and PRDM16), a decrease in the expression of lipogenic/adipogenic genes (Pparγ and Cd36), an increase in the number of brite/beige adipocytes, neurite outgrowth, blood vessels, and improvements in glucose homeostasis and mitochondrial homeostasis within adipose tissues [,,,]. S100b, on the other hand, stimulates neurite production and addresses deficient sympathetic innervation caused by Calsyntenin-3β deficiency, a mammal-specific endoplasmic reticulum membrane protein. This deficiency is implicated in functional sympathetic innervation and the maintenance of mitochondrial homeostasis in adipose tissues as a countermeasure against obesity [,,]. Nevertheless, the accumulation of oxidative stress-induced S100b promotes the transition of myoblasts into brown adipocytes. This is substantiated by the increased expression of PRDM16 [,], the upregulation of bone morphogenetic protein 7 (BMP-7), which enhances differentiation of brown preadipocytes, and the promotion of mitochondrial biogenesis []. Furthermore, hormones are released from BAT in response to cyclic AMP–mediated thermogenic activation. These hormones include adiponectin, adipsin, fatty acid–binding protein 4 (FABP4), retinol-binding protein 4 (RBP4), chemerin, clusterin, and macrophage migration inhibitory factor [] (Figure 4)
5. Conclusions
Disruption of mitochondrial homeostasis induced by obesity has adverse effects on lipid metabolism, adipocyte differentiation, oxidative capacity, inflammation, insulin sensitivity, and thermogenesis. Elevating mitochondrial homeostasis in thermogenic fat emerges as a promising avenue for developing treatments for metabolic diseases. The exerkines (myokines, adipokines, batokines) released during exercise have the potential to improve glucose and lipid metabolism, ameliorate mitochondrial homeostasis, and stimulate fat browning and thermogenesis in response to obesity-associated metabolic diseases. A thorough comprehension of the intricate interplay between mitochondrial homeostasis and thermogenesis in adipose tissue, along with the advantageous effects of exercise, could lead to the development of non-pharmacological therapeutic strategies to prevent obesity-related metabolic diseases.
Author Contributions
Literature search, H.S.; writing—original draft preparation, H.S. and H.Z.; writing—review and editing, H.Z. and D.J.; supervision, D.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work supported in part by a grant from research project of Shanghai University of Sport (2023STD023).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available within the article.
Conflicts of Interest
No conflicts of interests statement.
References
- Perdomo, C.M.; Cohen, R.V.; Sumithran, P.; Clement, K.; Fruhbeck, G. Contemporary medical, device, and surgical therapies for obesity in adults. Lancet 2023, 401, 1116–1130. [Google Scholar] [CrossRef] [PubMed]
- Watso, J.C.; Fancher, I.S.; Gomez, D.H.; Hutchison, Z.J.; Gutierrez, O.M.; Robinson, A.T. The damaging duo: Obesity and excess dietary salt contribute to hypertension and cardiovascular disease. Obes. Rev. 2023, 24, e13589. [Google Scholar] [CrossRef] [PubMed]
- Ryder, R.E.J.; Laubner, K.; Benes, M.; Haluzik, M.; Munro, L.; Frydenberg, H.; Teare, J.P.; Ruban, A.; Fishman, S.; Santo, E.; et al. Endoscopic Duodenal-Jejunal Bypass Liner Treatment for Type 2 Diabetes and Obesity: Glycemic and Cardiovascular Disease Risk Factor Improvements in 1022 Patients Treated Worldwide. Diabetes Care 2023, 46, e89–e91. [Google Scholar] [CrossRef] [PubMed]
- Brown, O.I.; Drozd, M.; McGowan, H.; Giannoudi, M.; Conning-Rowland, M.; Gierula, J.; Straw, S.; Wheatcroft, S.B.; Bridge, K.; Roberts, L.D.; et al. Relationship Among Diabetes, Obesity, and Cardiovascular Disease Phenotypes: A UK Biobank Cohort Study. Diabetes Care 2023, 46, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.T. Obesity: A certain and avoidable cause of cancer. Lancet 2014, 384, 727–728. [Google Scholar] [CrossRef]
- Li, L.; Liang, J.; Zhang, C.; Liu, T.; Zhang, C. Peripheral actions and direct central-local communications of melanocortin 4 receptor signaling. J. Sport. Health Sci. 2023, 12, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Brestoff, J.R.; Wilen, C.B.; Moley, J.R.; Li, Y.; Zou, W.; Malvin, N.P.; Rowen, M.N.; Saunders, B.T.; Ma, H.; Mack, M.R.; et al. Intercellular Mitochondria Transfer to Macrophages Regulates White Adipose Tissue Homeostasis and Is Impaired in Obesity. Cell Metab. 2021, 33, 270–282.e8. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, B.; Long, L.; Luo, P.; Xiang, W.; Li, X.; Wang, H.; Jiang, Q.; Tan, X.; Luo, S.; et al. Improvement of obesity-associated disorders by a small-molecule drug targeting mitochondria of adipose tissue macrophages. Nat. Commun. 2021, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Tysoe, O. Macrophage mitochondrial levels of iron affect adipose tissue function in obesity. Nat. Rev. Endocrinol. 2023, 19, 3. [Google Scholar] [CrossRef]
- Giroud, M.; Kotschi, S.; Kwon, Y.; Le Thuc, O.; Hoffmann, A.; Gil-Lozano, M.; Karbiener, M.; Higareda-Almaraz, J.C.; Khani, S.; Tews, D.; et al. The obesity-linked human lncRNA AATBC stimulates mitochondrial function in adipocytes. EMBO Rep. 2023, 24, e57600. [Google Scholar] [CrossRef]
- Pileggi, C.A.; Blondin, D.P.; Hooks, B.G.; Parmar, G.; Alecu, I.; Patten, D.A.; Cuillerier, A.; O’Dwyer, C.; Thrush, A.B.; Fullerton, M.D.; et al. Exercise training enhances muscle mitochondrial metabolism in diet-resistant obesity. EBioMedicine 2022, 83, 104192. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Faccenda, D.; Campanella, M. Pharmacological advances in mitochondrial therapy. EBioMedicine 2021, 65, 103244. [Google Scholar] [CrossRef] [PubMed]
