Analysis of the Urine Volatilome of COVID-19 Patients and the Possible Metabolic Alterations Produced by the Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Sample Preparation and Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
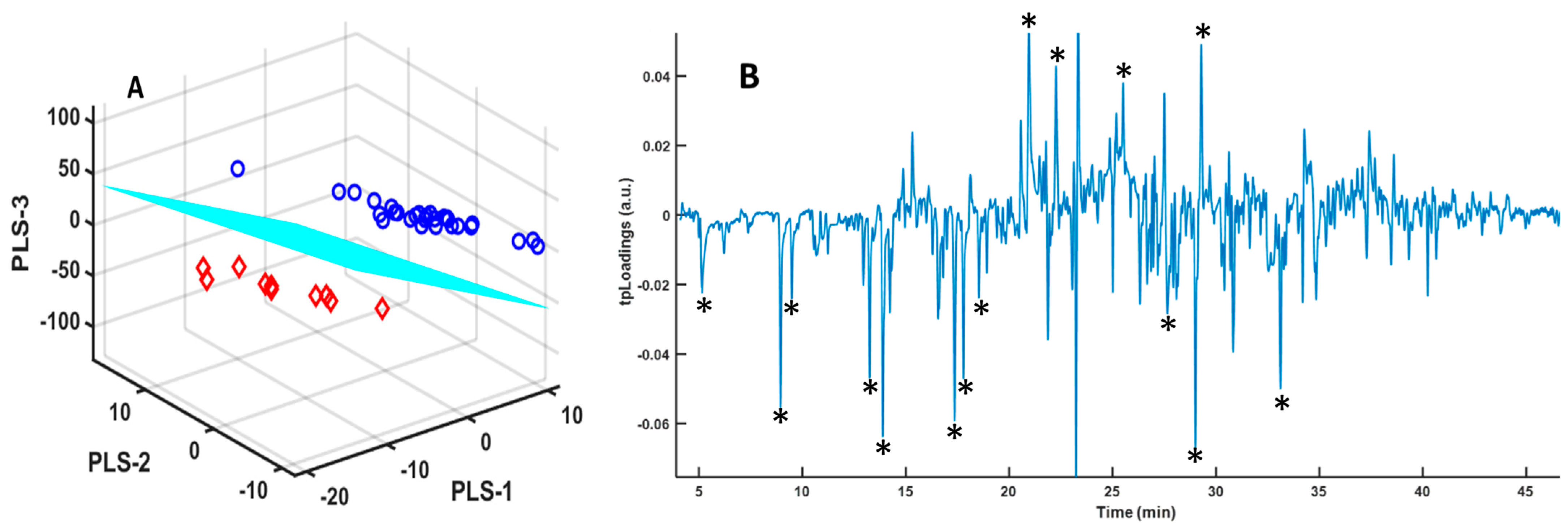
2.3. Statistical Analysis
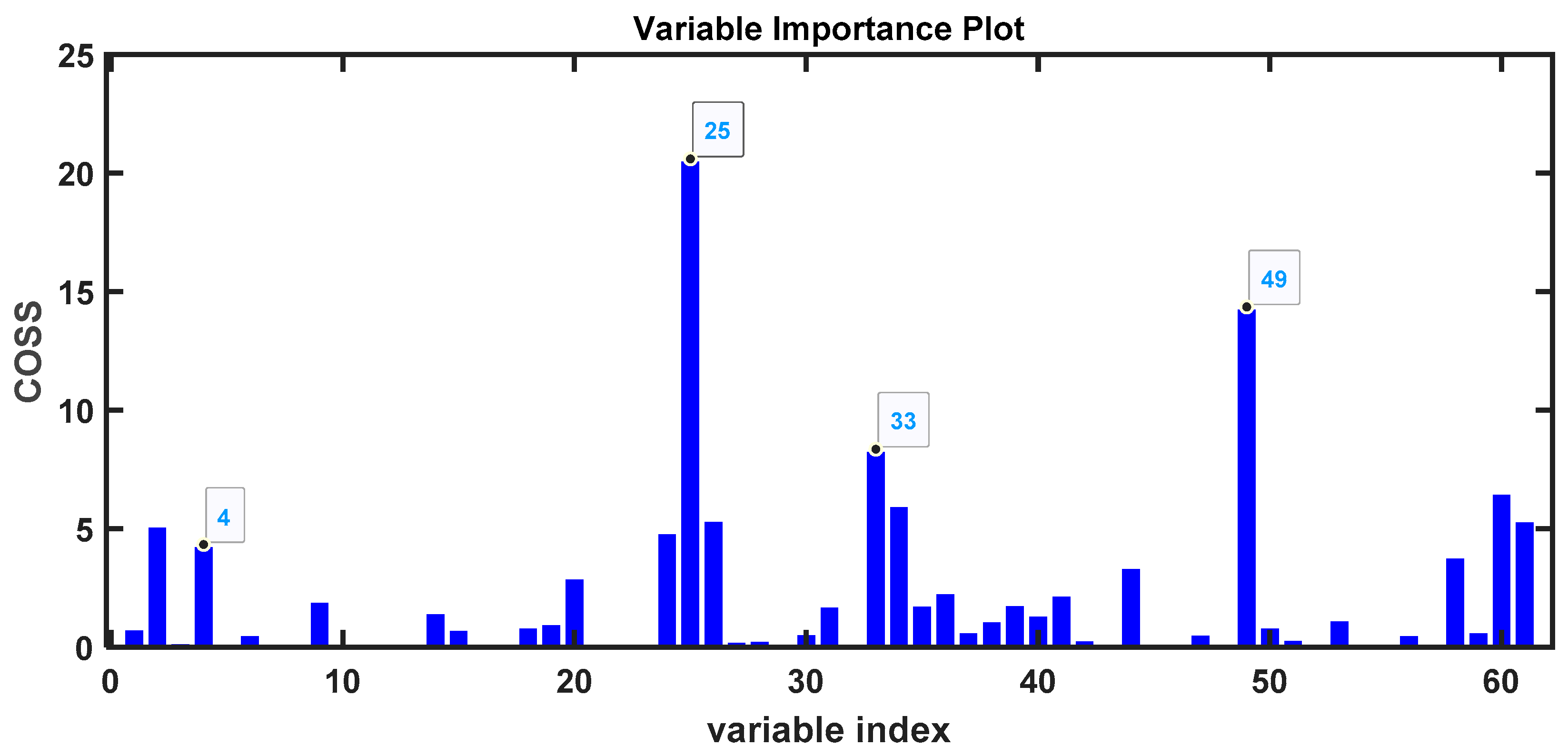
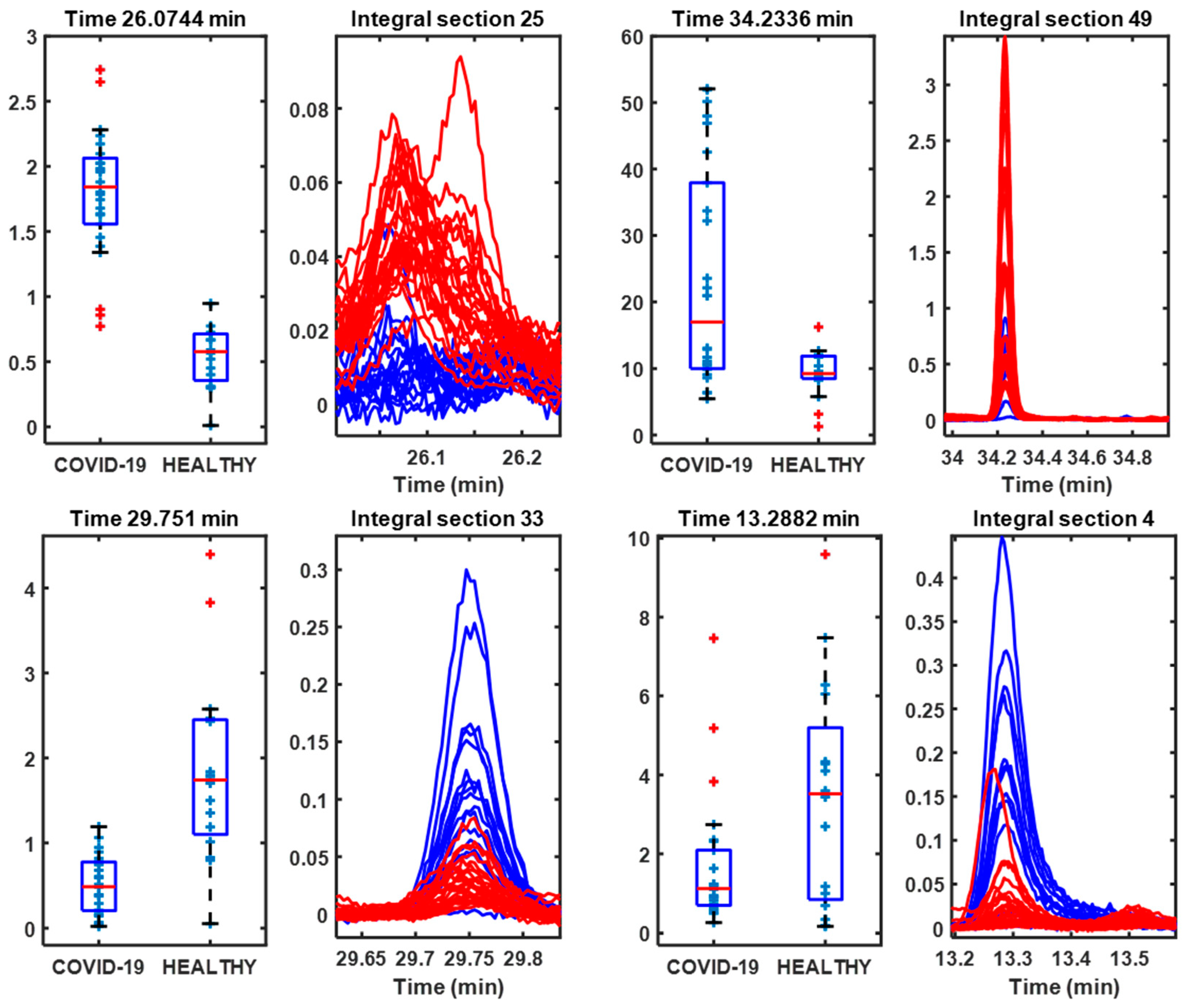
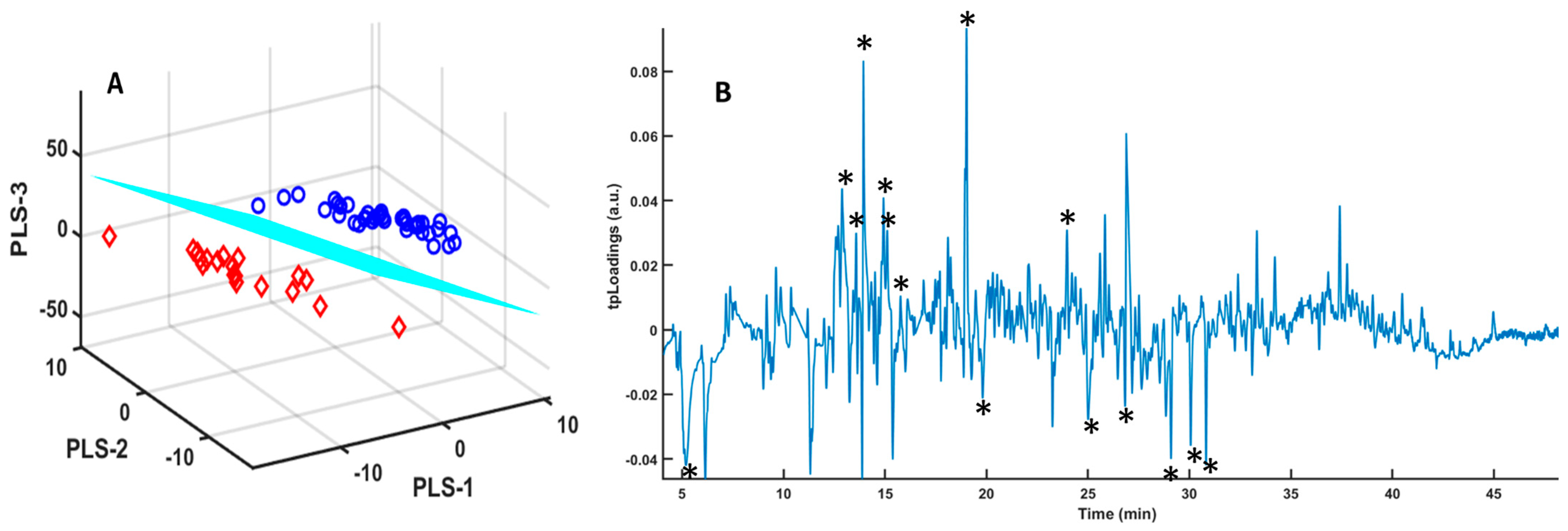
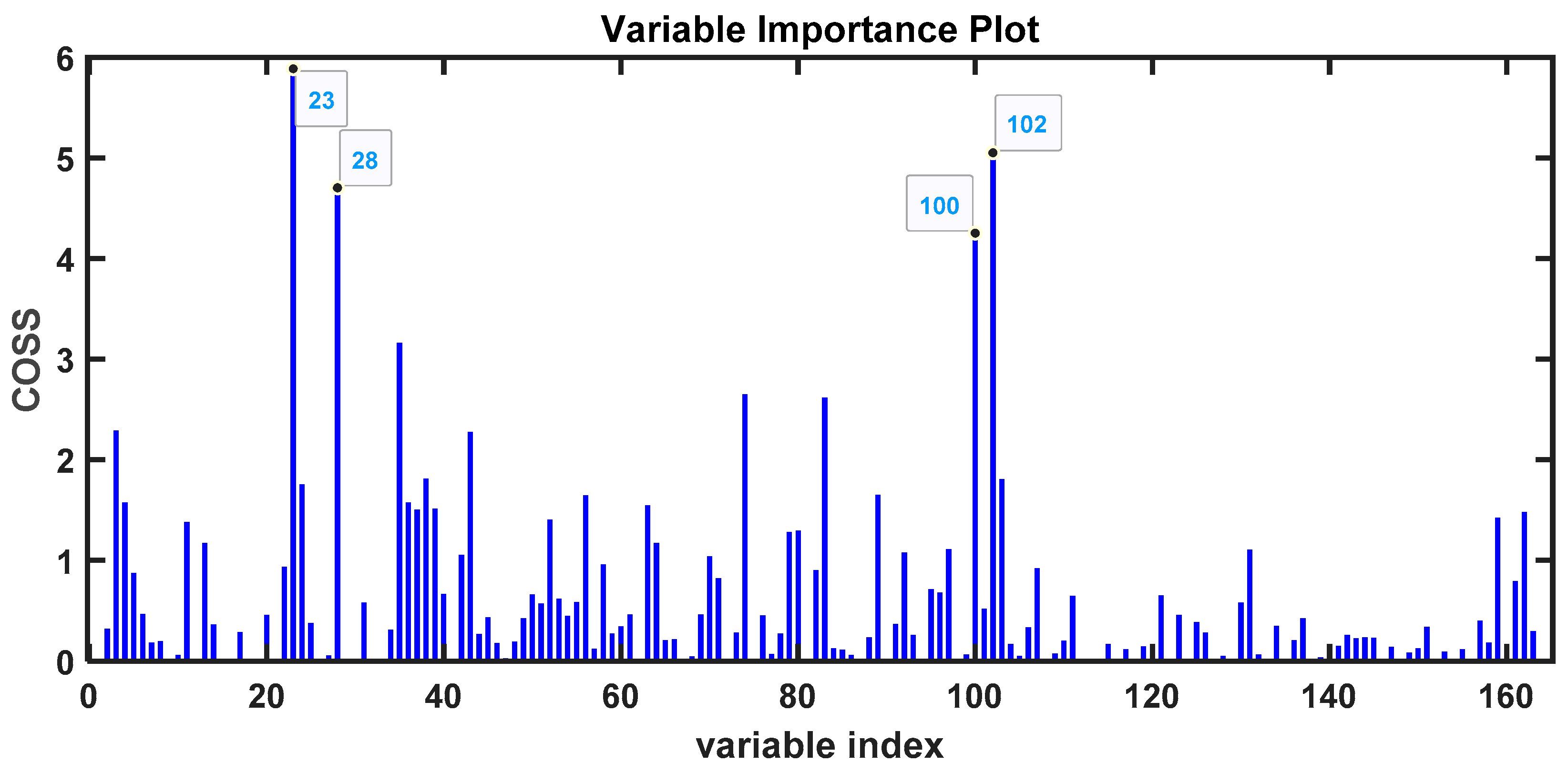
3. Results
3.1. Samples Without Any Treatment
| Time (min) | m/z | Compound | Confidence (%) | Higher in |
|---|---|---|---|---|
| 26.0744 | 164 | 2′-Hydroxy-4′,5′-dimethylacetophenona | 87 | COVID-19 |
| 34.2336 | 206 | Phenol, 2,4-bis(1,1-dimethylethyl) | 97 | COVID-19 |
