Exposure to OPFRs Is Associated with Obesity and Dysregulated Serum Lipid Profiles: Data from 2017–2018 NHANES
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Detection of Urinary Concentrations of OPFR Biomarkers
2.3. The Definition of Outcomes
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Study Participants
3.2. Overview of OPFR Metabolites in Urine Samples
3.3. Associations of Urinary OPFR Metabolites, BMI, and Obesity
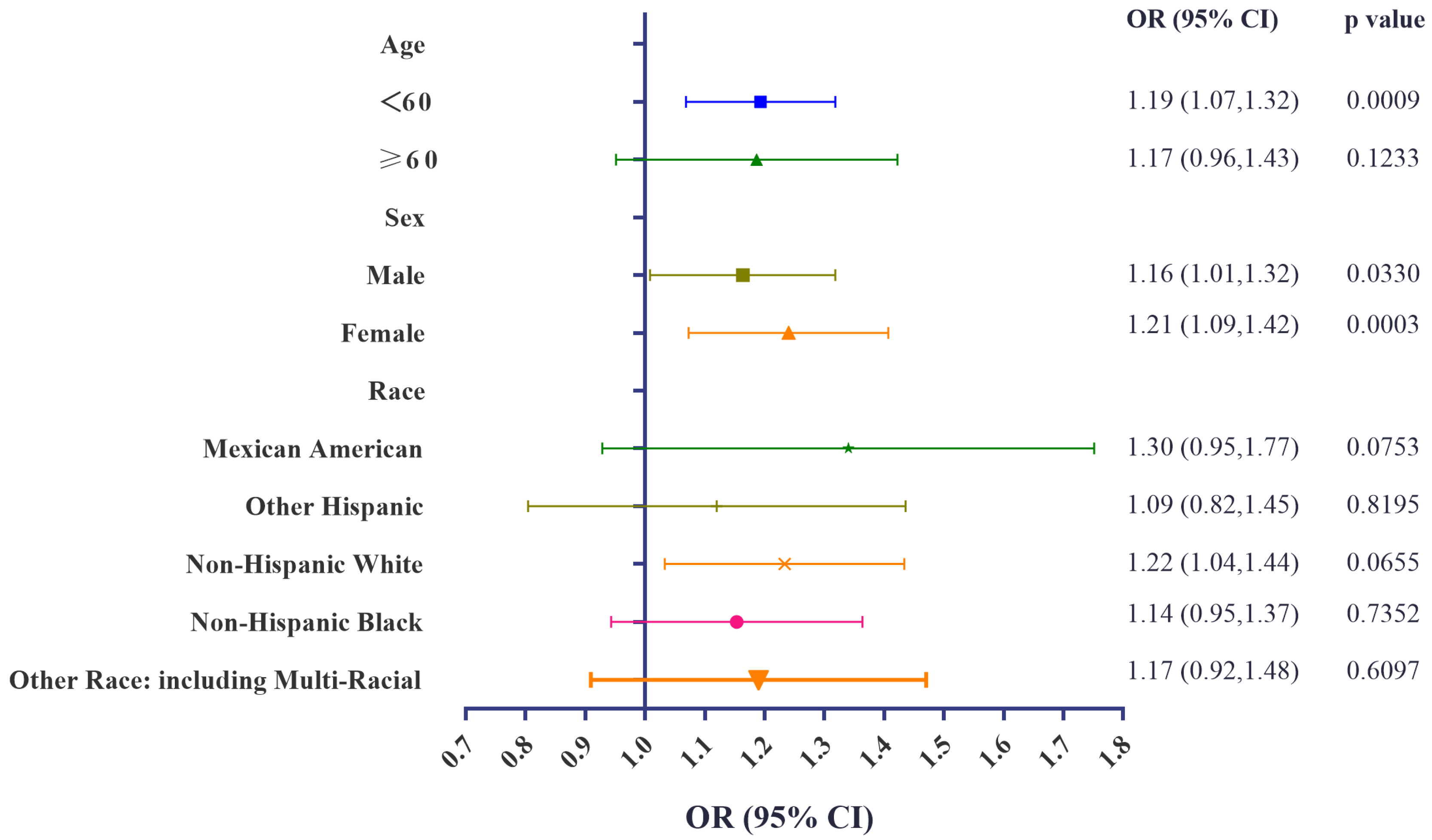
3.4. Stratification Analyses of Associations of Urinary BCEP/BDCPP Concentrations and Obesity
3.5. Association of Urinary Concentrations of OPFR Metabolites and Serum Lipid Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pantelaki, I.; Voutsa, D. Organophosphate flame retardants (OPFRs): A review on analytical methods and occurrence in wastewater and aquatic environment. Sci. Total Environ. 2019, 649, 247–263. [Google Scholar] [CrossRef]
- Cowell, W.J.; Sjödin, A.; Jones, R.; Wang, Y.; Wang, S.; Herbstman, J.B. Determinants of prenatal exposure to polybrominated diphenyl ethers (PBDEs) among urban, minority infants born between 1998 and 2006. Environ. Pollut. 2018, 233, 774–781. [Google Scholar] [CrossRef]
- Blum, A.; Behl, M.; Birnbaum, L.; Diamond, M.L.; Phillips, A.; Singla, V.; Sipes, N.S.; Stapleton, H.M.; Venier, M. Organophosphate Ester Flame Retardants: Are They a Regrettable Substitution for Polybrominated Diphenyl Ethers? Environ. Sci. Technol. Lett. 2019, 6, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, C.; Zheng, Q.; Yang, W.; Niu, X.; Zhang, Y.; Lu, G. Occurrence and ecological implications of organophosphate triesters and diester degradation products in wastewater, river water, and tap water. Environ. Pollut. 2020, 259, 113810. [Google Scholar] [CrossRef]
- Han, X.; Hao, Y.; Li, Y.; Yang, R.; Wang, P.; Zhang, G.; Zhang, Q.; Jiang, G. Occurrence and distribution of organophosphate esters in the air and soils of Ny-Ålesund and London Island, Svalbard, Arctic. Environ. Pollut. 2020, 263, 114495. [Google Scholar] [CrossRef] [PubMed]
- Giulivo, M.; Capri, E.; Kalogianni, E.; Milacic, R.; Majone, B.; Ferrari, F.; Eljarrat, E.; Barceló, D. Occurrence of halogenated and organophosphate flame retardants in sediment and fish samples from three European river basins. Sci. Total Environ. 2017, 586, 782–791. [Google Scholar] [CrossRef]
- Yao, C.; Yang, H.; Li, Y. A review on organophosphate flame retardants in the environment: Occurrence, accumulation, metabolism and toxicity. Sci. Total Environ. 2021, 795, 148837. [Google Scholar] [CrossRef]
- Tang, J.; Ma, S.; Hu, X.; Lin, M.; Li, G.; Yu, Y.; An, T. Handwipes as indicators to assess organophosphate flame retardants exposure and thyroid hormone effects in e-waste dismantlers. J. Hazard. Mater. 2023, 443, 130248. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, C.; Fu, L.; Gu, S.; Wang, C. New Insights in the Endocrine Disrupting Effects of Three Primary Metabolites of Organophosphate Flame Retardants. Environ. Sci. Technol. 2020, 54, 4465–4474. [Google Scholar] [CrossRef]
