The Metabolomics Changes in Luria–Bertani Broth Medium under Different Sterilization Methods and Their Effects on Bacillus Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Treatment of LB Broth Medium
2.2. 16S rRNA Gene Sequencing and Analysis
2.3. Measurement of the Protein Concentrations and Antioxidant Capacity
2.4. Bacterial Inoculation Experiment
2.5. Metabolite Extraction and Metabolomics Analysis
2.6. Statistical Analysis
3. Results
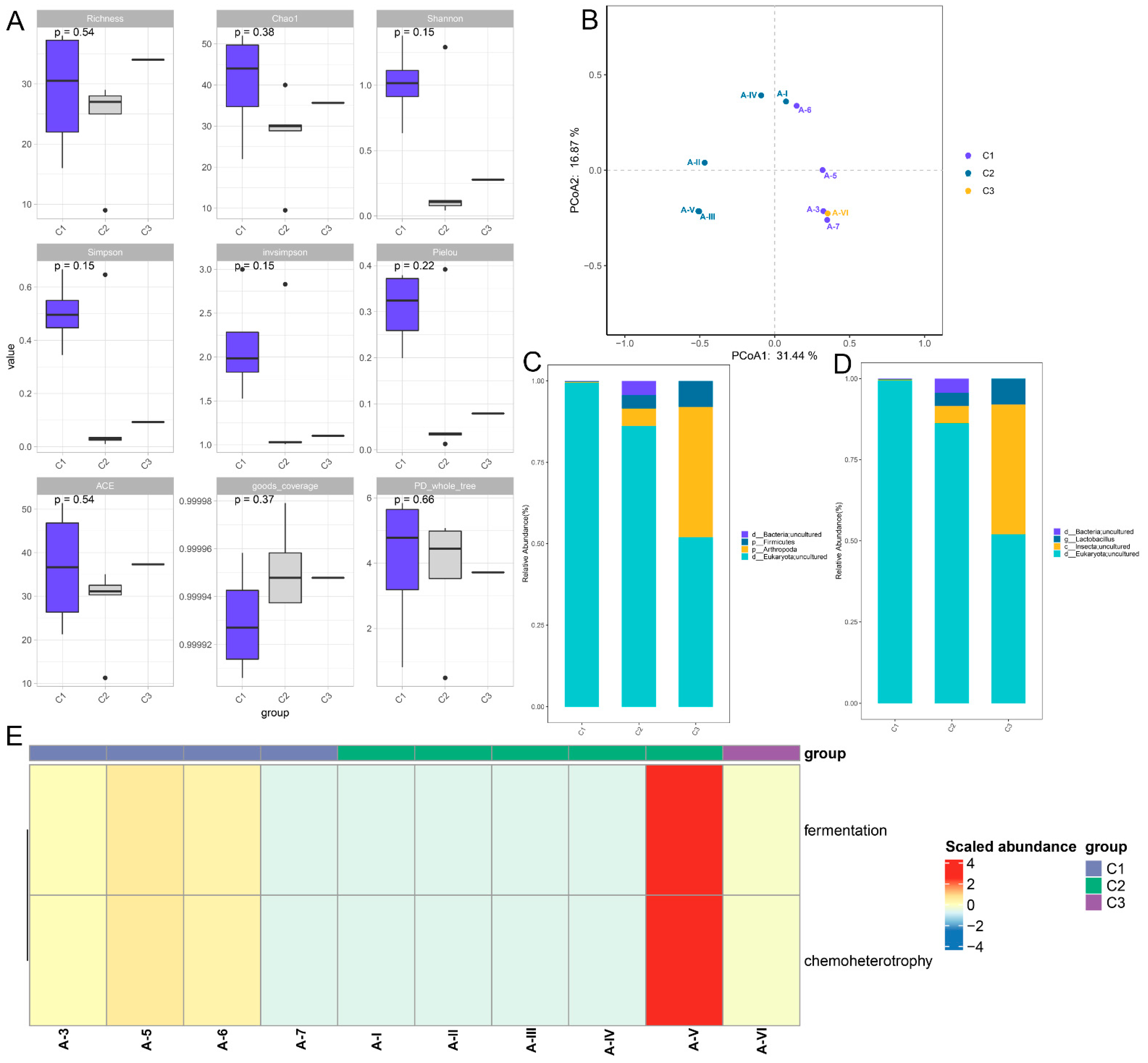
3.1. Microbiome Changes in the LB Medium with Different Treatments
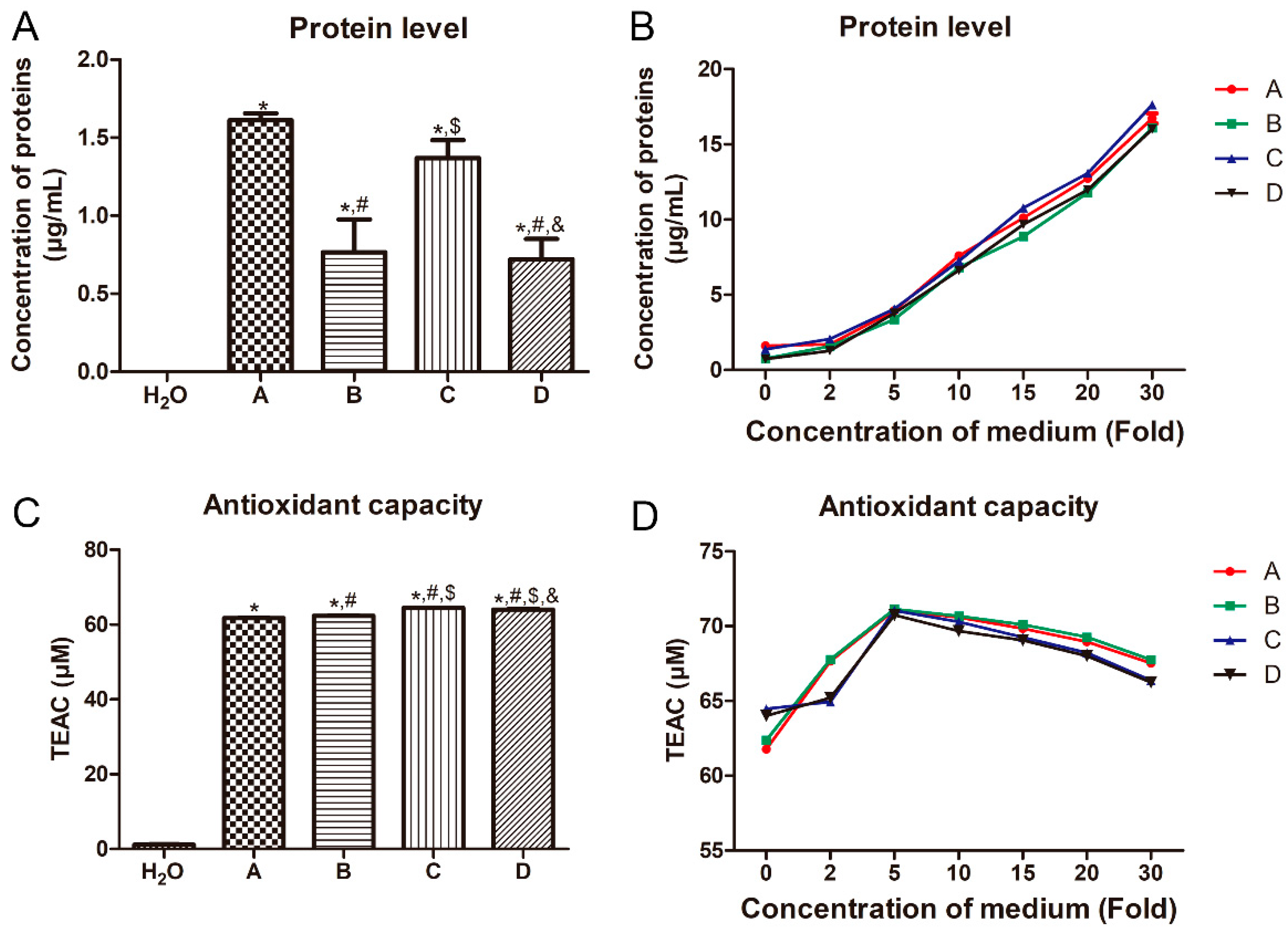
3.2. Effects of Different Treatments on the Protein Level and Antioxidant Capacity of LB Medium
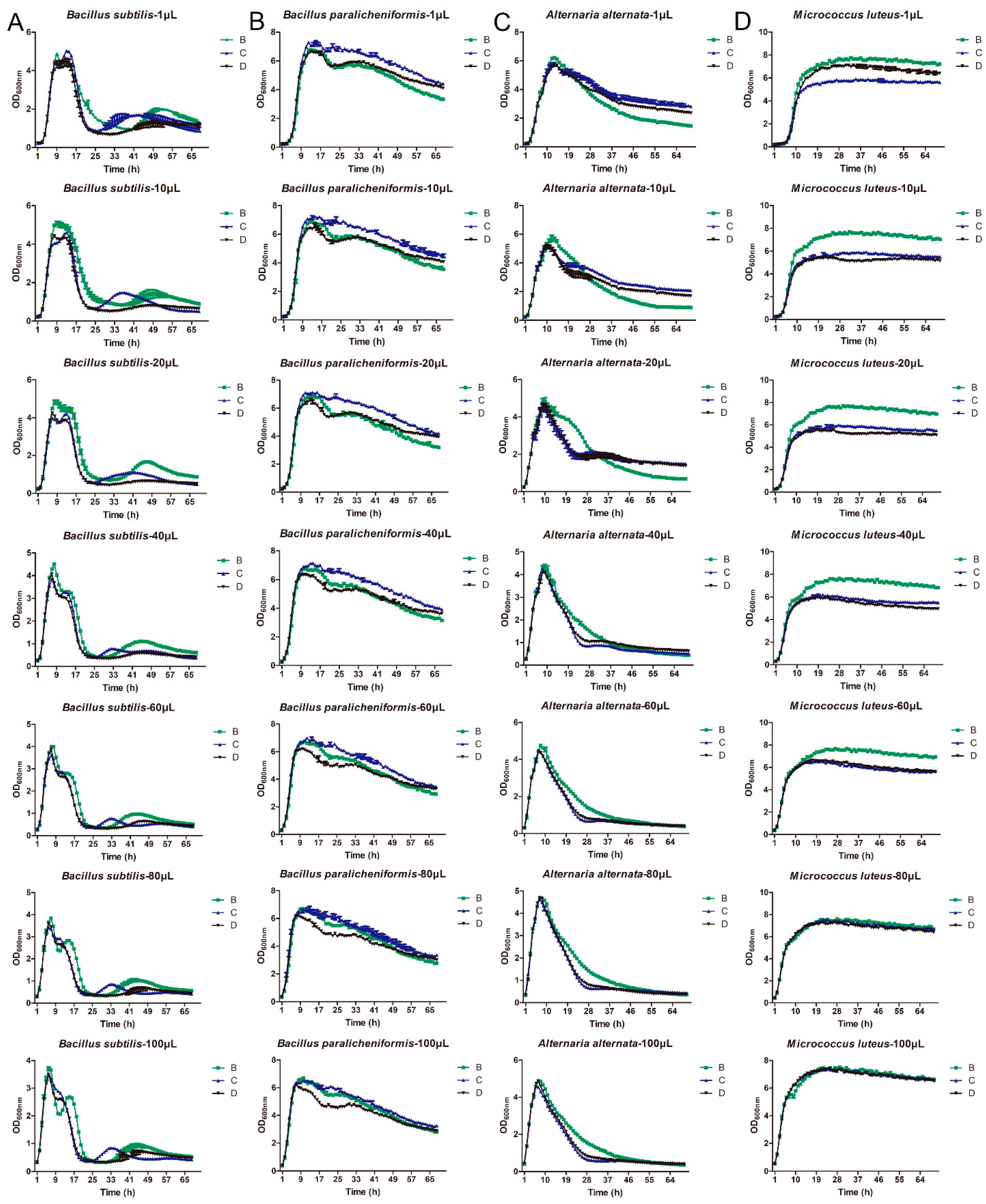
3.3. Growth Ability of Different Bacteria on the LB Broth Medium
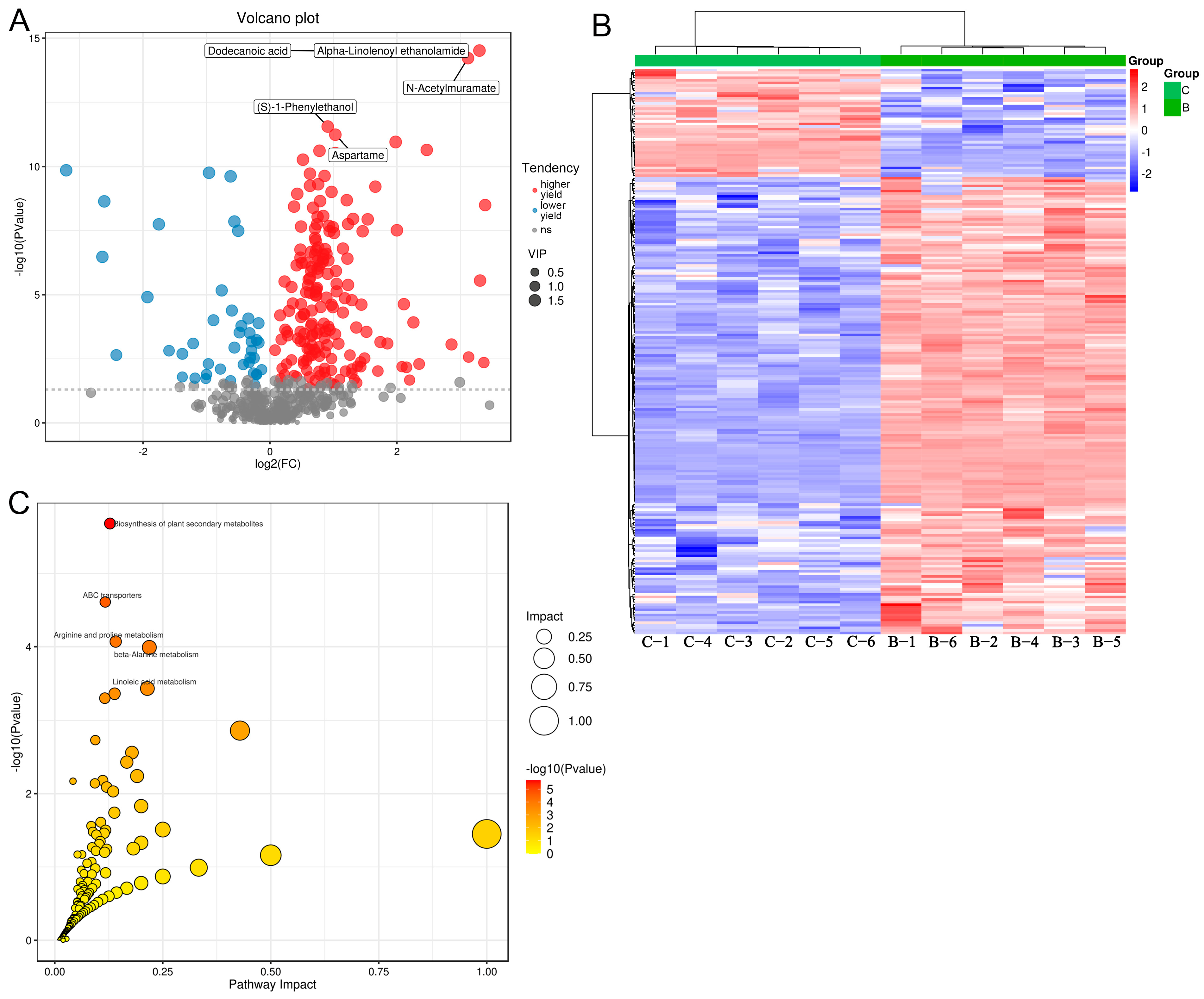
3.4. Differential Metabolites between the Medium in the B and C Groups, and Functional Analysis
3.5. Differential Metabolites between the Medium in the C and D Groups, and Functional Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nasiri, V.; Dalimi, A.; Ghaffarifar, F. LB broth-lyophilized Rabbit serum (LLR) as a new and suitable culture medium for cultivation of promastigotes of Leishmania major. J. Parasit. Dis. 2017, 41, 247–251. [Google Scholar] [CrossRef]
- Jira, J.; Rezek, B.; Kriha, V.; Artemenko, A.; Matolínová, I.; Skakalova, V.; Stenclova, P.; Kromka, A. Inhibition of E. coli Growth by Nanodiamond and Graphene Oxide Enhanced by Luria-Bertani Medium. Nanomaterials 2018, 8, 140. [Google Scholar] [CrossRef] [PubMed]
