Metabolomic Profiling of Blood Plasma in Females with Hyperplasia and Endometrial Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Population and Study Design
2.2. Metabolite Extraction
2.3. Metabolite Analysis
2.4. Data Handling and Processing
2.5. Bioinformatic Analysis
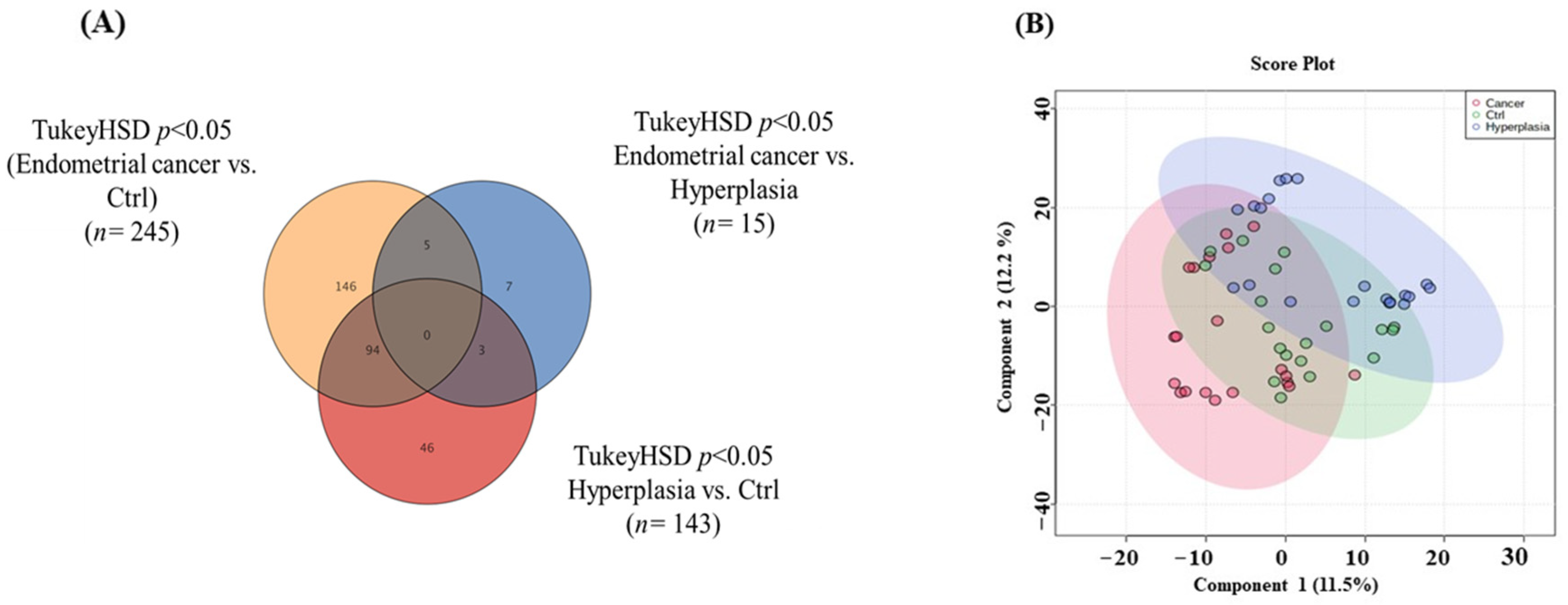
3. Results
3.1. Mass Ion Detection and Metabolite Identification
3.2. Overview of the Three Study Groups (Ctrl, HP, and EC)
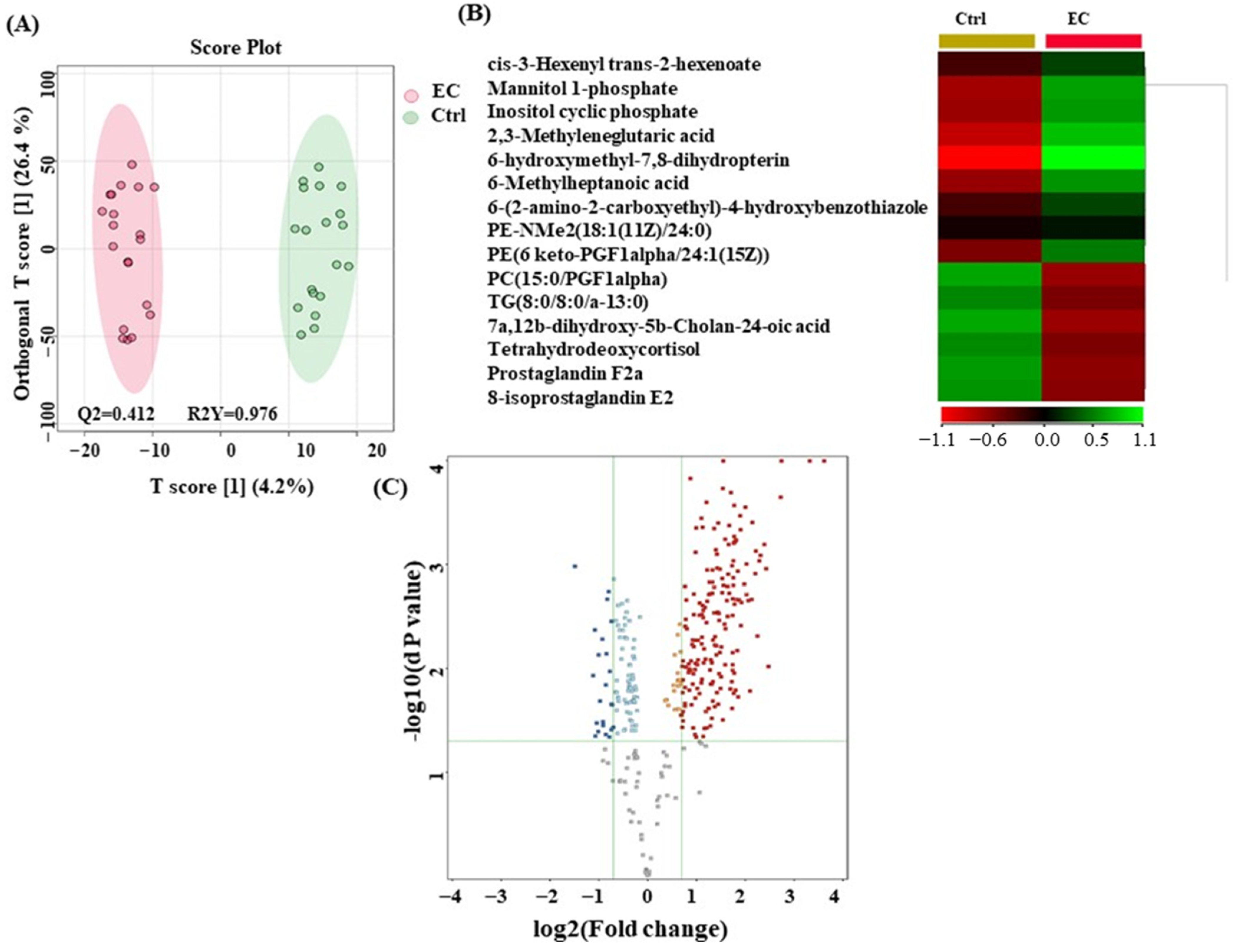
3.3. Metabolomic Profiling between EC and Ctrl
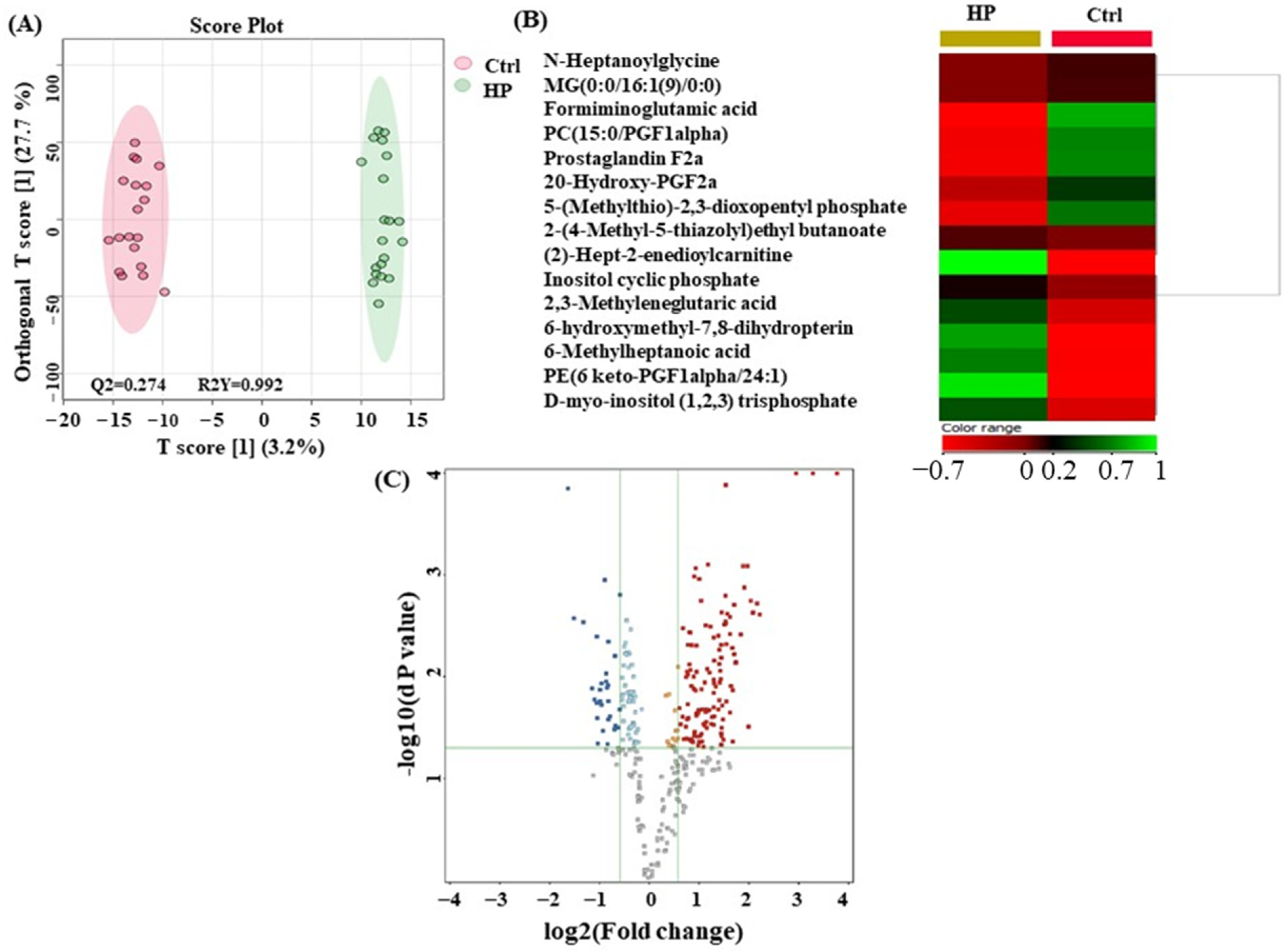
3.4. Metabolomics Profiling between HP and Ctrl
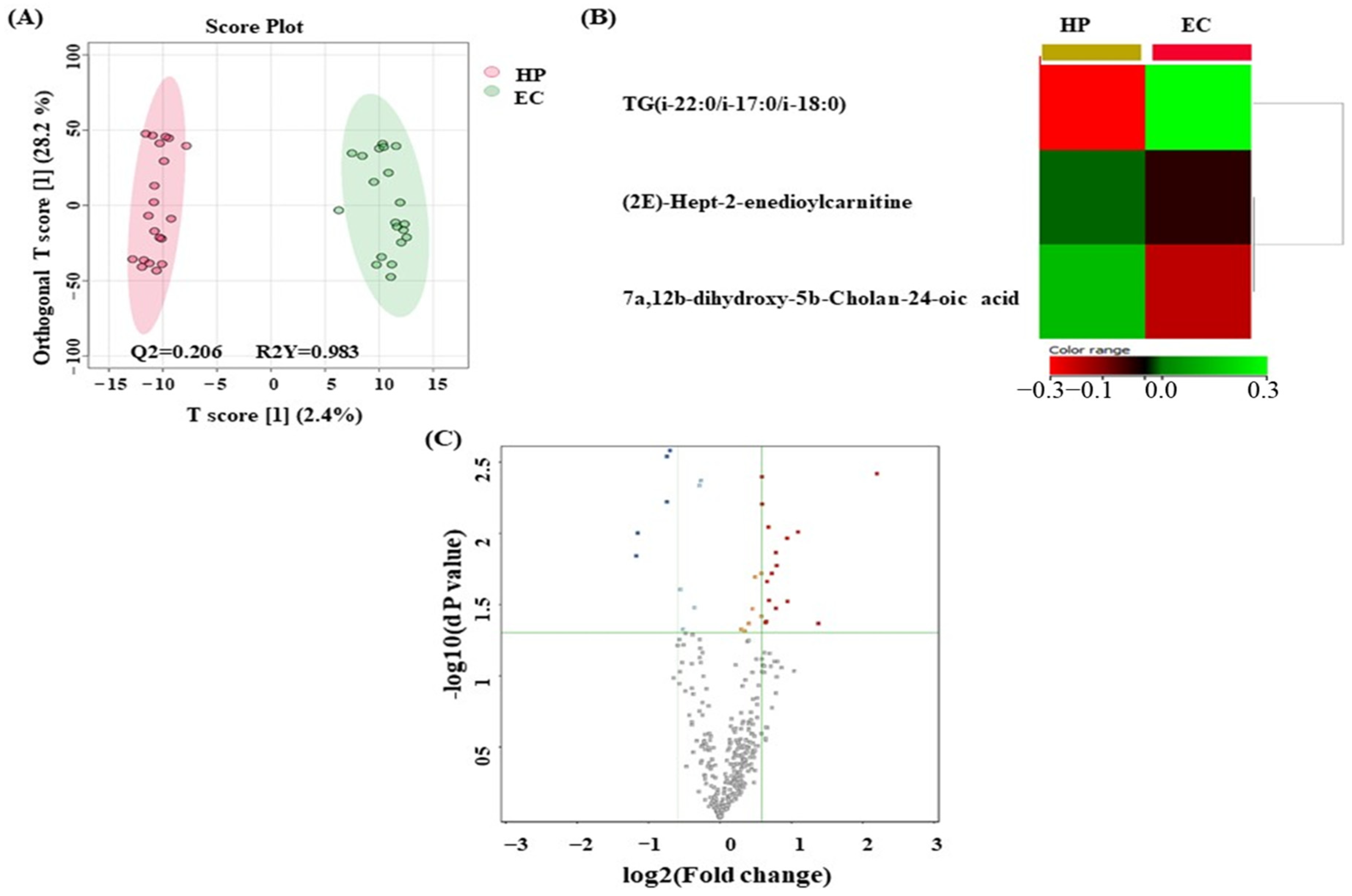
3.5. Metabolomics Profiling between EC and HP
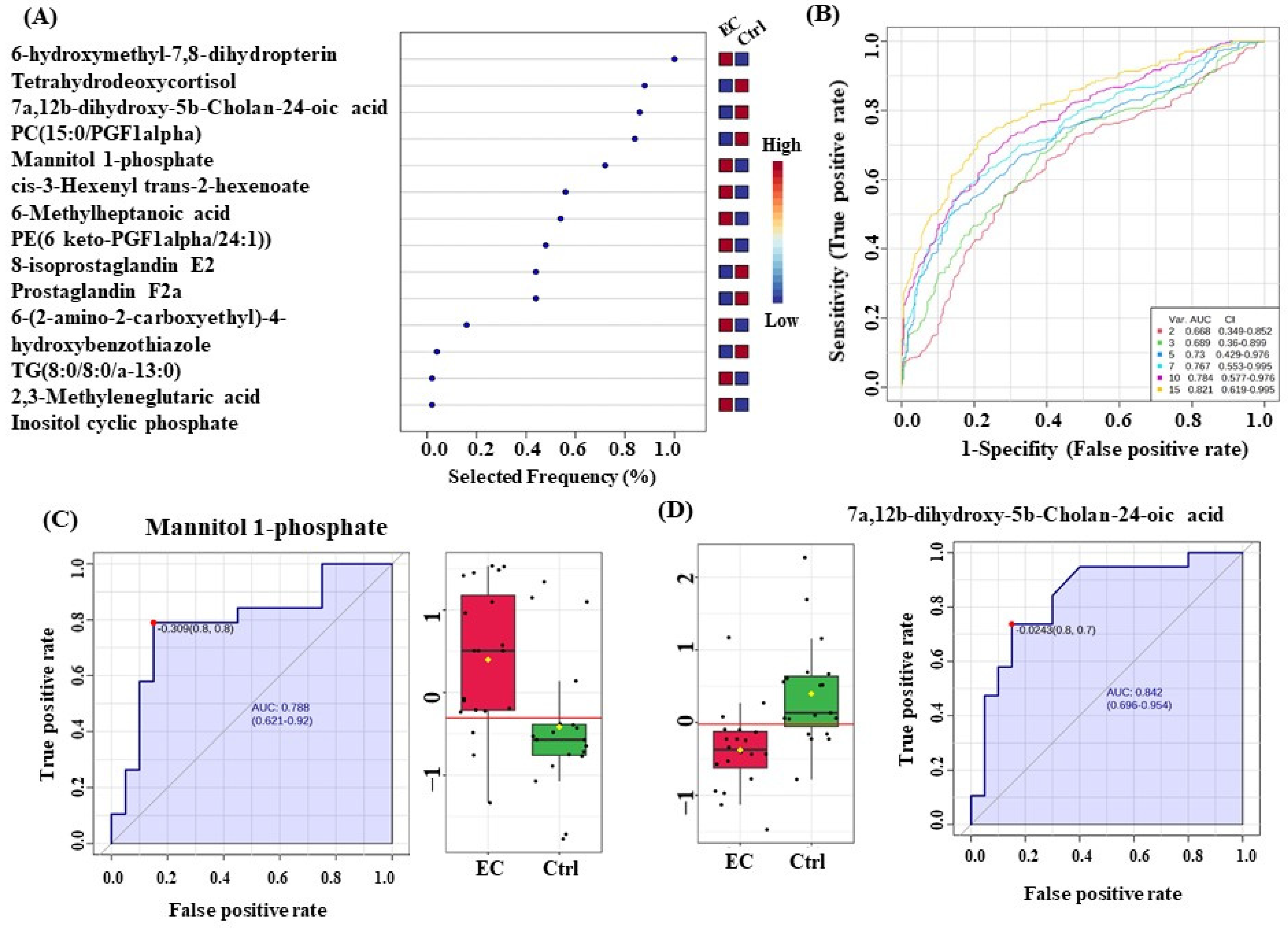
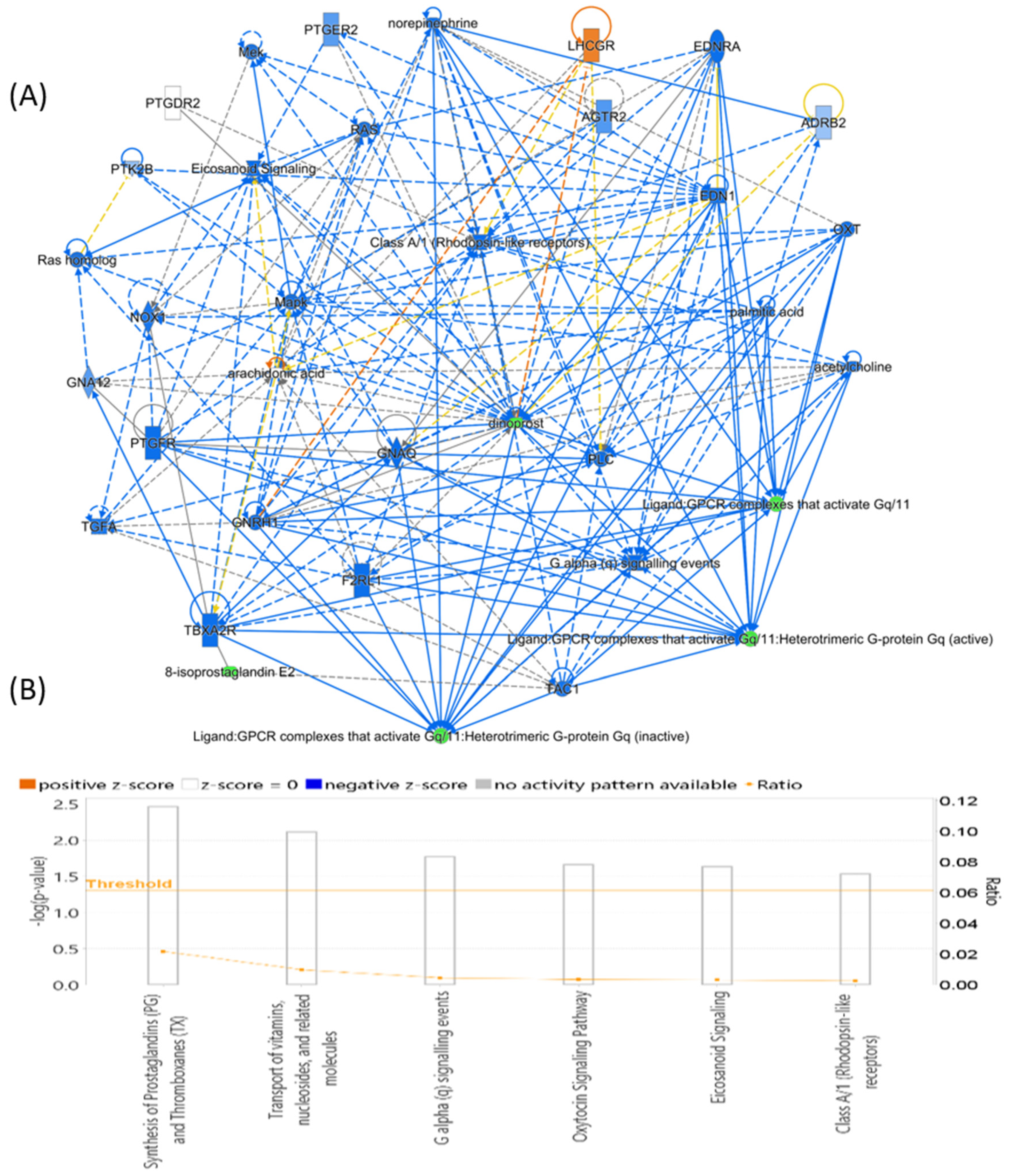
3.6. Evaluation of Metabolite Biomarkers between EC and Ctrl Groups and Network Pathway
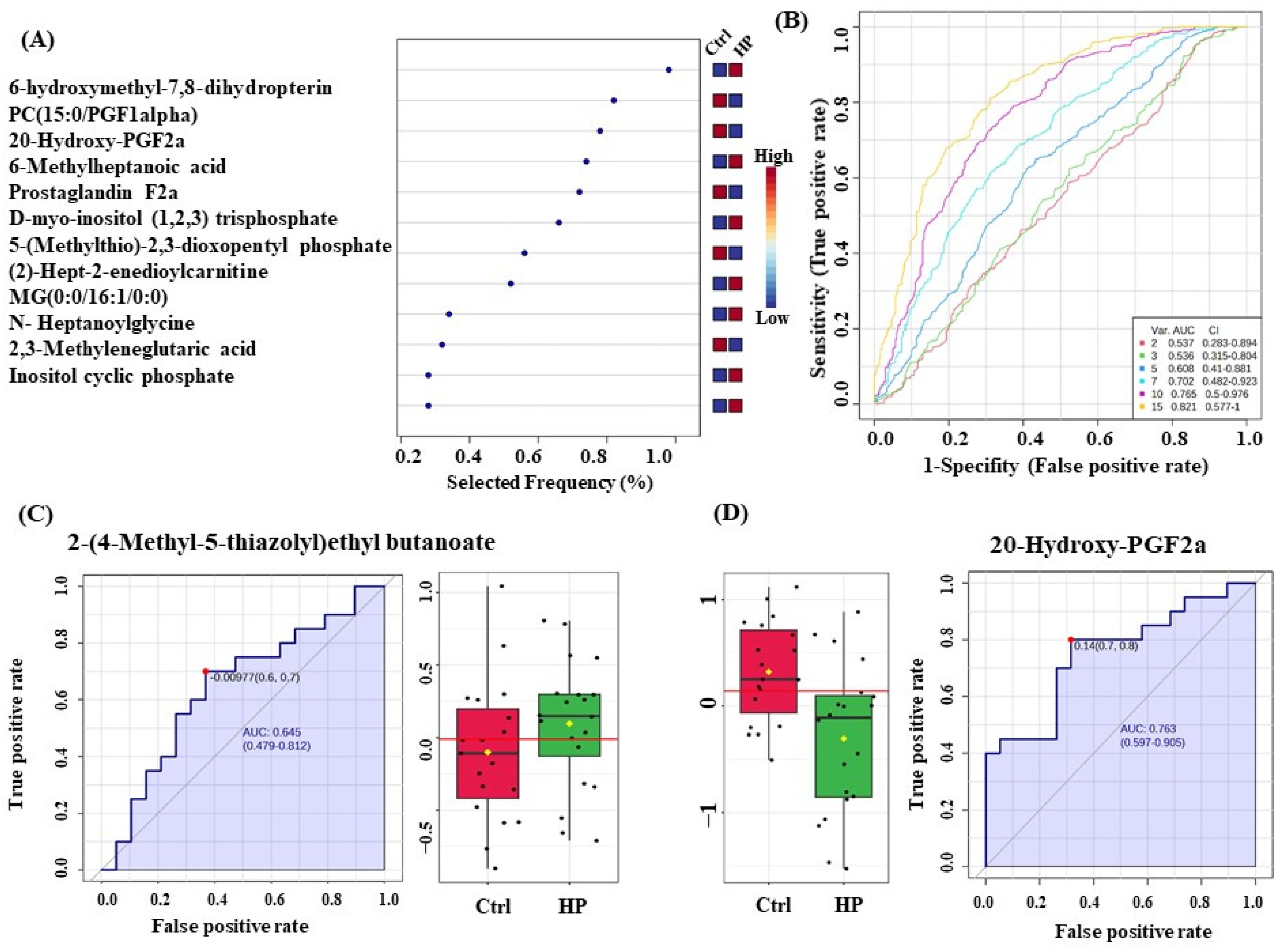
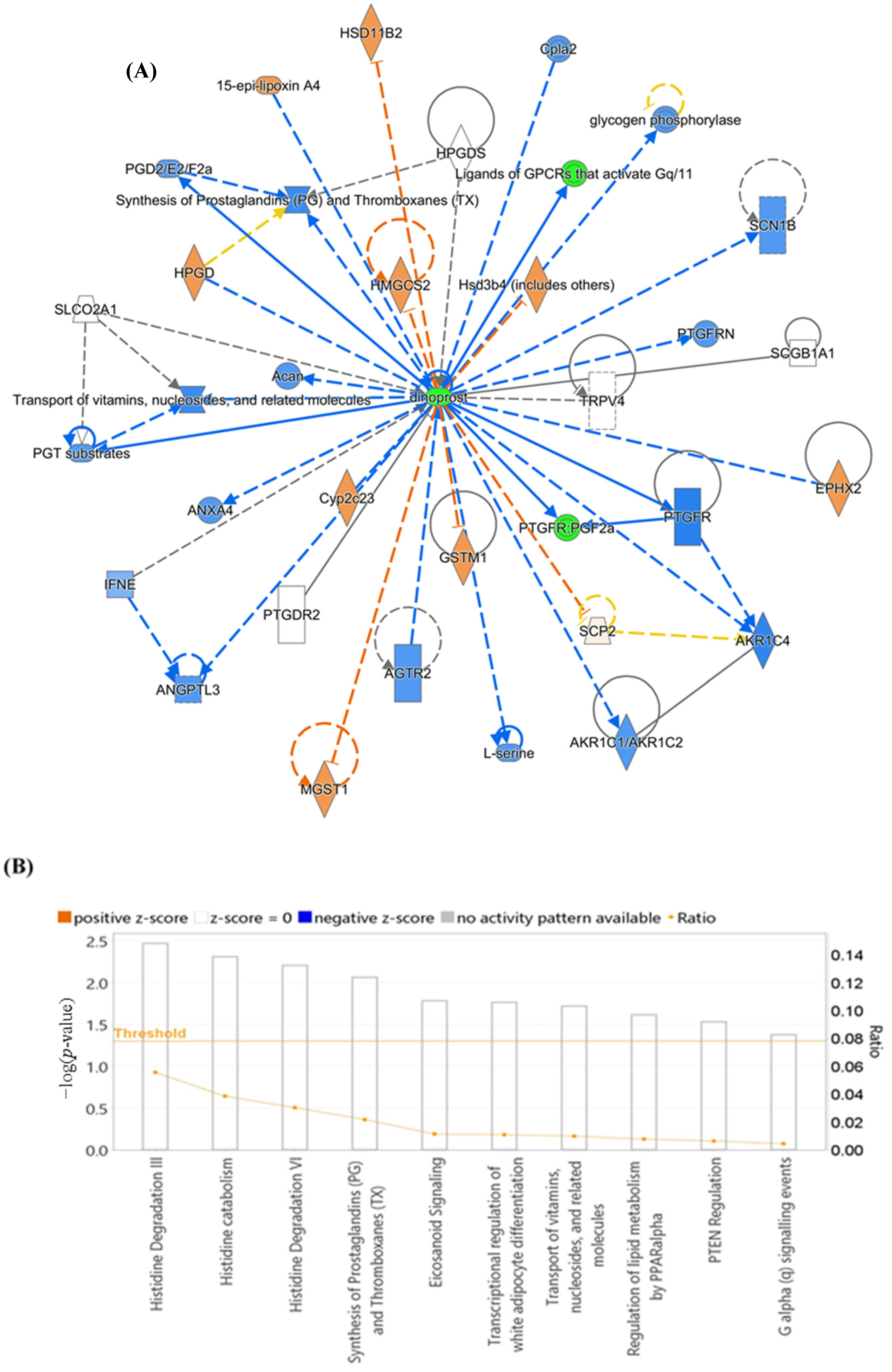
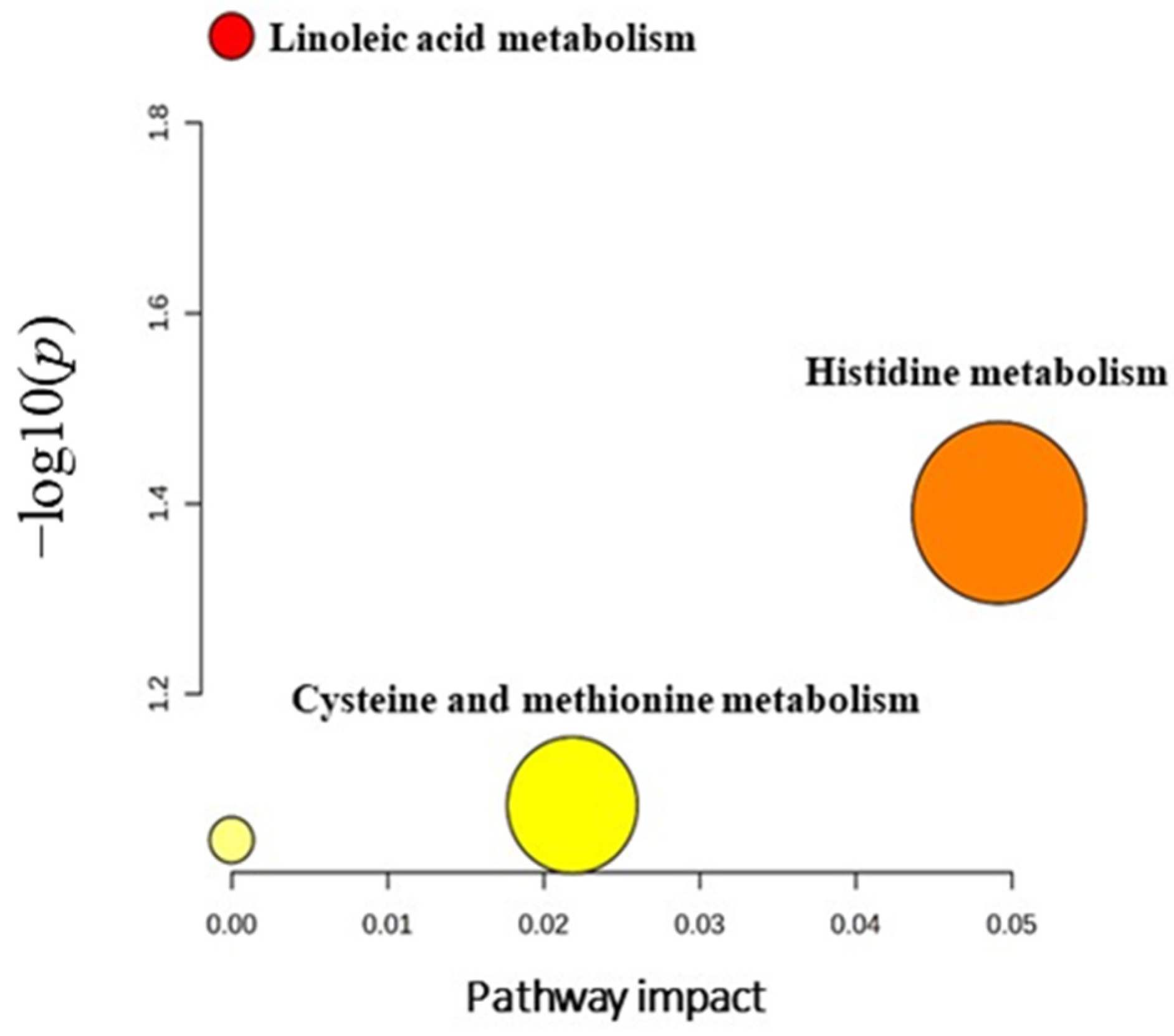
3.7. Evaluation of Metabolite Biomarkers between HP and Ctrl Groups and Network Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Kong, L.; Gao, G.; Luo, J. A brief introduction to web-based genome browsers. Brief. Bioinform. 2013, 14, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Corpus, C.U.; Vulva, U.O.V. Gynecologic Cancer Incidence, United States—2012–2016; Centers for Disease Control and Prevention, US Department of Health and Human Services: Washington, DC, USA, 2019; Volume 11. [Google Scholar]
- Board, E. Uterine Cancer: Statistics. 03/2023. Available online: https://www.cancer.net/cancer-types/uterine-cancer/statistics (accessed on 1 April 2023).
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- Cancer Research UK. Types and Grades of Womb Cancer. 2023. Available online: https://www.cancerresearchuk.org/about-cancer/womb-cancer/types-grades (accessed on 31 March 2023).
