Abstract
Trillions of diverse microbes reside in the gut and are deeply interwoven with the human physiological process, from food digestion, immune system maturation, and fighting invading pathogens, to drug metabolism. Microbial drug metabolism has a profound impact on drug absorption, bioavailability, stability, efficacy, and toxicity. However, our knowledge of specific gut microbial strains, and their genes that encode enzymes involved in the metabolism, is limited. The microbiome encodes over 3 million unique genes contributing to a huge enzymatic capacity, vastly expanding the traditional drug metabolic reactions that occur in the liver, manipulating their pharmacological effect, and, ultimately, leading to variation in drug response. For example, the microbial deactivation of anticancer drugs such as gemcitabine can lead to resistance to chemotherapeutics or the crucial role of microbes in modulating the efficacy of the anticancer drug, cyclophosphamide. On the other hand, recent findings show that many drugs can shape the composition, function, and gene expression of the gut microbial community, making it harder to predict the outcome of drug-microbiota interactions. In this review, we discuss the recent understanding of the multidirectional interaction between the host, oral medications, and gut microbiota, using traditional and machine-learning approaches. We analyze gaps, challenges, and future promises of personalized medicine that consider gut microbes as a crucial player in drug metabolism. This consideration will enable the development of personalized therapeutic regimes with an improved outcome, ultimately leading to precision medicine.
1. Introduction
Orally administered drugs encounter millions of microbial species in the gastrointestinal tract (GIT). With approximately 3.3 million unique genes, gut microbes are considered an invisible organ that vastly expands the human enzymatic capacity [1,2,3]. These microbes, their genetic components, and epigenetics regulation are unique to each individual and contribute to varied drug responses between individuals [3,4,5,6]. Several examples in the literature have noted the effect of microbes on drug metabolism [7,8,9]. Their effect is widespread over drugs used to treat cancer, depression, Parkinson’s disease, and cardiovascular diseases [10,11,12,13,14]. For example, Mycoplasma hyorhinis encodes an enzyme known as cytidine deaminase which deactivates the anticancer drug gemcitabine to 2′,2′difluorodeoxyuridine, contributing to chemotherapeutic resistance [15,16]. Other microbes such as Escherichia coli can metabolize multiple anticancer drugs such as gemcitabine, doxorubicin, and tretazicar [5,16,17]. A recent finding suggests that a single microbe, Bacteroides thetaiotaomicron, is capable of metabolizing more than 40 drugs including the widely used calcium channel blocker, diltiazem [7]. Computational software has been developed to predict the susceptibility of different drugs to microbial metabolism based on the presence of specific functional groups in the drug. Moreover, gut microbes might affect the efficacy of drugs in an indirect way, such as in the case of cyclophosphamide, where the activity of the drug is mediated by its damaging effect on the gut mucosa which enables the systemic translocation of gut microbes to lymph nodes and spleen, increasing the density of immune cells at the tumor microenvironment and activating an immune attack on cancer cells [18,19] (Figure 1). The interaction between gut microbes and oral medications is not a one-way interaction that makes these medications susceptible to microbial degradation, it is bidirectional, where drugs can also exert an effect on gut microbes shaping their composition and function [20]. It is generally acknowledged that gut microbes are affected by antibiotics, but more recent findings report that more than 24% of non-antibiotic drugs exert an antibiotic-like action on gut microbes [21]. For example, analysis of the gut microbial community in patients receiving metformin treatment revealed that metformin specifically decreases the abundance of Bacteroides fragilis [22], while atorvastatin promotes the growth of Akkermansia muciniphila and Faecalibacterium prausnitzii, and inhibits the growth of Proteobacteria and Enterobacteriacae. This interaction is also observed with supplements such as Vitamin D, which increases Streptococcus salivarius, Bacteroides sp., and Parabacteroides [23]. With increasing interest in pharmacomicrobiomics as an integral component of the personalized medicine approach [24,25], we present this overview of the current knowledge of microbial drug metabolism and its impact on drug bioavailability and therapeutic outcome. We discuss the bidirectional interactions between oral medications and gut microbes. We assess the recent development of computational tools to predict possible drug metabolism by microbes. Considering the unique microbiome signature of each individual in the planning of therapeutic regimes is a crucial component in the development of personalized and precision medicine [26,27].
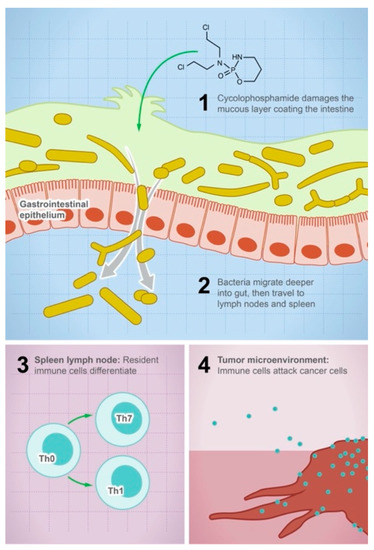
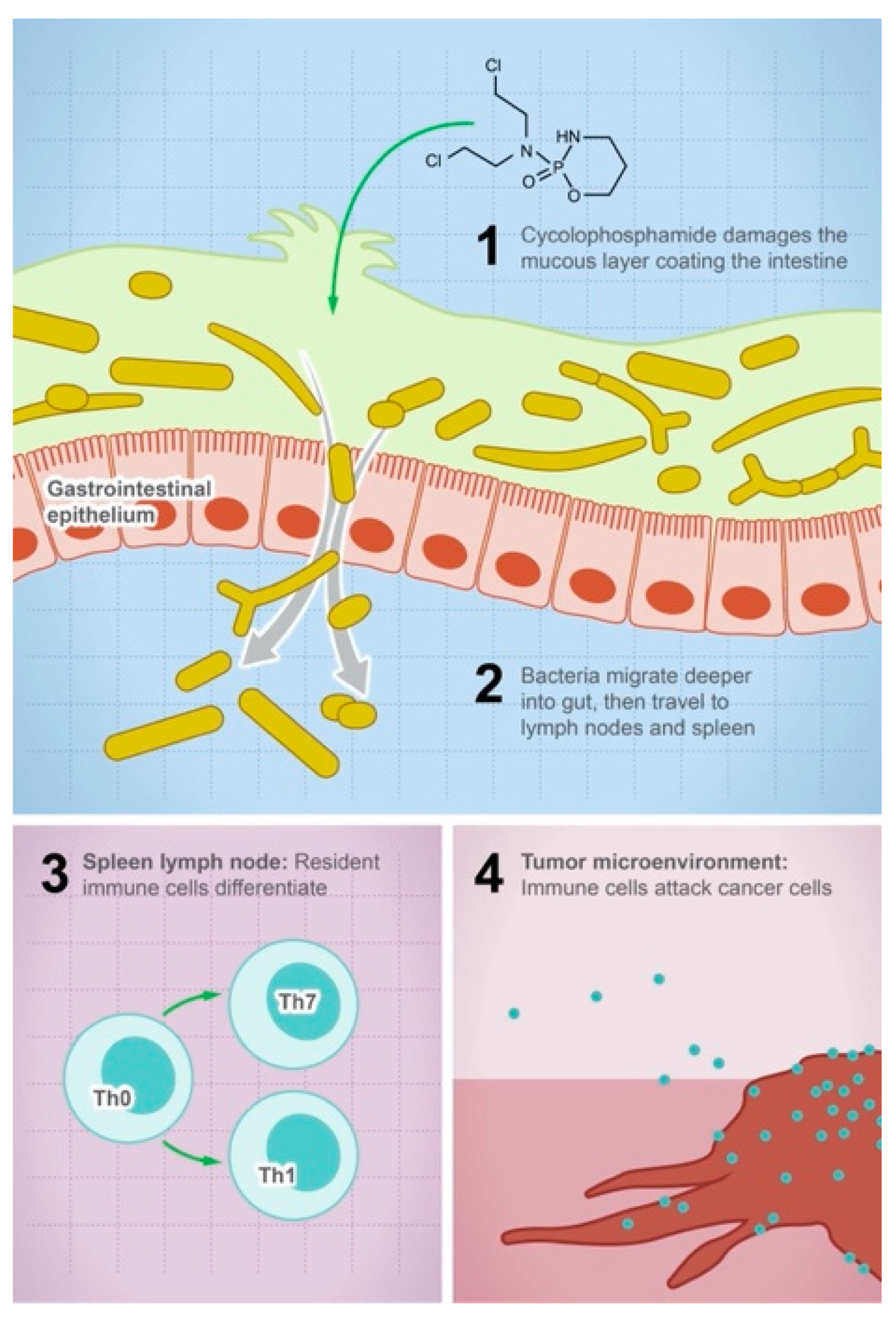
Figure 1.
Gut microbes mediate the efficacy of the anticancer drug cyclophosphamide. The illustration shows the steps leading to the anticancer activity of cyclophosphamide: (1) the drug damages the mucous layer coating the intestine, (2) bacteria can systemically migrate to lymph nodes and spleen, (3) activation of the immune cell differentiation and production of cytokines and toxic mediators, and (4) the density of immune cells in the tumor microenvironment will increase and attack tumor cells.
2. Susceptibility of Oral Medications to Microbial Metabolism
Multiple functional groups within oral medications are susceptible to microbial metabolism leading to drug activation, inactivation, or toxicity. Recent reports show that particular functional groups such as ester, amide, nitro, and azo, cause the drug to be more susceptible to microbial enzymatic degradation [7]. For example, albiflorin contains an ester group which causes it to be susceptible to hydrolysis by some species of Bifidobacteria [13]. Similarly, benzodiazepines are susceptible to metabolism due to the presence of a nitro group [28,29]. The antibacterial activities of prontosil, neoprontosil, sulfasalazine, balsalazide, and olsalazine are mediated by microbial azo reduction [30,31,32,33,34]. The antidiarrheal effect of Loperamide oxide is mediated by cleavages of N-oxide bonds, and the production of Loperamide by intestinal microbiota [35]. The laxative effect of sodium picosulfate requires the conversion of bisulfate to 4,4′-dihydroxydiphenyl-(2-pyridyl)-methane by bacteria residing in the gut [36]. The diarrheal side effect of the anticancer drug Irinotecan is attenuated by the glucuronidase enzyme produced by intestinal flora [37]. Anaerobic incubation of levamisole with human gut microbes results in the production of levametabol-I, II and III, thiazole ring-opened metabolites that have been implicated in anti-colon cancer activity [38]. Selected examples of common microbial drug metabolism based on particular functional groups are listed in Table 1 and/or illustrated in Figure 2.

Table 1.
Susceptible medications for microbial metabolism.