- Mendham, A.E.; Goedecke, J.H.; Zeng, Y.; Larsen, S.; George, C.; Hauksson, J.; Fortuin-de Smidt, M.C.; Chibalin, A.V.; Olsson, T.; Chorell, E. Exercise training improves mitochondrial respiration and is associated with an altered intramuscular phospholipid signature in women with obesity. Diabetologia 2021, 64, 1642–1659. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Wang, H.; Lin, K.; Wang, R.; Guo, S.; Chen, P.; Wu, H.; Liu, T.; Wang, R. Exercise-induced microbial changes in preventing type 2 diabetes. Sci. China Life Sci. 2023, 67, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Tian, Z.; Wang, R. Exercise mitigates age-related metabolic diseases by improving mitochondrial dysfunction. Ageing Res. Rev. 2023, 91, 102087. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Yao, T.; Zhou, P.; Kazak, L.; Tenen, D.; Lyubetskaya, A.; Dawes, B.A.; Tsai, L.; Kahn, B.B.; Spiegelman, B.M.; et al. Brown Adipose Tissue Controls Skeletal Muscle Function via the Secretion of Myostatin. Cell Metab. 2018, 28, 631–643.e3. [Google Scholar] [CrossRef]
- Jia, D.; Zhang, J.; Liu, X.; Andersen, J.P.; Tian, Z.; Nie, J.; Shi, Y. Insulin Resistance in Skeletal Muscle Selectively Protects the Heart in Response to Metabolic Stress. Diabetes 2021, 70, 2333–2343. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Cai, M.; Xi, Y.; Du, S.; Tian, Z. Interval exercise training increases LIF expression and prevents myocardial infarction-induced skeletal muscle atrophy in rats. Life Sci. 2018, 193, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Rosina, M.; Ceci, V.; Turchi, R.; Chuan, L.; Borcherding, N.; Sciarretta, F.; Sanchez-Diaz, M.; Tortolici, F.; Karlinsey, K.; Chiurchiu, V.; et al. Ejection of damaged mitochondria and their removal by macrophages ensure efficient thermogenesis in brown adipose tissue. Cell Metab. 2022, 34, 533–548.e12. [Google Scholar] [CrossRef]
- Borcherding, N.; Jia, W.; Giwa, R.; Field, R.L.; Moley, J.R.; Kopecky, B.J.; Chan, M.M.; Yang, B.Q.; Sabio, J.M.; Walker, E.C.; et al. Dietary lipids inhibit mitochondria transfer to macrophages to divert adipocyte-derived mitochondria into the blood. Cell Metab. 2022, 34, 1499–1513.e8. [Google Scholar] [CrossRef]
- Jia, D.; Zhang, J.; Nie, J.; Andersen, J.P.; Rendon, S.; Zheng, Y.; Liu, X.; Tian, Z.; Shi, Y. Cardiolipin remodeling by ALCAT1 links hypoxia to coronary artery disease by promoting mitochondrial dysfunction. Mol. Ther. 2021, 29, 3498–3511. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Guo, H.; Qiu, X.; Lin, T.Y.; Qin, C.; Celio, G.; Yong, P.; Senders, M.; Han, X.; Bernlohr, D.A.; et al. Lipocalin 2 regulates mitochondrial phospholipidome remodeling, dynamics, and function in brown adipose tissue in male mice. Nat. Commun. 2023, 14, 6729. [Google Scholar] [CrossRef] [PubMed]
- Chella Krishnan, K.; Vergnes, L.; Acin-Perez, R.; Stiles, L.; Shum, M.; Ma, L.; Mouisel, E.; Pan, C.; Moore, T.M.; Peterfy, M.; et al. Sex-specific genetic regulation of adipose mitochondria and metabolic syndrome by Ndufv2. Nat. Metab. 2021, 3, 1552–1568. [Google Scholar] [CrossRef]
- Fink, B.D.; Yu, L.; Sivitz, W.I. Modulation of complex II-energized respiration in muscle, heart, and brown adipose mitochondria by oxaloacetate and complex I electron flow. FASEB J. 2019, 33, 11696–11705. [Google Scholar] [CrossRef]
- Pedersen, J.S.; Rygg, M.O.; Chrois, K.; Sustarsic, E.G.; Gerhart-Hines, Z.; Wever Albrechtsen, N.J.; Serizawa, R.R.; Kristiansen, V.B.; Basse, A.L.; Boilesen, A.E.B.; et al. Influence of NAFLD and bariatric surgery on hepatic and adipose tissue mitochondrial biogenesis and respiration. Nat. Commun. 2022, 13, 2931. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Xu, H.Y.; Zhou, W.Y.; Xia, Y.F.; Li, B.Y.; Shi, Y.J.; Dou, X.; Yang, Q.Q.; Qian, S.W.; Tang, Y.; et al. CLCF1 signaling restrains thermogenesis and disrupts metabolic homeostasis by inhibiting mitochondrial biogenesis in brown adipocytes. Proc. Natl. Acad. Sci. USA 2023, 120, e2305717120. [Google Scholar] [CrossRef] [PubMed]
- Yau, W.W.; Singh, B.K.; Lesmana, R.; Zhou, J.; Sinha, R.A.; Wong, K.A.; Wu, Y.; Bay, B.H.; Sugii, S.; Sun, L.; et al. Thyroid hormone (T(3)) stimulates brown adipose tissue activation via mitochondrial biogenesis and MTOR-mediated mitophagy. Autophagy 2019, 15, 131–150. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y. Emerging roles of cardiolipin remodeling in mitochondrial dysfunction associated with diabetes, obesity, and cardiovascular diseases. J. Biomed. Res. 2010, 24, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Zhang, J.; Qi, S.; Liu, Z.; Zhang, X.; Zheng, Y.; Andersen, J.P.; Zhang, W.; Strong, R.; Martinez, P.A.; et al. Cardiolipin remodeling by ALCAT1 links mitochondrial dysfunction to Parkinson’s diseases. Aging Cell 2019, 18, e12941. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Wang, H.; Zhang, W.; Chan, D.C.; Shi, Y. Lysocardiolipin acyltransferase 1 (ALCAT1) controls mitochondrial DNA fidelity and biogenesis through modulation of MFN2 expression. Proc. Natl. Acad. Sci. USA 2012, 109, 6975–6980. [Google Scholar] [CrossRef]
- Han, X.; Yang, J.; Cheng, H.; Yang, K.; Abendschein, D.R.; Gross, R.W. Shotgun lipidomics identifies cardiolipin depletion in diabetic myocardium linking altered substrate utilization with mitochondrial dysfunction. Biochemistry 2005, 44, 16684–16694. [Google Scholar] [CrossRef] [PubMed]
