Abstract
Autoimmune diseases, characterized by the immune system’s loss of self-tolerance, lack definitive diagnostic tests, necessitating the search for reliable biomarkers. This systematic review aims to identify common metabolite changes across multiple autoimmune diseases. Following PRISMA guidelines, we conducted a systematic literature review by searching MEDLINE, ScienceDirect, Google Scholar, PubMed, and Scopus (Elsevier) using keywords “Metabolomics”, “Autoimmune diseases”, and “Metabolic changes”. Articles published in English up to March 2023 were included without a specific start date filter. Among 257 studies searched, 88 full-text articles met the inclusion criteria. The included articles were categorized based on analyzed biological fluids: 33 on serum, 21 on plasma, 15 on feces, 7 on urine, and 12 on other biological fluids. Each study presented different metabolites with indications of up-regulation or down-regulation when available. The current study’s findings suggest that amino acid metabolism may serve as a diagnostic biomarker for autoimmune diseases, particularly in systemic lupus erythematosus (SLE), multiple sclerosis (MS), and Crohn’s disease (CD). While other metabolic alterations were reported, it implies that autoimmune disorders trigger multi-metabolite changes rather than singular alterations. These shifts could be consequential outcomes of autoimmune disorders, representing a more complex interplay. Further studies are needed to validate the metabolomics findings associated with autoimmune diseases.
1. Introduction
Autoimmune diseases include a wide range of clinical disorders, including rheumatoid arthritis (RA), multiple sclerosis (MS), inflammatory bowel diseases (IBDs), autoimmune liver diseases, and systemic lupus erythematosus (SLE), characterized by loss of self-tolerance by the immune system. Autoimmune diseases may be systemic or organ-specific, resulting in various complications and disabilities. Incidence of autoimmune disease varies owing to the diversity of diseases, affecting 5–10% of the population around the globe [1,2]. Several factors (genetic, environmental, and epigenetic factors) are involved in the development of autoimmune diseases [3]. Autoimmune diseases are poorly diagnosed owing to obscure symptoms and overlapping symptoms of various diseases. The majority of autoimmune diseases are multi-genic, with multiple susceptibility genes interacting to create the abnormal phenotype [4]. Several gene variants have been discovered for autoimmune diseases; however, their relationship with disease susceptibility remains elusive. Hence, a novel approach is required for comprehensive understanding of autoimmune disease biology, especially underlying molecular mechanisms and treatment strategies. Metabolomics is an emerging technology that has drawn the attention of the scientific community in order to identify disease biomarkers due to its cost-effectiveness, short time period for repeated measurements, and very close observation of the metabolic state of patients [5,6]. Metabolomics is employed to assess metabolites, which are the end products of biochemical processes, in both a quantitative and qualitative manner. Metabolomics provides better information about the status of metabolites that occur due to changes in gene expression. It is widely used in pharmaceutical industries and R&D for detecting biomarkers for diseases, identifying their signaling pathways, and assessing their efficacy. Metabolomics is classified into two categories: targeted metabolomics and untargeted metabolomics. Targeted metabolomics analyses specific metabolites, whereas non-targeted metabolomics is utilized to analyze the metabolites extracted from organisms systematically and comprehensively [7]. Metabolomics consists of various steps to identify novel disease biomarkers. Several biological specimens, including urine, cerebrospinal fluids, fecal extracts, serum, cyst fluid blisters, synovial fluids, plasma, seminal fluids, tissue extracts, dialysis fluids, exhaled breath condensates, bile fluids, and tissue biopsy extracts (aqueous and lipid), are the most common specimens utilized in metabolomics [8]. Analytical techniques, specifically mass spectroscopy (MS) in combination with various separation techniques (gas chromatography, liquid chromatography, HPLC, UPLC, and capillary electrophoresis) and nuclear magnetic resonance (NMR), are utilized for metabolomics studies [9,10,11]. Compared to NMR, MS is preferred for metabolomics as it requires small sample volumes and has high sensitivity and simple sample preparation [12]. pH is one of the major disadvantages of NMR, especially when dealing with urine samples. Several lines of evidence show the importance of metabolomics in the detection of various autoimmune diseases. Evidence from clinical trials has shown that metabolites can act as potential biomarkers for various diseases [13,14,15,16]. Previous clinical studies have reported that oncometabolites may act as diagnostic biomarkers for various carcinomas [17,18,19,20]. In addition to blood glucose, phospholipid profiling is also useful in identification of type 2 diabetes mellitus [21]. Trimethylamine N-oxide (TMAO) can also be used as a prognostic marker for patients with acute ischemic stroke who are at an increased risk of unfavorable clinical outcomes [22,23]. Another study reported a link between heart failure and urobilin and sphingomyelin (30:1) [24]. An association between carcinoma and eicosanoid metabolites was also reported [25]. For autoimmune diseases, serum, plasma, fecal extracts, urine, and other biological samples differ depending on the specific disease. Few studies have investigated biomarkers for diagnosis [26,27,28]. However, there is currently only specific tests for diagnosis for some autoimmune diseases. As a result, the current study aims to identify common metabolite changes across multiple autoimmune diseases.
2. Materials and Methods
2.1. Literature Search and Data Curation
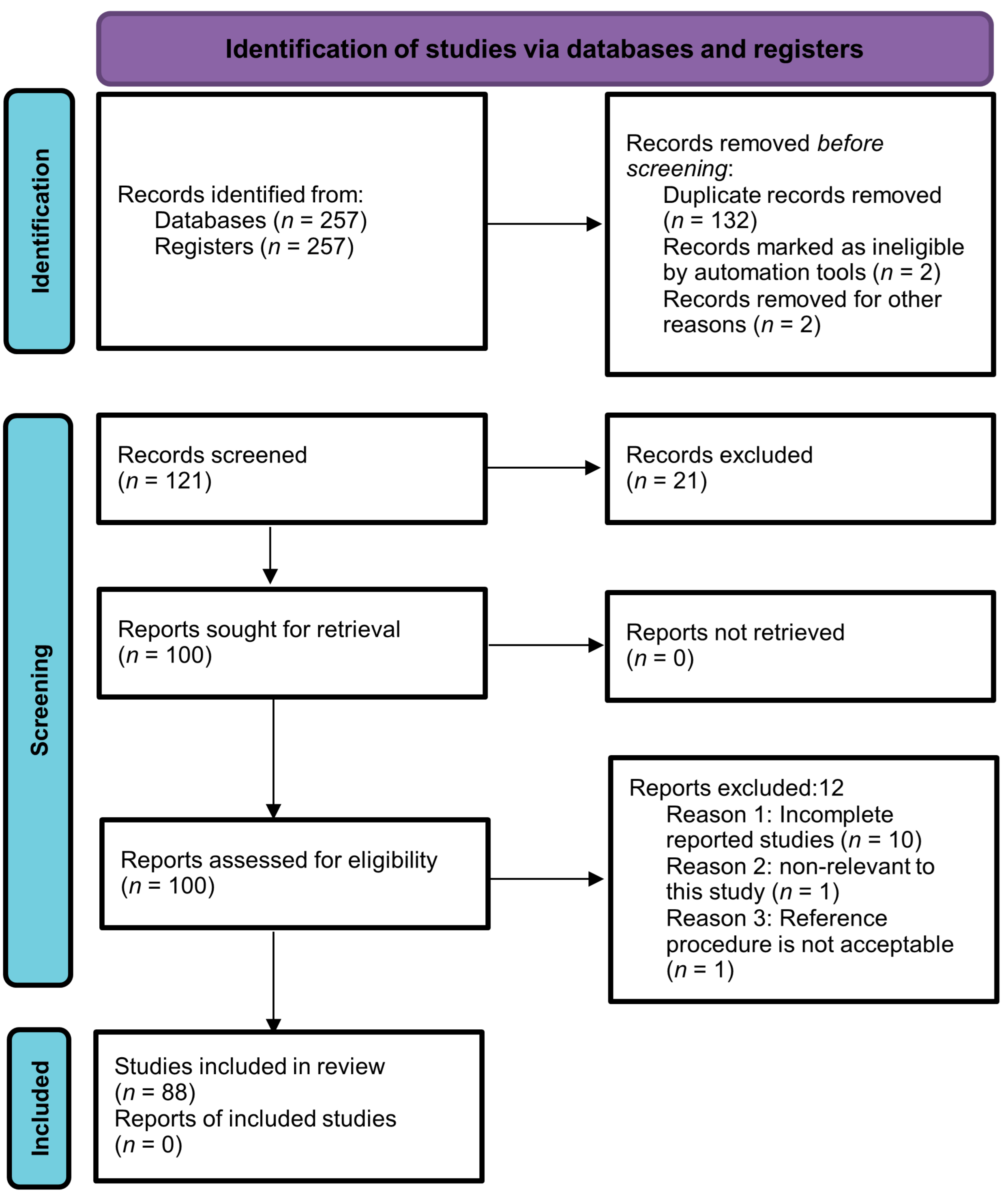
The current systematic review was performed by following the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). MEDLINE, ScienceDirect, Google Scholar, PubMed, and Scopus (Elsevier) were searched for articles by using the following terms: “Metabolomics”, “Autoimmune diseases”, “Biological samples”, and “Metabolic changes”. Articles published in the English language up to March 2023 were included with no specific start date filter. Two hundred and fifty-seven articles were retrieved through a search strategy. After careful assessment of titles and abstracts, a total of 136 studies were un-contextualized for this study. Thus, 121 abstracts were left for further scanning. Then, 21 out of 121 studies were excluded due to repetition, and an additional 12 articles did not meet the predefined inclusion criteria, specifically those that were not published in English and lacked control groups. As a result, a final collection of 88 full-text articles were eligible for analysis. The selection process is depicted in Figure 1 using a PRISMA flow chart, outlining the study’s progression through these stages. The protocol of the study was registered with PROSPERO (registration number: CRD42023447059).