| 29.751 | 208 | Decahydro-4,4,8,9,10-pentamethylnaphthalene | 49 | Control |
| 13.2882 | 106 | p-Xylene | 97 | Control |
| 29.948 | 152 | 3-(But-3-enyl)-cyclohexanone | 53 | COVID-19 |
| 26.5193 | 135 | Benzothiazole | 94 | COVID-19 |
| 9.5204 | 92 | Toluene | 95 | Control |
| 25.8843 | 72 | 1-(1-Propen-1-yl)-2-(2-thiopent-3-yl) disulfide | 72 | COVID-19 |
| 41.024 | 236 | 2,4-Diphenyl-4-methyl-2(E)-pentene | 95 | Control |
| 24.4244 | 154 | l-Menthone | 98 | Control |
| 30.4316 | 252 | 5-Octadecene, (E)- | 49 | COVID-19 |
| 32.0588 | 170 | Methacrylic acid, tetradecyl ester | 74 | COVID-19 |
| 17.4058 | 126 | Dimethyl trisulfide | 95 | COVID-19 |
| 31.4391 | 200 | 1-Dodecanol, 2-methyl-, (S)- | 80 | COVID-19 |
| 30.2871 | 138 | 2-(2,2-Dimethylvinyl)thiophene | 83 | COVID-19 |
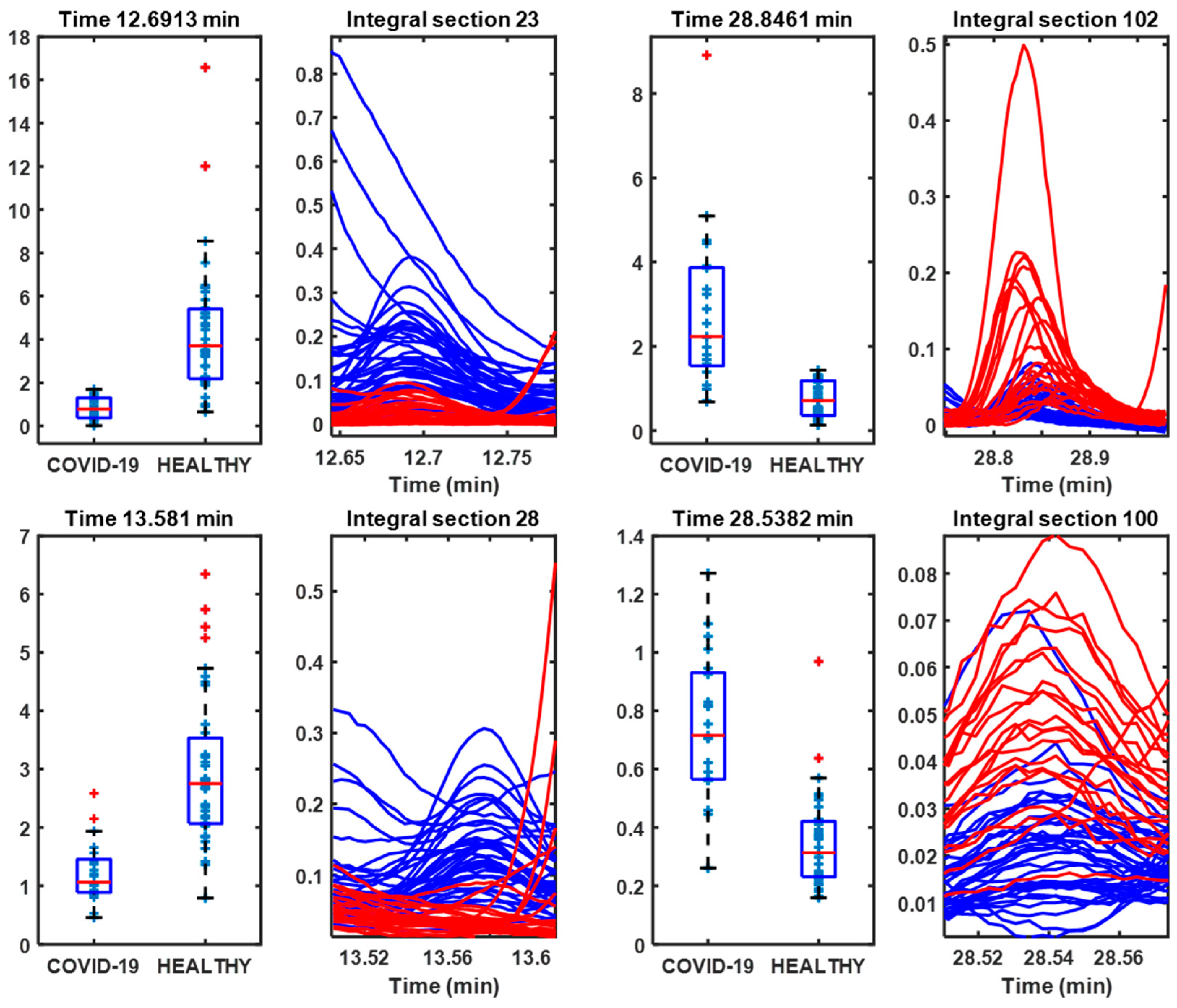
3.2. Lyophilized Samples
| Time (min) | m/z | Compound | Confidence (%) | Higher in |
|---|---|---|---|---|
| 12.6913 | 90 | 2,3-Butanediol | 80 | Control |
| 28.8461 | 113 | Caprolactam | 96 | COVID-19 |
| 13.581 | 98 | Cyclopentanone, 3-methyl- | 96 | Control |
| 28.5382 | 148 | Benzaldehyde, 2,4,5-trimethyl- | 93 | COVID-19 |
| 14.9345 | 78 | Dimethyl Sulfoxide (DMSO) | 96 | Control |
| 22.8465 | 42 | 2H-Pyran-2-one, tetrahydro- | 90 | Control |
| 25.162 | 71 | Oxalic acid, 2-ethylhexyl hexyl ester | 64 | COVID-19 |
| 5.1405 | 119 | Methane-d, trichloro- | 95 | COVID-19 |
| 16.9914 | 170 | Decane | 93 | Control |
