Obstructive Sleep Apnea, Metabolic Dysfunction, and Periodontitis—Machine Learning and Statistical Analyses of the Dental, Oral, Medical Epidemiological (DOME) Big Data Study
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. Ethical Approval
2.3. Criteria for Enrollment
2.4. Definition and Examination of the Variables
2.4.1. Sociodemographic Characteristics
2.4.2. Health-Related Habits and Attendance Patterns
2.4.3. Medical Diagnoses and Auxiliary Test Results Definitions
2.4.4. Dental Parameters
2.5. Methods of Statistical and Machine Learning Analytics
2.5.1. Statistical Analyses
2.5.2. Machine Learning (ML) Models
3. Results
3.1. The Associations between OSA and Socio-Demographic Parameters
3.2. The Associations of OSA with Health-Related Habits and Attendance Patterns
3.3. The Associations between OSA and Metabolic Morbidity
3.4. The Associations between OSA and Medical Indices
3.5. The Associations between OSA and the Dental Status
3.6. Multivariate Analysis for OSA as the Dependent Variable
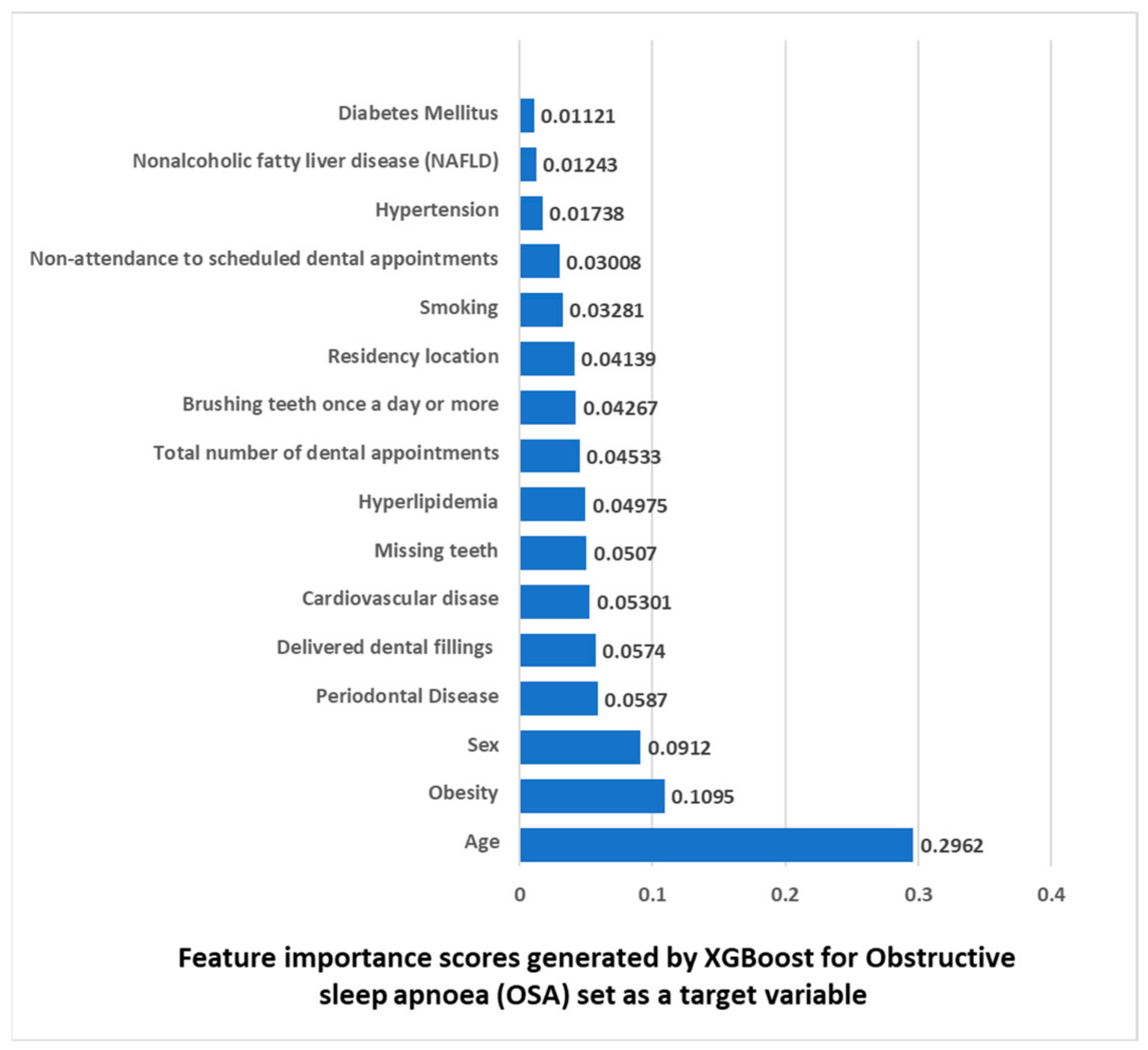
3.7. Features Importance Based on XGBoost Machine Learning Algorithm with OSA Set as a Target Variable
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jordan, A.S.; McSharry, D.G.; Malhotra, A. Adult obstructive sleep apnoea. Lancet 2014, 383, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Veasey, S.C.; Rosen, I.M. Obstructive Sleep Apnea in Adults. N. Engl. J. Med. 2019, 380, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.P.; Chen, Y.F.; Du, J.K. Obstructive sleep apnea treatment in adults. Kaohsiung J. Med. Sci. 2020, 36, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Yaggi, H.K.; Strohl, K.P. Adult obstructive sleep apnea/hypopnea syndrome: Definitions, risk factors, and pathogenesis. Clin. Chest Med. 2010, 31, 179–186. [Google Scholar] [CrossRef]
- Salari, N.; Khazaie, H.; Abolfathi, M.; Ghasemi, H.; Shabani, S.; Rasoulpoor, S.; Mohammadi, M.; Rasoulpoor, S.; Khaledi-Paveh, B. The effect of obstructive sleep apnea on the increased risk of cardiovascular disease: A systematic review and meta-analysis. Neurol. Sci. 2022, 43, 219–231. [Google Scholar] [CrossRef]
- Mitra, A.K.; Bhuiyan, A.R.; Jones, E.A. Association and Risk Factors for Obstructive Sleep Apnea and Cardiovascular Diseases: A Systematic Review. Diseases 2021, 9, 88. [Google Scholar] [CrossRef]
- Falkner, B.; Cossrow, N.D. Prevalence of metabolic syndrome and obesity-associated hypertension in the racial ethnic minorities of the United States. Curr. Hypertens. Rep. 2014, 16, 449. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S.; Participants, E.F.P.W.; Methodological, C. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 4–60. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), S173–S182. [Google Scholar] [CrossRef]
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef]
- Grigalauskiene, R.; Slabsinskiene, E.; Vasiliauskiene, I. Biological approach of dental caries management. Stomatologija 2015, 17, 107–112. [Google Scholar]