- Rosenmai, A.K.; Winge, S.B.; Möller, M.; Lundqvist, J.; Wedebye, E.B.; Nikolov, N.G.; Lilith Johansson, H.K.; Vinggaard, A.M. Organophosphate ester flame retardants have antiandrogenic potential and affect other endocrine related endpoints in vitro and in silico. Chemosphere 2021, 263, 127703. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.F.; Cheng, F.J.; Huang, W.T.; Kung, C.T.; Lee, C.T.; Cheng, B.C.; Chen, J.B.; Li, S.H.; Wang, C.C.; Wang, L.J.; et al. The associations between renal disease severity and exposure to organophosphate flame retardants in patients with chronic kidney disease. Environ. Int. 2022, 170, 107573. [Google Scholar] [CrossRef]
- Hogberg, H.T.; de Cássia da Silveira, E.S.R.; Kleensang, A.; Bouhifd, M.; Cemiloglu Ulker, O.; Smirnova, L.; Behl, M.; Maertens, A.; Zhao, L.; Hartung, T. Organophosphorus flame retardants are developmental neurotoxicants in a rat primary brainsphere in vitro model. Arch. Toxicol. 2021, 95, 207–228. [Google Scholar] [CrossRef] [PubMed]
- Saquib, Q.; Al-Salem, A.M.; Siddiqui, M.A.; Ansari, S.M.; Zhang, X.; Al-Khedhairy, A.A. Organophosphorus Flame Retardant TDCPP Displays Genotoxic and Carcinogenic Risks in Human Liver Cells. Cells 2022, 11, 195. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Kumanyika, S.; Dietz, W.H. Solving Population-wide Obesity—Progress and Future Prospects. N. Engl. J. Med. 2020, 383, 2197–2200. [Google Scholar] [CrossRef] [PubMed]
- Safaei, M.; Sundararajan, E.A.; Driss, M.; Boulila, W.; Shapi’i, A. A systematic literature review on obesity: Understanding the causes & consequences of obesity and reviewing various machine learning approaches used to predict obesity. Comput. Biol. Med. 2021, 136, 104754. [Google Scholar]
- Keys, A.; Fidanza, F.; Karvonen, M.J.; Kimura, N.; Taylor, H.L. Indices of relative weight and obesity. J. Chronic Dis. 1972, 25, 329–343. [Google Scholar] [CrossRef]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014, 129, S102–S138. [Google Scholar] [CrossRef]
- Heindel, J.J.; Vom Saal, F.S.; Blumberg, B.; Bovolin, P.; Calamandrei, G.; Ceresini, G.; Cohn, B.A.; Fabbri, E.; Gioiosa, L.; Kassotis, C.; et al. Parma consensus statement on metabolic disruptors. Environ. Health Glob. Access Sci. Source 2015, 14, 54. [Google Scholar] [CrossRef]
- Kahn, L.G.; Philippat, C.; Nakayama, S.F.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: Implications for human health. Lancet Diabetes Endocrinol. 2020, 8, 703–718. [Google Scholar] [CrossRef]
- Vekic, J.; Zeljkovic, A.; Stefanovic, A.; Jelic-Ivanovic, Z.; Spasojevic-Kalimanovska, V. Obesity and dyslipidemia. Metab. Clin. Exp. 2019, 92, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Wang, Q. PXR-mediated organophorous flame retardant tricresyl phosphate effects on lipid homeostasis. Chemosphere 2021, 284, 131250. [Google Scholar] [CrossRef]
- Bérubé, R.; LeFauve, M.K.; Heldman, S.; Chiang, Y.T.; Birbeck, J.; Westrick, J.; Hoffman, K.; Kassotis, C.D. Adipogenic and endocrine disrupting mixture effects of organic and inorganic pollutant mixtures. Sci. Total Environ. 2023, 876, 162587. [Google Scholar] [CrossRef]
- Maia, M.L.; Sousa, S.; Pestana, D.; Faria, A.; Teixeira, D.; Delerue-Matos, C.; Domingues, V.F.; Calhau, C. Impact of brominated flame retardants on lipid metabolism: An in vitro approach. Environ. Pollut. 2022, 294, 118639. [Google Scholar] [CrossRef]
- National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey: 2017–2018 Data Documentation, Codebook, and Frequencies[EB/OL]. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2017-2018/SSFR_J.htm (accessed on 16 March 2023).
