No Differences in Urine Bisphenol A Concentrations between Subjects Categorized with Normal Cognitive Function and Mild Cognitive Impairment Based on Montreal Cognitive Assessment Scores
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Oversight
2.2. Study Population
2.3. Montreal Cognitive Assessment Scale
2.4. Hamilton Depression Rating Scale
2.5. International Physical Activity Questionnaires
2.6. Anthropometric Parameters and Body Composition
2.7. Sociodemographic Questionnaire
2.8. Bisphenol A Levels
2.9. Minimum Sample Size Calculation
2.10. Statistical Analysis
3. Results
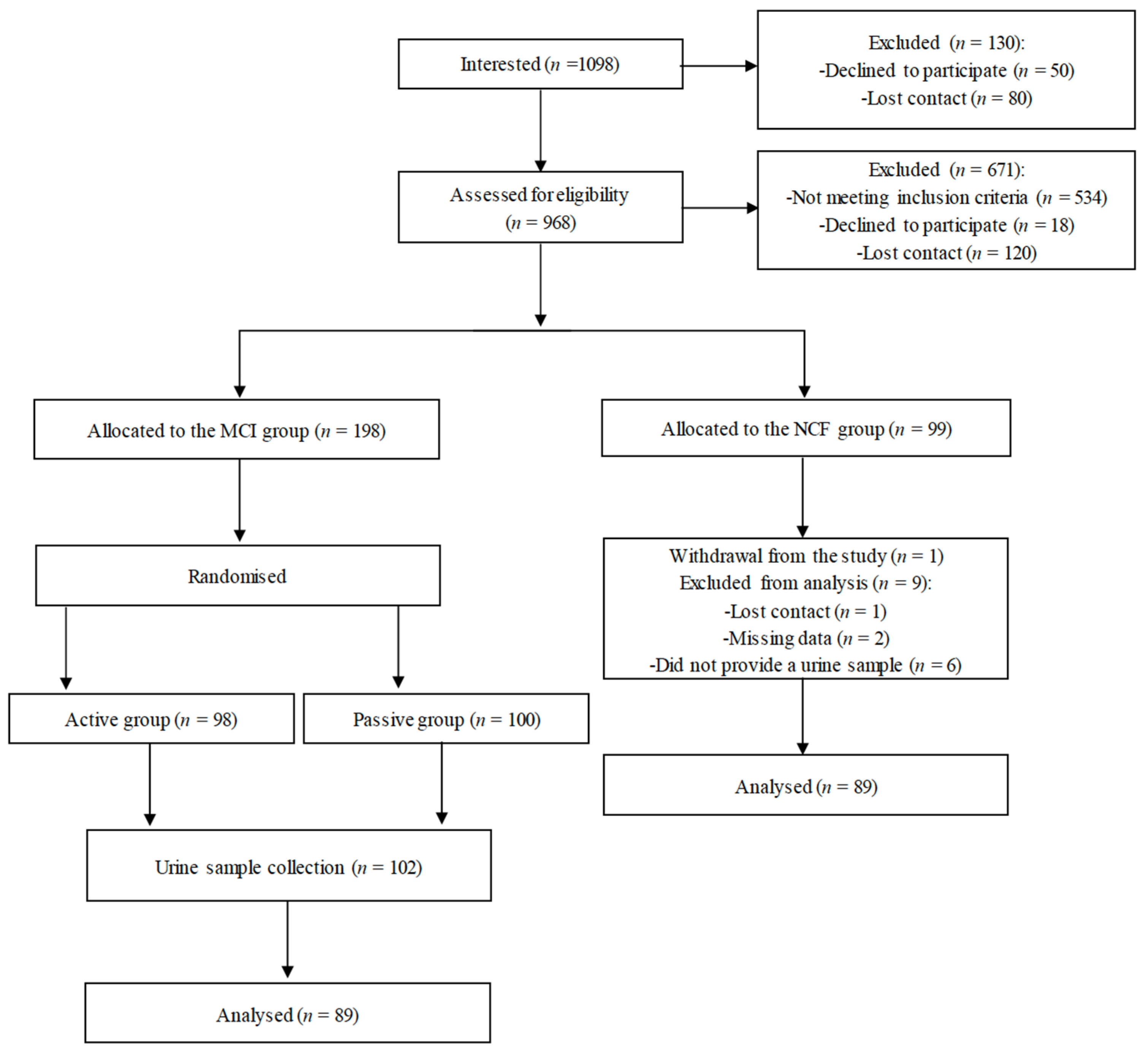
3.1. Study Workflow
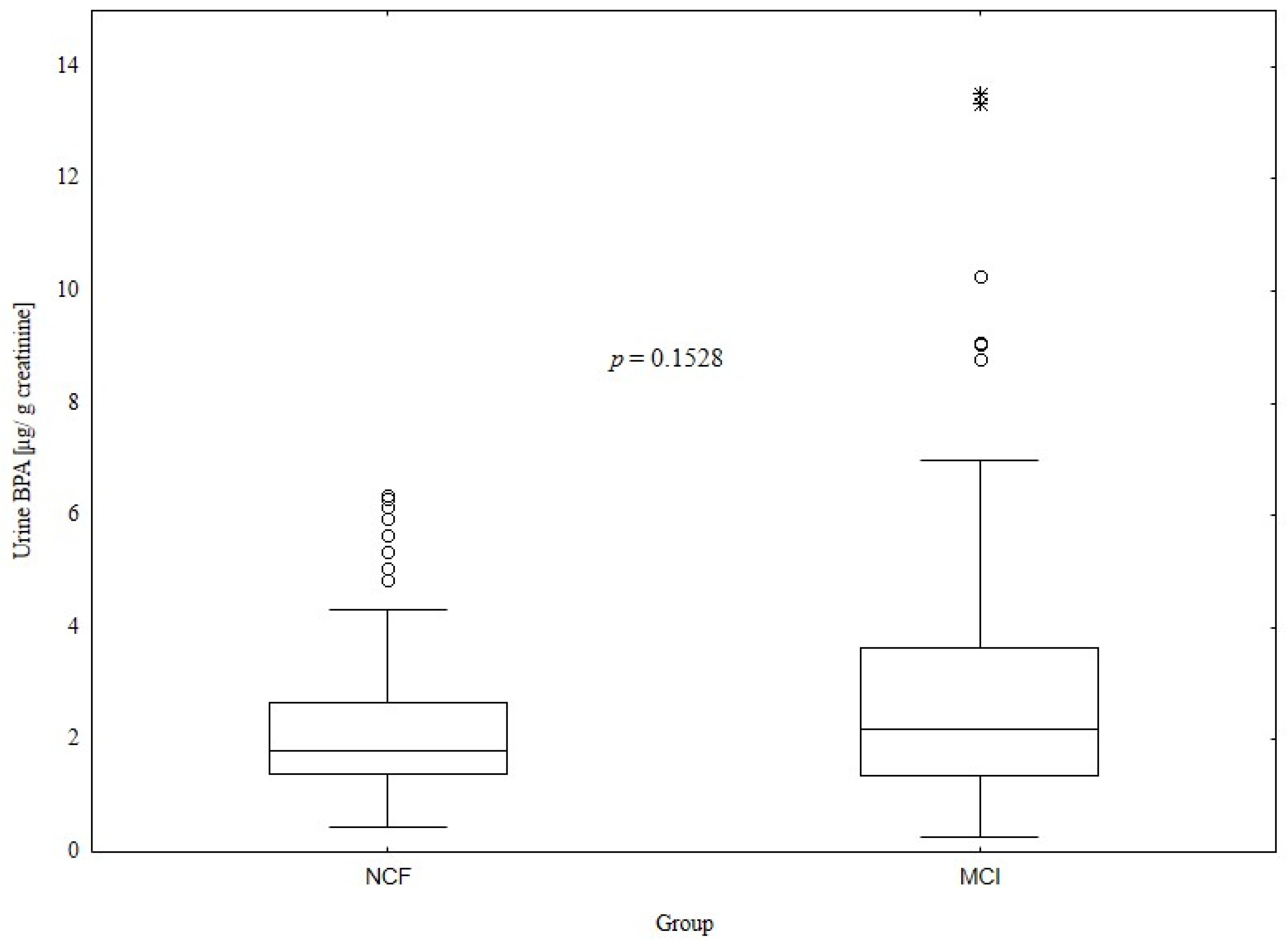
3.2. Comparison of Bisphenol A Levels between the Normal Cognitive Function and Mild Cognitive Impairment Groups
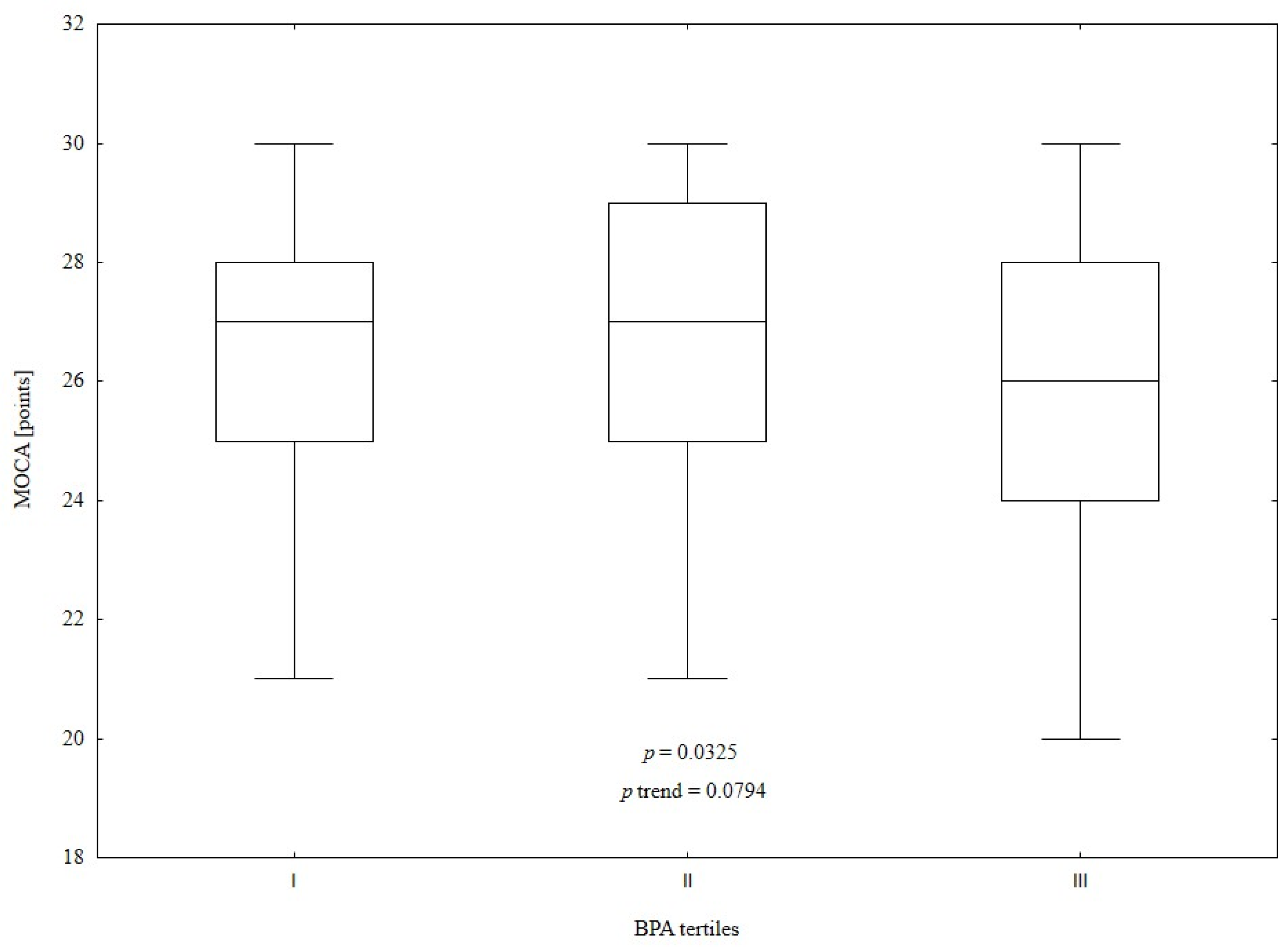
3.3. Comparison of Study Population According to Urine Bisphenol A Tertiles
3.4. Associations between Urine Bisphenol A Concentrations and Predictive Variables
3.5. Predictive Factors of Mild Cognitive Impairment Prevalence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations Department of Economic and Social Affairs. World Population Prospects: The 2017 Revision; Key Findings & Advance Tables. Working Paper no. ESA/P/WP/248 2017; United Nations Department of Economic and Social Affairs: New York, NY, USA, 2017. [Google Scholar]
- Song, W.X.; Wu, W.W.; Zhao, Y.Y.; Xu, H.L.; Chen, G.C.; Jin, S.Y.; Chen, J.; Xian, S.X.; Liang, J.H. Evidence from a Meta-Analysis and Systematic Review Reveals the Global Prevalence of Mild Cognitive Impairment. Front. Aging Neurosci. 2023, 15, 1227112. [Google Scholar] [CrossRef] [PubMed]
- Winblad, B.; Palmer, K.; Kivipelto, M.; Jelic, V.; Fratiglioni, L.; Wahlund, L.O.; Nordberg, A.; Bäckman, L.; Albert, M.; Almkvist, O.; et al. Mild Cognitive Impairment—Beyond Controversies, towards a Consensus: Report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 2004, 256, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Caracciolo, B.; Brayne, C.; Gauthier, S.; Jelic, V.; Fratiglioni, L. Mild Cognitive Impairment: A Concept in Evolution. J. Intern. Med. 2014, 275, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C. Mild Cognitive Impairment as a Diagnostic Entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Shiri-Feshki, M. Rate of Progression of Mild Cognitive Impairment to Dementia—Meta-Analysis of 41 Robust Inception Cohort Studies. Acta Psychiatr. Scand. 2009, 119, 252–265. [Google Scholar] [CrossRef]
