Metabolic Regulations of Smilax china L. against β-Amyloid Toxicity in Caenorhabditis elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Plants Material
2.2. Preparation of Sc Root n-BuOH Extracts
2.3. ScE Chemical Components Analysis via LC-HRMS
2.4. C. elegans Maintenance and Treatment
2.5. C. elegans Toxicity Assay
2.6. Body Bends
2.7. Reproductive Assay and Body Length
2.8. Life Span Assay on N2 and CL4176 C. elegans
2.9. Paralysis Assay
2.10. Aβ Deposits Observation
2.11. Preparation, LC-HRMS Analysis of C. elegans Metabolome, and Data Analysis
2.12. Real-Time Quantitative PCR (qRT-PCR)
2.13. Statistical Analysis
3. Results
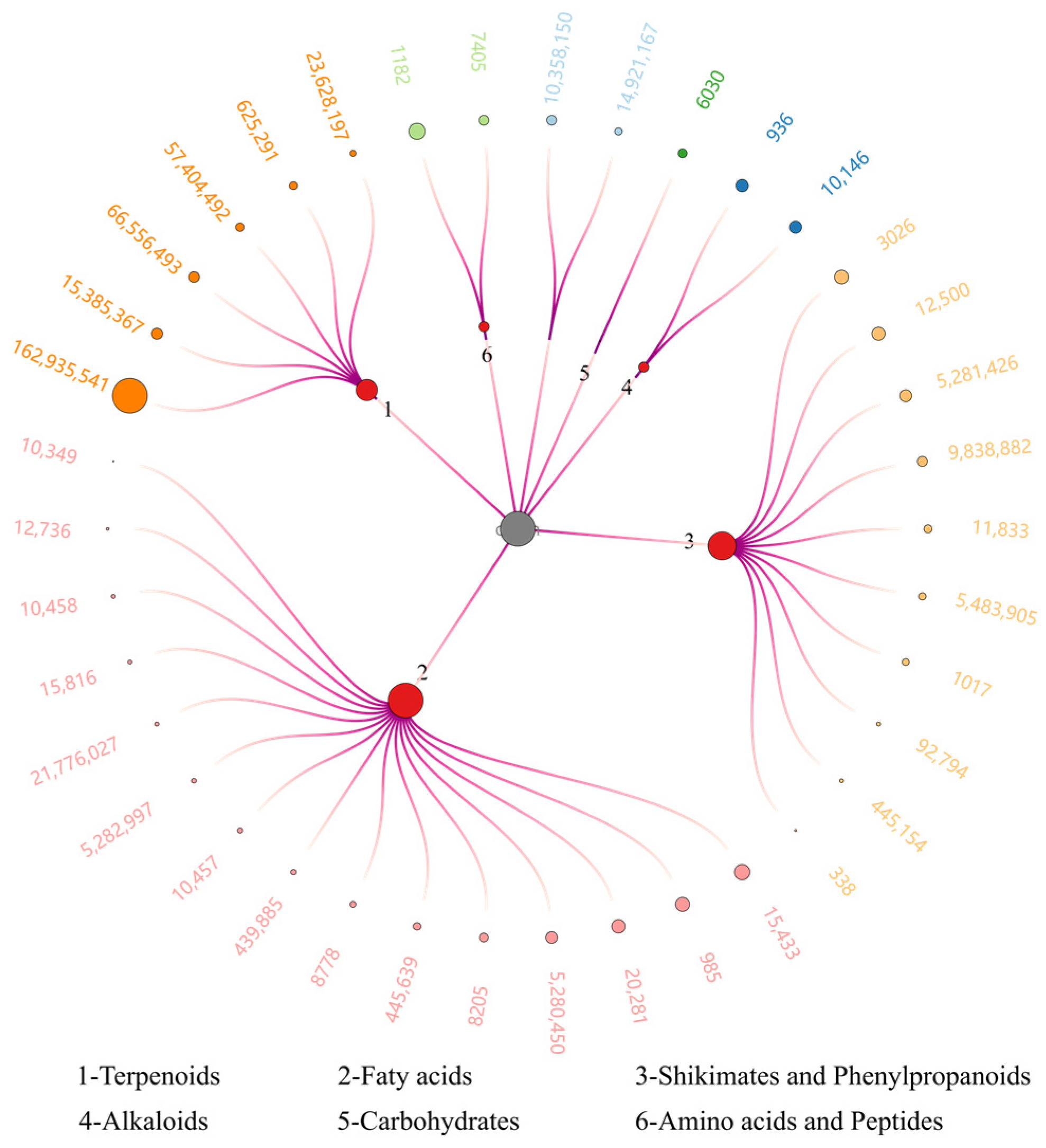
3.1. LC-HRMS Analysis of ScE
3.2. Effect of ScE on N2 C. elegans Growth Characteristics
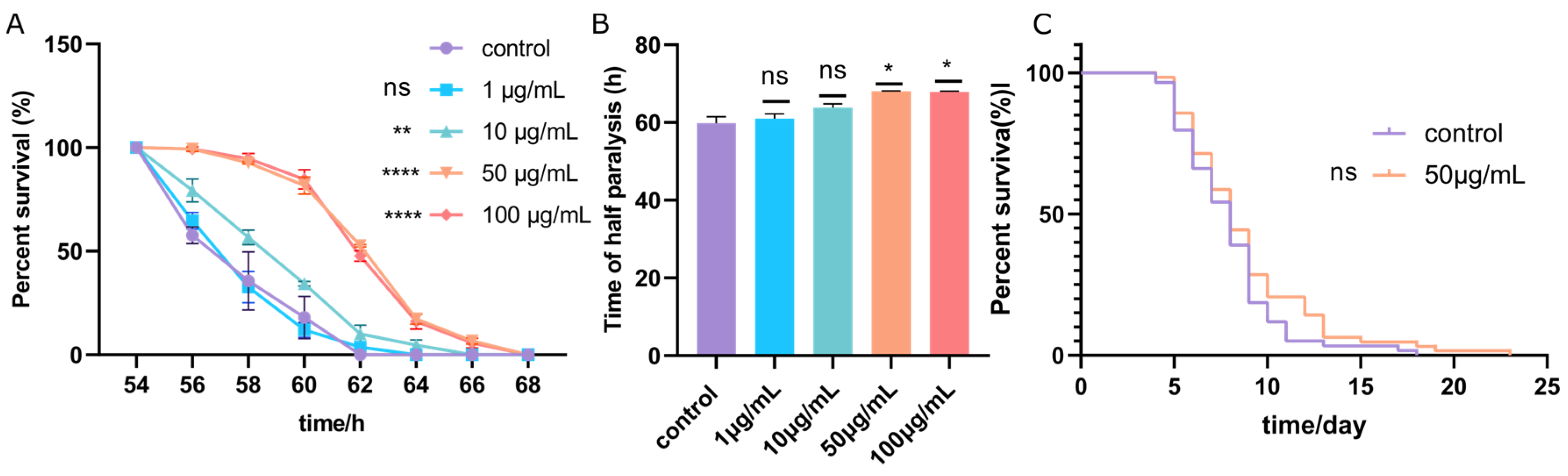
3.3. ScE Alleviated Symptoms of Paralysis of CL4176
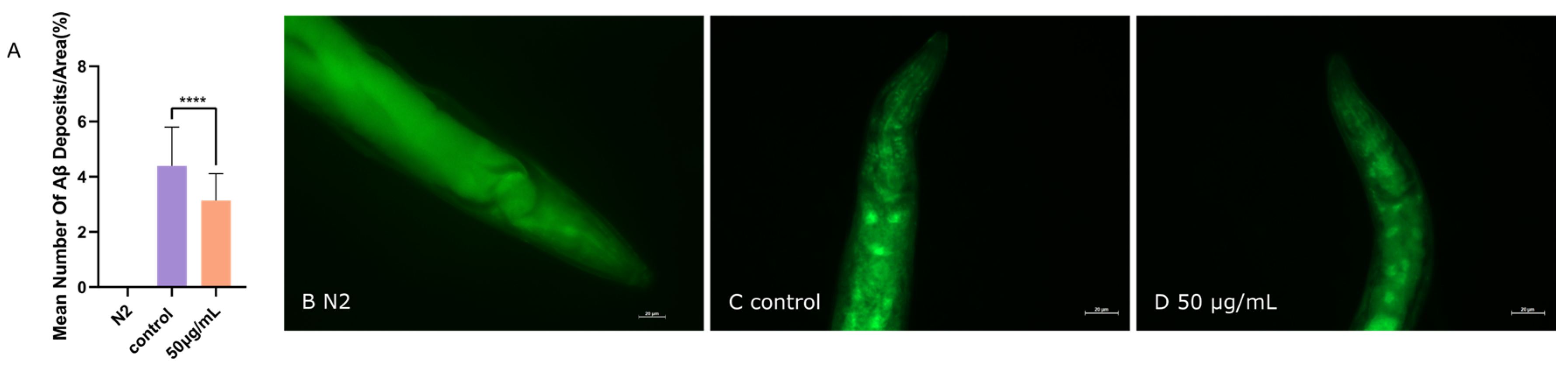
3.4. ScE Reduced Aβ Aggregation in C. elegans CL2006 Strain
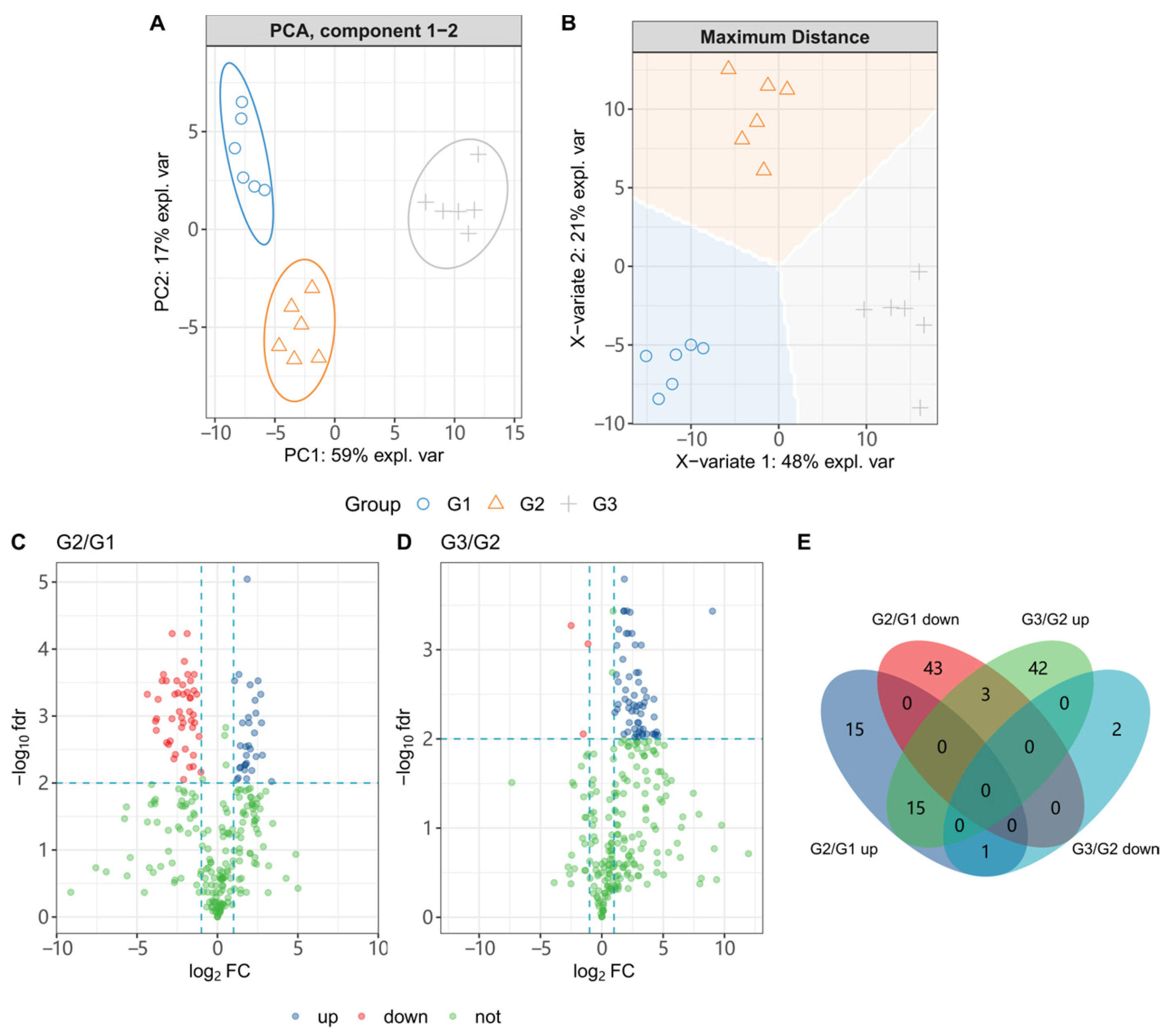
3.5. C. elegans CL4176 Metabolome Interfered by ScE
3.6. Validation of Metabolic Regulatory Mechanisms by qRT-PCR
4. Discussion
4.1. ScE Is a Highly Effective and Low-Toxicity Substance Group against Neurodegenerative Diseases
4.2. Metabolic Pathways Affected by ScE in CL4176
4.3. Regulatory Sites of ScE Repairing AD Metabolic Disorders
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Liu, J.; Dou, P.; Wu, Z.; Zheng, Z.; Pan, X.; Zhou, T.; Wang, K. Oral Absorption Characteristics and Mechanisms of a Pectin-Type Polysaccharide from Smilax china L. across the Intestinal Epithelium. Carbohydr. Polym. 2021, 270, 118383. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yin, H.; Lan, Z.; Ma, S.; Zhang, C.; Yang, Z.; Li, P.; Lin, B. Anti-Hyperuricemic and Nephroprotective Effects of Smilax china L. J. Ethnopharmacol. 2011, 135, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-T.; Zhao, Y.; Piao, X.-L.; Qiu, L.; Huang, Y.; Cheng, X.-G. The Active Components of Smilax china L. against Cancer by Interfering with the Interactions among Associated Proteins. J. Funct. Foods 2023, 106, 105591. [Google Scholar] [CrossRef]
- Song, L.; Tian, L.; Ma, Y.; Xie, Y.; Feng, H.; Qin, F.; Mo, L.; Lin, S.; Hou, L.; Wang, C. Protection of Flavonoids from Smilax china L. Rhizome on Phenol Mucilage-Induced Pelvic Inflammation in Rats by Attenuating Inflammation and Fibrosis. J. Funct. Foods 2017, 28, 194–204. [Google Scholar] [CrossRef]