- National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey [EB/OL]. Available online: https://www.cdc.gov/nchs/nhanes/ (accessed on 8 May 2023).
- Jayatilaka, N.K.; Restrepo, P.; Davis, Z.; Vidal, M.; Calafat, A.M.; Ospina, M. Quantification of 16 urinary biomarkers of exposure to flame retardants, plasticizers, and organophosphate insecticides for biomonitoring studies. Chemosphere 2019, 235, 481–491. [Google Scholar] [CrossRef]
- Caballero, B. Humans against Obesity: Who Will Win? Adv. Nutr. 2019, 10, S4–S9. [Google Scholar] [CrossRef]
- Guo, X.; Wu, B.; Xia, W.; Gao, J.; Xie, P.; Feng, L.; Sun, C.; Liang, M.; Ding, X.; Zhao, D.; et al. Association of organophosphate ester exposure with cardiovascular disease among US adults: Cross-sectional findings from the 2011–2018 National Health and Nutrition Examination Survey. Chemosphere 2022, 308, 136428. [Google Scholar] [CrossRef]
- Li, R.; Zhan, W.; Ren, J.; Gao, X.; Huang, X.; Ma, Y. Associations between organophosphate esters concentrations and markers of liver function in US adolescents aged 12–19 years: A mixture analysis. Environ. Pollut. 2022, 314, 120255. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, H.; Lu, K.; Yang, S.; Tang, X.; Zhou, M.; Sun, G.; Zhang, Z.; Chu, H. Chlorinated Organophosphate Flame Retardants Impair the Lung Function via the IL-6/JAK/STAT Signaling Pathway. Environ. Sci. Technol. 2022, 56, 17858–17869. [Google Scholar] [CrossRef] [PubMed]
- Hornung, R.W.; Reed, L.D. Estimation of Average Concentration in the Presence of Nondetectable Values. Appl. Occup. Environ. Hyg. 1990, 5, 46–51. [Google Scholar] [CrossRef]
- Johnson, C.L.; Paulose-Ram, R.; Ogden, C.L.; Carroll, M.D.; Kruszon-Moran, D.; Dohrmann, S.M.; Curtin, L.R. National health and nutrition examination survey: Analytic guidelines, 1999–2010. In Vital and Health Statistics. Series 2: Data Evaluation and Methods Research; Department of Health and Human Services Public Health Servic: Washington, DC, USA, 2013; pp. 1–24. [Google Scholar]