- Abdi Ghavidel, A.; Aghamiri, S.; Jajarmi, V.; Bandehpour, M.; Kazemi, B. The Influence of Different Culture Media on the Growth and Recombinant Protein Production of Iranian Lizard Leishmania Promastigote. Iran. J. Parasitol. 2022, 17, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Benocci, T.; de Vries, R.P.; Daly, P. A senescence-delaying pre-culture medium for transcriptomics of Podospora anserina. J. Microbiol. Methods 2018, 146, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Guo, J.; Yang, Q.; Huang, J. Diluted conventional media improve the microbial cultivability from aquarium seawater. J. Microbiol. 2019, 57, 759–768. [Google Scholar] [CrossRef]
- Yamamoto, K.; Toya, S.; Sabidi, S.; Hoshiko, Y.; Maeda, T. Diluted Luria-Bertani medium vs. sewage sludge as growth media: Comparison of community structure and diversity in the culturable bacteria. Appl. Microbiol. Biotechnol. 2021, 105, 3787–3798. [Google Scholar] [CrossRef]
- Smith, A.; Kaczmar, A.; Bamford, R.A.; Smith, C.; Frustaci, S.; Kovacs-Simon, A.; O’Neill, P.; Moore, K.; Paszkiewicz, K.; Titball, R.W.; et al. The Culture Environment Influences Both Gene Regulation and Phenotypic Heterogeneity in Escherichia coli. Front. Microbiol. 2018, 9, 1739. [Google Scholar] [CrossRef]
