Metabolite Biomarkers of Prolonged and Intensified Pain and Distress in Head and Neck Cancer Patients Undergoing Radio- or Chemoradiotherapy by Means of NMR-Based Metabolomics—A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of the Patient Groups
2.2. Distress and Pain Measurements
2.3. Serum Samples Collection
2.4. Sample Preparation for NMR Spectroscopy
2.5. Measurement Protocol and Quality Control
2.6. Spectra Post-Processing
2.7. Metabolite Identification
2.8. Metabolite Quantification
2.9. Data Analysis and the Validation of the Multivariate Model
3. Results
- -
- WOD (weeks of distress)—the number of weeks during RT/CHRT when the distress was >0;
- -
- MD (maximum of distress)—a maximum value of the distress during RT/CHRT;
- -
- WOP (weeks of pain)—the number of weeks during RT/CHRT when the pain was >0;
- -
- MP (maximum of pain)—a maximum value of the pain during RT/CHRT.
3.1. Multivariate Modelling
- -
- L: Low (<median value),
- -
- H: High (≥median value).
3.2. Correlations between Distress and Pain
- -
- The duration (WOD) and the intensity (MD) of the distress;
- -
- The duration (WOP) and the intensity (MP) of the pain;
- -
- The intensity of the distress (MD) and the pain (MP).
| R | RT | CHRT | ||||||
|---|---|---|---|---|---|---|---|---|
| WOD | MD | WOP | MP | WOD | MD | WOP | MP | |
| WOD | 0.42 | 0.35 | 0.18 | 0.57 | 0.23 | 0.36 | ||
| MD | 0.20 | 0.48 | 0.29 | 0.59 | ||||
| WOP | 0.32 | 0.45 | ||||||
4. Discussion
4.1. The Altered Metabolites Grouped According to Their Class and/or Participation in Specific Metabolic Processes
4.1.1. Lipids
4.1.2. Glutamine, Glucose and Other Metabolites of Energy Metabolism
4.1.3. Metabolites of One-Carbon Metabolism
4.1.4. Metabolites of Protein Metabolism and Oxidative Stress
4.1.5. Branched-Chain Amino Acids (BCAAs)
4.2. Correlations between the Analyzed Groups
4.3. Limitations of the Study
5. Conclusions
- -
- Primarily affect plasma lipids, and this effect, seen as an increase in the integral in-tensities of the lipid signals, is particularly intense during prolonged stress (OPLS-DA p(corr) values from 0.35 to 0.54);
- -
- Disturb energy metabolism by strong alterations in the glutamine levels;
- -
- Impact one-carbon metabolism (the prolonged distress and pain reduce the levels of glycine, serine and methanol, the intensified distress and prolonged pain alter the levels of threonine and histidine, while the intensified pain increases the levels of betaine).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cramer, J.D.; Johnson, J.T.; Nilsen, M.L. Pain in Head and Neck Cancer Survivors: Prevalence, Predictors, and Quality-of-Life Impact. Otolaryngol. Head Neck Surg. 2018, 159, 853–858. [Google Scholar] [CrossRef]
- Gane, E.M.; Michaleff, Z.A.; Cottrell, M.A.; McPhail, S.M.; Hatton, A.L.; Panizza, B.J. Prevalence, incidence, and risk factors for shoulder and neck dysfunction after neck dissection: A systematic review. Eur. J. Surg. Oncol. 2017, 43, 1199–1218. [Google Scholar] [CrossRef] [PubMed]
- Low, C.; Fullarton, M.; Parkinson, E.; O’Brien, K.; Jackson, S.R.; Lowe, D.; Rogers, S.N. Issues of intimacy and sexual dysfunction following major head and neck cancer treatment. Oral Oncol. 2009, 45, 898–903. [Google Scholar] [CrossRef]
- Wan Leung, S.; Lee, T.F.; Chien, C.Y.; Chao, P.J.; Tsai, W.L.; Fang, F.M. Health-related quality of life in 640 head and neck cancer survivors after radiotherapy using EORTC QLQ-C30 and QLQ-H&N35 questionnaires. BMC Cancer 2011, 11, 128. [Google Scholar] [CrossRef]
- Henry, M.; Rosberger, Z.; Bertrand, L.; Klassen, C.; Hier, M.; Zeitouni, A.; Kost, K.; Mlynarek, A.; Richardson, K.; Black, M.; et al. Prevalence and Risk Factors of Suicidal Ideation among Patients with Head and Neck Cancer: Longitudinal Study. Otolaryngol. Head Neck Surg. 2018, 159, 843–852. [Google Scholar] [CrossRef]
- Nayak, S.G.; Sharan, K.; Chakrabarty, J.; Devi, E.S.; Ravishankar, N.; George, A. Psychosocial Distress of Head Neck Cancer (HNC) Patients Receiving Radiotherapy: A Systematic Review. Asian Pac. J. Cancer Prev. 2022, 23, 1827–1835. [Google Scholar] [CrossRef]