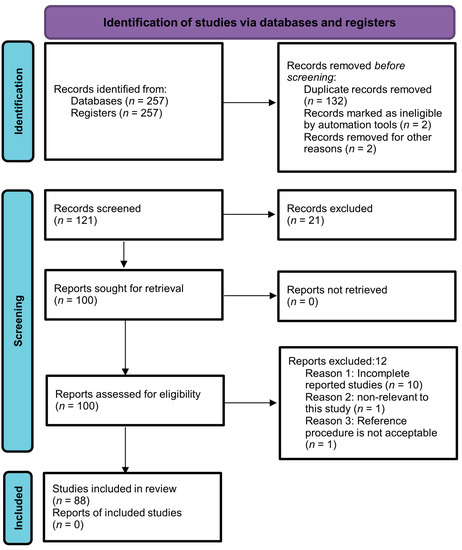
Figure 1.
PRISMA flow chart illustrating the selection process of the studies.
The initial screening of titles was conducted by the first reviewer (AM). Subsequently, two reviewers (AM and KSA) independently assessed the title and abstracts using an eligibility checklist to exclude irrelevant studies. Full texts of potentially eligible studies were retrieved for a comprehensive evaluation and final selection. Two reviewers (WFF and AAO) critically evaluated the quality and validity of the included studies. The first reviewer extracted the data, which were then verified by the second and third reviewers (KSA, AAO), and finally reviewed by the fourth reviewer (WFF) for accuracy and completeness. Consensus discussions were held to address any discrepancies and ensure study eligibility.
2.2. Data Synthesis
The outcomes of the included studies were summarized in tables mentioning the author, analytical technique, biological fluids, models, and the number of patients and controls. Metabolic changes with respect to different biological fluids were also summarized in tables.
2.3. Risk of Bias Assessment
For both the fluid samples and the studies, the risk of bias was assessed by using the AMSTAR 2 tool. We assessed the patient recruitment process and examined the information available/lack of information about the patients. Contrasting targeted and non-targeted metabolic analysis tactics were also evaluated and, finally, fluid sample collection techniques were also taken into consideration.
3. Results
The systematic search of different databases of published articles produced 88 studies. General characteristics of the included studies are shown in Table 1, which includes the author, biological fluids, analytical techniques, models, and sample sizes of different groups which allowed for further categorization of metabolomics changes in the biological fluids that were analyzed in the included studies: plasma, serum, feces, urine, and other biological fluids (synovial fluids, CSF, tears, peripheral blood monocytes, in vivo white matter, peripheral blood, and lymphocytes). In 12 out of 88 studies, other fluid samples were used in contrast to plasma, urine, feces, and serum [29,30,31,32,33,34,35,36,37,38,39,40].

Table 1.
General characteristics of the included studies.
3.1. Serum
A total of 33 studies assessed the metabolite changes in the serum of patients. In all studies, the analysis was performed on a human model. While changes were observed in various metabolites, not a single metabolite was found to be statistically significant across all the studies. In 11 studies, aromatic amino acids (tyrosine, tryptophan, and phenylalanine) were altered [44,45,49,51,53,57,79,80,105,110,111], four of which were found to be associated with SLE and PBC. Ten studies reported an alteration in branched amino acids (leucine, isoleucine and valine) [44,45,49,52,53,59,79,98,105,110], of which four studies found an association with SLE [49,52,53,59]. Alterations in fatty acids were observed in eight studies [44,50,53,57,61,104,106,108]. Among the eight studies, three were related to SLE [50,53,57]. The remaining metabolites that were shown to be significantly changed in the serum samples were linked to numerous metabolic pathways, including those related to lipid metabolism, ATP storage, nucleotide metabolism, oxidative stress, amino acid metabolism, and the TCA cycle (Table 2).

Table 2.
Metabolite changes found in serum.
3.2. Plasma
A total of 21 studies assessed the metabolite changes in the plasma of patients. The analysis was conducted on human models in all studies. In all 21 plasma-based metabolite studies, not a single metabolite exhibited a consistent statistical significance across all experiments. Eleven out of twenty-one studies showed alterations in amino acid metabolism [41,46,62,68,69,70,72,91,96,100,101], of which five were shown to be associated with MS [62,68,69,70,72] and four with T1D [91,96,100,101]. Seven out of twenty-one studies showed an alteration in aromatic amino acids [41,46,62,68,70,96,101]. The alteration of metabolite levels in lipid metabolism has been reported in seven studies [41,56,65,66,93,99,101]. Of these, three and two studies were associated with MS [65,66] and T1D [93,99,101]. Fang et al. reported the alteration in membrane phosphoproteins and dihydroceramides [42]. Åkesson et al. reported the metabolic alteration in kynurenine pathways [55]. The remaining metabolites that were shown to be significantly changed in the plasma samples were linked to numerous metabolic pathways. These pathways included nucleotide metabolism, oxidative stress, amino acid metabolism, glycolytic metabolism, and the TCA cycle (Table 3).

Table 3.
Metabolites changes found in plasma.
3.3. Feces
A total of 15 studies assessed the metabolite changes in the feces of patients. The analysis was conducted on human models in all studies. Seven out of fifteen studies showed metabolic alterations in amino acid metabolism [58,74,75,77,81,85,88]. Of these, five studies were linked to CD [74,77,81,85,88]. Five out of fifteen studies showed an alteration in aromatic amino acids [58,74,75,78,81]. Of these, three studies were linked to CD [74,78,81]. Eight out of fifteen studies showed an alteration in bile acids [78,83,84,85,86,87,88,89]. Of these, five studies were linked to CD [78,84,85,86,88]. The remaining metabolites that were shown to be significantly changed in the fecal samples were linked to numerous metabolic pathways. These pathways included nucleotide metabolism, lipid metabolism, amino acid metabolism, and the TCA cycle (Table 4). Nonetheless, it is important to highlight that none of the identified metabolites exhibited consistent and significant alterations throughout all analyses.

Table 4.
Metabolites changes found in feces.
3.4. Urine
A total of seven studies assessed the metabolite changes in the urine of patients. The analysis was conducted on human models in all studies. However, none of the identified metabolites showed consistent significant alterations across all studies. Four out of seven studies showed metabolic alterations in amino acid metabolism [54,71,92,94]. Of these, two studies were linked to T1D [92,94]. Three out of seven studies showed metabolic alterations in aromatic amino acids, especially tryptophan [54,71,94]. One of the studies reported a decrease in trigonelline and hippurate [111]. Deja et al. observed an increase in urea [92]. Another study reported an increase in bile acids [109]. The remaining metabolites that were shown to be significantly changed in the urine samples were linked to numerous metabolic pathways. These pathways included lipid metabolism, amino acid metabolism, and the TCA cycle (Table 5).

Table 5.
Metabolites changes found in urine.