- Jones, A.; Ali, M.U.; Kenny, M.; Mayhew, A.; Mokashi, V.; He, H.; Lin, S.; Yavari, E.; Paik, K.; Subramanian, D.; et al. Potentially Modifiable Risk Factors for Dementia and Mild Cognitive Impairment: An Umbrella Review and Meta-Analysis. Dement. Geriatr. Cogn. Disord. 2024. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; Singh, A.; Vellapandian, C. Bisphenol A Exposure Links to Exacerbation of Memory and Cognitive Impairment: A Systematic Review of the Literature. Neurosci. Biobehav. Rev. 2022, 143, 104939. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.F.; Tariq, T.; Fatima, B.; Sahar, A.; Tariq, F.; Munir, S.; Khan, S.; Nawaz Ranjha, M.M.A.; Sameen, A.; Zeng, X.A.; et al. An Insight into Bisphenol A, Food Exposure and Its Adverse Effects on Health: A Review. Front. Nutr. 2022, 9, 1047827. [Google Scholar] [CrossRef]
- Ginsberg, G.; Rice, D.C. Does Rapid Metabolism Ensure Negligible Risk from Bisphenol A? Environ. Health Perspect. 2009, 117, 1639–1643. [Google Scholar] [CrossRef]
- Völkel, W.; Colnot, T.; Csanády, G.A.; Filser, J.G.; Dekant, W. Metabolism and Kinetics of Bisphenol a in Humans at Low Doses Following Oral Administration. Chem. Res. Toxicol. 2002, 15, 1281–1287. [Google Scholar] [CrossRef]
- Calafat, A.M.; Ye, X.; Wong, L.Y.; Reidy, J.A.; Needham, L.L. Exposure of the U.S. Population to Bisphenol A and 4-Tertiary-Octylphenol: 2003-2004. Environ. Health Perspect. 2008, 116, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.H.; Lee, C.Y.; Chuang, Y.S.; Shih, C.L. Exposure to Bisphenol A Associated with Multiple Health-Related Outcomes in Humans: An Umbrella Review of Systematic Reviews with Meta-Analyses. Environ. Res. 2023, 237, 116900. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Liu, B.; Rong, S.; Dai, S.Y.; Trasande, L.; Lehmler, H.J. Association between Bisphenol A Exposure and Risk of All-Cause and Cause-Specific Mortality in US Adults. JAMA Netw. Open 2020, 3, e2011620. [Google Scholar] [CrossRef] [PubMed]
- Kawa, I.A.; Masood, A.; Fatima, Q.; Mir, S.A.; Jeelani, H.; Manzoor, S.; Rashid, F. Endocrine Disrupting Chemical Bisphenol A and Its Potential Effects on Female Health. Diabetes Metab. Syndr. 2021, 15, 803–811. [Google Scholar] [CrossRef]
- Ni, Y.; Hu, L.; Yang, S.; Ni, L.; Ma, L.; Zhao, Y.; Zheng, A.; Jin, Y.; Fu, Z. Bisphenol A Impairs Cognitive Function and 5-HT Metabolism in Adult Male Mice by Modulating the Microbiota-Gut-Brain Axis. Chemosphere 2021, 282, 130952. [Google Scholar] [CrossRef]
- Lin, C.C.; Chien, C.J.; Tsai, M.S.; Hsieh, C.J.; Hsieh, W.S.; Chen, P.C. Prenatal Phenolic Compounds Exposure and Neurobehavioral Development at 2 and 7 Years of Age. Sci. Total Environ. 2017, 605–606, 801–810. [Google Scholar] [CrossRef]