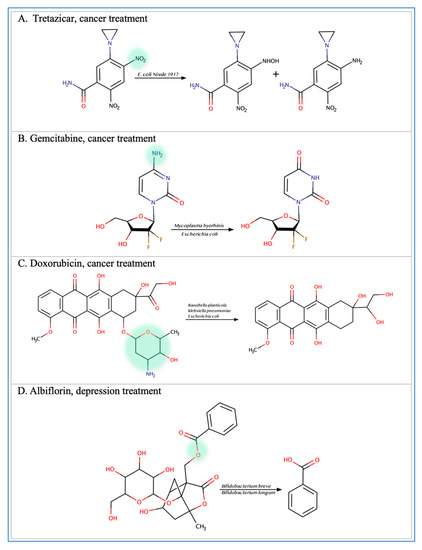
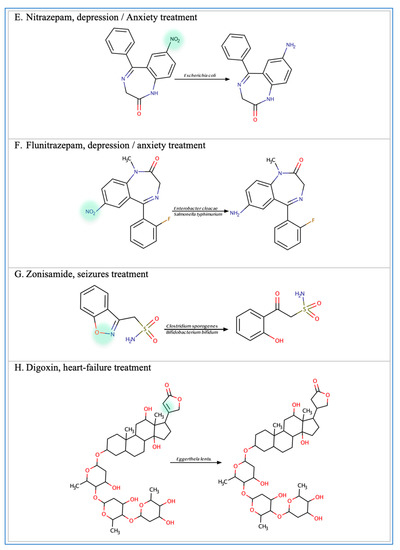
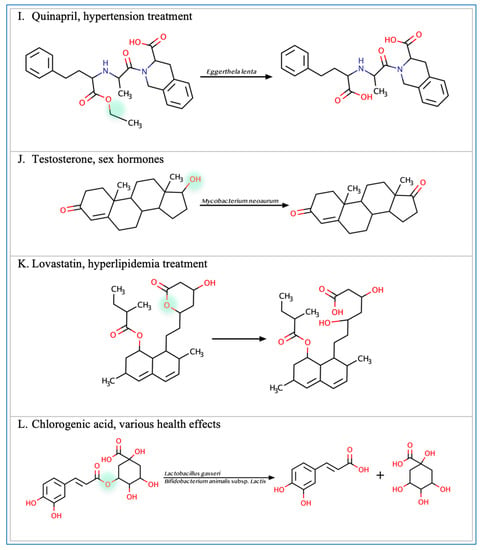
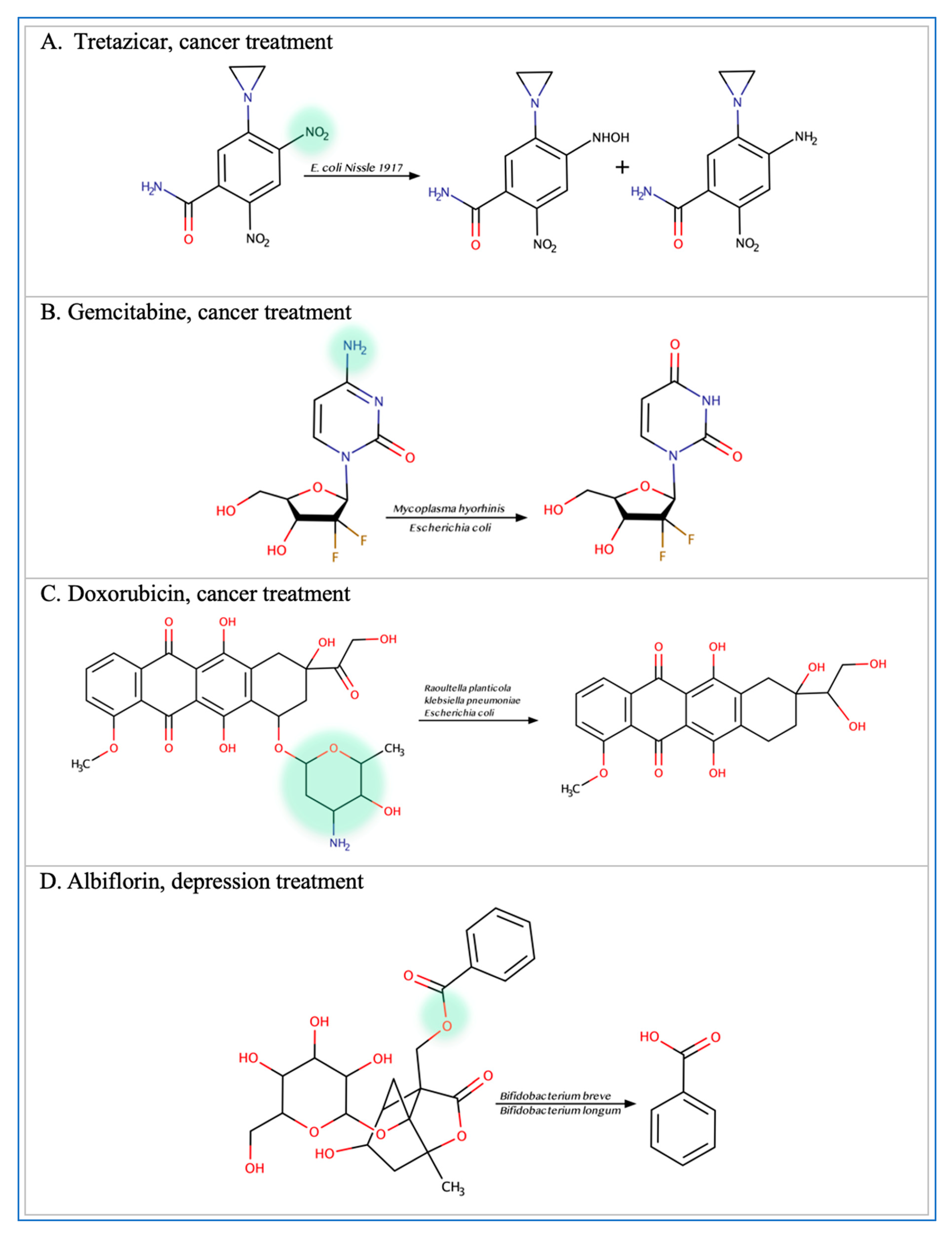
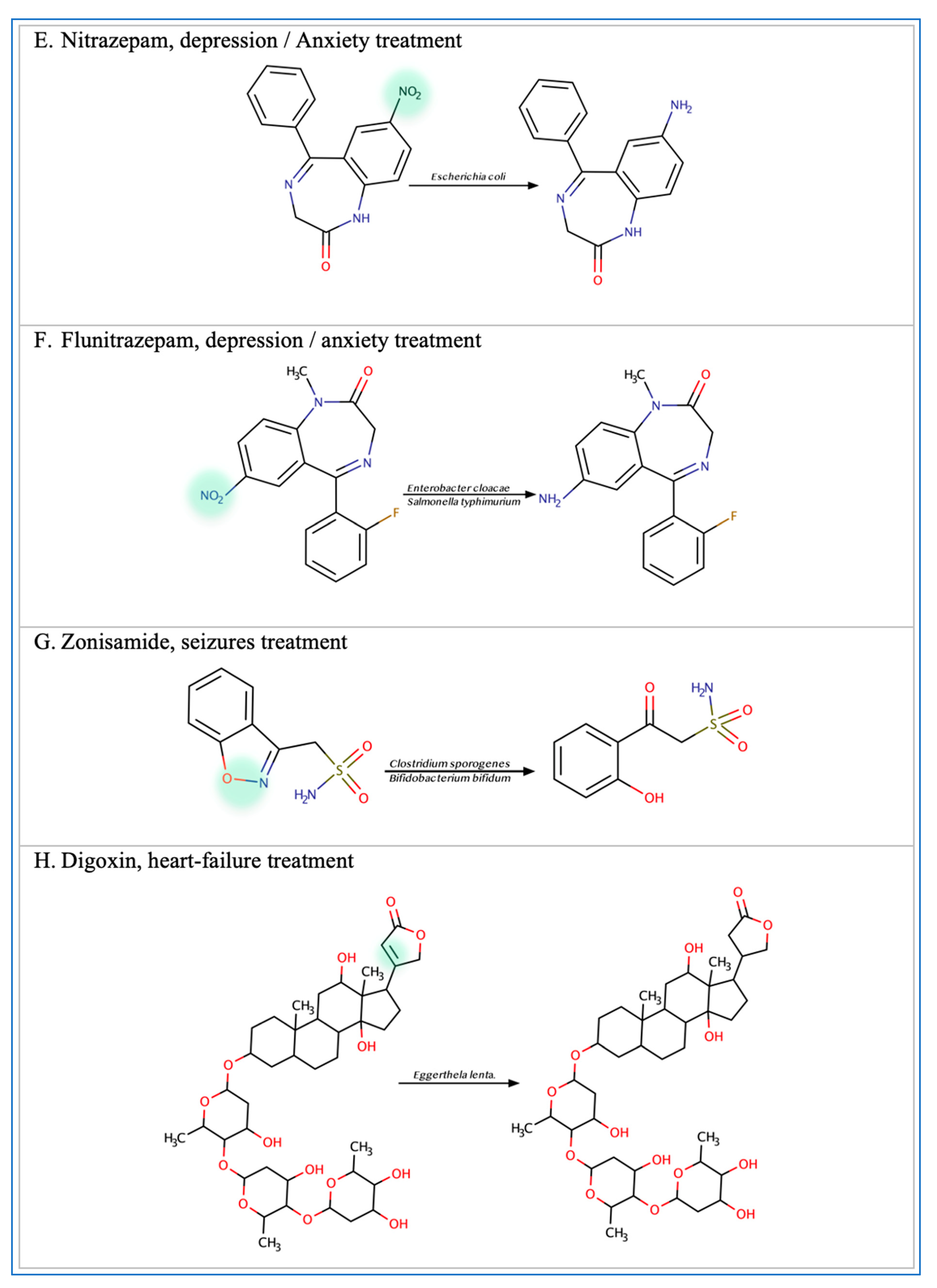
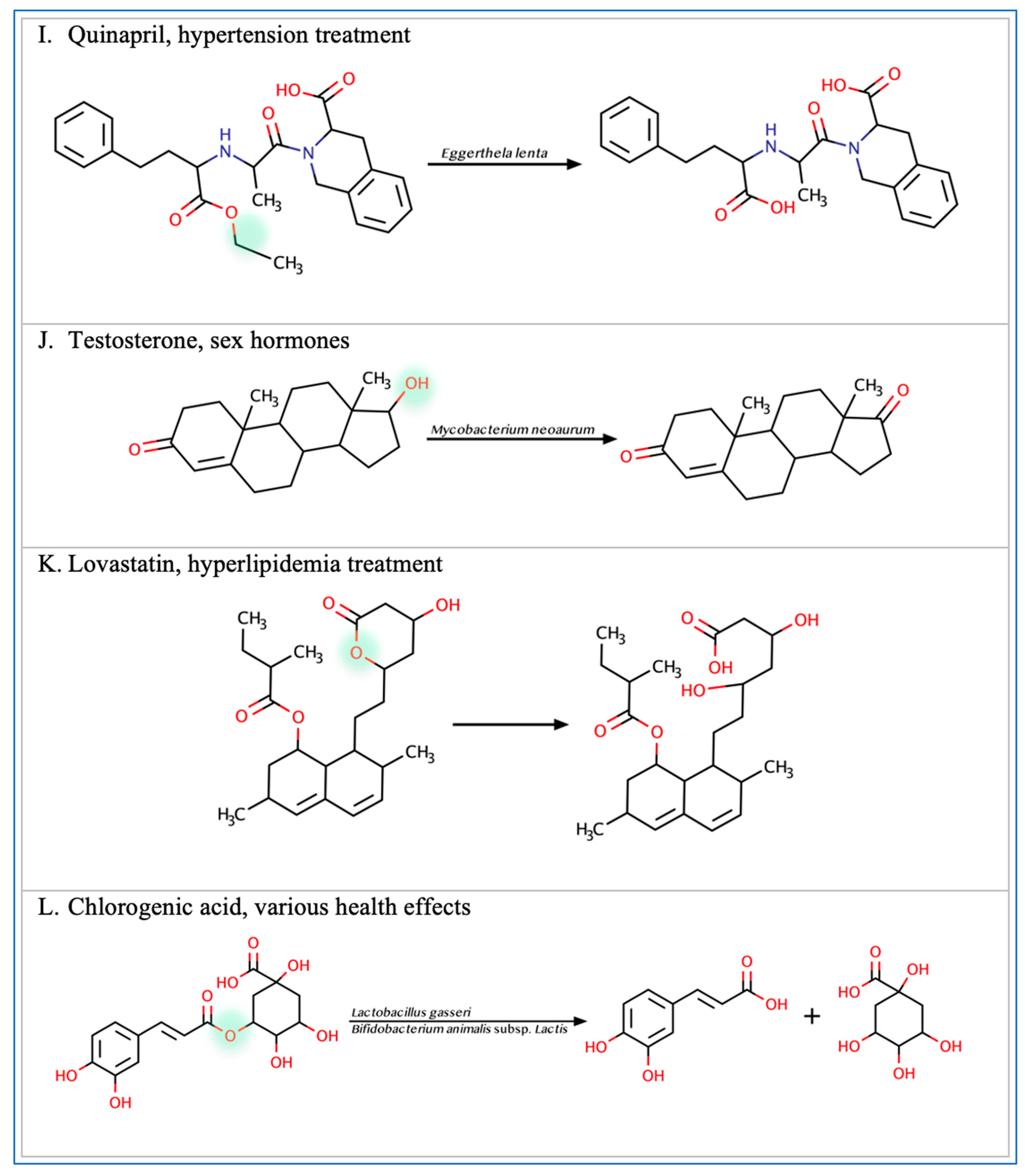
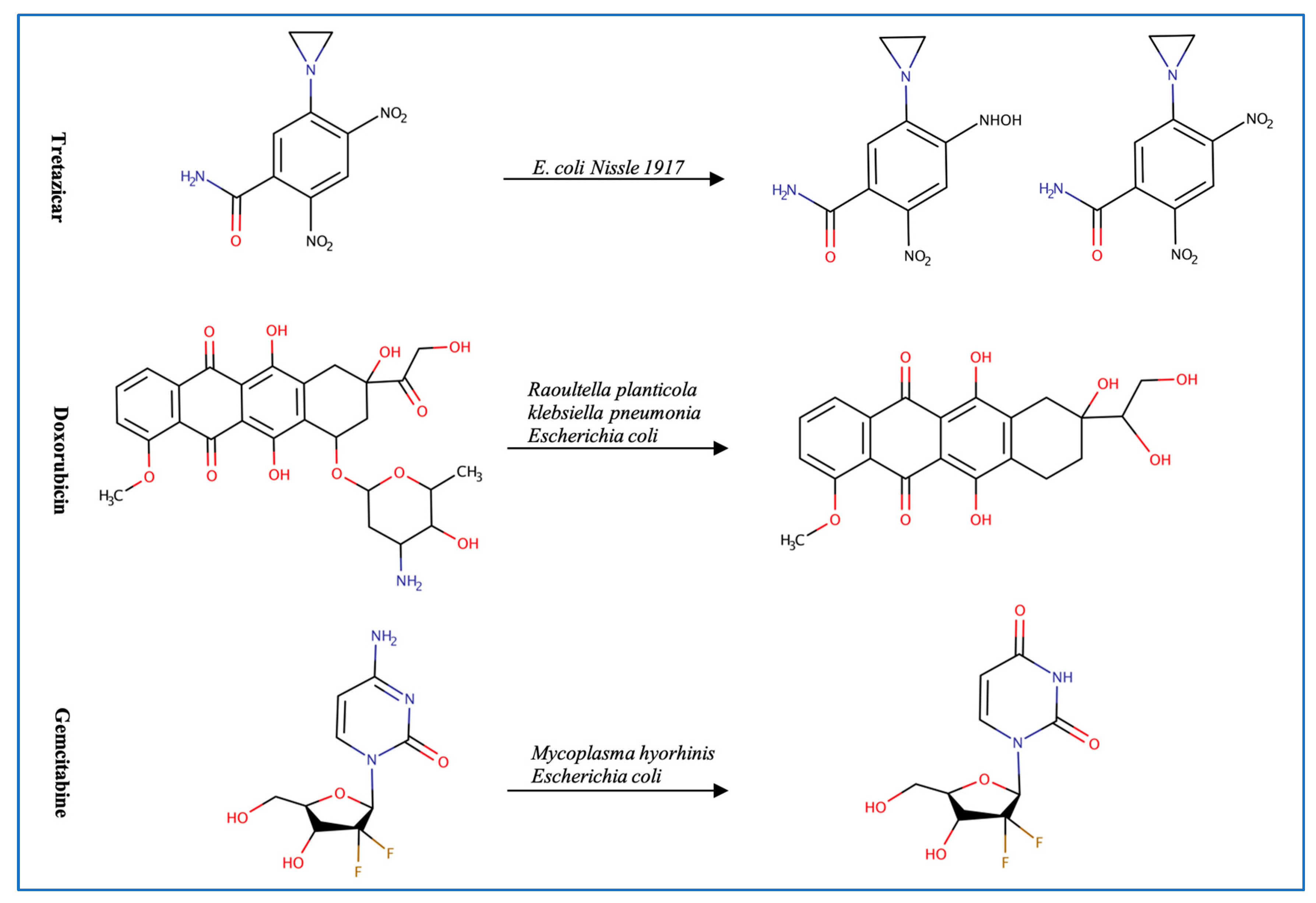
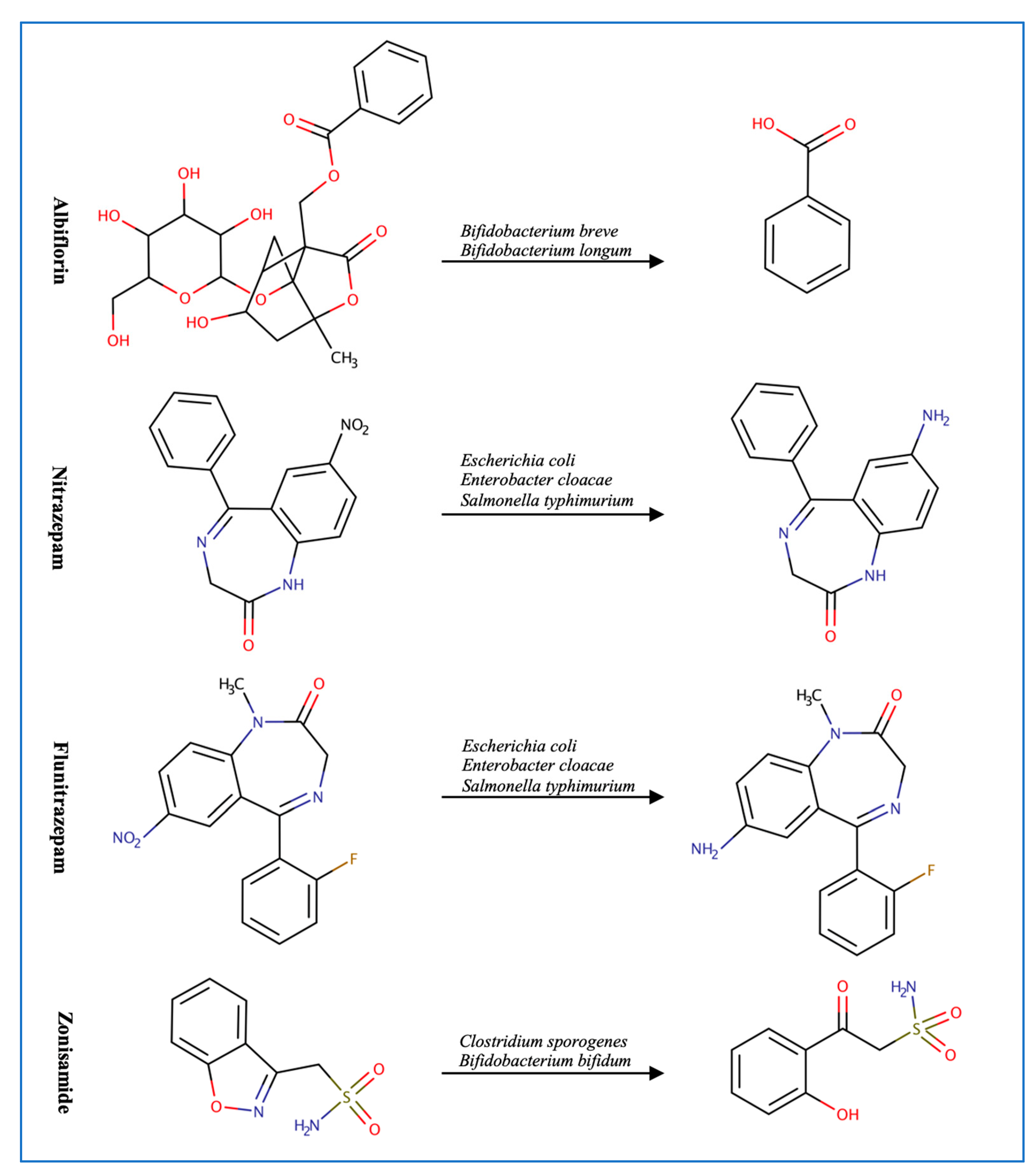
Figure 2.
Illustration of some examples of microbial drug metabolism. (A) Shows degradation of the nitro functional group of tretazicar used for the treatment of cancer by E. coli Nissle 1917. (B) Shows degradation of the amine functional group of the anticancer drug gemcitabine by Mycoplasma hyorihinis and E. coli. (C) Shows deglycosylation of doxorubicin used for the treatment of cancer by Raoultella planticola, Klebsiella pneumoniae, and E. coli. (D) Shows degradation of the ester functional group of albiflorin used for the treatment of depression by Bifidobacterium breve and Bifidobacterium longum. (E) Shows degradation of the nitro functional group of nitrazepam used for the treatment of depression and anxiety by E.coli. (F) Shows degradation of the nitro functional group of flunitrazepam used for the treatment of depression and anxiety by Enterobacter cloacae and Salmonella typhimurium. (G) Shows degradation of the benzisoxazole N-O bond of zonisamide used for the treatment of seizures by Clostridium sporogenes and Bifidobacterium bifidum. (H) Shows a reduction in the carbon 20 and carbon 22 bonds of digoxin used for the treatment of heart failure by Eggerthela lenta. (I) Shows degradation of the ester bond of quinapril used for the treatment of hypertension by Eggerthela lenta. (J) Shows dehydrogenation of the hydroxyl functional group at carbon 17 of testosterone by Eggerthela lenta. (K) Shows degradation of the lactone group of lovastatin used for the treatment of hyperlipidemia by a group of gut microbes. (L) Shows degradation of the ester functional group of chlorogenic acid by Lactobacillus gasseri and Bifidobacterium animalis subsp lactis.
3. Microbial Drug Metabolism for Different Oral Medications Classes
Here we discuss examples of microbial metabolism based on xenobiotic intended use, including cancer, central nervous system, cardiovascular, steroids, supplements, and natural products.
3.1. Anticancer Drugs
Inconsistent response and resistance to anticancer therapeutics is a major concern [4]. This resistance, in part, might be attributed to the microbiome microenvironment (Figure 3). Multiple microbial species have been linked to resistance to chemotherapeutics including E. coli and Listeria welshimeri [5]. E. coli is involved in the degradation of multiple anticancer drugs such as tretazicar, gemcitabine, and doxorubicin [5,16,17]. The strain E. coli Nissle 1917 reduces tretazicar to its active metabolite [5]. E. coli produces cytidine deaminase enzyme which converts gemcitabine to the inactive product 2′,2′difluorodeoxyuridine [16,17]. The bacterial cytidine deaminase is also found in M. hyorhinis which metabolizes gemcitabine to 2′,2′-difluoro-2′-deoxyuridine, reducing the drug’s activity by 10-60-fold. The presence of pyrimidine nucleoside phosphorylase further potentiates the metabolism of gemcitabine, because it catabolizes the products from gemcitabine metabolism, increasing the reaction rate towards gemcitabine degradation [15]. Similarly, Raoultella planticola can convert doxorubicin to 7-deoxydixirubicinlone and 7-deoxydoxorubicinol by reductive deglycosylation [17]. Of note is that a molybdenum cofactor is required to degrade doxorubicin by multiple microbes such as Raoultella