3.5. Other Biological Fluids
A total of 12 studies assessed the metabolite changes in the other biological fluids (synovial fluids, CSF, tears, peripheral blood monocytes, in vivo white matter, and peripheral blood and lymphocytes) of patients. The analysis was conducted on human models in all studies. In all 12 other biological fluid-based metabolite studies, not a single metabolite showed significant changes that were consistent across all experiments. Six out of twelve studies performed metabolomics analysis on CSF [32,34,35,36,38,39]. Two studies performed metabolomics analysis on synovial fluid [29,30]. Two studies performed metabolomics analysis on peripheral blood lymphocytes and monocytes [31,40]. One out of twelve studies performed metabolomics analysis on tears [37]. One study carried out metabolomics analysis on in vivo white matter [33]. Among the twelve studies, six reported metabolic changes in amino acid metabolism [30,31,33,37,38,39]. Of these, three studies were linked to MS [37,38,39]. A study conducted on blood samples from patients with MS observed a decrease in glucose and lactate levels [40]. There was another study conducted on CSF specimens from patients with MS, which observed a decrease in glycine, dimethylarginine, and glycerophospholipid PC-O (34:0), as well as hexoses [39]. Podlecka-Piętowska et al. analyzed the metabolic alteration in CSF from MS patients and observed a decrease in acetone, choline, urea, 1,3-dimethylurate, creatinine, isoleucine, myo-inositol, leucine, 3-OH butyrate, and acetyl-CoA [38]. A study conducted by Cicalini et al. reported an increase in amino acids and acylcarnitines in the tears of MS patients [37]. A study performed by Herman et al. reported a decrease in 3-methoxytyramine and caffeine in the CSF of MS patients [36]. Pieragostino et al. reported a decrease in phosphatydic acid and an increase in phosphatidylcholine and phosphatidylinositol in patients with MS [35]. Vingara et al. analyzed the metabolic alteration in in vivo white matter and reported a decrease in lipid metabolism in patients with MS [33]. Gonzalo et al. analyzed the metabolic alteration in CSF and reported a decrease in PPARϒ and an increase in 8-iso-prostaglandin F2α in patients with MS [32]. The remaining metabolites that were shown to be significantly changed in the other biological fluid samples were linked to numerous metabolic pathways. These pathways included nucleotide metabolism, lipid metabolism, amino acid metabolism, glycolytic metabolism, and the TCA cycle (Table 6).

Table 6.
Metabolite changes found in other biological fluids.
4. Discussion
The metabolomics approach is a continuously evolving approach in the field of “omics” technology that offers a molecular view of disease pathophysiology and identifies disease biomarkers. Metabolomics also provides early diagnosis of diseases, better intervention, and monitoring of the progression of disease and the potency of treatment. The term autoimmune disease refers to a group of chronic disorders that are associated with a variety of metabolic changes that vary with the disease type. Given the absence of definitive cures for autoimmune diseases, patients are confronted with enduring illness and ongoing treatment throughout their lives. Hence, early diagnosis and recognition of various autoimmune diseases are essential to lessen disease progression and prevent painful conditions as well as co-morbidity and mortality caused by autoimmune diseases. The studies included in this systematic review analyzed the metabolic changes in various autoimmune diseases (rheumatoid arthritis, multiple sclerosis, systemic lupus erythematosus, Crohn’s disease, primary sclerosing cholangitis, primary biliary cholangitis, inflammatory bowel disease, ulcerative colitis, and type 1 diabetes) in serum, plasma, feces, urine, and other biological fluids including synovial fluids, CSF, tears, in vivo white matter, and peripheral blood monocytes and lymphocytes. All studies were carried out on patients. Mass spectroscopy and nuclear magnetic resonance were used in these studies. In most of the studies, mass spectroscopy was utilized in combination with various separation techniques. Metabolites that are identified through metabolomics analysis of various biological fluids are either reported as increased or decreased in contrast to controls. Various metabolites were found to increase or decrease, belonging to various metabolic pathways including TCA, glycolytic, amino acid metabolism, ATP metabolism, nucleotide metabolism, oxidative stress, lipid metabolism, and carbohydrate metabolism. A relatively consistent change in the proportion of metabolites was observed. However, there were instances of variation between individual cases. For instance, one of the studies reported an increase in the level of phosphatidylcholine in CSF specimens [35], while some studies observed a decrease in the level of phosphatidylcholine in tears and plasma [37,90]. We observed similar findings for other metabolites. It is possible that this may be due to interspecies differences in the metabolic process of patients, suggesting that further studies are required about pathophysiology and metabolomics. Further, for metabolomics findings to be applicable across species, it is imperative to identify both similarities and differences between animals and humans. Additionally, human clinical populations must be evaluated in order to confirm the utility of identified biomarker candidates in animal models. Different studies have reported metabolic changes associated with various autoimmune diseases. The metabolism of acylcarnitine and carnitine, changes in fatty acid metabolism, as well as TCA cycle metabolites have been linked to mitochondrial dysfunction [26,57,61,91,109,111,113]. Reactive oxygen species, antioxidant metabolites, glucogenic amino acid metabolites [58,114,115], and the accumulation of signaling metabolites were also reported [116]. Developing metabolites associated with mitochondrial dysfunction may be a focus for future research. The metabolism of various amino acids and lipids has been found to be similar in a number of studies. However, an alteration in phosphorylcholine has only been reported in a limited number of studies. In many studies, altered amino acid metabolism and the ratio of aromatic to branched amino acids have been found to be diagnostic indicators of autoimmune diseases, particularly SLE, MS, and CD [58,117,118]. However, the metabolic changes in the level of amino acids across the studies were different. This suggests that further studies are required to validate the ratio of aromatic and branched amino acids as a diagnostic indicator of autoimmune diseases. Maintaining body homeostasis requires the synthesis and degradation of proteins. Amino acid metabolism plays a crucial role in this biochemical process, including regulation of the innate and adaptive immune systems [119,120]. The utilization of amino acid metabolism changes as diagnostic markers offers several compelling advantages [121]. Amino acids, being stable and easily measurable in biological fluids, present a feasible and practical option for clinical assessment. Their involvement in a wide array of metabolic pathways makes them valuable indicators of physiological changes. Several studies have shown that alteration in amino acid metabolism is linked with various disease conditions, including cardiovascular disease [122], cancer [123,124], and autoimmune diseases [100,101,102,119,120,121]. A case report has shown that serum levels of aspartic and glutamic acids are linked with the development of myasthenia gravis [125]. Reports conducted on dietary protein restriction have demonstrated that branched amino acids contribute to promoting metabolic health [126,127]. In the current study, there were changes in serum levels of aromatic amino acids in 11 studies and branched amino acids in 10. A significant alteration in amino acid metabolism was observed in 11 plasma reports. Seven studies reported significant alterations in amino acid metabolism in feces whereas four studies reported them in urine. The above findings indicated that branched amino acid metabolism may act as a diagnostic biomarker for autoimmune diseases, specifically SLE, CD, and MS. Altered amino acids in other biological fluids may be related to different stages or severity of autoimmune diseases. However, it is necessary to validate the method with a larger study sample before it can be applied to diagnostic practice, due to the multifactorial, heterogeneous, and complex nature of these diseases. Only 88 articles met the inclusion criteria for the current study. There are, however, several articles on metabolomics and autoimmune diseases that did not meet our inclusion criteria or did not appear in databases due to keywords or database limitations. Thus, these studies were not chosen for this systematic review. A study should identify and control for confounding factors (dietary habits, patient demographics, and concurrent medical conditions) since biological fluids, especially plasma, urine, and serum, all reflect systemic metabolism. These confounding factors may be involved in the metabolic alterations, indicating that statistical modeling is required for development of diagnostic biomarkers of autoimmune diseases.
5. Conclusions
The findings of the current study suggest that alterations in amino acid metabolism, particularly aromatic and branched amino acids, may serve as potential diagnostic biomarkers for autoimmune diseases such as SLE, MS, and CD. We also observed altered amino acid metabolism in various biological fluids including plasma, feces, urine, synovial fluids, CSF, tears, peripheral blood monocytes, in vivo white matter, peripheral blood, and lymphocytes. The study also emphasizes the complexity and heterogeneity of autoimmune disorders, since several other metabolic alterations have been reported. These alterations within various metabolic pathways were linked to energy metabolism, oxidative stress, lipid metabolism, and nucleotide metabolism, suggesting that these shifts are likely consequences of autoimmune disorders. However, biomarkers are changed owing to slight alterations in the experimental environment. Hence, metabolomics analyses must be carefully performed in the laboratory. While amino acid metabolism emerges as a promising diagnostic biomarker, the study emphasizes that further studies are required to validate the method with a larger study sample before it can be applied to diagnostic practice, due to the multifactorial, heterogeneous, and complex nature of these diseases. Researchers need to explore the correlation between the severity or stages of autoimmune disease and amino acid metabolism in different biological fluids. Furthermore, studies are required to evaluate the relationship between alterations in amino acid metabolism in various biological fluids and different autoimmune diseases. They are also required to investigate the potential therapeutic targets and conduct longitudinal studies to evaluate the efficacy of the identified biomarkers over time.