- Degirmencioglu Gok, D.; Tuygar Okutucu, F.; Ozturk, N.; Ceyhun, H.A. Association of Bisphenol A with Cognitive Functions and Functionality in Adult Attention Deficit Hyperactivity Disorder. J. Psychiatr. Res. 2024, 169, 64–72. [Google Scholar] [CrossRef]
- Zhang, H.; Kuang, H.; Luo, Y.; Liu, S.; Meng, L.; Pang, Q.; Fan, R. Low-Dose Bisphenol A Exposure Impairs Learning and Memory Ability with Alterations of Neuromorphology and Neurotransmitters in Rats. Sci. Total Environ. 2019, 697, 134036. [Google Scholar] [CrossRef]
- Kim, M.E.; Park, H.R.; Gong, E.J.; Choi, S.Y.; Kim, H.S.; Lee, J. Exposure to Bisphenol A Appears to Impair Hippocampal Neurogenesis and Spatial Learning and Memory. Food Chem. Toxicol. 2011, 49, 3383–3389. [Google Scholar] [CrossRef]
- Johnson, S.A.; Javurek, A.B.; Painter, M.S.; Ellersieck, M.R.; Welsh, T.H.; Camacho, L.; Lewis, S.M.; Vanlandingham, M.M.; Ferguson, S.A.; Rosenfeld, C.S. Effects of Developmental Exposure to Bisphenol A on Spatial Navigational Learning and Memory in Rats: A CLARITY-BPA Study. Horm. Behav. 2016, 80, 139–148. [Google Scholar] [CrossRef]
- Huang, Z.; Fu, W.; Dou, L.; Bao, H.; Wu, W.; Su, P.; Huang, K.; Zhu, P.; Sheng, J.; Xu, Y.; et al. Prenatal Bisphenol A Exposure and Early Childhood Behavior and Cognitive Function: A Chinese Birth Cohort Study. Neuroendocrinology 2022, 112, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yang, F.; Li, D.K.; Tian, Y.; Miao, M.; Zhang, Y.; Ji, H.; Yuan, W.; Liang, H. Prenatal Bisphenol A Exposure, Fetal Thyroid Hormones and Neurobehavioral Development in Children at 2 and 4 Years: A Prospective Cohort Study. Sci. Total Environ. 2020, 722, 137887. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Reynolds, J.E.; Long, M.; Ostertag, C.; Pollock, T.; Hamilton, M.; Dunn, J.F.; Liu, J.; Martin, J.; Grohs, M.; et al. The Effects of Prenatal Bisphenol A Exposure on Brain Volume of Children and Young Mice. Environ. Res. 2022, 214, 114040. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Sawicka-Gutaj, N.; Gruszczyński, D.; Guzik, P.; Mostowska, A.; Walkowiak, J. Publication Ethics of Human Studies in the Light of the Declaration of Helsinki—A Mini-Review. JMS 2022, 91, e700. [Google Scholar] [CrossRef]
- Jamka, M.; Chrobot, M.; Jaworska, N.; Brylak, J.; Makarewicz-Bukowska, A.; Popek, J.; Janicka, A.; Walkowiak, J. Comparison of Eating Habits, Body Composition and Densitometric Parameters between Subjects with Normal Cognitive Function and Mild Cognitive Impairment: An Observational Study. Nutrients 2024, 16, 644. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Rush, J.; First, M.; Blacker, D. (Eds.) Handbook of Psychiatric Measures; American Psychiatric Publishing Inc.: Arlington, TX, USA, 2008. [Google Scholar]
