Impact of Reperfusion on Plasma Oxylipins in ST-Segment Elevation Myocardial Infarction
Abstract
:1. Introduction
2. Materials and Methods
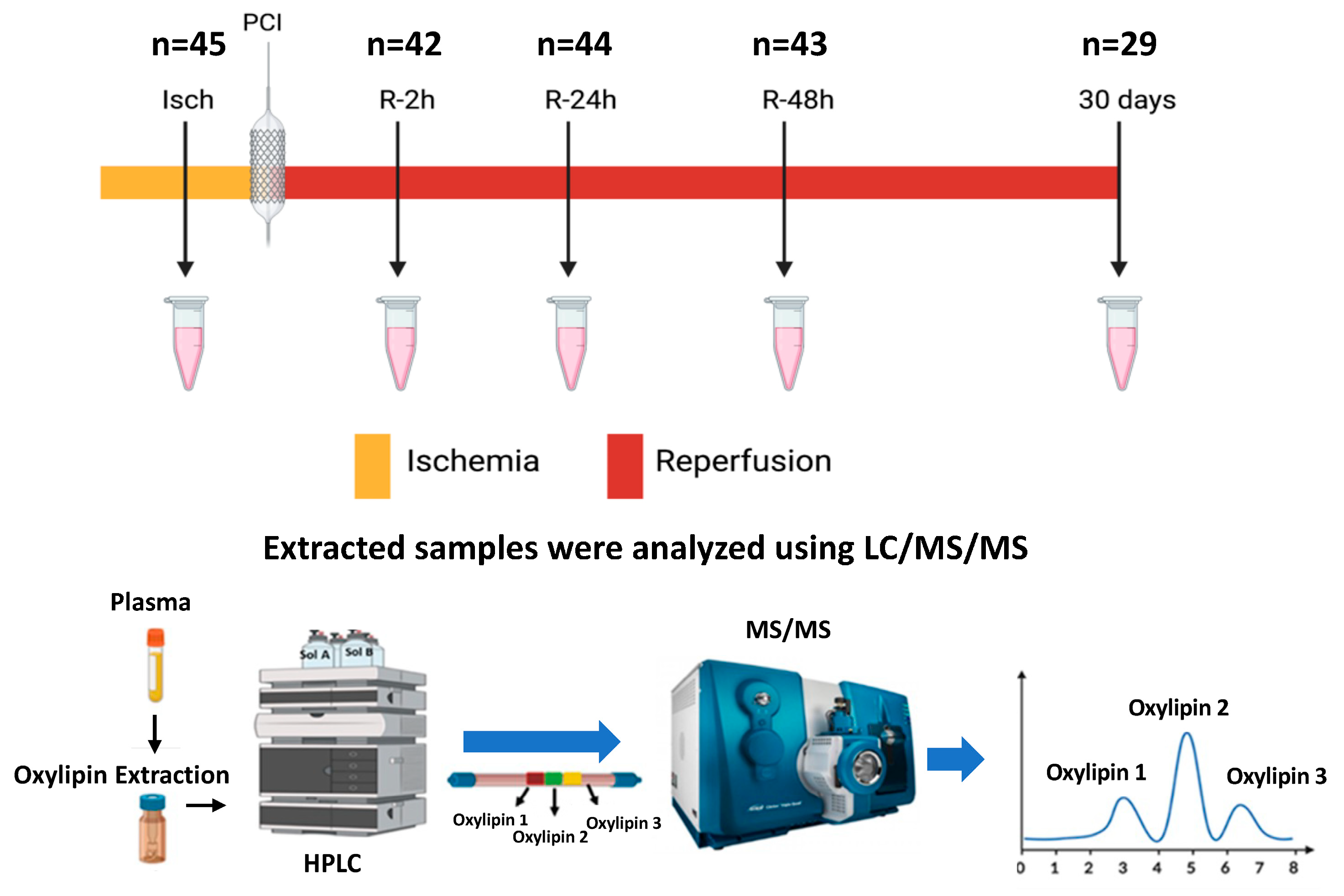
2.1. Study Population
2.2. Free Oxylipin Analysis
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
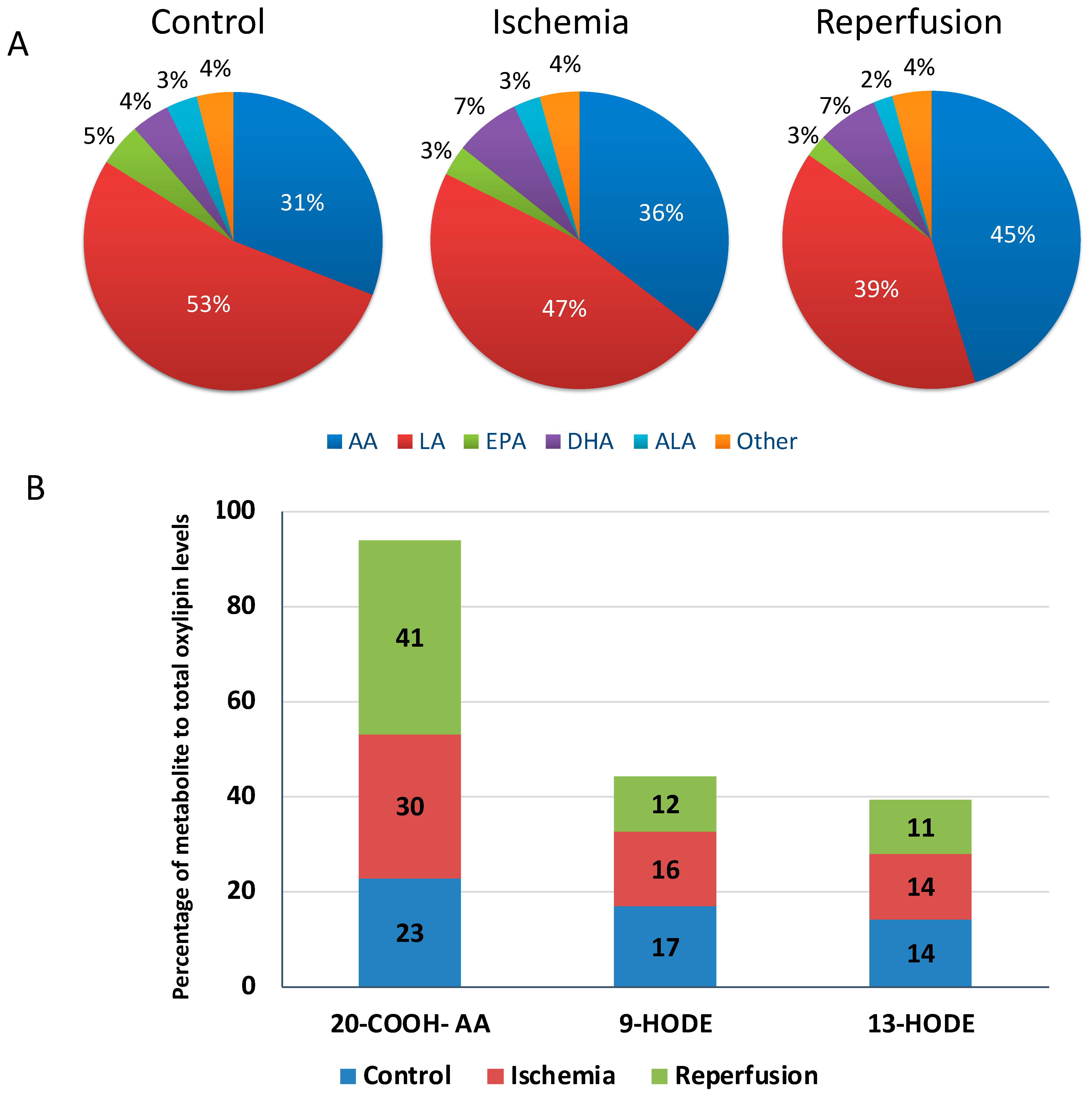
3.2. Overall Characterization of Plasma Oxylipins Levels in STEMI Patients and Controls
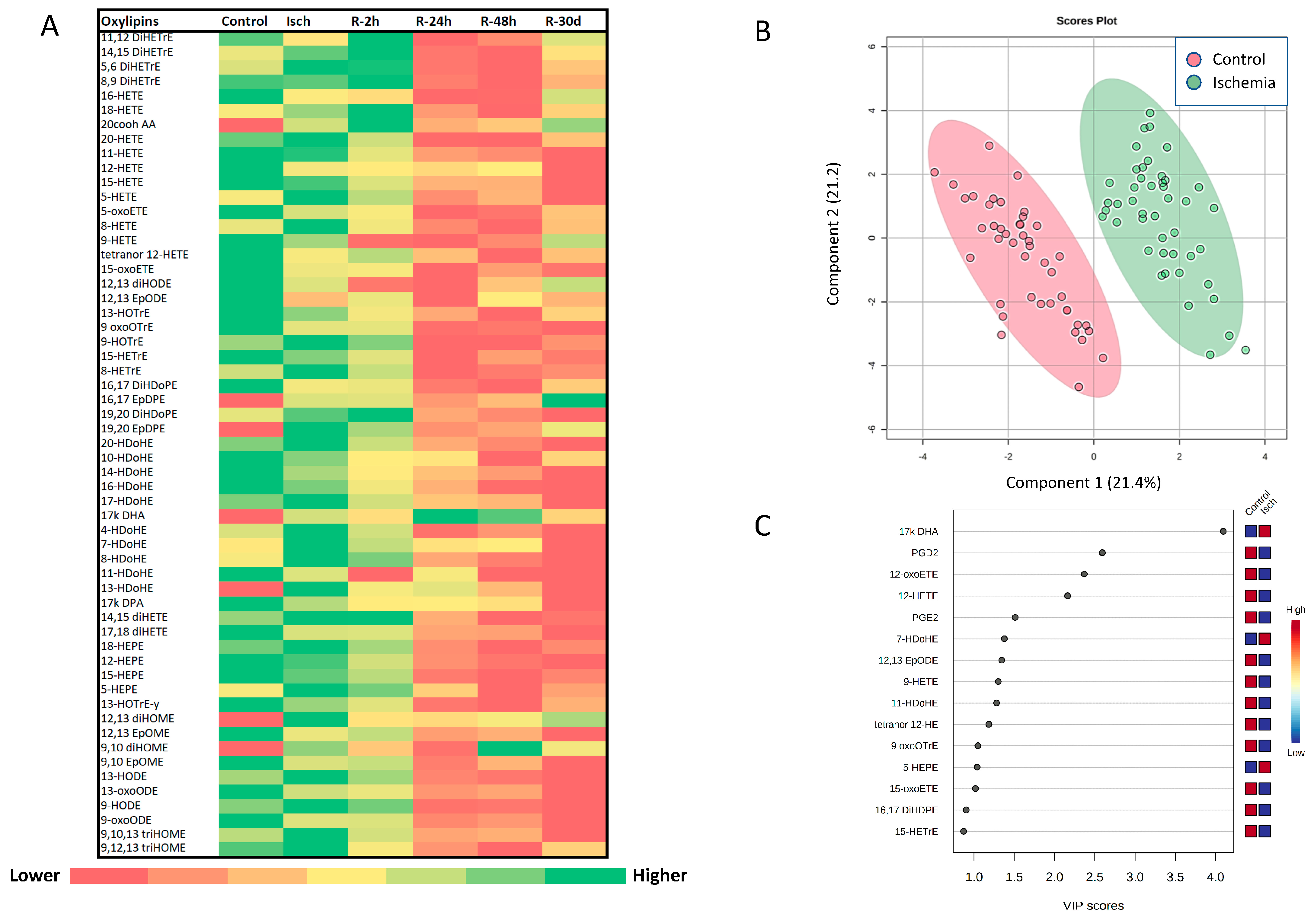
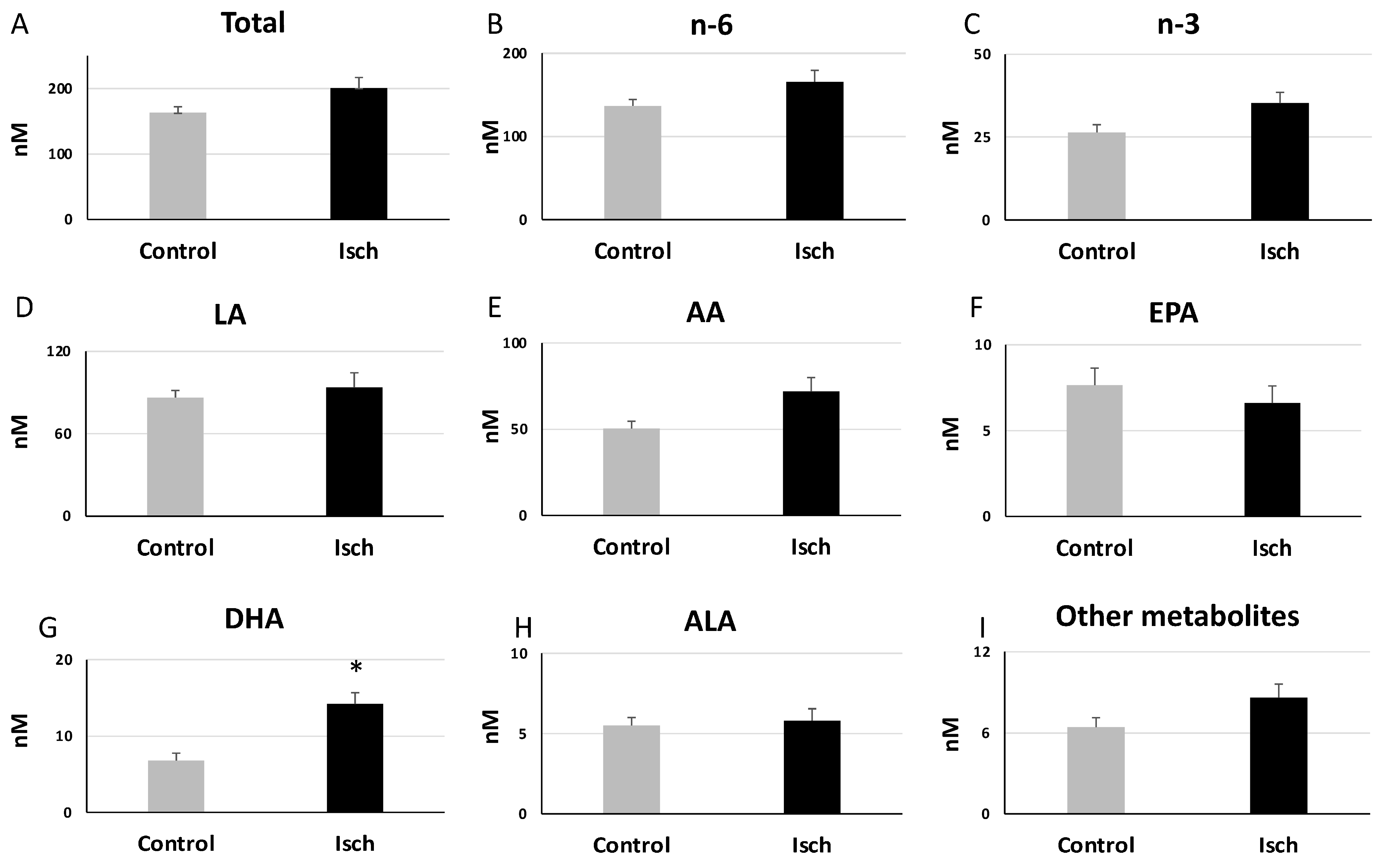
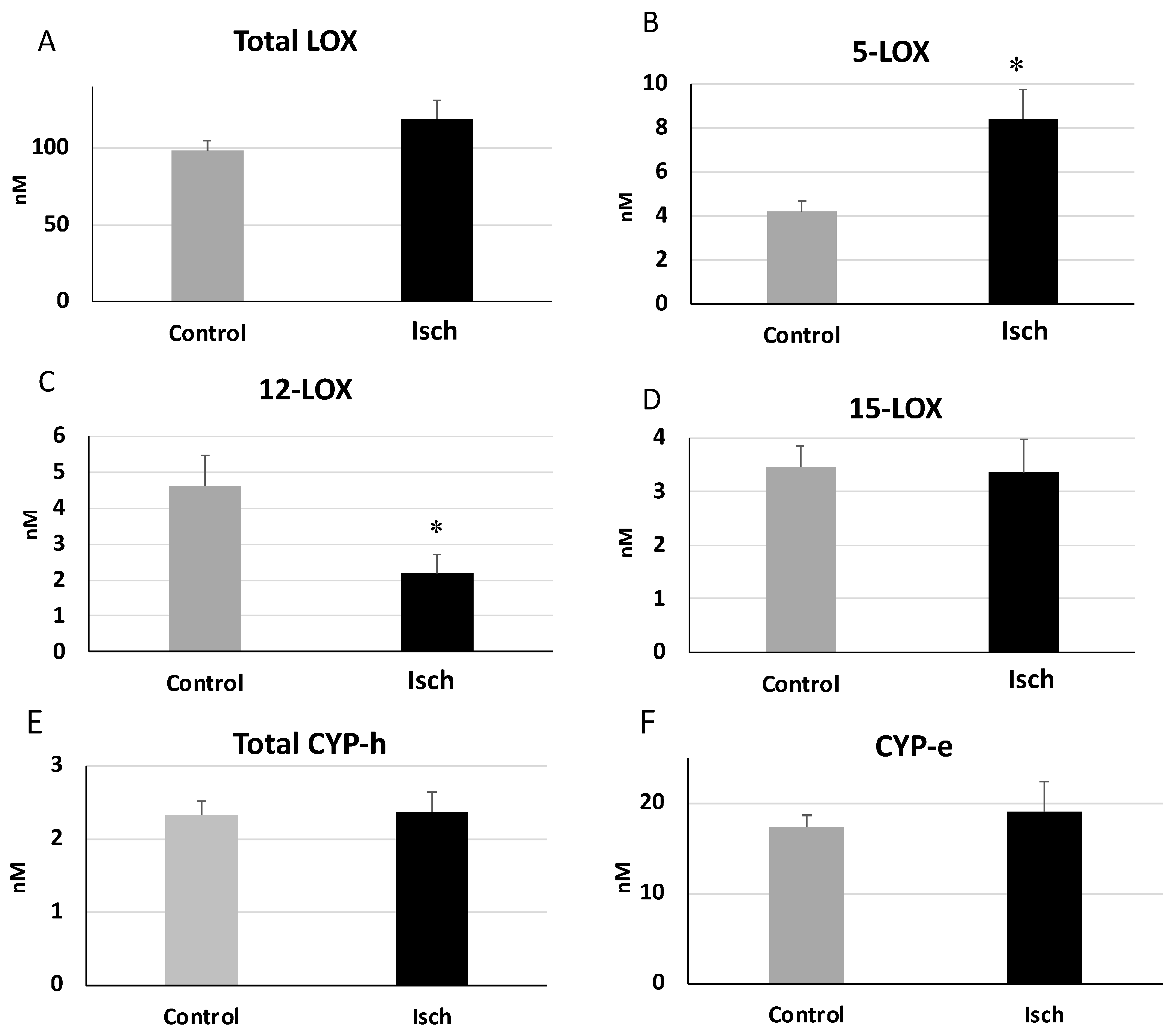
3.3. Alterations in the Plasma Oxylipin Profile of Ischemic STEMI Patients (Isch Group) Compared to Controls
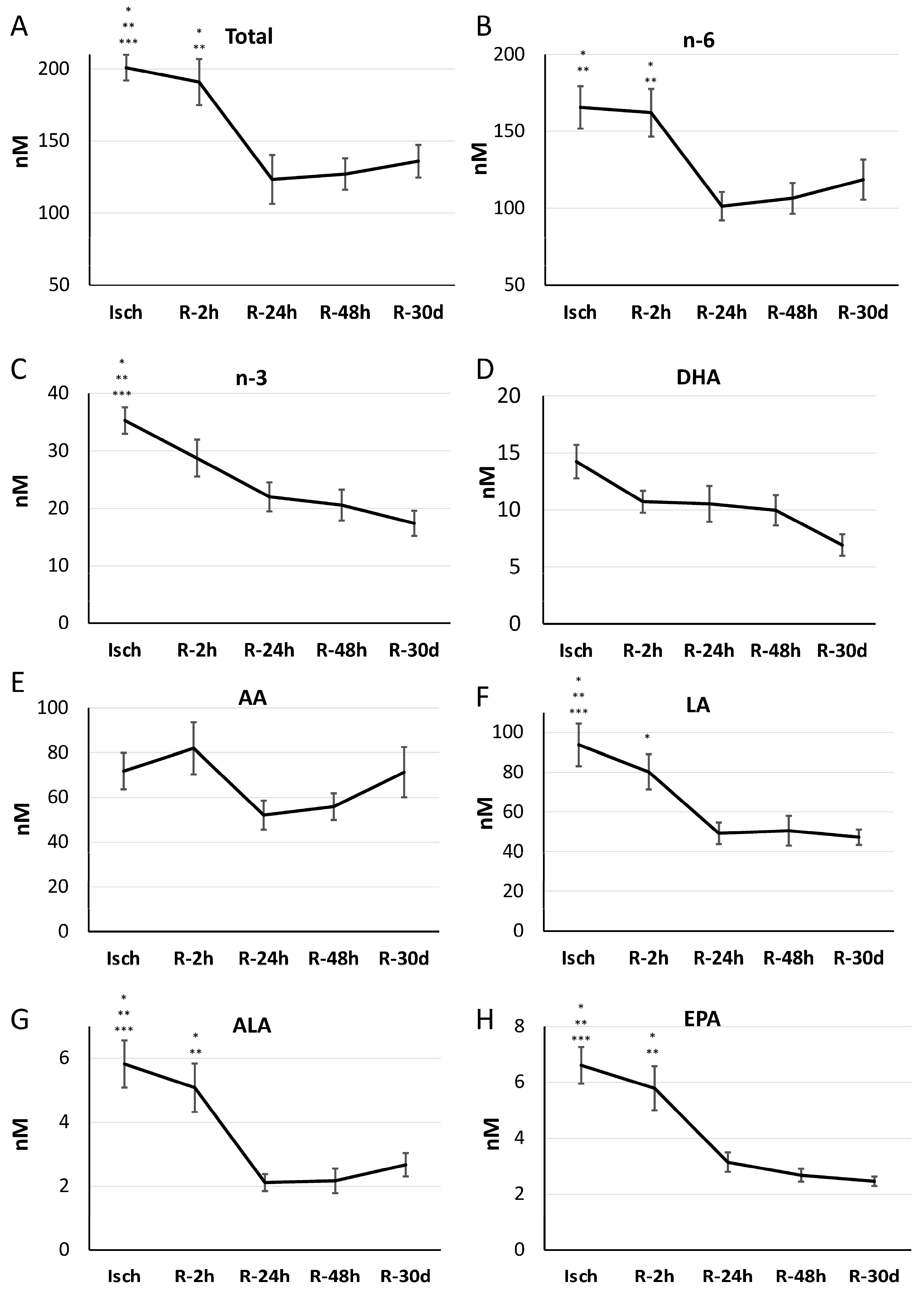
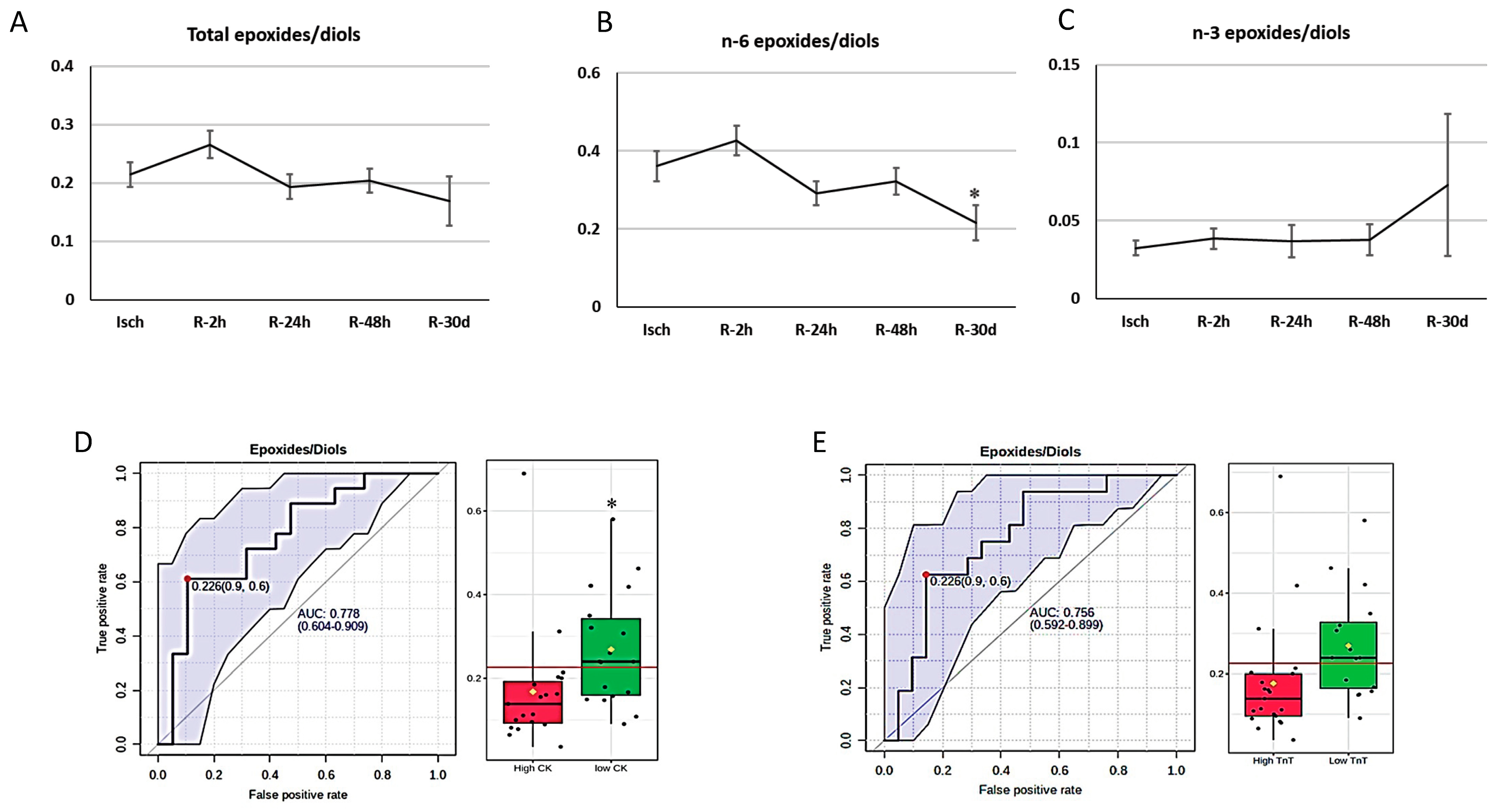
3.4. Impact of Reperfusion on Plasma Oxylipin Profile
3.5. Oxylipins and Cardiac Biomarkers
4. Discussion
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, A.; Cannon, C.P. Acute Coronary Syndromes: Diagnosis and Management, Part I; Elsevier: Amsterdam, The Netherlands, 2009; pp. 917–938. [Google Scholar]
- Akhtar, M.M.; Iqbal, Y.H.; Kaski, J.C. Managing unstable angina and non-ST elevation MI. Practitioner 2010, 254, 25–31. [Google Scholar]
- Kingma, J. Myocardial infarction: An overview of STEMI and NSTEMI physiopathology and treatment. World J. Cardiovasc. Dis. 2018, 8, 498. [Google Scholar] [CrossRef]
- Al-Furaih, N.; Janus, S.E.; Hackler, I.I.I.E.; Hajjari, J.; Al-Kindi, S.G. Cardiogenic shock complicating myocardial infarction: Mortality trends in the United States from the past two decades. J. Cardiovasc. Med. 2022, 23, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Morddarvanjoghi, F.; Abdolmaleki, A.; Rasoulpoor, S.; Khaleghi, A.A.; Hezarkhani, L.A.; Shohaimi, S.; Mohammadi, M. The global prevalence of myocardial infarction: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2023, 23, 206. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart disease and stroke statistics—2022 update: A report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Adeyomoye, O.; Olaniyan, O.T.; Adetunji, C.O. Production of Polyunsaturated Fatty Acids (PUFAs) and Their Biomedical Application. In Next-Generation Algae: Volume II: Applications in Medicine and the Pharmaceutical Industry; Wiley-Scrivener: Salem, MA, USA, 2023; pp. 125–137. [Google Scholar]
- Mitra, S.; Ray, S. Percutaneous Coronary Intervention in Acute Myocardial Infarction. In The Protocol Book for Intensive Care; Jp Medical Ltd.: London, UK, 2018; p. 277. [Google Scholar]
