Immunohistochemical Localization of Fibroblast Activation Protein in Coronary Arteries with Different Forms of Atherosclerosis
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. The FAP Expression in Human Coronaries Without and with Coronary Plaques with Different Levels of Atherosclerosis Progression Was Examined. FAP Staining Was Absent in Cross-Sections from Coronary Vessels with No Atherosclerosis, Whereas a Gradual Increase in Staining Was Observed with the Progression of Atherosclerotic Plaques
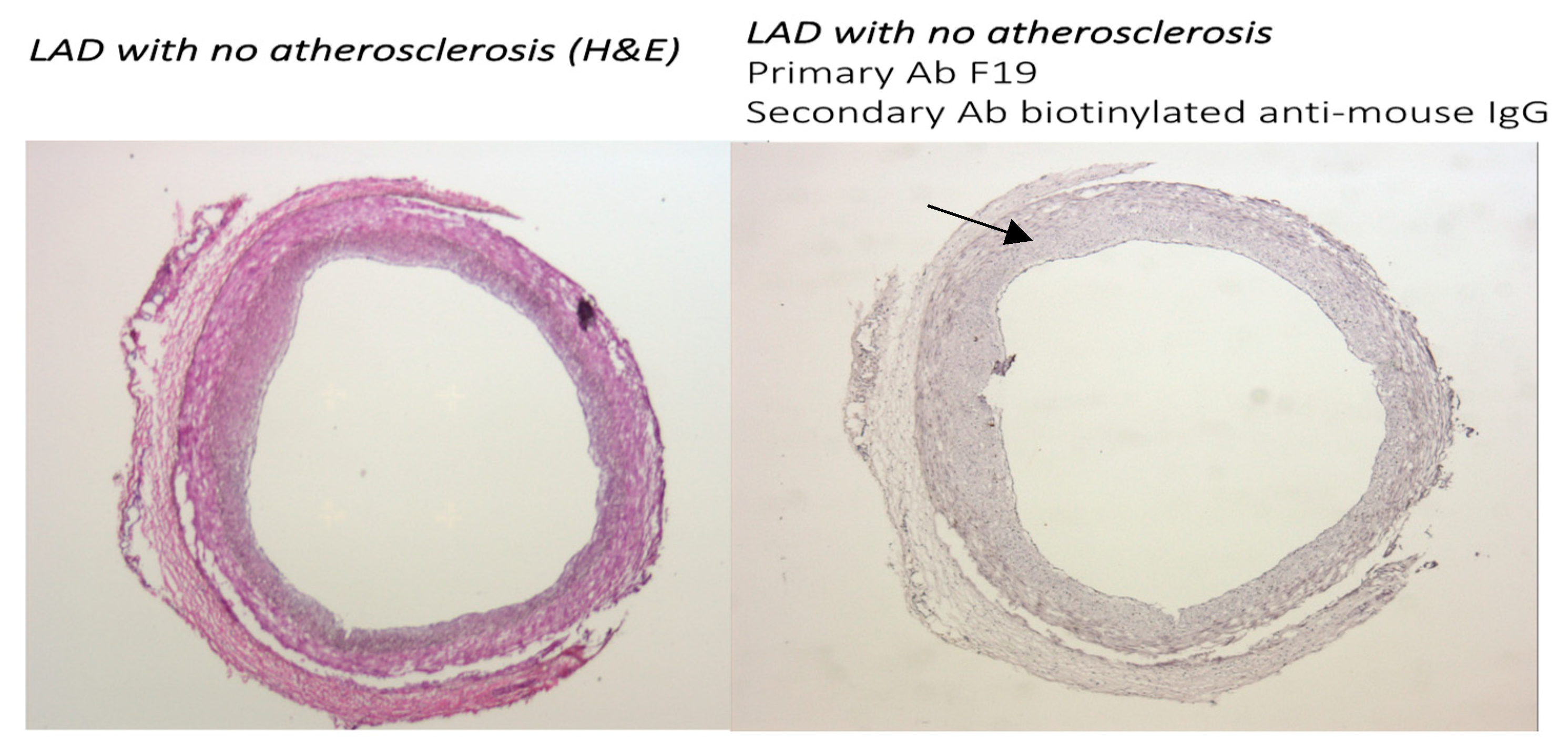
3.1.1. FAP Staining in Coronary Vessels with No Atherosclerosis and with Coronary Artery Disease
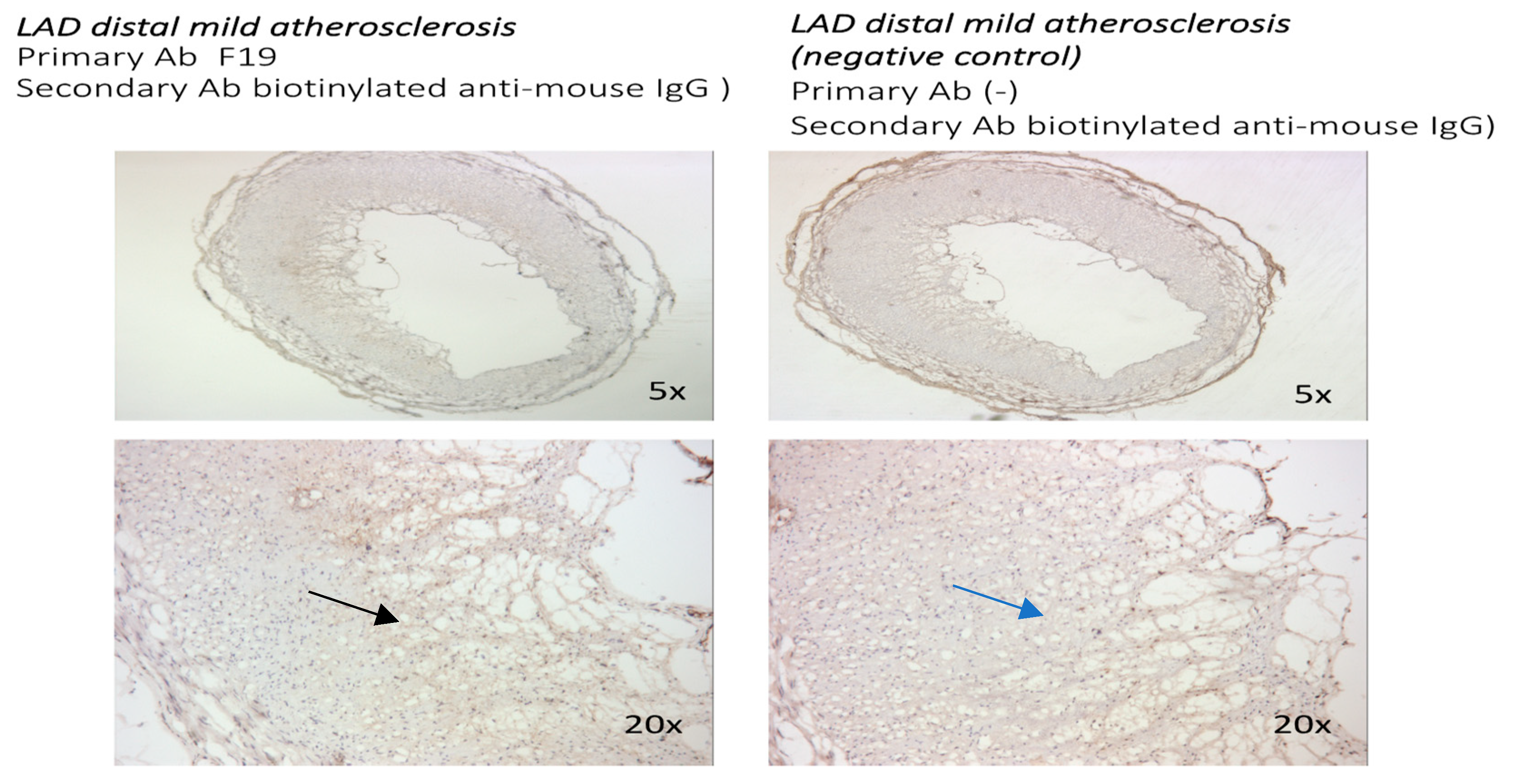
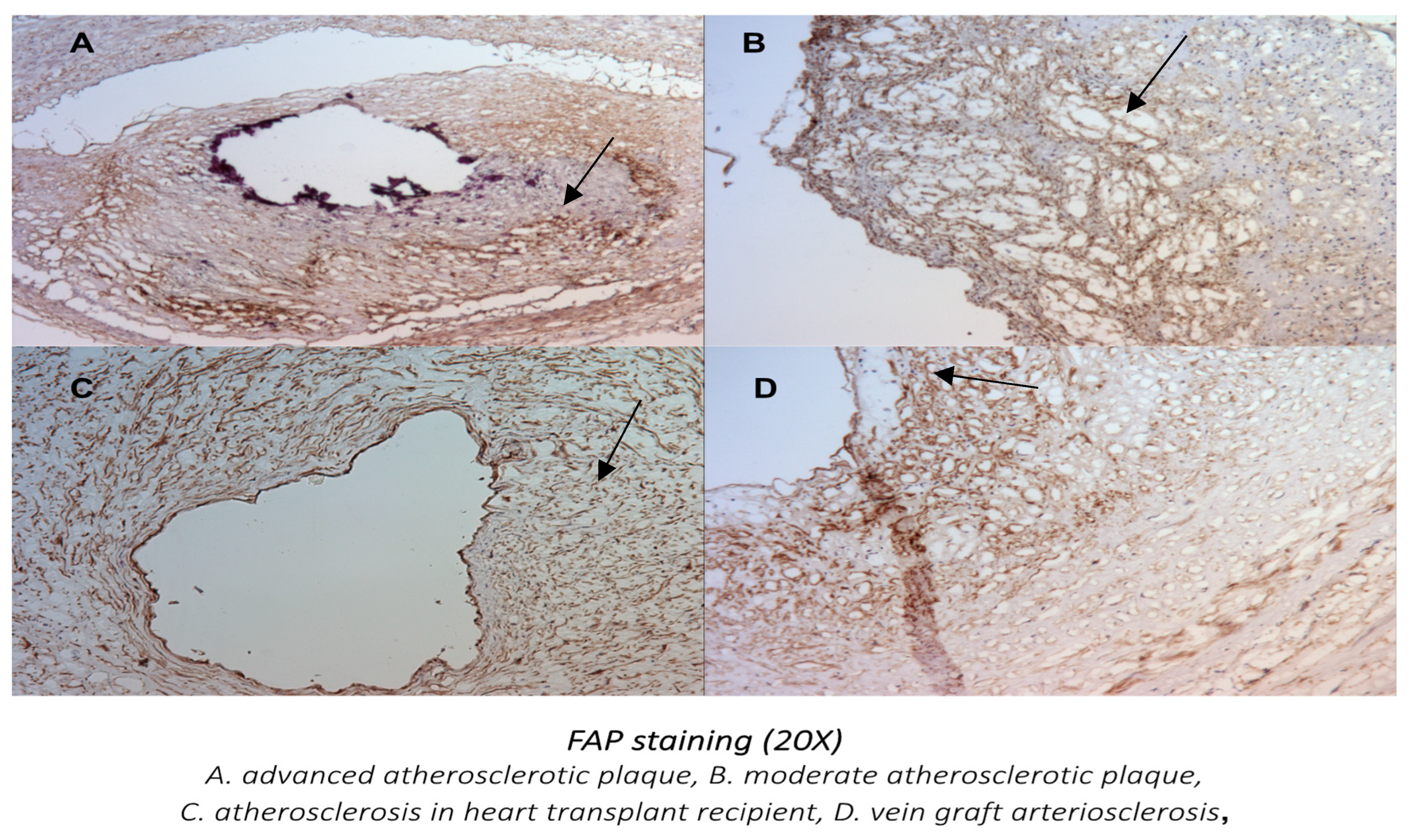
3.1.2. Localization of FAP Staining in Advanced Atherosclerotic Plaques
3.2. The Presence of FAP Was Also Examined in Other Distinct Models of Atherosclerosis: The Sections of Coronaries in Post Bypass Atherosclerosis (Figure 5) and Cardiac Allograph Vasculopathy (Figure 6) Both Showed Positive and Distinct FAP Staining Compared with Native Coronary Artery Disease
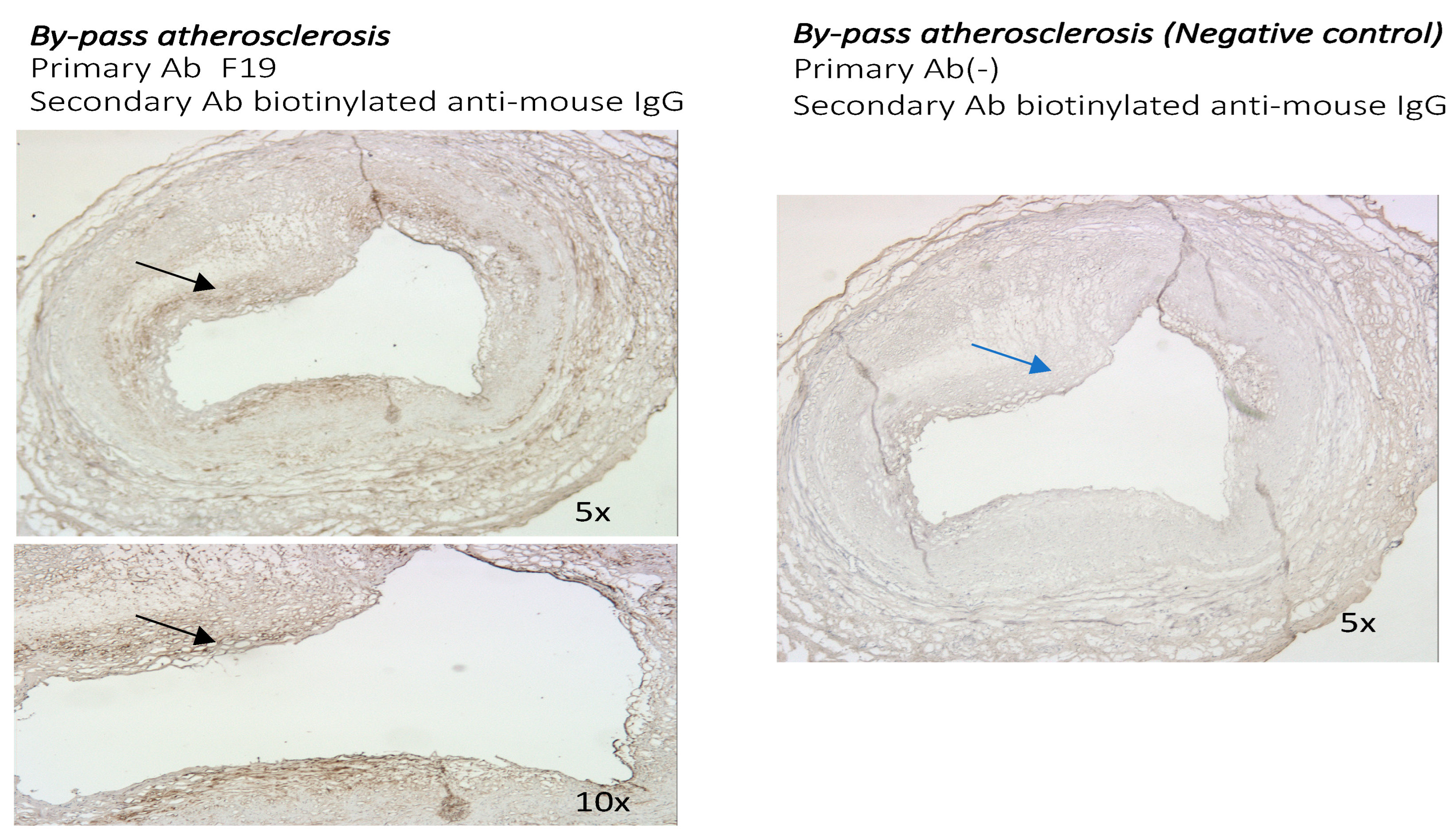
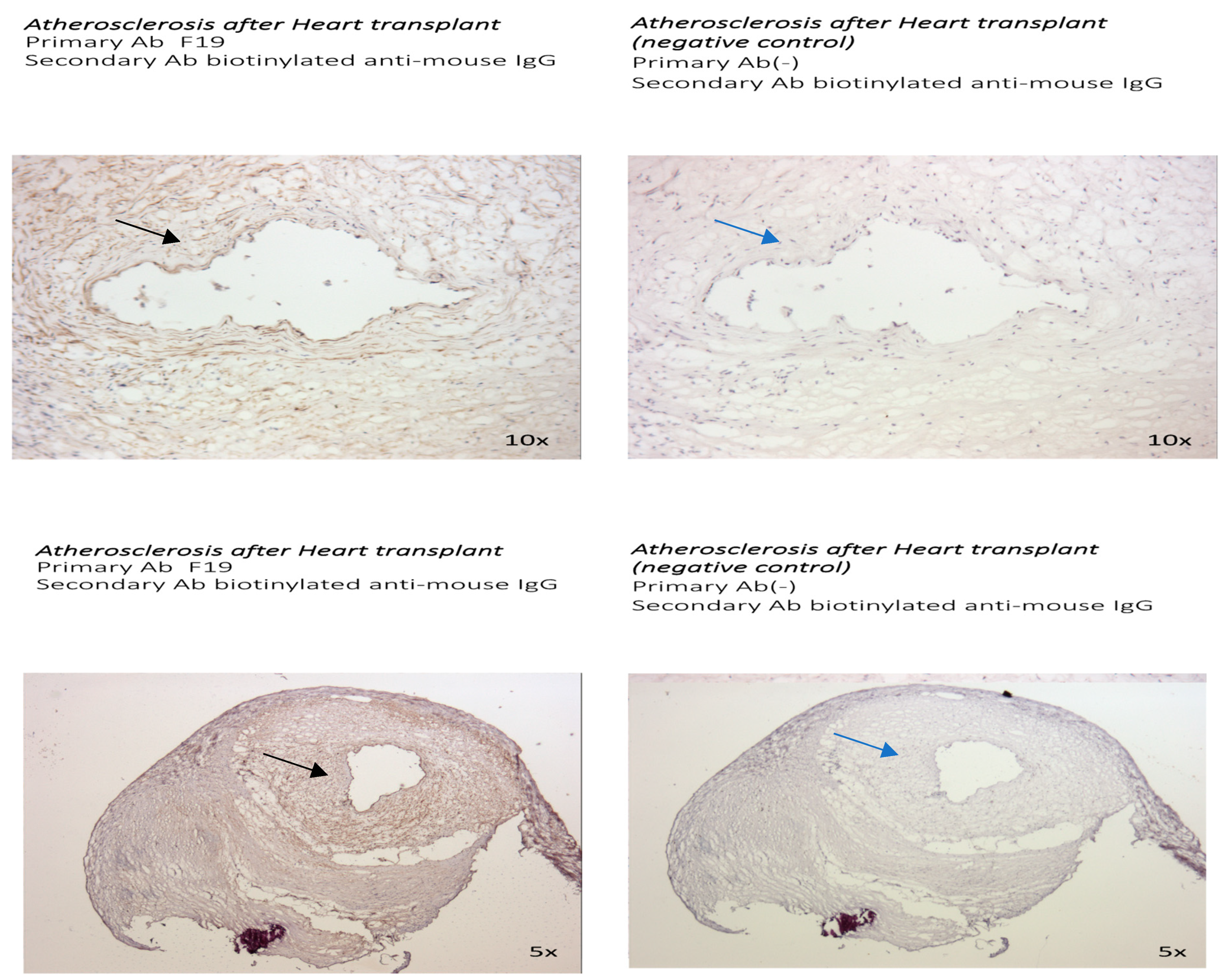
3.2.1. The Presence and Localization of FAP Staining in Postbypass Coronary Atherosclerosis and Heart Transplant Arteriosclerosis
3.2.2. Comparison of FAP Staining in Native Coronary Atherosclerosis with Other Forms of Coronary Artery Disease