| 15.7596 | 96 | 2-Cyclopenten-1-one, 2-methyl- | 94 | Control |
| 29.1009 | 158 | Formamide, N,N-dibutyl- | 97 | COVID-19 |
| 12.851 | 90 | 2,3-Butanediol | 90 | Control |
| 26.4242 | 135 | Benzothiazole | 93 | COVID-19 |
| 19.8201 | 134 | Benzene, 1,2,3,4-tetramethyl- | 80 | COVID-19 |
| 15.1208 | 108 | Pyrazine, 2,6-dimethyl- | 91 | Control |
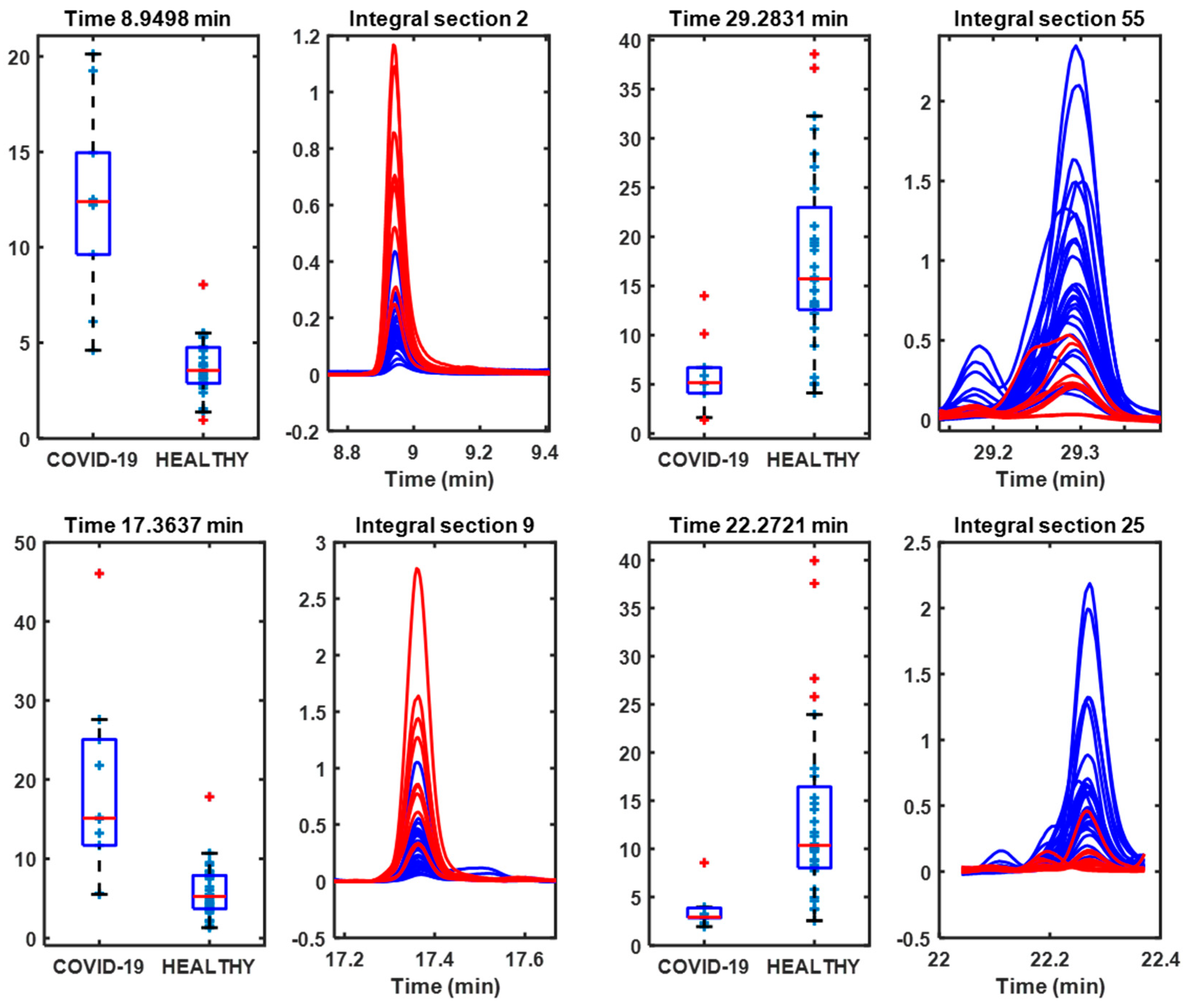
3.3. H2SO4 Samples
| Time (min) | m/z | Compound | Confidence (%) | Higher in |
|---|---|---|---|---|
| 8.9498 | 94 | Disulfide, dimethyl (DMDS) | 97 | COVID-19 |
| 29.2831 | 97 | 1,1,5-Trimethyl-1,2-dihydronaphthalene | 97 | Control |
| 17.3637 | 126 | Dimethyl trisulfide (DMTS) | 97 | COVID-19 |
| 22.2721 | 124 | Phenol, 2-methoxy- (guaiacol) | 94 | Control |
| 5.1212 | 84 | Methane-d, trichloro- | 94 | COVID-19 |
| 17.7782 | 105 | Benzene, 1,2,4-trimethyl- | 90 | COVID-19 |
| 13.8925 | 71 | 4-Heptanone | 91 | COVID-19 |
| 33.0889 | 204 | Spiro[5.5]undeca-1,8-diene, 1,5,5,9-tetramethyl-, (R)- | 98 | COVID-19 |
| 29.1006 | 224 | Cetene | 97 | COVID-19 |
| 25.4963 | 138 | 2-Methoxy-5-methylphenol | 80 | Control |
| 14.44 | 74 | Butanoic acid, 3-methyl- | 81 | COVID-19 |
| 9.4973 | 91 | Toluene | 95 | COVID-19 |
| 29.0055 | 159 | 1H-Inden-1-one, 2,3-dihydro-3,4,7-trimethyl- | 91 | COVID-19 |
| 20.9414 | 170 | trans-Linalool oxide (furanoid) | 90 | Control |
| 18.5347 | 68 | D-Limonene | 98 | COVID-19 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases?n=c (accessed on 24 September 2024).