- Prabhu, S.D.; Frangogiannis, N.G. The Biological Basis for Cardiac Repair after Myocardial Infarction: From Inflammation to Fibrosis. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Y.; Yiang, G.T.; Liao, W.T.; Po, A.; Tsai, Y.; Cheng, Y.L.; Cheng, P.W.; Li, C.Y.; Li, C.J. Cellular Physiology and Biochemistry Cellular Physiology and Biochemistry Review Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Melissa Gabbs Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in our understanding of oxylipins derived from dietary PUFAs. Adv. Nutr. 2015, 6, 513–540. [Google Scholar]
- Christie, W.W.; Harwood, J.L. Oxidation of polyunsaturated fatty acids to produce lipid mediators. Essays Biochem. 2020, 64, 401–421. [Google Scholar] [PubMed]
- Koch, E.; Mainka, M.; Dalle, C.; Ostermann, A.I.; Rund, K.M.; Kutzner, L.; Froehlich, L.F.; Bertrand-Michel, J.; Gladine, C.; Schebb, N.H. Stability of oxylipins during plasma generation and long-term storage. Talanta 2020, 217, 121074. [Google Scholar] [CrossRef]
- Narumiya, S. Physiology and pathophysiology of prostanoid receptors. Proc. Jpn. Acad. Ser. B 2007, 83, 296–319. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. “Cell Membrane Theory of Senescence” and the Role of Bioactive Lipids in Aging, and Aging Associated Diseases and Their Therapeutic Implications. Biomolecules 2021, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.C.; Wheelock, C.E.; Haeggström, J.Z. The Eicosanoids, Redox-Regulated Lipid Mediators in Immunometabolic Disorders. Antioxid. Redox Signal. 2018, 29, 275–296. [Google Scholar] [CrossRef] [PubMed]
- Oliw, E.H. Oxygenation of polyunsaturated fatty acids by cytochrome P450 monooxygenates. Prog. Lipid Res. 1994, 33, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Kytikova, O.; Denisenko, Y.; Novgorodtseva, T.; Bocharova, N.; Kovalenko, I. Fatty acid epoxides in the regulation of the inflammation. Biomeditsinskaya Khimiya 2022, 68, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Spector, A.A.; Kim, H.-Y. Cytochrome P450 epoxygenase pathway of polyunsaturated fatty acid metabolism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.L.; Edson, K.Z.; Totah, R.A.; Rettie, A.E. Cytochrome P450 ω-Hydroxylases in Inflammation and Cancer. Adv. Pharmacol. 2015, 74, 223–262. [Google Scholar]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.M.; Lein, P.J.; et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef]
- Jenkins, C.M.; Cedars, A.; Gross, R.W. Eicosanoid signalling pathways in the heart. Cardiovasc. Res. 2008, 82, 240–249. [Google Scholar] [CrossRef]
