Abstract
Several studies have detected a direct association between serum uric acid (SUA) and cardiovascular (CV) risk. In consideration that SUA largely depends on kidney function, some studies explored the role of the serum creatinine (sCr)-normalized SUA (SUA/sCr) ratio in different settings. Previously, the URRAH (URic acid Right for heArt Health) Study has identified a cut-off value of this index to predict CV mortality at 5.35 Units. Therefore, given that no SUA/sCr ratio threshold for CV risk has been identified for patients with diabetes, we aimed to assess the relationship between this index and CV mortality and to validate this threshold in the URRAH subpopulation with diabetes; the URRAH participants with diabetes were studied (n = 2230). The risk of CV mortality was evaluated by the Kaplan–Meier estimator and Cox multivariate analysis. During a median follow-up of 9.2 years, 380 CV deaths occurred. A non-linear inverse association between baseline SUA/sCr ratio and risk of CV mortality was detected. In the whole sample, SUA/sCr ratio > 5.35 Units was not a significant predictor of CV mortality in diabetic patients. However, after stratification by kidney function, values > 5.35 Units were associated with a significantly higher mortality rate only in normal kidney function, while, in participants with overt kidney dysfunction, values of SUA/sCr ratio > 7.50 Units were associated with higher CV mortality. The SUA/sCr ratio threshold, previously proposed by the URRAH Study Group, is predictive of an increased risk of CV mortality in people with diabetes and preserved kidney function. While, in consideration of the strong association among kidney function, SUA, and CV mortality, a different cut-point was detected for diabetics with impaired kidney function. These data highlight the different predictive roles of SUA (and its interaction with kidney function) in CV risk, pointing out the difference in metabolic- and kidney-dependent SUA levels also in diabetic individuals.
1. Introduction
Several studies have detected a direct association between serum uric acid (SUA) and cardiovascular (CV) risk. In particular, the URic acid Right for heArt Health (URRAH) Study, an Italian multicenter cohort study, has unequivocally shown that SUA is an independent risk factor for all-cause and CV mortality in different settings [1,2]. Given that SUA largely depends on kidney function, a number of studies explored the role of the kidney function-normalized SUA (SUA to creatinine ratio—SUA/sCr) [3,4,5,6,7]. This ratio is associated with metabolic diseases (e.g., metabolic syndrome, non-alcoholic fatty liver disease, β-cell dysfunction), chronic obstructive pulmonary disease, and also with increased all-cause mortality [4,6,7,8,9]. Recently, in the URRAH population, a threshold for CV risk was identified for the general population; in particular, a SUA/sCr ratio above 5.35 Units was an independent predictor of CV mortality both in men and women [10]. Therefore, given (i) the strong interaction among diabetes, kidney function, and SUA [11], (ii) few available data on the CV predictive role of SUA/sCr ratio in diabetic patients, (iii) the low cost of the SUA/sCr ratio and its reliability applicable to routine clinical practice of diabetes care, we aimed to assess the relationship between this index and CV mortality and to validate, the SUA/sCr ratio threshold of 5.35 Units (previously proposed for the risk of CV mortality in the general population [10]), in the subgroup of diabetic individuals of the URRAH study population.
2. Materials and Methods (See Supplemental Methods)
2.1. Study Population
The URRAH database is a multicenter retrospective, observational cohort study, which involves data from several cohorts distributed in almost all the Italian regions (age: 18–95 years). All the details of the URRAH project have been published previously [12]. From an updated URRAH database (a total of 3157 diabetic participants at baseline—12% of the whole population), 2230 diabetic participants were considered for the purpose of the present study after sequential exclusion of participants without a complete database (n = 903), and those on hypouricemic therapy at baseline (n = 24).
2.2. Examination Procedures and Outcomes Assessment
The URRAH study procedures have been extensively described [12]. In particular, hypertension was defined as office systolic blood pressure (BP) ≥ 140 and/or diastolic BP ≥ 90 mmHg or current antihypertensive drug treatment [13]. Diabetes was defined according to the history of diabetes (fasting plasma glucose ≥ 126 mg/dL or hemoglobin A1c ≥ 48 mmol/mol at baseline examination or treatment with antidiabetic drugs) [14]. The estimated glomerular filtration rate (eGFR) was calculated using the standard formula [15]. The overt kidney dysfunction was defined as eGFR equals below 60 mL/min per 1.73 m2 [16]. The SUA/sCr ratio (expressed in Units) was calculated according to the formula SUA (mg/dL) divided by serum creatinine (mg/dL). The triglycerides/HDL-cholesterol ratio (expressed in Units) was calculated using the following formula: triglycerides (mg/dL) divided by HDL-cholesterol (mg/dL). CV mortality for incident events was evaluated at the end of the follow-up [12].