- Hamilton, M. A Rating Scale for Depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Biernat, E. International Physical Activity Questionnaire—Polish Long Version. Polish J. Sport. Med. 2013, 29, 1–15. [Google Scholar]
- World Health Organization A Healthy Lifestyle—WHO Recommendations. Available online: https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle---who-recommendations (accessed on 1 April 2024).
- World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation; World Health Organization: Genewa, Switzerland, 2008. [Google Scholar]
- Minetto, M.A.; Busso, C.; Lalli, P.; Gamerro, G.; Massazza, G. DXA-Derived Adiposity and Lean Indices for Management of Cardiometabolic and Musculoskeletal Frailty: Data Interpretation Tricks and Reporting Tips. Front. Rehabil. Sci. 2021, 2, 712977. [Google Scholar] [CrossRef]
- Jamka, M.; Makarewicz, A.; Wasiewicz-Gajdzis, M.; Brylak, J.; Wielińska-Wiśniewska, H.; Pawlak, Z.; Nowak, J.K.; Herzig, K.-H.; Mądry, E.; Walkowiak, J. App-Assured Essential Physical Activity for the Prevention of Cognitive Decline: Changing Paradigms in Public Health—A Study Protocol for a Randomised Controlled Trial: A Study Protocol of the PA PROTECT Study. JMS 2021, 90, e530. [Google Scholar] [CrossRef]
- Calafat, A.M.; Kuklenyik, Z.; Reidy, J.A.; Caudill, S.P.; Ekong, J.; Needham, L.L. Urinary Concentrations of Bisphenol A and 4-Nonylphenol in a Human Reference Population. Environ. Health Perspect. 2005, 113, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.M.; Kolossa-Gehring, M.; Schröter-Kermani, C.; Angerer, J.; Brüning, T. Bisphenol A in 24 h Urine and Plasma Samples of the German Environmental Specimen Bank from 1995 to 2009: A Retrospective Exposure Evaluation. J Expo Sci Environ Epidemiol 2012, 22, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Hwang, M.S.; Ko, A.; Jeong, D.H.; Lee, J.M.; Moon, G.; Lee, K.S.; Kho, Y.H.; Shin, M.K.; Lee, H.S.; et al. Risk Assessment Based on Urinary Bisphenol A Levels in the General Korean Population. Environ. Res. 2016, 150, 606–615. [Google Scholar] [CrossRef] [PubMed]
- IndexBox Global Canned Food Market Report 2024—Prices, Size, Forecast, and Companies. Available online: https://www.indexbox.io/store/world-homogenized-composite-food-preparations-market-report-analysis-and-forecast-to-2020/ (accessed on 25 March 2024).