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Del Monte-Nieto, G.; Fischer, J.W.; Gorski, D.J.; Harvey, R.P.; Kovacic, J.C. Basic Biology of Extracellular Matrix in the Cardiovascular System, Part 1/4 JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 2169–2188. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G.; Kovacic, J.C. Extracellular Matrix in Ischemic Heart Disease, Part 4/4 JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 2219–2235. [Google Scholar] [CrossRef] [PubMed]
- Shami, A.; Gonçalves, I.; Hultgårdh-Nilsson, A. Collagen and related extracellular matrix proteins in atherosclerotic plaque development. Curr. Opin. Lipidol. 2014, 25, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Raines, E.W. The extracellular matrix can regulate vascular cell migration, proliferation, and survival: Relationships to vascular disease. Int. J. Exp. Pathol. 2000, 81, 173–182. [Google Scholar] [CrossRef]
- Rekhter, M.D. Collagen synthesis in atherosclerosis: Too much and not enough. Cardiovasc. Res. 1999, 41, 376–384. [Google Scholar] [CrossRef]
- Avery, D.; Govindaraju, P.; Jacob, M.; Todd, L.; Monslow, J.; Puré, E. Extracellular matrix directs phenotypic heterogeneity of activated fibroblasts. Matrix Biol. 2018, 67, 90–106. [Google Scholar] [CrossRef]
- MacLean, J.; Pasumarthi, K.B. Signaling mechanism regulating fibroblast activation, phenoconversion and fibrosis in the heart. Indian J. Biochem. Biophys. 2014, 51, 476–482. [Google Scholar]