planticola, K. pneumoniae, and E. coli BW25113. In addition, gut microbes might have an indirect effect in enhancing response to anticancer drugs. Data show that the administration of antibiotics with the immune checkpoint inhibitor (ICI) anti-PD-1, decreases the progression-free survival (PFS) and overall survival (OS) period [49] in patients, while administration of Alistipes indistinctus, Akkermansia muciniphila, and Enterococcus hirae, restores efficacy of the treatment [50]. These microbes are thought to activate an immune response against cancer cells by increasing the secretion of IL-2 from the dendritic cells and recruiting different types of T cells in the tumor microenvironment [50,51]. The gut microbial signature can also be used as a biomarker indicative of possible ICIs’ treatment response. For example, a high fecal microbial diversity and abundance of the bacterial species Enterococcus faecium, Veillonella parvula, Bifidobacterium longum, Bifidobacterium adolescentis, Lactobacillus sp., K. pneumoniae, Collinsella aerofaciens, and Parabacteroides merdae suggest a better response [52]. Additionally, a longer progression-free survival period is linked to a high diversity and abundance of Romunicoccaceae, Clostridales, and Faecalibacterium [53]. The baseline microbiome can predict poor ipilimumab treatment response in metastatic melanoma patients with an accuracy of 99%. For example, the bacterial OTUs (Bacteroides uniformis, Bacteroides vulgatus, Parabacteroides distasonis) can act as potential biomarkers for ipilimumab-colitis free patients [54]. While previous findings support the effect of gut microbes on ICIs, they also highlight their potential role as biomarkers for treatment response. Given the crucial impact of these metabolic reactions on chemotherapeutics resistance, it is essential to consider the microbiome inter-patient variation and potential metabolic activities that affect treatment outcome [17].
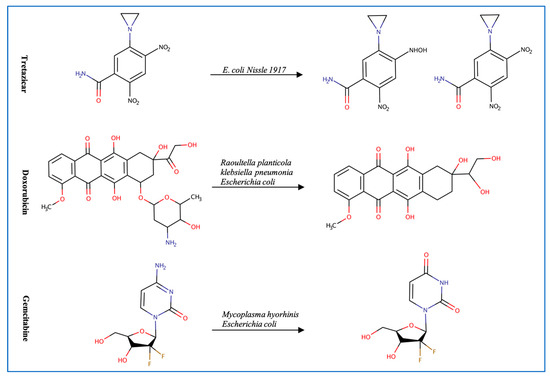
Figure 3.
Examples of anticancer drugs transformed by gut microbes.
3.2. Central Nervous System Drugs
Mounting evidence supports the link between gut microbes and neurodegenerative and psychological disorders [55,56,57] (Figure 4). For example, the bacterial strains Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium animalis, and Bifidobacterium adolescentis can metabolize the anti-depression drug, albiflorin. B. longum has the strongest ability at hydrolyzing albiflorin followed by B. breve since they are the only two microbes that significantly increase the concentration of albiflorin’s metabolite. After searching the genome of the strains for esterase enzymes and evaluating the 3D models of the proteins, similarities were found in the core domain, indicating that esterase enzymes could be responsible for albiflorin conversion [13]. It is important to highlight the common use of those hydrolyzing bacterial strains in probiotic supplements since it could interfere with treatment response. The metabolizing effect is not limited to Bifidobacteria since gut microbes can metabolize clonazepam, nitrazepam, and flunitrazepam through nitro-reduction [28,29,58]. The genes nfsB, nfnB, and nfsI are responsible for the metabolic effect of E. coli, Enterobacter cloacae, and Salmonella typhimurium, respectively [29]. Reports show that Clostridium sporogenes and Bifidobacterium bifidum can also reduce zonisamide and risperidone through the benzisoxazole N-O bond into inactive forms [59].
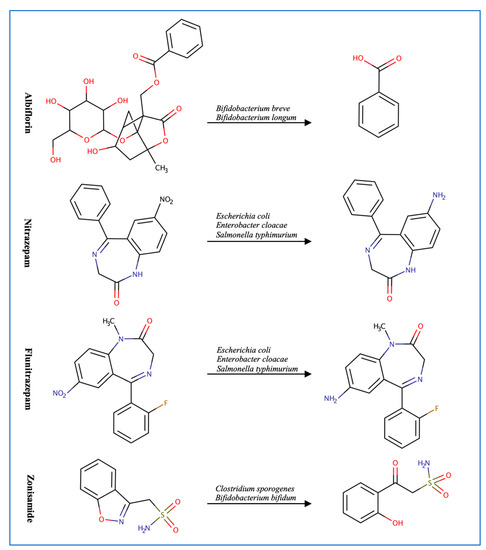
Figure 4.
Examples of CNS drugs transformed by gut microbes.