- Osazuwa-Peters, N.; Simpson, M.C.; Zhao, L.; Boakye, E.A.; Olomukoro, S.I.; Deshields, T.; Loux, T.M.; Varvares, M.A.; Schootman, M. Suicide risk among cancer survivors: Head and neck versus other cancers. Cancer 2018, 124, 4072–4079. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, X.; Kong, X.; Wang, Z.; Zhu, M.; Ren, Y.; Dong, H.; Fang, Y.; Wang, J. Subsequent risk of suicide among 9,300,812 cancer survivors in US: A population-based cohort study covering 40 years of data. eClinicalMedicine 2022, 44, 101295. [Google Scholar] [CrossRef] [PubMed]
- Oskam, I.M.; Verdonck-de Leeuw, I.M.; Aaronson, N.K.; Kuik, D.J.; de Bree, R.; Doornaert, P.; Langendijk, J.A.; Leemans, C.R. Quality of life as predictor of survival: A prospective study on patients treated with combined surgery and radiotherapy for advanced oral and oropharyngeal cancer. Radiother Oncol. 2010, 97, 258–262. [Google Scholar] [CrossRef]
- Scharpf, J.; Karnell, L.H.; Christensen, A.J.; Funk, G.F. The role of pain in head and neck cancer recurrence and survivorship. Arch. Otolaryngol. Head Neck Surg. 2009, 135, 789–794. [Google Scholar] [CrossRef]
- Karvonen-Gutierrez, C.A.; Ronis, D.L.; Fowler, K.E.; Terrell, J.E.; Gruber, S.B.; Duffy, S.A. Quality of life scores predict survival among patients with head and neck cancer. J. Clin. Oncol. 2008, 26, 2754–2760. [Google Scholar] [CrossRef] [PubMed]
- Morton, R.P.; Davies, A.D.; Baker, J.; Baker, G.A.; Stell, P.M. Quality of life in treated head and neck cancer patients: A preliminary report. Clin. Otolaryngol. Allied Sci. 1984, 9, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Van der Elst, S.; Bardash, Y.; Wotman, M.; Kraus, D.; Tham, T. The prognostic impact of depression or depressive symptoms on patients with head and neck cancer: A systematic review and meta-analysis. Head Neck 2021, 43, 3608–3617. [Google Scholar] [CrossRef] [PubMed]
- Jansen, F.; Verdonck-de Leeuw, I.M.; Cuijpers, P.; Leemans, C.R.; Waterboer, T.; Pawlita, M.; Penfold, C.; Thomas, S.J.; Waylen, A.; Ness, A.R. Depressive symptoms in relation to overall survival in people with head and neck cancer: A longitudinal cohort study. Psychooncology 2018, 27, 2245–2256. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, T.V.; Wirth, T.; Ranasinghe, S.; Ah-See, K.W.; Renny, N.; Hurman, D. Head and neck cancer pain: Systematic review of prevalence and associated factors. J. Oral Maxillofac. Res. 2012, 3, e1. [Google Scholar] [CrossRef] [PubMed]
- Løke, D.; Løvstad, M.; Andelic, N.; Andersson, S.; Ystrom, E.; Vassend, O. The role of pain and psychological distress in fatigue: A co-twin and within-person analysis of confounding and causal relations. Health Psychol. Behav. Med. 2022, 10, 160–179. [Google Scholar] [CrossRef] [PubMed]
- Thaker, P.H.; Lutgendorf, S.K.; Sood, A.K. The neuroendocrine impact of chronic stress on cancer. Cell Cycle 2007, 6, 430–433. [Google Scholar] [CrossRef]
- Glaser, R.; Kiecolt-Glaser, J.K. Stress-induced immune dysfunction: Implications for health. Nat. Rev. Immunol. 2005, 5, 243–251. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, L.; Zhang, X.; Yang, X.; Niedermann, G.; Xue, J. Psychological distress and eustress in cancer and cancer treatment: Advances and perspectives. Sci. Adv. 2022, 8, eabq7982. [Google Scholar] [CrossRef]
- Ting, E.Y.; Yang, A.C.; Tsai, S.J. Role of Interleukin-6 in Depressive Disorder. Int. J. Mol. Sci. 2020, 21, 2194. [Google Scholar] [CrossRef]
- Bernabé, D.G.; Tamae, A.C.; Biasoli, É.R.; Oliveira, S.H. Stress hormones increase cell proliferation and regulates interleukin-6 secretion in human oral squamous cell carcinoma cells. Brain Behav. Immun. 2011, 25, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Li, C.; He, Y.; Griffin, R.; Ye, Q.; Li, L. Chronic stress promotes oral cancer growth and angiogenesis with increased circulating catecholamine and glucocorticoid levels in a mouse model. Oral Oncol. 2015, 51, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Uz, U.; Eskiizmir, G. Association Between Interleukin-6 and Head and Neck Squamous Cell Carcinoma: A Systematic Review. Clin. Exp. Otorhinolaryngol. 2021, 14, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Deshields, T.L.; Wells-Di Gregorio, S.; Flowers, S.R.; Irwin, K.E.; Nipp, R.; Padgett, L.; Zebrack, B. Addressing distress management challenges: Recommendations from the consensus panel of the American Psychosocial Oncology Society and the Association of Oncology Social Work. CA Cancer J. Clin. 2021, 71, 407–436. [Google Scholar] [CrossRef]