- Colorado-Yohar, S.M.; Castillo-González, A.C.; Sánchez-Meca, J.; Rubio-Aparicio, M.; Sánchez-Rodríguez, D.; Salamanca-Fernández, E.; Ardanaz, E.; Amiano, P.; Fernández, M.F.; Mendiola, J.; et al. Concentrations of Bisphenol-A in Adults from the General Population: A Systematic Review and Meta-Analysis. Sci. Total Environ. 2021, 775, 145755. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Liu, J.; Wang, C.; Ouyang, Z.; Kuang, J.; Pang, Q.; Fan, R. Sex-Specific Oxidative Damage Effects Induced by BPA and Its Analogs on Primary Hippocampal Neurons Attenuated by EGCG. Chemosphere 2021, 264, 128450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Y.; Wang, T.; Zhang, X.; Zhang, J.; Ji, X.; Lu, L. Distribution and Potential Risk Factors of Bisphenol a in Serum and Urine among Chinese from 2004 to 2019. Front. Public Health 2024, 12, 1196248. [Google Scholar] [CrossRef] [PubMed]
- Valentino, R.; D’Esposito, V.; Ariemma, F.; Cimmino, I.; Beguinot, F.; Formisano, P. Bisphenol A Environmental Exposure and the Detrimental Effects on Human Metabolic Health: Is It Necessary to Revise the Risk Assessment in Vulnerable Population? J. Endocrinol. Investig. 2016, 39, 259–263. [Google Scholar] [CrossRef]
- Moon, S.; Yu, S.H.; Lee, C.B.; Park, Y.J.; Yoo, H.J.; Kim, D.S. Effects of Bisphenol A on Cardiovascular Disease: An Epidemiological Study Using National Health and Nutrition Examination Survey 2003-2016 and Meta-Analysis. Sci. Total Environ. 2021, 763, 142941. [Google Scholar] [CrossRef]
- Hwang, S.; Lim, J.E.; Choi, Y.; Jee, S.H. Bisphenol A Exposure and Type 2 Diabetes Mellitus Risk: A Meta-Analysis. BMC Endocr. Disord. 2018, 18, 81. [Google Scholar] [CrossRef]
- Wu, W.; Li, M.; Liu, A.; Wu, C.; Li, D.; Deng, Q.; Zhang, B.; Du, J.; Gao, X.; Hong, Y. Bisphenol A and the Risk of Obesity a Systematic Review With Meta-Analysis of the Epidemiological Evidence. Dose Response 2020, 18, 1559325820916949. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wen, S.; Yuan, D.; Peng, L.; Zeng, R.; Yang, Z.; Liu, Q.; Xu, L.; Kang, D. The Association between the Environmental Endocrine Disruptor Bisphenol A and Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Gynecol. Endocrinol. 2018, 34, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Makarewicz, A.; Jamka, M.; Wasiewicz-Gajdzis, M.; Bajerska, J.; Miśkiewicz-Chotnicka, A.; Kwiecień, J.; Lisowska, A.; Gagnon, D.; Herzig, K.H.; Mądry, E.; et al. Comparison of Subjective and Objective Methods to Measure the Physical Activity of Non-Depressed Middle-Aged Healthy Subjects with Normal Cognitive Function and Mild Cognitive Impairment-A Cross-Sectional Study. Int. J. Environ. Res. Public. Health 2021, 18, 8042. [Google Scholar] [CrossRef] [PubMed]
- Månsson, T.; Overton, M.; Pihlsgård, M.; Elmståhl, S. Impaired kidney function is associated with lower cognitive function in the elder general population. Results from the Good Aging in Skåne (GÅS) cohort study. BMC Geriatr. 2019, 9, 360. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carrillo, A.; Mustieles, V.; Pérez-Lobato, R.; Molina-Molina, J.M.; Reina-Pérez, I.; Vela-Soria, F.; Rubio, S.; Olea, N.; Fernández, M.F. Bisphenol A and Cognitive Function in School-Age Boys: Is BPA Predominantly Related to Behavior? Neurotoxicology 2019, 74, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Muckle, G.; Arbuckle, T.; Bouchard, M.F.; Fraser, W.D.; Ouellet, E.; Séguin, J.R.; Oulhote, Y.; Webster, G.M.; Lanphear, B.P. Associations of Penatal Urinary Bisphenol A Concentrations with Child Behaviors and Cognitive Abilities. Environ. Health Perspect. 2017, 125, 067008. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Chianese, R.; Troisi, J.; Richards, S.; Nori, S.L.; Fasano, S.; Guida, M.; Plunk, E.; Viggiano, A.; Pierantoni, R.; et al. Neuro-Toxic and Reproductive Effects of BPA. Curr. Neuropharmacol. 2019, 17, 1109–1132. [Google Scholar] [CrossRef] [PubMed]
- Costa, H.E.; Cairrao, E. Effect of Bisphenol A on the Neurological System: A Review Update. Arch. Toxicol. 2024, 98, 1–73. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, P.; Huang, Q.; Chi, Y.; Dong, S.; Fan, J. Bisphenol-A Induces Neurodegeneration through Disturbance of Intracellular Calcium Homeostasis in Human Embryonic Stem Cells-Derived Cortical Neurons. Chemosphere 2019, 229, 618–630. [Google Scholar] [CrossRef]
- Sillapachaiyaporn, C.; Chuchawankul, S.; Nilkhet, S.; Moungkote, N.; Sarachana, T.; Ung, A.T.; Baek, S.J.; Tencomnao, T. Ergosterol Isolated from Cloud Ear Mushroom (Auricularia polytricha) Attenuates Bisphenol A-Induced BV2 Microglial Cell Inflammation. Food Res. Int. 2022, 157, 111433. [Google Scholar] [CrossRef]