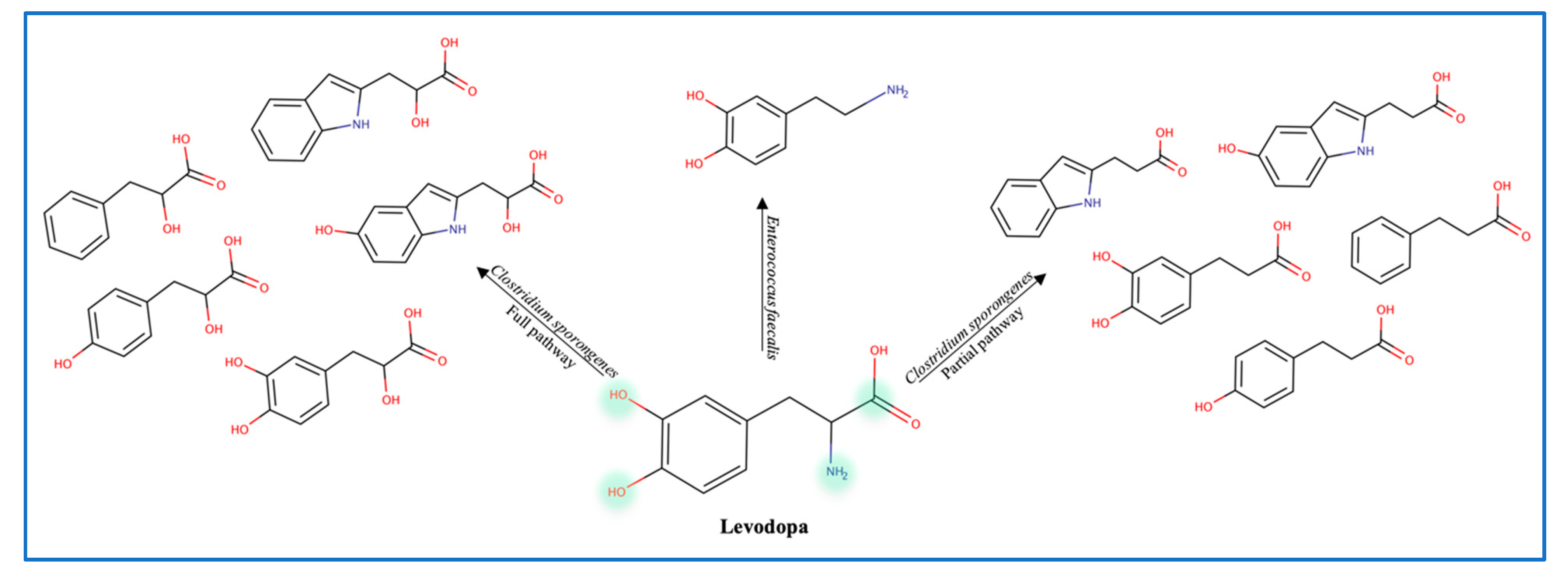
To explain the variations in levodopa (L-dopa) treatment response, the role of gut microbes was evaluated by administering antibiotics to patients receiving the treatment. Data show that antibiotics increase the efficacy of levodopa, which confirms the role of gut microbes in metabolizing L-dopa [29]. Balskus et al. identified a decarboxylase enzyme in Enterococcus faecalis as the main metabolizing enzyme. However, this decarboxylation is non-selective since both L-dopa and tyrosine are decarboxylated (Figure 5) [12]. Moreover, the tyrDc operon is deemed essential for the E. faecalis decarboxylating effect [60]. Further conversion of dopamine to m-tyramine is carried out by another gut microbe known as Eggerthela lenta which produces a dopamine dehydroxylase molybdenum-dependent enzyme. Interestingly, co-administration of carbidopa (a tyrosine decarboxylase inhibitor) was found to be partially effective against bacterial tyrosine decarboxylase (inhibiting 50% of E. faecalis enzyme) [12]. L-dopa is also deaminated by Clostridium sporongenes to propionic acid metabolites through the activity of aromatic amino transferase. Silencing of this enzyme resulted in partial deamination and reduction in propionic acid metabolites. Although even with partial deamination these metabolites can still inhibit ileal muscles motility [61,62]. Of note is that Levodopa metabolites produced by C. sporongenes are either fully deaminated or partially deaminated due to a mutated dehydratase enzyme in the deamination pathway. These findings emphasize the need to consider the microbiome variation between individuals and adjust the dose of levodopa accordingly.
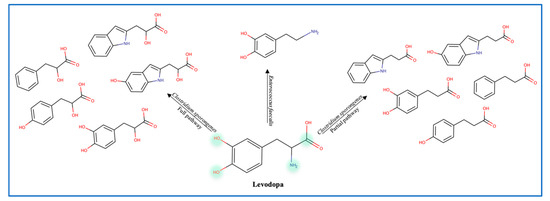
Figure 5.
Levodopa metabolites produced by gut microbes.
3.3. Cardiovascular Drugs
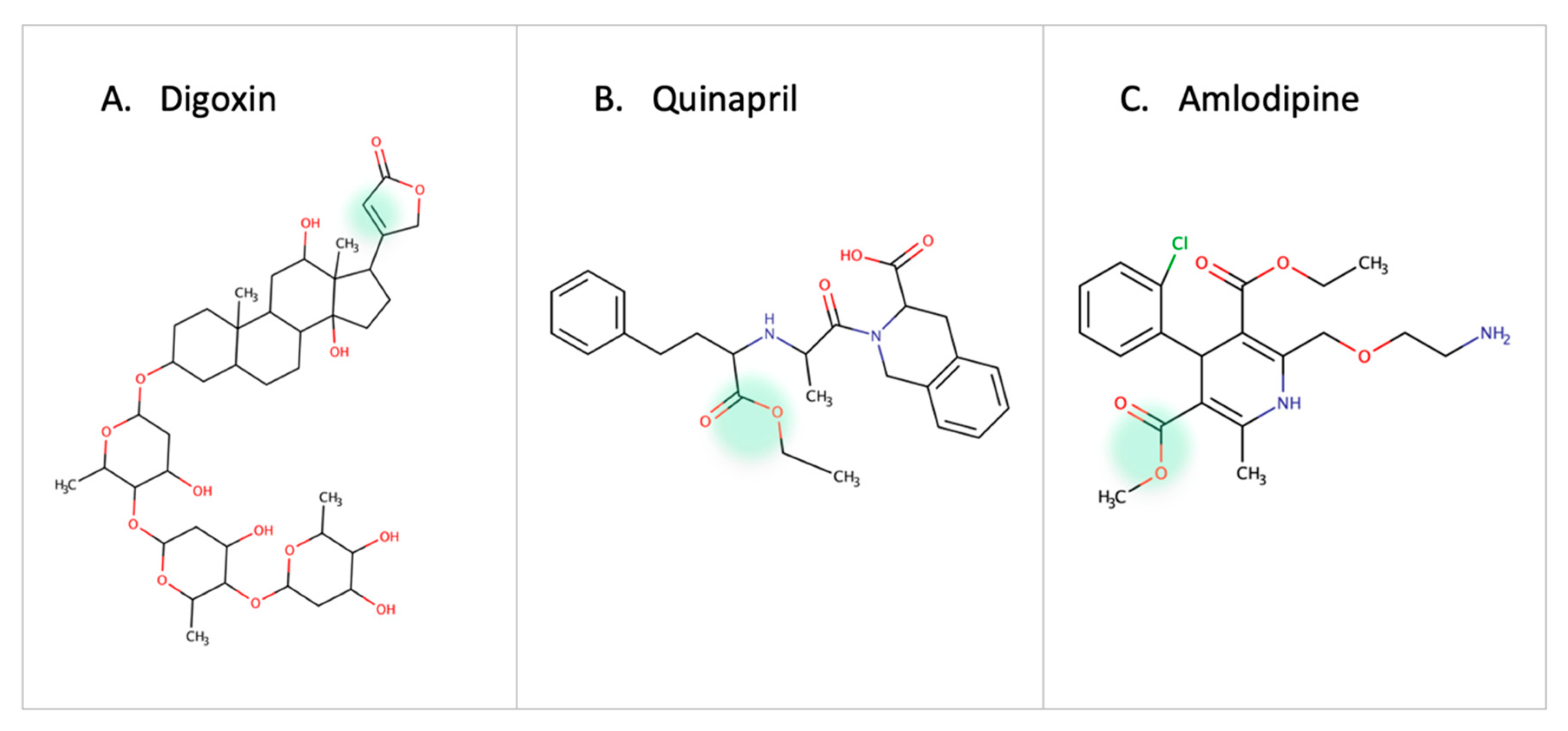
One of the earliest reports on drug-microbe metabolism was on Digoxin. It is reduced in the gut by Eggerthela lenta to dihydroxy digoxin through a mechanism similar to that of the natural substrate fumarate by a cardiac glycoside reductase enzyme [63,64,65]. In hypertension patients, the use of quinapril (ACEI, angiotensin converting enzyme inhibitor) or amlodipine (CCB, calcium channel blocker) is affected by esterase enzymes in gut microbes [10,66] (Figure 6). Administration of antibiotics in patients receiving Amlodipine led to an increase in the drug concentration which proved the role of microbes in amlodipine degradation. Accordingly, it is necessary to adjust the dose of quinapril and amlodipine in patients receiving antibiotics or with high/low abundance of the involved bacterial strain [66]. The treatment response of lovastatin is altered due to the presence of gut microbes. Administering the drug with antibiotics decreases the active hydroxy acid metabolite. This highlights the role of gut microbes in metabolizing statins and the indirect drug–drug interaction between statins and antibiotics [11].
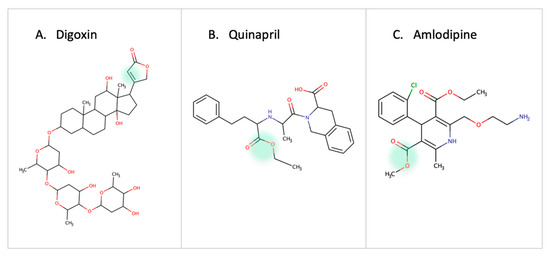
Figure 6.
Examples of cardiovascular drugs transformed by gut microbes.
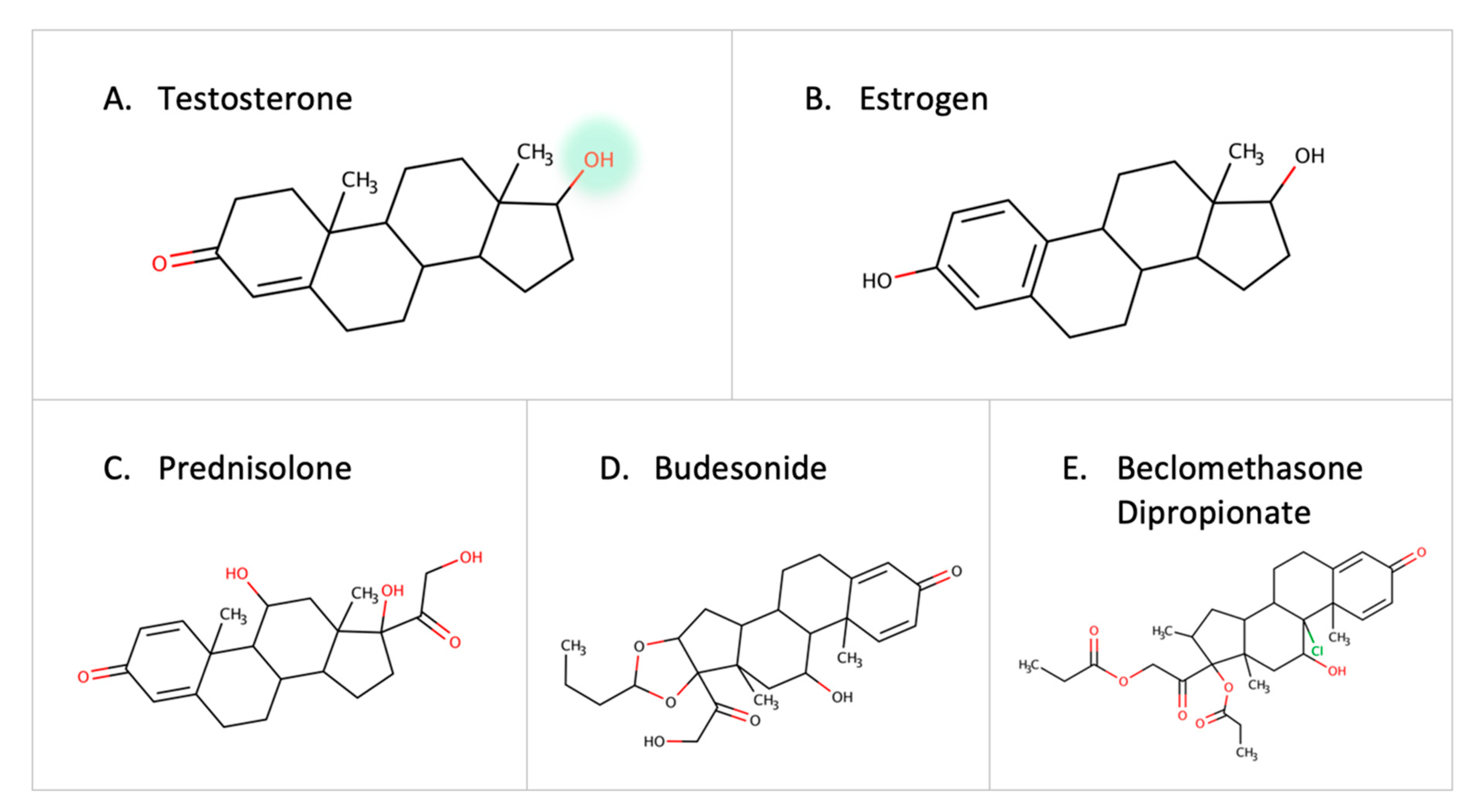
3.4. Steroids and Corticosteroids
Steroid-degrading enzymes are present in Proteobacteria and Actinobacteria [67]. Recently, the testosterone-degrading enzyme, 3-beta-hydroxysteroid dehydrogenase enzyme (3beta-HSD), was identified in Mycobacterium neoaurum and other microbes [68]. A low level of the natural testosterone can result in depression-like symptoms in males [68,69,70]. Thus, the abundance of microbes containing steroid-degrading enzymes can be a contributor to disease progression. Other steroid-degrading enzymes such as steroid reductase and hydroxysteroid dehydrogenase are expressed by Streptococcus mutans and Bacillus cereus [71]. Moreover, the desmolase enzyme in Clostridium scindens is capable of cleaving the side chain of 17-hydroxylatedcorticoids [72]. Estrogen is inactivated by glucuronidation, but gut microbes reactivate it by eliminating the glucuronic moiety due to the effect of B-glucuronidase [73]. Since estrogen is implicated in breast cancer, it is necessary to further investigate the role of gut microbes in estrogen metabolism [74]. In addition to native hormones, reports show that colonic bacteria can metabolize external corticosteroids such as prednisolone, budesonide, and beclomethasone dipropionate before they reach the site of action [75] (Figure 7). With the established links between steroid hormones and certain diseases [76], gut microbes can play a major role in degrading drugs, leading to a decrease in drug response or contributing to the pathogenesis of certain diseases.