- Jadoon, N.A.; Munir, W.; Shahzad, M.A.; Choudhry, Z.S. Assessment of depression and anxiety in adult cancer outpatients: A cross-sectional study. BMC Cancer 2010, 10, 594. [Google Scholar] [CrossRef] [PubMed]
- Park, E.M.; Deal, A.M.; Check, D.K.; Hanson, L.C.; Reeder-Hayes, K.E.; Mayer, D.K.; Yopp, J.M.; Song, M.K.; Muriel, A.C.; Rosenstein, D.L. Parenting concerns, quality of life, and psychological distress in patients with advanced cancer. Psychooncology 2016, 25, 942–948. [Google Scholar] [CrossRef]
- Park, S.; Kang, C.H.; Hwang, Y.; Seong, Y.W.; Lee, H.J.; Park, I.K.; Kim, Y.T. Risk factors for postoperative anxiety and depression after surgical treatment for lung cancer. Eur. J. Cardio-Thorac. Surg. 2016, 49, e16–e21. [Google Scholar] [CrossRef]
- Floriou-Servou, A.; von Ziegler, L.; Waag, R.; Schläppi, C.; Germain, P.L.; Bohacek, J. The Acute Stress Response in the Multiomic Era. Biol. Psychiatry 2021, 89, 1116–1126. [Google Scholar] [CrossRef]
- Rabasa, C.; Dickson, S.L. Impact of stress on metabolism and energy balance. Curr. Opin. Behav. Sci. 2016, 9, 71–77. [Google Scholar] [CrossRef]
- van der Kooij, M.A. The impact of chronic stress on energy metabolism. Mol. Cell. Neurosci. 2020, 107, 103525. [Google Scholar] [CrossRef]
- Preiser, J.C.; Ichai, C.; Orban, J.C.; Groeneveld, A.B. Metabolic response to the stress of critical illness. Br. J. Anaesth. 2014, 113, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Marazziti, D.; Rutigliano, G.; Baroni, S.; Landi, P.; Dell’Osso, L. Metabolic syndrome and major depression. CNS Spectr. 2014, 19, 293–304. [Google Scholar] [CrossRef]
- Reveille, J.D. Biomarkers in axial spondyloarthritis and low back pain: A comprehensive review. Clin. Rheumatol. 2022, 41, 617–634. [Google Scholar] [CrossRef] [PubMed]
- Sibille, K.T.; Steingrímsdóttir, Ó.A.; Fillingim, R.B.; Stubhaug, A.; Schirmer, H.; Chen, H.; McEwen, B.S.; Nielsen, C.S. Investigating the Burden of Chronic Pain: An Inflammatory and Metabolic Composite. Pain Res. Manag. 2016, 2016, 7657329. [Google Scholar] [CrossRef] [PubMed]
- Loevinger, B.L.; Muller, D.; Alonso, C.; Coe, C.L. Metabolic syndrome in women with chronic pain. Metabolism 2007, 56, 87–93. [Google Scholar] [CrossRef]
- Jha, M.K.; Song, G.J.; Lee, M.G.; Jeoung, N.H.; Go, Y.; Harris, R.A.; Park, D.H.; Kook, H.; Lee, I.K.; Suk, K. Metabolic Connection of Inflammatory Pain: Pivotal Role of a Pyruvate Dehydrogenase Kinase-Pyruvate Dehydrogenase-Lactic Acid Axis. J. Neurosci. 2015, 35, 14353–14369. [Google Scholar] [CrossRef]
- Aroke, E.N.; Powell-Roach, K.L. The Metabolomics of Chronic Pain Conditions: A Systematic Review. Biol. Res. Nurs. 2020, 22, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Jha, S.C.; Shutta, K.H.; Huang, T.; Balasubramanian, R.; Clish, C.B.; Hankinson, S.E.; Kubzansky, L.D. Psychological distress and metabolomic markers: A systematic review of posttraumatic stress disorder, anxiety, and subclinical distress. Neurosci. Biobehav. Rev. 2022, 143, 104954. [Google Scholar] [CrossRef]
- Piras, C.; Conte, S.; Pibiri, M.; Rao, G.; Muntoni, S.; Leoni, V.P.; Finco, G.; Atzori, L. Metabolomics and psychological features in fibromyalgia and electromagnetic sensitivity. Sci. Rep. 2020, 10, 20418. [Google Scholar] [CrossRef]
- Adachi, Y.; Toyoshima, K.; Nishimoto, R.; Ueno, S.; Tanaka, T.; Imaizumi, A.; Arashida, N.; Nakamura, M.; Abe, Y.; Hakamada, T.; et al. Association between plasma α-aminobutyric acid and depressive symptoms in older community-dwelling adults in Japan. Geriatr. Gerontol. Int. 2019, 19, 254–258. [Google Scholar] [CrossRef]