2.3. Statistical Analysis
The statistical analyses were performed using the SPSS software (version 23, SPSS Inc., Chicago, IL, USA) and the statistical package R (version 4.3.1). Because eGFR, SUA, creatinine, glucose, total cholesterol, triglycerides, and HDL-cholesterol were non-normal distributed, log-transformed values were used in the analyses. Bivariate relationships between the variables under investigation were evaluated by Spearman’s correlation analysis. To analyze the type of association between SUA/sCr (as a continuous variable) and CV mortality, restricted cubic splines (RCS) regression models with 4 knots (5th-reference, 35th, 65th, and 95th percentiles) were utilized. For the present study, the sample was stratified by participants with SUA/sCr ratio above or below 5.35 Units, according to the previously detected threshold for CV mortality [10]. To evaluate differences among groups’ characteristics, the analysis of variance (ANOVA) was used for continuous data, and the chi-squared test was utilized to evaluate differences between categorical variables. To assess the role of baseline SUA/sCr ratio on the risk of CV mortality, Kaplan–Meier survival curves, log-rank tests, and Cox proportional-hazards models were used. The impact of traditional risk factors and that of potential confounding factors of the sample (p-value < 0.2, relating to the comparison between those who died and those who did not) was explored by multivariate models adjusted for baseline age, gender, cigarette smoking, body mass index (BMI), hypertension status, total cholesterol, HDL-cholesterol, triglycerides, blood glucose, and statin use. The proportional hazard (PH) assumption was assessed by visual inspection of Kaplan-Meyer curves. Furthermore, separate analyses stratified by baseline kidney function were also carried out after the identification of two groups: one including participants with evidence of overt kidney dysfunction (KD[+], defined as eGFR ≤ 60 mL/min per 1.73 m2) and the other one including participants without evidence of overt kidney dysfunction (KD[−], defined as eGFR > 60 mL/min per 1.73 m2). Finally, in consideration of the results and the strong interaction among kidney function, SUA and CV mortality rate, we identified an additional cut-off point (Youden’s index) for KD[+] by the receiver-operating characteristic (ROC) analysis and the area under the curve (AUC), with its 95% confidence interval (CI), to assess the ability of SUA/sCr ratio on CV mortality in this subgroup (cut-point: 7.50 Units). The results are reported as mean (or geometric mean) with SD, percentages, or hazard ratio (HR) and 95%CI (Bootstrap CI, 1000 iterations) unless otherwise indicated. Two-sided p values < 0.05 were considered statistically significant.
3. Results
The baseline characteristics of the 2230 participants included are reported in Table 1.

Table 1.
Baseline characteristics of all the study participants and stratified according to the SUA/sCr ratio threshold predictive for cardiovascular mortality.
The mean age at baseline was 65.0 years; 51.2% were men, 45.7% of the participants were overweight, 32.9% were obese, 18.3% were smokers, and 76.2% were hypertensive (47% on regular antihypertensive treatment). The prevalence of high SUA/sCr ratio was 53.5% according to the 5.35 Units threshold. The analysis of the correlation between the SUA/sCr ratio and the most relevant characteristics of participants at baseline showed a significant association with BMI (r = 0.18), eGFR (r = 0.30), blood glucose (r = −0.06) and triglycerides (r = 0.08), and as expected with SUA (r = 0.70) and creatinine (r = −0.36); by contrast, no association was found with age, systolic and diastolic BP, total cholesterol, HDL-cholesterol, and triglycerides/HDL-cholesterol ratio (p > 0.05) . During a median follow-up of 9.2 years (110 months, 25th–75th: 60–144 months), 671 (30.1%) all-cause deaths occurred, 380 of which were due to primary CV causes (acute myocardial infarction = 113, heart failure = 98, stroke = 86, and hypertensive complications = 83). RCS regression model detected a non-linear relationship between SUA/sCr ratio and CV mortality (test for overall: p < 0.001, test for non-linearity: p < 0.001) (Figure 1A). The shape of the association was also confirmed after adjustment for main confounders (Figure 1B).