- Sustarsic, E.G.; Ma, T.; Lynes, M.D.; Larsen, M.; Karavaeva, I.; Havelund, J.F.; Nielsen, C.H.; Jedrychowski, M.P.; Moreno-Torres, M.; Lundh, M.; et al. Cardiolipin Synthesis in Brown and Beige Fat Mitochondria Is Essential for Systemic Energy Homeostasis. Cell Metab. 2018, 28, 159–174.e11. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, F.H.; Han, C.; Wang, Z.Z.; Gao, K.K.; Qiao, Y.B.; Ma, S.; Xie, T.; Wang, J. Alterations in mitochondrial biogenesis and respiratory activity, inflammation of the senescence-associated secretory phenotype, and lipolysis in the perirenal fat and liver of rats following lifelong exercise and detraining. FASEB J. 2021, 35, e21890. [Google Scholar] [CrossRef]
- Drake, J.C.; Wilson, R.J.; Laker, R.C.; Guan, Y.; Spaulding, H.R.; Nichenko, A.S.; Shen, W.; Shang, H.; Dorn, M.V.; Huang, K.; et al. Mitochondria-localized AMPK responds to local energetics and contributes to exercise and energetic stress-induced mitophagy. Proc. Natl. Acad. Sci. USA 2021, 118, e2025932118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Goldman, S.; Baerga, R.; Zhao, Y.; Komatsu, M.; Jin, S. Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 19860–19865. [Google Scholar] [CrossRef] [PubMed]
- Mottillo, E.P.; Desjardins, E.M.; Crane, J.D.; Smith, B.K.; Green, A.E.; Ducommun, S.; Henriksen, T.I.; Rebalka, I.A.; Razi, A.; Sakamoto, K.; et al. Lack of Adipocyte AMPK Exacerbates Insulin Resistance and Hepatic Steatosis through Brown and Beige Adipose Tissue Function. Cell Metab. 2016, 24, 118–129. [Google Scholar] [CrossRef]
- Martinez-Lopez, N.; Garcia-Macia, M.; Sahu, S.; Athonvarangkul, D.; Liebling, E.; Merlo, P.; Cecconi, F.; Schwartz, G.J.; Singh, R. Autophagy in the CNS and Periphery Coordinate Lipophagy and Lipolysis in the Brown Adipose Tissue and Liver. Cell Metab. 2016, 23, 113–127. [Google Scholar] [CrossRef]
- Sinha, R.A.; Singh, B.K.; Zhou, J.; Wu, Y.; Farah, B.L.; Ohba, K.; Lesmana, R.; Gooding, J.; Bay, B.H.; Yen, P.M. Thyroid hormone induction of mitochondrial activity is coupled to mitophagy via ROS-AMPK-ULK1 signaling. Autophagy 2015, 11, 1341–1357. [Google Scholar] [CrossRef] [PubMed]
- Lesmana, R.; Sinha, R.A.; Singh, B.K.; Zhou, J.; Ohba, K.; Wu, Y.; Yau, W.W.; Bay, B.H.; Yen, P.M. Thyroid Hormone Stimulation of Autophagy Is Essential for Mitochondrial Biogenesis and Activity in Skeletal Muscle. Endocrinology 2016, 157, 23–38. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Zhang, Z.; Wang, R.; Bo, H.; Zhang, Y. Exercise Improves the Coordination of the Mitochondrial Unfolded Protein Response and Mitophagy in Aging Skeletal Muscle. Life 2023, 13, 1006. [Google Scholar] [CrossRef]
- Taylor, D.; Gottlieb, R.A. Parkin-mediated mitophagy is downregulated in browning of white adipose tissue. Obesity 2017, 25, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Xu, Z.; Liu, L.; Guo, Q.; Wu, H.; Liang, X.; Zhou, D.; Xiao, L.; Liu, L.; Liu, Y.; et al. Mitophagy Directs Muscle-Adipose Crosstalk to Alleviate Dietary Obesity. Cell Rep. 2018, 23, 1357–1372. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Liu, Y.; Sun, C.; Yin, H. Transient p53 inhibition sensitizes aged white adipose tissue for beige adipocyte recruitment by blocking mitophagy. FASEB J. 2019, 33, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Demine, S.; Renard, P.; Arnould, T. Mitochondrial Uncoupling: A Key Controller of Biological Processes in Physiology and Diseases. Cells 2019, 8, 795. [Google Scholar] [CrossRef] [PubMed]
- Lyamzaev, K.G.; Tokarchuk, A.V.; Panteleeva, A.A.; Mulkidjanian, A.Y.; Skulachev, V.P.; Chernyak, B.V. Induction of autophagy by depolarization of mitochondria. Autophagy 2018, 14, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Kanemoto, N.; Okamoto, T.; Tanabe, K.; Shimada, T.; Minoshima, H.; Hidoh, Y.; Aoyama, M.; Ban, T.; Kobayashi, Y.; Ando, H.; et al. Antidiabetic and cardiovascular beneficial effects of a liver-localized mitochondrial uncoupler. Nat. Commun. 2019, 10, 2172. [Google Scholar] [CrossRef] [PubMed]
- Kenwood, B.M.; Weaver, J.L.; Bajwa, A.; Poon, I.K.; Byrne, F.L.; Murrow, B.A.; Calderone, J.A.; Huang, L.; Divakaruni, A.S.; Tomsig, J.L.; et al. Identification of a novel mitochondrial uncoupler that does not depolarize the plasma membrane. Mol. Metab. 2014, 3, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Jian, C.S.; Fu, J.J.; Cheng, X.; Shen, L.J.; Ji, Y.X.; Wang, X.M.; Pan, S.; Tian, H.; Tian, S.; Liao, R.F.; et al. Low-Dose Sorafenib Acts as a Mitochondrial Uncoupler and Ameliorates Nonalcoholic Steatohepatitis. Cell Metab. 2020, 31, 892–908.e11. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, S.J.; Chen, S.Y.; Brandon, A.E.; Salamoun, J.M.; Byrne, F.L.; Garcia, C.J.; Beretta, M.; Olzomer, E.M.; Shah, D.P.; Philp, A.M.; et al. Mitochondrial uncoupler BAM15 reverses diet-induced obesity and insulin resistance in mice. Nat. Commun. 2020, 11, 2397. [Google Scholar] [CrossRef]
- Perry, R.J.; Kim, T.; Zhang, X.M.; Lee, H.Y.; Pesta, D.; Popov, V.B.; Zhang, D.; Rahimi, Y.; Jurczak, M.J.; Cline, G.W.; et al. Reversal of hypertriglyceridemia, fatty liver disease, and insulin resistance by a liver-targeted mitochondrial uncoupler. Cell Metab. 2013, 18, 740–748. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Johnson, J.; Fang, W.; Halpern, J.; Marosi, K.; Liu, D.; Geisler, J.G.; Mattson, M.P. A mitochondrial uncoupler prodrug protects dopaminergic neurons and improves functional outcome in a mouse model of Parkinson’s disease. Neurobiol. Aging 2020, 85, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, D.G. The hunt for the molecular mechanism of brown fat thermogenesis. Biochimie 2017, 134, 9–18. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Kazak, L.; Spiegelman, B.M. New Advances in Adaptive Thermogenesis: UCP1 and Beyond. Cell Metab. 2019, 29, 27–37. [Google Scholar] [CrossRef]