- Hoffman, K.; Daniels, J.L.; Stapleton, H.M. Urinary metabolites of organophosphate flame retardants and their variability in pregnant women. Environ. Int. 2014, 63, 169–172. [Google Scholar] [CrossRef]
- Hoffman, K.; Lorenzo, A.; Butt, C.M.; Adair, L.; Herring, A.H.; Stapleton, H.M.; Daniels, J.L. Predictors of urinary flame retardant concentration among pregnant women. Environ. Int. 2017, 98, 96–101. [Google Scholar] [CrossRef]
- Green, A.J.; Graham, J.L.; Gonzalez, E.A.; La Frano, M.R.; Petropoulou, S.E.; Park, J.S.; Newman, J.W.; Stanhope, K.L.; Havel, P.J.; La Merrill, M.A. Perinatal triphenyl phosphate exposure accelerates type 2 diabetes onset and increases adipose accumulation in UCD-type 2 diabetes mellitus rats. Reprod. Toxicol. 2017, 68, 119–129. [Google Scholar] [CrossRef]
- Patisaul, H.B.; Roberts, S.C.; Mabrey, N.; McCaffrey, K.A.; Gear, R.B.; Braun, J.; Belcher, S.M.; Stapleton, H.M. Accumulation and endocrine disrupting effects of the flame retardant mixture Firemaster® 550 in rats: An exploratory assessment. J. Biochem. Mol. Toxicol. 2013, 27, 124–136. [Google Scholar] [CrossRef]
- Boyle, M.; Buckley, J.P.; Quirós-Alcalá, L. Associations between urinary organophosphate ester metabolites and measures of adiposity among U.S. children and adults: NHANES 2013–2014. Environ. Int. 2019, 127, 754–763. [Google Scholar] [CrossRef]
- Luo, K.; Zhang, R.; Aimuzi, R.; Wang, Y.; Nian, M.; Zhang, J. Exposure to Organophosphate esters and metabolic syndrome in adults. Environ. Int. 2020, 143, 105941. [Google Scholar] [CrossRef] [PubMed]
- van der Schyff, V.; Kalina, J.; Govarts, E.; Gilles, L.; Schoeters, G.; Castaño, A.; Esteban-López, M.; Kohoutek, J.; Kukučka, P.; Covaci, A.; et al. Exposure to flame retardants in European children—Results from the HBM4EU aligned studies. Int. J. Hyg. Environ. Health 2023, 247, 114070. [Google Scholar] [CrossRef]
- Su, G.; Crump, D.; Letcher, R.J.; Kennedy, S.W. Rapid in vitro metabolism of the flame retardant triphenyl phosphate and effects on cytotoxicity and mRNA expression in chicken embryonic hepatocytes. Environ. Sci. Technol. 2014, 48, 13511–13519. [Google Scholar] [CrossRef] [PubMed]