- Wang, M.; Bai, Q.-X.; Zheng, X.-X.; Hu, W.-J.; Wang, S.; Tang, H.-P.; Yu, A.-Q.; Yang, B.-Y.; Kuang, H.-X. Smilax china L.: A Review of Its Botany, Ethnopharmacology, Phytochemistry, Pharmacological Activities, Actual and Potential Applications. J. Ethnopharmacol. 2024, 318, 116992. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Pei, T.; Song, L.; Zhu, D.; Han, Z.; Zhang, J.; Huang, X.; Qiu, X.; Xiao, W. Flavonoids from Smilax china L. Rhizome Improve Chronic Pelvic Inflammatory Disease by Promoting Macrophage Reprogramming via the NLRP3 Inflammasome-Autophagy Pathway. J. Funct. Foods 2023, 109, 105802. [Google Scholar] [CrossRef]
- Ban, J.Y.; Cho, S.O.; Choi, S.-H.; Ju, H.S.; Kim, J.Y.; Bae, K.; Song, K.-S.; Seong, Y.H. Neuroprotective Effect of Smilacis Chinae Rhizome on NMDA-Induced Neurotoxicity in vitro and Focal Cerebral Ischemia in vivo. J. Pharmacol. Sci. 2008, 106, 68–77. [Google Scholar] [CrossRef]
- Ban, J.Y.; Cho, S.O.; Koh, S.B.; Song, K.-S.; Bae, K.; Seong, Y.H. Protection of Amyloid β Protein (25–35)-Induced Neurotoxicity by Methanol Extract of Smilacis Chinae Rhizome in Cultured Rat Cortical Neurons. J. Ethnopharmacol. 2006, 106, 230–237. [Google Scholar] [CrossRef]
- Li, X.; Yang, L.; Xu, M.; Qiao, G.; Li, C.; Lin, L.; Zheng, G. Smilax china L. Polyphenols Alleviates Obesity and Inflammation by Modulating Gut Microbiota in High Fat/High Sucrose Diet-Fed C57BL/6J Mice. J. Funct. Foods 2021, 77, 104332. [Google Scholar] [CrossRef]
- Cai, Y.; Du, J.; Li, A.; Zhu, Y.; Xu, L.; Sun, K.; Ma, S.; Guo, T. Initial Levels of β-Amyloid and Tau Deposition Have Distinct Effects on Longitudinal Tau Accumulation in Alzheimer’s Disease. Alzheimer’s Res. Ther. 2023, 15, 30. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2023 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2023, 19, 1598–1695. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-Based Therapy for Alzheimer’s Disease: Challenges, Successes and Future. Signal Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Sato, W.; Watanabe-Takahashi, M.; Murata, T.; Utsunomiya-Tate, N.; Motoyama, J.; Anzai, M.; Ishihara, S.; Nishioka, N.; Uchiyama, H.; Togashi, J.; et al. A Tailored Tetravalent Peptide Displays Dual Functions to Inhibit Amyloid β Production and Aggregation. Commun. Biol. 2023, 6, 383. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Lv, Z.; Guo, M.; Sun, C.; Li, X.; Jiang, Z.; Zhang, W.; Chen, C. A Lycium barbarum Extract Inhibits Β-amyloid Toxicity by Activating the Antioxidant System and mtUPR in a Caenorhabditis elegans Model of Alzheimer’s Disease. FASEB J. 2022, 36, e22156. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Qin, Q.; Zhang, Y.; Xie, L.; Xiao, J.; Cao, Y.; Su, Z.; Chen, Y. Carnosic Acid Ameliorated Aβ-Mediated (Amyloid-β Peptide) Toxicity, Cholinergic Dysfunction and Mitochondrial Defect in Caenorhabditis elegans of Alzheimer’s Model. Food Funct. 2022, 13, 4624–4640. [Google Scholar] [CrossRef] [PubMed]
- Aydoğan, C. Recent Advances and Applications in LC-HRMS for Food and Plant Natural Products: A Critical Review. Anal. Bioanal. Chem. 2020, 412, 1973–1991. [Google Scholar] [CrossRef] [PubMed]
- Arjunan, A.; Sah, D.K.; Woo, M.; Song, J. Identification of the Molecular Mechanism of Insulin-like Growth Factor-1 (IGF-1): A Promising Therapeutic Target for Neurodegenerative Diseases Associated with Metabolic Syndrome. Cell Biosci. 2023, 13, 16. [Google Scholar] [CrossRef]
- Strefeler, A.; Jan, M.; Quadroni, M.; Teav, T.; Rosenberg, N.; Chatton, J.-Y.; Guex, N.; Gallart-Ayala, H.; Ivanisevic, J. Molecular Insights into Sex-Specific Metabolic Alterations in Alzheimer’s Mouse Brain Using Multi-Omics Approach. Alzheimer’s Res. Ther. 2023, 15, 8. [Google Scholar] [CrossRef]
- Ding, J.; Ji, J.; Rabow, Z.; Shen, T.; Folz, J.; Brydges, C.R.; Fan, S.; Lu, X.; Mehta, S.; Showalter, M.R.; et al. A Metabolome Atlas of the Aging Mouse Brain. Nat. Commun. 2021, 12, 6021. [Google Scholar] [CrossRef]
- Odom, J.D.; Sutton, V.R. Metabolomics in Clinical Practice: Improving Diagnosis and Informing Management. Clin. Chem. 2021, 67, 1606–1617. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Sun, Y.-P.; Luo, Y.-M.; Peng, D.-H.; Li, X.; Yang, B.-Y.; Wang, Q.-H.; Kuang, H.-X. Biomarkers for the Clinical Diagnosis of Alzheimer’s Disease: Metabolomics Analysis of Brain Tissue and Blood. Front. Pharmacol. 2021, 12, 700587. [Google Scholar] [CrossRef]
- Zhu, G.; Guo, M.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Integrative Metabolomic Characterization Reveals the Mediating Effect of Bifidobacterium Breve on Amino Acid Metabolism in a Mouse Model of Alzheimer’s Disease. Nutrients 2022, 14, 735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Deng, Y.; Zhang, A.; Yan, L.; Zhang, Z.; Wei, J.; Zhang, Q. LC-MS/MS Insight into Vitamin C Restoration to Metabolic Disorder Evoked by Amyloid-β in Caenorhabditis elegans CL2006. Metabolites 2022, 12, 841. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xiao, C.; Wang, F. Comparison of Two Varieties Fig (Peggy Red and Green) Peel Extracts by Liquid Chromatography–Tandem Mass Spectrometry Analysis and for Neuroprotective Efficacy in Caenorhabditis elegans. J. Med. Food. 2023, 26, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Sun, Y.; Wang, Z.; Su, Y.; Wang, Y.; Wang, X. Exendin-4 Alleviates β-Amyloid Peptide Toxicity via DAF-16 in a Caenorhabditis elegans Model of Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 955113. [Google Scholar] [CrossRef]
- Wang, E.; Wang, N.; Zou, Y.; Fahim, M.; Zhou, Y.; Yang, H.; Liu, Y.; Li, H. Black Mulberry (Morus nigra) Fruit Extract Alleviated AD-Like Symptoms Induced by Toxic Aβ Protein in Transgenic Caenorhabditis elegans via Insulin DAF-16 Signaling Pathway. Food Res. Int. 2022, 160, 111696. [Google Scholar] [CrossRef]
- Du, F.; Zhao, H.; Yao, M.; Yang, Y.; Jiao, J.; Li, C. Deer Antler Extracts Reduce Amyloid-Beta Toxicity in a Caenorhabditis elegans Model of Alzheimer’s Disease. J. Ethnopharmacol. 2022, 285, 114850. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; Zou, J.; Li, Y.; Zhang, X.-G. Chondroitin Sulfate E Alleviates β-Amyloid Toxicity in Transgenic Caenorhabditis elegans by Inhibiting Its Aggregation. Int. J. Biol. Macromol. 2022, 209, 1280–1287. [Google Scholar] [CrossRef]
- Sánchez-Martínez, J.D.; Cifuentes, A.; Valdés, A. Omics Approaches to Investigate the Neuroprotective Capacity of a Citrus sinensis (Sweet Orange) Extract in a Caenorhabditis elegans Alzheimer’s Model. Food Res. Int. 2023, 172, 113128. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Li, X.; Miao, Y.; Gao, J.; Zhang, Q. A New Utilization of Total Flavonoids from Acer Truncatum Samara and Leaves: Anti-Aging and Metabolic Regulation. Ind. Crops Prod. 2023, 203, 117207. [Google Scholar] [CrossRef]
- Bai, X.; Liu, C.-M.; Li, H.-J.; Zhang, Z.-P.; Cui, W.-B.; An, F.-L.; Zhang, Z.-X.; Wang, D.-S.; Fei, D.-Q. Ethyl Caffeate Attefnuates Aβ-Induced Toxicity in Caenorhabditis elegans AD Models via the Insulin/Insulin-like Growth Factor-1 Signaling Pathway. Bioorg. Chem. 2023, 139, 106714. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yuan, Y.; Zhang, R.; Song, Y.; Sui, T.; Wang, J.; Wang, C.; Chen, Y.; Guan, S.; Wang, L. A Deuterohemin Peptide Protects a Transgenic Caenorhabditis elegans Model of Alzheimer’s Disease by Inhibiting Aβ1–42 Aggregation. Bioorg. Chem. 2019, 82, 332–339. [Google Scholar] [CrossRef]