- Arnaud, C.; Bochaton, T.; Pepin, J.L.; Belaidi, E. Obstructive sleep apnoea and cardiovascular consequences: Pathophysiological mechanisms. Arch. Cardiovasc. Dis. 2020, 113, 350–358. [Google Scholar] [CrossRef]
- Khodadadi, N.; Khodadadi, M.; Zamani, M. Is periodontitis associated with obstructive sleep apnea? A systematic review and meta-analysis. J. Clin. Exp. Dent. 2022, 14, e359–e365. [Google Scholar] [CrossRef]
- Al-Jewair, T.S.; Al-Jasser, R.; Almas, K. Periodontitis and obstructive sleep apnea’s bidirectional relationship: A systematic review and meta-analysis. Sleep Breath. 2015, 19, 1111–1120. [Google Scholar] [CrossRef]
- Gunaratnam, K.; Taylor, B.; Curtis, B.; Cistulli, P. Obstructive sleep apnoea and periodontitis: A novel association? Sleep Breath. 2009, 13, 233–239. [Google Scholar] [CrossRef]
- Zhang, Z.; Ge, S.; Zhai, G.; Yu, S.; Cui, Z.; Si, S.; Chou, X. Incidence and risk of periodontitis in obstructive sleep apnea: A meta-analysis. PLoS ONE 2022, 17, e0271738. [Google Scholar] [CrossRef]
- Lembo, D.; Caroccia, F.; Lopes, C.; Moscagiuri, F.; Sinjari, B.; D’Attilio, M. Obstructive Sleep Apnea and Periodontal Disease: A Systematic Review. Medicina 2021, 57, 640. [Google Scholar] [CrossRef]
- Loke, W.; Girvan, T.; Ingmundson, P.; Verrett, R.; Schoolfield, J.; Mealey, B.L. Investigating the association between obstructive sleep apnea and periodontitis. J. Periodontol. 2015, 86, 232–243. [Google Scholar] [CrossRef]
- Sales-Peres, S.H.; Groppo, F.C.; Rojas, L.V.; de Sales-Peres, C.M.; Sales-Peres, A. Periodontal Status in Morbidly Obese Patients With and Without Obstructive Sleep Apnea Syndrome Risk: A Cross-Sectional Study. J. Periodontol. 2016, 87, 772–782. [Google Scholar] [CrossRef]
- Davidovich, E.; Hevroni, A.; Gadassi, L.T.; Spierer-Weil, A.; Yitschaky, O.; Polak, D. Dental, oral pH, orthodontic and salivary values in children with obstructive sleep apnea. Clin. Oral. Investig. 2022, 26, 2503–2511. [Google Scholar] [CrossRef]
- Pico-Orozco, J.; Silvestre, F.J.; Carrasco-Llatas, M.; Silvestre-Rangil, J. Dental caries status in adults with sleep apnea-hypopnea syndrome. J. Clin. Exp. Dent. 2022, 14, e274–e279. [Google Scholar] [CrossRef] [PubMed]
- Acar, M.; Turkcan, I.; Ozdas, T.; Bal, C.; Cingi, C. Obstructive sleep apnoea syndrome does not negatively affect oral and dental health. J. Laryngol. Otol. 2015, 129, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.A.; Njimbouom, S.N.; Kim, J.D. Optimal Feature Selection-Based Dental Caries Prediction Model Using Machine Learning for Decision Support System. Bioengineering 2023, 10, 245. [Google Scholar] [CrossRef] [PubMed]
- Ertas, K.; Pence, I.; Cesmeli, M.S.; Ay, Z.Y. Determination of the stage and grade of periodontitis according to the current classification of periodontal and peri-implant diseases and conditions (2018) using machine learning algorithms. J. Periodontal Implant. Sci. 2023, 53, 38–53. [Google Scholar] [CrossRef]
- Ben-Assuli, O.; Bar, O.; Geva, G.; Siri, S.; Tzur, D.; Almoznino, G. Body Mass Index and Caries: Machine Learning and Statistical Analytics of the Dental, Oral, Medical Epidemiological (DOME) Nationwide Big Data Study. Metabolites 2022, 13, 37. [Google Scholar] [CrossRef]