- Chang, S.H.; Song, N.J.; Choi, J.H.; Yun, U.J.; Park, K.W. Mechanisms underlying UCP1 dependent and independent adipocyte thermogenesis. Obes. Rev. 2019, 20, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.I.; Middelbeek, R.J.; Goodyear, L.J. Exercise Effects on White Adipose Tissue: Beiging and Metabolic Adaptations. Diabetes 2015, 64, 2361–2368. [Google Scholar] [CrossRef] [PubMed]
- Trevellin, E.; Scorzeto, M.; Olivieri, M.; Granzotto, M.; Valerio, A.; Tedesco, L.; Fabris, R.; Serra, R.; Quarta, M.; Reggiani, C.; et al. Exercise training induces mitochondrial biogenesis and glucose uptake in subcutaneous adipose tissue through eNOS-dependent mechanisms. Diabetes 2014, 63, 2800–2811. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, Y.J.; Seong, J.K. AMP-activated protein kinase activation in skeletal muscle modulates exercise-induced uncoupled protein 1 expression in brown adipocyte in mouse model. J. Physiol. 2022, 600, 2359–2376. [Google Scholar] [CrossRef]
- Felix-Soriano, E.; Sainz, N.; Gil-Iturbe, E.; Castilla-Madrigal, R.; Celay, J.; Fernandez-Galilea, M.; Pejenaute, A.; Lostao, M.P.; Martinez-Climent, J.A.; Moreno-Aliaga, M.J. Differential remodeling of subcutaneous white and interscapular brown adipose tissue by long-term exercise training in aged obese female mice. J. Physiol. Biochem. 2023, 79, 451–465. [Google Scholar] [CrossRef]
- Dewal, R.S.; Stanford, K.I. Effects of exercise on brown and beige adipocytes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 71–78. [Google Scholar] [CrossRef]
- Luo, Z.; Ma, L.; Zhao, Z.; He, H.; Yang, D.; Feng, X.; Ma, S.; Chen, X.; Zhu, T.; Cao, T.; et al. TRPV1 activation improves exercise endurance and energy metabolism through PGC-1alpha upregulation in mice. Cell Res. 2012, 22, 551–564. [Google Scholar] [CrossRef]
- Ringseis, R.; Mooren, F.C.; Keller, J.; Couturier, A.; Wen, G.; Hirche, F.; Stangl, G.I.; Eder, K.; Kruger, K. Regular endurance exercise improves the diminished hepatic carnitine status in mice fed a high-fat diet. Mol. Nutr. Food Res. 2011, 55 (Suppl. S2), S193–S202. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Wu, Z.; Zhang, B.; Wang, C.; Mao, F.; Liu, X.; Hu, K.; Sun, X.; Jin, W.; Kuang, S. Fndc5 loss-of-function attenuates exercise-induced browning of white adipose tissue in mice. FASEB J. 2019, 33, 5876–5886. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, X.; Huang, C.; Liu, H.; Zhang, Q.; Sun, Q.; Jia, Y.; Liu, S.; Dong, M.; Hou, M.; et al. FGF2 disruption enhances thermogenesis in brown and beige fat to protect against adiposity and hepatic steatosis. Mol. Metab. 2021, 54, 101358. [Google Scholar] [CrossRef] [PubMed]
- Khalagi, K.; Ansarifar, A.; Fahimfar, N.; Sanjari, M.; Gharibzdeh, S.; Sharifi, F.; Shafiee, G.; Heshmat, R.; Nabipour, I.; Larijani, B.; et al. Cardio-metabolic and socio-demographic risk factors associated with dependency in basic and instrumental activities of daily living among older Iranian adults: Bushehr elderly health program. BMC Geriatr. 2021, 21, 172. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chen, X.; Hu, G.; Li, C.; Guo, L.; Zhang, L.; Sun, F.; Xia, Y.; Yan, W.; Cui, Z.; et al. Small Extracellular Vesicles From Brown Adipose Tissue Mediate Exercise Cardioprotection. Circ. Res. 2022, 130, 1490–1506. [Google Scholar] [CrossRef] [PubMed]
- Rossato, M. Aging and brown adipose tissue activity decline in human: Does the brain extinguish the fire? Aging Clin. Exp. Res. 2016, 28, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Enerback, S. Human brown adipose tissue. Cell Metab. 2010, 11, 248–252. [Google Scholar] [CrossRef]
- Nedergaard, J.; Cannon, B. The changed metabolic world with human brown adipose tissue: Therapeutic visions. Cell Metab. 2010, 11, 268–272. [Google Scholar] [CrossRef]
- Harb, E.; Kheder, O.; Poopalasingam, G.; Rashid, R.; Srinivasan, A.; Izzi-Engbeaya, C. Brown adipose tissue and regulation of human body weight. Diabetes Metab. Res. Rev. 2023, 39, e3594. [Google Scholar] [CrossRef]
- Vatner, D.E.; Oydanich, M.; Zhang, J.; Campbell, S.C.; Vatner, S.F. Exercise enhancement by RGS14 disruption is mediated by brown adipose tissue. Aging Cell 2023, 22, e13791. [Google Scholar] [CrossRef]
- Brenmoehl, J.; Ohde, D.; Walz, C.; Langhammer, M.; Schultz, J.; Hoeflich, A. Analysis of Activity-Dependent Energy Metabolism in Mice Reveals Regulation of Mitochondrial Fission and Fusion mRNA by Voluntary Physical Exercise in Subcutaneous Fat from Male Marathon Mice (DUhTP). Cells 2020, 9, 2697. [Google Scholar] [CrossRef]
- Jia, D.; Zhang, H.; Liu, T.; Wang, R. Exercise Alleviates Aging of Adipose Tissue through Adipokine Regulation. Metabolites 2024, 14, 135. [Google Scholar] [CrossRef]
- Sambeat, A.; Gulyaeva, O.; Dempersmier, J.; Sul, H.S. Epigenetic Regulation of the Thermogenic Adipose Program. Trends Endocrinol. Metab. 2017, 28, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Galvez, I.; Hinchado, M.D.; Martin-Cordero, L.; Moran-Plata, F.J.; Graham, G.; Francisco-Morcillo, J.; Ortega, E. The anti-inflammatory and bioregulatory effects of habitual exercise in high-fat diet-induced obesity involve crown-like structures and MCP-1 in white adipose tissue. Exerc. Immunol. Rev. 2023, 29, 111–120. [Google Scholar] [PubMed]