- Westphal, C.; Konkel, A.; Schunck, W.H. Cytochrome p450 enzymes in the bioactivation of polyunsaturated Fatty acids and their role in cardiovascular disease. In Monooxygenase, Peroxidase and Peroxygenase Properties and Mechanisms of Cytochrome P450; Springer: Berlin/Heidelberg, Germany, 2015; pp. 151–187. [Google Scholar]
- Fosshaug, L.E.; Colas, R.A.; Anstensrud, A.K.; Gregersen, I.; Nymo, S.; Sagen, E.L.; Michelsen, A.; Vinge, L.E.; Øie, E.; Gullestad, L.; et al. Early increase of specialized pro-resolving lipid mediators in patients with ST-elevation myocardial infarction. EBioMedicine 2019, 46, 264–273. [Google Scholar] [CrossRef]
- Horii, Y.; Nakaya, M.; Ohara, H.; Nishihara, H.; Watari, K.; Nagasaka, A.; Nakaya, T.; Sugiura, Y.; Okuno, T.; Koga, T.; et al. Leukotriene B4 receptor 1 exacerbates inflammation following myocardial infarction. FASEB J. 2020, 34, 8749–8763. [Google Scholar] [CrossRef]
- Monirujjaman, M.; Devassy, J.G.; Yamaguchi, T.; Sidhu, N.; Kugita, M.; Gabbs, M.; Nagao, S.; Zhou, J.; Ravandi, A.; Aukema, H.M. Distinct oxylipin alterations in diverse models of cystic kidney diseases. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2017, 1862, 1562–1574. [Google Scholar] [CrossRef] [PubMed]
- Leng, S.; Winter, T.; Aukema, H.M. Dietary LA and sex effects on oxylipin profiles in rat kidney, liver, and serum differ from their effects on PUFAs. J. Lipid Res. 2017, 58, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Deems, R.; Buczynski, M.W.; Bowers-Gentry, R.; Harkewicz, R.; Dennis, E.A. Detection and Quantitation of Eicosanoids via High Performance Liquid Chromatography-Electrospray Ionization-Mass Spectrometry. Methods Enzymol. 2007, 432, 59–82. [Google Scholar] [PubMed]
- Dennis, E.A.; Cao, J.; Hsu, Y.H.; Magrioti, V.; Kokotos, G. Phospholipase A2 enzymes: Physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 2011, 111, 6130–6185. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Lin, J.; Gong, Y.; Bi, X.; Hu, S.; Lv, Q.; Chen, J.; Li, X.; Chen, J.; Zhang, W.; et al. iPLA2β contributes to ER stress-induced apoptosis during myocardial ischemia/reperfusion injury. Cells 2021, 10, 1446. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P.; Libby, P.; Bhatt, D.L. Emerging Mechanisms of Cardiovascular Protection for the Omega-3 Fatty Acid Eicosapentaenoic Acid. Arter. Thromb. Vasc. Biol. 2020, 40, 1135–1147. [Google Scholar] [CrossRef]
- Fang, X.; Dillon, J.S.; Hu, S.; Harmon, S.D.; Yao, J.; Anjaiah, S.; Falck, J.R.; Spector, A.A. 20-Carboxy-arachidonic acid is a dual activator of peroxisome proliferator-activated receptors α and γ. Prostaglandins Other Lipid Mediat. 2007, 82, 175–184. [Google Scholar] [CrossRef]