- Murali, R.; Soslow, R.A.; Weigelt, B. Classification of endometrial carcinoma: More than two types. Lancet Oncol. 2014, 15, e268–e278. [Google Scholar] [CrossRef] [PubMed]
- American Society Cancer. Endometrial Cancer Early Detection, Diagnosis, and Staging. 2023. Available online: https://www.cancer.org/cancer/types/endometrial-cancer/detection-diagnosis-staging.html (accessed on 11 July 2023).
- Yen, T.-T.; Wang, T.-L.; Fader, A.N.; Shih, I.-M.M.; Gaillard, S.M. Molecular Classification and Emerging Targeted Therapy in Endometrial Cancer. Int. J. Gynecol. Pathol. 2020, 39, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Zagouri, F.; Bozas, G.; Kafantari, E.; Tsiatas, M.; Nikitas, N.; Dimopoulos, M.A.; Papadimitriou, C.A. Endometrial cancer: What is new in adjuvant and molecularly targeted therapy? Obstet. Gynecol. Int. 2010, 2010, 749579. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Douville, C.; Cohen, J.D.; Yen, T.T.; Kinde, I.; Sundfelt, K.; Kjær, S.K.; Hruban, R.H.; Shih, I.M.; et al. Evaluation of liquid from the Papanicolaou test and other liquid biopsies for the detection of endometrial and ovarian cancers. Sci. Transl. Med. 2018, 10, eaap8793. [Google Scholar] [CrossRef]
- Kinde, I.; Bettegowda, C.; Wang, Y.; Wu, J.; Agrawal, N.; Shih, I.M.; Kurman, R.; Dao, F.; Levine, D.A.; Giuntoli, R.; et al. Evaluation of DNA from the Papanicolaou test to detect ovarian and endometrial cancers. Sci. Transl. Med. 2013, 5, 167ra4. [Google Scholar] [CrossRef]
- Jacob, M.; Lopata, A.L.; Dasouki, M.; Rahman, A.M.A. Metabolomics toward personalized medicine. Mass. Spectrom. Rev. 2019, 38, 221–238. [Google Scholar] [CrossRef]