Author Contributions
A.M.: Conceptualized and designed the study. K.S.A. and A.A.O.: Conducted the literature search and initial screening of titles and abstracts to identify relevant studies. A.M. and K.S.A.: Performed the full-text screening of selected studies to determine their eligibility for inclusion. A.A.O. and W.F.F.: Critically assessed the quality and validity of the included studies. All authors: Verified extracted data. A.M. and A.A.O.: Analyzed and interpreted the synthesized data. A.M. and W.F.F.: Wrote the original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, L.; Wang, F.-S.; Gershwin, M.E. Human autoimmune diseases: A comprehensive update. J. Intern. Med. 2015, 278, 369–395. [Google Scholar] [CrossRef]
- Tsoukalas, D.; Fragoulakis, V.; Papakonstantinou, E.; Antonaki, M.; Vozikis, A.; Tsatsakis, A.; Buga, A.M.; Mitroi, M.; Calina, D. Prediction of autoimmune diseases by targeted metabolomic assay of urinary organic acids. Metabolites 2020, 10, 502. [Google Scholar] [CrossRef]
- Hewagama, A.; Richardson, B. The genetics and epigenetics of autoimmune diseases. J. Autoimmun. 2009, 33, 3–11. [Google Scholar] [CrossRef]
- Gregersen, P.K.; Behrens, T.W. Genetics of autoimmune diseases—Disorders of immune homeostasis. Nat. Rev. Genet. 2006, 7, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Tsoukalas, D.; Sarandi, E.; Thanasoula, M.; Docea, A.O.; Tsilimidos, G.; Calina, D.; Tsatsakis, A. Metabolic fingerprint of chronic obstructive lung diseases: A new diagnostic perspective. Metabolites 2019, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, D.K.; Hollywood, K.A.; Goodacre, R. Metabolomics for the masses: The future of metabolomics in a personalized world. New Horiz. Transl. Med. 2017, 3, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Lelli, V.; Belardo, A.; Timperio, A.M.; Lelli, V.; Belardo, A.; Timperio, A.M. From targeted quantification to untargeted metabolomics. In Metabolomics—Methodology and Applications in Medical Sciences and Life Sciences; IntechOpen: London, UK, 2021; ISBN 978-1-83969-084-6. [Google Scholar]
- Kang, J.; Zhu, L.; Lu, J.; Zhang, X. Application of metabolomics in autoimmune diseases: Insight into biomarkers and pathology. J. Neuroimmunol. 2015, 279, 25–32. [Google Scholar] [CrossRef]
- Naz, S.; Vallejo, M.; García, A.; Barbas, C. Method validation strategies involved in non-targeted metabolomics. J. Chromatogr. A 2014, 1353, 99–105. [Google Scholar] [CrossRef]
- Gowda, G.A.N.; Raftery, D. NMR Based Metabolomics. Adv. Exp. Med. Biol. 2021, 1280, 19–37. [Google Scholar] [CrossRef]
- Letertre, M.P.M.; Dervilly, G.; Giraudeau, P. Combined nuclear magnetic resonance spectroscopy and mass spectrometry approaches for metabolomics. Anal. Chem. 2021, 93, 500–518. [Google Scholar] [CrossRef]
- Rist, M.J.; Muhle-Goll, C.; Görling, B.; Bub, A.; Heissler, S.; Watzl, B.; Luy, B. Influence of Freezing and Storage Procedure on Human Urine Samples in NMR-Based Metabolomics. Metabolites 2013, 3, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef]
- Li, C.; Zheng, S.; You, H.; Liu, X.; Lin, M.; Yang, L.; Li, L. Sphingosine 1-phosphate (S1P)/S1P receptors are involved in human liver fibrosis by action on hepatic myofibroblasts motility. J. Hepatol. 2011, 54, 1205–1213. [Google Scholar] [CrossRef]
- Castelino, F.V. Lipids and eicosanoids in fibrosis: Emerging targets for therapy. Curr. Opin. Rheumatol. 2012, 24, 649–655. [Google Scholar] [CrossRef]
- Herranz, D.; Ambesi-Impiombato, A.; Sudderth, J.; Sánchez-Martín, M.; Belver, L.; Tosello, V.; Xu, L.; Wendorff, A.A.; Castillo, M.; Haydu, J.E.; et al. Metabolic reprogramming induces resistance to anti-NOTCH1 therapies in T cell acute lymphoblastic leukemia. Nat. Med. 2015, 21, 1182–1189. [Google Scholar] [CrossRef]
- Pera, B.; Krumsiek, J.; Assouline, S.E.; Marullo, R.; Patel, J.; Phillip, J.M.; Román, L.; Mann, K.K.; Cerchietti, L. Metabolomic profiling reveals cellular reprogramming of b-cell lymphoma by a lysine deacetylase inhibitor through the choline pathway. EBioMedicine 2018, 28, 80–89. [Google Scholar] [CrossRef]
- Hashim, N.A.A.; Ab-Rahim, S.; Suddin, L.S.; Saman, M.S.A.; Mazlan, M. Global serum metabolomics profiling of colorectal cancer. Mol. Clin. Oncol. 2019, 11, 3–14. [Google Scholar] [CrossRef]
- Donnelly, D.; Aung, P.P.; Jour, G. The “-OMICS” facet of melanoma: Heterogeneity of genomic, proteomic and metabolomic biomarkers. Semin. Cancer Biol. 2019, 59, 165–174. [Google Scholar] [CrossRef]
- Wang, C.; Kong, H.; Guan, Y.; Yang, J.; Gu, J.; Yang, S.; Xu, G. Plasma phospholipid metabolic profiling and biomarkers of type 2 diabetes mellitus based on high-performance liquid chromatography/electrospray mass spectrometry and multivariate statistical analysis. Anal. Chem. 2005, 77, 4108–4116. [Google Scholar] [CrossRef]
- Zhai, Q.; Wang, X.; Chen, C.; Tang, Y.; Wang, Y.; Tian, J.; Zhao, Y.; Liu, X. Prognostic value of plasma trimethylamine n-oxide levels in patients with acute ischemic stroke. Cell Mol. Neurobiol. 2019, 39, 1201–1206. [Google Scholar] [CrossRef]
- Rexidamu, M.; Li, H.; Jin, H.; Huang, J. Serum levels of Trimethylamine-N-oxide in patients with ischemic stroke. Biosci. Rep. 2019, 39, BSR20190515. [Google Scholar] [CrossRef] [PubMed]
- Stenemo, M.; Ganna, A.; Salihovic, S.; Nowak, C.; Sundström, J.; Giedraitis, V.; Broeckling, C.D.; Prenni, J.E.; Svensson, P.; Magnusson, P.K.E.; et al. The metabolites urobilin and sphingomyelin (30:1) are associated with incident heart failure in the general population. ESC Heart Fail. 2019, 6, 764–773. [Google Scholar] [CrossRef]
- Wang, D.; Dubois, R.N. Eicosanoids and cancer. Nat. Rev. Cancer 2010, 10, 181–193. [Google Scholar] [CrossRef]
- Van Pevenage, P.M.; Birchmier, J.T.; June, R.K. Utilizing metabolomics to identify potential biomarkers and perturbed metabolic pathways in osteoarthritis: A systematic review. Semin. Arthritis Rheum. 2023, 59, 152163. [Google Scholar] [CrossRef]
- Huang, T.; Pu, Y.; Wang, X.; Li, Y.; Yang, H.; Luo, Y.; Liu, Y. Metabolomic analysis in spondyloarthritis: A systematic review. Front. Microbiol. 2022, 13, 965709. [Google Scholar] [CrossRef] [PubMed]
- Marrie, R.A.; Reider, N.; Cohen, J.; Stuve, O.; Sorensen, P.S.; Cutter, G.; Reingold, S.C.; Trojano, M. A systematic review of the incidence and prevalence of autoimmune disease in multiple sclerosis. Mult. Scler. 2015, 21, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Zheng, K.D.; Lin, K.; Zheng, G.; Zou, H.; Wang, J.M.; Lin, Y.Y.; Chuka, C.M.; Ge, R.S.; Zhai, W.; et al. Energy metabolism disorder as a contributing factor of rheumatoid arthritis: A comparative proteomic and metabolomic study. PLoS ONE 2015, 10, e0132695. [Google Scholar] [CrossRef]