- Whelan, J.; Fritsche, K. Linoleic acid. Adv. Nutr. 2013, 4, 311–312. [Google Scholar] [CrossRef]
- Blasbalg, T.L.; Hibbeln, J.R.; Ramsden, C.E.; Majchrzak, S.F.; Rawlings, R.R. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr. 2011, 93, 950–962. [Google Scholar] [CrossRef] [PubMed]
- Quehenberger, O.; Armando, A.M.; Brown, A.H.; Milne, S.B.; Myers, D.S.; Merrill, A.H.; Bandyopadhyay, S.; Jones, K.N.; Kelly, S.; Shaner, R.L.; et al. Lipidomics reveals a remarkable diversity of lipids in human plasma 1 [S]. J. Lipid Res. 2010, 51, 3299–3305. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Quehenberger, O.; Armando, A.; Dennis, E.A. Polyunsaturated fatty acid metabolites as novel lipidomic biomarkers for noninvasive diagnosis of nonalcoholic steatohepatitis1. J. Lipid Res. 2015, 56, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Petasis, N.A. Resolvins and protectins in inflammation resolution. Chem. Rev. 2011, 111, 5922–5943. [Google Scholar] [CrossRef] [PubMed]
- Ravingerova, T.; Adameova, A.; Carnicka, S.; Nemcekova, M.; Kelly, T.; Matejikova, J.; Galatou, E.; Barlaka, E.; Lazou, A. The role of PPAR in myocardial response to ischemia in normal and diseased heart. Gen. Physiol. Biophys. 2011, 30, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Yue T li Bao, W.; Jucker, B.M.; Gu J li Romanic, A.M.; Brown, P.J.; Cui, J.; Thudium, D.T.; Boyce, R.; Burns-Kurtis, C.L.; Mirabile, R.C. Activation of peroxisome proliferator–activated receptor-α protects the heart from ischemia/reperfusion injury. Circulation 2003, 108, 2393–2399. [Google Scholar]
- Lotz, C.; Lazariotto, M.; Redel, A.; Smul, T.M.; Stumpner, J.; Blomeyer, C.; Tischer-Zeitz, T.; Schmidt, J.; Pociej, J.; Roewer, N.; et al. Activation of peroxisome-proliferator-activated receptors α and γ mediates remote ischemic preconditioning against myocardial infarction in vivo. Exp. Biol. Med. 2011, 236, 113–122. [Google Scholar] [CrossRef]
- Egawa, D.; Itoh, T.; Akiyama, Y.; Saito, T.; Yamamoto, K. 17-OxoDHA is a PPARα/γ dual covalent modifier and agonist. ACS Chem. Biol. 2016, 11, 2447–2455. [Google Scholar] [CrossRef]
- Heydari, B.; Abdullah, S.; Pottala, J.V.; Shah, R.; Abbasi, S.; Mandry, D.; Francis, S.A.; Lumish, H.; Ghoshhajra, B.B.; Hoffmann, U.; et al. Effect of omega-3 acid ethyl esters on left ventricular remodeling after acute myocardial infarction: The OMEGA-REMODEL randomized clinical trial. Circulation 2016, 134, 378–391. [Google Scholar] [CrossRef]