- Han, J.; Li, Q.; Chen, Y.; Yang, Y. Recent Metabolomics Analysis in Tumor Metabolism Reprogramming. Front. Mol. Biosci. 2021, 8, 763902. [Google Scholar] [CrossRef]
- Schmidt, D.R.; Patel, R.; Kirsch, D.G.; Lewis, C.A.; Vander Heiden, M.G.; Locasale, J.W. Metabolomics in cancer research and emerging applications in clinical oncology. CA Cancer J. Clin. 2021, 71, 333–358. [Google Scholar] [CrossRef]
- Troisi, J.; Mollo, A.; Lombardi, M.; Scala, G.; Richards, S.M.; Symes, S.J.K.; Travaglino, A.; Neola, D.; de Laurentiis, U.; Insabato, L.; et al. The Metabolomic Approach for the Screening of Endometrial Cancer: Validation from a Large Cohort of Women Scheduled for Gynecological Surgery. Biomolecules 2022, 12, 1229. [Google Scholar] [CrossRef] [PubMed]
- Dossus, L.; Kouloura, E.; Biessy, C.; Viallon, V.; Siskos, A.P.; Dimou, N.; Rinaldi, S.; Merritt, M.A.; Allen, N.; Fortner, R.; et al. Prospective analysis of circulating metabolites and endometrial cancer risk. Gynecol. Oncol. 2021, 162, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Audet-Delage, Y.; Villeneuve, L.; Grégoire, J.; Plante, M.; Guillemette, C. Identification of Metabolomic Biomarkers for Endometrial Cancer and Its Recurrence after Surgery in Postmenopausal Women. Front. Endocrinol. 2018, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Njoku, K.; Sutton, C.J.; Whetton, A.D.; Crosbie, E.J. Metabolomic Biomarkers for Detection, Prognosis and Identifying Recurrence in Endometrial Cancer. Metabolites 2020, 10, 314. [Google Scholar] [CrossRef] [PubMed]
- Raffone, A.; Troisi, J.; Boccia, D.; Travaglino, A.; Capuano, G.; Insabato, L.; Mollo, A.; Guida, M.; Zullo, F. Metabolomics in endometrial cancer diagnosis: A systematic review. Acta Obs. Gynecol. Scand. 2020, 99, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Jaber, M.A.; Benabdelkamel, H.; Dahabiyeh, L.A.; Masood, A.; AlMalki, R.H.; Musambil, M.; Alfadda, A.A.; Rahman, A.M.A. The metabolomics approach revealed a distinctive metabolomics pattern associated with hyperthyroidism treatment. Front. Endocrinol. 2022, 13, 1050201. [Google Scholar] [CrossRef] [PubMed]