- Díaz-Araya, G.; Vivar, R.; Humeres, C.; Boza, P.; Bolivar, S.; Muñoz, C. Cardiac fibroblasts as sentinel cells in cardiac tissue: Receptors, signaling pathways and cellular functions. Pharmacol. Res. 2015, 101, 30–40. [Google Scholar] [CrossRef]
- Christiansen, V.J.; Jackson, K.W.; Lee, K.N.; KcKnee, P. Effect of fibroblast activation protein and al-pha2-antiplasmin cleaving enzyme on collagen types I, III and IV. Arch. Biochem. Biophys. 2007, 457, 177–186. [Google Scholar] [CrossRef]
- Edosada, C.Y.; Quan, C.; Wiesmann, C.; Tran, T.; Sutherlin, D.; Reynolds, M.; Elliott, J.M.; Raab, H.; Fairbrother, W.; Wolf, B.B. Selective inhibition of fibroblast acti-vation protein protease based on dipeptide substrate specificity. J. Biol. Chem. 2006, 281, 7437–7444. [Google Scholar] [CrossRef]
- Zhang, H.E.; Gorrell, M. Identification of Novel Natural Substrates of Fibroblast Activation Protein by Differential Degradomics and Proteomics. Mol. Cell. Proteom. 2019, 18, 65–85. [Google Scholar] [CrossRef] [PubMed]
- Ghersi, G.; Dong, H.; Goldstein, L.A.; Yunyun, Y. Regulation of fibroblast migration on collagenous matrix by a cell surface peptidase complex. J. Biol. Chem. 2002, 277, 29231–29241. [Google Scholar] [CrossRef]