- Yuan, M.; Zhang, Y.; Hua, T.; Liu, X.L.; Liu, T.; Yuan, R.Y.; Li, G.P.; Zhu, Y.; Zhang, X. Omega-3 polyunsaturated fatty acid supplementation improves lipid metabolism and endothelial function by providing a beneficial eicosanoid-pattern in patients with acute myocardial infarction: A randomized, controlled trial. Clin. Nutr. 2021, 40, 445–459. [Google Scholar] [CrossRef]
- Zirpoli, H.; Abdillahi, M.; Quadri, N.; Ananthakrishnan, R.; Wang, L.; Rosario, R.; Zhu, Z.; Deckelbaum, R.J.; Ramasamy, R. Acute administration of n-3 rich triglyceride emulsions provides cardioprotection in murine models after ischemia-reperfusion. PLoS ONE 2015, 10, e0116274. [Google Scholar] [CrossRef]
- Blömer, N.; Pachel, C.; Hofmann, U.; Nordbeck, P.; Bauer, W.; Mathes, D.; Frey, A.; Bayer, B.; Vogel, B.; Ertl, G.; et al. 5-Lipoxygenase facilitates healing after myocardial infarction. Basic Res. Cardiol. 2013, 108, 367. [Google Scholar] [CrossRef] [PubMed]
- Maskrey, B.H.; Rushworth, G.F.; Law, M.H.; Treweeke, A.T.; Wei, J.; Leslie, S.J.; Megson, I.L.; Whitfield, P.D. 12-hydroxyeicosatetraenoic acid is associated with variability in aspirin-induced platelet inhibition. J. Inflamm. Lond. Engl. 2014, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Eynard, A.R.; Galli, G.; Tremoli, E.; Maderna, P.; Magni, F.; Paoletti, R. Aspirin inhibits platelet 12-hydroxy-eicosatetraenoic acid formation. J. Lab. Clin. Med. 1986, 107, 66–72. [Google Scholar]
- Tremoli, E.; Maderna, P.; Eynard, A.; Gregori, M.; Galli, G. In vitro effects of aspirin and non steroidal anti-inflammatory drugs on the formation of 12-hydroxyeicosatetraenoic acid by platelets. Prostaglandins Leukot. Med. 1986, 23, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Maderna, P.; Caruso, D.; Tremoli, E.; Galli, G.; Grossi, E. Differential effects of oral administrations to human volunteers of acetylsalicylic acid, sodium salicylate and indomethacin on 12-hydroxyeicosatetraenoic acid formation by stimulated platelets. Thromb. Res. 1988, 52, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, A.; Sandstedt, M.; Sandstedt, J.; Wickelgren, R.; Hansson, G.I.; Jeppsson, A.; Hultén, L.M. The arachidonate 15-lipoxygenase enzyme product 15-HETE is present in heart tissue from patients with ischemic heart disease and enhances clot formation. PLoS ONE 2016, 11, e0161629. [Google Scholar] [CrossRef] [PubMed]
- Kaduce, T.L.; Fang, X.; Harmon, S.D.; Oltman, C.L.; Dellsperger, K.C.; Teesch, L.M.; Gopal, V.R.; Falck, J.R.; Campbell, W.B.; Weintraub, N.L.; et al. 20-hydroxyeicosatetraenoic acid (20-HETE) metabolism in coronary endothelial cells. J. Biol. Chem. 2004, 279, 2648–2656. [Google Scholar] [CrossRef] [PubMed]