- Benabdelkamel, H.; Jaber, M.A.; Dahabiyeh, L.A.; Masood, A.; AlMalki, R.H.; Musambil, M.; Rahman, A.M.A.; Alfadda, A.A. Metabolomic profile of overweight patients on LT4 treatment for hypothyroidism. Eur. Thyroid J. 2023, 12, e230062. [Google Scholar] [CrossRef]
- AlMalki, R.H.; Sebaa, R.; Al-Ansari, M.M.; Al-Alwan, M.; Alwehaibi, M.A.; Rahman, A.M.A. E. coli Secretome Metabolically Modulates MDA-MB-231 Breast Cancer Cells’ Energy Metabolism. Int. J. Mol. Sci. 2023, 24, 4219. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. Multivariate analysis in metabolomics. Curr. Metabolomics 2013, 1, 92–107. [Google Scholar]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The human metabolome database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Kim, S.Y. Cancer Energy Metabolism: Shutting Power off Cancer Factory. Biomol. Ther. 2018, 26, 39–44. [Google Scholar] [CrossRef]
- Warburg, O. On respiratory impairment in cancer cells. Science 1956, 124, 269–270. [Google Scholar] [CrossRef]
- Sonveaux, P.; Vegran, F.; Schroeder, T.; Wergin, M.C.; Verrax, J.; Rabbani, Z.N.; De Saedeleer, C.J.; Kennedy, K.M.; Diepart, C.; Jordan, B.F.; et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Investig. 2008, 118, 3930–3942. [Google Scholar] [CrossRef]
- Li, J.T.; Yang, H.; Lei, M.Z.; Zhu, W.P.; Su, Y.; Li, K.Y.; Zhu, W.Y.; Wang, J.; Zhang, L.; Qu, J.; et al. Dietary folate drives methionine metabolism to promote cancer development by stabilizing MAT IIA. Signal Transduct. Target. Ther. 2022, 7, 192. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Mancuso, A.; Daikhin, E.; Nissim, I.; Yudkoff, M.; Wehrli, S.; Thompson, C.B. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 19345–19350. [Google Scholar] [CrossRef]
