Role of the Adrenal Medulla in Hypoglycaemia-Associated Autonomic Failure—A Diabetic Perspective
Abstract
1. Introduction
2. Pancreatic Responses to Hypoglycaemia and the Relevance of the Adrenal Gland
3. Adrenal Medullary Responses to Hypoglycaemia
3.1. Overview of Adrenal Medulla Biology
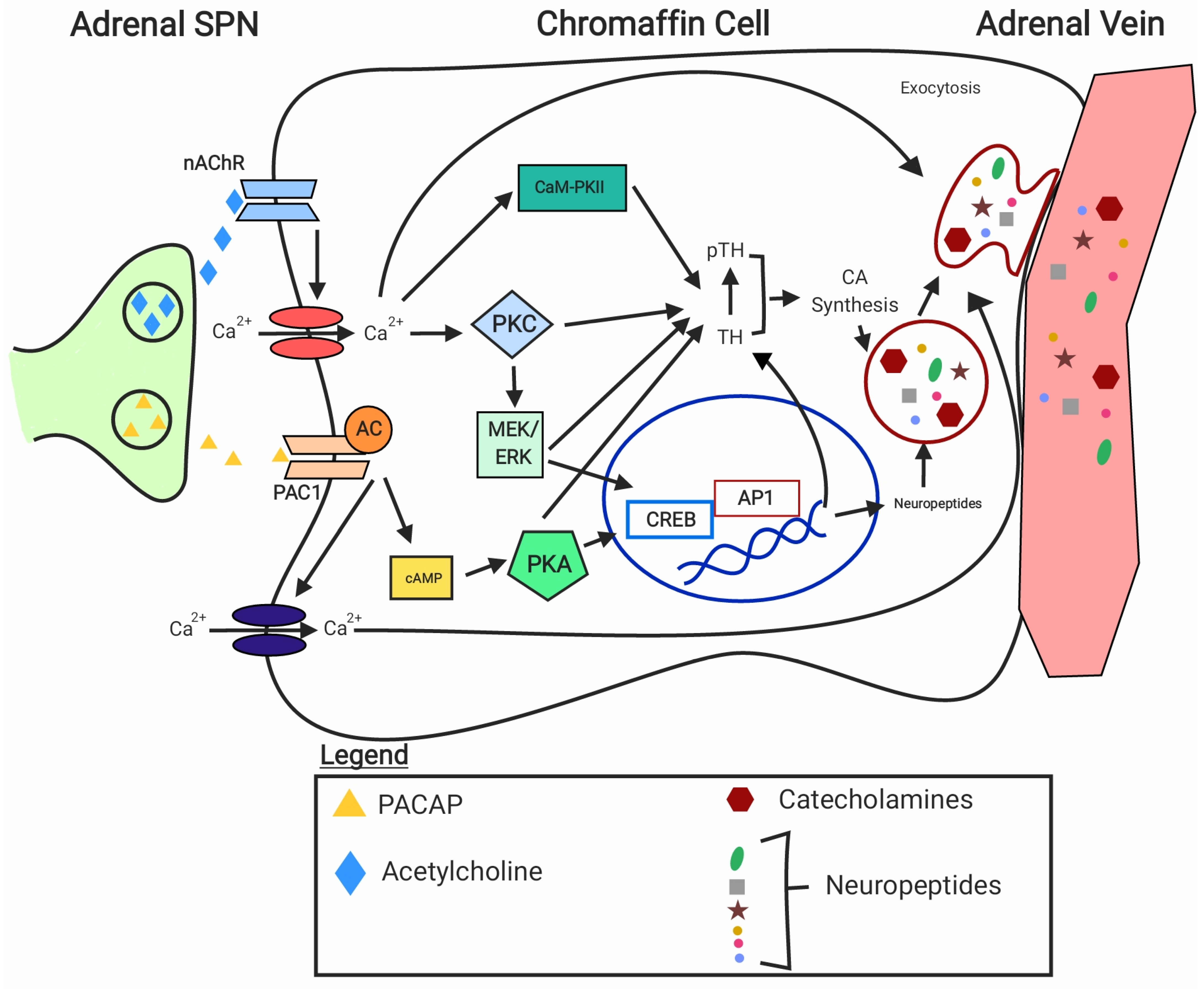
3.2. Chromaffin Cell Activation, Signalling, and Catecholamine Release
3.2.1. General Mechanisms
3.2.2. Impact of Hypoglycaemia
2-Deoxy Glucose and Insulin as Experimental Tools to Investigate the Effects of Hypoglycaemia and Glucoprivation
The Effects of 2DG and Insulin on Adrenaline Release in Humans and Rodents
The Effects of 2DG and Insulin on Adrenal Sympathetic Nerve Activity and Adrenomedullary Cell Activation
The Effects of 2DG and Insulin on the Adrenomedullary Cell Signalling
The Effects of 2DG and Insulin on Synaptic Proteins/Exocytosis
3.3. Catecholamine Synthesis
3.3.1. General Mechanisms
3.3.2. Impact of Hypoglycaemia in Diabetic and Non-Diabetic Rodents
Short-Term Changes—TH Phosphorylation
Mid-Term Changes—Gene Expression
Long-Term Changes—Total Protein Expression
| Alterations in the Adrenal Gland | Species | Recurrent 2DG/Insulin, Dose, and Route of Administration | Number of Antecedent Episodes | Finding in Response to Subsequent Glucoprivation/Hypoglycaemia | Time of Measurement | Plasma Adrenaline in Response to Single/Recurrent Hypoglycaemia |
|---|---|---|---|---|---|---|
| Catecholamine release | Non-diabetic mice | Insulin, 2.5 U/kg; i.p. [89] | Once a day for 5 days [89] | ↓ adrenaline response [89] | 30 min from the last injection [89] | ~16/7 ng/mL [89] |
| Non-diabetic rats | Insulin, 2 U/kg; i.p. [37] | Twice a day for 3 days [37] | ↓ expression of genes of proteins associated with exocytosis [37] | 60 min from the last injection [37] | Did not measure [37] | |
| Insulin, 5 U/kg; i.p. [88] | Once a day for 3 days [88] | ↓ adrenaline response [88] | 2 h from the last injection [88] | 1317/170.7 pg/mL [88] | ||
| Diabetic rats | Insulin, 30–40 IU/kg; i.p. [87] | Once a day for 3 days [87] | ↓ adrenaline response [87] | 90 min from the last injection [87] | 5000/2500 pg/mL [87] | |
| Insulin, 5 U/kg; i.p. [88] | Once a day for 3 days [88] | ↔ adrenaline response [88] | 2 h from the last injection [88] | 2958/3804 pg/mL [88] | ||
| Adrenal sympathetic nerve activity/ stimulation | Non-diabetic rats | Insulin, 1.5 U/kg; s.c. [36] | Once a day for 2 days [36] | ↔ASNA [36] | During the subsequent hypoglycaemic episode [36] | ~2800/1200 pg/mL [36] |
| Insulin, 2 U/kg; s.c. [31] | Once a day for 2 days [31] | ↔ASNA [31] | During the subsequent hypotensive episode [31] | ~1100/700 pg/mL [31] | ||
| Insulin, 1 U/kg; s.c. [35] | Once a day for 3 days [35] | a. ↓ Ad and NAd release in response to nerve stimulation; b. ↔ Ad and NAd release in response to AChR agonists [35] | a. Day 4; b. Day 5 [35] | 707 ± 89/458 ± 135 pg/mL [35] | ||
| Chromaffin cell activation | Non-diabetic rats | 2DG, 200 mg/kg; s.c. [76] | Once a day for 10 days [76] | Reduced Fos expression [76] | 2 h from the last injection [76] | Did not measure [76] |
| Intracellular signalling | Non-diabetic rats | Insulin, 10 U/kg; i.p. [98] | Once a day for 2 days [98] | ↔ ERK 1/2 activation [98] | 60 min from the last injection [98] | 4417 ± 594/2617 ± 185 pg/mL [98] |
| Insulin, 2 U/kg; i.p. [32] | Once a day (RH) and twice a day (2RH) for 3 days [32] | ↔ PKA activation [32] | 60 min from the last injection [32] | ~2/2 ng/mL (RH) ~2.8/1.5 ng/mL (2RH) [32] | ||
| Insulin, 2 U/kg; i.p. [37] | Twice a day for 3 days [37] | ↓ PKC gene expression [37] | 60 min from the last injection [37] | Did not measure [37] | ||
| Adrenal gland catecholamine content | Non-diabetic rats | Insulin, 2 U/kg; s.c. [31] | Once a day for 2 days [31] | ↓ Adrenaline content of the adrenal gland [31] | Measured on the day after the second hypoglycaemic episode [31] | ~1100/700 pg/mL [31] |
| Insulin, 1 U/kg; s.c. [35] | Once a day for 3 days [35] | ↔ Adrenaline content of the adrenal gland [35] | Measured on the day after the subsequent hypoglycaemic episode [35] | 707 ± 89/458 ± 135 pg/mL [35] | ||
| TH mRNA, protein, and phosphorylation | Non-diabetic rats [31,32,79,98,110,112] | 2DG, 500 mg/kg and Insulin, 5 U/kg; i.p. [79] | Once a day for 6 days [79] | ↑ TH mRNA [79] [110] | 5 h after the seventh injection [79] | Did not measure [79] |
| 2DG, 500 mg/kg and Insulin, 5 U/kg; i.p. [110] | Once a day for 5 and 6 days [110] | ↑ TH mRNA ↑ TH protein [110] | 24 h after the sixth injection and 5 h after the seventh injection [110] | Did not measure [110] | ||
| Insulin, 2 U/kg; i.p. [32] | Once a day (RH) and twice a day (2RH) for 3 days [32] | ↓ TH mRNA (2RH) ↓ Ser40TH phosphorylation ↑ TH protein (2RH) [32] | TH mRNA: 3.5 h from the end of hypoglycaemic clamp on the last day; TH protein and pSer40TH at 60 min from the last injection [32] | ~2/2 ng/mL (RH) ~2.8/1.5 ng/mL (2RH) [32] | ||
| 2DG, 500 mg/kg and Insulin, 2 U/kg; s.c. [112] | Once a day for 5 days [112] | ↑ TH protein [112] | 6 h after the administration of the last dose of 2DG/insulin [112] | Did not measure [112] | ||
| Insulin, 10 U/kg; i.p. [98] | Once a day for 2 days [98] | ↑ TH protein ↑ Ser40TH phosphorylation [98] | 60 min from the subsequent episode [98] | 4417 ± 594/2617 ± 185 pg/mL [98] | ||