- Grapov, D.; Fiehn, O.; Campbell, C.; Chandler, C.J.; Burnett, D.J.; Souza, E.C.; Casazza, G.A.; Keim, N.L.; Hunter, G.R.; Fernandez, J.R.; et al. Impact of a weight loss and fitness intervention on exercise-associated plasma oxylipin patterns in obese, insulin-resistant, sedentary women. Physiol. Rep. 2020, 8, e14547. [Google Scholar] [CrossRef]
- Wendell, S.G.; Golin-Bisello, F.; Wenzel, S.; Sobol, R.W.; Holguin, F.; Freeman, B.A. 15-Hydroxyprostaglandin dehydrogenase generation of electrophilic lipid signaling mediators from hydroxy ω-3 fatty acids. J. Biol. Chem. 2015, 290, 5868–5880. [Google Scholar] [CrossRef]
- Groeger, A.L.; Cipollina, C.; Cole, M.P.; Woodcock, S.R.; Bonacci, G.; Rudolph, T.K.; Rudolph, V.; Freeman, B.A.; Schopfer, F.J. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat. Chem. Biol. 2010, 6, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Hanif, A.; Edin, M.L.; Zeldin, D.C.; Morisseau, C.; Nayeem, M.A. Effect of soluble epoxide hydrolase on the modulation of coronary reactive hyperemia: Role of oxylipins and PPARγ. PloS ONE 2016, 11, e0162147. [Google Scholar] [CrossRef] [PubMed]
- Nayeem, M.A. Role of oxylipins in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1142–1154. [Google Scholar] [CrossRef] [PubMed]
- Edin, M.L.; Wang, Z.; Bradbury, J.A.; Graves, J.P.; Lih, F.B.; DeGraff, L.M.; Foley, J.F.; Torphy, R.; Ronnekleiv, O.K.; Tomer, K.B.; et al. Endothelial expression of human cytochrome P450 epoxygenase CYP2C8 increases susceptibility to ischemia-reperfusion injury in isolated mouse heart. FASEB J. 2011, 25, 3436. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, M.F.; Grant, D.F.; Cheek, J.M.; Greene, J.F.; Williamson, K.C.; Hammock, B.D. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat. Med. 1997, 3, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Koerner, I.P.; Jacks, R.; DeBarber, A.E.; Koop, D.; Mao, P.; Grant, D.F.; Alkayed, N.J. Polymorphisms in the human soluble epoxide hydrolase gene EPHX2 linked to neuronal survival after ischemic injury. J. Neurosci. 2007, 27, 4642–4649. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; North, K.E.; Bray, M.S.; Fornage, M.; Seubert, J.M.; Newman, J.W.; Hammock, B.D.; Couper, D.J.; Heiss, G.; Zeldin, D.C. Genetic variation in soluble epoxide hydrolase (EPHX2) and risk of coronary heart disease: The Atherosclerosis Risk in Communities (ARIC) study. Hum. Mol. Genet. 2006, 15, 1640–1649. [Google Scholar] [CrossRef]
- Solati, Z. Oxolipidomic Characterization of Ischemia-Reperfusion Injury. 2021. Available online: https://mspace.lib.umanitoba.ca/bitstreams/505ea4f7-c1f8-420e-9644-05f6fa36d59d/download (accessed on 22 December 2023).