- Ishtiaq, A.; Ali, T.; Bakhtiar, A.; Bibi, R.; Bibi, K.; Mushtaq, I.; Li, S.; Khan, W.; Khan, U.; Anis, R.A.; et al. Melatonin Abated Bisphenol A-Induced Neurotoxicity via P53/PUMA/Drp-1 Signaling. Environ. Sci. Pollut. Res. Int. 2021, 28, 17789–17801. [Google Scholar] [CrossRef] [PubMed]
- El-Missiry, M.A.; Othman, A.I.; Al-Abdan, M.A.; El-Sayed, A.A. Melatonin Ameliorates Oxidative Stress, Modulates Death Receptor Pathway Proteins, and Protects the Rat Cerebrum against Bisphenol-A-Induced Apoptosis. J. Neurol. Sci. 2014, 347, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Yadav, A.; Tiwari, S.K.; Seth, B.; Chauhan, L.K.S.; Khare, P.; Ray, R.S.; Chaturvedi, R.K. Dynamin-Related Protein 1 Inhibition Mitigates Bisphenol A-Mediated Alterations in Mitochondrial Dynamics and Neural Stem Cell Proliferation and Differentiation. J. Biol. Chem. 2016, 291, 15923–15939. [Google Scholar] [CrossRef]
- Khan, J.; Salhotra, S.; Goswami, P.; Akhtar, J.; Jahan, S.; Gupta, S.; Sharma, S.; Banerjee, B.D.; Parvez, S.; Gupta, S.; et al. Bisphenol A Triggers Axonal Injury and Myelin Degeneration with Concomitant Neurobehavioral Toxicity in C57BL/6J Male Mice. Toxicology 2019, 428, 152299. [Google Scholar] [CrossRef] [PubMed]
- Chesnut, M.; Paschoud, H.; Repond, C.; Smirnova, L.; Hartung, T.; Zurich, M.G.; Hogberg, H.T.; Pamies, D. Human IPSC-Derived Model to Study Myelin Disruption. Int. J. Mol. Sci. 2021, 22, 9473. [Google Scholar] [CrossRef] [PubMed]
- Moyano, P.; Flores, A.; García, J.; García, J.M.; Anadon, M.J.; Frejo, M.T.; Sola, E.; Pelayo, A.; del Pino, J. Bisphenol A Single and Repeated Treatment Increases HDAC2, Leading to Cholinergic Neurotransmission Dysfunction and SN56 Cholinergic Apoptotic Cell Death through AChE Variants Overexpression and NGF/TrkA/P75NTR Signaling Disruption. Food Chem. Toxicol. 2021, 157, 112614. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhou, W.; Sun, S.; Du, X.; Han, Y.; Shi, W.; Liu, G. Immunotoxicity and Neurotoxicity of Bisphenol A and Microplastics Alone or in Combination to a Bivalve Species, Tegillarca granosa. Environ. Pollut. 2020, 265, 115115. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Meng, L.; Kuang, H.; Liu, J.; Lv, X.; Pang, Q.; Fan, R. Maternal Exposure to Environmental Bisphenol A Impairs the Neurons in Hippocampus across Generations. Toxicology 2020, 432, 152393. [Google Scholar] [CrossRef]
- Khadrawy, Y.A.; Noor, N.A.; Mourad, I.M.; Ezz, H.S.A. Neurochemical Impact of Bisphenol A in the Hippocampus and Cortex of Adult Male Albino Rats. Toxicol. Ind. Health 2016, 32, 1711–1719. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Agarwal, S.; Tripathi, A.; Chaturvedi, R.K. Bisphenol-A Mediated Inhibition of Hippocampal Neurogenesis Attenuated by Curcumin via Canonical Wnt Pathway. Mol. Neurobiol. 2016, 53, 3010–3029. [Google Scholar] [CrossRef]
- Hu, F.; Li, T.; Gong, H.; Chen, Z.; Jin, Y.; Xu, G.; Wang, M. Bisphenol A Impairs Synaptic Plasticity by Both Pre- and Postsynaptic Mechanisms. Adv. Sci. 2017, 4, 1600493. [Google Scholar] [CrossRef]
- Di Pietro, P.; D’Auria, R.; Viggiano, A.; Ciaglia, E.; Meccariello, R.; Russo, R.D.; Puca, A.A.; Vecchione, C.; Nori, S.L.; Santoro, A. Bisphenol A Induces DNA Damage in Cells Exerting Immune Surveillance Functions at Peripheral and Central Level. Chemosphere 2020, 254, 126819. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, P.; Qian, W.; Li, Y.; Zhao, J.; Huan, F.; Wang, J.; Xiao, H. Perinatal Bisphenol A Exposure and Adult Glucose Homeostasis: Identifying Critical Windows of Exposure. PLoS ONE 2013, 8, e64143. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Fang, F.; Chen, D.; Gao, Y.; Liu, J.; Gao, R.; Wang, J.; Xiao, H. Bisphenol A Disrupts Glucose Transport and Neurophysiological Role of IR/IRS/AKT/GSK3β Axis in the Brain of Male Mice. Environ. Toxicol. Pharmacol. 2016, 43, 7–12. [Google Scholar] [CrossRef]
- Klenke, U.; Constantin, S.; Wray, S. BPA Directly Decreases GnRH Neuronal Activity via Noncanonical Pathway. Endocrinology 2016, 157, 1980–1990. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xie, C.; Yu, P.; Fang, F.; Zhu, J.; Cheng, J.; Gu, A.; Wang, J.; Xiao, H. Involvement of Insulin Signaling Disturbances in Bisphenol A-Induced Alzheimer’s Disease-like Neurotoxicity. Sci. Rep. 2017, 7, 7497. [Google Scholar] [CrossRef] [PubMed]
- Blondell, S.J.; Hammersley-Mather, R.; Veerman, J.L. Does Physical Activity Prevent Cognitive Decline and Dementia?: A Systematic Review and Meta-Analysis of Longitudinal Studies. BMC Public Health 2014, 14, 510. [Google Scholar] [CrossRef] [PubMed]