- Deng, Y.; Yao, H.; Chen, W.; Wei, H.; Li, X.; Zhang, F.; Gao, S.; Man, H.; Chen, J.; Tao, X.; et al. Profiling of polar urine metabolite extracts from Chinese colorectal cancer patients to screen for potential diagnostic and adverse-effect biomarkers. J. Cancer 2020, 11, 6925. [Google Scholar] [CrossRef] [PubMed]
- Pandit, M.; Kil, Y.-S.; Ahn, J.-H.; Pokhrel, R.H.; Gu, Y.; Mishra, S.; Han, Y.; Ouh, Y.-T.; Kang, B.; Jeong, M.S.; et al. Methionine consumption by cancer cells drives a progressive upregulation of PD-1 expression in CD4 T cells. Nat. Commun. 2023, 14, 2593. [Google Scholar] [CrossRef] [PubMed]
- Vinnars, E.; Bergstöm, J.; Fürst, P. Influence of the postoperative state on the intracellular free amino acids in human muscle tissue. Ann. Surg. 1975, 182, 665. [Google Scholar] [CrossRef] [PubMed]
- Hammoudi, N.; Ahmed, K.B.R.; Garcia-Prieto, C.; Huang, P. Metabolic alterations in cancer cells and therapeutic implications. Chin. J. Cancer 2011, 30, 508. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Du, W.; Cao, S.; Wu, Y.; Dong, N.; Wang, Y.; Xu, Y. Systematic analyses of glutamine and glutamate metabolisms across different cancer types. Chin. J. Cancer 2017, 36, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, L. The metabolic processes of folic acid and Vitamin B12 deficiency. J. Health Res. Rev. 2014, 1, 5–9. [Google Scholar] [CrossRef]
- Kwarteng, D.O.; Gangoda, M.; Kooijman, E.E. The effect of methylated phosphatidylethanolamine derivatives on the ionization properties of signaling phosphatidic acid. Biophys. Chem. 2023, 296, 107005. [Google Scholar] [CrossRef]