- Altmaier, E.; Emeny, R.T.; Krumsiek, J.; Lacruz, M.E.; Lukaschek, K.; Häfner, S.; Kastenmüller, G.; Römisch-Margl, W.; Prehn, C.; Mohney, R.P.; et al. Metabolomic profiles in individuals with negative affectivity and social inhibition: A population-based study of Type D personality. Psychoneuroendocrinology 2013, 38, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Balasubramanian, R.; Yao, Y.; Clish, C.B.; Shadyab, A.H.; Liu, B.; Tworoger, S.S.; Rexrode, K.M.; Manson, J.E.; Kubzansky, L.D.; et al. Associations of depression status with plasma levels of candidate lipid and amino acid metabolites: A meta-analysis of individual data from three independent samples of US postmenopausal women. Mol. Psychiatry 2021, 26, 3315–3327. [Google Scholar] [CrossRef] [PubMed]
- Onderwater, G.L.J.; Ligthart, L.; Bot, M.; Demirkan, A.; Fu, J.; van der Kallen, C.J.H.; Vijfhuizen, L.S.; Pool, R.; Liu, J.; Vanmolkot, F.H.M.; et al. Large-scale plasma metabolome analysis reveals alterations in HDL metabolism in migraine. Neurology 2019, 92, e1899–e1911. [Google Scholar] [CrossRef] [PubMed]
- Eddington, H.S.; McLeod, M.; Trickey, A.W.; Barreto, N.; Maturen, K.; Morris, A.M. Patient-reported distress and age-related stress biomarkers among colorectal cancer patients. Cancer Med. 2021, 10, 3604–3612. [Google Scholar] [CrossRef] [PubMed]
- Boguszewicz, Ł.; Bieleń, A.; Ciszek, M.; Wendykier, J.; Szczepanik, K.; Skorupa, A.; Mrochem-Kwarciak, J.; Składowski, K.; Sokół, M. NMR-Based Metabolomics in Investigation of the Radiation Induced Changes in Blood Serum of Head and Neck Cancer Patients and Its Correlation with the Tissue Volumes Exposed to the Particulate Doses. Int. J. Mol. Sci. 2021, 22, 6310. [Google Scholar] [CrossRef] [PubMed]
- Boguszewicz, Ł.; Bieleń, A.; Jarczewski, J.D.; Ciszek, M.; Skorupa, A.; Składowski, K.; Sokół, M. Molecular response to induction chemotherapy and its correlation with treatment outcome in head and neck cancer patients by means of NMR-based metabolomics. BMC Cancer 2021, 21, 410. [Google Scholar] [CrossRef]
- Boguszewicz, Ł.; Bieleń, A.; Mrochem-Kwarciak, J.; Skorupa, A.; Ciszek, M.; Heyda, A.; Wygoda, A.; Kotylak, A.; Składowski, K.; Sokół, M. NMR-based metabolomics in real-time monitoring of treatment induced toxicity and cachexia in head and neck cancer: A method for early detection of high risk patients. Metabolomics 2019, 15, 110. [Google Scholar] [CrossRef]
- Boguszewicz, Ł.; Hajduk, A.; Mrochem-Kwarciak, J.; Skorupa, A.; Ciszek, M.; Heyda, A.; Skladowski, K.; Sokół, M. 1H NMR based metabolomic approach to monitoring of the head and neck cancer treatment toxicity. Metabolomics 2016, 12, 102. [Google Scholar] [CrossRef]
- Jensen, M.P.; McFarland, C.A. Increasing the reliability and validity of pain intensity measurement in chronic pain patients. Pain 1993, 55, 195–203. [Google Scholar] [CrossRef]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011, 63 (Suppl. 11), 240–252. [Google Scholar] [CrossRef]
- Ferraz, M.B.; Quaresma, M.R.; Aquino, L.R.; Atra, E.; Tugwell, P.; Goldsmith, C. Reliability of pain scales in the assessment of literate and illiterate patients with rheumatoid arthritis. J. Rheumatol. 1990, 17, 1022–1024. [Google Scholar] [PubMed]
- Riba, M.B.; Donovan, K.A.; Ahmed, K.; Andersen, B.; Braun, I.; Breitbart, W.S.; Brewer, B.W.; Corbett, C.; Fann, J.; Fleishman, S.; et al. NCCN Guidelines® Insights: Distress Management, Version 2.2023. J. Natl. Compr. Cancer Netw. 2023, 21, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Bultz, B.D.; Holland, J.C. Emotional distress in patients with cancer: The sixth vital sign. Community Oncol. 2006, 5, 311–314. [Google Scholar] [CrossRef]
- Cutillo, A.; O’Hea, E.; Person, S.; Lessard, D.; Harralson, T.; Boudreaux, E. The Distress Thermometer: Cutoff Points and Clinical Use. Oncol. Nurs. Forum 2017, 44, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Donovan, K.A.; Grassi, L.; McGinty, H.L.; Jacobsen, P.B. Validation of the distress thermometer worldwide: State of the science. Psychooncology 2014, 23, 241–250. [Google Scholar] [CrossRef]
- Gascon, B.; Panjwani, A.A.; Mazzurco, O.; Li, M. Screening for Distress and Health Outcomes in Head and Neck Cancer. Curr. Oncol. 2022, 29, 3793–3806. [Google Scholar] [CrossRef]
- Peters, A.; McEwen, B.S.; Friston, K. Uncertainty and stress: Why it causes diseases and how it is mastered by the brain. Prog. Neurobiol. 2017, 156, 164–188. [Google Scholar] [CrossRef]