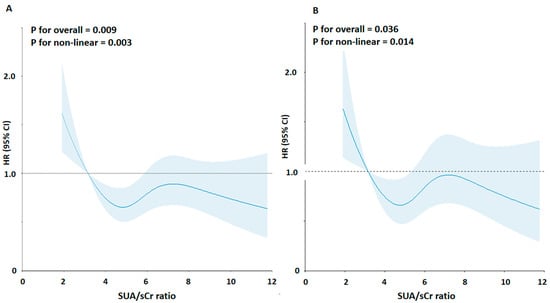
Figure 1.
Association between the SUA/sCr ratio and risk of cardiovascular mortality using a Restricted Cubic Spline Regression Model. Solid lines indicate hazard ratios (HRs), and shadow shapes indicate 95% confidence intervals (CIs). (A) Unadjusted; (B) Adjusted for age, gender, body mass index, cigarette smoking, hypertension status, total cholesterol, triglycerides, blood glucose, and statin use.
The differences in baseline characteristics between participants stratified by the SUA/sCr ratio, defined according to thresholds of 5.35 Units, are also reported in Table 1. Diabetic individuals with SUA/sCrea ratio > 5.35 Units had significantly higher BMI, SUA, triglycerides and eGFR, and higher prevalence of obesity, and hypertension, than those with SUA/sCr ratio below 5.35 Units. In addition, individuals with SUA/sCr ratio above 5.35 Units had significantly lower serum creatinine and prevalence of kidney dysfunction and cigarette smoking.
Participants with SUA/sCr ratio of more than 5.35 had a non-significant higher incidence of CV mortality than participants with an SUA/sCr ratio below 5.35 (18.1% vs. 15.9%, p = 0.17). To assess the multivariate-adjusted models, we also evaluated the differences in baseline characteristics between those who died and those not (Table 2).

Table 2.
Baseline characteristics stratified by cardiovascular mortality.
As expected, those who died had a worse cardio-metabolic profile. The Kaplan-Meier curves showed an overlap of the curves (log-rank test: 1.288, p = 0.26) (Supplemental Figure S1). The Cox regression analysis confirmed the non-significant difference in the risk of CV mortality between the two groups (>5.35 vs. ≤5.35 Units: HR: 1.12, 95%CI: 0.91–1.38).
Next, in consideration of these results, the interaction among SUA, kidney function, and CV mortality rate, and the shape of the association between SUA/sCr ratio and CV mortality risk, we explored the relationship between SUA/sCr ratio and CV mortality stratifying by kidney function (Figure 2) (Table 3).
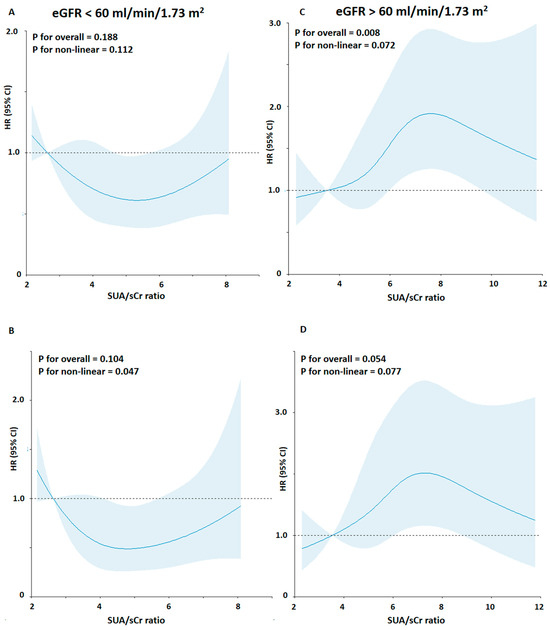
Figure 2.
Association between the SUA/sCr ratio and risk of cardiovascular mortality using a Restricted Cubic Spline Regression Model stratified by kidney function. Solid lines indicate hazard ratios (HRs), and shadow shapes indicate 95% confidence intervals (CIs). (A,C) Unadjusted; (B,D) Adjusted for age, gender, body mass index, cigarette smoking, hypertension status, total cholesterol, triglycerides, blood glucose, and statin use.

Table 3.
Baseline characteristics of the study participants stratified by kidney dysfunction (KD) and according to the SUA/sCr ratio threshold, which is predictive for cardiovascular mortality.