| Insulin, 2 U/kg; s.c. [31] | Once a day for 2 days [31] | ↔ TH protein ↔ Ser40TH phosphorylation [31] | Day after the second hypoglycaemic episode [31] | ~1100/700 pg/mL [31] | ||
| Non-diabetic mice [34] | Insulin, 2.5 U/kg; i.p. [34] | Once a day for 3 days [34] | ↓ TH immunoreactivity [20,34] | 24 h after the last injection [34] | ~7/4 ng/mL [34] | |
| Untreated diabetic rats [111] | Insulin, 2 U/kg; s.c. [111] | Twice a day for 4 days [111] | ↔ TH mRNA [111] | Not given [111] | Did not measure [111] | |
| Insulin-treated diabetic rats [113] | Insulin, 2 U/kg; s.c. [113] | Twice a day for 3 days and one episode on day 4 [113] | ↔ TH mRNA ↑ TH protein [113] | At 90 min during the subsequent episode [113] | ~30/25 nM [113] | |
| DBH Protein expression | Non-diabetic rats [98,112] | Insulin, 10 U/kg; i.p. [98] | Once a day for 2 days [98] | ↔ DBH protein [98] | 60 min from the subsequent episode [98] | 4417 ± 594/2617 ± 185 pg/mL [98] |
| 2DG, 500 mg/kg and Insulin, 2 U/kg; s.c. [112] | Once a day for 5 days [112] | ↑ DBH protein [112] | 6 h after the administration of the last dose of 2DG/insulin [112] | Did not measure [112] | ||
| Untreated diabetic rats [111] | 2DG, 500 mg/kg and Insulin, 2 U/kg; s.c. [111] | Twice a day for 4 days [111] | ↑ DBH mRNA [111] | Not given [111] | Did not measure [111] | |
| Insulin-treated diabetic rats [113] | 2DG, 500 mg/kg and Insulin, 2 U/kg; s.c. [113] | Twice a day for 3 days and one episode on day 4 [113] | ↓ DBH protein [113] | At 90 min during the subsequent episode [113] | ~30/25 nM [113] | |
| PNMT mRNA and protein expression | Non-diabetic rats [31,32,98,112] | Insulin, 2 U/kg; s.c. [31] | Once a day for 2 days [31,98] | ↔ PNMT protein [31] | Day after the second hypoglycaemic episode [31] | ~1100/700 pg/mL [31] |
| Insulin, 2 U/kg; i.p. [32] | Once a day (RH) and twice a day (2RH) for 3 days [32] | ↔ PNMT mRNA [32] | 3.5 h from the end of hypoglycaemic clamp on the last day [32] | ~2/2 ng/mL (RH) ~2.8/1.5 ng/mL (2RH) [32] | ||
| Insulin, 10 U/kg; i.p. [98] | Once a day for 2 days [98] | ↔ PNMT protein [98] | 60 min from the subsequent episode [98] | 4417 ± 594/2617 ± 185 pg/mL [98] | ||
| 2DG, 500 mg/kg and Insulin, 2 U/kg; s.c. [112] | Once a day for 5 days [112] | ↑ PNMT protein (insulin) ↔ PNMT protein (2DG) [112] | 6 h after the administration of the last dose of 2DG/insulin [112] | Did not measure [112] | ||
| Untreated diabetic rats [111] | 2DG, 500 mg/kg and Insulin, 2 U/kg; s.c. [111] | Twice a day for 4 days [111] | ↓ PNMT mRNA [111] | Not given [111] | Did not measure [111] | |
| Insulin-treated diabetic rats [113] | 2DG, 500 mg/kg and Insulin, 2 U/kg; s.c. [113] | Twice a day for 3 days and one episode on day 4 [113] | ↔ PNMT mRNA and PNMT protein [113] | At 90 min during the subsequent episode [113] | ~30/25 nM [113] |
4. Possible Contribution of Adrenomedullary Peptides to HAAF
4.1. Overview of the Adrenomedullary Peptides and Their Role in Modulating Catecholamine Release
4.2. Effects of Single and Recurrent Hypoglycaemia on the Gene Expression and Abundance of the Adrenomedullary Peptides
4.3. Effects of Peptide Antagonists
5. Challenges in Investigating the HAAF phenomenon
6. Summary and Conclusions
- The effects of recurrent hypoglycaemia or diabetes on the adrenomedullary signalling, synaptic proteins, and catecholamine release are not established, and the studies are very limited in this area.
- The catecholamine synthetic capacity of the adrenal medulla in response to recurrent hypoglycaemia has been extensively studied in non-diabetic animals, but produced inconsistent results. Short-term changes in relation to TH phosphorylation, specifically at Ser31 and Ser40 has not been consistently shown. Findings of long-term changes in relation to TH protein expression show that rats have increased TH protein expression following single or recurrent hypoglycaemia, while studies in mice show that TH protein levels return to basal levels in the context of recurrent hypoglycaemia, indicating that the catecholamine synthetic response of the adrenal medulla to recurrent hypoglycaemia may be species specific or experimental design specific. Studies in diabetic rodents are very limited.
- While there are very few studies which have investigated the effects of insulin-induced hypoglycaemia on neuropeptides in the adrenal gland, current evidence suggests that recurrent insulin-induced hypoglycaemia increases protein expression of neuropeptides such as NPY, galanin, and proenkephalin. Moreover, this phenomenon may occur across different species since consistent findings have been reported in both rats and mice. Peptide antagonism, particularly for NPY and opioids, could be a key strategy in preventing the short-term and long-term changes to TH expression and activity. Despite some variations in the reported expression of the neuropeptides covered in this review, their collective impact on adrenaline secretion in HAAF warrants further investigation.
- Animal models of HAAF in diabetic rodents are still lacking.
7. Future Directions
- How catecholamine synthesis and release from the adrenal medulla are altered in response to recurrent hypoglycaemia in diabetic and non-diabetic subjects?
- Do adrenomedullary peptides contribute to HAAF?