- Sánchez-Clemente, R.; Guijo, M.I.; Nogales, J.; Blasco, R. Carbon Source Influence on Extracellular pH Changes along Bacterial Cell-Growth. Genes 2020, 11, 1292. [Google Scholar] [CrossRef]
- Zhang, X.; Ruan, Y.; Liu, W.; Chen, Q.; Gu, L.; Guo, A. Transcriptome Analysis of Gene Expression in Dermacoccus abyssi HZAU 226 under Lysozyme Stress. Microorganisms 2020, 8, 707. [Google Scholar] [CrossRef]
- Watts, G.S.; Youens-Clark, K.; Slepian, M.J.; Wolk, D.M.; Oshiro, M.M.; Metzger, G.S.; Dhingra, D.; Cranmer, L.D.; Hurwitz, B.L. 16S rRNA gene sequencing on a benchtop sequencer: Accuracy for identification of clinically important bacteria. J. Appl. Microbiol. 2017, 123, 1584–1596. [Google Scholar] [CrossRef]
- Sanschagrin, S.; Yergeau, E. Next-generation sequencing of 16S ribosomal RNA gene amplicons. J. Vis. Exp. 2014, 90, 51709. [Google Scholar] [CrossRef]
- Johnson, J.S.; Spakowicz, D.J.; Hong, B.Y.; Petersen, L.M.; Demkowicz, P.; Chen, L.; Leopold, S.R.; Hanson, B.M.; Agresta, H.O.; Gerstein, M.; et al. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat. Commun. 2019, 10, 5029. [Google Scholar] [CrossRef]
- Wang, Y.; Sheng, H.F.; He, Y.; Wu, J.Y.; Jiang, Y.X.; Tam, N.F.; Zhou, H.W. Comparison of the levels of bacterial diversity in freshwater, intertidal wetland, and marine sediments by using millions of illumina tags. Appl. Environ. Microbiol. 2012, 78, 8264–8271. [Google Scholar] [CrossRef]