In the KD[−] group (n = 1697), participants with SUA/sCrea ratio > 5.35 Units had significantly higher age, BMI, systolic BP, SUA, and eGFR, higher prevalence of hypertension and statin use, and lower serum creatinine and prevalence of smokers than those with SUA/sCr ratio below 5.35 Units.
Moreover, the participants of the KD[−] group with a SUA/sCr ratio of more than 5.35 Units had a significantly higher incidence of CV mortality than participants with a SUA/sCr ratio below 5.35 Units (16.0% vs. 10.4%, p = 0.001) (Figure 3).
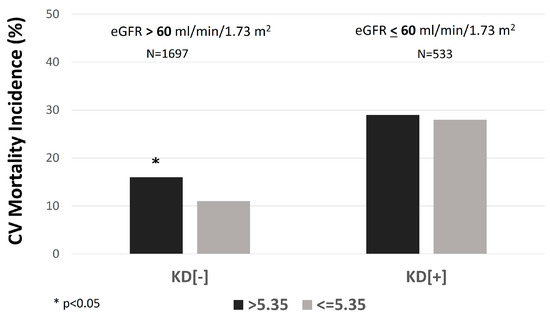
Figure 3.
The incidence rate of cardiovascular (CV) mortality in individuals with serum uric acid (SUA)/creatinine (sCr) ratio > 5.35 vs. ≤5.35 mg/dL, stratified by kidney function. KD[+]: eGFR ≤ 60 mL/min per 1.73 m2; KD[−]: eGFR > 60 mL/min per 1.73 m2.
In the KD[+] group (n = 533), individuals with a SUA/sCr ratio above 5.35 Units had significantly higher BMI, SUA, total cholesterol, and eGFR and lower serum creatinine than those with a ratio below 5.35 Units. Moreover, the contrary of the other group, in the KD[+] group, there was no significantly higher rate of CV mortality in participants with a SUA/sCr ratio of more than 5.35 Units compared with the other group (28.9% vs. 27.5%, p = 0.72) (Figure 3). The Kaplan-Meier curves for CV mortality in KD[−] showed that participants with values above 5.35 Units had a significantly higher probability of CV mortality than those with SUA/sCr ratio below 5.35 Units (log-rank test: 11.927, p = 0.001) (Figure 4).
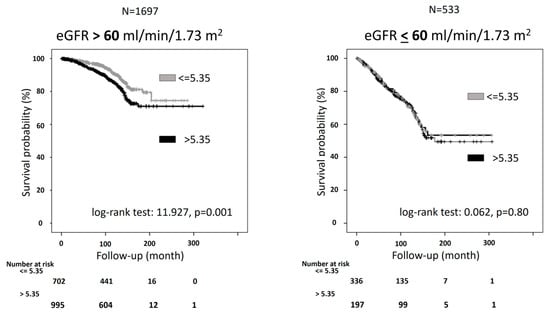
Figure 4.
Kaplan-Meier curves for cardiovascular (CV) mortality for people with serum uric acid (SUA)/Creatinine (sCr) ratio lower or above 5.35 Units stratified by kidney function. KD[+]: eGFR ≤ 60 mL/min per 1.73 m2; KD[−]: eGFR > 60 mL/min per 1.73 m2.
The inspection of Kaplan-Meyer curves did not detect a PH assumption violation. The Cox regression analysis confirmed the predictive role of the CV mortality cut-off, which showed a greater risk of CV mortality in participants with SUA/sCr ratio above with respect to below 5.35 Units (Table 4).

Table 4.
Cox-regression analysis of the risk of cardiovascular mortality according to kidney dysfunction (KD).
This predictive role was also detected after adjustment for some potential confounders (Table 4). The relationship was also confirmed in a model including insulin resistance, expressed by triglyceride/HDL-cholesterol ratio instead of triglycerides and HDL-cholesterol (HR: 1.58, 95%CI: 1.11–2.25). On the other hand, as expected, the Kaplan-Meier curves for CV mortality in KD[+] showed an overlap of the two curves of the survival (log-rank test: 0.062, p = 0.80) (Figure 3). The trend was confirmed by the Cox regression analysis, which indicated no significant risk difference between the two groups (Table 4).
Moreover, also the results from further stratification by different levels of overt kidney dysfunction confirmed this trend in KD[+] (eGFR 45–60 mL/min/1.73 m2, n = 387, 27.2% vs. 22.8%, p = 0.32; eGFR < 45 mL/min/1.73 m2, n = 146, 41.7% vs. 35.8%, p = 0.58).