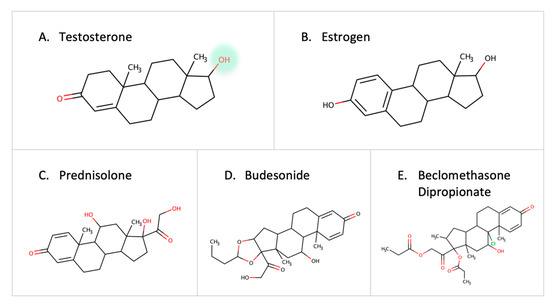
Figure 7.
Examples of steroids and corticosteroids transformed by gut microbes.
3.5. Miscellaneous Xenobiotics and Natural Substances
Gut microbes can exert an effect on supplements, such as vitamin D. Gut microbes have been linked to low vitamin D levels since germ-free mice have shown high levels of fibroblast growth factor 23 (FGF23), while microbial transplantation restored vitamin D and FGF23 balance, which might suggest an indirect effect of gut microbes [77] although results are conflicting [78]. Additionally, gut microbes also affect natural substances such as chlorogenic, gallic, ferulic, and caffeic acids. Chlorogenic acid has various effects such as antioxidant [79], anti-inflammatory [79], anti-hypertensive [80], and glucose regulation [81]. An ongoing debate is whether chlorogenic acid can inhibit the growth of certain microbes or if the microbes are the ones capable of degrading the compound using enzymes such as cinnamoyl esterase. Faulds et al. reported that Lactobacillus gasseri can use phenolic compounds as its carbon source, which further stimulates its microbial growth. Upon testing microbes’ ability to degrade phenolic compounds, L. gasseri and Bifidobacterium animalis subsp. Lactis have been identified as the microbes with the greatest chlorogenic acid degrading effect. The mentioned strains contain cinnamoyl esterase that degrades chlorogenic acid to caffeic acid and quinic acid [82]. Munoz et al. reported that Lactobacillus brevis isolated from human feces CECT 4121, human mouth CECT 5354, and wine fermentation RM84, can decarboxylate p-coumaric acid, ferulic acid, caffeic acid, gallic acid, and protocatechuic acid, to vinyl phenol, vinyl guaiaol, vinyl catechol, pyrogallol, and catechol, respectively, but subsequent modification of the vinyl derivatives did not occur. Although p-coumaric acid and caffeic acid were completely decarboxylated, ferulic acid was still present, indicating non-complete decarboxylation. After searching the genome of L. brevis CECT 5354, a sequence encoding a phenolic acid decarboxylase explained the observed effect [83].
4. Effect of Oral Medications on Gut Microbes
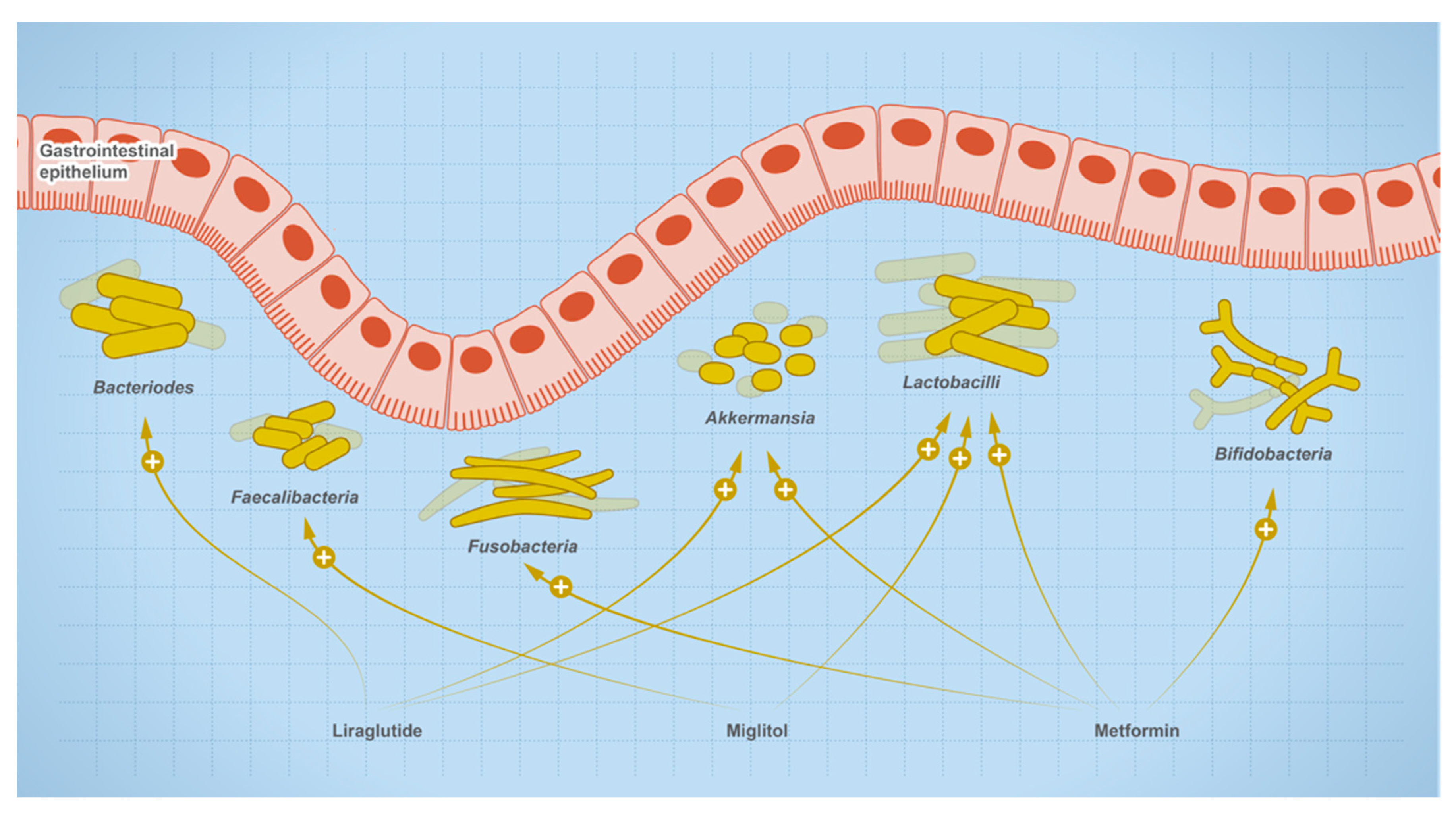
The relationship between gut microbes and drugs is bidirectional, where drugs are susceptible to microbial metabolism and can shape the composition of the microbial community, such as drugs used for the treatment of type 2 diabetes (Figure 8). Users of metformin showed elevated levels of butanoate, quinone, and sugar breakdown metabolites with species of Enterobacteriaceae such as E. coli being the main contributors to these functional shifts in metformin users. Other research shows that mice on a high-fat diet (HFD) experienced dysbiosis when treated with metformin, while untreated HFD mice showed an increase in the genera Akkermansia and Alistipes, and a decrease in Lactonifactor, Lactococcus, Anaerotrotruncus, Parabacteroides, Odoribacter, Lawsonia, and Blautia. In addition, one of the experienced metformin treatment outcomes can be due to the role of Akkermansia as an anti-inflammatory. It reduces the expression of IL-6, IL-1b, mRNA, and normalizes the levels of regulatory T cells [84]. In newly treated T2D patients, microbial analysis of fecal samples collected after 3 days of metformin treatment showed a reduction in the Bacteroides genus. Other research showed metformin also reduces the growth of B. fragilis and this effect is mediated through the folate and methionine pathway [22]. Metformin increases the levels of 5-methyl tetrahydrofolate, 5,10-methylene tetrahydrofolate, S-adenosylmethionine, and S-adenosylhomocysteine, but reduces the levels of methionine and tetrahydrofolate [85]. The observed effect was consistent with methionine synthase inhibition in which 5-methyl tetrahydrofolate is accumulated [86]. Accordingly, metformin may cause its effect through a similar inhibition.
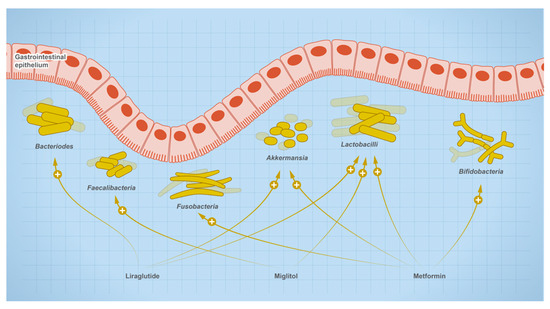
Figure 8.
Effect of therapeutics used for the treatment of type-2 diabetic on gut microbial abundance.
Interestingly, in a meta-analysis study, it was found that changes in the abundance of specific individual microbial taxa were associated with multiple independent drugs, while individual microbial features were also associated with single drugs [87,88]. For example, Streptococcus salivarius abundance increased in opiates, oral steroids, platelet aggregation inhibitors, proton pump inhibitors (PPIs), SSRI antidepressants, and vitamin D supplements [88], while benzodiazepine was found to be associated with an increase in the abundance of Haemophilus parainfluenzae, a bacterium that has been reported to be more common in patients with irritable bowel syndrome [87]. Increased abundances of Bifidobacterium dentium were found selectively in PPI users, while Eubacterium ramulus was in abundance within participants on SSRI antidepressants, and tricyclic antidepressant users showed higher abundances of Clostridium leptum. Individuals taking laxatives showed higher abundances of Alistipes and Bacteroides species [87] and steroid inhaler users had Streptococcus mutans and Bifidobacterium dentium in greater abundance. The use of drugs was also associated with shifts in gut function profiles [88]. For example, individuals taking high doses of PPIs demonstrated a significant decrease in a pathway involved in amino acid biosynthesis. In a group of patients treated with atorvastatin, the drug decreased the relative abundance of the following taxa and genera: Proteobacteria, Enterobacteriaceae, Desulfovibrio, Prevotella, Collinsella, and Streptococcus, while it increased the abundance of Firmicutes, Akkermansia muciniphila, and Faecalibacterium prausnitzii [20]. Moreover, the administration of methotrexate resulted in various alterations in the gut microbial community, especially in Bacteroidetes. Methotrexate consistently decreased Bacteroidetes in bacterial isolate, mice, and the gut community [89]. Analysis of fecal samples of subclinical thyroidism patients receiving L-thyroxine indicated the presence of a dose-dependent relationship between L-thyroxine and gut microbes. The drug changed the relative abundance of microbial species that have a role in hydrolysis and carbohydrate metabolism functions performed in the gut. Increasing the dose of L-thyroxine increased the genera odoribacter, Enterococcus, and Ruminococcus. Since the Enterococcus species is an antibiotic-resistant strain, it may increase the chance of patients developing infections. It is noteworthy to mention that Ruminococcus increased in patients with Hashimoto’s type of hypothyroidism [90].
A meta-analysis of cohort studies revealed that several drugs affect the gut microbial composition of patients. There was an association between certain drugs and the abundance of specific microbial species. The use of SSRIs, oral steroids, platelet aggregation inhibitors, opiates, and vitamin D increased the abundance of Streptococcus salivarus. The increase in Eubacterium ramulus was specifically associated with the use of SSRIs. This category of medication consisted of six drugs with paroxetine comprising 32% of it. Additionally, the use of laxatives was associated with an increase in the abundance of Alistipes and Bacteroides. Moreover, using Tricyclic antidepressants increased the abundance of Clostridium leptum and Actinomyces. The use of steroid inhalers was associated with an increase in the abundance of Streptococcus mutans and Bifidobacterium dentium. Unlike inhaled steroids, oral steroids increased the abundance of Methanobrevibacter smithii and the methanogenesis pathways. To explain the weight gain associated with oral steroids administration, researchers found an association between Methanobrevibacter smithii, obesity, and high BMI. One drawback to the study was the fact that some changes in the microbiome profile could be due to the disease itself, not just the drug [88]. Moreover, researchers reported an increase in the relative abundance of Bacteroides spp. and Parabacteroides spp. upon vitamin D supplementation, and a decrease in the Firmicutes/Bacteroides ratio. However, for the results to be conclusive, assessment of a larger sample size would be necessary to confirm the association [23]. Table 2 summarizes the effects of several drugs on gut microbes. Table 2 summarizes selected examples of drugs and their effect on the microbiota composition.