- Samokhvalov, V.; Jamieson, K.L.; Darwesh, A.M.; Keshavarz-Bahaghighat, H.; Lee, T.Y.; Edin, M.; Lih, F.; Zeldin, D.C.; Seubert, J.M. Deficiency of soluble epoxide hydrolase protects cardiac function impaired by LPS-induced acute inflammation. Front. Pharmacol. 2019, 9, 1572. [Google Scholar] [CrossRef]
- Calder, P.C.; Deckelbaum, R.J. Intravenous fish oil in hospitalized adult patients: Reviewing the reviews. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 119–123. [Google Scholar] [CrossRef]
- Berger, M.M.; Delodder, F.; Liaudet, L.; Tozzi, P.; Schlaepfer, J.; Chiolero, R.L.; Tappy, L. Three short perioperative infusions of n-3 PUFAs reduce systemic inflammation induced by cardiopulmonary bypass surgery: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 97, 246–254. [Google Scholar] [CrossRef]
- Carpentier, Y.A.; Hacquebard, M.; Portois, L.; Dupont, I.E.; Deckelbaum, R.J.; Malaisse, W.J. Rapid cellular enrichment of eicosapentaenoate after a single intravenous injection of a novel medium-chain triacylglycerol: Fish-oil emulsion in humans. Am. J. Clin. Nutr. 2010, 91, 875–882. [Google Scholar] [CrossRef]
- Motoki, A.; Merkel, M.J.; Packwood, W.H.; Cao, Z.; Liu, L.; Iliff, J.; Alkayed, N.J.; Van Winkle, D.M. Soluble epoxide hydrolase inhibition and gene deletion are protective against myocardial ischemia-reperfusion injury in vivo. Am. J. Physiol.-Heart Circ. Physiol. 2008, 295, H2128–H2134. [Google Scholar] [CrossRef]
- Chaudhary, K.R.; Zordoky, B.N.; Edin, M.L.; Alsaleh, N.; El-Kadi, A.O.; Zeldin, D.C.; Seubert, J.M. Differential effects of soluble epoxide hydrolase inhibition and CYP2J2 overexpression on postischemic cardiac function in aged mice. Prostaglandins Other Lipid Mediat. 2013, 104, 8–17. [Google Scholar] [CrossRef]
- Darwesh, A.M.; Keshavarz-Bahaghighat, H.; Jamieson, K.L.; Seubert, J.M. Genetic deletion or pharmacological inhibition of soluble epoxide hydrolase ameliorates cardiac ischemia/reperfusion injury by attenuating NLRP3 inflammasome activation. Int. J. Mol. Sci. 2019, 20, 3502. [Google Scholar] [CrossRef]

| Characteristics | STEMI Patients (n = 45) | Controls (n = 44) | p-Value |
|---|---|---|---|
| Male % | 66.60 | 56.30 | 0.08 |
| Age, yr (mean ± SEM) | 65.20 ± 2.08 | 60.20 ± 1.49 | 0.06 |
| Body mass index (BMI) (mean ± SEM) | 25.80 ± 1.14 | 30.2 ± 1.05 | 0.001 * |
| Left ventricular ejection fraction (LVEF) % | 62.00 | - | |
| Time (min), onset of chest pain to reperfusion (Median (min-max)) | 150 (52–738) | NA | |
| Left anterior descending coronary artery (LAD) Infarct (%) | 41.60 | NA | |
| Right coronary artery (RCA) Infarct (%) | 50.00 | NA | |
| Circumflex Infarct (%) | 8.00 | NA | |
| Peak CK (Median (min-max)) (units/L) | 1105 (141–10,655) | NA | |
| Peak TnT (Median (min-max)) (ng/L) | 1093 (1–10,000) | NA | |
| Co-morbidity | |||
| Type 2 diabetes mellitus (%) | 20.80 | 14.50 | 0.40 |
| Smoker (%) | 12.70 | 18.70 | 0.30 |
| Hypertension (%) | 41.60 | 48.10 | 0.50 |
| Dyslipidemia (%) | 41.60 | 25.90 | 0.08 |
| Lipids | |||
| Triglyceride (TG) (mean ± SEM) (mmol/L) | 1.70 ± 1.40 | - | |
| High-density lipoprotein (HDL) (mean ± SEM) (mmol/L) | 1.20 ± 0.40 | - | |
| Low-density lipoprotein (LDL) (mean ± SEM) (mmol/L) | 2.80 ± 0.90 | - | |
| Total cholesterol (mean ± SEM) (mmol/L) | 4.20 ± 1.20 | - | |
| Medications at baseline | |||
| ACEI/ARB (%) | 20.80 | 21.80 | 0.90 |
| Betablocker (%) | 6.20 | 16.30 | 0.10 |
| Statin (%) | 16.60 | 14.50 | 0.70 |
| Metabolite | Fatty Acid Precursor | Pathway | CK (r) | p Value | TnT (r) | p Value |
|---|---|---|---|---|---|---|
| DHA-derived metabolites (total) | NA | NA | 0.33 | 0.046 * | 0.50 | 1.00 × 10−3 ** |
| 11-HDoHE | DHA | LOX/non-enz | 0.56 | 0.014 ** | 0.46 | 0.004 ** |
| 13-HDoHE | DHA | LOX/non-enz | 0.45 | 0.005 ** | - | - |
| 17-keto DHA | DHA | LOX | - | 0.56 | 0.0003 ** | |
| 10-HDoHE | DHA | LOX | 0.40 | 0.015 * | 0.34 | 1.00 × 10−3 * |
| 16-HDoHE | DHA | LOX | 0.42 | 0.010 * | - | - |
| 17-HDoHE | DHA | LOX | 0.37 | 0.023 * | - | - |
| 8-HDoHE | DHA | LOX | 0.33 | 0.045 * | - | - |
| 16,17 EpDPE | DHA | CYP-e | 0.34 | 0.040 * | 0.68 | 0.000001 ** |
| 19,20 DiHDoPE | DHA | CYP-e | - | 0.52 | 0.001 ** | |
| 20-HDoHE | DHA | CYP-h | 0.37 | 0.025 * | - | - |
| 17-Keto DPA | DPA | LOX | - | 0.84 | 1.00 × 10−5 ** | |
| 5-HETE | AA | LOX | 0.40 | 0.019 * | - | - |
| 11-HETE | AA | LOX | 0.43 | 0.007 ** | - | - |