Given these results and the reasons mentioned above, we assessed a different cut-off point for KD[+] (7.50 Units). In this group, the Kaplan-Meier curves for CV mortality showed that participants with values above 7.50 Units had a significantly higher probability of CV mortality than those with SUA/sCr ratio below 7.50 Units (log-rank test: 4.165, p = 0.04) (Supplemental Figure S2). The inspection of Kaplan-Meyer curves did not detect a PH assumption violation. The Cox regression analysis confirmed the predictive role of this CV mortality cut-off value, which showed a greater risk of CV mortality in participants with SUA/sCr ratio above with respect to below 7.50 Units (Table 5).

Table 5.
Cox-regression analysis of the risk of cardiovascular mortality patients with kidney dysfunction (KD).
This predictive role was also detected after adjustment for some potential confounders (Table 5). The association was also confirmed in a model including insulin resistance, expressed by triglyceride/HDL-cholesterol ratio instead of triglycerides and HDL-cholesterol (HR: 2.83, 95%CI: 1.41–5.69).
4. Discussion
The main findings of this study showed a non-linear association between baseline SUA/sCr ratio and risk of CV mortality in diabetic participants. Moreover, the SUA/sCr ratio threshold previously proposed by the URRAH study group [10] was not predictive of CV mortality in the whole diabetic population. In consideration of this result and the strong relevance of kidney function on SUA and CV mortality rate, we analyzed the sample stratified into two groups by the presence or absence of kidney dysfunction. The SUA/sCr ratio of 5.35 Units is predictive for CV mortality in a diabetic population with normal kidney function (eGFR > 60 mL/min per 1.73 m2), revealing that values of SUA/sCr ratio higher than 5.35 were associated with a 39% increased risk of CV mortality in this group, independently of potential confounders, among which lipid and glucose profile, and insulin resistance. By contrast, this cut-point was not predictive of CV mortality in diabetic participants with kidney dysfunction (<60 mL/min per 1.73 m2). However, in this group, a newly detected cut-point (7.50 Units) was associated with the rate of CV mortality and values of the SUA/sCr ratio above 7.50 were associated with a 79% increased risk, independently of hypertension, lipid, and glucose profile, and insulin resistance. These results are strengthened by the large study population being highly representative of the general population with diabetes, the long follow-up period, and no bias due to the pharmacological treatment of hyperuricemia.
These results are in agreement with our previous data on diabetic participants of the URRAH study, which showed a direct relationship between SUA and CV mortality risk [14]. In addition, our results are in line with data on the relationship between the SUA/sCr ratio and CV risk in diabetic populations. Indeed, SUA/sCr was an independent risk factor for the progression of diabetic nephropathy in a large diabetic Chinese population [17]. Similar results were found in a study involving a sample of rural elderly diabetic patients, in which the SUA/sCr ratio had a predictive role in the decline in kidney function during a follow-up of 6 months [18]; likewise, SUA/sCr ratio was also an independent risk factor for kidney disease in diabetic patients with normo-albuminuria [19]. On the other hand, although the values above 5.35 were associated with a slightly higher rate of CV mortality in diabetic participants with kidney dysfunction, these values were not predictive of CV mortality in this group. This inconsistency in the predictive role of SUA/sCr ratio between KD[−] and KD[+] may be due to the strong influence of kidney function on the index and the rate of CV mortality, thus to the subsequent substantial inverse non-linear relationship between the SUA/sCr ratio and CV mortality risk in this sample, to the small sample of participants with kidney dysfunction and to the difference in the index values (as continuous item) between KD[+] and KD[−]. Indeed, in the whole sample, the SUA/sCr ratio was significantly higher in KD[−] than in KD[+], as expected. Noteworthy, after stratification by kidney dysfunction, the SUA/sCr ratio was significantly higher in those who died than not in KD[−], whereas the index distribution was not different in KD[+]. Furthermore, these data are in agreement with those of a recent study on a general population that found an elevated risk effect of hyperuricemia associated with CV mortality in individuals with normal kidney function rather than with reduced kidney function [20]. Likely, in normal kidney function, hyperuricemia due to the overproduction of uric acid is a more harmful CV risk factor than hyperuricemia resulting from reduced kidney excretion of SUA; the interaction between diabetes and kidney dysfunction would explain a high mortality rate in participants with kidney dysfunction. Hence, in KD[+], the direct effect of SUA on CV mortality was affected by kidney dysfunction. Moreover, the biological mechanism(s) linking SUA with CV disease have been hypothesized and include oxidative stress, inflammation, and arterial stiffness [21]. SUA is also an indicator of kidney function: SUA levels are influenced by kidney clearance function, and higher SUA levels are observed in patients with poor kidney function. Therefore, the SUA/sCr ratio, since it reflects SUA levels normalized to kidney function, could serve as a more accurate indicator of CV disease.