- Selmi-Ruby, S.; Marín-Sáez, J.; Fildier, A.; Buleté, A.; Abdallah, M.; Garcia, J.; Deverchère, J.; Spinner, L.; Giroud, B.; Ibanez, S.; et al. In Vivo Characterization of the Toxicological Properties of DPhP, One of the Main Degradation Products of Aryl Phosphate Esters. Environ. Health Perspect. 2020, 128, 127006. [Google Scholar] [CrossRef] [PubMed]
- Le, Y.; Shen, H.; Yang, Z.; Lu, D.; Wang, C. Comprehensive analysis of organophosphorus flame retardant-induced mitochondrial abnormalities: Potential role in lipid accumulation. Environ. Pollut. 2021, 274, 116541. [Google Scholar] [CrossRef]
- Belcher, S.M.; Cookman, C.J.; Patisaul, H.B.; Stapleton, H.M. In vitro assessment of human nuclear hormone receptor activity and cytotoxicity of the flame retardant mixture FM 550 and its triarylphosphate and brominated components. Toxicol. Lett. 2014, 228, 93–102. [Google Scholar] [CrossRef]
- Pillai, H.K.; Fang, M.; Beglov, D.; Kozakov, D.; Vajda, S.; Stapleton, H.M.; Webster, T.F.; Schlezinger, J.J. Ligand binding and activation of PPARγ by Firemaster® 550: Effects on adipogenesis and osteogenesis in vitro. Environ. Health Perspect. 2014, 122, 1225–1232. [Google Scholar] [CrossRef]
- Arukwe, A.; Carteny, C.C.; Eggen, T. Lipid peroxidation and oxidative stress responses in juvenile salmon exposed to waterborne levels of the organophosphate compounds tris(2-butoxyethyl)- and tris(2-chloroethyl) phosphates. J. Toxicol. Environ. Health Part A 2016, 79, 515–525. [Google Scholar] [CrossRef]
- Yang, D.; Wei, X.; Zhang, Z.; Chen, X.; Zhu, R.; Oh, Y.; Gu, N. Tris (2-chloroethyl) phosphate (TCEP) induces obesity and hepatic steatosis via FXR-mediated lipid accumulation in mice: Long-term exposure as a potential risk for metabolic diseases. Chem. Biol. Interact. 2022, 363, 110027. [Google Scholar] [CrossRef]