- Gaspar, R.C.; Munoz, V.R.; Kuga, G.K.; Nakandakari, S.; Minuzzi, L.G.; Botezelli, J.D.; da Silva, A.S.R.; Cintra, D.E.; de Moura, L.P.; Ropelle, E.R.; et al. Acute physical exercise increases leptin-induced hypothalamic extracellular signal-regulated kinase1/2 phosphorylation and thermogenesis of obese mice. J. Cell Biochem. 2019, 120, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.R.; Micheletti, T.O.; Pimentel, G.D.; Katashima, C.K.; Lenhare, L.; Morari, J.; Mendes, M.C.; Razolli, D.S.; Rocha, G.Z.; de Souza, C.T.; et al. Hypothalamic S1P/S1PR1 axis controls energy homeostasis. Nat. Commun. 2014, 5, 4859. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.R.; Katashima, C.K.; Bueno Silva, C.G.; Lenhare, L.; Micheletti, T.O.; Camargo, R.L.; Ghezzi, A.C.; Camargo, J.A.; Assis, A.M.; Tobar, N.; et al. Hypothalamic S1P/S1PR1 axis controls energy homeostasis in Middle-Aged Rodents: The reversal effects of physical exercise. Aging 2016, 9, 142–155. [Google Scholar] [CrossRef]
- Shirkhani, S.; Marandi, S.M.; Kazeminasab, F.; Esmaeili, M.; Ghaedi, K.; Esfarjani, F.; Shiralian-Esfahani, H.; Nasr-Esfahani, M.H. Comparative studies on the effects of high-fat diet, endurance training and obesity on Ucp1 expression in male C57BL/6 mice. Gene 2018, 676, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Bostrom, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostrom, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Pollard, A.E.; Martins, L.; Muckett, P.J.; Khadayate, S.; Bornot, A.; Clausen, M.; Admyre, T.; Bjursell, M.; Fiadeiro, R.; Wilson, L.; et al. AMPK activation protects against diet induced obesity through Ucp1-independent thermogenesis in subcutaneous white adipose tissue. Nat. Metab. 2019, 1, 340–349. [Google Scholar] [CrossRef]
- Kazak, L.; Chouchani, E.T.; Jedrychowski, M.P.; Erickson, B.K.; Shinoda, K.; Cohen, P.; Vetrivelan, R.; Lu, G.Z.; Laznik-Bogoslavski, D.; Hasenfuss, S.C.; et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 2015, 163, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Knuth, C.M.; Peppler, W.T.; Townsend, L.K.; Miotto, P.M.; Gudiksen, A.; Wright, D.C. Prior exercise training improves cold tolerance independent of indices associated with non-shivering thermogenesis. J. Physiol. 2018, 596, 4375–4391. [Google Scholar] [CrossRef]
- Wang, S.; Gopinath, T.; Larsen, E.K.; Weber, D.K.; Walker, C.; Uddigiri, V.R.; Mote, K.R.; Sahoo, S.K.; Periasamy, M.; Veglia, G. Structural basis for sarcolipin’s regulation of muscle thermogenesis by the sarcoplasmic reticulum Ca2+-ATPase. Sci. Adv. 2021, 7, eabi7154. [Google Scholar] [CrossRef] [PubMed]
- Rowland, L.A.; Maurya, S.K.; Bal, N.C.; Kozak, L.; Periasamy, M. Sarcolipin and uncoupling protein 1 play distinct roles in diet-induced thermogenesis and do not compensate for one another. Obesity 2016, 24, 1430–1433. [Google Scholar] [CrossRef] [PubMed]
- Gamu, D.; Bombardier, E.; Smith, I.C.; Fajardo, V.A.; Tupling, A.R. Sarcolipin provides a novel muscle-based mechanism for adaptive thermogenesis. Exerc. Sport. Sci. Rev. 2014, 42, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Bal, N.C.; Maurya, S.K.; Sopariwala, D.H.; Sahoo, S.K.; Gupta, S.C.; Shaikh, S.A.; Pant, M.; Rowland, L.A.; Bombardier, E.; Goonasekera, S.A.; et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat. Med. 2012, 18, 1575–1579. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Kang, Q.; Yoneshiro, T.; Camporez, J.P.; Maki, H.; Homma, M.; Shinoda, K.; Chen, Y.; Lu, X.; Maretich, P.; et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat. Med. 2017, 23, 1454–1465. [Google Scholar] [CrossRef]
- Chen, M.; Wilson, E.A.; Cui, Z.; Sun, H.; Shrestha, Y.B.; Podyma, B.; Le, C.H.; Naglieri, B.; Pacak, K.; Gavrilova, O.; et al. G(s)alpha deficiency in the dorsomedial hypothalamus leads to obesity, hyperphagia, and reduced thermogenesis associated with impaired leptin signaling. Mol. Metab. 2019, 25, 142–153. [Google Scholar] [CrossRef]
- Fischer, A.W.; Hoefig, C.S.; Abreu-Vieira, G.; de Jong, J.M.A.; Petrovic, N.; Mittag, J.; Cannon, B.; Nedergaard, J. Leptin Raises Defended Body Temperature without Activating Thermogenesis. Cell Rep. 2016, 14, 1621–1631. [Google Scholar] [CrossRef]
- Young, C.N.; Morgan, D.A.; Butler, S.D.; Rahmouni, K.; Gurley, S.B.; Coffman, T.M.; Mark, A.L.; Davisson, R.L. Angiotensin type 1a receptors in the forebrain subfornical organ facilitate leptin-induced weight loss through brown adipose tissue thermogenesis. Mol. Metab. 2015, 4, 337–343. [Google Scholar] [CrossRef]
- May, F.J.; Baer, L.A.; Lehnig, A.C.; So, K.; Chen, E.Y.; Gao, F.; Narain, N.R.; Gushchina, L.; Rose, A.; Doseff, A.I.; et al. Lipidomic Adaptations in White and Brown Adipose Tissue in Response to Exercise Demonstrate Molecular Species-Specific Remodeling. Cell Rep. 2017, 18, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- Lehnig, A.C.; Dewal, R.S.; Baer, L.A.; Kitching, K.M.; Munoz, V.R.; Arts, P.J.; Sindeldecker, D.A.; May, F.J.; Lauritzen, H.; Goodyear, L.J.; et al. Exercise Training Induces Depot-Specific Adaptations to White and Brown Adipose Tissue. iScience 2019, 11, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Sepa-Kishi, D.M.; Ceddia, R.B. Exercise-Mediated Effects on White and Brown Adipose Tissue Plasticity and Metabolism. Exerc. Sport. Sci. Rev. 2016, 44, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Tellez, B.; Sanchez-Delgado, G.; Acosta, F.M.; Alcantara, J.M.A.; Amaro-Gahete, F.J.; Martinez-Avila, W.D.; Merchan-Ramirez, E.; Munoz-Hernandez, V.; Osuna-Prieto, F.J.; Jurado-Fasoli, L.; et al. No evidence of brown adipose tissue activation after 24 weeks of supervised exercise training in young sedentary adults in the ACTIBATE randomized controlled trial. Nat. Commun. 2022, 13, 5259. [Google Scholar] [CrossRef] [PubMed]