- Drager, L.F.; Togeiro, S.M.; Polotsky, V.Y.; Lorenzi-Filho, G. Obstructive sleep apnea: A cardiometabolic risk in obesity and the metabolic syndrome. J. Am. Coll. Cardiol. 2013, 62, 569–576. [Google Scholar] [CrossRef]
- Fietze, I.; Laharnar, N.; Obst, A.; Ewert, R.; Felix, S.B.; Garcia, C.; Glaser, S.; Glos, M.; Schmidt, C.O.; Stubbe, B.; et al. Prevalence and association analysis of obstructive sleep apnea with gender and age differences—Results of SHIP-Trend. J. Sleep Res. 2019, 28, e12770. [Google Scholar] [CrossRef]
- Almoznino, G.; Kedem, R.; Turgeman, R.; Bader, T.; Yavnai, N.; Zur, D.; Shay, B. The Dental, Oral, Medical Epidemiological (DOME) Study: Protocol and Study Methods. Methods Inf. Med. 2020, 59, 119–130. [Google Scholar] [CrossRef]
- Abramovitz, I.; Zini, A.; Atzmoni, M.; Kedem, R.; Zur, D.; Protter, N.E.; Almoznino, G. Cognitive Performance and Its Associations with Dental Caries: Results from the Dental, Oral, Medical Epidemiological (DOME) Records-Based Nationwide Study. Biology 2021, 10, 178. [Google Scholar] [CrossRef]
- Abramovitz, I.; Zini, A.; Kessler Baruch, O.; Kedem, R.; Protter, N.E.; Shay, B.; Yavnai, N.; Zur, D.; Mijiritsky, E.; Almoznino, G. SOS teeth with advanced caries and sociodemographic indicators, health-related habits and dental attendance patterns: Data from the Dental, Oral, Medical Epidemiological (DOME) nationwide records-based study. BMC Oral. Health 2021, 21, 389. [Google Scholar] [CrossRef]
- Abramovitz, I.; Zini, A.; Pribluda, P.; Kedem, R.; Zur, D.; Protter, N.E.; Almoznino, G. “Dental Cluster” Versus “Metabolic Cluster”: Analyzing the Associations of Planned and Delivered Dental Procedures with Metabolic Syndrome, Utilizing Data from the Dental, Oral, Medical Epidemiological (DOME) Cross-Sectional Record-Based Nationwide Study. Biology 2021, 10, 608. [Google Scholar] [CrossRef]
- Almoznino, G.; Kessler Baruch, O.; Kedem, R.; Protter, N.E.; Shay, B.; Yavnai, N.; Zur, D.; Mijiritsky, E.; Abramovitz, I. SOS Teeth: First Priority Teeth with Advanced Caries and Its Associations with Metabolic Syndrome among a National Representative Sample of Young and Middle-Aged Adults. J. Clin. Med. 2020, 9, 3170. [Google Scholar] [CrossRef]
- Almoznino, G.; Zini, A.; Kedem, R.; Protter, N.E.; Zur, D.; Abramovitz, I. Hypertension and Its Associations with Dental Status: Data from the Dental, Oral, Medical Epidemiological (DOME) Nationwide Records-Based Study. J. Clin. Med. 2021, 10, 176. [Google Scholar] [CrossRef]
- Ram, D.; Wilensky, A.; Zur, D.; Almoznino, G. The Triangle of Nonalcoholic Fatty Liver Disease, Metabolic Dysfunction, and Periodontitis: Analysis of the Dental, Oral, Medical and Epidemiological (DOME) Records-Based Nationwide Research. Metabolites 2022, 12, 1212. [Google Scholar] [CrossRef]
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed.; American Academy of Sleep Medicine: Darien, IL, USA, 2014. [Google Scholar]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-Learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Friedman, J. Greedy function approximation: A gradient boosting machine. Ann. Statist. 2001, 29, 1189–1232. [Google Scholar] [CrossRef]