In this context, our results in the diabetic sample with preserved kidney function confirmed the predictive role of the ratio threshold found in the whole URRAH sample [10], highlighting the role of kidney-independent metabolic overproduction of SUA on CV risk. The apparent discrepancies with participants with kidney dysfunction may be justified by the substantial superiority of the kidney function on CV mortality rate in this subgroup of diabetic participants and the negligible prevalence of kidney dysfunction in the large sample of the general population participating in the URRAH study [10]. Furthermore, despite the cardio-metabolic risk profile being worse in those with a ratio above 5.35 Units, the potential independent predictive role of the SUA/sCr ratio on CV mortality may not be disentangled in this sample at high CV risk also for the strong interaction among diabetes, kidney dysfunction, and SUA [11,17,19].
Nevertheless, our study has some limitations: (i) the URRAH study enrolled only white participants. Thus, its results are not generalizable to other ethnicities; (ii) the study design is observational. Hence, it did not allow the establishment of the cause-relationship; (iii) the analysis was based on a single SUA and sCr measurement; however, these parameters have little pre-analytical biological variability.
5. Conclusions
The results of the present study show for the first time that the SUA/sCr ratio is predictive of CV mortality in people with diabetes. In particular, because the kidney function strongly affects the values of SUA/sCr ratio and the rate of CV mortality, the main findings of the study indicate two different thresholds: 5.35 Units (previously proposed by the URRAH study group [10]) for diabetic participants with preserved kidney function, and 7.50 Units for diabetic participants with overt kidney dysfunction. Although this population is already at high risk for the presence of diabetes, these cut-offs allow us to identify those at greater CV risk in addition to the risk due to diabetes. These data highlight the different predictive roles of SUA (and its interaction with kidney function) in the CV risk, pointing out the difference in metabolic- and kidney-dependent SUA levels also in diabetic individuals, hence the importance of the stratification by kidney function. Given the strong interaction between SUA and kidney function, this index is more complete than SUA alone in predicting CV mortality. For these reasons, these results make the SUA/sCr ratio a low-cost, reliable marker applicable to routine clinical practice of diabetes care. Nonetheless, further studies are needed to support our conclusions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/metabo14030164/s1, Supplemental Methods: Additional information on methods; Figure S1: Kaplan-Meier curves for cardiovascular (CV) mortality for people with serum uric acid (SUA)/Creatinine (sCr) ratio lower or above 5.35 Units; Figure S2: Kaplan-Meier curves for cardiovascular (CV) mortality for people with kidney dysfunction and stratified by serum uric acid (SUA)/Creatinine (sCr) ratio lower or above 7.50 Units.
Author Contributions
Conceptualization, L.D., M.M., A.V., C.B. and F.G.; Data curation, E.C. and V.T.; Formal analysis, L.D.; Funding acquisition, A.V., C.B., V.T. and E.C.; Methodology, L.D.; Project administration, A.V., C.B., V.T. and E.C.; Resources, A.V., C.B., E.C. and V.T.; Software, L.D., V.T. and E.C.; Supervision, F.G.; Validation, F.G.; Visualization, C.B., E.C., V.T. and L.D.; Writing—original draft, L.D., M.M. and F.G.; Writing—review and editing, L.D., M.M., P.C., A.V., E.C., V.T., C.M.B., F.A., M.B., R.C., F.C., M.C. (Michele Ciccarelli), A.F.G.C., R.D., M.C. (Massimo Cirillo), G.D., C.F., L.G., C.G., G.G., G.I., L.L., F.M., A.M. (Alessandro Maloberti), S.M., A.M. (Alberto Mazza), A.M. (Alessandro Mengozzi), P.P., M.L.M., P.N., G.P., R.P., F.Q.-T., M.R., E.R., G.R. (Gianpaolo Reboldi), G.R. (Giulia Rivasi), M.S., M.V., G.T., F.V., A.U., P.V., C.B. and F.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by an unrestricted grant from the Fondazione of the Italian Society of Hypertension (grant: MIOL).