- What are the cellular and molecular mechanisms that are involved in adrenal medullary responses to single and recurrent hypoglycaemia in diabetic and non-diabetic subjects?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Diedrich, L.; Sandoval, D.; Davis, S.N. Hypoglycemia associated autonomic failure. Clin. Auton. Res. 2002, 12, 358–365. [Google Scholar] [CrossRef]
- Heller, S.R. Hypoglycaemia: Its pathophysiology in insulin treated diabetes and hypoglycaemia unawareness. Br. J. Diabetes Vasc. Dis. 2011, 11, 6–9. [Google Scholar] [CrossRef]
- Kalra, S.; Mukherjee, J.J.; Venkataraman, S.; Bantwal, G.; Shaikh, S.; Saboo, B.; Das, A.K.; Ramachandran, A. Hypoglycemia: The neglected complication. Indian J. Endocrinol. Metab. 2013, 17, 819–834. [Google Scholar] [CrossRef]
- Cryer, P.E. Hypoglycemia in type 1 diabetes mellitus. Endocrinol. Metab. Clin. N. Am. 2010, 39, 641–654. [Google Scholar] [CrossRef]
- Cryer, P.E.; Davis, S.N.; Shamoon, H. Hypoglycemia in diabetes. Diabetes Care 2003, 26, 1902–1912. [Google Scholar] [CrossRef]
- Leese, G.P.; Wang, J.; Broomhall, J.; Kelly, P.; Marsden, A.; Morrison, W.; Frier, B.M.; Morris, A.D. Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: A population-based study of health service resource use. Diabetes Care 2003, 26, 1176–1180. [Google Scholar] [CrossRef]
- Unger, J. Uncovering undetected hypoglycemic events. Diabetes Metab. Syndr. Obes. Targets Ther. 2012, 5, 57. [Google Scholar] [CrossRef]
- Senthilkumaran, M.; Zhou, X.-F.; Bobrovskaya, L. Challenges in modelling hypoglycaemia-associated autonomic failure: A review of human and animal studies. Int. J. Endocrinol. 2016, 2016, 9801640. [Google Scholar] [CrossRef]
- Sprague, J.E.; Arbelaez, A.M. Glucose counterregulatory responses to hypoglycemia. Pediatr. Endocrinol. Rev. PER 2011, 9, 463–473; quiz 474–465. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3755377/ (accessed on 1 December 2023).
- DeRosa, M.A.; Cryer, P.E. Hypoglycemia and the sympathoadrenal system: Neurogenic symptoms are largely the result of sympathetic neural, rather than adrenomedullary, activation. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E32–E41. [Google Scholar] [CrossRef]
- Cryer, P.E. The barrier of hypoglycemia in diabetes. Diabetes 2008, 57, 3169–3176. [Google Scholar] [CrossRef]
- Arbelaez, A.M.; Xing, D.; Cryer, P.E.; Kollman, C.; Beck, R.W.; Sherr, J.; Ruedy, K.J.; Tamborlane, W.V.; Mauras, N.; Tsalikian, E. Blunted glucagon but not epinephrine responses to hypoglycemia occurs in youth with less than 1 yr duration of type 1 diabetes mellitus. Pediatr. Diabetes 2014, 15, 127–134. [Google Scholar] [CrossRef]
- Siafarikas, A.; Johnston, R.J.; Bulsara, M.K.; O’leary, P.; Jones, T.W.; Davis, E.A. Early loss of the glucagon response to hypoglycemia in adolescents with type 1 diabetes. Diabetes Care 2012, 35, 1757–1762. [Google Scholar] [CrossRef]
- Bisgaard Bengtsen, M.; Møller, N. Mini-review: Glucagon responses in type 1 diabetes—A matter of complexity. Physiol. Rep. 2021, 9, e15009. [Google Scholar] [CrossRef]
- Demirbilek, H.; Vuralli, D.; Haris, B.; Hussain, K. Managing severe hypoglycaemia in patients with diabetes: Current challenges and emerging therapies. Diabetes Metab. Syndr. Obes. 2023, 16, 259–273. [Google Scholar] [CrossRef]
- Heller, S.R.; Peyrot, M.; Oates, S.K.; Taylor, A.D. Hypoglycemia in patient with type 2 diabetes treated with insulin: It can happen. BMJ Open Diabetes Res. Care 2020, 8, e001194. [Google Scholar] [CrossRef]
- Amiel, S.A.; Cryer, P.E. Attenuated sympathoadrenal responses, but not severe hypoglycemia, during aggressive glycemic therapy of early type 2 diabetes. Diabetes 2009, 58, 515–517. [Google Scholar] [CrossRef][Green Version]
- Bisgaard Bengtsen, M.; Møller, N. Experimentally induced hypoglycemia-associated autonomic failure in humans: Determinants, designs, and drawbacks. J. Endocr. Soc. 2022, 6, bvac123. [Google Scholar] [CrossRef]
- Mitrakou, A.; Fanelli, C.; Veneman, T.; Perriello, G.; Calderone, S.; Platanisiotis, D.; Rambotti, A.; Raptis, S.; Brunetti, P.; Cryer, P.; et al. Reversibility of unawareness of hypoglycemia in patients with insulinomas. N. Engl. J. Med. 1993, 329, 834–839. [Google Scholar] [CrossRef]
- Yale, J.F.; Paty, B.; Senior, P.A. Hypoglycemia. Can J Diabetes 2018, 42 (Suppl S1), S104–S108. [Google Scholar] [CrossRef]
- Macon, E.L.; Devore, M.H.; Lin, Y.K.; Music, M.B.; Wooten, M.; McMullen, C.A.; Woodcox, A.M.; Marksbury, A.R.; Beckner, Z.; Patel, B.V.; et al. Current and future therapies to treat impaired awareness of hypoglycemia. Front. Pharmacol. 2023, 14, 1271814. [Google Scholar] [CrossRef]
- Rickels, M.R. Hypoglycemia-associated autonomic failure, counterregulatory responses, and therapeutic options in type 1 diabetes. Ann. N. Y. Acad. Sci. 2019, 1454, 68–79. [Google Scholar] [CrossRef]
- Burckhardt, M.A.; Abraham, M.B.; Dart, J.; Smith, G.; Paramalingam, N.; O’Dea, J.; de Bock, M.I.; Davis, E.A.; Jones, T.W. Impact of hybrid closed loop therapy on hypoglycemia awareness in individuals with type 1 diabetes and impaired hypoglycemia awareness. Diabetes Technol. Ther. 2021, 23, 482–490. [Google Scholar] [CrossRef]
- Lin, Y.K.; Richardson, C.R.; Dobrin, I.; DeJonckheere, M.J.; Mizokami-Stout, K.; Fetters, M.D.; Aikens, J.E.; Fisher, S.J.; Ye, W.; Pop-Busui, R. Beliefs around hypoglycemia and their impacts on hypoglycemia outcomes in individuals with type 1 diabetes and high risks for hypoglycemia despite using advanced diabetes technologies. Diabetes Care 2022, 45, 520–528. [Google Scholar] [CrossRef]
- Lin, Y.K.; Hung, M.; Sharma, A.; Chan, O.; Varner, M.W.; Staskus, G.; Fisher, S.J. Impaired awareness of hypoglycemia continues to be a risk factor for severe hypoglycemia despite the use of continuous glucose monitoring system in type 1 diabetes. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2019, 25, 517–525. [Google Scholar] [CrossRef]
- Cryer, P.E. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N. Engl. J. Med. 2013, 369, 362–374. [Google Scholar] [CrossRef]
- Litvin, M.; Clark, A.L.; Fisher, S.J. Recurrent hypoglycemia: Boosting the brain’s metabolic flexibility. J. Clin. Investig. 2013, 123, 1922–1924. [Google Scholar] [CrossRef]
- Reno, C.M.; Litvin, M.; Clark, A.L.; Fisher, S.J. Defective counterregulation and hypoglycemia unawareness in diabetes: Mechanisms and emerging treatments. Endocrinol. Metab. Clin. N. Am. 2013, 42, 15–38. [Google Scholar] [CrossRef]
- Stanley, S.; Moheet, A.; Seaquist, E.R. Central mechanisms of glucose sensing and counterregulation in defense of hypoglycemia. Endocr. Rev. 2019, 40, 768–788. [Google Scholar] [CrossRef]
- Herlein, J.A.; Morgan, D.A.; Phillips, B.G.; Haynes, W.G.; Sivitz, W.I. Antecedent hypoglycemia, catecholamine depletion, and subsequent sympathetic neural responses. Endocrinology 2006, 147, 2781–2788. [Google Scholar] [CrossRef] [PubMed]