- Liu, H.Z.M.; Lu, X.S.; Yao, C. Weighted Gini index feature selection method for imbalanced data. In Proceedings of the ICNSC 2018—15th IEEE International Conference on Networking, Sensing and Control, Zhuhai, China, 27–29 March 2018; pp. 1–6. [Google Scholar]
- Huang, N.; Lu, G.; Cai, G.; Xu, D.; Xu, J.; Li, F.; Zhang, L. Feature selection of power quality disturbance signals with an entropy-importance-based random forest. Entropy 2016, 18, 44. [Google Scholar] [CrossRef]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef]
- Tufik, S.; Santos-Silva, R.; Taddei, J.A.; Bittencourt, L.R. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010, 11, 441–446. [Google Scholar] [CrossRef]
- Heinzer, R.; Marti-Soler, H.; Marques-Vidal, P.; Tobback, N.; Andries, D.; Waeber, G.; Preisig, M.; Vollenweider, P.; Haba-Rubio, J. Impact of sex and menopausal status on the prevalence, clinical presentation, and comorbidities of sleep-disordered breathing. Sleep Med. 2018, 51, 29–36. [Google Scholar] [CrossRef]
- Punjabi, N.M. The epidemiology of adult obstructive sleep apnea. Proc. Am. Thorac. Soc. 2008, 5, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, D.; Kikemeni, A.; Skourti, A.; Amfilochiou, A. The influence of socio-economic status on the severity of obstructive sleep apnea: A cross-sectional observational study. Sleep Sci. 2018, 11, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, O.; Lanteri, P.; Garbarino, S. Association between socioeconomic status, belonging to an ethnic minority and obstructive sleep apnea: A systematic review of the literature. Sleep Med. 2019, 57, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Etindele Sosso, F.A.; Matos, E. Socioeconomic disparities in obstructive sleep apnea: A systematic review of empirical research. Sleep Breath. 2021, 25, 1729–1739. [Google Scholar] [CrossRef]
- Hnin, K.; Mukherjee, S.; Antic, N.A.; Catcheside, P.; Chai-Coetzer, C.L.; McEvoy, D.; Vakulin, A. The impact of ethnicity on the prevalence and severity of obstructive sleep apnea. Sleep Med. Rev. 2018, 41, 78–86. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef]
- Esen, A.D.; Akpinar, M. Relevance of obstructive sleep apnea and smoking: Obstructive sleep apnea and smoking. Fam. Pract. 2021, 38, 181–186. [Google Scholar] [CrossRef]
- Bielicki, P.; Trojnar, A.; Sobieraj, P.; Wasik, M. Smoking status in relation to obstructive sleep apnea severity (OSA) and cardiovascular comorbidity in patients with newly diagnosed OSA. Adv. Respir. Med. 2019, 87, 103–109. [Google Scholar] [CrossRef]
- Taveira, K.V.M.; Kuntze, M.M.; Berretta, F.; de Souza, B.D.M.; Godolfim, L.R.; Demathe, T.; De Luca Canto, G.; Porporatti, A.L. Association between obstructive sleep apnea and alcohol, caffeine and tobacco: A meta-analysis. J. Oral. Rehabil. 2018, 45, 890–902. [Google Scholar] [CrossRef]
- Hou, H.; Zhao, Y.; Yu, W.; Dong, H.; Xue, X.; Ding, J.; Xing, W.; Wang, W. Association of obstructive sleep apnea with hypertension: A systematic review and meta-analysis. J. Glob. Health 2018, 8, 010405. [Google Scholar] [CrossRef]