- Peres Valgas da Silva, C.; Hernandez-Saavedra, D.; White, J.D.; Stanford, K.I. Cold and Exercise: Therapeutic Tools to Activate Brown Adipose Tissue and Combat Obesity. Biology 2019, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Aldiss, P.; Betts, J.; Sale, C.; Pope, M.; Budge, H.; Symonds, M.E. Exercise-induced ‘browning’ of adipose tissues. Metabolism 2018, 81, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Montanari, T.; Poscic, N.; Colitti, M. Factors involved in white-to-brown adipose tissue conversion and in thermogenesis: A review. Obes. Rev. 2017, 18, 495–513. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Gao, M.; Sun, H.; Liu, D. Interleukin-6 gene transfer reverses body weight gain and fatty liver in obese mice. Biochim. Biophys. Acta 2015, 1852, 1001–1011. [Google Scholar] [CrossRef]
- Giralt, M.; Gavalda-Navarro, A.; Villarroya, F. Fibroblast growth factor-21, energy balance and obesity. Mol. Cell Endocrinol. 2015, 418 Pt 1, 66–73. [Google Scholar] [CrossRef]
- Son, J.S.; Zhao, L.; Chen, Y.; Chen, K.; Chae, S.A.; de Avila, J.M.; Wang, H.; Zhu, M.J.; Jiang, Z.; Du, M. Maternal exercise via exerkine apelin enhances brown adipogenesis and prevents metabolic dysfunction in offspring mice. Sci. Adv. 2020, 6, eaaz0359. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.R.; Long, J.Z.; White, J.P.; Svensson, K.J.; Lou, J.; Lokurkar, I.; Jedrychowski, M.P.; Ruas, J.L.; Wrann, C.D.; Lo, J.C.; et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 2014, 157, 1279–1291. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jia, S.; Yang, Y.; Piao, L.; Wang, Z.; Jin, Z.; Bai, L. Exercise induced meteorin-like protects chondrocytes against inflammation and pyroptosis in osteoarthritis by inhibiting PI3K/Akt/NF-kappaB and NLRP3/caspase-1/GSDMD signaling. Biomed. Pharmacother. 2023, 158, 114118. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, H.; Alizadeh, A. Association of Meteorin-Like Hormone with insulin resistance and body composition in healthy Iranian adults. Diabetes Metab. Syndr. 2020, 14, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Carriere, A.; Jeanson, Y.; Berger-Muller, S.; Andre, M.; Chenouard, V.; Arnaud, E.; Barreau, C.; Walther, R.; Galinier, A.; Wdziekonski, B.; et al. Browning of white adipose cells by intermediate metabolites: An adaptive mechanism to alleviate redox pressure. Diabetes 2014, 63, 3253–3265. [Google Scholar] [CrossRef]
- Kammoun, H.L.; Febbraio, M.A. Come on BAIBA light my fire. Cell Metab. 2014, 19, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.D.; Bostrom, P.; O’Sullivan, J.F.; Schinzel, R.T.; Lewis, G.D.; Dejam, A.; Lee, Y.K.; Palma, M.J.; Calhoun, S.; Georgiadi, A.; et al. beta-Aminoisobutyric acid induces browning of white fat and hepatic beta-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 2014, 19, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Sandrini, L.; Ieraci, A.; Amadio, P.; Zara, M.; Mitro, N.; Lee, F.S.; Tremoli, E.; Barbieri, S.S. Physical Exercise Affects Adipose Tissue Profile and Prevents Arterial Thrombosis in BDNF Val66Met Mice. Cells 2019, 8, 875. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, M.; Horii, N.; Fujie, S.; Inoue, K.; Hasegawa, N.; Iemitsu, K.; Uchida, M.; Iemitsu, M. Decreased muscle-derived musclin by chronic resistance exercise is associated with improved insulin resistance in rats with type 2 diabetes. Physiol. Rep. 2021, 9, e14823. [Google Scholar] [CrossRef]
- Lund, J.; Breum, A.W.; Gil, C.; Falk, S.; Sass, F.; Isidor, M.S.; Dmytriyeva, O.; Ranea-Robles, P.; Mathiesen, C.V.; Basse, A.L.; et al. The anorectic and thermogenic effects of pharmacological lactate in male mice are confounded by treatment osmolarity and co-administered counterions. Nat. Metab. 2023, 5, 677–698. [Google Scholar] [CrossRef]
- Lin, Z.; Tian, H.; Lam, K.S.; Lin, S.; Hoo, R.C.; Konishi, M.; Itoh, N.; Wang, Y.; Bornstein, S.R.; Xu, A.; et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 2013, 17, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Subbotina, E.; Sierra, A.; Zhu, Z.; Gao, Z.; Koganti, S.R.; Reyes, S.; Stepniak, E.; Walsh, S.A.; Acevedo, M.R.; Perez-Terzic, C.M.; et al. Musclin is an activity-stimulated myokine that enhances physical endurance. Proc. Natl. Acad. Sci. USA 2015, 112, 16042–16047. [Google Scholar] [CrossRef]
- Takahashi, H.; Alves, C.R.R.; Stanford, K.I.; Middelbeek, R.J.W.; Nigro, P.; Ryan, R.E.; Xue, R.; Sakaguchi, M.; Lynes, M.D.; So, K.; et al. TGF-beta2 is an exercise-induced adipokine that regulates glucose and fatty acid metabolism. Nat. Metab. 2019, 1, 291–303. [Google Scholar] [CrossRef]
- Pervin, S.; Reddy, S.T.; Singh, R. Novel Roles of Follistatin/Myostatin in Transforming Growth Factor-beta Signaling and Adipose Browning: Potential for Therapeutic Intervention in Obesity Related Metabolic Disorders. Front. Endocrinol. 2021, 12, 653179. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Chang, B.; Yu, Q.L.; Xu, S.T.; Yi, X.J.; Cao, S.C. Adiponectin treatment improves insulin resistance in mice by regulating the expression of the mitochondrial-derived peptide MOTS-c and its response to exercise via APPL1-SIRT1-PGC-1alpha. Diabetologia 2020, 63, 2675–2688. [Google Scholar] [CrossRef] [PubMed]
- Kon, M.; Ebi, Y.; Nakagaki, K. Effects of acute sprint interval exercise on follistatin-like 1 and apelin secretions. Arch. Physiol. Biochem. 2021, 127, 223–227. [Google Scholar] [CrossRef] [PubMed]