- Young, S.P.; Kapoor, S.R.; Viant, M.R.; Byrne, J.J.; Filer, A.; Buckley, C.D.; Kitas, G.D.; Raza, K. The impact of inflammation on metabolomic profiles in patients with arthritis. Arthritis Rheum. 2013, 65, 2015–2023. [Google Scholar] [CrossRef]
- Perl, A.; Hanczko, R.; Lai, Z.-W.; Oaks, Z.; Kelly, R.; Borsuk, R.; Asara, J.M.; Phillips, P.E. Comprehensive metabolome analyses reveal N-acetylcysteine-responsive accumulation of kynurenine in systemic lupus erythematosus: Implications for activation of the mechanistic target of rapamycin. Metabolomics 2015, 11, 1157–1174. [Google Scholar] [CrossRef]
- Gonzalo, H.; Brieva, L.; Tatzber, F.; Jové, M.; Cacabelos, D.; Cassanye, A.; Lanau-Angulo, L.; Boada, J.; Serrano, J.C.; González, C.; et al. Lipidome analysis in multiple sclerosis reveals protein lipoxidative damage as a potential pathogenic mechanism. J. Neurochem. 2012, 123, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Vingara, L.K.; Yu, H.J.; Wagshul, M.E.; Serafin, D.; Christodoulou, C.; Pelczer, I.; Krupp, L.B.; Maletić-Savatić, M. Metabolomic approach to human brain spectroscopy identifies associations between clinical features and the frontal lobe metabolome in multiple sclerosis. Neuroimage 2013, 82, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Reinke, S.N.; Broadhurst, D.I.; Sykes, B.D.; Baker, G.B.; Catz, I.; Warren, K.G.; Power, C. Metabolomic profiling in multiple sclerosis: Insights into biomarkers and pathogenesis. Mult. Scler. J. 2014, 20, 1396–1400. [Google Scholar] [CrossRef] [PubMed]
- Pieragostino, D.; D’Alessandro, M.; di Ioia, M.; Rossi, C.; Zucchelli, M.; Urbani, A.; Di Ilio, C.; Lugaresi, A.; Sacchetta, P.; Del Boccio, P. An integrated metabolomics approach for the research of new cerebrospinal fluid biomarkers of multiple sclerosis. Mol. Biosyst. 2015, 11, 1563–1572. [Google Scholar] [CrossRef]
- Herman, S.; Khoonsari, P.E.; Tolf, A.; Steinmetz, J.; Zetterberg, H.; Åkerfeldt, T.; Jakobsson, P.-J.; Larsson, A.; Spjuth, O.; Burman, J.; et al. Integration of magnetic resonance imaging and protein and metabolite CSF measurements to enable early diagnosis of secondary progressive multiple sclerosis. Theranostics 2018, 8, 4477–4490. [Google Scholar] [CrossRef]
- Cicalini, I.; Rossi, C.; Pieragostino, D.; Agnifili, L.; Mastropasqua, L.; di Ioia, M.; De Luca, G.; Onofrj, M.; Federici, L.; Del Boccio, P. Integrated Lipidomics and Metabolomics Analysis of Tears in Multiple Sclerosis: An Insight into Diagnostic Potential of Lacrimal Fluid. Int. J. Mol. Sci. 2019, 20, 1265. [Google Scholar] [CrossRef]
- Podlecka-Piętowska, A.; Kacka, A.; Zakrzewska-Pniewska, B.; Nojszewska, M.; Zieminska, E.; Chalimoniuk, M.; Toczylowska, B. Altered Cerebrospinal Fluid Concentrations of Hydrophobic and Hydrophilic Compounds in Early Stages of Multiple Sclerosis—Metabolic Profile Analyses. J. Mol. Neurosci. 2019, 69, 94–105. [Google Scholar] [CrossRef]
- Carlsson, H.; Abujrais, S.; Herman, S.; Khoonsari, P.E.; Åkerfeldt, T.; Svenningsson, A.; Burman, J.; Kultima, K. Targeted metabolomics of CSF in healthy individuals and patients with secondary progressive multiple sclerosis using high-resolution mass spectrometry. Metabolomics 2020, 16, 26. [Google Scholar] [CrossRef]
- Zahoor, I.; Suhail, H.; Datta, I.; Ahmed, M.E.; Poisson, L.M.; Waters, J.; Rashid, F.; Bin, R.; Singh, J.; Cerghet, M.; et al. Blood-based untargeted metabolomics in relapsing-remitting multiple sclerosis revealed the testable therapeutic target. Proc. Natl. Acad. Sci. USA 2022, 119, e2123265119. [Google Scholar] [CrossRef]
- Madsen, R.K.; Lundstedt, T.; Gabrielsson, J.; Sennbro, C.J.; Alenius, G.M.; Moritz, T.; Rantapää-Dahlqvist, S.; Trygg, J. Diagnostic properties of metabolic perturbations in rheumatoid arthritis. Arthritis Res. Ther. 2011, 13, R19. [Google Scholar] [CrossRef]
- Fang, L.; Mundra, P.A.; Fan, F.; Galvin, A.; Weir, J.M.; Wong, G.; Chin-Dusting, J.; Cicuttini, F.; Meikle, P.; Dart, A.M. Plasma lipidomic profiling in patients with rheumatoid arthritis. Metabolomics 2016, 12, 136. [Google Scholar] [CrossRef]
- Zabek, A.; Swierkot, J.; Malak, A.; Zawadzka, I.; Deja, S.; Bogunia-Kubik, K.; Mlynarz, P. Application of (1) H NMR-based serum metabolomic studies for monitoring female patients with rheumatoid arthritis. J. Pharm. Biomed. Anal. 2016, 117, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, J.; Hu, C.; Xie, Z.; Li, H.; Wei, S.; Wang, D.; Wen, C.; Xu, G. Exploration of the serum metabolite signature in patients with rheumatoid arthritis using gas chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2016, 127, 60–67. [Google Scholar] [CrossRef]
- Li, J.; Che, N.; Xu, L.; Zhang, Q.; Wang, Q.; Tan, W.; Zhang, M. LC-MS-based serum metabolomics reveals a distinctive signature in patients with rheumatoid arthritis. Clin. Rheumatol. 2018, 37, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, C.; Hiraishi, T.; Oku, T.; Okuma, K.; Suzumura, K.; Hashimoto, M.; Ito, H.; Aramori, I.; Hirayama, Y. Metabolomic approach to the exploration of biomarkers associated with disease activity in rheumatoid arthritis. PLoS ONE 2019, 14, e0219400. [Google Scholar] [CrossRef]
- Takahashi, S.; Saegusa, J.; Onishi, A.; Morinobu, A. Biomarkers identified by serum metabolomic analysis to predict biologic treatment response in rheumatoid arthritis patients. Rheumatology 2019, 58, 2153–2161. [Google Scholar] [CrossRef]
- Hur, B.; Gupta, V.K.; Huang, H.; Wright, K.A.; Warrington, K.J.; Taneja, V.; Davis, J.M.; Sung, J. Plasma metabolomic profiling in patients with rheumatoid arthritis identifies biochemical features predictive of quantitative disease activity. Arthritis Res. Ther. 2021, 23, 164. [Google Scholar] [CrossRef]
- Ouyang, X.; Dai, Y.; Wen, J.L.; Wang, L.X. 1H NMR-based metabolomic study of metabolic profiling for systemic lupus erythematosus. Lupus 2011, 20, 1411–1420. [Google Scholar] [CrossRef]
- Wu, T.; Xie, C.; Han, J.; Ye, Y.; Weiel, J.; Li, Q.; Blanco, I.; Ahn, C.; Olsen, N.; Putterman, C.; et al. Metabolic disturbances associated with systemic lupus erythematosus. PLoS ONE 2012, 7, e37210. [Google Scholar] [CrossRef]
- Bengtsson, A.A.; Trygg, J.; Wuttge, D.M.; Sturfelt, G.; Theander, E.; Donten, M.; Moritz, T.; Sennbro, C.J.; Torell, F.; Lood, C.; et al. Metabolic profiling of systemic lupus erythematosus and comparison with primary sjögren’s syndrome and systemic sclerosis. PLoS ONE 2016, 11, e0159384. [Google Scholar] [CrossRef]