- Fouts, D.E.; Szpakowski, S.; Purushe, J.; Torralba, M.; Waterman, R.C.; MacNeil, M.D.; Alexander, L.J.; Nelson, K.E. Next generation sequencing to define prokaryotic and fungal diversity in the bovine rumen. PLoS ONE 2012, 7, e48289. [Google Scholar] [CrossRef] [PubMed]
- Kielkopf, C.L.; Bauer, W.; Urbatsch, I.L. Bradford Assay for Determining Protein Concentration. Cold Spring Harb. Protoc. 2020, 2020, 102269. [Google Scholar] [CrossRef]
- Huang, L.; Nazarova, E.V.; Tan, S.; Liu, Y.; Russell, D.G. Growth of Mycobacterium tuberculosis in vivo segregates with host macrophage metabolism and ontogeny. J. Exp. Med. 2018, 215, 1135–1152. [Google Scholar] [CrossRef]
- Rinschen, M.M.; Ivanisevic, J.; Giera, M.; Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 2019, 20, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, J. Untargeted metabolomic analysis of metabolites related to body dysmorphic disorder (BDD). Funct. Integr. Genom. 2023, 23, 70. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef] [PubMed]
- Lanza, V.F.; Tedim, A.P.; Martínez, J.L.; Baquero, F.; Coque, T.M. The Plasmidome of Firmicutes: Impact on the Emergence and the Spread of Resistance to Antimicrobials. Microbiol. Spectr. 2015, 3, 379–419. [Google Scholar] [CrossRef]
- Gavande, P.V.; Basak, A.; Sen, S.; Lepcha, K.; Murmu, N.; Rai, V.; Mazumdar, D.; Saha, S.P.; Das, V.; Ghosh, S. Functional characterization of thermotolerant microbial consortium for lignocellulolytic enzymes with central role of Firmicutes in rice straw depolymerization. Sci. Rep. 2021, 11, 3032. [Google Scholar] [CrossRef]