- Tenlep, S.Y.N.; Weaver, M.; Chen, J.; Vsevolozhskaya, O.; Morris, A.J.; Rashid, C.S. Tris(1,3-dichloro-2-propyl) phosphate is a metabolism-disrupting chemical in male mice. Toxicol. Lett. 2023, 374, 31–39. [Google Scholar] [CrossRef]
- Cui, H.; Chang, Y.; Cao, J.; Jiang, X.; Li, M. Liver immune and lipid metabolism disorders in mice induced by triphenyl phosphate with or without high fructose and high fat diet. Chemosphere 2022, 308, 136543. [Google Scholar] [CrossRef]
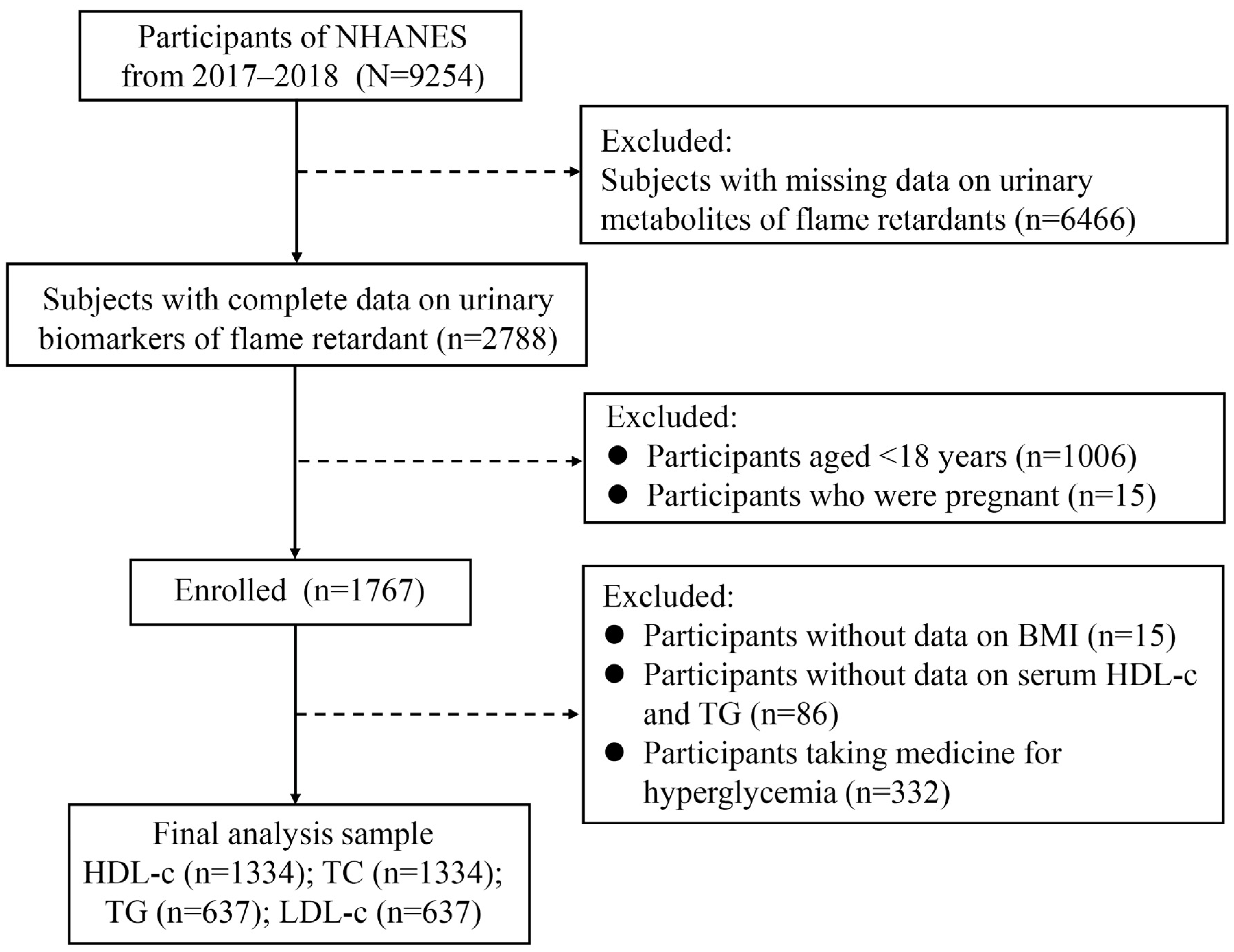
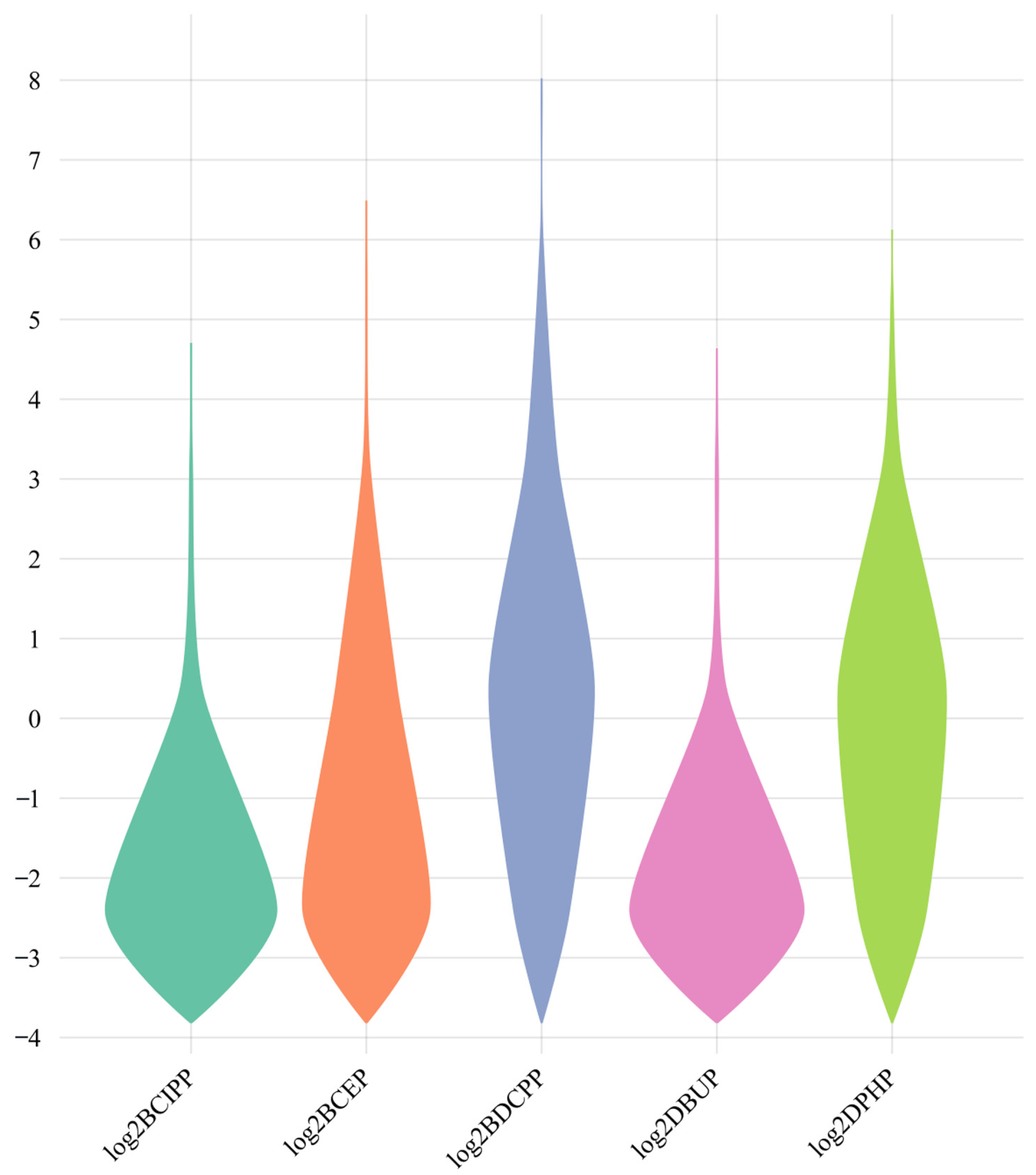
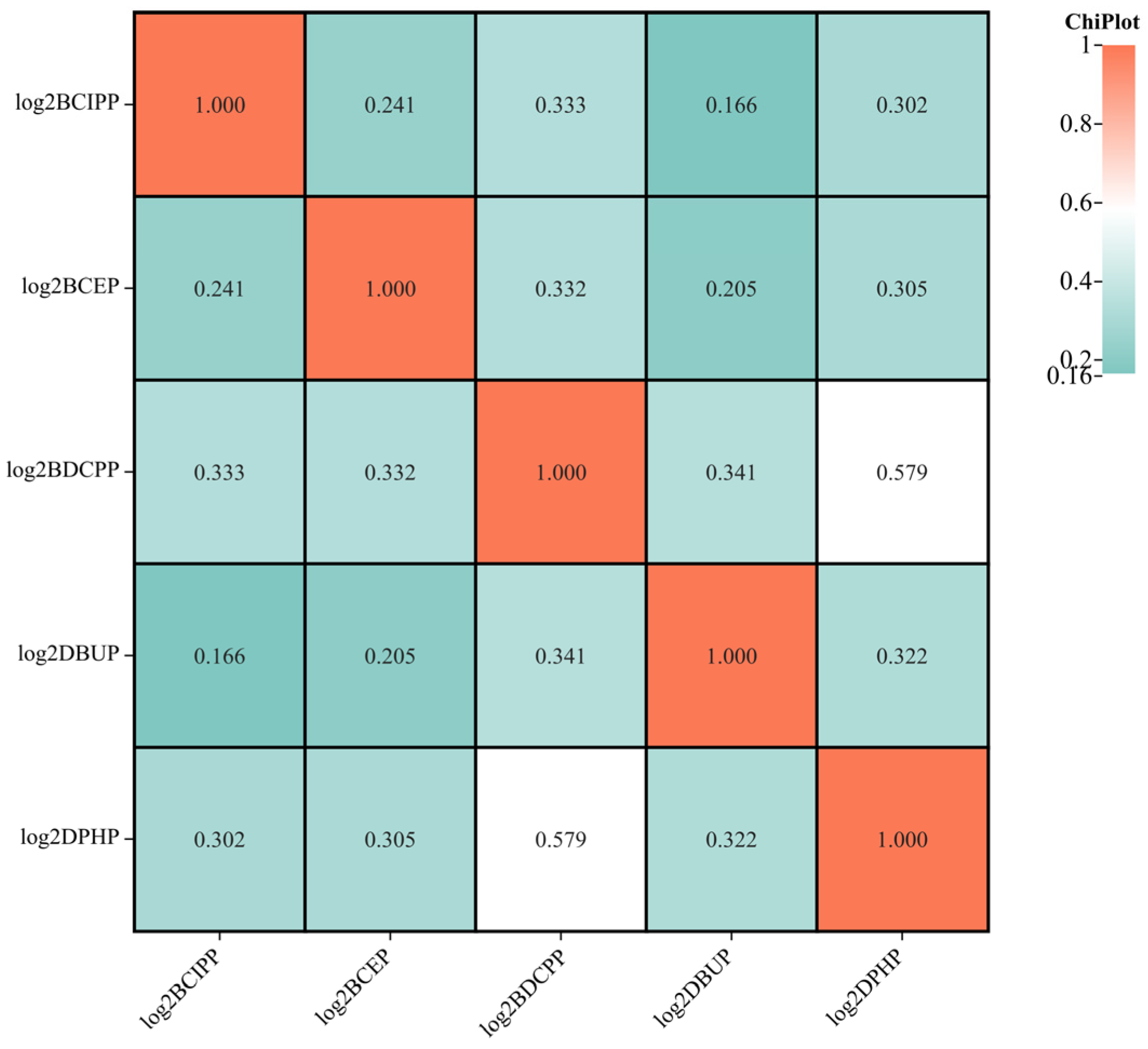
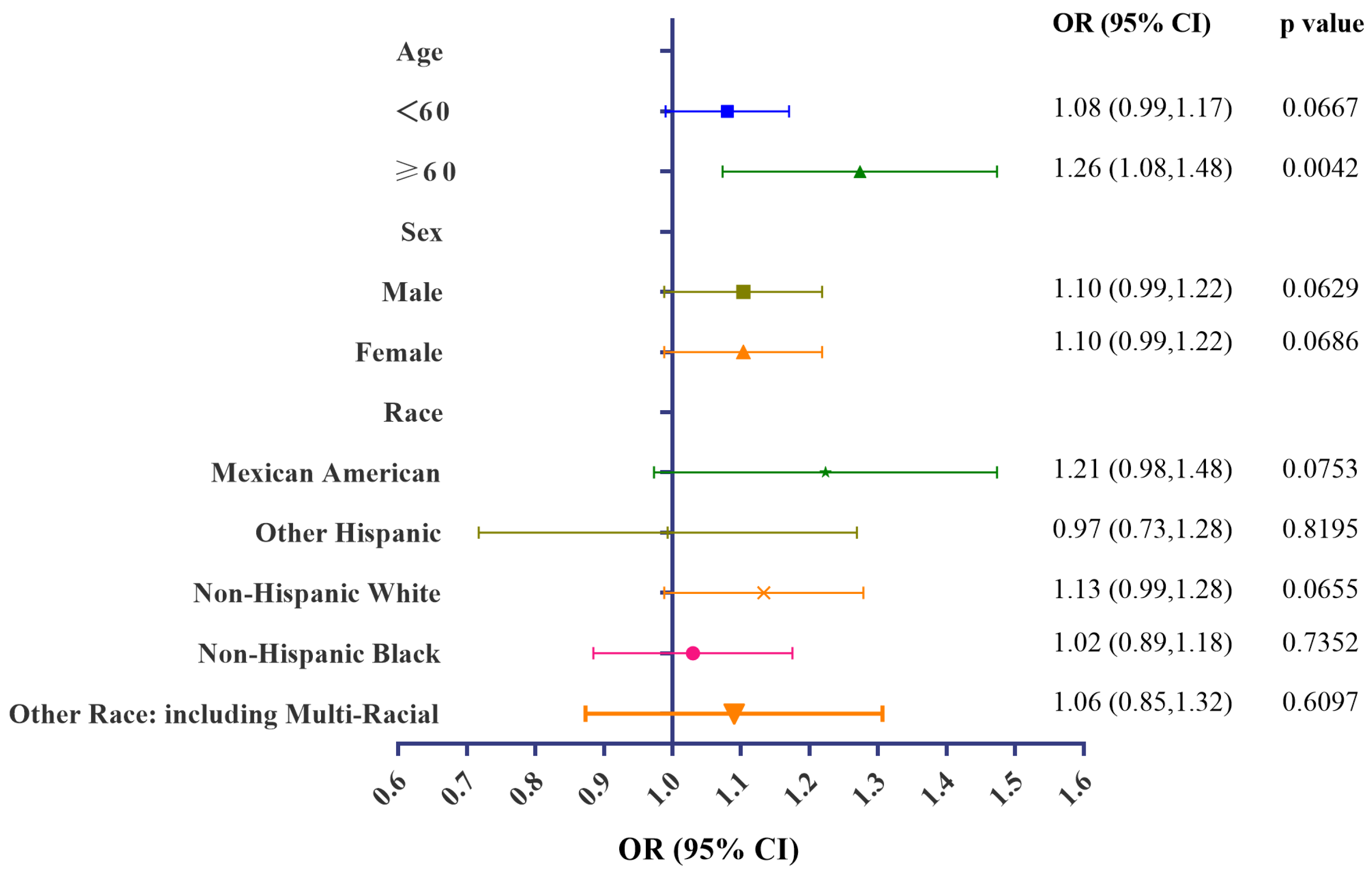
| Variables | All Participants (n = 1334) |
|---|---|
| Age, years, mean ± SD | 45.19 ± 17.59 |
| Sex, n (%) | |
| Male | 656 (49.18) |
| Female | 678 (50.82) |
| BMI, kg/m2, mean ± SD | 29.42 ± 7.88 |
| Race, n (%) | |
| Mexican American | 206 (15.44%) |
| Other Hispanic | 122 (9.15%) |
| Non-Hispanic White | 449 (33.66%) |
| Non-Hispanic Black | 297 (22.26%) |
| Other race: including multiracial | 260 (19.49%) |
| Smoking status, n (%) | |
| Current smoker | 839 (62.89%) |
| Former smoker | 206 (15.44%) |
| Non-smoker | 289 (21.66%) |
| Drinking, n (%) | 158 (11.84%) |
| Physical activity, n (%) | |
| None | 675 (50.60%) |
| Moderate activity | 299 (22.41%) |
| Vigorous activity | 360 (26.99%) |
| Education level, n (%) | |
| Less than 9th grade | 103 (8.25%) |
| 9–11th grade | 146 (11.70%) |
| High school graduate/GED or equivalent | 281 (22.52%) |
| Some college or AA degree | 406 (32.53%) |
| College graduate or above | 312 (25.00%) |
| Marital status, n (%) | |
| Married/living with partner | 747 (59.76%) |
| Widowed/divorced/separated | 241 (19.28%) |
| Never married | 262 (20.96%) |
| Family PIR, n (%) | |
| <1 | 248 (18.59%) |
| 1–1.99 | 322 (24.14%) |
| 2–3.99 | 296 (22.19%) |
| ≥4 | 289 (21.66%) |
| Missing | 179 (13.42%) |
| Hypertension, n (%) | 334 (25.04%) |
| Diabetes, n (%) | 105 (7.87%) |
| Stroke, n (%) | 32 (2.40%) |
| Coronary artery disease, n (%) | 20 (1.50%) |
| Heart failure, n (%) | 20 (1.50%) |