Table 2.
The impact of various drugs on the abundance of microbiota.
5. Computational Prediction of Microbial Drug Metabolism
There are some available tools that can predict possible drug metabolism but they face several obstacles since the microbiome is specific to each individual and changes with multiple factors such as diseases, drugs, and lifestyle [92]. Zeng et al. (2019) created a database to include information on the transformation of bioactive substances by gut microbes along with disease and microbe interactions. Only experimentally determined interactions are referenced in the database. Using the NCBI taxonomy database, the authors collected information on the identified microbial strains, drugs, bioactive substances, herbal medicines, traditional medicines, and environmental pollutants. Finally, they developed a prediction web server called “MASI: Microbiota Active-Substance Interaction database”. Based on the input in the search engine, related data from the literature are listed. For instance, if a drug name is searched, all microbes that metabolize this drug are listed [93]. MASI was followed by the database “MagMD: Metabolic Action of gut Microbiota to Drugs” which includes information on enzymes and their impact on drug efficacy. The database was developed in a process similar to MASI [94].
To predict the possibility of drug degradation by gut microbes, Zhao et al. (2017) developed a machine-learning prediction tool called “Drug Bug”. They analyzed 491 gut bacterial genomes for metabolic enzymes, leading to a total of 324,697 metabolic enzymes. The enzymes were distributed into specific metabolic enzyme (EC) classes that perform certain metabolic modifications. The substrates of the database metabolic enzymes were used to predict the drug molecules that may be degraded. After modifications of the prediction tool, a web server called “DrugBug” was developed, where three steps are performed to predict a molecule’s bacterial degradation. In the first step, the molecule’s PubChem ID is entered, or the mol/sdf file uploaded, then the model, sampling, and probability threshold are determined to predict the major EC class (Oxidoreductase EC1, Transferases EC2, Hydrolases EC3, Lyases EC4, Isomerases EC5, Ligases EC6). In the second step, the molecule’s EC-subclass is determined. In the third step, the fingerprint, Tanimoto index, and best protein hits parameters are selected. Lastly, microbes that have metabolic enzymes capable of degrading the molecule are listed [95]. Additionally, researchers evaluated the literature for drug-microbe interactions and collected around 455 confirmed interactions. Then they used machine learning to develop 11 models utilizing different techniques to predict drug and microbe interactions. The model sorts drugs into either depleted, or not depleted [96]. Another machine learning model, that includes 40 bacterial strains from the gut, trained over 18,600 drug/microbiome interactions that could predict the effect of administered drugs on the microbiome. Such a model is essential since variations in microbiome diversity are related to certain diseases [97]. The mentioned prediction software and databases are summarized in Table 3. Further development of machine learning tools to accurately predict possible microbial drug metabolism is crucial to advance the field of pharmacomicrobiomics.

Table 3.
Database and Prediction Software.
6. Discussion
The gut microbial community encodes a plethora of enzymes that metabolize most drugs with various chemical structures, functional groups, and intended use. Moreover, the microbial community is also manipulated by oral medications that can promote or inhibit the growth of certain microbial strains, resulting in an indirect or unknown effect on body functions. Multiple databases and software have been developed to predict this complex relationship toward advancing personalized and precision medicine. The establishment of a systematically curated approach to defining the therapeutic outcome of each prescription drug based on the patient-microbiome signature will be the next frontier in advancing therapeutics’ efficacy and outcome. Furthermore, it is crucial to include the evaluation of drug–microbe interactions in the Food and Drug Administration (FDA) drug approval. This can be implemented through the evaluation of drug degradation by gut colonizing microbes, such as by using a fecal microbial extract, before the drug is approved for clinical trials [98].
6.1. The Implementation of Pharmacomicrobiomic in Therapeutic Regimes
Individuals differ widely in their genes that metabolize drugs and genes that encode for cellular receptors, resulting in varied pharmacokinetics and pharmacodynamics of prescribed drugs. The implementation of pharmacogenomics in drug prescription, although sporadic and not comprehensive for all patients and all drugs, shows promise in advancing healthcare. There is now another major player that has never been accounted for, namely, the microbiome. The development of personalized prescriptions based on an individual’s microbiome signature to predict response and resistance to specific drugs is referred to as pharmacomicrobiomics [24,25], which is a challenging yet promising advance in precision medicine. The availability of genomic and proteomic data will have a profound impact on accelerating the discovery of machine learning software to predict if a particular gene or a signature metabolite could be linked to a specific reaction leading to drug metabolism. Although multiple algorithms have been developed, they still need further improvement to address concerns such as accuracy and validity for translational medicine [99,100,101,102].
6.1.1. Challenges of Microbiome-Based Personalized Medicine
The microbiome is very dynamic, even within the same individual, and is shaped by multiple factors including diet, lifestyle, diseases, drugs, stress, and host genetics [103,104]. This dynamic makes the drug–microbiota interaction harder to predict or evaluate based on species variability. Prediction of drug–microbiota interaction should be based on an encoded gene, not species, because bacterial genes are redundant among human microbiomes. Here, for example, we screened the presence of cytidine deaminase, originally identified in Mycoplasma sp., in other microbes. We identified 78 distinct species that harbor the same gene and, thus, can result in the same effect on gemcitabine or other drugs that share the same functional groups. Additionally, genes could be transferred from one species to another, especially under stress conditions, and the chronic use of drugs might be considered a stress-like condition for microbes [105]. Moreover, bacterial gene expression could also be induced in response to chronic drug administration. For example, research shows that microbial metabolism for the calcium channel blocker, diltiazem, is enhanced with repeated administration [7]. To add to the challenge, we must consider the possibility of cooperation between different microbes which leads to drug metabolism. For example, incubation of the steroid drug dexamethasone with 28 fecal cultures obtained from healthy human donors resulted in different metabolic products that were not dependent on culture density or the presence of the main dexamethasone metabolizing bacteria, Clostridium scindens [7]. Resources such as the second version of the assembly of gut organisms through reconstruction and analysis (AGORA-2) are very useful if utilized to create patient-personalized microbiome models. This will aid in predicting the possible degradation of administered drugs prior to starting the treatment plan [106].
6.1.2. Promises of Considering the Microbiome Signature in Prescriptions
A simple modification to the prescription regime can result in a huge impact, for example, the use of enzyme inhibitors to suppress an undesirable microbial metabolism and thus decrease drug resistance. Further examples are the use of cytidine deaminase inhibitors in conjunction with gemcitabine to decrease resistance to chemotherapy [107] or the use of protease inhibitors with peptide drugs [108]. Another intervention might include the use of antibiotics to eliminate metabolizing microbes, for example, Helicobacter pylori decarboxylates levodopa in the gut before reaching the central nervous system, thus decreasing brain exposure and drug efficacy [109]. Studies showed that the elimination of H. pylori improved the absorption and pharmacokinetic properties of Levodopa [110]. On the other hand, to enable a desirable microbial metabolism, we can use probiotics, fecal transplants, or purified enzymes [111]. Another interesting approach is the manipulation of the microbiome through diet modification or the implementation of prebiotics to indirectly affect drug response, especially for chronically used drugs [112]. It is interesting to speculate that drug metabolism could also be affected by quorum sensing, the process that mediates microbial communication through secretion of small diffusible molecules known as autoinducers. Quorum sensing is affected by population density and might show an impact on drug metabolism either by its direct effect on some microbial cellular processes or indirectly thorough controlling expression of genes that encodes enzymes catalyzing drug breakdown. Manipulation of quorum sensing in microbes is now seen as a promising approach to control virulence, and it will also be interesting to investigate in the future how this process might precisely affect microbe–drug interactions [113,114,115,116]. To enable these interventions, the scientific community must advance the development of multi-omics-based machine learning models to accurately predict microbiota–xenobiotic interactions and further enable translational precision medicine [117].
7. Conclusions
Given the wide variation in microbiome signature between individuals, and their profound impact on metabolism of medications, implementation of pharmacomicrobiomics in medicine should be considered. Moreover, early pharmacokinetics studies should be revised to include microbiome testing. The explosion of multi-omics data will enable the development of novel algorithms to predict the impact of the individual’s microbiome on chronic medications, to achieve the best therapeutic outcome [25,96,118,119].
Author Contributions
S.M. and W.K.M. collected data, developed figures and tables, and wrote the manuscript. M.S. contributed to the review design. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, H.; Cai, X.; Zhong, H.; Feng, Q.; Sunagawa, S.; Arumugam, M.; Kultima, J.R.; Prifti, E.; Nielsen, T.; et al. An integrated catalog of reference genes in the human gut microbiome. Nat. Biotechnol. 2014, 32, 834–841. [Google Scholar] [CrossRef]
- Koppel, N.; Rekdal, V.M.; Balskus, E.P. Chemical transformation of xenobiotics by the human gut microbiota. Science 2017, 356, 1246–1257. [Google Scholar] [CrossRef] [PubMed]
- Garajová, I.; Balsano, R.; Wang, H.; Leonardi, F.; Giovannetti, E.; Deng, D.; Peters, G.J. The role of the microbiome in drug resistance in gastrointestinal cancers. Expert Rev. Anticancer. Ther. 2020, 21, 165–176. [Google Scholar] [CrossRef]
- Lehouritis, P.; Cummins, J.; Stanton, M.; Murphy, C.T.; McCarthy, F.O.; Reid, G.; Tangney, M. Local bacteria affect the efficacy of chemo-therapeutic drugs. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A.L. Separating host and microbiome contributions to drug pharmacokinetics and toxicity. Science 2019, 363, eaat9931. [Google Scholar] [CrossRef]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A.L. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 2019, 570, 462–467. [Google Scholar] [CrossRef]
- Pant, A.; Maiti, T.K.; Mahajan, D.; Das, B. Human Gut Microbiota and Drug Metabolism. Microb. Ecol. 2022, 1–15. [Google Scholar] [CrossRef]
- Collins, S.L.; Patterson, A.D. The gut microbiome: An orchestrator of xenobiotic metabolism. Acta Pharm. Sin. B 2020, 10, 19–32. [Google Scholar] [CrossRef]