- Tejedor-Sanz, S.; Stevens, E.T.; Li, S.; Finnegan, P.; Nelson, J.; Knoesen, A.; Light, S.H.; Ajo-Franklin, C.M.; Marco, M.L. Extracellular electron transfer increases fermentation in lactic acid bacteria via a hybrid metabolism. eLife 2022, 11, e70684. [Google Scholar] [CrossRef] [PubMed]
- Zunga, M.; Yebra, M.J.; Monedero, V. Complex Oligosaccharide Utilization Pathways in Lactobacillus. Curr. Issues Mol. Biol. 2021, 40, 49–80. [Google Scholar] [CrossRef] [PubMed]
- Ayivi, R.D.; Ibrahim, S.A.; Krastanov, A.; Somani, A.; Siddiqui, S.A. The impact of alternative nitrogen sources on the growth and viability of Lactobacillus delbrueckii ssp. bulgaricus. J. Dairy Sci. 2022, 105, 7986–7997. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A. Where in the world do bacteria experience oxidative stress? Environ. Microbiol. 2019, 21, 521–530. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant Properties of Probiotic Bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Choi, G.H.; Lee, N.K.; Paik, H.D. Optimization of Medium Composition for Biomass Production of Lactobacillus plantarum 200655 Using Response Surface Methodology. J. Microbiol. Biotechnol. 2021, 31, 717–725. [Google Scholar] [CrossRef]
- Skoda, A.M.; Simovic, D.; Karin, V.; Kardum, V.; Vranic, S.; Serman, L. The role of the Hedgehog signaling pathway in cancer: A comprehensive review. Bosn. J. Basic Med. Sci. 2018, 18, 8–20. [Google Scholar] [CrossRef]