- Kong, Y.-Z.; Yu, X.; Tang, J.-J.; Ouyang, X.; Huang, X.-R.; Fingerle-Rowson, G.; Bacher, M.; Scher, L.A.; Bucala, R.; Lan, H.Y. Macrophage migration inhibitory factor induces mmp-9 expression: Implications for destabilization of human atherosclerotic plaques. Atherosclerosis 2005, 178, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Brokopp, C.E.; Schoenauer, R.; Richards, P.; Bauer, S.; Lohmann, C.; Emmert, M.Y.; Weber, B.; Winnik, S.; Aikawa, E.; Graves, K.; et al. Fibroblast activation protein is induced by inflammation and de-grades type I collagen in thin cap fibroatheromata. Eur. Heart J. 2011, 32, 2713–2722. [Google Scholar] [CrossRef] [PubMed]
- Mohmand-Borkowski, A.; Rozmyslowicz, T. Expression of fibroblast activation protein in human coronary vessels. Pol. Med. J. 2021, 49, 5–8. [Google Scholar]
- Meletta, R.; Muller Herde, A.; Chiotellis, A.; Isa, M.; Rancic, Z.; Borel, N.; Ametamey, S.M.; Krämer, S.D.; Schibli, R. Evaluation of the Radiolabeled Boronic Acid-Based FAP Inhibitor MIP-1232 for Atherosclerotic Plaque Imaging. Molecules 2015, 20, 2081–2099. [Google Scholar] [CrossRef]
- Diekmann, J.; Koenig, T.; Thackeray, J.T.; Derlin, T.; Czerner, C.; Neuser, J.; Ross, T.L.; Schäfer, A.; Tillmanns, J.; Bauersachs, J.; et al. Cardiac Fibroblast Activation in Patients Early After Acute Myocardial Infarction: Integration with MR Tissue Characterization and Subsequent Functional Outcome. J. Nucl. Med. 2022, 63, 1415–1423. [Google Scholar] [CrossRef]
- Vukovic, I.; Arsenijevic, N.; Lackovic, V.; Todorovic, V. The origin and differentiation potential of smooth muscle cells in coronary atherosclerosis. Exp. Clin. Cardiol. 2006, 11, 123–128. [Google Scholar]