- Law, C.K.; Lam, F.M.; Chung, R.C.; Pang, M.Y. Physical Exercise Attenuates Cognitive Decline and Reduces Behavioural Problems in People with Mild Cognitive Impairment and Dementia: A Systematic Review. J. Physiother. 2020, 66, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, Y.; Peng, W.; Wang, M.; Chen, X.; Li, M.; Ruan, Y.; Sun, S.; Yang, T.; Yang, Y.; et al. Association between Physical Activity and Mild Cognitive Impairment in Community-Dwelling Older Adults: Depression as a Mediator. Front. Aging Neurosci. 2022, 14, 964886. [Google Scholar] [CrossRef]
- Bai, W.; Chen, P.; Cai, H.; Zhang, Q.; Su, Z.; Cheung, T.; Jackson, T.; Sha, S.; Xiang, Y.T. Worldwide Prevalence of Mild Cognitive Impairment among Community Dwellers Aged 50 Years and Older: A Meta-Analysis and Systematic Review of Epidemiology Studies. Age Ageing 2022, 51, afac173. [Google Scholar] [CrossRef]
- Xue, H.P.; Hou, P.; Li, Y.N.; Mao, X.; Wu, L.F.; Liu, Y.B. Factors for Predicting Reversion from Mild Cognitive Impairment to Normal Cognition: A Meta-Analysis. Int. J. Geriatr. Psychiatry 2019, 34, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Vadikolias, K.; Tsiakiri-Vatamidis, A.; Tripsianis, G.; Tsivgoulis, G.; Ioannidis, P.; Serdari, A.; Heliopoulos, J.; Livaditis, M.; Piperidou, C. Mild Cognitive Impairment: Effect of Education on the Verbal and Nonverbal Tasks Performance Decline. Brain Behav. 2012, 2, 627. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Tan, L.; Wang, H.F.; Tan, M.S.; Tan, L.; Li, J.Q.; Zhao, Q.F.; Yu, J.T. Education and Risk of Dementia: Dose-Response Meta-Analysis of Prospective Cohort Studies. Mol. Neurobiol. 2016, 53, 3113–3123. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.M.; Calafat, A.M. Human Body Burdens of Chemicals Used in Plastic Manufacture. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2078. [Google Scholar] [CrossRef]
- Anastasio, P.; Cirillo, M.; Spitali, L.; Frangiosa, A.; Pollastro, R.M.; De Santo, N.G. Level of Hydration and Renal Function in Healthy Humans. Kidney Int. 2001, 60, 748–756. [Google Scholar] [CrossRef]
| Total (n = 178) | NCF (n = 89) | MCI (n = 89) | p | |
|---|---|---|---|---|
| Median (Q1–Q3) | ||||
| Age [years] | 58 (54–62) | 56 (54–61) | 59 (54–62) | 0.5385 |
| Weight [kg] | 75.50 (64.40–87.50) | 76.0 (64.4–91.0) | 75.00 (64.50–84.60) | 0.4750 |
| BMI [kg/m2] | 27.07 (23.88–30.39) | 26.70 (23.53–30.75) | 27.12 (24.56–30.00) | 0.6605 |
| Waist circumference [cm] | 90 (80–100) | 89 (80–102) | 93 (80–100) | 0.8728 |
| LMI [kg/m2] | 16.05 (14.20–18.00) | 16.00 (14.10–18.30) | 16.10 (14.40–17.70) | 0.8958 |
| ALMI [kg/m2] | 6.73 (5.87–7.88) | 6.91 (5.85–8.00) | 6.66 (5.91–7.61) | 0.5326 |
| Total physical activity [min/day] | 99 (56–155) | 105 (59–163) | 81 (54–150) | 0.1259 |
| HAM-D [points] | 3 (1–6) | 4 (1–6) | 3 (1–5) | 0.8846 |
| Total (n = 178) | NCF (n = 89) | MCI (n = 89) | p | ||
|---|---|---|---|---|---|
| n (%) | |||||
| Sex | Women | 130 (73.0%) | 64 (71.9%) | 66 (74.2%) | 0.8660 |
| Men | 48 (27.0%) | 25 (28.1%) | 23 (25.8%) | ||
| Place of residence | Village | 29 (16.3%) | 13 (14.6%) | 16 (18.0%) | 0.8504 |
| City < 50.000 inhabitants | 14 (7.9%) | 6 (6.7%) | 8 (9.0%) | ||
| City of 50.000–500.000 inhabitants | 15 (8.4%) | 8 (9.0%) | 7 (7.9%) | ||
| City > 500.000 inhabitants | 120 (67.4%) | 62 (69.7%) | 58 (65.2%) | ||
| Relationship status | Formal/informal relationship | 137 (77.0%) | 72 (80.9%) | 65 (73.0%) | 0.2631 |
| Single | 40 (22.5%) | 17 (19.1%) | 23 (25.8%) | ||
| No information | 1 (0.6%) | 0 (0.0%) | 1 (1.1%) | ||
| Education | Primary | 2 (1.1%) | 0 (0.0%) | 2 (2.2%) | 0.0562 |
| Vocational | 6 (3.4%) | 1 (1.1%) | 5 (5.6%) | ||
| Secondary | 29 (16.3%) | 11 (12.4%) | 18 (20.2%) | ||
| High | 141 (79.2%) | 77 (86.5%) | 64 (71.9%) | ||
| Socio-occupational status | Employed | 144 (80.9%) | 74 (83.1%) | 70 (78.7%) | 0.7367 |
| Unemployed | 2 (1.1%) | 1 (1.1%) | 1 (1.1%) | ||
| Pensioner | 32 (18.0%) | 14 (15.7%) | 18 (20.2%) | ||
| Financial situation | Very good | 16 (9.0%) | 7 (7.9%) | 9 (10.1%) | 0.5156 |
| Good | 121 (66.0%) | 60 (67.4%) | 61 (68.5%) | ||
| Mediocre | 39 (21.9%) | 20 (22.5%) | 19 (21.3%) | ||
| Bad | 2 (1.1%) | 2 (2.2%) | 0 (0.0%) | ||
| Formerly smoking | Yes | 70 (39.3%) | 31 (34.8%) | 39 (43.8%) | 0.2827 |
| No | 108 (60.7%) | 58 (65.2%) | 50 (56.2%) | ||
| Currently smoking | Yes | 17 (9.6%) | 7 (7.9%) | 10 (11.2%) | 0.6112 |
| No | 161 (90.4%) | 82 (92.1%) | 79 (88.8%) | ||
| Alcohol consumption | Once a day | 4 (2.2%) | 3 (3.4%) | 1 (1.1%) | 0.3085 |
| Several times a week | 22 (12.4%) | 15 (16.9%) | 7 (7.9%) | ||
| Once a week | 45 (25.3%) | 21 (23.6%) | 24 (27.0%) | ||
| 1–3 times a month | 71 (39.9%) | 32 (36.0%) | 39 (43.8%) | ||
| Never | 36 (20.2%) | 18 (20.2%) | 18 (20.2%) | ||
| Antihypertensive drugs | Yes | 50 (28.1%) | 23 (25.8%) | 27 (30.3%) | 0.6171 |