- Fu, J.; Li, L.; Huo, D.; Yang, R.; Yang, B.; Xu, B.; Yang, X.; Dai, M.; Tan, C.; Chen, H.; et al. Meningitic Escherichia coli α-hemolysin aggravates blood-brain barrier disruption via targeting TGFβ1-triggered hedgehog signaling. Mol. Brain 2021, 14, 116. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Yang, Q.; Zhao, W.; Cui, L.; Wang, B.; Zhang, L.; Cheng, H.; Song, S.; Zhang, L. Genomics and LC-MS Reveal Diverse Active Secondary Metabolites in Bacillus amyloliquefaciens WS-8. J. Microbiol. Biotechnol. 2020, 30, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Beis, K. Structural basis for the mechanism of ABC transporters. Biochem. Soc. Trans. 2015, 43, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.F.; Wang, Y.; Cai, Z.; Bai, S.; Yao, Z.; Awan, U.A.; Zhang, Z.; Zheng, W.; Zhang, H. Gut microbiota promotes host resistance to low-temperature stress by stimulating its arginine and proline metabolism pathway in adult Bactrocera dorsalis. PLoS Pathog. 2020, 16, e1008441. [Google Scholar] [CrossRef] [PubMed]
- Senizza, A.; Callegari, M.L.; Senizza, B.; Minuti, A.; Rocchetti, G.; Morelli, L.; Patrone, V. Effects of Linoleic Acid on Gut-Derived Bifidobacterium breve DSM 20213: A Transcriptomic Approach. Microorganisms 2019, 7, 710. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Tabashsum, Z.; Patel, P.; Bernhardt, C.; Biswas, D. Linoleic Acids Overproducing Lactobacillus casei Limits Growth, Survival, and Virulence of Salmonella Typhimurium and Enterohaemorrhagic Escherichia coli. Front. Microbiol. 2018, 9, 2663. [Google Scholar] [CrossRef]