- Kudrick, N.; Chan, O.; La Gamma, E.F.; Kim, J.L.; Tank, A.W.; Sterling, C.; Nankova, B.B. Posttranscriptional regulation of adrenal th gene expression contributes to the maladaptive responses triggered by insuli—Induced recurrent hypoglycemia. Physiol. Rep. 2015, 3, e12307. [Google Scholar] [CrossRef]
- LaGamma, E.F.; Kirtok, N.; Chan, O.; Nankova, B.B. Partial blockade of nicotinic acetylcholine receptors improves the counterregulatory response to hypoglycemia in recurrently hypoglycemic rats. Am. J. Physiol.-Endocrinol. Metab. 2014, 307, E580–E588. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Q.; Joe, D.; Wang, M.; Whim, M.D. Recurrent hypoglycemia inhibits the counterregulatory response by suppressing adrenal activity. J. Clin. Investig. 2018, 128, 3866–3871. [Google Scholar] [CrossRef]
- Orban, B.O.; Routh, V.H.; Levin, B.E.; Berlin, J.R. Direct effects of recurrent hypoglycaemia on adrenal catecholamine release. Diabetes Vasc. Dis. Res. 2015, 12, 2–12. [Google Scholar] [CrossRef]
- Sivitz, W.I.; Herlein, J.A.; Morgan, D.A.; Fink, B.D.; Phillips, B.G.; Haynes, W.G. Effect of acute and antecedent hypoglycemia on sympathetic neural activity and catecholamine responsiveness in normal rats. Diabetes 2001, 50, 1119–1125. [Google Scholar] [CrossRef]
- Kim, J.L.; La Gamma, E.F.; Estabrook, T.; Kudrick, N.; Nankova, B.B. Correction: Whole genome expression profiling associates activation of unfolded protein response with impaired production and release of epinephrine after recurrent hypoglycemia. PLoS ONE 2017, 12, e0173839. [Google Scholar] [CrossRef]
- Kreutzenberg, S.V.D.; Riccio, A.; Dorella, M.; Avogaro, A.; Marescotti, M.C.; Tiengo, A.; Prato, S.D. Surgical removal of insulinoma restores glucose recovery from hypoglycaemia but does not normalize insulin action. Eur. J. Clin. Investig. 1995, 25, 360–367. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Hsieh, M.-C.; Hsin, S.-C.; Shin, S.-J.; Lin, K.-D. Insulinoma-associated transient hypothalamus—Pituitary—Adrenal axis impairment and amelioration by steroid therapy and surgical intervention: A case report. Kaohsiung J. Med. Sci. 2007, 23, 526–530. [Google Scholar] [CrossRef]
- Higgs, J.A.; Quinn, A.P.; Seely, K.D.; Richards, Z.; Mortensen, S.P.; Crandall, C.S.; Brooks, A.E. Pathophysiological link between insulin resistance and adrenal incidentalomas. Int. J. Mol. Sci. 2022, 23, 4340. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Berger, I.; Scriba, L.; Santambrogio, A.; Steenblock, C. Adrenal cortex–medulla interactions in adaptation to stress and disease. Curr. Opin. Endocr. Metab. Res. 2019, 8, 9–14. [Google Scholar] [CrossRef]
- Yamamoto, T.J.I.J.o.E.; Disorders, M. Opinion: The collaboration of adrenal cortex and medulla from clinical viewpoint. Int. J. Endocrinol. Metab. Disord. 2020, 6, 1–4. [Google Scholar]
- Ahmed, S.; Soliman, A.; De Sanctis, V.; Alyafie, F.; Alaaraj, N.; Hamed, N.; Ali, H.A.; Kamal, A. Defective cortisol secretion in response to spontaneous hypoglycemia but normal cortisol response to acth stimulation in neonates with hyperinsulinemic hypoglycemia (hh). Acta Bio-Medica Atenei Parm. 2021, 92, e2021182. [Google Scholar] [CrossRef]
- Unsicker, K.; Huber, K.; Schütz, G.; Kalcheim, C. The chromaffin cell and its development. Neurochem. Res. 2005, 30, 921–925. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Ehrhart-Bornstein, M.; Androutsellis-Theotokis, A.; Eisenhofer, G.; Vukicevic, V.; Licinio, J.; Wong, M.L.; Calissano, P.; Nisticò, G.; Preziosi, P.; et al. Chromaffin cells: The peripheral brain. Mol. Psychiatry 2012, 17, 354–358. [Google Scholar] [CrossRef]
- Verhofstad, A.A.; Coupland, R.E.; Parker, T.R.; Goldstein, M. Immunohistochemical and biochemical study on the development of the noradrenaline- and adrenaline-storing cells of the adrenal medulla of the rat. Cell Tissue Res. 1985, 242, 233–243. Available online: https://www.ncbi.nlm.nih.gov/pubmed/3902244 (accessed on 1 December 2023). [CrossRef]
- Vollmer, R.R.; Baruchin, A.; Kolibal-Pegher, S.S.; Corey, S.P.; Stricker, E.M.; Kaplan, B.B. Selective activation of norepinephrine- and epinephrine-secreting chromaffin cells in rat adrenal medulla. Am. J. Physiol. 1992, 263, R716–R721. Available online: http://ajpregu.physiology.org/content/263/3/R716. (accessed on 1 December 2023). [CrossRef]
- Sherwin, R.S.; Sacca, L. Effect of epinephrine on glucose metabolism in humans: Contribution of the liver. Am. J. Physiol. -Endocrinol. Metab. 1984, 247, E157–E165. [Google Scholar] [CrossRef]
- Kesse, W.; Parker, T.; Coupland, R. The innervation of the adrenal gland. I. The source of pre-and postganglionic nerve fibres to the rat adrenal gland. J. Anat. 1988, 157, 33. [Google Scholar]
- Strack, A.M.; Sawyer, W.B.; Marubio, L.M.; Loewy, A.D. Spinal origin of sympathetic preganglionic neurons in the rat. Brain Res. 1988, 455, 187–191. [Google Scholar] [CrossRef]
- Douglas, W.W.; Rubin, R.P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J. Physiol. 1961, 159, 40–57. [Google Scholar] [CrossRef]
- Wakade, A. Studies on secretion of catecholamines evoked by acetylcholine or transmural stimulation of the rat adrenal gland. J. Physiol. 1981, 313, 463. [Google Scholar] [CrossRef]
- Wakade, A.; Wakade, T. Contribution of nicotinic and muscarinic receptors in the secretion of catecholamines evoked by endogenous and exogenous acetylcholine. Neuroscience 1983, 10, 973–978. [Google Scholar] [CrossRef]
- Murabayashi, H.; Kuramoto, H.; Kawano, H.; Sasaki, M.; Kitamura, N.; Miyakawa, K.; Tanaka, K.; Oomori, Y. Immunohistochemical features of substance p-immunoreactive chromaffin cells and nerve fibers in the rat adrenal gland. Arch. Histol. Cytol. 2007, 70, 183–196. [Google Scholar] [CrossRef]
- Eiden, L.E.; Jiang, S.Z. What’s new in endocrinology: The chromaffin cell. Front. Endocrinol. 2018, 9, 711. [Google Scholar] [CrossRef]
- Kuri, B.A.; Chan, S.A.; Smith, C.B. Pacap regulates immediate catecholamine release from adrenal chromaffin cells in an activity-dependent manner through a protein kinase c-dependent pathway. J. Neurochem. 2009, 110, 1214–1225. [Google Scholar] [CrossRef]
- Morales, A.; Mohan, R.; Chen, X.; Coffman, B.L.; Bendahmane, M.; Watch, L.; West, J.L.; Bakshi, S.; Traynor, J.R.; Giovannucci, D.R.; et al. Pacap and acetylcholine cause distinct ca2+ signals and secretory responses in chromaffin cells. J. Gen. Physiol. 2022, 155, e202213180. [Google Scholar] [CrossRef]
- Parker, L.M.; Kumar, N.N.; Lonergan, T.; Goodchild, A.K. Neurochemical codes of sympathetic preganglionic neurons activated by glucoprivation. J. Comp. Neurol. 2013, 521, 2703–2718. [Google Scholar] [CrossRef]
- Verberne, A.J.; Korim, W.S.; Sabetghadam, A.; Llewellyn-Smith, I.J. Adrenaline: Insights into its metabolic roles in hypoglycaemia and diabetes. Br. J. Pharmacol. 2016, 173, 1425–1437. [Google Scholar] [CrossRef]
- Guérineau, N.C. Cholinergic and peptidergic neurotransmission in the adrenal medulla: A dynamic control of stimulus-secretion coupling. IUBMB Life 2020, 72, 553–567. [Google Scholar] [CrossRef]