- Anderson, J.; Slade, A.N.; McDonagh, P.R.; Burton, W.; Fields, E.C. The long-lasting relationship of distress on radiation oncology-specific clinical outcomes. Adv. Radiat. Oncol. 2018, 4, 354–361. [Google Scholar] [CrossRef]
- Fernández, L.P.; Gómez de Cedrón, M.; Ramírez de Molina, A. Alterations of Lipid Metabolism in Cancer: Implications in Prognosis and Treatment. Front. Oncol. 2020, 10, 577420. [Google Scholar] [CrossRef]
- Arlauckas, S.P.; Browning, E.A.; Poptani, H.; Delikatny, E.J. Imaging of cancer lipid metabolism in response to therapy. NMR Biomed. 2019, 32, e4070. [Google Scholar] [CrossRef]
- Lee, K.; Kim, S.; Jo, J.K. The Relationships between Abnormal Serum Lipid Levels, Depression, and Suicidal Ideation According to Sex. J. Clin. Med. 2022, 11, 2119. [Google Scholar] [CrossRef]
- Anni, N.S.; Jung, S.J.; Shim, J.S.; Jeon, Y.W.; Lee, G.B.; Kim, H.C. Stressful life events and serum triglyceride levels: The Cardiovascular and Metabolic Diseases Etiology Research Center cohort in Korea. Epidemiol. Health. 2021, 43, e2021042. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Y.; Li, J.; Jin, C.; Zhong, D. The Effect of Psychological Burden on Dyslipidemia Moderated by Greenness: A Nationwide Study from China. Int. J. Environ. Res. Public Health 2022, 19, 14287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ma, C.; Wang, X.; Wang, Q.; Song, W.; Li, C.; Zhai, C.; Qi, Y.; Fan, S.; Cheng, F. Impact of chronic psychological stress on nonalcoholic fatty liver disease. Int. J. Clin. Exp. Med. 2019, 12, 7991–7998. [Google Scholar]
- Motoyama, K.; Nakai, Y.; Miyashita, T.; Fukui, Y.; Morita, M.; Sanmiya, K.; Sakakibara, H.; Matsumoto, I.; Abe, K.; Yakabe, T.; et al. Isolation stress for 30 days alters hepatic gene expression profiles, especially with reference to lipid metabolism in mice. Physiol. Genom. 2009, 37, 79–87. [Google Scholar] [CrossRef]
- Patti, G.J.; Yanes, O.; Shriver, L.P.; Courade, J.P.; Tautenhahn, R.; Manchester, M.; Siuzdak, G. Metabolomics implicates altered sphingolipids in chronic pain of neuropathic origin. Nat. Chem. Biol. 2012, 8, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Schaller, A.K.C.S.; Peterson, A.; Bäckryd, E. Pain management in patients undergoing radiation therapy for head and neck cancer—A descriptive study. Scand. J. Pain. 2020, 21, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Esteve, E.; Ricart, W.; Fernández-Real, J.M. Dyslipidemia and inflammation: An evolutionary conserved mechanism. Clin. Nutr. 2005, 24, 16–31. [Google Scholar] [CrossRef]
- Yamamotová, A.; Srámková, T.; Rokyta, R. Intensity of pain and biochemical changes in blood plasma in spinal cord trauma. Spinal Cord 2010, 48, 21–26. [Google Scholar] [CrossRef]
- Krikava, K.; Kalla, K.; Yamamotová, A.; Rokyta, R. Blood serum changes in patients with pain during bone fractures and acute pancreatitis. Neuroendocrinol. Lett. 2004, 25, 62–69. [Google Scholar]
- Osthues, T.; Sisignano, M. Oxidized Lipids in Persistent Pain States. Front. Pharmacol. 2019, 10, 1147. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Dai, L.; Crooks, D.R.; Neckers, L.M.; Higashi, R.M.; Fan, T.W.-M.; Lane, L.N. NMR Methods for Determining Lipid Turnover via Stable Isotope Resolved Metabolomics. Metabolites 2021, 11, 202. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine reliance in cell metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Kurkinen, K.; Kärkkäinen, O.; Lehto, S.M.; Luoma, I.; Kraav, S.L.; Nieminen, A.I.; Kivimäki, P.; Therman, S.; Tolmunen, T. One-carbon and energy metabolism in major depression compared to chronic depression in adolescent outpatients: A metabolomic pilot study. J. Affect. Disord. 2021, 6, 100261. [Google Scholar] [CrossRef]
- Chen, Q.; Kirk, K.; Shurubor, Y.I.; Zhao, D.; Arreguin, A.J.; Shahi, I.; Valsecchi, F.; Primiano, G.; Calder, E.L.; Carelli, V.; et al. Rewiring of Glutamine Metabolism Is a Bioenergetic Adaptation of Human Cells with Mitochondrial DNA Mutations. Cell Metab. 2018, 27, 1007–1025.e5. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhang, B.; Lin, X.; Fu, X.; An, Y.; Zou, Y.; Wang, J.X.; Wang, Z.; Yu, T. Lactate metabolism in human health and disease. Signal Transduct. Target. Ther. 2022, 7, 305. [Google Scholar] [CrossRef]