- Ahmad, M.; Makati, D.; Akbar, S. Review of and Updates on Hypertension in Obstructive Sleep Apnea. Int. J. Hypertens. 2017, 2017, 1848375. [Google Scholar] [CrossRef]
- Gunduz, C.; Basoglu, O.K.; Hedner, J.; Bonsignore, M.R.; Hein, H.; Staats, R.; Bouloukaki, I.; Roisman, G.; Pataka, A.; Sliwinski, P.; et al. Hyperlipidaemia prevalence and cholesterol control in obstructive sleep apnoea: Data from the European sleep apnea database (ESADA). J. Intern. Med. 2019, 286, 676–688. [Google Scholar] [CrossRef]
- Gunduz, C.; Basoglu, O.K.; Hedner, J.; Zou, D.; Bonsignore, M.R.; Hein, H.; Staats, R.; Pataka, A.; Barbe, F.; Sliwinski, P.; et al. Obstructive sleep apnoea independently predicts lipid levels: Data from the European Sleep Apnea Database. Respirology 2018, 23, 1180–1189. [Google Scholar] [CrossRef]
- Yaggi, H.K.; Concato, J.; Kernan, W.N.; Lichtman, J.H.; Brass, L.M.; Mohsenin, V. Obstructive sleep apnea as a risk factor for stroke and death. N. Engl. J. Med. 2005, 353, 2034–2041. [Google Scholar] [CrossRef]
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e56–e67. [Google Scholar] [CrossRef]
- Imani, M.M.; Sadeghi, M.; Farokhzadeh, F.; Khazaie, H.; Brand, S.; Dursteler, K.M.; Bruhl, A.; Sadeghi-Bahmani, D. Evaluation of Blood Levels of C-Reactive Protein Marker in Obstructive Sleep Apnea: A Systematic Review, Meta-Analysis and Meta-Regression. Life 2021, 11, 362. [Google Scholar] [CrossRef]
- Jin, S.; Jiang, S.; Hu, A. Association between obstructive sleep apnea and non-alcoholic fatty liver disease: A systematic review and meta-analysis. Sleep Breath. 2018, 22, 841–851. [Google Scholar] [CrossRef]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef]
- Maniaci, A.; Riela, P.M.; Iannella, G.; Lechien, J.R.; La Mantia, I.; De Vincentiis, M.; Cammaroto, G.; Calvo-Henriquez, C.; Di Luca, M.; Chiesa Estomba, C.; et al. Machine Learning Identification of Obstructive Sleep Apnea Severity through the Patient Clinical Features: A Retrospective Study. Life 2023, 13, 702. [Google Scholar] [CrossRef]
- Bitners, A.C.; Arens, R. Evaluation and Management of Children with Obstructive Sleep Apnea Syndrome. Lung 2020, 198, 257–270. [Google Scholar] [CrossRef]
- Sheiham, A.; Watt, R.G. The common risk factor approach: A rational basis for promoting oral health. Community Dent. Oral. Epidemiol. 2000, 28, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhou, N.; Lobbezoo, F.; Almeida, F.R.; Cistulli, P.A.; Dieltjens, M.; Huynh, N.T.; Kato, T.; Lavigne, G.J.; Masse, J.F.; et al. Dental sleep-related conditions and the role of oral healthcare providers: A scoping review. Sleep Med. Rev. 2023, 67, 101721. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhou, N.; Chattrattrai, T.; van Selms, M.K.A.; de Vries, R.; Hilgevoord, A.A.J.; de Vries, N.; Aarab, G.; Lobbezoo, F. Associations between snoring and dental sleep conditions: A systematic review. J. Oral. Rehabil. 2023, 50, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Cammaroto, G.; Stringa, L.M.; Iannella, G.; Meccariello, G.; Zhang, H.; Bahgat, A.Y.; Calvo-Henriquez, C.; Chiesa-Estomba, C.; Lechien, J.R.; Barillari, M.R.; et al. Manipulation of Lateral Pharyngeal Wall Muscles in Sleep Surgery: A Review of the Literature. Int. J. Environ. Res. Public Health 2020, 17, 5315. [Google Scholar] [CrossRef]