- Guleria, A.; Pratap, A.; Dubey, D.; Rawat, A.; Chaurasia, S.; Sukesh, E.; Phatak, S.; Ajmani, S.; Kumar, U.; Khetrapal, C.L.; et al. NMR based serum metabolomics reveals a distinctive signature in patients with Lupus Nephritis. Sci. Rep. 2016, 6, 35309. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Huang, J.; Zhang, C.; Hu, X.; Gao, M.; Shi, A.; Zha, W.; Shi, L.; Huang, C.; Yang, L. Serum metabolomic profiling in patients with systemic lupus erythematosus by GC/MS. Mod. Rheumatol. 2016, 26, 914–922. [Google Scholar] [CrossRef]
- Yan, B.; Huang, J.; Dong, F.; Yang, L.; Huang, C.; Gao, M.; Shi, A.; Zha, W.; Shi, L.; Hu, X. Urinary metabolomic study of systemic lupus erythematosus based on gas chromatography/mass spectrometry. Biomed. Chromatogr. 2016, 30, 1877–1881. [Google Scholar] [CrossRef] [PubMed]
- Åkesson, K.; Pettersson, S.; Ståhl, S.; Surowiec, I.; Hedenström, M.; Eketjäll, S.; Trygg, J.; Jakobsson, P.J.; Gunnarsson, I.; Svenungsson, E.; et al. Kynurenine pathway is altered in patients with SLE and associated with severe fatigue. Lupus Sci. Med. 2018, 5, e000254. [Google Scholar] [CrossRef]
- Shin, T.H.; Kim, H.A.; Jung, J.Y.; Baek, W.Y.; Lee, H.S.; Park, H.J.; Min, J.; Paik, M.J.; Lee, G.; Suh, C.H. Analysis of the free fatty acid metabolome in the plasma of patients with systemic lupus erythematosus and fever. Metabolomics 2017, 14, 14. [Google Scholar] [CrossRef]
- Li, Y.; Liang, L.; Deng, X.; Zhong, L. Lipidomic and metabolomic profiling reveals novel candidate biomarkers in active systemic lupus erythematosus. Int. J. Clin. Exp. Pathol. 2019, 12, 857–866. [Google Scholar] [PubMed]
- Zhang, Q.; Yin, X.; Wang, H.; Wu, X.; Li, X.; Li, Y.; Zhang, X.; Fu, C.; Li, H.; Qiu, Y. Fecal metabolomics and potential biomarkers for systemic lupus erythematosus. Front. Immunol. 2019, 10, 976. [Google Scholar] [CrossRef]
- Zhang, Y.; Gan, L.; Tang, J.; Liu, D.; Chen, G.; Xu, B. Metabolic profiling reveals new serum signatures to discriminate lupus nephritis from systemic lupus erythematosus. Front. Immunol. 2022, 13, 967371. [Google Scholar] [CrossRef]
- Mehrpour, M.; Kyani, A.; Tafazzoli, M.; Fathi, F.; Joghataie, M.T. A metabonomics investigation of multiple sclerosis by nuclear magnetic resonance. Magn. Reson. Chem. 2013, 51, 102–109. [Google Scholar] [CrossRef]
- Dickens, A.M.; Larkin, J.R.; Griffin, J.L.; Cavey, A.; Matthews, L.; Turner, M.R.; Wilcock, G.K.; Davis, B.G.; Claridge, T.D.W.; Palace, J.; et al. A type 2 biomarker separates relapsing-remitting from secondary progressive multiple sclerosis. Neurology 2014, 83, 1492–1499. [Google Scholar] [CrossRef]
- Cocco, E.; Murgia, F.; Lorefice, L.; Barberini, L.; Poddighe, S.; Frau, J.; Fenu, G.; Coghe, G.; Murru, M.R.; Murru, R.; et al. 1H-NMR analysis provides a metabolomic profile of patients with multiple sclerosis. Neurol.—Neuroimmunol. Neuroinflamm. 2016, 3, e185. [Google Scholar] [CrossRef] [PubMed]
- Gebregiworgis, T.; Nielsen, H.H.; Massilamany, C.; Gangaplara, A.; Reddy, J.; Illes, Z.; Powers, R. A urinary metabolic signature for multiple sclerosis and neuromyelitis optica. J. Proteome Res. 2016, 15, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.K.; Bilgin, A.; Lovejoy, D.B.; Tan, V.; Bustamante, S.; Taylor, B.V.; Bessede, A.; Brew, B.J.; Guillemin, G.J. Kynurenine pathway metabolomics predicts and provides mechanistic insight into multiple sclerosis progression. Sci. Rep. 2017, 7, 41473. [Google Scholar] [CrossRef]
- Stoessel, D.; Stellmann, J.P.; Willing, A.; Behrens, B.; Rosenkranz, S.C.; Hodecker, S.C.; Stürner, K.H.; Reinhardt, S.; Fleischer, S.; Deuschle, C.; et al. Metabolomic profiles for primary progressive Multiple Sclerosis stratification and disease course monitoring. Front. Hum. Neurosci. 2018, 12, 226. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, P.; Fitzgerald, K.C.; Venkata, S.L.V.; Smith, M.D.; Kornberg, M.D.; Mowry, E.M.; Haughey, N.J.; Calabresi, P.A. Dimethyl fumarate treatment induces lipid metabolism alterations that are linked to immunological changes. Ann. Clin. Transl. Neurol. 2019, 6, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.L.; Briggs, F.B.S.; Winnike, J.H.; Natanzon, Y.; Maichle, S.; Knagge, K.J.; Newby, L.K.; Gregory, S.G. Metabolome-based signature of disease pathology in MS. Mult. Scler. Relat. Disord. 2019, 31, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Lorefice, L.; Murgia, F.; Fenu, G.; Frau, J.; Coghe, G.; Murru, M.R.; Tranquilli, S.; Visconti, A.; Marrosu, M.G.; Atzori, L.; et al. Assessing the metabolomic profile of multiple sclerosis patients treated with interferon beta 1a by 1H-NMR Spectroscopy. Neurotherapeutics 2019, 16, 797–807. [Google Scholar] [CrossRef]
- Kasakin, M.F.; Rogachev, A.D.; Predtechenskaya, E.V.; Zaigraev, V.J.; Koval, V.V.; Pokrovsky, A.G. Targeted metabolomics approach for identification of relapsing–remitting multiple sclerosis markers and evaluation of diagnostic models. MedChemComm 2019, 10, 1803–1809. [Google Scholar] [CrossRef]
- Sylvestre, D.A.; Slupsky, C.M.; Aviv, R.I.; Swardfager, W.; Taha, A.Y. Untargeted metabolomic analysis of plasma from relapsing-remitting multiple sclerosis patients reveals changes in metabolites associated with structural changes in brain. Brain Res. 2020, 1732, 146589. [Google Scholar] [CrossRef]
- Gaetani, L.; Boscaro, F.; Pieraccini, G.; Calabresi, P.; Romani, L.; Di Filippo, M.; Zelante, T. Host and Microbial Tryptophan metabolic profiling in multiple sclerosis. Front. Immunol. 2020, 11, 157. [Google Scholar] [CrossRef]
- Murgia, F.; Lorefice, L.; Noto, A.; Spada, M.; Frau, J.; Fenu, G.; Coghe, G.; Gagliano, A.; Atzori, L.; Cocco, E. Metabolomic changes in patients affected by multiple sclerosis and treated with fingolimod. Metabolites 2023, 13, 428. [Google Scholar] [CrossRef] [PubMed]
- De Preter, V.; Machiels, K.; Joossens, M.; Arijs, I.; Matthys, C.; Vermeire, S.; Rutgeerts, P.; Verbeke, K. Faecal metabolite profiling identifies medium-chain fatty acids as discriminating compounds in IBD. Gut 2015, 64, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Bjerrum, J.T.; Wang, Y.; Hao, F.; Coskun, M.; Ludwig, C.; Günther, U.; Nielsen, O.H. Metabonomics of human fecal extracts characterize ulcerative colitis, Crohn’s disease and healthy individuals. Metabolomics 2015, 11, 122–133. [Google Scholar] [CrossRef]
- Lamas, B.; Richard, M.L.; Leducq, V.; Pham, H.P.; Michel, M.L.; Da Costa, G.; Bridonneau, C.; Jegou, S.; Hoffmann, T.W.; Natividad, J.M.; et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 2016, 22, 598–605. [Google Scholar] [CrossRef]