- de Castro, C.A.; da Silva, K.A.; Rocha, M.C.; Sene-Fiorese, M.; Nonaka, K.O.; Malavazi, I.; Anibal, F.F.; Duarte, A. Exercise and Omentin: Their Role in the Crosstalk Between Muscle and Adipose Tissues in Type 2 Diabetes Mellitus Rat Models. Front. Physiol. 2018, 9, 1881. [Google Scholar] [CrossRef] [PubMed]
- Valenca-Pereira, F.; Fang, Q.; Marie, I.J.; Giddings, E.L.; Fortner, K.A.; Yang, R.; Villarino, A.V.; Huang, Y.H.; Frank, D.A.; Wen, H.; et al. IL-6 enhances CD4 cell motility by sustaining mitochondrial Ca2+ through the noncanonical STAT3 pathway. Proc. Natl. Acad. Sci. USA 2021, 118, e2103444118. [Google Scholar] [CrossRef]
- Cuevas-Ramos, D.; Mehta, R.; Aguilar-Salinas, C.A. Fibroblast Growth Factor 21 and Browning of White Adipose Tissue. Front. Physiol. 2019, 10, 37. [Google Scholar] [CrossRef]
- Attane, C.; Foussal, C.; Le Gonidec, S.; Benani, A.; Daviaud, D.; Wanecq, E.; Guzman-Ruiz, R.; Dray, C.; Bezaire, V.; Rancoule, C.; et al. Apelin treatment increases complete Fatty Acid oxidation, mitochondrial oxidative capacity, and biogenesis in muscle of insulin-resistant mice. Diabetes 2012, 61, 310–320. [Google Scholar] [CrossRef]
- Alizadeh, H. Meteorin-like protein (Metrnl): A metabolic syndrome biomarker and an exercise mediator. Cytokine 2022, 157, 155952. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, L.; Jin, B.; Wu, Y.; Xu, L.; Chang, X.; Hu, L.; Wang, G.; Huang, Y.; Song, L.; et al. Metrnl Alleviates Lipid Accumulation by Modulating Mitochondrial Homeostasis in Diabetic Nephropathy. Diabetes 2023, 72, 611–626. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, W.; Yu, M.; Liu, J.; Sun, H.; Yang, P. Exercise-Generated beta-Aminoisobutyric Acid (BAIBA) Reduces Cardiomyocyte Metabolic Stress and Apoptosis Caused by Mitochondrial Dysfunction Through the miR-208b/AMPK Pathway. Front. Cardiovasc. Med. 2022, 9, 803510. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, P.; Ng, C.F.; Pang, B.P.S.; Chan, W.S.; Tse, M.C.L.; Bi, X.; Kwan, H.R.; Brobst, D.; Herlea-Pana, O.; Yang, X.; et al. Muscle-generated BDNF (brain derived neurotrophic factor) maintains mitochondrial quality control in female mice. Autophagy 2022, 18, 1367–1384. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Sathiakumar, D.; Lua, B.J.; Kukreti, H.; Lee, M.; McFarlane, C. Myostatin signals through miR-34a to regulate Fndc5 expression and browning of white adipocytes. Int. J. Obes. 2017, 41, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Khalafi, M.; Hossein Sakhaei, M.; Kheradmand, S.; Symonds, M.E.; Rosenkranz, S.K. The impact of exercise and dietary interventions on circulating leptin and adiponectin in individuals who are overweight and those with obesity: A systematic review and meta-analysis. Adv. Nutr. 2023, 14, 128–146. [Google Scholar] [CrossRef]
- Martinez-Huenchullan, S.F.; Tam, C.S.; Ban, L.A.; Ehrenfeld-Slater, P.; McLennan, S.V.; Twigg, S.M. Skeletal muscle adiponectin induction in obesity and exercise. Metabolism 2020, 102, 154008. [Google Scholar] [CrossRef]
- Porter, J.W.; Rowles, J.L., 3rd; Fletcher, J.A.; Zidon, T.M.; Winn, N.C.; McCabe, L.T.; Park, Y.M.; Perfield, J.W., 2nd; Thyfault, J.P.; Rector, R.S.; et al. Anti-inflammatory effects of exercise training in adipose tissue do not require FGF21. J. Endocrinol. 2017, 235, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Norheim, F.; Langleite, T.M.; Gulseth, H.L.; Birkeland, K.I.; Drevon, C.A. Effects of long-term exercise on plasma adipokine levels and inflammation-related gene expression in subcutaneous adipose tissue in sedentary dysglycaemic, overweight men and sedentary normoglycaemic men of healthy weight. Diabetologia 2019, 62, 1048–1064. [Google Scholar] [CrossRef]
- Amouzad Mahdirejei, H.; Fadaei Reyhan Abadei, S.; Abbaspour Seidi, A.; Eshaghei Gorji, N.; Rahmani Kafshgari, H.; Ebrahim Pour, M.; Bagheri Khalili, H.; Hajeizad, F.; Khayeri, M. Effects of an eight-week resistance training on plasma vaspin concentrations, metabolic parameters levels and physical fitness in patients with type 2 diabetes. Cell J. 2014, 16, 367–374. [Google Scholar]
- Golbidi, S.; Laher, I. Exercise induced adipokine changes and the metabolic syndrome. J. Diabetes Res. 2014, 2014, 726861. [Google Scholar] [CrossRef]
- Zadeh, M.A.M.; Afrasyabi, S.; Mohamadi, Z.A. The effects of exercise training induced calories expenditure on type 2 diabetes related cardio metabolic physiological parameters and adipocytokines. J. Diabetes Metab. Disord. 2022, 21, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- Moayedi, F.; Taghian, F.; Jalali Dehkordi, K.; Hosseini, S.A. Cumulative effects of exercise training and consumption of propolis on managing diabetic dyslipidemia in adult women: A single-blind, randomized, controlled trial with pre-post-intervention assessments. J. Physiol. Sci. 2023, 73, 17. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.I.; Middelbeek, R.J.; Townsend, K.L.; Lee, M.Y.; Takahashi, H.; So, K.; Hitchcox, K.M.; Markan, K.R.; Hellbach, K.; Hirshman, M.F.; et al. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes 2015, 64, 2002–2014. [Google Scholar] [CrossRef]
- Kawanishi, N.; Yano, H.; Yokogawa, Y.; Suzuki, K. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc. Immunol. Rev. 2010, 16, 105–118. [Google Scholar]
- Kajimura, S.; Spiegelman, B.M.; Seale, P. Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metab. 2015, 22, 546–559. [Google Scholar] [CrossRef]