- Gulotta, G.; Iannella, G.; Meccariello, G.; Cammaroto, G.; Visconti, I.C.; de Vincentiis, M.; Greco, A.; Pelucchi, S.; Magliulo, G.; Ruoppolo, G.; et al. Barbed suture Extrusion and Exposure in palatoplasty for OSA: What does it mean? Am. J. Otolaryngol. 2021, 42, 102994. [Google Scholar] [CrossRef]
- Wetselaar, P.; Manfredini, D.; Ahlberg, J.; Johansson, A.; Aarab, G.; Papagianni, C.E.; Reyes Sevilla, M.; Koutris, M.; Lobbezoo, F. Associations between tooth wear and dental sleep disorders: A narrative overview. J. Oral. Rehabil. 2019, 46, 765–775. [Google Scholar] [CrossRef]
- Mukherjee, S.; Galgali, S.R. Obstructive sleep apnea and periodontitis: A cross-sectional study. Indian. J. Dent. Res. 2021, 32, 44–50. [Google Scholar] [CrossRef]
- Nadeem, R.; Molnar, J.; Madbouly, E.M.; Nida, M.; Aggarwal, S.; Sajid, H.; Naseem, J.; Loomba, R. Serum inflammatory markers in obstructive sleep apnea: A meta-analysis. J. Clin. Sleep Med. 2013, 9, 1003–1012. [Google Scholar] [CrossRef]
- Nizam, N.; Basoglu, O.K.; Tasbakan, M.S.; Lappin, D.F.; Buduneli, N. Is there an association between obstructive sleep apnea syndrome and periodontal inflammation? Clin. Oral. Investig. 2016, 20, 659–668. [Google Scholar] [CrossRef]
- Koutsourelakis, I.; Vagiakis, E.; Roussos, C.; Zakynthinos, S. Obstructive sleep apnoea and oral breathing in patients free of nasal obstruction. Eur. Respir. J. 2006, 28, 1222–1228. [Google Scholar] [CrossRef]
- Carra, M.C.; Thomas, F.; Schmitt, A.; Pannier, B.; Danchin, N.; Bouchard, P. Oral health in patients treated by positive airway pressure for obstructive sleep apnea: A population-based case-control study. Sleep Breath. 2016, 20, 405–411. [Google Scholar] [CrossRef]
- Dioguardi, A.; Al-Halawani, M. Oral Appliances in Obstructive Sleep Apnea. Otolaryngol. Clin. N. Am. 2016, 49, 1343–1357. [Google Scholar] [CrossRef]
| Parameters | Variables | Without OSA | OSA | p Value | OR (95% CI) # |
|---|---|---|---|---|---|
| No. (%) % | No. (%) % | ||||
| Sex | Women | 33,045 (99.9) | 18 (0.1) | <0.001 * | 1 |
| Men | 99,166 (99.7) | 300 (0.3) | 5.52 (3.45–8.93) | ||
| Educational level | High school | 112,956 (100) | 56 (0.0) | <0.001 ^ | 0.04 (0.03–0.05) |
| Technicians | 7319 (98.6) | 107 (1.4) | 1.19 (0.93–1.53) | ||
| Academic | 12,661 (98.8) | 155 (1.2) | 1 | ||
| Residency location | Urban non-Jewish | 17,887 (99.8) | 31 (0.2) | 0.002 ^ | 0.70 (0.48–1.02) |
| Rural | 577 (99.0) | 6 (1.0) | 4.20 (1.86–9.48) | ||
| Urban Jewish | 113,188 (99.8) | 280 (0.2) | 1 | ||
| Socio-economic status (SES) | Low | 5710 (99.8) | 9 (0.2) | 0.270 ^ | 1 |
| Medium | 68,460 (99.8) | 159 (0.2) | 1.47 (0.75–2.89) | ||
| High | 56,562 (99.7) | 145 (0.3) | 1.63 (0.83–3.19) | ||
| Rings of a city/town | Central rings—midtown | 118,164 (99.8) | 286 (0.2) | 0.745 * | 1.06 (0.74–1.53) |