- Kubera, B.; Hubold, C.; Otte, S.; Lindenberg, A.S.; Zeiss, I.; Krause, R.; Steinkamp, M.; Klement, J.; Entringer, S.; Pellerin, L.; et al. Rise in plasma lactate concentrations with psychosocial stress: A possible sign of cerebral energy demand. Obes. Facts 2012, 5, 384–392. [Google Scholar] [CrossRef]
- Vione, B.; Ramacieri, G.; Zavaroni, G.; Piano, A.; La Rocca, G.; Caracausi, M.; Vitale, L.; Piovesan, A.; Gori, C.; Pirazzoli, G.L.; et al. One-carbon pathway metabolites are altered in the plasma of subjects with Down syndrome: Relation to chromosomal dosage. Front. Med. 2022, 9, 1006891. [Google Scholar] [CrossRef]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef]
- Sugden, C. One-carbon metabolism in psychiatric illness. Nutr. Res. Rev. 2006, 19, 117–136. [Google Scholar] [CrossRef] [PubMed]
- Jeyhoonabadi, M.; Alimoahmmadi, S.; Hassanpour, S.; Hashemnia, M. Betaine Ameliorates Depressive-Like Behaviors in Zinc Oxide Nanoparticles Exposed Mice. Biol. Trace Elem. Res. 2002, 200, 4771–4781. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Du, J.; Che, B.; Guo, Y.; Zhang, J.; Ju, Z.; Xu, T.; Zhong, X.; Zhang, Y.; Zhong, C. Circulating choline pathway nutrients and depression after ischemic stroke. Eur. J. Neurol. 2022, 29, 459–468. [Google Scholar] [CrossRef]
- Bjelland, I.; Tell, G.S.; Vollset, S.E.; Konstantinova, S.; Ueland, P.M. Choline in anxiety and depression: The Hordaland Health Study. Am. J. Clin. Nutr. 2009, 90, 0002–9165. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Su, D.M.; Wang, R.H.; Liu, Y.; Wang, H. Antinociceptive effects of choline against acute and inflammatory pain. Neuroscience 2005, 132, 49–56. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, K.; Krishnan, A.; Cervenka, E.; Hu, G.; Guadagno, E.; Trakadis, Y. Biomarkers for major depressive and bipolar disorders using metabolomics: A systematic review. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2019, 180, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Maes, M. “Functional” or “psychosomatic” symptoms, e.g. a flu-like malaise, aches and pain and fatigue, are major features of major and in particular of melancholic depression. Neuroendocrinol. Lett. 2009, 30, 564–573. [Google Scholar]
- Altamura, C.; Maes, M.; Dai, J.; Meltzer, H.Y. Plasma concentrations of excitatory amino acids, serine, glycine, taurine and histidine in major depression. Eur. Neuropsychopharmacol. 1995, 5 (Suppl. 1), 71–75. [Google Scholar] [CrossRef]
- Matthews, D.E. An overview of phenylalanine and tyrosine kinetics in humans. J. Nutr. 2007, 137 (Suppl. 1), 1549S–1555S, discussion 1573S–1575S. [Google Scholar] [CrossRef]
- Ipson, B.R.; Green, R.A.; Wilson, J.T.; Watson, J.N.; Faull, K.F.; Fisher, A.L. Tyrosine aminotransferase is involved in the oxidative stress response by metabolizing meta-tyrosine in Caenorhabditis elegans. J. Biol. Chem. 2019, 294, 9536–9554. [Google Scholar] [CrossRef]
- Kim, E.; Zhao, Z.; Rzasa, J.R.; Glassman, M.; Bentley, W.E.; Chen, S.; Kelly, D.L.; Payne, G.F. Association of acute psychosocial stress with oxidative stress: Evidence from serum analysis. Redox Biol. 2021, 47, 102138. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Fan, S.; Liu, M.; Zhong, J.; Cao, D.; Zheng, P.; Wang, Y.; Wei, Y.; Fang, L.; Xie, P. Objective diagnosis of post-stroke depression using NMR-based plasma metabonomics. Neuropsychiatr. Dis. Treat. 2019, 15, 867–881. [Google Scholar] [CrossRef]
- Alexander, G.M.; Reichenberger, E.; Peterlin, B.L.; Perreault, M.J.; Grothusen, J.R.; Schwartzman, R.J. Plasma amino acids changes in complex regional pain syndrome. Pain Res. Treat. 2013, 2013, 742407. [Google Scholar] [CrossRef]
- Wesseldijk, F.; Fekkes, D.; Huygen, F.J.P.M.; van de Heide-Mulder, M.; Zijlstra, F.J. Increased plasma glutamate, glycine, and arginine levels in complex regional pain syndrome type 1. Acta Anaesthesiol. Scand. 2008, 52, 688–694. [Google Scholar] [CrossRef]