| No | 128 (71.9%) | 66 (74.2%) | 62 (69.7%) | ||
| Hypolipemic drugs | Yes | 17 (9.6%) | 7 (7.9%) | 10 (11.2%) | 0.6112 |
| No | 161 (90.4%) | 82 (92.1%) | 79 (88.8%) | ||
| Hypoglycaemic drugs | Yes | 8 (4.5%) | 2 (2.2%) | 6 (6.7%) | 0.2778 |
| No | 170 (95.5%) | 87 (97.8%) | 83 (93.3%) | ||
| Hypothyroidism drugs | Yes | 29 (16.3%) | 17 (19.1%) | 12 (13.5%) | 0.4173 |
| No | 149 (83.7%) | 72 (80.9%) | 77 (86.5%) | ||
| β | SE | t | p | |
|---|---|---|---|---|
| Age [years] | 0.1900 | 0.0740 | 2.5669 | 0.0111 |
| BMI [kg/m2] | −0.0582 | 0.0753 | −0.7734 | 0.4403 |
| LMI [kg/m2] | −0.1147 | 0.0748 | −1.5340 | 0.1268 |
| ALMI [kg/m2] | −0.0373 | 0.0753 | −0.4955 | 0.6208 |
| Total physical activity [min/day] | 0.0553 | 0.0753 | 0.7351 | 0.4633 |
| MOCA [points] | −0.0891 | 0.0751 | −1.1870 | 0.2368 |
| HAM-D [points] | 0.0294 | 0.0753 | 0.3902 | 0.6969 |
| Sex 1 | 0.0955 | 0.0750 | 1.2724 | 0.2049 |
| Place of residence 2 | −0.0558 | 0.0753 | −0.7416 | 0.4593 |
| Relationship status 3 | −0.0738 | 0.0754 | −0.9791 | 0.3289 |
| Education 4 | −0.0655 | 0.0752 | −0.8713 | 0.3848 |
| Socio-occupational status 5 | −0.1520 | 0.0745 | −2.0401 | 0.0428 |
| Financial situation 6 | −0.0989 | 0.0750 | −1.3185 | 0.1891 |
| Formerly smoking 7 | 0.1152 | 0.0749 | 1.5386 | 0.1257 |
| Currently smoking 7 | 0.0632 | 0.0752 | 0.8396 | 0.4023 |
| Alcohol consumption 7 | 0.0708 | 0.0752 | 0.9414 | 0.3478 |
| Antihypertensive drugs 7 | −0.0161 | 0.0754 | −0.2131 | 0.8315 |
| Hypolipemic drugs 7 | −0.0646 | 0.0752 | −0.8590 | 0.3915 |
| Hypoglycaemic drugs 7 | 0.0059 | 0.0754 | 0.0780 | 0.9379 |
| Hypothyroidism drugs 7 | 0.0109 | 0.0754 | 0.1446 | 0.8852 |
| β | SE | t | p | |
|---|---|---|---|---|
| Age [years] | 0.1532 | 0.0905 | 1.6921 | 0.0924 |
| Socio-occupational status 1 | −0.0640 | 0.0905 | −0.7064 | 0.4809 |
| OR | 95% CI | p | |
|---|---|---|---|
| Age [years] | 1.016 | 0.961–1.075 | 0.5690 |
| BMI [kg/m2] | 0.998 | 0.945–1.055 | 0.9537 |
| LMI [kg/m2] | 0.966 | 0.862–1.081 | 0.5444 |
| ALMI [kg/m2] | 1.000 | 0.997–1.003 | 0.9004 |
| Total physical activity [min/day] | 0.996 | 0.993–1.000 | 0.0437 |
| HAM-D [points] | 0.989 | 0.888–1.102 | 0.8474 |
| Urine BPA [µg/g creatinine] | 1.032 | 0.946–1.127 | 0.4764 |
| Sex 1 | 1.121 | 0.578–2.174 | 0.7356 |
| Place of residence 2 | 1.281 | 0.576–2.850 | 0.5432 |
| Relationship status 3 | 0.667 | 0.328–1.359 | 0.2647 |
| Education 4 | 0.399 | 0.186–0.857 | 0.0184 |
| Socio-occupational status 5 | 1.339 | 0.631–2.840 | 0.4465 |
| Financial situation 6 | 1.210 | 0.601–2.434 | 0.5936 |
| Formerly smoking 7 | 0.685 | 0.374–1.254 | 0.2204 |
| Currently smoking 7 | 0.674 | 0.245–1.859 | 0.4464 |
| Alcohol consumption 7 | 1.000 | 0.481–2.078 | 1.0000 |
| Antihypertensive drugs 7 | 1.250 | 0.649–2.407 | 0.5051 |
| Hypolipemic drugs 7 | 1.483 | 0.538–4.088 | 0.4464 |
| Hypoglycaemic drugs 7 | 3.145 | 0.617–16.023 | 0.1679 |
| Hypothyroidism drugs 7 | 0.660 | 0.295–1.478 | 0.3123 |
| OR | 95% CI | p | |
|---|---|---|---|
| Education 1 | 0.478 | 0.217–1.053 | 0.0220 |
| Total physical activity [min/day] | 1.000 | 0.999–1.000 | 0.0501 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamka, M.; Kurek, S.; Makarewicz-Bukowska, A.; Miśkiewicz-Chotnicka, A.; Wasiewicz-Gajdzis, M.; Walkowiak, J. No Differences in Urine Bisphenol A Concentrations between Subjects Categorized with Normal Cognitive Function and Mild Cognitive Impairment Based on Montreal Cognitive Assessment Scores. Metabolites 2024, 14, 271. https://doi.org/10.3390/metabo14050271
Jamka M, Kurek S, Makarewicz-Bukowska A, Miśkiewicz-Chotnicka A, Wasiewicz-Gajdzis M, Walkowiak J. No Differences in Urine Bisphenol A Concentrations between Subjects Categorized with Normal Cognitive Function and Mild Cognitive Impairment Based on Montreal Cognitive Assessment Scores. Metabolites. 2024; 14(5):271. https://doi.org/10.3390/metabo14050271
Chicago/Turabian StyleJamka, Małgorzata, Szymon Kurek, Aleksandra Makarewicz-Bukowska, Anna Miśkiewicz-Chotnicka, Maria Wasiewicz-Gajdzis, and Jarosław Walkowiak. 2024. "No Differences in Urine Bisphenol A Concentrations between Subjects Categorized with Normal Cognitive Function and Mild Cognitive Impairment Based on Montreal Cognitive Assessment Scores" Metabolites 14, no. 5: 271. https://doi.org/10.3390/metabo14050271
APA StyleJamka, M., Kurek, S., Makarewicz-Bukowska, A., Miśkiewicz-Chotnicka, A., Wasiewicz-Gajdzis, M., & Walkowiak, J. (2024). No Differences in Urine Bisphenol A Concentrations between Subjects Categorized with Normal Cognitive Function and Mild Cognitive Impairment Based on Montreal Cognitive Assessment Scores. Metabolites, 14(5), 271. https://doi.org/10.3390/metabo14050271