- Coburn, L.A.; Horst, S.N.; Allaman, M.M.; Brown, C.T.; Williams, C.S.; Hodges, M.E.; Druce, J.P.; Beaulieu, D.B.; Schwartz, D.A.; Wilson, K.T. L-Arginine availability and metabolism is altered in ulcerative colitis. Inflamm. Bowel Dis. 2016, 22, 1847–1858. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Clavel, T.; Smirnov, K.; Schmidt, A.; Lagkouvardos, I.; Walker, A.; Lucio, M.; Michalke, B.; Schmitt-Kopplin, P.; Fedorak, R.; et al. Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD. Gut 2017, 66, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.P.; Goudarzi, M.; Singh, N.; Tong, M.; McHardy, I.H.; Ruegger, P.; Asadourian, M.; Moon, B.H.; Ayson, A.; Borneman, J.; et al. A disease-associated microbial and metabolomics state in relatives of pediatric inflammatory bowel disease patients. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 750–766. [Google Scholar]
- Kolho, K.L.; Pessia, A.; Jaakkola, T.; de Vos, W.M.; Velagapudi, V. Faecal and serum metabolomics in paediatric inflammatory bowel disease. J. Crohns Colitis 2017, 11, 321–334. [Google Scholar] [CrossRef]
- Nikolaus, S.; Schulte, B.; Al-Massad, N.; Thieme, F.; Schulte, D.M.; Bethge, J.; Rehman, A.; Tran, F.; Aden, K.; Häsler, R.; et al. Increased Tryptophan metabolism is associated with activity of inflammatory bowel diseases. Gastroenterology 2017, 153, 1504–1516.e2. [Google Scholar] [CrossRef]
- Santoru, M.L.; Piras, C.; Murgia, A.; Palmas, V.; Camboni, T.; Liggi, S.; Ibba, I.; Lai, M.A.; Orrù, S.; Blois, S.; et al. Cross sectional evaluation of the gut-microbiome metabolome axis in an Italian cohort of IBD patients. Sci. Rep. 2017, 7, 9523. [Google Scholar] [CrossRef]
- Scoville, E.A.; Allaman, M.M.; Brown, C.T.; Motley, A.K.; Horst, S.N.; Williams, C.S.; Koyama, T.; Zhao, Z.; Adams, D.W.; Beaulieu, D.B.; et al. Alterations in lipid, amino acid, and energy metabolism distinguish Crohn’s disease from ulcerative colitis and control subjects by serum metabolomic profiling. Metabolomics 2017, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Marcišauskas, S.; Ji, B.; Nielsen, J. Metagenomic analysis of bile salt biotransformation in the human gut microbiome. BMC Genom. 2019, 20, 517. [Google Scholar] [CrossRef]
- Weng, Y.J.; Gan, H.Y.; Li, X.; Huang, Y.; Li, Z.C.; Deng, H.M.; Chen, S.Z.; Zhou, Y.; Wang, L.S.; Han, Y.P.; et al. Correlation of diet, microbiota and metabolite networks in inflammatory bowel disease. J. Dig. Dis. 2019, 20, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J.; et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 2019, 4, 293–305. [Google Scholar] [CrossRef]
- Diederen, K.; Li, J.V.; Donachie, G.E.; Meij, T.G.; Waart, D.R.; Hakvoort, T.; Kindermann, A.; Wagner, J.; Auyeung, V.; Te Velde, A.A.; et al. Exclusive enteral nutrition mediates gut microbial and metabolic changes that are associated with remission in children with Crohn’s disease. Sci. Rep. 2020, 10, 18879. [Google Scholar]
- Bushman, F.D.; Conrad, M.; Ren, Y.; Zhao, C.; Gu, C.; Petucci, C.; Kim, M.S.; Abbas, A.; Downes, K.J.; Devas, N.; et al. Multi-omic Analysis of the Interaction between Clostridioides difficile Infection and Pediatric Inflammatory Bowel Disease. Cell Host Microbe 2020, 28, 422–433.e7. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.; Zhang, X.; Xiao, F.; Hu, H.; Li, X.; Dong, F.; Sun, M.; Xiao, Y.; Ge, T.; et al. Microbial and metabolic features associated with outcome of infliximab therapy in pediatric Crohn’s disease. Gut Microbes 2021, 13, 1865708. [Google Scholar] [CrossRef]
- Yang, Z.H.; Liu, F.; Zhu, X.R.; Suo, F.Y.; Jia, Z.; Yao, S.K. Altered profiles of fecal bile acids correlate with gut microbiota and inflammatory responses in patients with ulcerative colitis. World J. Gastroenterol. 2021, 27, 3609–3629. [Google Scholar] [CrossRef]
- Wu, X.; Liu, K.; Wu, Q.; Wang, M.; Chen, X.; Li, Y.; Qian, L.; Li, C.; Dai, G.; Zhang, Q.; et al. Biomarkers of metabolomics in inflammatory bowel disease and damp-heat syndrome: A preliminary study. Evid.-Based Complement. Altern. Med. 2022, 2022, 3319646. [Google Scholar] [CrossRef] [PubMed]
- Dutta, T.; Chai, H.S.; Ward, L.E.; Ghosh, A.; Persson, X.M.; Ford, G.C.; Kudva, Y.C.; Sun, Z.; Asmann, Y.W.; Kocher, J.P.A.; et al. Concordance of changes in metabolic pathways based on plasma metabolomics and skeletal muscle transcriptomics in type 1 diabetes. Diabetes 2012, 61, 1004–1016. [Google Scholar] [CrossRef]
- Deja, S.; Barg, E.; Młynarz, P.; Basiak, A.; Willak-Janc, E. 1H NMR-based metabolomics studies of urine reveal differences between type 1 diabetic patients with high and low HbAc1 values. J. Pharm. Biomed. Anal. 2013, 83, 43–48. [Google Scholar] [CrossRef]
- Balderas, C.; Rupérez, F.J.; Ibañez, E.; Señorans, J.; Guerrero-Fernández, J.; Casado, I.G.; Gracia-Bouthelier, R.; García, A.; Barbas, C. Plasma and urine metabolic fingerprinting of type 1 diabetic children. Electrophoresis 2013, 34, 2882–2890. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, A.; Pirillo, P.; Moret, V.; Stocchero, M.; Gucciardi, A.; Perilongo, G.; Moretti, C.; Monciotti, C.; Giordano, G.; Baraldi, E. Metabolomics reveals new metabolic perturbations in children with type 1 diabetes. Pediatr. Diabetes 2018, 19, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Frohnert, B.I.; Webb-Robertson, B.J.; Bramer, L.M.; Reehl, S.M.; Waugh, K.; Steck, A.K.; Norris, J.M.; Rewers, M. Predictive modeling of type 1 diabetes stages using disparate data sources. Diabetes 2020, 69, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Lanza, I.R.; Zhang, S.; Ward, L.E.; Karakelides, H.; Raftery, D.; Nair, K.S. Quantitative metabolomics by 1H-NMR and LC-MS/MS confirms altered metabolic pathways in diabetes. PLoS ONE 2010, 5, e10538. [Google Scholar] [CrossRef]
- Dutta, T.; Kudva, Y.C.; Persson, X.M.; Schenck, L.A.; Ford, G.C.; Singh, R.J.; Carter, R.; Nair, K.S. Impact of long-term poor and good glycemic control on metabolomics alterations in type 1 diabetic people. J. Clin. Endocrinol. Metab. 2016, 101, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Brugnara, L.; Vinaixa, M.; Murillo, S.; Samino, S.; Rodriguez, M.A.; Beltran, A.; Lerin, C.; Davison, G.; Correig, X.; Novials, A. Metabolomics approach for analyzing the effects of exercise in subjects with type 1 diabetes mellitus. PLoS ONE 2012, 7, e40600. [Google Scholar] [CrossRef]
- Knebel, B.; Strassburger, K.; Szendroedi, J.; Kotzka, J.; Scheer, M.; Nowotny, B.; Müssig, K.; Lehr, S.; Pacini, G.; Finner, H.; et al. Specific metabolic profiles and their relationship to insulin resistance in recent-onset type 1 and type 2 diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 2130–2140. [Google Scholar] [CrossRef]