- Fernandez-De-Las-Peñas, C.; Notarte, K.I.; Macasaet, R.; Velasco, J.V.; Catahay, J.A.; Ver, A.T.; Chung, W.; Valera-Calero, J.A.; Navarro-Santana, M. Persistence of post-COVID symptoms in the general population two years after SARS-CoV-2 infection: A systematic review and meta-analysis. J. Infect. 2024, 88, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- Ansone, L.; Rovite, V.; Brīvība, M.; Jagare, L.; Pelcmane, L.; Borisova, D.; Thews, A.; Leiminger, R.; Kloviņš, J. Longitudinal NMR-Based Metabolomics Study Reveals How Hospitalized COVID-19 Patients Recover: Evidence of Dyslipidemia and Energy Metabolism Dysregulation. Int. J. Mol. Sci. 2024, 25, 1523. [Google Scholar] [CrossRef] [PubMed]
- Marhuenda-Egea, F.C.; Narro-Serrano, J.; Shalabi-Benavent, M.J.; Álamo-Marzo, J.M.; Amador-Prous, C.; Algado-Rabasa, J.T.; Garijo-Saiz, A.M.; Marco-Escoto, M. A metabolic readout of the urine metabolome of COVID-19 patients. Metabolomics 2023, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Ghini, V.; Maggi, L.; Mazzoni, A.; Spinicci, M.; Zammarchi, L.; Bartoloni, A.; Annunziato, F.; Turano, P. Serum NMR Profiling Reveals Differential Alterations in the Lipoproteome Induced by Pfizer-BioNTech Vaccine in COVID-19 Recovered Subjects and Naïve Subjects. Front. Mol. Biosci. 2022, 9, 839809. [Google Scholar] [CrossRef]
- Holmes, E.; Wist, J.; Masuda, R.; Lodge, S.; Nitschke, P.; Kimhofer, T.; Loo, R.L.; Begum, S.; Boughton, B.; Yang, R.; et al. Incomplete Systemic Recovery and Metabolic Phenoreversion in Post-Acute-Phase Nonhospitalized COVID-19 Patients: Implications for Assessment of Post-Acute COVID-19 Syndrome. J. Proteome Res. 2021, 20, 3315–3329. [Google Scholar] [CrossRef]
- Bruzzone, C.; Bizkarguenaga, M.; Gil-Redondo, R.; Diercks, T.; Arana, E.; de Vicuña, A.G.; Seco, M.; Bosch, A.; Palazón, A.; Juan, I.S.; et al. SARS-CoV-2 Infection Dysregulates the Metabolomic and Lipidomic Profiles of Serum. iScience 2020, 23, 101645. [Google Scholar] [CrossRef]
- Frankevich, N.; Tokareva, A.; Chagovets, V.; Starodubtseva, N.; Dolgushina, N.; Shmakov, R.; Sukhikh, G.; Frankevich, V. COVID-19 Infection during Pregnancy: Disruptions in Lipid Metabolism and Implications for Newborn Health. Int. J. Mol. Sci. 2023, 24, 13787. [Google Scholar] [CrossRef]
- Lomova, N.; Dolgushina, N.; Tokareva, A.; Chagovets, V.; Starodubtseva, N.; Kulikov, I.; Sukhikh, G.; Frankevich, V. Past COVID-19: The Impact on IVF Outcomes Based on Follicular Fluid Lipid Profile. Int. J. Mol. Sci. 2022, 24, 10. [Google Scholar] [CrossRef]
- Lomova, N.; Chagovets, V.; Dolgopolova, E.; Novoselova, A.; Petrova, U.; Shmakov, R.; Frankevich, V. Altered amino acid profiles of the “mother–fetus” system in COVID-19. Bull. Russ. State Med Univ. 2022, 3, 51–60. [Google Scholar] [CrossRef]
- Lionetto, L.; Ulivieri, M.; Capi, M.; De Bernardini, D.; Fazio, F.; Petrucca, A.; Pomes, L.M.; De Luca, O.; Gentile, G.; Casolla, B.; et al. Increased kynurenine-to-tryptophan ratio in the serum of patients infected with SARS-CoV-2: An observational cohort study. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1867, 166042. [Google Scholar] [CrossRef]
- Chuachaina, S.; Thaveesangsakulthai, I.; Sinsukudomchai, P.; Somboon, P.; Traipattanakul, J.; Torvorapanit, P.; Chatdarong, K.; Kulsing, C.; Nhujak, T. Identification of Volatile Markers in Sweat for COVID-19 Screening by Gas Chromatography-Mass Spectrometry. ChemistrySelect 2024, 9, e202304388. [Google Scholar] [CrossRef]
- Chen, X.; Gu, X.; Yang, J.; Jiang, Z.; Deng, J. Gas Chromatography–Mass Spectrometry Technology: Application in the Study of Inflammatory Mechanism in COVID-19 Patients. Chromatographia 2023, 86, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Di Gilio, A.; Palmisani, J.; Picciariello, A.; Zambonin, C.; Aresta, A.; De Vietro, N.; Franchini, S.A.; Ventrella, G.; Nisi, M.R.; Licen, S.; et al. Identification of a characteristic VOCs pattern in the exhaled breath of post-COVID subjects: Are metabolic alterations induced by the infection still detectable? J. Breath Res. 2023, 17, 047101. [Google Scholar] [CrossRef] [PubMed]
- Woollam, M.; Angarita-Rivera, P.; Siegel, A.P.; Kalra, V.; Kapoor, R.; Agarwal, M. Exhaled VOCs can discriminate subjects with COVID-19 from healthy controls. J. Breath Res. 2022, 16, 036002. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Jiang, H.; Chen, Y.; Gu, S.; Xia, J.; Zhang, H.; Lu, Y.; Yan, R.; Li, L. The faecal metabolome in COVID-19 patients is altered and associated with clinical features and gut microbes. Anal. Chim. Acta 2021, 1152, 338267. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Yan, R.; Lv, L.; Jiang, H.; Lu, Y.; Sheng, J.; Xie, J.; Wu, W.; Xia, J.; Xu, K.; et al. The serum metabolome of COVID-19 patients is distinctive and predictive. Metabolism 2021, 118, 154739. [Google Scholar] [CrossRef]
- Angeletti, S.; Travaglino, F.; Spoto, S.; Pascarella, M.C.; Mansi, G.; De Cesaris, M.; Sartea, S.; Giovanetti, M.; Fogolari, M.; Plescia, D.; et al. COVID-19 sniffer dog experimental training: Which protocol and which implications for reliable sidentification? J. Med. Virol. 2021, 93, 5924–5930. [Google Scholar] [CrossRef]