- Shen, T.; Liu, J.L.; Wang, C.Y.; Rixiati, Y.; Li, S.; Cai, L.D.; Zhao, Y.Y.; Li, J.M. Targeting Erbin in B cells for therapy of lung metastasis of colorectal cancer. Signal Transduct. Target. Ther. 2021, 6, 115. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Guo, J.; Chen, X.; He, H. The Metabolomics Changes in Luria–Bertani Broth Medium under Different Sterilization Methods and Their Effects on Bacillus Growth. Metabolites 2023, 13, 958. https://doi.org/10.3390/metabo13080958
Wang H, Guo J, Chen X, He H. The Metabolomics Changes in Luria–Bertani Broth Medium under Different Sterilization Methods and Their Effects on Bacillus Growth. Metabolites. 2023; 13(8):958. https://doi.org/10.3390/metabo13080958
Chicago/Turabian StyleWang, Haifeng, Juan Guo, Xing Chen, and Hongxuan He. 2023. "The Metabolomics Changes in Luria–Bertani Broth Medium under Different Sterilization Methods and Their Effects on Bacillus Growth" Metabolites 13, no. 8: 958. https://doi.org/10.3390/metabo13080958
APA StyleWang, H., Guo, J., Chen, X., & He, H. (2023). The Metabolomics Changes in Luria–Bertani Broth Medium under Different Sterilization Methods and Their Effects on Bacillus Growth. Metabolites, 13(8), 958. https://doi.org/10.3390/metabo13080958