- Besametur, G.L.; Joegensen, H.F.; Clarke, M.C.H.; Bennett, M.; Mallat, Z. Vascular smooth muscle cells in atherosclerosis. Nat. Rev. Cardiol. 2019, 16, 727–744. [Google Scholar]
- Bennett, M.; Sinha, S.; Owen, G. Vascular smooth muscle cells in atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef]
- Hu, D.; Yin, C.; Luo, S.; Habenicht, A.J.R.; Mohanta, S.K. Vascular Smooth Muscle Cells Contribute to Atherosclerosis Immunity. Front. Immunol. 2019, 10, 1101. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.; Owens, G.K. Reconciling smooth muscle cell oligoclonality and proliferative capacity in experimental atherosclerosis. Circ. Res. 2016, 119, 1262–1264. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.; Bernardo, A.S.; Trotter, M.; Pedersen, R.A.; Sinha, S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin–dependent disease susceptibility. Nat. Biotechnol. 2012, 30, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Uryga, A.; Reinhold, J.; Figg, N.; Baker, L.; Finigan, A.; Gray, K.; Kumar, S.; Clarke, M.; Bennett, M. Vascular smooth muscle cell senescence promotes atherosclerosis and features of plaque vulnerability. Circulation 2015, 132, 1909–1919. [Google Scholar] [CrossRef]