- David, P.; Shoenfeld, Y. The Smell in COVID-19 Infection: Diagnostic Opportunities. Isr. Med. Assoc. J. 2020, 22, 401–403. [Google Scholar]
- Dickey, T.; Junqueira, H. Toward the use of medical scent detection dogs for COVID-19 screening. J. Am. Osteopat. Assoc. 2021, 121, 141–148. [Google Scholar] [CrossRef]
- Giovannini, G.; Haick, H.; Garoli, D. Detecting COVID-19 from Breath: A Game Changer for a Big Challenge. ACS Sensors 2021, 6, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, W.; Cordell, R.L.; Wilde, M.J.; Richardson, M.; Carr, L.; Dasi, A.S.D.; Hargadon, B.; Free, R.C.; Monks, P.S.; Brightling, C.E.; et al. Diagnosis of COVID-19 by exhaled breath analysis using gas chromatography-mass spectrometry. ERJ Open Res. 2021, 7, 00139–2021. [Google Scholar] [CrossRef] [PubMed]
- Snitz, K.; Andelman-Gur, M.; Pinchover, L.; Weissgross, R.; Weissbrod, A.; Mishor, E.; Zoller, R.; Linetsky, V.; Medhanie, A.; Shushan, S.; et al. Proof of concept for real-time detection of SARS-CoV-2 infection with an electronic nose. PLoS ONE 2021, 16, e0252121. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Baker, J.; Boyd, M.T.; Coyle, S.; Probert, C.; Chapman, E.A. Optimisation of Urine Sample Preparation for Headspace-Solid Phase Microextraction Gas Chromatography-Mass Spectrometry: Altering Sample pH, Sulphuric Acid Concentration and Phase Ratio. Metabolites 2020, 10, 482. [Google Scholar] [CrossRef] [PubMed]
- Aggio, R.B.M.; Mayor, A.; Coyle, S.; Reade, S.; Khalid, T.; Ratcliffe, N.M.; Probert, C.S.J. Freeze-drying: An alternative method for the analysis of volatile organic compounds in the headspace of urine samples using solid phase micro-extraction coupled to gas chromatography—Mass spectrometry. Chem. Cent. J. 2016, 10, 9. [Google Scholar] [CrossRef]
- Wiley Science Solutions. Wiley Registry/NIST Mass Spectral Library 2023; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2023. [Google Scholar]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- The MathWorks Inc. MATLAB, version: 24.2 (R2024a); The MathWorks Inc.: Natick, MA, USA, 2022. [Google Scholar]
- Li, H.; Liang, Y.; Xu, Q.; Cao, D. Key wavelengths screening using competitive adaptive reweighted sampling method for multivariate calibration. Anal. Chim. Acta 2009, 648, 77–84. [Google Scholar] [CrossRef]
- Tang, L.; Peng, S.; Bi, Y.; Shan, P.; Hu, X. A New Method Combining LDA and PLS for Dimension Reduction. PLoS ONE 2014, 9, e96944. [Google Scholar] [CrossRef]
- Li, H.-D.; Zeng, M.-M.; Tan, B.-B.; Liang, Y.-Z.; Xu, Q.-S.; Cao, D.-S. Recipe for revealing informative metabolites based on model population analysis. Metabolomics 2010, 6, 353–361. [Google Scholar] [CrossRef]
- Li, H.-D.; Xu, Q.-S.; Liang, Y.-Z. libPLS: An integrated library for partial least squares regression and linear discriminant analysis. Chemom. Intell. Lab. Syst. 2018, 176, 34–43. [Google Scholar] [CrossRef]
- Amann, A.; Costello, B.d.L.; Miekisch, W.; Schubert, J.; Buszewski, B.; Pleil, J.; Ratcliffe, N.; Risby, T. The human volatilome: Volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J. Breath Res. 2014, 8, 034001. [Google Scholar] [CrossRef] [PubMed]
- de Lacy Costello, B.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A review of the volatiles from the healthy human body. J. Breath Res. 2014, 8, 014001. [Google Scholar] [CrossRef] [PubMed]
- Daulton, E.; Wicaksono, A.N.; Tiele, A.; Kocher, H.M.; Debernardi, S.; Crnogorac-Jurcevic, T.; Covington, J.A. Volatile organic compounds (VOCs) for the non-invasive detection of pancreatic cancer from urine. Talanta 2021, 221, 121604. [Google Scholar] [CrossRef] [PubMed]
- Drabińska, N.; Flynn, C.; Ratcliffe, N.; Belluomo, I.; Myridakis, A.; Gould, O.; Fois, M.; Smart, A.; Devine, T.; Costello, B.P.J.d.L. A literature survey of all volatiles from healthy human breath and bodily fluids: The human volatilome. J. Breath Res. 2021, 15, 034001. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, R.; Varadwaj, P. Smelling the Disease: Diagnostic Potential of Breath Analysis. Mol. Diagn. Ther. 2023, 27, 321–347. [Google Scholar] [CrossRef]
- Grandjean, D.; Sarkis, R.; Lecoq-Julien, C.; Benard, A.; Roger, V.; Levesque, E.; Bernes-Luciani, E.; Maestracci, B.; Morvan, P.; Gully, E.; et al. Can the detection dog alert on COVID-19 positive persons by sniffing axillary sweat samples? A proof-of-concept study. PLoS ONE 2020, 15, e0243122. [Google Scholar] [CrossRef]
- Lamote, K.; Brinkman, P.; Vandermeersch, L.; Vynck, M.; Sterk, P.J.; Van Langenhove, H.; Thas, O.; Van Cleemput, J.; Nackaerts, K.; van Meerbeeck, J.P. Breath analysis by gas chromatography-mass spectrometry and electronic nose to screen for pleural mesothelioma: A cross-sectional case-control study. Oncotarget 2017, 8, 91593–91602. [Google Scholar] [CrossRef]
- Teresa, R.-C.M.; Rosaura, V.-G.; Elda, C.-M.; Ernesto, G.-P. The avocado defense compound phenol-2,4-bis (1,1-dimethylethyl) is induced by arachidonic acid and acts via the inhibition of hydrogen peroxide production by pathogens. Physiol. Mol. Plant Pathol. 2014, 87, 32–41. [Google Scholar] [CrossRef]
- Marais, J. 1, 1,6-Trimethyl-1,2-dihydronaphthalene (TDN): A Possible Degradation Product of Lutein and beta-Carotene. S. Afr. J. Enol. Vitic. 1992, 13, 52–55. [Google Scholar] [CrossRef][Green Version]