- Blackwell, T.K.; Steinbaugh, M.J.; Hourihan, J.M.; Ewald, C.Y.; Isik, M. SKN-1/Nrf, Stress Responses, and Aging in Caenorhabditis elegans. Free Radic. Biol. Med. 2015, 88, 290–301. [Google Scholar] [CrossRef]
- Baumanns, S.; Muehlemeyer, F.; Miesbauer, L.C.; Baake, J.; Roloff, E.M.; Beis, D.M.; Wenzel, U. 4-Phenylbutyric Acid Attenuates Amyloid-β Proteotoxicity through Activation of HSF-1 in an Alzheimer’s Disease Model of the Nematode Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2023, 673, 16–22. [Google Scholar] [CrossRef]
- Thakral, S.; Yadav, A.; Singh, V.; Kumar, M.; Kumar, P.; Narang, R.; Sudhakar, K.; Verma, A.; Khalilullah, H.; Jaremko, M.; et al. Alzheimer’s Disease: Molecular Aspects and Treatment Opportunities Using Herbal Drugs. Ageing Res. Rev. 2023, 88, 101960. [Google Scholar] [CrossRef] [PubMed]
- Yokeshwaran, A.; Yokeshwaran, V.; Sabarisenthil, B.; Kalaichelvan, V.K. Anticholinesterase Activity of Plant Extracts of Smilax zeylanica and Smilax china. Int. J. Pharm. Sci. Res. 2020, 11, 4370–4374. [Google Scholar] [CrossRef]
- Ban, J.Y.; Cho, S.O.; Jeon, S.-Y.; Bae, K.; Song, K.-S.; Seong, Y.H. 3,4-Dihydroxybenzoic Acid from Smilacis Chinae Rhizome Protects Amyloid-β Protein (25–35)-Induced Neurotoxicity in Cultured Rat Cortical Neurons. Neurosci. Lett. 2007, 420, 184–188. [Google Scholar] [CrossRef]
- Ahmad, H.I.; Shoaib Khan, H.M.; Akhtar, N.; Ijaz, S. Phenolic, Flavonoid Content and Radical Scavenging Activity of Smilax china with Its Inhibitory Potential against Clinically Important Enzymes. Nat. Prod. Res. 2021, 35, 2066–2071. [Google Scholar] [CrossRef]
- Suelves, N.; Saleki, S.; Ibrahim, T.; Palomares, D.; Moonen, S.; Koper, M.J.; Vrancx, C.; Vadukul, D.M.; Papadopoulos, N.; Viceconte, N.; et al. Senescence-Related Impairment of Autophagy Induces Toxic Intraneuronal Amyloid-β Accumulation in a Mouse Model of Amyloid Pathology. Acta Neuropathol. Commun. 2023, 11, 82. [Google Scholar] [CrossRef]
- Ullah, N.; Lee, H.Y.; Naseer, M.I.; Ullah, I.; Suh, J.W.; Kim, M.O. Nicotinamide Inhibits Alkylating Agent-Induced Apoptotic Neurodegeneration in the Developing Rat Brain. PLoS ONE 2011, 6, e27093. [Google Scholar] [CrossRef]