- Bobrovskaya, L.; Damanhuri, H.A.; Ong, L.K.; Schneider, J.J.; Dickson, P.W.; Dunkley, P.R.; Goodchild, A.K. Signal transduction pathways and tyrosine hydroxylase regulation in the adrenal medulla following glucoprivation: An in vivo analysis. Neurochem. Int. 2010, 57, 162–167. [Google Scholar] [CrossRef]
- Fischer-Colbrie, R.; Eskay, R.L.; Eiden, L.E.; Maas, D. Transsynaptic regulation of galanin, neurotensin, and substance p in the adrenal medulla: Combinatorial control by second-messenger signaling pathways. J. Neurochem. 1992, 59, 780–783. [Google Scholar] [CrossRef]
- Malhotra, R.K.; Wakade, T.D.; Wakade, A.R. Cross-communication between acetylcholine and vip in controlling catecholamine secretion by affecting camp, inositol triphosphate, protein kinase c, and calcium in rat adrenal medulla. J. Neurosci. 1989, 9, 4150–4157. [Google Scholar] [CrossRef]
- Chheda, M.G.; Ashery, U.; Thakur, P.; Rettig, J.; Sheng, Z.H. Phosphorylation of snapin by pka modulates its interaction with the snare complex. Nat. Cell Biol. 2001, 3, 331–338. [Google Scholar] [CrossRef]
- Gao, J.; Hirata, M.; Mizokami, A.; Zhao, J.; Takahashi, I.; Takeuchi, H.; Hirata, M. Differential role of snap-25 phosphorylation by protein kinases a and c in the regulation of snare complex formation and exocytosis in pc12 cells. Cell. Signal. 2016, 28, 425–437. [Google Scholar] [CrossRef]
- Snyder, D.; Kelly, M.; Woodbury, D. Snare complex regulation by phosphorylation. Cell Biochem. Biophys. 2006, 45, 111–123. [Google Scholar] [CrossRef]
- Dhara, M.; Mohrmann, R.; Bruns, D. V-snare function in chromaffin cells. Pflug. Arch 2018, 470, 169–180. [Google Scholar] [CrossRef]
- Kvetnansky, R.; Sabban, E.L.; Palkovits, M. Catecholaminergic systems in stress: Structural and molecular genetic approaches. Physiol. Rev. 2009, 89, 535–606. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Kopin, I.J.; Goldstein, D.S. Catecholamine metabolism: A contemporary view with implications for physiology and medicine. Pharmacol. Rev. 2004, 56, 331–349. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Pecorella, W.; Pacak, K.; Hooper, D.; Kopin, I.J.; Goldstein, D.S. The neuronal and extraneuronal origins of plasma 3-methoxy-4-hydroxyphenylglycol in rats. J. Auton. Nerv. Syst. 1994, 50, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, W.S.; Smythe, G.A.; Storlien, L.H. 2-deoxy-d-glucose-induced hyperglycemia: Role for direct sympathetic nervous system activation of liver glucose output. Brain Res. 1989, 505, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Ritter, S.; Taylor, J.S. Capsaicin abolishes lipoprivic but not glucoprivic feeding in rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1989, 256, R1232–R1239. [Google Scholar] [CrossRef] [PubMed]
- Damanhuri, H.A.; Burke, P.G.; Ong, L.K.; Bobrovskaya, L.; Dickson, P.W.; Dunkley, P.R.; Goodchild, A.K. Tyrosine hydroxylase phosphorylation in catecholaminergic brain regions: A marker of activation following acute hypotension and glucoprivation. PLoS ONE 2012, 7, e50535. [Google Scholar] [CrossRef]
- Korim, W.S.; Bou Farah, L.; McMullan, S.; Verberne, A.J. Orexinergic activation of medullary premotor neurons modulates the adrenal sympathoexcitation to hypothalamic glucoprivation. Diabetes 2014, 63, 1895–1906. [Google Scholar] [CrossRef]
- Parker, L.M.; Kumar, N.N.; Lonergan, T.; McMullan, S.; Goodchild, A.K. Distribution and neurochemical characterization of neurons in the rat ventrolateral medulla activated by glucoprivation. Brain Struct. Funct. 2015, 220, 117–134. [Google Scholar] [CrossRef]
- Sanders, N.M.; Ritter, S. Repeated 2-deoxy-d-glucose-induced glucoprivation attenuates fos expression and glucoregulatory responses during subsequent glucoprivation. Diabetes 2000, 49, 1865–1874. [Google Scholar] [CrossRef][Green Version]
- Sanders, N.M.; Ritter, S. Acute 2dg-induced glucoprivation or dexamethasone abolishes 2dg-induced glucoregulatory responses to subsequent glucoprivation. Diabetes 2001, 50, 2831–2836. [Google Scholar] [CrossRef]
- Sanders, N.M.; Taborsky, G.J., Jr.; Wilkinson, C.W.; Daumen, W.; Figlewicz, D.P. Antecedent hindbrain glucoprivation does not impair the counterregulatory response to hypoglycemia. Diabetes 2007, 56, 217–223. [Google Scholar] [CrossRef]
- Rusnak, M.; Jelokova, J.; Vietor, I.; Sabban, E.L.; Kvetnansky, R. Different effects of insulin and 2-deoxy-d-glucose administration on tyrosine hydroxylase gene expression in the locus coeruleus and the adrenal medulla in rats—Molecular genetic and neurobiological advances. Brain Res. Bull. 1998, 46, 447–452. [Google Scholar] [CrossRef]
- Pillion, D.J.; Arnold, P.; Yang, M.; Stockard, C.R.; Grizzle, W.E. Receptors for insulin and insulin-like growth factor-i in the human adrenal gland. Biochem. Biophys. Res. Commun. 1989, 165, 204–211. [Google Scholar] [CrossRef]
- Morgan, D.A.; Balon, T.W.; Ginsberg, B.H.; Mark, A.L. Nonuniform regional sympathetic nerve responses to hyperinsulinemia in rats. Am. J. Physiol. 1993, 264, R423–R427. [Google Scholar] [CrossRef]
- Galassetti, P.; Davis, S.N. Effects of insulin per se on neuroendocrine and metabolic counter-regulatory responses to hypoglycaemia. Clin. Sci. 2000, 99, 351–362. [Google Scholar] [CrossRef]
- Shum, K.; Inouye, K.; Chan, O.; Mathoo, J.; Bilinski, D.; Matthews, S.G.; Vranic, M. Effects of antecedent hypoglycemia, hyperinsulinemia, and excess corticosterone on hypoglycemic counterregulation. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E455–E465. [Google Scholar] [CrossRef]
- Inouye, K.; Shum, K.; Chan, O.; Mathoo, J.; Matthews, S.G.; Vranic, M. Effects of recurrent hyperinsulinemia with and without hypoglycemia on counterregulation in diabetic rats. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E1369–E1379. [Google Scholar] [CrossRef][Green Version]
- Sankar, A.; Khodai, T.; McNeilly, A.D.; McCrimmon, R.J.; Luckman, S.M. Experimental models of impaired hypoglycaemia-associated counter-regulation. Trends Endocrinol. Metab. 2020, 31, 691–703. [Google Scholar] [CrossRef]
- Chan, O.; Chan, S.; Inouye, K.; Shum, K.; Matthews, S.G.; Vranic, M. Diabetes impairs hypothalamo-pituitary-adrenal (hpa) responses to hypoglycemia, and insulin treatment normalizes hpa but not epinephrine responses. Diabetes 2002, 51, 1681–1689. [Google Scholar] [CrossRef]
- Farhat, R.; de Santana-Van Vliet, E.; Su, G.; Neely, L.; Benally, T.; Chan, O. Carvedilol prevents impairment of the counterregulatory response in recurrently hypoglycaemic diabetic rats. Endocrinol. Diabetes Metab. 2021, 4, e00226. [Google Scholar] [CrossRef]
- Nedoboy, P.E.; Farnham, M.M.-J. Still excited, but less aroused—the effects of nutritional ketosis on epinephrine response and hypothalamic orexin neuron activation following recurrent hypoglycemia in diabetic rats. Metabolites 2023, 13, 42. [Google Scholar]
- Shankar, K.; Varshney, S.; Gupta, D.; Mani, B.K.; Osborne-Lawrence, S.; Metzger, N.P.; Richard, C.P.; Zigman, J.M. Ghrelin does not impact the blunted counterregulatory response to recurrent hypoglycemia in mice. Front. Endocrinol. 2023, 14, 1181856. [Google Scholar] [CrossRef]