| 12-HETE | AA | LOX | 0.41 | 0.011 * | - | - |
| Compounds | Fatty Acid | Pathway | Control (nM) | Isch (nM) |
|---|---|---|---|---|
| PGD2 | AA | COX | 0.36 ± 0.09 | 0.00 ± 0.00 * |
| PGE2 | AA | COX | 0.09 ± 0.02 | 0.00 ± 0.00 |
| 11,12 DiHETrE | AA | CYP-e | 0.35 ± 0.02 | 0.32 ± 0.04 |
| 14,15 DiHETrE | AA | CYP-e | 0.35 ± 0.02 | 0.39 ± 0.05 |
| 5,6 DiHETrE | AA | CYP-e | 0.15 ± 0.01 | 0.20 ± 0.03 |
| 8,9 DiHETrE | AA | CYP-e | 0.20 ± 0.01 | 0.19 ± 0.03 |
| 16-HETE | AA | CYP-h | 0.47 ± 0.03 | 0.29 ± 0.03 * |
| 18-HETE | AA | CYP-h | 0.07 ± 0.01 | 0.11 ± 0.02 |
| 20-COOH- AA | AA | CYP-h | 37.23 ± 4.11 | 60.69 ± 7.59 * |
| 20-HETE | AA | CYP-h | 0.81 ± 0.09 | 0.87 ± 0.14 |
| 17-HETE | AA | CYP-h | 0.04 ± 0.01 | 0.00 ± 00 * |
| 12-HETE | AA | LOX | 2.72 ± 0.43 | 0.75 ± 0.30 * |
| 5-HETE | AA | LOX | 1.80 ± 0.12 | 3.95 ± 0.92 * |
| 5-oxoETE | AA | LOX | 0.21 ± 0.02 | 0.10 ± 0.01 * |
| 8-HETE | AA | LOX | 0.72 ± 0.06 | 1.00 ± 0.15 |
| tetranor 12-HETE | AA | LOX | 0.99 ± 0.10 | 0.47 ± 0.07 * |
| 12-oxoETE | AA | LOX | 0.81 ± 0.19 | 0.00 ± 0.00 * |
| 15-oxoETE | AA | LOX | 0.18 ± 0.02 | 0.06 ± 0.00 * |
| 15-HETE | AA | LOX/COX | 1.73 ± 0.18 | 1.61 ± 0.32 |
| 11-HETE | AA | LOX/COX/non-enz | 0.47 ± 0.05 | 0.44 ± 0.12 |
| 9-HETE | AA | LOX/non-enz | 0.40 ± 0.07 | 0.30 ± 0.20 |
| 12,13 diHODE | ALA | CYP-e | 0.22 ± 0.04 | 0.16 ± 0.05 |
| 12,13 EpODE | ALA | CYP-e | 0.20 ± 0.01 | 0.07 ± 0.00 * |
| 9-oxoOTrE | ALA | LOX | 0.70 ± 0.08 | 0.28 ± 0.03 * |
| 9-HOTrE | ALA | LOX | 4.38 ± 0.39 | 5.29 ± 0.71 |
| 15-HETrE | DGLA | LOX | 0.39 ± 0.02 | 0.31 ± 0.06 |
| 8-HETrE | DGLA | LOX | 0.17 ± 0.01 | 0.26 ± 0.06 |
| 19,20 EpDPE | DHA | CYP-e | 0.00 ± 0.00 | 0.02 ± 0.00 * |
| 19,20 DiHDPE | DHA | CYP-e | 1.17 ± 0.09 | 1.59 ± 0.17 |
| 16,17 DiHDPE | DHA | CYP-e | 0.07 ± 0.00 | 0.04 ± 0.00 |
| 20-HDoHE | DHA | CYP-h | 0.44 ± 0.07 | 0.52 ± 0.11 |
| 10-HDoHE | DHA | LOX | 0.25 ± 0.03 | 0.18 ± 0.04 |
| 14-HDoHE | DHA | LOX | 0.98 ± 0.22 | 0.63 ± 0.12 |
| 16-HDoHE | DHA | LOX | 0.34 ± 0.04 | 0.27 ± 0.06 |
| 17-HDoHE | DHA | LOX | 1.02 ± 0.19 | 1.17 ± 0.23 |
| 4-HDoHE | DHA | LOX | 1.61 ± 0.28 | 2.67 ± 0.45 |
| 7-HDoHE | DHA | LOX | 0.26 ± 0.06 | 0.57 ± 0.07 * |
| 8-HDoHE | DHA | LOX | 0.38 ± 0.07 | 0.86 ± 0.16 * |
| 17-Keto DHA | DHA | LOX | 0.00 ± 0.00 | 5.30 ± 0.92 * |
| 11-HDoHE | DHA | LOX/non-enz | 0.22 ± 0.05 | 0.06 ± 0.04 * |
| 13-HDoHE | DHA | LOX/non-enz | 0.00 ± 0.00 | 0.24 ± 0.10 |
| 17-keto DPA | DPA | LOX | 5.21 ± 0.64 | 4.55 ± 0.82 |
| 14,15 diHETE | EPA | CYP-e | 1.02 ± 0.10 | 1.12 ± 0.12 |
| 17,18 diHETE | EPA | CYP-e | 4.44 ± 0.94 | 2.67 ± 0.26 |
| 18-HEPE | EPA | CYP-h | 0.48 ± 0.06 | 0.56 ± 0.08 |
| 12-HEPE | EPA | LOX | 0.9 ± 0.23 | 0.8 ± 0.23 |
| 5-HEPE | EPA | LOX | 0.49 ± 0.09 | 1.18 ± 0.17 * |
| 15-HEPE | EPA | LOX/COX | 0.29 ± 0.04 | 0.25 ± 0.04 |
| 13-HOTrE | GLA | LOX | 3.59 ± 0.40 | 3.02 ± 0.39 |
| 13-HOTrE-y | GLA | LOX | 0.65 ± 0.06 | 0.47 ± 0.06 |
| 12,13 diHOME | LA | CYP-e | 2.85 ± 0.16 | 5.99 ± 2.38 |
| 12,13 EpOME | LA | CYP-e | 3.21 ± 0.22 | 1.84 ± 0.16 * |
| 9,10 diHOME | LA | CYP-e | 1.89 ± 0.12 | 3.59 ± 0.84 |
| 9,10 EpOME | LA | CYP-e | 1.22 ± 0.11 | 0.78 ± 0.10 * |
| 13-HODE | LA | LOX | 23.16 ± 1.46 | 27.48 ± 3.60 |
| 13-oxoODE | LA | LOX | 5.88 ± 0.45 | 3.58 ± 0.27 * |
| 9-HODE | LA | LOX | 27.53 ± 1.77 | 31.68 ± 3.61 |
| 9-oxoODE | LA | LOX | 7.75 ± 0.65 | 4.05 ± 0.32 * |
| 9,12,13 triHOME | LA | LOX/CYP-e | 3.42 ± 0.25 | 3.58 ± 0.37 |
| 9,10,13 triHOME | LA | LOX/CYP-e | 9.44 ± 0.70 | 11.83 ± 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solati, Z.; Surendran, A.; Aukema, H.M.; Ravandi, A. Impact of Reperfusion on Plasma Oxylipins in ST-Segment Elevation Myocardial Infarction. Metabolites 2024, 14, 19. https://doi.org/10.3390/metabo14010019
Solati Z, Surendran A, Aukema HM, Ravandi A. Impact of Reperfusion on Plasma Oxylipins in ST-Segment Elevation Myocardial Infarction. Metabolites. 2024; 14(1):19. https://doi.org/10.3390/metabo14010019
Chicago/Turabian StyleSolati, Zahra, Arun Surendran, Harold M. Aukema, and Amir Ravandi. 2024. "Impact of Reperfusion on Plasma Oxylipins in ST-Segment Elevation Myocardial Infarction" Metabolites 14, no. 1: 19. https://doi.org/10.3390/metabo14010019
APA StyleSolati, Z., Surendran, A., Aukema, H. M., & Ravandi, A. (2024). Impact of Reperfusion on Plasma Oxylipins in ST-Segment Elevation Myocardial Infarction. Metabolites, 14(1), 19. https://doi.org/10.3390/metabo14010019