| Heart attack, n (%) | 237 (13.41%) |
| Variables | All Participants (n = 1334) |
|---|---|
| Urinary creatinine (mg/dL), median (Q1–Q3) | 118.00 (63.00–181.00) |
| Urinary metabolites of OPFRs (ng/mL), GM (95% CI) | |
| BCIPP | 0.335 (0.313–0.357) |
| BCEP | 1.046 (0.979–1.118) |
| BDCPP | 0.136 (0.129–0.143) |
| DBUP | 0.132 (0.125–0.138) |
| DPHP | 0.764 (0.718–0.812) |
| Variables | Model 1 | Model 2 | ||
|---|---|---|---|---|
| β (95% CI) | p Value | β (95% CI) | p Value | |
| BCIPP | 0.32 (0.03, 0.62) | 0.0323 | 0.17 (−0.15, 0.49) | 0.3030 |
| BCEP | 0.33 (0.09, 0.57) | 0.0068 | 0.27 (0.02, 0.52) | 0.0338 |
| BDCPP | 0.71 (0.46, 0.96) | <0.0001 | 0.56 (0.25, 0.87) | 0.0004 |
| DBUP | 0.35 (0.03, 0.67) | 0.0310 | 0.07 (−0.28, 0.41) | 0.6987 |
| DPHP | 0.26 (−0.00, 0.52) | 0.0528 | −0.08 (−0.40, 0.24) | 0.6195 |
| Variables | Models | OR (95% CI) | p Value |
|---|---|---|---|
| BCIPP | Model 1 | 1.05 (0.97, 1.13) | 0.2557 |
| Model 2 | 1.02 (0.93, 1.11) | 0.7364 | |
| BCEP | Model 1 | 1.12 (1.05, 1.20) | 0.0005 |
| Model 2 | 1.10 (1.02, 1.18) | 0.0096 | |
| BDCPP | Model 1 | 1.21 (1.13, 1.30) | <0.0001 |
| Model 2 | 1.19 (1.09, 1.30) | 0.0001 | |
| DBUP | Model 1 | 1.10 (1.01, 1.20) | 0.0273 |
| Model 2 | 1.04 (0.95, 1.14) | 0.4258 | |
| DPHP | Model 1 | 1.09 (1.01, 1.17) | 0.0206 |
| Model 2 | 1.00 (0.92, 1.09) | 0.9820 |
| Variables | Models | TG | TC | HDL-c | LDL-c | ||||
|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p Value | β (95% CI) | p Value | β (95% CI) | p Value | β (95% CI) | p Value | ||
| BCIPP | Model 1 | −2.74 (−6.65, 1.18) | 0.1714 | −0.08 (−1.59, 1.42) | 0.9125 | −0.09 (−0.66, 0.48) | 0.7540 | −0.70 (−2.55, 1.16) | 0.4628 |
| Model 2 | −3.31 (−7.75, 1.13) | 0.1444 | 0.05 (−1.63, 1.73) | 0.9519 | 0.24 (−0.36, 0.84) | 0.4345 | −1.22 (−3.31, 0.86) | 0.2499 | |
| BCEP | Model 1 | 0.61 (−2.62, 3.84) | 0.7117 | −0.51 (−1.71, 0.70) | 0.4090 | −0.54 (−1.00, −0.09) | 0.0199 | −0.41 (−1.94, 1.12) | 0.6018 |
| Model 2 | 0.16 (−3.43, 3.75) | 0.9305 | −0.39 (−1.71, 0.92) | 0.5580 | −0.32 (−0.79, 0.16) | 0.1887 | −1.20 (−3.37, 0.97) | 0.2794 | |