- Yang, T.; Mei, X.; Tackie-Yarboi, E.; Akere, M.T.; Kyoung, J.; Mell, B.; Yeo, J.-Y.; Cheng, X.; Zubcevic, J.; Richards, E.M.; et al. Identification of a Gut Commensal That Compromises the Blood Pressure-Lowering Effect of Ester Angiotensin-Converting Enzyme Inhibitors. Hypertension 2022, 79, 1591–1601. [Google Scholar] [CrossRef]
- Yoo, D.H.; Kim, I.S.; Van Le, T.K.; Jung, I.H.; Yoo, H.H.; Kim, D.H. Gut Microbiota-Mediated Drug. Interactions between Lovastatin and Antibiotics. Drug Metab. Dis. 2016, 42, 1508–1513. [Google Scholar]
- Rekdal, V.M.; Bess, E.N.; Bisanz, J.E.; Turnbaugh, P.J.; Balskus, E.P. Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science 2019, 364, eaau6323. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Han, P.; Fu, J.; Zhang, Z.-W.; Ma, S.-R.; Pan, L.-B.; Xia, Y.-Y.; Yu, H.; Xu, H.; Liu, C.-X.; et al. Esterases from Bifidobacteria Exhibit the Conversion of Albiflorin in Gut Microbiota. Front. Microbiol. 2022, 13, 1174. [Google Scholar] [CrossRef] [PubMed]
- Hashim, H.; Azmin, S.; Razlan, H.; Yahya, N.W.; Tan, H.J.; Manaf, M.R.A.; Ibrahim, N.M. Eradication of Helicobacter pylori infection improves levodopa action, clinical symptoms and quality of life in patients with parkinson’s disease. PLoS ONE 2014, 9, e112330. [Google Scholar] [CrossRef]
- Voorde, J.V.; Sabuncuoğlu, S.; Noppen, S.; Hofer, A.; Ranjbarian, F.; Fieuws, S.; Balzarini, J.; Liekens, S. Nucleoside-catabolizing Enzymes in Mycoplasma-infected Tumor Cell Cultures Compromise the Cytostatic Activity of the Anticancer Drug Gemcitabine. J. Biol. Chem. 2014, 289, 13054–13065. [Google Scholar] [CrossRef]
- Wurdeman, S.R.; Stevens, P.M.; Campbell, J.H.; Davie-Smith, F.; Coulter, E.; Kennon, B.; Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Physiol. Behav. 2017, 176, 498–503. [Google Scholar]
- Yan, A.; Culp, E.; Perry, J.; Lau, J.T.; MacNeil, L.T.; Surette, M.G.; Wright, G.D. Transformation of the Anticancer Drug Doxorubicin in the Human Gut Microbiome. ACS Infect. Dis. 2018, 4, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillère, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The Intestinal Microbiota Modulates the Anticancer Immune Effects of Cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X. Effects of cyclophosphamide on immune system and gut microbiota in mice. Microbiol. Res. 2015, 171, 97–106. [Google Scholar] [CrossRef]
- Khan, T.J.; Ahmed, Y.M.; Zamzami, M.A.; Siddiqui, A.M.; Khan, I.; Baothman, O.A.S.; Mehanna, M.G.; Kuerban, A.; Kaleemuddin, M.; Yasir, M. Atorvastatin Treatment Modulates the Gut Microbiota of the Hypercholesterolemic Patients. OMICS A J. Integr. Biol. 2018, 22, 154–163. [Google Scholar] [CrossRef]
- Maier, L.; Pruteanu, M.; Kuhn, M.; Zeller, G.; Telzerow, A.; Anderson, E.E.; Brochado, A.R.; Fernandez, K.C.; Dose, H.; Mori, H.; et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018, 555, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xie, C.; Wang, G.; Wu, Y.; Wu, Q.; Wang, X.; Jiang, C. Gut microbiota and intestinal FXR mediate the clinical benefits of met-formin. Nat. Med. 2018, 24, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Shirvani, A.; Kalajian, T.A.; Song, A.; Holick, M.F. The effect of various doses of oral Vitamin D3 supplementation on gut microbiota in healthy adults: A Randomized, Double-blinded, Dose-response Study. Anticancer. Res. 2020, 40, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Dikeocha, I.J.; Al-Kabsi, A.M.; Miftahussurur, M.; Alshawsh, M.A. Pharmacomicrobiomics: Influence of gut microbiota on drug and xenobiotic metabolism. FASEB J. 2022, 36, e22350. [Google Scholar] [CrossRef]
- Chen, H.-Q.; Gong, J.-Y.; Xing, K.; Liu, M.-Z.; Ren, H.; Luo, J.-Q. Pharmacomicrobiomics: Exploiting the Drug-Microbiota Interactions in Antihypertensive Treatment. Front. Med. 2022, 8, 3110. [Google Scholar] [CrossRef]
- Nkera-Gutabara, C.K.; Kerr, R.; Scholefield, J.; Hazelhurst, S.; Naidoo, J. Microbiomics: The Next Pillar of Precision Medicine and Its Role in African Healthcare. Front. Genet. 2022, 13, 616. [Google Scholar] [CrossRef]
- Petrosino, J.F. The microbiome in precision medicine: The way forward. Genome Med. 2018, 10, 12. [Google Scholar] [CrossRef]
- Takeno, S.; Sakai, T. Involvement of the intestinal microflora in nitrazepam-induced teratogenicity in rats and its relationship to nitroreduction. Teratology 1991, 44, 209–214. [Google Scholar] [CrossRef]
- LinWu, S.-W.; Syu, C.-J.; Chen, Y.-L.; Wang, A.H.-J.; Peng, F.-C. Characterization of Escherichia coli nitroreductase NfsB in the metabolism of nitrobenzodiazepines. Biochem. Pharmacol. 2009, 78, 96–103. [Google Scholar] [CrossRef]
- Xie, Y.; Hu, F.; Xiang, D.; Lu, H.; Li, W.; Zhao, A.; Huang, L.; Wang, R. The metabolic effect of gut microbiota on drugs. Drug. Metab. Rev. 2020, 52, 139–156. [Google Scholar] [CrossRef]
- Gingell, R.; Bridges, J.W.; Williams, R.T. The Role of the Gut Flora in the Metabolism of Prontosil and Neoprontosil in the Rat. Xenobiotica 1971, 129, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Hayllar, J.; Bjarnason, I. Sulphasalazine in ulcerative colitis: In memoriam? Gut 1991, 32, 462–463. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.P.; Pope, D.J.; Gilbert, A.P.; Sacra, P.J.; Baron, J.H.; Lennard-Jones, J.E. Studies of two novel sulfasalazine analogs, ipsalazide and balsalazide. Dig. Dis. Sci. 1983, 28, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Wadword, A.N.; Fitton, A. Olsalazine: A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in inflammatory bowel disease. Drugs 1991, 41, 647–664. [Google Scholar] [CrossRef]
- Basit, A.W.; Newton, J.; Lacey, L.F. Susceptibility of the H2-receptor antagonists cimetidine, famotidine and nizatidine, to metabolism by the gastrointestinal microflora. Int. J. Pharm. 2002, 237, 23–33. [Google Scholar] [CrossRef]
- Kim, D.-H.; Hyun, S.-H.; Shim, S.-B.; Kobashi, K. The Role of Intestinal Bacteria in the Transformation of Sodium Picosulfate. Jpn. J. Pharmacol. 1992, 59, 1–5. [Google Scholar] [CrossRef]
- Kodawara, T.; Higashi, T.; Negoro, Y.; Kamitani, Y.; Igarashi, T.; Watanabe, K.; Tsukamoto, H.; Yano, R.; Masada, M.; Iwasaki, H.; et al. The Inhibitory Effect of Ciprofloxacin on the β-Glucuronidase-mediated Deconjugation of the Irinotecan Metabolite SN-38-G. Basic Clin. Pharmacol. Toxicol. 2016, 118, 333–337. [Google Scholar]
- Shu, Y.Z.; Kingston, D.G.I.; Van Tassell, R.L.; Wilkins, T.D. Metabolism of levamisole, an anti-colon cancer drug, by human intestinal bacteria. Xenobiotica 1991, 21, 737–750. [Google Scholar] [CrossRef]
- Rafii, F.; Sutherland, J.B.; Hansen, J.E.B.; Cerniglia, C.E.; Hansen, E.B. Reduction of nitrazepam by Clostridium leptum, a nitroreductase-producing bacterium isolated from the human intestinal tract. Clin. Infect. Dis. 1997, 25, S121–S122. [Google Scholar] [CrossRef]
- Magnusson, J.; Bergdahl, B.; Bogentoft, C.; Jonsson, U. Metabolism of digoxin and absorption site. Br. J. Clin. Pharmacol. 1982, 14, 284–285. [Google Scholar] [CrossRef]
- Tozaki, H.; Emi, Y.; Horisaka, E.; Fujita, T.; Yamamoto, A.; Muranishi, S. Degradation of Insulin and Calcitonin and Their Protection by Various Protease Inhibitors in Rat Caecal Contents: Implications in Peptide Delivery to the Colon. J. Pharm. Pharmacol. 1997, 49, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Sousa, T.; Paterson, R.; Moore, V.; Carlsson, A.; Abrahamsson, B.; Basit, A.W. The gastrointestinal microbiota as a site for the bio-transformation of drugs. Int. J. Pharm. 2008, 363, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.; Kang, Y.R.; Nepal, M.R.; Shakya, R.; Kang, M.J.; Kang, W.; Lee, S.; Jeong, H.G.; Jeong, T.C. Impact of gut microbiota on drug metabolism: An update for safe and effective use of drugs. Arch. Pharmacal Res. 2017, 40, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Trinh, H.-T.; Joh, E.-H.; Kwak, H.-Y.; Baek, N.-I.; Kim, D.-H. Anti-pruritic effect of baicalin and its metabolites, baicalein and oroxylin A, in mice. Acta Pharmacol. Sin. 2010, 31, 718–724. [Google Scholar] [CrossRef]
- Zhang, M.; Peng, C.S.; Li, X.B. In vivo and in vitro metabolites from the main diester and monoester diterpenoid alkaloids in a traditional Chinese herb, the aconitum species. Evid. Based Complement. Altern. Med. 2015, 252434. [Google Scholar]
- Roowi, S.; Mullen, W.; Edwards, C.A.; Crozier, A. Yoghurt impacts on the excretion of phenolic acids derived from colonic breakdown of orange juice flavanones in humans. Mol. Nutr. Food Res. 2009, 53, S68–S75. [Google Scholar] [CrossRef]
- Liu, X.; Suzuki, N.; Laxmi, Y.S.; Okamoto, Y.; Shibutani, S. Anti-breast cancer potential of daidzein in rodents. Life Sci. 2012, 91, 415–419. [Google Scholar] [CrossRef]
- Park, E.-K.; Shin, J.; Bae, E.-A.; Lee, Y.-C.; Kim, D.-H. Intestinal Bacteria Activate Estrogenic Effect of Main Constituents Puerarin and Daidzin of Pueraria thunbergiana. Biol. Pharm. Bull. 2006, 29, 2432–2435. [Google Scholar] [CrossRef]
- Derosa, L.; Hellmann, M.D.; Spaziano, M.; Halpenny, D.; Fidelle, M.; Rizvi, H.; Long, N.; Plodkowski, A.J.; Arbour, K.C.; Chaft, J.E.; et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann. Oncol. 2018, 29, 1437–1444. [Google Scholar] [CrossRef]
- Routy, B.; le Chatelier, E.; DeRosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2018, 97, 91–97. [Google Scholar] [CrossRef]
- Huang, J.; Liu, W.; Kang, W.; He, Y.; Yang, R.; Mou, X.; Zhao, W. Effects of microbiota on anticancer drugs: Current knowledge and potential applications. eBioMedicine 2022, 83, 104197. [Google Scholar] [CrossRef]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.-L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2017, 28, 1368–1379, Erratum in Ann. Oncol. 2019, 30, 2012. [Google Scholar] [CrossRef] [PubMed]
- Capuco, A.; Urits, I.; Hasoon, J.; Chun, R.; Gerald, B.; Wang, J.K.; Kassem, H.; Ngo, A.L.; Abd-Elsayed, A.; Simopoulos, T.; et al. Current Perspectives on Gut Microbiome Dysbiosis and Depression. Adv. Ther. 2020, 37, 1328–1346. [Google Scholar] [CrossRef]
- Wingfield, B.; Lapsley, C.; McDowell, A.; Miliotis, G.; McLafferty, M.; O’neill, S.M.; Coleman, S.; McGinnity, T.M.; Bjourson, A.J.; Murray, E.K. Variations in the oral microbiome are associated with depression in young adults. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Lach, G.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics 2018, 15, 36–59. [Google Scholar] [CrossRef]
- Elmer, G.W.; Remmel, R.P. Role of the intestinal microflora in clonazepam metabolism in the rat. Xenobiotica 1984, 14, 829–840. [Google Scholar] [CrossRef]
- Kitamura, S.; Sugihara, K.; Kuwasako, M.; Tatsumi, K. The Role of Mammalian Intestinal Bacteria in the Reductive Metabolism of Zonisamide. J. Pharm. Pharmacol. 1997, 49, 253–256. [Google Scholar] [CrossRef]
- Connil, N.; Le Breton, Y.; Dousset, X.; Auffray, Y.; Rincé, A.; Prévost, H. Identification of the Enterococcus faecalis tyrosine decarboxylase operon involved in tyramine production. Appl. Environ. Microbiol. 2002, 68, 3537–3544. [Google Scholar] [CrossRef]
- Fasano, A.; Bove, F.; Gabrielli, M.; Petracca, M.; Zocco, M.A.; Ragazzoni, E.; Barbaro, F.; Piano, C.; Fortuna, S.; Tortora, A.; et al. The role of small intestinal bacterial overgrowth in Parkinson’s disease. Mov. Disord. 2013, 28, 1241–1249. [Google Scholar] [CrossRef]
- Van Kessel, S.P.; de Jong, H.R.; Winkel, S.L.; van Leeuwen, S.S.; Nelemans, S.A.; Permentier, H.; Keshavarzian, A.; El Aidy, S. Gut bacterial deamination of residual levodopa medication for Parkinson’s disease. BMC Biol. 2020, 18, 137. [Google Scholar] [CrossRef]
- Haiser, H.J.; Seim, K.L.; Balskus, E.P.; Turnbaugh, P.J. Mechanistic insight into digoxin inactivation by Eggerthella lenta augments our understanding of its pharmacokinetics. Gut Microbes. 2014, 5, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Jaiswal, S.K.; Dhoke, G.V.; Srivastava, G.N.; Sharma, A.K.; Sharma, V.K. Mechanistic and structural insight into promis-cuity based metabolism of cardiac drug digoxin by gut microbial enzyme. J. Cell. Biochem. 2018, 119, 5287–5296. [Google Scholar] [CrossRef]