- Rensen, S.S.M.; Doevendans, P.A.F.M.; van Eys, G.J.J.M. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth. Heart J. 2007, 15, 100–108. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Xie, B.-D.; Sun, L.; Chen, W.; Jiang, S.-L.; Liu, W.; Bian, F.; Tian, H.; Li, R.-K. Phenotypic Switching of Vascular Smooth Muscle Cells in the ‘Normal Region’ of Aorta From Atherosclerosis Patients Is Regulated by miR-145. Cell. Mol. Med. 2016, 20, 1049–1061. [Google Scholar] [CrossRef]
- Allahverdian, S.; Chaabene, C.h.; Boukais, K. Smooth muscle cell fate and plasticity in atherosclerosis. Cardiovasc. Res. 2018, 114, 540–550. [Google Scholar] [CrossRef]
- Diekmann, J.; Koenig, T.; Zwadlo, C.; Derlin, T.; Neuser, J.; Thackeray, J.T.; Schäfer, A.; Ross, T.L.; Bauersachs, J.; Bengel, F.M. Molecular Imaging Identifies Fibroblast Activation Beyond the Infarct Region After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2021, 77, 1835–1837. [Google Scholar] [CrossRef]
- Hernandez, J.; Rosas, E.A.; Juarez, E.I.C.; Ferro-Flores, G.; Cuevas, C.L.S.; Luna-Gutiérrez, M.; Ramírez, G.M.; Ahumada, J.V.; Sandoval, S.H.; León, N.C.; et al. Cardiac fibroblast activation after acute coronary syndrome detected by 99MTCIFAP spect, multimodality approach and follow up. J. Am. Coll. Cardiol. 2024, 83, 4402. [Google Scholar] [CrossRef]
- Diekmann, J.; Bengel, F.M. Cardiac Applications of Fibroblast Activation Protein Imaging. PET Clin. 2023, 18, 389–396. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Y.; Wang, S.; Du, B.; Li, X.; Li, Y. Highlighting Fibroblasts Activation in Fibrosis: The State-of-The-Art Fibroblast Activation Protein Inhibitor PET Imaging in Cardiovascular Diseases. J. Clin. Med. 2023, 12, 6033. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, D.B.; Fraccarollo, D.; Galuppo, P.; Frantz, S.; Bauersachs, J.; Tillmanns, J. Genetic ablation of fibroblast activation protein alpha attenuates left ventricular dilation after myocardial infarction. PLoS ONE 2021, 16, e0248196. [Google Scholar] [CrossRef] [PubMed]
- Rurik, J.G.; Tombácz, I.; Yadegari, A.; Fernández, P.O.M.; Shewale, S.V.; Li, L.; Kimura, T.; Soliman, O.Y.; Papp, T.E.; Tam, Y.K.; et al. CAR T cells produced in vivo to treat cardiac injury. Science 2022, 375, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ma, M.; Cao, D.; Zheng, A.; Zhang, Y.; Su, Y.; Wang, J.; Xu, Y.; Zhou, M.; Tang, Y.; et al. Inhibition of Fap Promotes Cardiac Repair by Stabilizing BNP. Circ. Res. 2023, 132, 586–600. [Google Scholar] [CrossRef] [PubMed]
- Wadey, K.; Lopes, J.; Bendeck, M.; George, S. Role of smooth muscle cells in coronary artery bypass grafting failure. Cardiovasc. Res. 2018, 114, 601–610. [Google Scholar] [CrossRef]
- Abazid, R.M.; Romsa, J.G.; Akincioglu, C.; Warrington, J.C.; Bureau, Y.; Kiaii, B.; Vezina, W.C. Coronary calcium progression after bypass grafting surgery. Open Heart 2021, 8, e001684. [Google Scholar] [CrossRef]