- Lontchi-Yimagou, E.; Aleksic, S.; Hulkower, R.; Gospin, R.; Goyal, A.; Kuo, B.; Mitchell, W.G.; You, J.Y.; Upadhyay, L.; Carey, M.; et al. Plasma epinephrine contributes to the development of experimental hypoglycemia-associated autonomic failure. J. Clin. Endocrinol. Metab. 2020, 105, 3416–3427. [Google Scholar] [CrossRef] [PubMed]
- Moheet, A.; Kumar, A.; Eberly, L.E.; Kim, J.; Roberts, R.; Seaquist, E.R. Hypoglycemia-associated autonomic failure in healthy humans: Comparison of two vs three periods of hypoglycemia on hypoglycemia-induced counterregulatory and symptom response 5 days later. J. Clin. Endocrinol. Metab. 2014, 99, 664–670. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Galan, B.E.; Tack, C.J.; Willemsen, J.J.; Sweep, C.G.; Smits, P.; Lenders, J.W. Plasma metanephrine levels are decreased in type 1 diabetic patients with a severely impaired epinephrine response to hypoglycemia, indicating reduced adrenomedullary stores of epinephrine. J. Clin. Endocrinol. Metab. 2004, 89, 2057–2061. [Google Scholar] [CrossRef][Green Version]
- Ritter, S.; Scheurink, A.; Singer, L.K. 2-deoxy-d-glucose but not 2-mercaptoacetate increases fos-like immunoreactivity in adrenal medulla and sympathetic preganglionic neurons. Obes. Res. 1995, 3, 729S–734S. [Google Scholar] [CrossRef]
- Senthilkumaran, M.; Bobrovskaya, L.; Verberne, A.J.M.; Llewellyn-Smith, I.J. Insulin-responsive autonomic neurons in rat medulla oblongata. J. Comp. Neurol. 2018, 526, 2665–2682. [Google Scholar] [CrossRef]
- Greenberg, M.E.; Ziff, E.B.; Greene, L.A. Stimulation of neuronal acetylcholine receptors induces rapid gene transcription. Science 1986, 234, 80–83. [Google Scholar] [CrossRef]
- Morgan, J.I.; Curran, T. Stimulus-transcription coupling in the nervous system: Involvement of the inducible proto-oncogenes fos and jun. Annu. Rev. Neurosci. 1991, 14, 421–451. [Google Scholar] [CrossRef]
- Kakall, Z.M.; Kavurma, M.M.; Cohen, E.M.; Howe, P.R.; Nedoboy, P.E.; Pilowsky, P.M. Repetitive hypoglycemia reduces activation of glucose-responsive neurons in c1 and c3 medullary brain regions to subsequent hypoglycemia. Am. J. Physiol.-Endocrinol. Metab. 2019, 317, E388–E398. [Google Scholar] [CrossRef]
- Senthilkumaran, M.; Bobrovskaya, L. The effects of recurrent hypoglycaemia and opioid antagonists on the adrenal catecholamine synthetic capacity in a rat model of haaf. Auton. Neurosci. Basic Clin. 2018, 210, 76–80. [Google Scholar] [CrossRef]
- Berends, A.M.A.; Eisenhofer, G.; Fishbein, L.; Horst-Schrivers, A.; Kema, I.P.; Links, T.P.; Lenders, J.W.M.; Kerstens, M.N. Intricacies of the molecular machinery of catecholamine biosynthesis and secretion by chromaffin cells of the normal adrenal medulla and in pheochromocytoma and paraganglioma. Cancers 2019, 11, 1121. [Google Scholar] [CrossRef]
- Bobrovskaya, L.; Gilligan, C.; Bolster, E.K.; Flaherty, J.J.; Dickson, P.W.; Dunkley, P.R. Sustained phosphorylation of tyrosine hydroxylase at serine 40: A novel mechanism for maintenance of catecholamine synthesis. J. Neurochem. 2007, 100, 479–489. [Google Scholar] [CrossRef]
- Dunkley, P.R.; Bobrovskaya, L.; Graham, M.E.; Von Nagy-Felsobuki, E.I.; Dickson, P.W. Tyrosine hydroxylase phosphorylation: Regulation and consequences. J. Neurochem. 2004, 91, 1025–1043. [Google Scholar] [CrossRef] [PubMed]
- Bobrovskaya, L.; Gelain, D.P.; Gilligan, C.; Dickson, P.W.; Dunkley, P.R. Pacap stimulates the sustained phosphorylation of tyrosine hydroxylase at serine 40. Cell. Signal. 2007, 19, 1141–1149. [Google Scholar] [CrossRef]
- Haycock, J.W. Multiple signaling pathways in bovine chromaffin cells regulate tyrosine hydroxylase phosphorylation at ser19, ser31, and ser40. Neurochem. Res. 1993, 18, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Ong, L.K.; Sominsky, L.; Dickson, P.W.; Hodgson, D.M.; Dunkley, P.R. The sustained phase of tyrosine hydroxylase activation in vivo. Neurochem. Res. 2012, 37, 1938–1943. [Google Scholar] [CrossRef] [PubMed]
- Bevilaqua, L.R.; Graham, M.E.; Dunkley, P.R.; von Nagy-Felsobuki, E.I.; Dickson, P.W. Phosphorylation of ser(19) alters the conformation of tyrosine hydroxylase to increase the rate of phosphorylation of ser(40). J. Biol. Chem. 2001, 276, 40411–40416. [Google Scholar] [CrossRef] [PubMed]
- Bobrovskaya, L.; Dunkley, P.R.; Dickson, P.W. Phosphorylation of ser19 increases both ser40 phosphorylation and enzyme activity of tyrosine hydroxylase in intact cells. J. Neurochem. 2004, 90, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, I.T.; Bobrovskaya, L.; Gordon, S.L.; Dunkley, P.R.; Dickson, P.W. Differential regulation of the human tyrosine hydroxylase isoforms via hierarchical phosphorylation. J. Biol. Chem. 2006, 281, 17644–17651. [Google Scholar] [CrossRef] [PubMed]
- Nunez, C.; Laorden, M.L.; Milanes, M.V. Regulation of serine (ser)-31 and ser40 tyrosine hydroxylase phosphorylation during morphine withdrawal in the hypothalamic paraventricular nucleus and nucleus tractus solitarius-a2 cell group: Role of erk1/2. Endocrinology 2007, 148, 5780–5793. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumaran, M.; Johnson, M.E.; Bobrovskaya, L. The effects of insulin-induced hypoglycaemia on tyrosine hydroxylase phosphorylation in rat brain and adrenal gland. Neurochem. Res. 2016, 41, 1612–1624. [Google Scholar] [CrossRef] [PubMed]
- Vietor, I.; Rusnak, M.; Viskupic, E.; Blazicek, P.; Sabban, E.L.; Kvetnansky, R. Glucoprivation by insulin leads to trans-synaptic increase in rat adrenal tyrosine hydroxylase mrna levels. Eur. J. Pharmacol. 1996, 313, 119–127. [Google Scholar] [CrossRef]
- Inouye, K.E.; Chan, O.; Yue, J.T.Y.; Matthews, S.G.; Vranic, M. Effects of diabetes and recurrent hypoglycemia on the regulation of the sympathoadrenal system and hypothalamo-pituitary-adrenal axis. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E422–E429. [Google Scholar] [CrossRef]
- Silbergeld, S.; Kvetňanský, R.; Weise, V.K.; Kopin, I.J. Effect of repeated administration of 2-deoxy-d-glucose or insulin on catechol- amine-synthesizing enzymes in rat adrenals. Biochem. Pharmacol. 1971, 20, 1763–1768. [Google Scholar] [CrossRef]
- Inouye, K.E.; Yue, J.T.; Chan, O.; Kim, T.; Akirav, E.M.; Park, E.; Riddell, M.C.; Burdett, E.; Matthews, S.G.; Vranic, M. Effects of insulin treatment without and with recurrent hypoglycemia on hypoglycemic counterregulation and adrenal catecholamine-synthesizing enzymes in diabetic rats. Endocrinology 2006, 147, 1860–1870. [Google Scholar] [CrossRef][Green Version]
- Eiden, L.E.; Emery, A.C.; Zhang, L.; Smith, C.B. Pacap signaling in stress: Insights from the chromaffin cell. Pflügers Arch. Eur. J. Physiol. 2018, 470, 79–88. [Google Scholar] [CrossRef]