- Sakamoto, T.; Cansev, M.; Wurtman, R.J. Oral Supplementation with Docosahexaenoic Acid and Uridine-5′-Monophosphate Increases Dendritic Spine Density in Adult Gerbil Hippocampus. Brain Res. 2007, 1182, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Lista, S.; González-Domínguez, R.; López-Ortiz, S.; González-Domínguez, Á.; Menéndez, H.; Martín-Hernández, J.; Lucia, A.; Emanuele, E.; Centonze, D.; Imbimbo, B.P.; et al. Integrative Metabolomics Science in Alzheimer’s Disease: Relevance and Future Perspectives. Ageing Res. Rev. 2023, 89, 101987. [Google Scholar] [CrossRef] [PubMed]
- Jasbi, P.; Shi, X.; Chu, P.; Elliott, N.; Hudson, H.; Jones, D.; Serrano, G.; Chow, B.; Beach, T.G.; Liu, L.; et al. Metabolic Profiling of Neocortical Tissue Discriminates Alzheimer’s Disease from Mild Cognitive Impairment, High Pathology Controls, and Normal Controls. J. Proteome Res. 2021, 20, 4303–4317. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, T.; Kanda, M.; Kitamura, H.; Morikawa, F.; Toru, S.; Nishimura, C.; Kasuga, K.; Tokutake, T.; Takahashi, T.; Kuroha, Y.; et al. Decreased Circulating Branched-Chain Amino Acids Are Associated with Development of Alzheimer’s Disease in Elderly Individuals with Mild Cognitive Impairment. Front. Nutr. 2022, 9, 1040476. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liu, P.; Li, S.; Li, B.; Li, Y.; Ma, L. Lysophospholipids and Branched Chain Amino Acids Are Associated with Aging: A Metabolomics-Based Study of Chinese Adults. Eur. J. Med. Res. 2023, 28, 58. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Regmi, D.; Islam, M.; Raja Somu, D.; Merk, V.; Du, D. Effects of Zinc and Carnosine on Aggregation Kinetics of Amyloid-Β40 Peptide. Arch. Biochem. Biophys. 2022, 32, 101333. [Google Scholar] [CrossRef] [PubMed]
- Hata, J.; Ohara, T.; Katakura, Y.; Shimizu, K.; Yamashita, S.; Yoshida, D.; Honda, T.; Hirakawa, Y.; Shibata, M.; Sakata, S.; et al. Association between Serum β-Alanine and Risk of Dementia: The Hisayama Study. Am. J. Epidemiol. 2019, 188, 1637–1645. [Google Scholar] [CrossRef]
- Kong, Y.; Wu, J.; Yuan, L. MicroRNA Expression Analysis of Adult-Onset Drosophila Alzheimer’s Disease Model. Curr. Alzheimer Res. 2014, 11, 882–891. [Google Scholar] [CrossRef]
- Altiné-Samey, R.; Antier, D.; Mavel, S.; Dufour-Rainfray, D.; Balageas, A.-C.; Beaufils, E.; Emond, P.; Foucault-Fruchard, L.; Chalon, S. The Contributions of Metabolomics in the Discovery of New Therapeutic Targets in Alzheimer’s Disease. Fundam. Clin. Pharmacol. 2021, 35, 582–594. [Google Scholar] [CrossRef]