- Tomé, D.; Bos, C. Lysine requirement through the human life cycle. J. Nutr. 2007, 137 (Suppl. 2), 1642S–1645S. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, N.; Shinoda, K.; Sato, H.; Sasaki, K.; Suzuki, M.; Yamaki, K.; Fujimori, T.; Yamamoto, H.; Osei-Hyiaman, D.; Ohashi, Y. Plasma metabolome analysis of patients with major depressive disorder. Psychiatry Clin. Neurosci. 2018, 72, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Tanaka, M.; Nozaki, S.; Yamaguti, K.; Mizuma, H.; Sasabe, T.; Sugino, T.; Shirai, T.; Kataoka, Y.; Kajimoto, Y.; et al. Mental fatigue-induced decrease in levels of several plasma amino acids. J. Neural Transm. 2007, 114, 555–561. [Google Scholar] [CrossRef]
- Holeček, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef]
- Le Couteur, D.G.; Solon-Biet, S.M.; Cogger, V.C.; Ribeiro, R.; de Cabo, R.; Raubenheimer, D.; Cooney, G.J.; Simpson, S.J. Branched chain amino acids, aging and age-related health. Ageing Res. Rev. 2020, 64, 101198. [Google Scholar] [CrossRef]
- Ogawa, S.; Koga, N.; Hattori, K.; Matsuo, J.; Ota, M.; Hori, H.; Sasayama, D.; Teraishi, T.; Ishida, I.; Yoshida, F.; et al. Plasma amino acid profile in major depressive disorder: Analyses in two independent case-control sample sets. J. Psychiatr. Res. 2018, 96, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Baranyi, A.; Amouzadeh-Ghadikolai, O.; Von Lewinski, D.; Rothenhäusler, H.B.; Theokas, S.; Robier, C.; Mangge, H.; Reicht, G.; Hlade, P.; Meinitzer, A. Branched-Chain Amino Acids as New Biomarkers of Major Depression—A Novel Neurobiology of Mood Disorder. PLoS ONE 2016, 4, e0160542. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M. Potential Role of Neuroactive Tryptophan Metabolites in Central Fatigue: Establishment of the Fatigue Circuit. Int. J. Tryptophan Res. 2020, 13, 1178646920936279. [Google Scholar] [CrossRef] [PubMed]


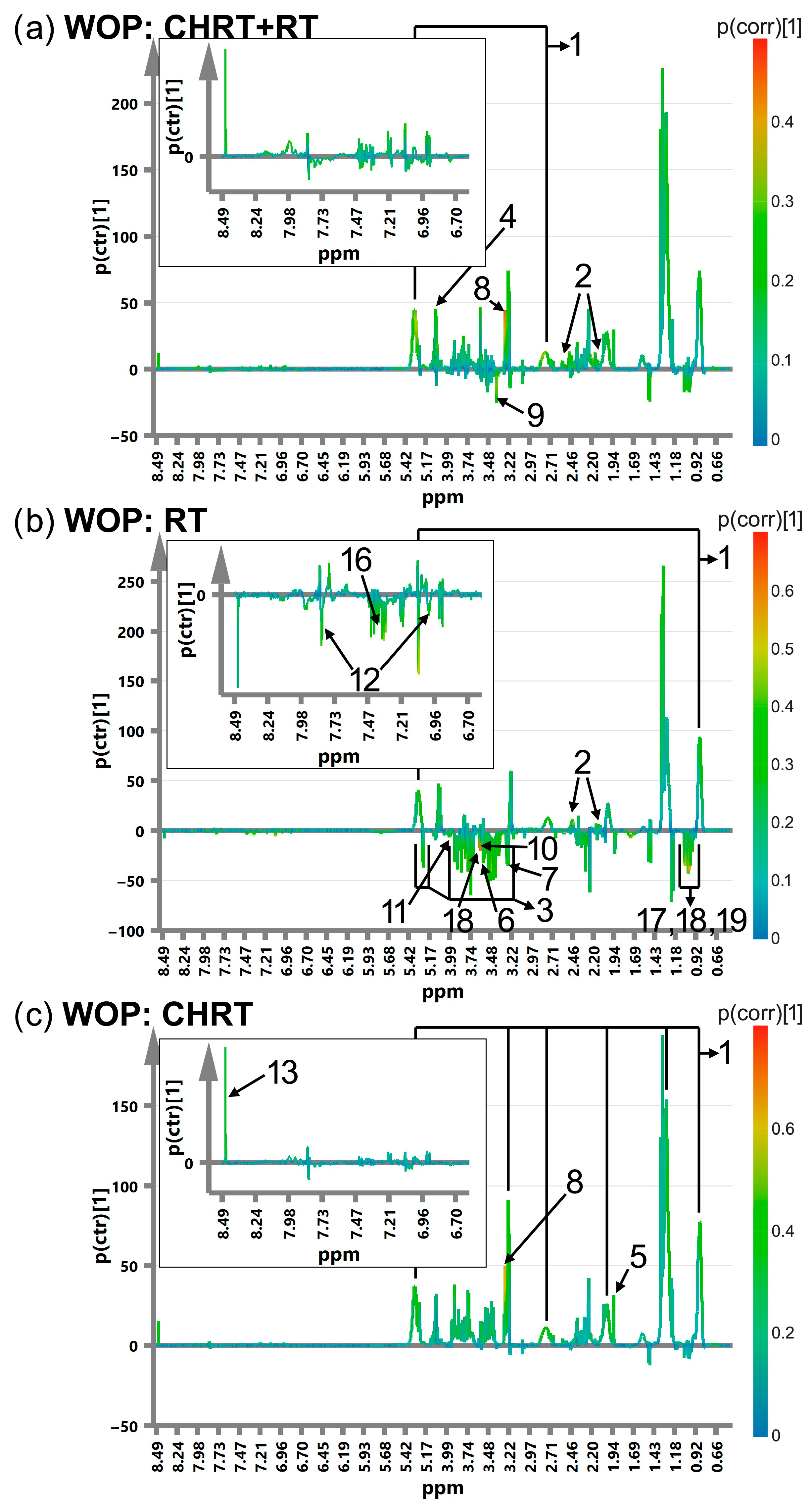
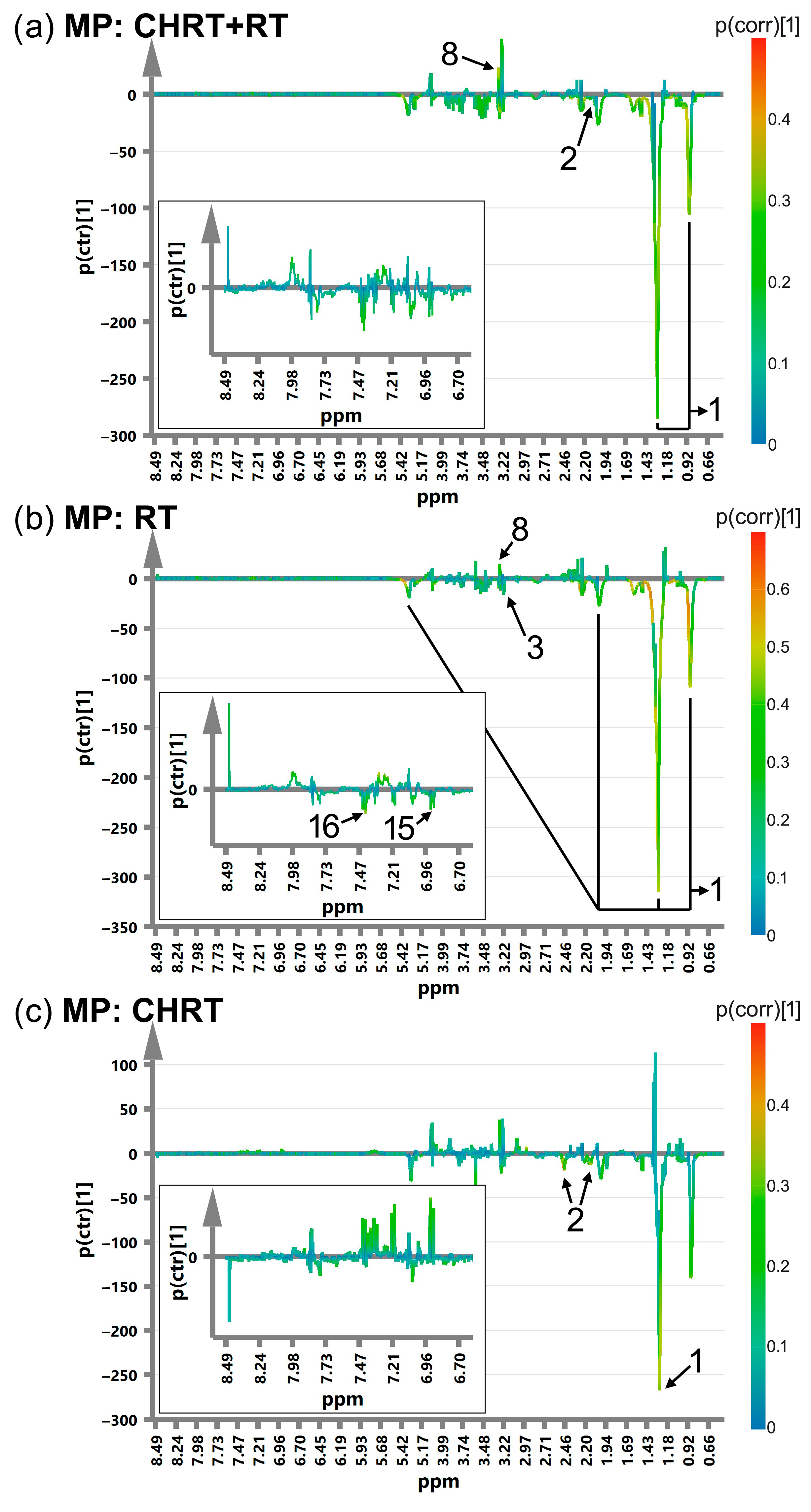

| Treatment Modality | RT | CHRT | Between Group Difference (p Value from MWU or χ2 Test) |
|---|---|---|---|
| Age | 0.055 | ||
| Range | 46–73 | 46–74 | |
| Median age | 61 | 58 | |
| Sex | 0.54 | ||
| Males | 26 | 26 | |
| Females | 10 | 7 | |
| Tumor localization | 0.001 | ||
| Hypopharynx | 3 | 7 | |
| Larynx | 26 | 14 | |
| Nasopharynx | 1 | 2 | |
| Oropharynx | 6 | 10 | |
| Tumor staging | |||
| T (primary tumor stage) | 0.045 | ||
| 1 | 3 | 2 | |
| 2 | 22 | 10 | |
| 3 | 7 | 11 | |
| 4 | 4 | 10 | |
| N (nodal stage) | 0.000 | ||
| 0 | 30 | 10 | |
| 1 | 2 | 5 | |
| 2 | 4 | 17 | |
| 3 | 0 | 1 | |
| TNM (tumor, nodes, metastases) | 0.000 | ||
| I | 2 | 0 | |
| II | 21 | 2 | |
| III | 6 | 8 | |
| IVa | 7 | 22 | |
| IVb | 0 | 1 | |
| WOD | WOP | ||||||
| No. of Weeks When Distress > 0 | No. of Patients | No of Weeks When Pain > 0 | No. of Patients | ||||
| Wholestudy Group | RT | CHRT | Wholestudy Group | RT | CHRT | ||
| 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| 1 | 6 | 1 | 5 | 1 | 4 | 2 | 2 |
| 2 | 9 | 5 | 4 | 2 | 2 | 1 | 1 |
| 3 | 11 | 6 | 5 | 3 | 1 | 1 | 0 |
| 4 | 3 | 3 | 0 | 4 | 6 | 5 | 1 |
| 5 | 9 | 7 | 2 | 5 | 9 | 7 | 2 |
| 6 | 6 | 4 | 2 | 6 | 18 | 8 | 10 |
| 7 | 18 | 10 | 8 | 7 | 23 | 11 | 12 |
| 8 | 7 | - | 7 | 8 | 5 | - | 5 |
| Median of weeks when distress > 0 | Median of weeks when pain > 0 | ||||||
| 5 | 5 | 6 | 6 | 6 | 7 | ||
| MD | MP | ||||||
| Max Distress Value | No. of Patients | Max Pain Value | No. of Patients | ||||
| Whole Study Group | RT | CHRT | Whole Study Group | RT | CHRT | ||
| 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| 1 | 18 | 6 | 12 | 1 | 3 | 2 | 1 |
| 2 | 14 | 10 | 4 | 2 | 7 | 2 | 5 |
| 3 | 9 | 5 | 4 | 3 | 17 | 8 | 9 |
| 4 | 7 | 2 | 5 | 4 | 13 | 9 | 4 |
| 5 | 10 | 8 | 2 | 5 | 13 | 6 | 7 |
| 6 | 3 | 2 | 1 | 6 | 5 | 4 | 1 |
| 7 | 4 | 2 | 2 | 7 | 6 | 4 | 2 |
| 8 | 2 | 0 | 2 | 8 | 2 | 0 | 2 |
| 9 | 0 | 0 | 0 | 9 | 1 | 0 | 1 |
| 10 | 2 | 1 | 1 | 10 | 1 | 0 | 1 |
| Median of max distress value | Median of max pain value | ||||||
| 3 | 3 | 3 | 4 | 4 | 4 | ||
| OPLS-DA Model Quality | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WOD | MD | WOP | MP | |||||||||
| CHRT+RT | RT | CHRT | CHRT+RT | RT | CHRT | CHRT+RT | RT | CHRT | CHRT+RT | RT | CHRT | |
| R2X predictive | 0.08 | 0.13 | 0.04 | 0.01 | 0.03 | 0.01 | 0.02 | 0.03 | 0.05 | 0.05 | 0.09 | 0.02 |
| R2Y | 0.54 | 0.65 | 0.62 | 0.37 | 0.51 | 0.63 | 0.50 | 0.30 | 0.66 | 0.43 | 0.56 | 0.47 |