- Lamichhane, S.; Kemppainen, E.; Trošt, K.; Siljander, H.; Hyöty, H.; Ilonen, J.; Toppari, J.; Veijola, R.; Hyötyläinen, T.; Knip, M.; et al. Circulating metabolites in progression to islet autoimmunity and type 1 diabetes. Diabetologia 2019, 62, 2287–2297. [Google Scholar] [CrossRef]
- Bervoets, L.; Massa, G.; Guedens, W.; Louis, E.; Noben, J.P.; Adriaensens, P. Metabolic profiling of type 1 diabetes mellitus in children and adolescents: A case-control study. Diabetol. Metab. Syndr. 2017, 9, 48. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, W.; Huang, K.; Dong, G.; Chen, X.; Xu, C.; Ni, Y.; Fu, J. Untargeted metabolomics reveals gender-and age-independent metabolic changes of type 1 diabetes in Chinese children. Front. Endocrinol. 2022, 13, 1037289. [Google Scholar] [CrossRef]
- Noso, S.; Babaya, N.; Hiromine, Y.; Taketomo, Y.; Niwano, F.; Yoshida, S.; Ikegami, H. Metabolic signatures of β-cell destruction in type 1 diabetes. J. Diabetes Investig. 2023, 14, 48–57. [Google Scholar] [CrossRef]
- Haukka, J.K.; Sandholm, N.; Forsblom, C.; Cobb, J.E.; Groop, P.H.; Ferrannini, E. Metabolomic profile predicts development of microalbuminuria in individuals with type 1 diabetes. Sci. Rep. 2018, 8, 13853. [Google Scholar] [CrossRef]
- Wang, J.B.; Pu, S.B.; Sun, Y.; Li, Z.F.; Niu, M.; Yan, X.Z.; Zhao, Y.L.; Wang, L.F.; Qin, X.M.; Ma, Z.J.; et al. Metabolomic profiling of autoimmune hepatitis: The diagnostic utility of nuclear magnetic resonance spectroscopy. J. Proteome Res. 2014, 13, 3792–3801. [Google Scholar] [CrossRef]
- Lian, J.S.; Liu, W.; Hao, S.R.; Chen, D.Y.; Wang, Y.Y.; Yang, J.L.; Jia, H.Y.; Huang, J.R. A serum metabolomic analysis for diagnosis and biomarker discovery of primary biliary cirrhosis and autoimmune hepatitis. Hepatobiliary Pancreat. Dis. Int. 2015, 14, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Trottier, J.; Białek, A.; Caron, P.; Straka, R.J.; Heathcote, J.; Milkiewicz, P.; Barbier, O. Metabolomic profiling of 17 bile acids in serum from patients with primary biliary cirrhosis and primary sclerosing cholangitis: A pilot study. Dig. Liver Dis. 2012, 44, 303–310. [Google Scholar] [CrossRef]
- Bell, L.N.; Wulff, J.; Comerford, M.; Vuppalanchi, R.; Chalasani, N. Serum metabolic signatures of primary biliary cirrhosis and primary sclerosing cholangitis. Liver Int. 2015, 35, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.M.; Wang, J.P.; Bao, W.M.; Yang, J.H.; Ma, L.K.; Yang, J.; Chen, H.; Xu, Y.; Yang, L.H.; Li, W.; et al. Urine and serum metabolomic profiling reveals that bile acids and carnitine may be potential biomarkers of primary biliary cirrhosis. Int. J. Mol. Med. 2015, 36, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Yang, T.; Zhou, Y.; Gao, G.Y.; Xing, F.; Peng, Y.; Tao, Y.Y.; Liu, C.H. Serum metabolomics analysis reveals a distinct metabolic profile of patients with primary biliary cholangitis. Sci. Rep. 2017, 7, 784. [Google Scholar] [CrossRef]
- Vignoli, A.; Orlandini, B.; Tenori, L.; Biagini, M.R.; Milani, S.; Renzi, D.; Luchinat, C.; Calabrò, A.S. Metabolic signature of primary biliary cholangitis and its comparison with celiac disease. J. Proteome Res. 2019, 18, 1228–1236. [Google Scholar] [CrossRef]
- Banales, J.M.; Iñarrairaegui, M.; Arbelaiz, A.; Milkiewicz, P.; Muntané, J.; Muñoz-Bellvis, L.; Casta, A.; Gonzalez, L.M.; Arretxe, E.; Alonso, C.; et al. Serum metabolites as diagnostic biomarkers for cholangiocarcinoma, hepatocellular carcinoma, and primary sclerosing cholangitis. Hepatology 2019, 70, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Regenold, W.T.; Phatak, P.; Makley, M.J.; Stone, R.D.; Kling, M.A. Cerebrospinal fluid evidence of increased extra-mitochondrial glucose metabolism implicates mitochondrial dysfunction in multiple sclerosis disease progression. J. Neurol. Sci. 2008, 275, 106–112. [Google Scholar] [CrossRef]
- Weyand, C.M.; Goronzy, J.J. Immunometabolism in early and late stages of rheumatoid arthritis. Nat. Rev. Rheumatol. 2017, 13, 291–301. [Google Scholar] [CrossRef]
- Blanco, L.P.; Kaplan, M.J. Metabolic alterations of the immune system in the pathogenesis of autoimmune diseases. PLoS Biol. 2023, 21, e3002084. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.; Li, W.; Cornaby, C.; Morel, L. Immune cell metabolism in autoimmunity. Clin. Exp. Immunol. 2019, 197, 181–192. [Google Scholar] [CrossRef]
- Porter, L.; Shoushtarizadeh, A.; Jelinek, G.A.; Brown, C.R.; Lim, C.K.; de Livera, A.M.; Jacobs, K.R.; Weiland, T.J. Metabolomic Biomarkers of Multiple Sclerosis: A Systematic Review. Front. Mol. Biosci. 2020, 7, 574133. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Liu, H.; Wei, P.; He, Q.; Zhang, J.; Shi, Q.; Liu, T.; Liu, S. Amino acids-targeted metabolomics reveals novel diagnostic biomarkers for ulcerative colitis and Crohn’s disease. Amino Acids 2023, 55, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Kono, M.; Yoshida, N.; Tsokos, G.C. Amino Acid Metabolism in Lupus. Front. Immunol. 2021, 12, 623844. [Google Scholar] [CrossRef] [PubMed]
- Mondanelli, G.; Iacono, A.; Carvalho, A.; Orabona, C.; Volpi, C.; Pallotta, M.T.; Matino, D.; Esposito, S.; Grohmann, U. Amino acid metabolism as drug target in autoimmune diseases. Autoimmun. Rev. 2019, 18, 334–348. [Google Scholar] [CrossRef]
- Piranavan, P.; Bhamra, M.; Perl, A. Metabolic targets for treatment of autoimmune diseases. Immunometabolism 2020, 2, e200012. [Google Scholar] [CrossRef]
- Müller, J.; Bertsch, T.; Volke, J.; Schmid, A.; Klingbeil, R.; Metodiev, Y.; Karaca, B.; Kim, S.H.; Lindner, S.; Schupp, T.; et al. Narrative review of metabolomics in cardiovascular disease. J. Thorac. Dis. 2021, 13, 2532–2550. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Liu, X.; Cheng, C.; Yu, W.; Yi, P. Metabolism of Amino Acids in Cancer. Front. Cell Dev. Biol. 2020, 8, 603837. [Google Scholar] [CrossRef]
- Schmidt, D.R.; Patel, R.; Kirsch, D.G.; Lewis, C.A.; Vander Heiden, M.G.; Locasale, J.W. Metabolomics in cancer research and emerging applications in clinical oncology. CA Cancer J. Clin. 2021, 71, 333–358. [Google Scholar] [CrossRef] [PubMed]
- Kośliński, P.; Rzepiński, Ł.; Koba, M.; Gackowski, M.; Maciejek, Z. Amino acids levels as a potential biomarker in myasthenia gravis. Folia Neuropathol. 2022, 60, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Arany, Z.; Neinast, M. Branched Chain Amino Acids in Metabolic Disease. Curr. Diab Rep. 2018, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, F.; Lavigne, C.; Jacques, H.; Marette, A. Role of dietary proteins and amino acids in the pathogenesis of insulin resistance. Annu. Rev. Nutr. 2007, 27, 293–310. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).