- Shiotani, Y.; Kimura, S.; Ohshige, Y.; Yanaihara, C.; Yanaihara, N. Immunohistochemical localization of pituitary adenylate cyclase-activating polypeptide (pacap) in the adrenal medulla of the rat. Peptides 1995, 16, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Tabarin, A.; Chen, D.; Håkanson, R.; Sundler, F. Pituitary adenylate cyclase-activating peptide in the adrenal gland of mammals: Distribution, characterization and responses to drugs. Neuroendocrinology 1994, 59, 113–119. [Google Scholar] [CrossRef]
- Fukushima, Y.; Hikichi, H.; Mizukami, K.; Nagayama, T.; Yoshida, M.; Suzuki-Kusaba, M.; Hisa, H.; Kimura, T.; Satoh, S. Role of endogenous pacap in catecholamine secretion from the rat adrenal gland. American journal of physiology. Regulatory, integrative and comparative physiology. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 2001, 281, R1562–R1567. [Google Scholar] [CrossRef]
- Mazzocchi, G.; Malendowicz, L.K.; Rebuffat, P.; Gottardo, L.; Nussdorfer, G.G. Expression and function of vasoactive intestinal peptide, pituitary adenylate cyclase-activating polypeptide, and their receptors in the human adrenal gland. J. Clin. Endocrinol. Metab. 2002, 87, 2575–2580. [Google Scholar] [CrossRef]
- Tanaka, K.; Shibuya, I.; Nagamoto, T.; Yamashita, H.; Kanno, T. Pituitary adenylate cyclase-activating polypeptide causes rapid ca2+ release from intracellular stores and long lasting ca2+ influx mediated by Na+ influx-dependent membrane depolarization in bovine adrenal chromaffin cells. Endocrinology 1996, 137, 956–966. [Google Scholar] [CrossRef]
- Isobe, K.; Nakai, T.; Takuwa, Y. Ca2+-dependent stimulatory effect of pituitary adenylate cyclase-activating polypeptide on catecholamine secretion from cultured porcine adrenal medullary chromaffin cells. Endocrinology 1993, 132, 1757–1765. [Google Scholar] [CrossRef]
- Isobe, K.; Yukimasa, N.; Nakai, T.; Takuwa, Y. Pituitary adenylate cyclase-activating polypeptide induces gene expression of the catecholamine synthesizing enzymes, tyrosine hydroxylase and dopamine β hydroxylase, through 3′, 5′-cyclic adenosine monophosphate- and protein kinase c-dependent mechanisms in cultured porcine adrenal medullary chromaffin cells. Neuropeptides 1996, 30, 167–175. [Google Scholar] [CrossRef]
- Malhotra, R.K.; Wakade, A.R. Vasoactive intestinal polypeptide stimulates the secretion of catecholamines from the rat adrenal gland. J. Physiol. 1987, 388, 285–294. [Google Scholar] [CrossRef]
- Oomori, Y.; Okuno, S.; Fujisawa, H.; Iuchi, H.; Ishikawa, K.; Satoh, Y.; Ono, K. Ganglion cells immunoreactive for catecholamine-synthesizing enzymes, neuropeptide y and vasoactive intestinal polypeptide in the rat adrenal gland. Cell Tissue Res. 1994, 275, 201–213. [Google Scholar] [CrossRef]
- Cavadas, C.; Silva, A.P.; Mosimann, F.; Cotrim, M.D.; Ribeiro, C.A.; Brunner, H.R.; Grouzmann, E. Npy regulates catecholamine secretion from human adrenal chromaffin cells. J. Clin. Endocrinol. Metab. 2001, 86, 5956–5963. [Google Scholar] [CrossRef]
- Higuchi, H.; Costa, E.; Yang, H.Y. Neuropeptide y inhibits the nicotine-mediated release of catecholamines from bovine adrenal chromaffin cells. J. Pharmacol. Exp. Ther. 1988, 244, 468–474. [Google Scholar]
- Bastiaensen, E.; De Block, J.; De Potter, W.P. Neuropeptide y is localized together with enkephalins in adrenergic granules of bovine adrenal medulla. Neuroscience 1988, 25, 679–686. [Google Scholar] [CrossRef]
- Bommer, M.; Herz, A. Neurotensin affects metabolism of opioid peptides, catecholamines and inositol phospholipids in bovine chromaffin cells. Life Sci. 1989, 44, 327–335. [Google Scholar] [CrossRef]
- Lundberg, J.M.; Rokaeus, A.; Hokfelt, T.; Rosell, S.; Brown, M.; Goldstein, M. Neurotensin-like immunoreactivity in the preganglionic sympathetic nerves and in the adrenal medulla of the cat. Acta Physiol. Scand. 1982, 114, 153–155. [Google Scholar] [CrossRef]
- Andreis, P.G.; Tortorella, C.; Ziolkowska, A.; Spinazzi, R.; Malendowicz, L.K.; Neri, G.; Nussdorfer, G.G. Evidence for a paracrine role of endogenous adrenomedullary galanin in the regulation of glucocorticoid secretion in the rat adrenal gland. Int. J. Mol. Med. 2007, 19, 511–515. [Google Scholar] [CrossRef][Green Version]
- Jarry, H.; Dietrich, M.; Barthel, A.; Giesler, A.; Wuttke, W. In vivo demonstration of a paracrine, inhibitory action of met-enkephalin on adrenomedullary catecholamine release in the rat. Endocrinology 1989, 125, 624–629. [Google Scholar] [CrossRef]
- Pelto-Huikko, M.; Salminen, T.; Hervonen, A. Localization of enkephalins in adrenaline cells and the nerves innervating adrenaline cells in rat adrenal medulla. Histochemistry 1985, 82, 377–383. [Google Scholar] [CrossRef]
- Livett, B.G.; Day, R.; Elde, R.P.; Howe, P.R. Co-storage of enkephalins and adrenaline in the bovine adrenal medulla. Neuroscience 1982, 7, 1323–1332. [Google Scholar] [CrossRef]
- Role, L.W.; Leeman, S.E.; Perlman, R.L. Somatostatin and substance p inhibit catecholamine secretion from isolated cells of guinea-pig adrenal medulla. Neuroscience 1981, 6, 1813–1821. [Google Scholar] [CrossRef]
- Zhou, X.F.; Marley, P.D.; Livett, B.G. Substance p modulates the time course of nicotinic but not muscarinic catecholamine secretion from perfused adrenal glands of rat. Br. J. Pharmacol. 1991, 104, 159–165. [Google Scholar] [CrossRef][Green Version]
- Kuramoto, H.; Kondo, H.; Fujita, T. Calcitonin gene-related peptide (cgrp)-like immunoreactivity in scattered chromaffin cells and nerve fibers in the adrenal gland of rats. Cell Tissue Res. 1987, 247, 309–315. [Google Scholar] [CrossRef]
- Di Angelantonio, S.; Giniatullin, R.; Costa, V.; Sokolova, E.; Nistri, A. Modulation of neuronal nicotinic receptor function by the neuropeptides cgrp and substance p on autonomic nerve cells. Br. J. Pharmacol. 2003, 139, 1061–1073. [Google Scholar] [CrossRef]
- Watanabe, T.; Masuo, Y.; Matsumoto, H.; Suzuki, N.; Ohtaki, T.; Masuda, Y.; Kitada, C.; Tsuda, M.; Fujino, M. Pituitary adenylate cyclase activating polypeptide provokes cultured rat chromaffin cells to secrete adrenaline. Biochemical and Biophysical Research Communications 1992, 182, 403–411. [Google Scholar] [CrossRef]
- Mazzocchi, G.; Malendowicz, L.K.; Neri, G.; Andreis, P.G.; Ziolkowska, A.; Gottardo, L.; Nowak, K.W.; Nussdorfer, G.G. Pituitary adenylate cyclase-activating polypeptide and pacap receptor expression and function in the rat adrenal gland. Int J Mol Med 2002, 9, 233–243. [Google Scholar] [CrossRef]
- Holgert, H.; Holmberg, K.; Hannibal, J.; Fahrenkrug, J.; Brimijoin, S.; Hartman, B.K.; Hokfelt, T. Pacap in the adrenal gland--relationship with choline acetyltransferase, enkephalin and chromaffin cells and effects of immunological sympathectomy. Neuroreport 1996, 8, 297–301. [Google Scholar] [CrossRef]
- Kumar, N.N.; Allen, K.; Parker, L.; Damanhuri, H.; Goodchild, A.K. Neuropeptide coding of sympathetic preganglionic neurons; focus on adrenally projecting populations. Neuroscience 2010, 170, 789–799. [Google Scholar] [CrossRef]