Institutional Review Board Statement
The URRAH study was performed according to the Declaration of Helsinki for Human Research (41st World Medical Assembly, 1990). Approval was sought from the Ethics Committee of the seven coordinating centers at the Division of Internal Medicine of the University of Bologna (no. 77/2018/Oss/AOUBo).
Informed Consent Statement
Informed consent was obtained from all subjects included in the study.
Data Availability Statement
The data presented in this study are available on a specific request from the corresponding author. The data are not publicly available.
Acknowledgments
The Uric Acid Right for Heart Health (URRAH) Project is a working group of Italian Society of Hypertension (SIIA). URRAH Study Group: Claudio Borghi (Coordinator); Fabio Angeli, Carlo Maria Barbagallo, Michele Bombelli, Federica Cappelli, Edoardo Casiglia, Rosario Cianci, Michele Ciccarelli, Arrigo F G Cicero, Massimo Cirillo, Pietro Cirillo, Lanfranco D’Elia, Raffaella Dell’Oro, Giovambattista Desideri, Claudio Ferri, Ferruccio Galletti, Loreto Gesualdo, Cristina Giannattasio, Guido Grassi, Guido Iaccarino, Luciano Lippa, Francesca Mallamaci, Alessandro Maloberti, Stefano Masi, Maria Masulli, Alberto Mazza, Alessandro Mengozzi, Maria Lorenza Muiesan, Pietro Nazzaro, Paolo Palatini, Gianfranco Parati, Roberto Pontremoli, Fosca Quarti-Trevano, Marcello Rattazzi, Gianpaolo Reboldi, Giulia Rivasi, Elisa Russo, Massimo Salvetti, Valerie Tikhonoff, Giuliano Tocci, Andrea Ungar, Paolo Verdecchia, Francesca Viazzi, Agostino Virdis, Massimo Volpe.
Conflicts of Interest
Borghi C. has received research grant support from Menarini Corporate and Novartis Pharma; has served as a consultant for Novartis Pharma, Alfasigma, Grunenthal, Menarini Corporate, and Laboratoires Servier; and received lecturing fees from Laboratoires Servier, Takeda, Astellas, Teijin, Novartis Pharma, Berlin Chemie, and Sanofi. The remaining authors have no disclosures to report.
References
- Maloberti, A.; Mengozzi, A.; Russo, E.; Cicero, A.F.G.; Angeli, F.; Agabiti Rosei, E.; Barbagallo, C.M.; Bernardino, B.; Bombelli, M.; Cappelli, F.; et al. The Results of the URRAH (Uric Acid Right for Heart Health) Project: A Focus on Hyperuricemia in Relation to Cardiovascular and Kidney Disease and its Role in Metabolic Dysregulation. High Blood Press Cardiovasc. Prev. 2023, 30, 411–425. [Google Scholar] [CrossRef]
- Saito, Y.; Tanaka, A.; Node, K.; Kobayashi, Y. Uric acid and cardiovascular disease: A clinical review. J. Cardiol. 2021, 78, 51–57. [Google Scholar] [CrossRef]
- Gu, L.; Huang, L.; Wu, H.; Lou, Q.; Bian, R. Serum uric acid to creatinine ratio: A predictor of incident chronic kidney disease in type 2 diabetes mellitus patients with preserved kidney function. Diab. Vasc. Dis. Res. 2017, 14, 221–225. [Google Scholar] [CrossRef]
- Kawamoto, R.; Kikuchi, A.; Ninomiya, D.; Tokumoto, Y.; Kumagi, T. Serum uric acid to creatinine ratio is a useful predictor of all-cause mortality among hypertensive patients. Clin. Hypertens. 2023, 29, 10. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.R.; Gonçalves, C.E.T.; Gonçalves, D.L.N.; Cotta, R.M.M.; da Silva, L.S. Association of uric acid and uric acid to creatinine ratio with chronic kidney disease in hypertensive patients. BMC Nephrol. 2021, 22, 311. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Tian, X.; Wu, S.; Zuo, Y.; Chen, S.; Mo, D.; Luo, Y.; Wang, Y. Metabolic factors mediate the association between serum uric acid to serum creatinine ratio and cardiovascular disease. J. Am. Heart Assoc. 2021, 10, e023054. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Shen, X.; Li, J.; Cha, E.; Gu, P.P.; Liu, J.; Zhu, W.; He, L.-L.; Li, G.-Q.; Wang, Z. Serum uric acid to creatinine ratio and metabolic syndrome in postmenopausal Chinese women. Medicine 2020, 17, e19959. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gu, L.; Yang, J.; Lou, Q. Serum uric acid to creatinine ratio correlates with β-cell function in type 2 diabetes. Diabetes Metab. Res. Rev. 2018, 34, e3001. [Google Scholar] [CrossRef] [PubMed]