- Bohm, A.; Hoffmann, C.; Irmler, M.; Schneeweiss, P.; Schnauder, G.; Sailer, C.; Schmid, V.; Hudemann, J.; Machann, J.; Schick, F.; et al. TGF-beta Contributes to Impaired Exercise Response by Suppression of Mitochondrial Key Regulators in Skeletal Muscle. Diabetes 2016, 65, 2849–2861. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Tian, F.; Wang, Z.; Song, H.; Chen, H.; Wu, B. Omentin-1 promotes mitochondrial biogenesis via PGC1alpha-AMPK pathway in chondrocytes. Arch. Physiol. Biochem. 2023, 129, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Paik, I.Y.; Ryu, J.H.; Lee, T.H.; Kim, D.E. Effects of aerobic and resistance exercises on circulating apelin-12 and apelin-36 concentrations in obese middle-aged women: A randomized controlled trial. BMC Women’s Health 2019, 19, 23. [Google Scholar] [CrossRef]
- Ghanbari-Niaki, A.; Saeidi, A.; Gharahcholo, L.; Moradi-Dehbaghi, K.; Zare-Kookandeh, N.; Ahmadian, M.; Zouhal, H.; Hackney, A.C. Influence of resistance training and herbal supplementation on plasma apelin and metabolic syndrome risk factors in postmenopausal women. Sci. Sports 2020, 35, 109.e1–109.e5. [Google Scholar] [CrossRef]
- Kim, S.; Park, D.H.; Lee, S.H.; Kwak, H.B.; Kang, J.H. Contribution of High-Intensity Interval Exercise in the Fasted State to Fat Browning: Potential Roles of Lactate and beta-hydroxybutyrate. Med. Sci. Sports Exerc. 2023, 55, 1160. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.J.; Zhu, J.Y.; Chen, M.; Guo, L. Exercise-Mediated Browning of White Adipose Tissue: Its Significance, Mechanism and Effectiveness. Int. J. Mol. Sci. 2021, 22, 11512. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Ye, M.; Resch, J.M.; Jedrychowski, M.P.; Hu, B.; Lowell, B.B.; Ginty, D.D.; Spiegelman, B.M. Innervation of thermogenic adipose tissue via a calsyntenin 3beta-S100b axis. Nature 2019, 569, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Delgado, G.; Martinez-Tellez, B.; Olza, J.; Aguilera, C.M.; Gil, A.; Ruiz, J.R. Role of Exercise in the Activation of Brown Adipose Tissue. Ann. Nutr. Metab. 2015, 67, 21–32. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, R.; Lucertini, F.; Guescini, M.; Polidori, E.; Zeppa, S.; Stocchi, V.; Cinti, S.; Cuppini, R. Exercise as a new physiological stimulus for brown adipose tissue activity. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, M. Neuregulin 4 as a novel adipokine in energy metabolism. Front. Physiol. 2022, 13, 1106380. [Google Scholar] [CrossRef]
- Bluher, M. Neuregulin 4: A “Hotline” Between Brown Fat and Liver. Obesity 2019, 27, 1555–1557. [Google Scholar] [CrossRef] [PubMed]
- Tutunchi, H.; Ostadrahimi, A.; Hosseinzadeh-Attar, M.J.; Miryan, M.; Mobasseri, M.; Ebrahimi-Mameghani, M. A systematic review of the association of neuregulin 4, a brown fat-enriched secreted factor, with obesity and related metabolic disturbances. Obes. Rev. 2020, 21, e12952. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Meyers, C.A.; Guerra, N.K.; King, M.A.; Meyer, E.M. The effects of rAAV2-mediated NGF gene delivery in adult and aged rats. Mol. Ther. 2004, 9, 262–269. [Google Scholar] [CrossRef]
- Camerino, C.; Conte, E.; Cannone, M.; Caloiero, R.; Fonzino, A.; Tricarico, D. Nerve Growth Factor, Brain-Derived Neurotrophic Factor and Osteocalcin Gene Relationship in Energy Regulation, Bone Homeostasis and Reproductive Organs Analyzed by mRNA Quantitative Evaluation and Linear Correlation Analysis. Front. Physiol. 2016, 7, 456. [Google Scholar] [CrossRef]
- Morozzi, G.; Beccafico, S.; Bianchi, R.; Riuzzi, F.; Bellezza, I.; Giambanco, I.; Arcuri, C.; Minelli, A.; Donato, R. Oxidative stress-induced S100B accumulation converts myoblasts into brown adipocytes via an NF-kappaB/YY1/miR-133 axis and NF-kappaB/YY1/BMP-7 axis. Cell Death Differ. 2017, 24, 2077–2088. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Song, R.; Du, Y.; Liu, H.; Li, X. Adtrp regulates thermogenic activity of adipose tissue via mediating the secretion of S100b. Cell Mol. Life Sci. 2022, 79, 407. [Google Scholar] [CrossRef] [PubMed]
- Saeidi, A.; Shishvan, S.R.; Soltani, M.; Tarazi, F.; Doyle-Baker, P.K.; Shahrbanian, S.; Mollabashi, S.S.; Khosravi, N.; Laher, I.; Moriarty, T.A.; et al. Differential Effects of Exercise Programs on Neuregulin 4, Body Composition and Cardiometabolic Risk Factors in Men With Obesity. Front. Physiol. 2021, 12, 797574. [Google Scholar] [CrossRef] [PubMed]
- Tayebi, S.M.; Ghanbari-Niaki, A.; Saeidi, A.; Hackney, A.C. Exercise Training, Neuregulin 4 and Obesity. Ann. Appl. Sport. Sci. 2017, 5, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Guma, A.; Diaz-Saez, F.; Camps, M.; Zorzano, A. Neuregulin, an Effector on Mitochondria Metabolism That Preserves Insulin Sensitivity. Front. Physiol. 2020, 11, 696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Y.; Deng, S.; Mo, Y.; Huang, Y.; Li, W.; Ge, C.; Ren, X.; Zhang, H.; Zhang, X.; et al. S100B/RAGE/Ceramide signaling pathway is involved in sepsis-associated encephalopathy. Life Sci. 2021, 277, 119490. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, P.; Krishnan, V.; Fettel, K.; Gao, P.; Zhu, Z.; Ren, J.; Thyagarajan, B. TRPV1 activation counters diet-induced obesity through sirtuin-1 activation and PRDM-16 deacetylation in brown adipose tissue. Int. J. Obes. 2017, 41, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Kajimura, S.; Yang, W.; Chin, S.; Rohas, L.M.; Uldry, M.; Tavernier, G.; Langin, D.; Spiegelman, B.M. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007, 6, 38–54. [Google Scholar] [CrossRef]
- Tseng, Y.H.; Kokkotou, E.; Schulz, T.J.; Huang, T.L.; Winnay, J.N.; Taniguchi, C.M.; Tran, T.T.; Suzuki, R.; Espinoza, D.O.; Yamamoto, Y.; et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 2008, 454, 1000–1004. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).