| Q2 | 0.41 | 0.49 | 0.52 | 0.23 | 0.31 | 0.43 | 0.32 | 0.17 | 0.53 | 0.30 | 0.39 | 0.32 |
| NOOC | 6 | 5 | 4 | 4 | 5 | 4 | 7 | 3 | 6 | 5 | 5 | 4 |
| R2X orthogonal | 0.70 | 0.64 | 0.67 | 0.70 | 0.76 | 0.64 | 0.78 | 0.68 | 0.72 | 0.68 | 0.70 | 0.70 |
| cv-ANOVA p value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| OPLS-DA Results | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # | Metabolite | ppm | p(corr) | |||||||||||
| WOD | MD | WOP | MP | |||||||||||
| CHRT+RT | RT | CHRT | CHRT+RT | RT | CHRT | CHRT+RT | RT | CHRT | CHRT+RT | RT | CHRT | |||
| Lipids | ||||||||||||||
| 1 | Lipids | 0.9 | 0.42 | 0.52 | 0.31 | 0.3 | 0.37 | −0.34 | −0.48 | |||||
| Lipids | 1.3 | 0.43 | 0.48 | 0.35 | 0.32 | 0.37 | −0.31 | −0.44 | −0.34 | |||||
| Lipids | 1.6 | 0.4 | 0.47 | 0.38 | ||||||||||
| Lipids | 2.0 | 0.4 | 0.51 | 0.32 | 0.33 | −0.34 | ||||||||
| Lipids | 2.2 | 0.4 | 0.45 | 0.4 | ||||||||||
| Lipids | 2.7 | 0.37 | 0.54 | 0.32 | 0.4 | |||||||||
| Lipids | 3.2 | 0.42 | ||||||||||||
| Lipids | 5.3 | 0.37 | 0.54 | 0.33 | 0.33 | 0.33 | 0.4 | −0.41 | ||||||
| Glutamine, glucose and other metabolites of energy metabolism | ||||||||||||||
| 2 | Glutamine | 2.1 | 0.36 | 0.34 | 0.32 | 0.34 | −0.31 | −0.38 | ||||||
| Glutamine | 2.47 | 0.32 | 0.35 | 0.32 | 0.31 | 0.4 | −0.42 | |||||||
| 3 | Glucose | * | −0.33 | 0.37 | −0.33 | −0.31 | ||||||||
| 4 | Lactate | 1.33 | 0.37 | |||||||||||
| Lactate | 4.13 | 0.4 | 0.3 | |||||||||||
| 5 | Acetate | 1.93 | 0.35 | |||||||||||
| Metabolites of one-carbon metabolism | ||||||||||||||
| 6 | Glycine | 3.57 | −0.5 | −0.31 | ||||||||||
| 7 | Choline | −0.31 | ||||||||||||
| 8 | Betaine | 3.27 | 0.46 | 0.63 | 0.34 | 0.33 | ||||||||
| 9 | Methanol | 3.38 | −0.31 | −0.4 | −0.31 | |||||||||
| 10 | Threonine | 3.6 | −0.35 | −0.3 | ||||||||||
| 11 | Serine | 3.99 | −0.3 | −0.34 | −0.34 | −0.35 | ||||||||
| 12 | Histidine | 7.03 | 0.33 | −0.39 | ||||||||||
| Histidine | 7.84 | 0.33 | −0.33 | |||||||||||
| 13 | Formate | 8.5 | 0.3 | |||||||||||
| Metabolites of protein metabolism and oxidative stress | ||||||||||||||
| 14 | Lysine | 3.0 | 0.31 | 0.35 | ||||||||||
| 15 | Tyrosine | 6.9 | 0.44 | −0.32 | ||||||||||
| Tyrosine | 7.2 | 0.41 | ||||||||||||
| 16 | Phenylalanine | 7.4 | 0.35 | −0.36 | −0.34 | |||||||||
| Branched-chain amino acids (BCAAs) | ||||||||||||||
| 17 | Leucine | 0.97 | −0.35 | |||||||||||
| Leucine | 1.7 | −0.39 | ||||||||||||
| 18 | Valine | 1.0 | 0.3 | −0.39 | ||||||||||
| Valine | 1.05 | 0.4 | −0.41 | |||||||||||
| Valine | 3.63 | −0.48 | ||||||||||||
| 19 | Isoleucine | 1.03 | −0.38 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boguszewicz, Ł.; Heyda, A.; Ciszek, M.; Bieleń, A.; Skorupa, A.; Mrochem-Kwarciak, J.; Składowski, K.; Sokół, M. Metabolite Biomarkers of Prolonged and Intensified Pain and Distress in Head and Neck Cancer Patients Undergoing Radio- or Chemoradiotherapy by Means of NMR-Based Metabolomics—A Preliminary Study. Metabolites 2024, 14, 60. https://doi.org/10.3390/metabo14010060
Boguszewicz Ł, Heyda A, Ciszek M, Bieleń A, Skorupa A, Mrochem-Kwarciak J, Składowski K, Sokół M. Metabolite Biomarkers of Prolonged and Intensified Pain and Distress in Head and Neck Cancer Patients Undergoing Radio- or Chemoradiotherapy by Means of NMR-Based Metabolomics—A Preliminary Study. Metabolites. 2024; 14(1):60. https://doi.org/10.3390/metabo14010060
Chicago/Turabian StyleBoguszewicz, Łukasz, Alicja Heyda, Mateusz Ciszek, Agata Bieleń, Agnieszka Skorupa, Jolanta Mrochem-Kwarciak, Krzysztof Składowski, and Maria Sokół. 2024. "Metabolite Biomarkers of Prolonged and Intensified Pain and Distress in Head and Neck Cancer Patients Undergoing Radio- or Chemoradiotherapy by Means of NMR-Based Metabolomics—A Preliminary Study" Metabolites 14, no. 1: 60. https://doi.org/10.3390/metabo14010060
APA StyleBoguszewicz, Ł., Heyda, A., Ciszek, M., Bieleń, A., Skorupa, A., Mrochem-Kwarciak, J., Składowski, K., & Sokół, M. (2024). Metabolite Biomarkers of Prolonged and Intensified Pain and Distress in Head and Neck Cancer Patients Undergoing Radio- or Chemoradiotherapy by Means of NMR-Based Metabolomics—A Preliminary Study. Metabolites, 14(1), 60. https://doi.org/10.3390/metabo14010060