- Kocak, N.D.; Sasak, G.; Akturk, U.A.; Akgun, M.; Boga, S.; Sengul, A.; Gungor, S.; Arinc, S. Serum uric acid levels and uric acid/creatinine ratios in stable chronic obstructive pulmonary disease (COPD) patients: Are these parameters efficient predictors of patients at risk for exacerbation and/or severity of disease? Med. Sci. Monit. 2016, 22, 4169–4176. [Google Scholar] [CrossRef] [PubMed]
- Casiglia, E.; Tikhonoff, V.; Virdis, A.; Grassi, G.; Angeli, F.; Barbagallo, C.M.; Bombelli, M.; Cicero, A.F.G.; Cirillo, M.; Cirillo, P.; et al. Serum uric acid/serum creatinine ratio as a predictor of cardiovascular events. Detection of prognostic cardiovascular cut-off values. J. Hypertens. 2023, 41, 180–186. [Google Scholar] [CrossRef]
- Koenig, W.; Meisinger, C. Uric acid, type 2 diabetes, and cardiovascular diseases: Fueling the common soil hypothesis? Clin. Chem. 2008, 54, 231–233. [Google Scholar] [CrossRef]
- Virdis, A.; Masi, S.; Casiglia, E.; Tikhonoff, V.; Cicero, A.F.G.; Ungar, A.; Rivasi, G.; Salvetti, M.; Barbagallo, C.M.; Bombelli, M.; et al. Identification of the Uric Acid Thresholds Predicting an Increased Total and Cardiovascular Mortality over 20 Years. Hypertension 2020, 75, 302–308. [Google Scholar] [CrossRef]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [CrossRef]
- Masulli, M.; D’Elia, L.; Angeli, F.; Barbagallo, C.M.; Bilancio, G.; Bombelli, M.; Bruno, B.; Casiglia, E.; Cianci, R.; Cicero, A.F.G.; et al. Serum uric acid levels threshold for mortality in diabetic individuals: The URic acid Right for heArt Health (URRAH) project. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1245–1252. [Google Scholar] [CrossRef]
- Levey, A.S.; Inker, L.A.; Coresh, J. GFR estimation: From physiology to public health. Am. J. Kidney Dis. 2014, 63, 820–834. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Stevens, P.E. Summary of Recommendation Statements. Kidney Int. Suppl. 2013, 3, 5–14. [Google Scholar] [CrossRef]
- Yao, C.; Gu, L.; Wang, T.; Xing, C. The association between serum uric acid to creatinine ratio and renal disease progression in type 2 diabetic patients in Chinese communities. J. Diabetes Complicat. 2019, 33, 473–476. [Google Scholar] [CrossRef]
- Kawamoto, R.; Ninomiya, D.; Kikuchi, A.; Akase, T.; Kasai, Y.; Ohtsuka, N.; Kumagi, T. Serum uric acid to creatinine ratio is a useful predictor of renal dysfunction among diabetic persons. Diabetes Metab. Synd. 2019, 13, 1851–1856. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhu, Z.; Ye, S.; Zheng, M. The Serum Uric Acid to Serum Creatinine Ratio is an Independent Risk Factor for Diabetic Kidney Disease. Diabetes Metab. Syndr. Obes. 2022, 28, 3693–3703. [Google Scholar] [CrossRef] [PubMed]
- Timsans, J.; Kauppi, J.E.; Kerola, A.M.; Lehto, T.M.; Kautiainen, H.J.; Kauppi, M.J. Hyperuricaemiaassociated all-cause mortality risk effect is increased by non-impaired kidney function—Is renal hyperuricaemia less dangerous? Eur. J. Intern. Med. 2023, 121, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Agnoletti, D.; Cicero, A.F.G.; Borghi, C. The Impact of Uric Acid and Hyperuricemia on Cardiovascular and Renal Systems. Cardiol. Clin. 2021, 39, 365–376. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).