- Atkinson, C.; Horsley, J.; Rhind-Tutt, S.; Charman, S.; Phillpotts, C.J.; Wallwork, J.; Goddard, M.J. Neointimal smooth muscle cells in human cardiac allograft coronary artery vasculopathy are of donor origin. J. Heart Lung Transplant. 2004, 23, 427–435. [Google Scholar] [CrossRef]
- Habibi, S.; Ghaffarpasand, E.; Shojaei, F.; Alihashemi, M.; Kahe, F.; Tajrishi, F.Z.; Chi, G. Prognostic value of Biomarkers in Cardiac Vasculopathy following Heart Transplantation: A Literature Review. Cardiology 2020, 145, 693–702. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohmand-Borkowski, A.; Rozmyslowicz, T. Immunohistochemical Localization of Fibroblast Activation Protein in Coronary Arteries with Different Forms of Atherosclerosis. Metabolites 2024, 14, 573. https://doi.org/10.3390/metabo14110573
Mohmand-Borkowski A, Rozmyslowicz T. Immunohistochemical Localization of Fibroblast Activation Protein in Coronary Arteries with Different Forms of Atherosclerosis. Metabolites. 2024; 14(11):573. https://doi.org/10.3390/metabo14110573
Chicago/Turabian StyleMohmand-Borkowski, Adam, and Tomasz Rozmyslowicz. 2024. "Immunohistochemical Localization of Fibroblast Activation Protein in Coronary Arteries with Different Forms of Atherosclerosis" Metabolites 14, no. 11: 573. https://doi.org/10.3390/metabo14110573
APA StyleMohmand-Borkowski, A., & Rozmyslowicz, T. (2024). Immunohistochemical Localization of Fibroblast Activation Protein in Coronary Arteries with Different Forms of Atherosclerosis. Metabolites, 14(11), 573. https://doi.org/10.3390/metabo14110573