- Gluck, M.R.; Thomas, R.G.; Davis, K.L.; Haroutunian, V. Implications for Altered Glutamate and GABA Metabolism in the Dorsolateral Prefrontal Cortex of Aged Schizophrenic Patients. Am. J. Psychiatry 2002, 159, 1165–1173. [Google Scholar] [CrossRef]
- Vesga-Jiménez, D.J.; Martin, C.; Barreto, G.E.; Aristizábal-Pachón, A.F.; Pinzón, A.; González, J. Fatty Acids: An Insight into the Pathogenesis of Neurodegenerative Diseases and Therapeutic Potential. Int. J. Mol. Sci. 2022, 23, 2577. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Khan, Z.A.; Shoeb, F.; Fatima, G.; Khan, R.H.; Khan, M.M. Role of de Novo Lipogenesis in Inflammation and Insulin Resistance in Alzheimer’s Disease. Int. J. Biol. Macromol. 2023, 242, 124859. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, J.; Jiang, F.; Xu, Y.-J.; Liu, Y. Metabolomics Reveals the Impact of the Saturation of Dietary Lipids on the Aging and Longevity of C. elegans. Mol. Omics 2022, 18, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Khurana, N.; Kaur, S.; Ali, N.; AlAsmari, A.F.; Waseem, M.; Iqbal, M.; Alzahrani, F.M.; Sharma, N. The Multifactorial Role of Vanillin in Amelioration of Aluminium Chloride and D-Galactose Induced Alzheimer’s Disease in Mice. Eur. J. Pharmacol. 2023, 954, 175832. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Yan, S.; Zheng, J.; Gao, Y.; Zhang, S.; Liu, Z.; Liu, X.; Xiao, C. Eriodictyol Attenuates LPS-Induced Neuroinflammation, Amyloidogenesis, and Cognitive Impairments via the Inhibition of NF-κB in Male C57BL/6J Mice and BV2 Microglial Cells. J. Agric. Food Chem. 2018, 66, 10205–10214. [Google Scholar] [CrossRef]
- Li, L.; Li, W.-J.; Zheng, X.-R.; Liu, Q.-L.; Du, Q.; Lai, Y.-J.; Liu, S.-Q. Eriodictyol Ameliorates Cognitive Dysfunction in APP/PS1 Mice by Inhibiting Ferroptosis via Vitamin D Receptor-Mediated Nrf2 Activation. Mol. Med. 2022, 28, 11. [Google Scholar] [CrossRef]
- Mett, J.; Müller, U. The Medium-Chain Fatty Acid Decanoic Acid Reduces Oxidative Stress Levels in Neuroblastoma Cells. Sci. Rep. 2021, 11, 6135. [Google Scholar] [CrossRef]
- Ozlu, C.; Chelliah, P.; Dahshi, H.; Horton, D.; Edgar, V.B.; Messahel, S.; Kayani, S. ECHS1 Deficiency and Its Biochemical and Clinical Phenotype. Am. J. Med. Genet. Part A 2022, 188, 2908–2919. [Google Scholar] [CrossRef]
- François-Heude, M.-C.; Lebigot, E.; Roze, E.; Warde, M.T.A.; Cances, C.; Damaj, L.; Espil, C.; Fluss, J.; de Lonlay, P.; Kern, I.; et al. Movement Disorders in Valine Métabolism Diseases Caused by HIBCH and ECHS1 Deficiencies. Eur. J. Neurol. 2022, 29, 3229–3242. [Google Scholar] [CrossRef]