| Peripheral rings—suburbs | 14,047 (99.8) | 32 (0.2) | 1 | ||
| Birth country | Western Europe | 10,538 (99.7) | 33 (0.3) | 0.096 ^ | 1.34 (0.93–1.92) |
| Eastern Europe | 1708 (99.6) | 7 (0.4) | 1.75 (0.82–3.71) | ||
| Asia | 505 (99.2) | 4 (0.8) | 3.38 (0.25–9.11) | ||
| Africa Ethiopia | 2527 (99.99) | 3 (0.001) | 0.59 (0.19–1.83) | ||
| North America | 2855 (99.9) | 4 (0.1) | 0.60 (0.22–1.61) | ||
| South America | 955 (99.8) | 2 (0.2) | 0.89 (0.22–3.60) | ||
| Israel | 113,094 (99.8) | 265 (0.2) | 1 | ||
| Parameter | OSA | Mean ± SD | p value ** | OR (95% CI) ## | |
| Age | No | 21.85 ± 5.97 | <0.001 | 1.19 (1.18–1.21) | |
| Yes | 37.38 ± 8.08 | ||||
| Length of service | No | 3.09 ± 6.23 | <0.001 | 1.09 (1.08–1.10) | |
| Yes | 19.19 ± 9.61 | ||||
| Parameter | Variable | Without OSA | OSA | p Value * | OR (95% CI) ** |
|---|---|---|---|---|---|
| No. (%) % | No. (%) % | ||||
| Current smoking | No | 125,445 (99.8) | 200 (0.2) | <0.001 | 1 |
| Yes | 6766 (98.3) | 118 (1.7) | 10.75 (8.62–13.51) | ||
| Teeth brushing at least once a day or more | No | 17,736 (99.5) | 84 (0.5) | <0.001 | 1 |
| Yes | 39,573 (99.7) | 103 (0.3) | 0.54 (0.41–0.73) | ||
| Parameter | OSA | Mean ± SD | p * value * | OR (95% CI) ** | |
| Total number of dental appointments | No | 5.86 ± 10.21 | <0.001 | 1.03 (1.02–1.03) | |
| Yes | 16.17 ± 19.84 | ||||
| Non-attendance to scheduled dental appointments | No | 0.99 ± 2.66 | <0.001 | 1.06 (1.05–1.08) | |
| Yes | 2.33 ± 4.46 | ||||
| Total number of appointments with a general physician | No | 14.21 ± 11.98 | <0.001 | 1.02 (1.01–1.03) | |
| Yes | 18.09 ± 15.86 | ||||
| Parameter | Variable | Without OSA | OSA | p * Value | OR (95% CI) ** |
|---|---|---|---|---|---|
| No. (%) % | No. (%) % | ||||
| Hypertension | No | 128,906 (99.8) | 260 (0.2) | <0.001 | 1 |
| Yes | 3305 (98.3) | 58 (1.7) | 8.55 (6.45–11.36) | ||
| Hyperlipidemia | No | 131,263 (99.8) | 305 (0.2) | <0.001 | 1 |
| Yes | 948 (98.6) | 13 (1.4) | 5.85 (3.37–10.10) | ||
| Diabetes Mellitus | No | 131,880 (99.8) | 304 (0.2) | <0.001 | 1 |
| Yes | 331 (95.9) | 14 (4.1) | 17.54 (10.42–29.41) | ||
| Obesity | No | 124,949 (99.9) | 132 (0.1) | <0.001 | 1 |
| Yes | 7262 (97.5) | 186 (2.5) | 23.81 (18.87–29.41) | ||
| Cardiovascular disease | No | 128,669 (99.8) | 262 (0.2) | <0.001 | 1 |
| Yes | 3542 (98.4) | 56 (1.6) | 7.63 (5.75–10.20) | ||
| Non-alcoholic fatty liver disease (NAFLD) | No | 131,308 (99.8) | 283 (0.2) | <0.001 | 1 |
| Yes | 903 (96.3) | 35 (3.7) | 17.24 (12.34–24.39) | ||
| Stroke | No | 132,121 (99.8) | 316 (0.2) | <0.001 | 1 |
| Yes | 90 (97.8) | 2 (2.2) | 9.09 (2.30–35.71) | ||
| Transient ischemic attack (TIA) | No | 132,114 (99.8) | 316 (0.2) | <0.001 | 1 |
| Yes | 97 (98.0) | 2 (2.0) | 8.47 (2.14–33.33) |
| Parameter | OSA | N | Mean ± SD | p Value * | OR (95% CI) ** |
|---|---|---|---|---|---|
| Weight (kilograms) | No | 66,371 | 73.23 ± 32.41 | <0.001 | 1.004 (1.003–1.005) |
| Yes | 246 | 90.65 ± 18.14 | |||
| Body mass index (BMI) | No | 66,149 | 24.26 ± 4.29 | <0.001 | 1.188 (1.165–1.212) |