- Dobkin, J.F.; Saha, J.R.; Butler, V.P., Jr.; Neu, H.C.; Lindenbaum, J. Digoxin-Inactivating Bacteria: Identification in Human Gut Flora. Science 1982, 5, 5–7. [Google Scholar] [CrossRef]
- Yoo, H.H.; Kim, I.S.; Yoo, D.H.; Kim, D.H. Effects of orally administered antibiotics on the bioavailability of amlodipine: Gut microbio-ta-mediated drug interaction. J. Hypertens. 2016, 34, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Holert, J.; Cardenas, E.; Bergstrand, L.H.; Zaikova, E.; Hahn, A.S.; Hallam, S.J.; Mohn, W.W. Metagenomes Reveal Global Distribution of Bacterial Steroid Catabolism in Natural, Engineered, and Host Environments. mBio 2018, 9, e02345-17. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, R.; Wang, M.; Peng, R.; Fu, S.; Fu, A.; Liu, T. 3β-Hydroxysteroid dehydrogenase expressed by gut microbes degrades tes-tosterone and is linked to depression in males. Cell Host Microbe 2022, 30, 329–339.e5. [Google Scholar] [CrossRef]
- Bhasin, S.; Seidman, S. Testosterone Treatment of Depressive Disorders in Men: Too Much Smoke, Not Enough High-Quality Evidence. JAMA Psychiatry 2019, 76, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.C.; Tajar, A.; Beynon, J.M.; Pye, S.R.; Silman, A.J.; Finn, J.D.; O’Neill, T.W.; Bartfai, G.; Casanueva, F.F.; Forti, G.; et al. Identification of Late-Onset Hypogonadism in Middle-Aged and Elderly Men. N. Engl. J. Med. 2010, 363, 123–135. [Google Scholar] [CrossRef]
- García-Gómez, E.; González-Pedrajo, B.; Camacho-Arroyo, I. Role of Sex Steroid Hormones in Bacterial-Host Interactions. BioMed Res. Int. 2013, 2013, 928290. [Google Scholar] [CrossRef]
- Morris, G.N.; Winter, J.; Cato, E.P. Clostridium scindens sp. nov., a human intestinal bacterium with desmolytic activity on cor-ticoids. Int. J. Syst. Bacteriol. 1985, 35, 478–481. [Google Scholar] [CrossRef]
- Flores, R.; Shi, J.; Fuhrman, B.; Xu, X.; Veenstra, T.D.; Gail, M.H.; Gajer, P.; Ravel, J.; Goedert, J.J. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: A cross-sectional study. J. Transl. Med. 2012, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Wu, J.; Chen, J. The Role of Gut Microbial β-Glucuronidase in Estrogen Reactivation and Breast Cancer. Front. Cell Dev. Biol. 2021, 9, 631552. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Gaisford, S.; Merchant, H.A.; Basit, A.W. Colonic bacterial metabolism of corticosteroids. Int. J. Pharm. 2013, 457, 268–274. [Google Scholar] [CrossRef]
- Needham, B.D.; Kaddurah-Daouk, R.; Mazmanian, S.K. Gut microbial molecules in behavioural and neurodegenerative condi-tions. Nat. Rev. Neurosci. 2020, 21, 717–731. [Google Scholar] [CrossRef]
- Bora, S.A.; Kennett, M.J.; Smith, P.B.; Patterson, A.D.; Cantorna, M.T. The Gut Microbiota Regulates Endocrine Vitamin D Metabolism through Fibroblast Growth Factor 23. Front. Immunol. 2018, 9, 408. [Google Scholar] [CrossRef]
- Ran, L.; Liu, A.B.; Lee, M.; Xie, P.; Lin, Y.; Yang, C.S. Effects of antibiotics on degradation and bioavailability of different vitamin E forms in mice. Biofactors 2019, 45, 450–462. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Ballevre, O.; Luo, H.; Zhang, W. Antihypertensive effects and mechanisms of chlorogenic acids. Hypertens. Res. 2012, 35, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of Chlorogenic Acid on Regulating Glucose and Lipids Metabolism: A Review. Evidence-Based Complement. Altern. Med. 2013, 2013, 801457. [Google Scholar] [CrossRef]
- Couteau, D.; McCartney, A.L.; Gibson, G.R.; Williamson, G. Isolation and characterization of human colonic bacteria able to hy-drolyse chlorogenic acid. J. Appl. Microbiol. 2001, 90, 873–881. [Google Scholar] [CrossRef]
- Curiel, J.A.; Rodríguez, H.; Landete, J.M.; de las Rivas, B.; Muñoz, R. Ability of Lactobacillus brevis strains to degrade food phenolic acids. Food Chem. 2010, 120, 225–229. [Google Scholar] [CrossRef]
- Shin, N.R.; Lee, J.C.; Lee, H.Y.; Kim, M.S.; Whon, T.W.; Lee, M.S.; Bae, J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef]
- Cabreiro, F.; Au, C.; Leung, K.-Y.; Vergara-Irigaray, N.; Cochemé, H.M.; Noori, T.; Weinkove, D.; Schuster, E.; Greene, N.D.; Gems, D. Metformin Retards Aging in C. elegans by Altering Microbial Folate and Methionine Metabolism. Cell 2013, 153, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Mato, J.M.; Martínez-Chantar, M.L.; Lu, S.C. Methionine Metabolism and Liver Disease. Annu. Rev. Nutr. 2008, 28, 273–293. [Google Scholar] [CrossRef] [PubMed]
- Weersma, R.K.; Zhernakova, A.; Fu, J. Interaction between drugs and the gut microbiome. Gut 2020, 69, 1510–1519. [Google Scholar] [CrossRef]
- Vich Vila, A.; Collij, V.; Sanna, S.; Sinha, T.; Imhann, F.; Bourgonje, A.; Mujagic, Z.; Jonkers, D.M.A.E.; Masclee, A.A.M.; Fu, J.; et al. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat. Commun. 2020, 11, 362. [Google Scholar] [CrossRef]
- Nayak, R.R.; Alexander, M.; Deshpande, I.; Stapleton-Gray, K.; Rimal, B.; Patterson, A.D.; Ubeda, C.; Scher, J.U.; Turnbaugh, P.J. Methotrexate impacts conserved pathways in diverse human gut bacteria leading to decreased host immune activation. Cell Host Microbe 2021, 29, 362–377.e11. [Google Scholar] [CrossRef]
- Yao, Z.; Zhao, M.; Gong, Y.; Chen, W.; Wang, Q.; Fu, Y.; Guo, T.; Zhao, J.; Gao, L.; Bo, T. Relation of Gut Microbes and L-Thyroxine Through Altered Thyroxine Metabolism in Subclinical Hypothyroidism Subjects. Front. Cell. Infect. Microbiol. 2020, 10, 495. [Google Scholar] [CrossRef]
- Jackson, M.A.; Verdi, S.; Maxan, M.-E.; Shin, C.M.; Zierer, J.; Bowyer, R.C.E.; Martin, T.; Williams, F.M.K.; Menni, C.; Bell, J.T.; et al. Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nat. Commun. 2018, 9, 2655. [Google Scholar] [CrossRef]
- ElRakaiby, M.; Dutilh, B.E.; Rizkallah, M.R.; Boleij, A.; Cole, J.N.; Aziz, R.K. Pharmacomicrobiomics: The Impact of Human Microbiome Variations on Systems Pharmacology and Personalized Therapeutics. OMICS: A J. Integr. Biol. 2014, 18, 402–414. [Google Scholar] [CrossRef]
- Zeng, X.; Yang, X.; Fan, J.; Tan, Y.; Ju, L.; Shen, W.; Wang, Y.; Wang, X.; Chen, W.; Ju, D.; et al. MASI: Microbiota—Active substance interactions database. Nucleic Acids Res. 2021, 49, D776–D782. [Google Scholar] [CrossRef]
- Zhou, J.; Ouyang, J.; Gao, Z.; Qin, H.; Jun, W.; Shi, T. MagMD: Database summarizing the metabolic action of gut microbiota to drugs. Comput. Struct. Biotechnol. J. 2022, 20, 6427–6430. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Jaiswal, S.K.; Chaudhary, N.; Sharma, V.K. A novel approach for the prediction of species-specific biotransformation of xenobiotic/drug molecules by the human gut microbiota. Sci. Rep. 2017, 7, 9751. [Google Scholar] [CrossRef] [PubMed]
- McCoubrey, L.E.; Thomaidou, S.; Elbadawi, M.; Gaisford, S.; Basit, A.W.; Orlu, M. Machine Learning Predicts Drug Metabolism and Bioaccumulation by Intestinal Microbiota. Pharmaceutics 2021, 13, 2001. [Google Scholar] [CrossRef] [PubMed]
- McCoubrey, L.E.; Elbadawi, M.; Orlu, M.; Gaisford, S.; Basit, A.W. Machine Learning Uncovers Adverse Drug Effects on Intestinal Bacteria. Pharmaceutics 2021, 13, 1026. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, L.; Kelly, L. Bringing microbiome-drug interaction research into the clinic. eBioMedicine 2019, 44, 708–715. [Google Scholar] [CrossRef]
- Sabater, A.; Ciudad, C.J.; Cendros, M.; Dobrokhotov, D.; Sabater-Tobella, J. g-Nomic: A new pharmacogenetics interpretation software. Pharmacogenomics Pers. Med. 2019, 12, 75–85. [Google Scholar] [CrossRef]
- Karp, P.D.; Paley, S.; Krummenacker, M.; Kothari, A.; Wannemuehler, M.J.; Phillips, G.J. Pathway Tools Management of Path-way/Genome Data for Microbial Communities. Front. Bioinform. 2022, 2, 43. [Google Scholar] [CrossRef]
- Kim, J.; Choi, J.-P.; Kim, M.S.; Bhak, J. PharmaKoVariome database for supporting genetic testing. Database 2022, 2022, baac092. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Tran, P.Q.; Breister, A.M.; Liu, Y.; Kieft, K.; Cowley, E.S.; Karaoz, U.; Anantharaman, K. METABOLIC: High-throughput profiling of microbial genomes for functional traits, metabolism, biogeochemistry, and community-scale functional networks. Microbiome 2022, 10, 33. [Google Scholar] [CrossRef]
- Bajinka, O.; Tan, Y.; Abdelhalim, K.A.; Özdemir, G.; Qiu, X. Extrinsic factors influencing gut microbes, the immediate consequences and restoring eubiosis. AMB Express 2020, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Kurilshikov, A.; Medina-Gomez, C.; Bacigalupe, R.; Radjabzadeh, D.; Wang, J.; Demirkan, A.; Le Roy, C.I.; Garay, J.A.R.; Finnicum, C.T.; Liu, X.; et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat. Genet. 2021, 53, 156–165. [Google Scholar] [CrossRef]
- Johnsen, A.R.; Kroer, N. Effects of stress and other environmental factors on horizontal plasmid transfer assessed by direct quantification of discrete transfer events. FEMS Microbiol. Ecol. 2007, 59, 718–728. [Google Scholar] [CrossRef]
- Heinken, A.; Hertel, J.; Acharya, G.; Ravcheev, D.A.; Nyga, M.; Okpala, O.E.; Hogan, M.; Magnúsdóttir, S.; Martinelli, F.; Nap, B.; et al. Genome-scale metabolic reconstruction of 7,302 human microorganisms for personalized medicine. Nat. Biotechnol. 2023, 1–12. [Google Scholar] [CrossRef]
- Pakti, M.; Saraswat, A.; Bhutkar, S.; Dukhande, V.; Patel, K. Combination of gemcitabine and cytidine deaminase inhibitor: A breakthrough cocktail for the treatment of pancreatic ductal adenocarcinom. Am. Assoc. Cancer Res. 2021, 81, 1059. [Google Scholar]
- Rudzińska, M.; Daglioglu, C.; Savvateeva, L.V.; Kaci, F.N.; Antoine, R.; Zamyatnin, A.A. Status and perspectives of pro-tease inhibitors and their combination with nanosized drug delivery systems for targeted cancer therapy. Drug Des. Devel. Ther. 2021, 15, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Nyholm, D.; Hellström, P.M. Effects of helicobacter pylori on levodopa pharmacokinetics. J. Parkinsons Dis. 2021, 11, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Lolekha, P.; Sriphanom, T.; Vilaichone, R.-K. Helicobacter pylori eradication improves motor fluctuations in advanced Parkinson’s disease patients: A prospective cohort study (HP-PD trial). PLoS ONE 2021, 16, e0251042. [Google Scholar] [CrossRef] [PubMed]
- Purdel, C.; Ungurianu, A.; Adam-Dima, I.; Margină, D. Exploring the potential impact of probiotic use on drug metabolism and efficacy. Biomed. Pharmacother. 2023, 161, 114468. [Google Scholar] [CrossRef]
- Olendzki, B.; Bucci, V.; Cawley, C.; Maserati, R.; McManus, M.; Olednzki, E.; Madziar, C.; Chiang, D.; Ward, D.V.; Pellish, R.; et al. Dietary manipulation of the gut microbiome in inflammatory bowel disease patients: Pilot study. Gut Microbes 2022, 14, 2046244. [Google Scholar] [CrossRef] [PubMed]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal–response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 9, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Falà, A.K.; Álvarez-Ordóñez, A.; Filloux, A.; Gahan, C.G.M.; Cotter, P.D. Quorum sensing in human gut and food microbiomes: Sig-nificance and potential for therapeutic targeting. Front. Microbiol. 2022, 13, 4389. [Google Scholar] [CrossRef]
- Rutherford, S.T.; Bassler, B.L. Bacterial Quorum Sensing: Its Role in Virulence and Possibilities for Its Control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar]
- Wu, S.; Yang, S.; Wang, M.; Song, N.; Feng, J.; Wu, H.; Yang, A.; Liu, C.; Li, Y.; Guo, F.; et al. Quorum sensing-based interactions among drugs, microbes, and diseases. Sci. China Life Sci. 2022, 66, 137–151. [Google Scholar] [CrossRef]
- Lam, K.N.; Alexander, M.; Turnbaugh, P.J. Precision Medicine Goes Microscopic: Engineering the Microbiome to Improve Drug Outcomes. Cell Host Microbe 2019, 26, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Steiner, H.E.; Patterson, H.K.; Giles, J.B.; Karnes, J.H. Bringing pharmacomicrobiomics to the clinic through well-designed studies. Clin. Transl. Sci. 2022, 15, 2303–2315. [Google Scholar] [CrossRef]
- Van Tran, T.T.; Tayara, H.; Chong, K.T. Artificial Intelligence in Drug Metabolism and Excretion Prediction: Recent Advances, Challenges, and Future Perspectives. Pharmaceutics 2023, 15, 1260. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).