- Merchant, T.E.; Minsky, B.D.; Lauwers, G.Y.; Diamantis, P.M.; Haida, T.; Glonek, T. Esophageal cancer phospholipids correlated with histopathologic findings: A 31P NMR study. NMR Biomed. Int. J. Devoted Dev. Appl. Magn. Reson. In Vivo 1999, 12, 184–188. [Google Scholar] [CrossRef]
- Lane, D.J.; Metselaar, B.; Greenough, M.; Bush, A.I.; Ayton, S.J. Ferroptosis and NRF2: An emerging battlefield in the neurodegeneration of Alzheimer’s disease. Essays Biochem. 2021, 65, 925–940. [Google Scholar]
- Patel, D.; Witt, S.N. Ethanolamine and phosphatidylethanolamine: Partners in health and disease. Oxidative Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef]
- Milne, G.L.; Dai, Q.; Roberts, L.J., II. The isoprostanes—25 years later. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2015, 1851, 433–445. [Google Scholar] [CrossRef]
- Bauer, J.; Ripperger, A.; Frantz, S.; Ergün, S.; Schwedhelm, E.; Benndorf, R.A. Pathophysiology of isoprostanes in the cardiovascular system: Implications of isoprostane-mediated thromboxane A 2 receptor activation. Br. J. Pharmacol. 2014, 171, 3115–3131. [Google Scholar] [CrossRef] [PubMed]
- Benndorf, R.A.; Schwedhelm, E.; Gnann, A.; Taheri, R.; Kom, G.; Didié, M.; Steenpass, A.; Ergün, S.; Böger, R.H. Isoprostanes Inhibit Vascular Endothelial Growth Factor–Induced Endothelial Cell Migration, Tube Formation, and Cardiac Vessel Sprouting In Vitro, As Well As Angiogenesis In Vivo via Activation of the Thromboxane A2 Receptor: A Potential Link Between Oxidative Stress and Impaired Angiogenesis. Circ. Res. 2008, 103, 1037–1046. [Google Scholar] [PubMed]
- Weber, T.J. Isoprostane-Mediated Tumor Promotion. Lab. Dir. Res. Dev. Annu. Rep.-Fisc. Year 2000, 2001, 81. [Google Scholar]