- Tehranian, R.; Montoya, S.E.; Van Laar, A.D.; Hastings, T.G.; Perez, R.G. Alpha-synuclein inhibits aromatic amino acid decarboxylase activity in dopaminergic cells. J. Neurochem. 2006, 99, 1188–1196. [Google Scholar] [CrossRef]
- Laslop, A.; Wohlfarter, T.; Fischer-Colbrie, R.; Steiner, H.J.; Humpel, C.; Saria, A.; Schmid, K.W.; Sperk, G.; Winkler, H. Insulin hypoglycemia increases the levels of neuropeptide y and calcitonin gene-related peptide, but not of chromogranins a and b, in rat chromaffin granules. Regul. Pept. 1989, 26, 191–202. [Google Scholar] [CrossRef]
- Han, S.; Chen, X.; Wu, Y.M.; Naes, L.; Westfall, T. Elevated neuropeptide y gene expression and release during hypoglycemic stress. Peptides 1997, 18, 1335–1340. [Google Scholar] [CrossRef]
- Kanamatsu, T.; Unsworth, C.D.; Diliberto, E.J., Jr.; Viveros, O.H.; Hong, J.S. Reflex splanchnic nerve stimulation increases levels of proenkephalin a mrna and proenkephalin a-related peptides in the rat adrenal medulla. Proc. Natl. Acad. Sci. USA 1986, 83, 9245–9249. [Google Scholar] [CrossRef]
- Fischer-Colbrie, R.; Iacangelo, A.; Eiden, L.E. Neural and humoral factors separately regulate neuropeptide y, enkephalin, and chromogranin a and b mrna levels in rat adrenal medulla. Proc. Natl. Acad. Sci. USA 1988, 85, 3240–3244. [Google Scholar] [CrossRef]
- Anouar, Y.; Eiden, L.E. Rapid and long-lasting increase in galanin mrna levels in rat adrenal medulla following insulin-induced reflex splanchnic nerve stimulation. Neuroendocrinology 1995, 62, 611–618. [Google Scholar] [CrossRef]
- Vaupel, R.; Jarry, H.; Schlomer, H.T.; Wuttke, W. Differential response of substance p-containing subtypes of adrenomedullary cells to different stressors. Endocrinology 1988, 123, 2140–2145. [Google Scholar] [CrossRef]
- Hamelink, C.; Tjurmina, O.; Damadzic, R.; Young, W.; Weihe, E.; Lee, H.-W.; Eiden, L. Pituitary adenylate cyclase-activating polypeptide is a sympathoadrenal neurotransmitter involved in catecholamine regulation and glucohomeostasis. PNAS 2002, 99, 461–466. [Google Scholar] [CrossRef]
- Saiani, L.; Guidotti, A. Opiate receptor-mediated inhibition of catecholamine release in primary cultures of bovine adrenal chromaffin cells. J. Neurochem. 1982, 39, 1669–1676. [Google Scholar] [CrossRef]
- Lemaire, S.; Livett, B.; Tseng, R.; Mercier, P.; Lemaire, I. Studies on the inhibitory action of opiate compounds in isolated bovine adrenal chromaffin cells: Noninvolvement of stereospecific opiate binding sites. J. Neurochem. 1981, 36, 886–892. [Google Scholar] [CrossRef]
- Vele, S.; Milman, S.; Shamoon, H.; Gabriely, I. Opioid receptor blockade improves hypoglycemia-associated autonomic failure in type 1 diabetes mellitus. J. Clin. Endocrinol. Metab. 2011, 96, 3424–3431. [Google Scholar] [CrossRef]
- Leu, J.; Cui, M.H.; Shamoon, H.; Gabriely, I. Hypoglycemia-associated autonomic failure is prevented by opioid receptor blockade. J. Clin. Endocrinol. Metab. 2009, 94, 3372–3380. [Google Scholar] [CrossRef]
- Bentsen, M.A.; Mirzadeh, Z.; Schwartz, M.W. Revisiting how the brain senses glucose—And why. Cell Metab. 2019, 29, 11–17. [Google Scholar] [CrossRef]
- Donovan, C.M.; Watts, A.G. Peripheral and central glucose sensing in hypoglycemic detection. Physiology 2014, 29, 314–324. [Google Scholar] [CrossRef]


| Peptide | Species | Model System | Immunoreactivity in Adrenaline Chromaffin Cells | Immunoreactivity in Noradrenaline Chromaffin Cells | Effects on Catecholamine Release |
|---|---|---|---|---|---|
| PACAP | Rat [115,116,117] Mouse [116] Human [118] Bovine [119] Porcine [120,121] | Adrenal gland [115,116,117,118] Adrenal medullary chromaffin cells [119,120,121] | + [118] − [115,116] | + [115,116,118] | ↑ [117,118,119,120,121] |
| VIP | Rat [122] Human [118] | Adrenal gland [118,122] | + [118] | + [118] | ↑ [118,122] |
| NPY | Rat [116,123] Human [124] Bovine [125,126] | Adrenal gland [116,123] Adrenal medullary chromaffin cells [124,125,126] | + [116,123,126] | + [123] − [116,126] | ↑ [124] ↓ [125] |
| Neurotensin | Bovine [127] Cat [128] | Adrenal medullary chromaffin cells [127] Adrenal medulla [128] | − [128] | + [128] | ↑ [127] |
| Galanin | Rat [129] | Adrenal gland [129] | + [129] | + [129] | ↑ [129] |
| ENK | Rat [130,131] Bovine [132] | Adrenal gland [130,131] Adrenal medulla [132] | + [131,132] | − [131,132] | ↓ [130] |
| SP | Guinea pig [133] Rat [134] | Adrenal medullary chromaffin cells [133] Adrenal gland [134] | + [133] | + [133] | ↓ [133,134] |
| CGRP | Rat [135] | Adrenal gland [135] | + [135] | − [135] | ↓ [136] |
| Somatostatin | Guinea pig [133] | Adrenal medullary chromaffin cells [133] | + [133] | + [133] | ↓ [133] |
| Peptide | Species | Stimulus | Effects on Gene/Peptide Expression in the Adrenal Gland/Medulla |
|---|---|---|---|
| NPY | Rats [37,142,143] | Recurrent hypoglycaemia (2 U/kg twice daily for three days) [37] | ↑ ProNPY gene [37] |
| Insulin (2 U/kg) [142] | ↑ mRNA and peptide [142] | ||
| Insulin (0.7, 3.5, 7 U/kg) [143] | Dose-dependent increase in mRNA; ↑ NPY-ir after 7 U/kg insulin [143] | ||
| Mice [34] | Recurrent hypoglycaemia (2.5 U/kg once daily for 3 consecutive days) [34] | ↑ NPY-ir [34] | |
| Enkephalin | Rats [37,144,145,146] | Recurrent hypoglycaemia (2 U/kg twice daily for three days) [37] | ↑ Proenkephalin gene [37] |
| Insulin (10 U/kg) [144] | ↑ PreproENK mRNA [144] | ||
| Insulin (8.5 U/kg) [145] | ↑ PreproENK mRNA [145] | ||
| Insulin (10 U/kg) [146] | ↑ PreproENK mRNA [146] | ||
| CGRP | Rats [142] | Insulin (2 U/kg) [142] | ↑ CGRP mRNA and Peptide [142] |
| SP | Rats [62] | Insulin (5 U/kg) [62] | ↑ SP peptide [62] |
| Galanin | Rats [37,62,146] | Recurrent hypoglycaemia (2 U/kg twice daily for three days) [37] | ↑ Galanin prepropeptide gene [37] |
| Insulin (5 U/kg) [62] | ↑ Galanin peptide [62] | ||
| Insulin (10 U/kg) [146] | ↑ Galanin mRNA [146] | ||
| Neurotensin | Rats [37,62] | Recurrent hypoglycaemia (2 U/kg twice daily for three days) [37] | ↑ Neurotensin gene [37] |
| Insulin (5 U/kg) [62] | ↑ Neurotensin peptide [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senthilkumaran, M.; Koch, C.; Herselman, M.F.; Bobrovskaya, L. Role of the Adrenal Medulla in Hypoglycaemia-Associated Autonomic Failure—A Diabetic Perspective. Metabolites 2024, 14, 100. https://doi.org/10.3390/metabo14020100
Senthilkumaran M, Koch C, Herselman MF, Bobrovskaya L. Role of the Adrenal Medulla in Hypoglycaemia-Associated Autonomic Failure—A Diabetic Perspective. Metabolites. 2024; 14(2):100. https://doi.org/10.3390/metabo14020100
Chicago/Turabian StyleSenthilkumaran, Manjula, Coen Koch, Mauritz Frederick Herselman, and Larisa Bobrovskaya. 2024. "Role of the Adrenal Medulla in Hypoglycaemia-Associated Autonomic Failure—A Diabetic Perspective" Metabolites 14, no. 2: 100. https://doi.org/10.3390/metabo14020100
APA StyleSenthilkumaran, M., Koch, C., Herselman, M. F., & Bobrovskaya, L. (2024). Role of the Adrenal Medulla in Hypoglycaemia-Associated Autonomic Failure—A Diabetic Perspective. Metabolites, 14(2), 100. https://doi.org/10.3390/metabo14020100