| BDCPP | Model 1 | −0.88 (−4.45, 2.70) | 0.6304 | −0.78 (−2.06, 0.50) | 0.2309 | −0.55 (−1.04, −0.07) | 0.0250 | −0.41 (−2.11, 1.28) | 0.6315 |
| Model 2 | −2.25 (−6.88, 2.38) | 0.3418 | −0.82 (−2.46, 0.82) | 0.3279 | 0.29 (−0.30, 0.89) | 0.3299 | −1.20 (−3.37, 0.97) | 0.2794 | |
| DBUP | Model 1 | 1.04 (−3.56, 5.64) | 0.6579 | −0.45 (−2.05, 1.15) | 0.5831 | −0.40 (−1.01, 0.21) | 0.1998 | −0.31 (−2.49, 1.86) | 0.7787 |
| Model 2 | 0.69 (−4.55, 5.93) | 0.7961 | −0.76 (−2.55, 1.03) | 0.4055 | 0.10 (−0.54, 0.75) | 0.7587 | −1.30 (−3.75, 1.15) | 0.2994 | |
| DPHP | Model 1 | −5.67 (−9.34, −2.00) | 0.0025 | −0.67 (−1.99, 0.66) | 0.3221 | −0.59 (−1.09, −0.08) | 0.0226 | −1.03 (−2.78, 0.73) | 0.2527 |
| Model 2 | −7.41 (−12.13, −2.68) | 0.0022 | −0.17 (−1.83, 1.49) | 0.8420 | −0.06 (−0.65, 0.54) | 0.8545 | −1.20 (−3.44, 1.03) | 0.2925 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Li, F.; Zhou, C.; Bu, J.; Yang, H.; Zhong, L.; Xing, W.; Li, L. Exposure to OPFRs Is Associated with Obesity and Dysregulated Serum Lipid Profiles: Data from 2017–2018 NHANES. Metabolites 2024, 14, 124. https://doi.org/10.3390/metabo14020124
Li H, Li F, Zhou C, Bu J, Yang H, Zhong L, Xing W, Li L. Exposure to OPFRs Is Associated with Obesity and Dysregulated Serum Lipid Profiles: Data from 2017–2018 NHANES. Metabolites. 2024; 14(2):124. https://doi.org/10.3390/metabo14020124
Chicago/Turabian StyleLi, He, Fenglin Li, Chaoyi Zhou, Jifan Bu, Hao Yang, Liangchen Zhong, Weilong Xing, and Liangzhong Li. 2024. "Exposure to OPFRs Is Associated with Obesity and Dysregulated Serum Lipid Profiles: Data from 2017–2018 NHANES" Metabolites 14, no. 2: 124. https://doi.org/10.3390/metabo14020124
APA StyleLi, H., Li, F., Zhou, C., Bu, J., Yang, H., Zhong, L., Xing, W., & Li, L. (2024). Exposure to OPFRs Is Associated with Obesity and Dysregulated Serum Lipid Profiles: Data from 2017–2018 NHANES. Metabolites, 14(2), 124. https://doi.org/10.3390/metabo14020124