- Zhang, L.-J.; Chen, B.; Zhang, J.-J.; Li, J.; Yang, Q.; Zhong, Q.-S.; Zhan, S.; Liu, H.; Cai, C. Serum polyunsaturated fatty acid metabolites as useful tool for screening potential biomarker of colorectal cancer. Prostaglandins Leukot. Essent. Fat. Acids 2017, 120, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.B.; Laekeman, G.M.; Keersmaekers, G.H.; Uyttenbroeck, F.L.; Vanderheyden, J.S.; Albertyn, G.P.; Haensch, C.F.; De Roy, G.J.; Herman, A.G. Prostaglandin F2 alpha in benign and malignant breast tumours. Br. J. Cancer 1985, 51, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Jara-Gutiérrez, Á.; Baladrón, V. The Role of Prostaglandins in Different Types of Cancer. Cells 2021, 10, 1487. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-J.; Chung, M.K.; Hwang, D.; Kim, W.-U. Proteomics in Rheumatoid Arthritis Research. Immune Netw. 2015, 15, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.M.; Kelly, R.W.; A Hawkins, R.; Miller, W.R. Prostaglandins in human mammary cancer. Br. J. Cancer 1984, 49, 459–464. [Google Scholar] [CrossRef]
- Corbet, C.; Pinto, A.; Martherus, R.; de Jesus, J.P.S.; Polet, F.; Feron, O. Acidosis Drives the Reprogramming of Fatty Acid Metabolism in Cancer Cells through Changes in Mitochondrial and Histone Acetylation. Cell Metab. 2016, 24, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Zhelev, Z.; Aoki, I.; Lazarova, D.; Vlaykova, T.; Higashi, T.; Bakalova, R. A “Weird” Mitochondrial Fatty Acid Oxidation as a Metabolic “Secret” of Cancer. Oxid. Med. Cell Longev. 2022, 2022, 2339584. [Google Scholar] [CrossRef] [PubMed]
- Arioz, D.T.; Kanat-Pektas, M.; Tuncer, N.; Koken, T.; Unlu, B.S.; Koken, G.; Yilmazer, M. L-Carnitine: A new insight into the pathogenesis of endometrial cancer. Arch. Gynecol. Obstet. 2015, 291, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.I.; Malik, S.A. Plasma lipid profile in gynecologic cancers. Eur. J. Gynaecol. Oncol. 2008, 29, 158–161. [Google Scholar]