- Ramos, G.; Antón, A.P.; Sánchez, M.d.N.; Pavón, J.L.P.; Cordero, B.M. Urinary volatile fingerprint based on mass spectrometry for the discrimination of patients with lung cancer and controls. Talanta 2017, 174, 158–164. [Google Scholar] [CrossRef]
- Wu, R.; Waidyanatha, S.; Henderson, A.P.; Serdar, B.; Zheng, Y.; Rappaport, S.M. Determination of dihydroxynaphthalenes in human urine by gas chromatography–mass spectrometry. J. Chromatogr. B 2005, 826, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Agapiou, A.; Amann, A.; Mochalski, P.; Statheropoulos, M.; Thomas, C. Trace detection of endogenous human volatile organic compounds for search, rescue and emergency applications. TrAC Trends Anal. Chem. 2015, 66, 158–175. [Google Scholar] [CrossRef]
- Broza, Y.Y.; Mochalski, P.; Ruzsanyi, V.; Amann, A.; Haick, H. Hybrid Volatolomics and Disease Detection. Angew. Chem. Int. Ed. 2015, 54, 11036–11048. [Google Scholar] [CrossRef] [PubMed]
- Lett, L.; George, M.; Slater, R.; Costello, B.D.L.; Ratcliffe, N.; García-Fiñana, M.; Lazarowicz, H.; Probert, C. Investigation of urinary volatile organic compounds as novel diagnostic and surveillance biomarkers of bladder cancer. Br. J. Cancer 2022, 127, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Fujita, A.; Ihara, K.; Kawai, H.; Obuchi, S.; Watanabe, Y.; Hirano, H.; Fujiwara, Y.; Takeda, Y.; Tanaka, M.; Kato, K. A novel set of volatile urinary biomarkers for late-life major depressive and anxiety disorders upon the progression of frailty: A pilot study. Discov. Ment. Health 2022, 2, 20. [Google Scholar] [CrossRef]
- Veeravalli, S.; Scott, F.H.; Varshavi, D.; Pullen, F.S.; Veselkov, K.; Phillips, I.R.; Everett, J.R.; Shephard, E.A. Treatment of wild-type mice with 2,3-butanediol, a urinary biomarker of Fmo5−/− mice, decreases plasma cholesterol and epididymal fat deposition. Front. Physiol. 2022, 13, 859681. [Google Scholar] [CrossRef]
- Tang, C.; Wang, M.; Liu, J.; Zhang, C.; Li, L.; Wu, Y.; Chu, Y.; Wu, D.; Liu, H.; Yuan, X. A Cyclopentanone Compound Attenuates the Over-Accumulation of Extracellular Matrix and Fibrosis in Diabetic Nephropathy via Downregulating the TGF-β/p38MAPK Axis. Biomedicines 2022, 10, 3270. [Google Scholar] [CrossRef]
- Barupal, D.K.; Fiehn, O. Generating the Blood Exposome Database Using a Comprehensive Text Mining and Database Fusion Approach. Environ. Health Perspect. 2019, 127, 97008. [Google Scholar] [CrossRef]
- Meldau, D.G.; Meldau, S.; Hoang, L.H.; Underberg, S.; Wunsche, H.; Baldwin, I.T. Dimethyl disulfide produced by the naturally associated bacterium bacillus sp b55 promotes nicotiana attenuata growth by enhancing sulfur nutrition. Plant Cell 2013, 25, 2731–2747. [Google Scholar] [CrossRef]
- Thorn, R.M.S.; Greenman, J. Microbial volatile compounds in health and disease conditions. J. Breath Res. 2012, 6, 024001. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, J.; Jin, X.; You, J.; Feng, K.; Ye, J.; Chen, J.; Zhang, S. Superior dimethyl disulfide degradation in a microbial fuel cell: Extracellular electron transfer and hybrid metabolism pathways. Environ. Pollut. 2022, 315, 120469. [Google Scholar] [CrossRef] [PubMed]
- Taunk, K.; Porto-Figueira, P.; Pereira, J.A.M.; Taware, R.; da Costa, N.L.; Barbosa, R.; Rapole, S.; Câmara, J.S. Urinary Volatomic Expression Pattern: Paving the Way for Identification of Potential Candidate Biosignatures for Lung Cancer. Metabolites 2022, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, S.; Li, Y.; Yu, L.; Zhao, Y.; Wu, Z.; Fan, Y.; Li, X.; Wang, Y.; Zhang, X.; et al. Identification of urinary volatile organic compounds as a potential non-invasive biomarker for esophageal cancer. Sci. Rep. 2023, 13, 18587. [Google Scholar] [CrossRef] [PubMed]


| Healthy Controls (n = 32) | COVID-19 Patients (n = 35) | |
|---|---|---|
| Age [Median (IQR)] | 52.5 (19.1) | 59 (20.0) |
| Sex, distribution | ||
| Male [n (%)] | 9 (28.1) | 11 (31.4) |
| Female [n (%)] | 23 (71.9) | 24 (68.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narro-Serrano, J.; Shalabi-Benavent, M.; Álamo-Marzo, J.M.; Seijo-García, Á.M.; Marhuenda-Egea, F.C. Analysis of the Urine Volatilome of COVID-19 Patients and the Possible Metabolic Alterations Produced by the Disease. Metabolites 2024, 14, 638. https://doi.org/10.3390/metabo14110638
Narro-Serrano J, Shalabi-Benavent M, Álamo-Marzo JM, Seijo-García ÁM, Marhuenda-Egea FC. Analysis of the Urine Volatilome of COVID-19 Patients and the Possible Metabolic Alterations Produced by the Disease. Metabolites. 2024; 14(11):638. https://doi.org/10.3390/metabo14110638
Chicago/Turabian StyleNarro-Serrano, Jennifer, Maruan Shalabi-Benavent, José María Álamo-Marzo, Álvaro Maximiliam Seijo-García, and Frutos Carlos Marhuenda-Egea. 2024. "Analysis of the Urine Volatilome of COVID-19 Patients and the Possible Metabolic Alterations Produced by the Disease" Metabolites 14, no. 11: 638. https://doi.org/10.3390/metabo14110638
APA StyleNarro-Serrano, J., Shalabi-Benavent, M., Álamo-Marzo, J. M., Seijo-García, Á. M., & Marhuenda-Egea, F. C. (2024). Analysis of the Urine Volatilome of COVID-19 Patients and the Possible Metabolic Alterations Produced by the Disease. Metabolites, 14(11), 638. https://doi.org/10.3390/metabo14110638