- Olgiati, S.; Skorvanek, M.; Quadri, M.; Minneboo, M.; Graafland, J.; Breedveld, G.J.; Bonte, R.; Ozgur, Z.; van den Hout, M.C.G.N.; Schoonderwoerd, K.; et al. Paroxysmal Exercise-Induced Dystonia within the Phenotypic Spectrum of ECHS1 Deficiency. Mov. Disord 2016, 31, 1041–1048. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, X.; Duan, X. Effect of Decanoic Acid on the Cognitive Function of AD Mice. Planta Med. 2017, 8, 48–51. [Google Scholar] [CrossRef]
- Seppälä, I.; Kleber, M.E.; Bevan, S.; Lyytikäinen, L.-P.; Oksala, N.; Hernesniemi, J.A.; Mäkelä, K.-M.; Rothwell, P.M.; Sudlow, C.; Dichgans, M.; et al. Associations of Functional Alanine-Glyoxylate Aminotransferase 2 Gene Variants with Atrial Fibrillation and Ischemic Stroke. Sci. Rep. 2016, 6, 23207. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Li, Y.; Wang, L.; Fang, X.; Zhang, J.; He, L.; Yang, L.; Li, D.; Geng, H. CRISPR/Cas9–Mediated Metabolic Pathway Reprogramming in a Novel Humanized Rat Model Ameliorates Primary Hyperoxaluria Type 1. Kidney Int. 2020, 98, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Vignoli, A.; Paciotti, S.; Tenori, L.; Eusebi, P.; Biscetti, L.; Chiasserini, D.; Scheltens, P.; Turano, P.; Teunissen, C.; Luchinat, C.; et al. Fingerprinting Alzheimer’s Disease by 1H Nuclear Magnetic Resonance Spectroscopy of Cerebrospinal Fluid. J. Proteome Res. 2020, 19, 1696–1705. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, L.; Deng, Y.; Du, Y.; Fang, X.; Fang, X.; Zhang, Q. Metabolic Regulations of Smilax china L. against β-Amyloid Toxicity in Caenorhabditis elegans. Metabolites 2024, 14, 49. https://doi.org/10.3390/metabo14010049
Yan L, Deng Y, Du Y, Fang X, Fang X, Zhang Q. Metabolic Regulations of Smilax china L. against β-Amyloid Toxicity in Caenorhabditis elegans. Metabolites. 2024; 14(1):49. https://doi.org/10.3390/metabo14010049
Chicago/Turabian StyleYan, Lili, Yuchan Deng, Yulan Du, Xutong Fang, Xin Fang, and Qiang Zhang. 2024. "Metabolic Regulations of Smilax china L. against β-Amyloid Toxicity in Caenorhabditis elegans" Metabolites 14, no. 1: 49. https://doi.org/10.3390/metabo14010049
APA StyleYan, L., Deng, Y., Du, Y., Fang, X., Fang, X., & Zhang, Q. (2024). Metabolic Regulations of Smilax china L. against β-Amyloid Toxicity in Caenorhabditis elegans. Metabolites, 14(1), 49. https://doi.org/10.3390/metabo14010049