- Mao, X.; Lei, H.; Yi, T.; Su, P.; Tang, S.; Tong, Y.; Dong, B.; Ruan, G.; Mustea, A.; Sehouli, J.; et al. Lipid reprogramming induced by the TFEB-ERRα axis enhanced membrane fluidity to promote EC progression. J. Exp. Clin. Cancer Res. 2022, 41, 28. [Google Scholar] [CrossRef]
- Bielecka-Dąbrowa, A.; Hannam, S.; Rysz, J.; Banach, M. Malignancy-associated dyslipidemia. Open Cardiovasc. Med. J. 2011, 5, 35–40. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benabdelkamel, H.; Jaber, M.A.; Akkour, K.; AlMalki, R.H.; Alfadda, A.A.; Masood, A.; Joy, S.S.; Alhalal, H.; Alwehaibi, M.A.; Arafah, M.; et al. Metabolomic Profiling of Blood Plasma in Females with Hyperplasia and Endometrial Cancer. Metabolites 2024, 14, 109. https://doi.org/10.3390/metabo14020109
Benabdelkamel H, Jaber MA, Akkour K, AlMalki RH, Alfadda AA, Masood A, Joy SS, Alhalal H, Alwehaibi MA, Arafah M, et al. Metabolomic Profiling of Blood Plasma in Females with Hyperplasia and Endometrial Cancer. Metabolites. 2024; 14(2):109. https://doi.org/10.3390/metabo14020109
Chicago/Turabian StyleBenabdelkamel, Hicham, Malak A. Jaber, Khalid Akkour, Reem H. AlMalki, Assim A. Alfadda, Afshan Masood, Salini Scaria Joy, Hani Alhalal, Moudi A. Alwehaibi, Maria Arafah, and et al. 2024. "Metabolomic Profiling of Blood Plasma in Females with Hyperplasia and Endometrial Cancer" Metabolites 14, no. 2: 109. https://doi.org/10.3390/metabo14020109
APA StyleBenabdelkamel, H., Jaber, M. A., Akkour, K., AlMalki, R. H., Alfadda, A. A., Masood, A., Joy, S. S., Alhalal, H., Alwehaibi, M. A., Arafah, M., Alshehri, E., & Abdel Rahman, A. M. (2024). Metabolomic Profiling of Blood Plasma in Females with Hyperplasia and Endometrial Cancer. Metabolites, 14(2), 109. https://doi.org/10.3390/metabo14020109