| Yes | 245 | 29.17 ± 5.00 | |||
| C-reactive protein (CRP) (mg/L) | No | 30,269 | 3.76 ± 10.16 | 0.007 | 1.011 (1.003–1.019) |
| Yes | 149 | 6.02 ± 14.19 | |||
| Cholesterol (mg/dL) | No | 27,895 | 175.76 ± 35.62 | <0.001 | 1.005 (1.002–1.008) |
| Yes | 285 | 182.60 ± 36.47 | |||
| High-density lipoprotein (HDL) (mg/dL) | No | 27,888 | 48.33 ± 11.79 | <0.001 | 0.958 (0.947–0.970) |
| Yes | 285 | 43.54 ± 9.17 | |||
| Low-density lipoprotein (LDL) (mg/dL) | No | 19,945 | 108.28 ± 29.99 | 0.012 | 1.005 (1.001–1.009) |
| Yes | 268 | 112.92 ± 32.83 | |||
| LDL cholesterol calculated (mg/dL) | No | 17,254 | 108.31 ± 30.41 | 0.018 | 1.005 (1.001–1.010) |
| Yes | 204 | 113.38 ± 30.46 | |||
| Non-HDL cholesterol (mg/dL) | No | 16,588 | 129.37 ± 34.98 | <0.001 | 1.007 (1.004–1.011) |
| Yes | 234 | 139.11 ± 35.77 | |||
| Very-low-density lipoprotein (VLDL) (mg/dL) | No | 27,846 | 20.56 ± 11.17 | <0.001 | 1.030 (1.022–1.038) |
| Yes | 285 | 25.64 ± 13.29 | |||
| Triglycerides | No | 27,898 | 104.23 ± 63.97 | <0.001 | 1.003 (1.002–1.004) |
| Yes | 285 | 128.20 ± 66.38 | |||
| Glycated hemoglobin (HbA1c) (%) | No | 1896 | 5.40 ± 0.97 | 0.092 | 1.207 (0.967–1.505) |
| Yes | 47 | 5.64 ± 0.98 | |||
| Fasting blood glucose (mg/dL) | No | 2486 | 87.10 ± 11.97 | 0.369 | 1.010 (0.989–1.032) |
| Yes | 41 | 88.79 ± 9.90 |
| Parameter | Variable | No OSA | OSA | p * Value | OR (95% CI) ** |
|---|---|---|---|---|---|
| No. (%) | No. (%) | ||||
| Periodontal Disease | No | 51,730 (99.7) | 136 (0.3) | <0.001 | 1 |
| Yes | 5579 (99.1) | 51 (0.9) | 3.46 (2.51–4.76) | ||
| The number of delivered dental procedures | Without OSA (Mean ± SD) | OSA (Mean ± SD) | p value * | OR (95% confidence interval) ** | |
| Fillings | 1.01 ± 1.97 | 1.27 ± 1.91 | 0.018 | 1.05 (1.01–1.10) | |
| Endodontic treatments | 0.08 ± 0.34 | 0.16 ± 0.50 | 0.004 | 1.43 (1.22–1.68) | |
| Renewal of endodontic treatment | 0.02 ± 0.17 | 0.08 ± 0.35 | <0.001 | 1.43 (1.18–1.72) | |
| Regular extractions | 0.10 ± 0.43 | 0.20 ± 0.51 | <0.001 | 1.19 (1.09–1.30) | |
| Surgical extractions | 0.05 ± 0.28 | 0.09 ± 0.32 | 0.035 | 1.37 (1.07–1.75) | |
| Indirect post and core | 0.10 ± 0.40 | 0.23 ± 0.59 | <0.001 | 1.38 (1.23–1.54) | |
| Direct post and core | 0.00 ± 0.09 | 0.04 ± 0.29 | 0.014 | 1.59 (1.29–1.95) | |
| Crowns | 0.05 ± 0.54 | 0.40 ± 1.74 | <0.001 | 1.25 (1.04–1.45) | |
| The number of missing teeth | 0.58 ± 1.29 | 1.21 ± 1.67 | <0.001 | 1.13 (1.09–1.18) | |
| Multivariate Binary Logistic Regression Analysis * | Collinearity Statistics Using Linear Regression Analysis ** | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | B | Std. Error | p Value | OR (95% CI) * | Tolerance | VIF | |
| (Intercept) | 9.845 | 0.738 | <0.001 | 0.002 (0.001–0.004) | |||
| Age | 0.134 | 0.010 | <0.001 | 1.14 (1.12–1.17) | 0.509 | 1.966 | |
| Sex: men/women | 0.880 | 0.333 | 0.008 | 2.41 (1.25–4.63) | 0.955 | 1.047 | |
| Residency location | Urban Jewish | −0.178 | 0.519 | 0.731 | 0.84 (0.30–2.32) | 0.979 | 1.021 |
| Urban non-Jewish | −0.443 | 0.563 | 0.432 | 0.64 (0.21–1.94) | 0.994 | 1.006 | |
| Smoking | 0.372 | 0.162 | 0.022 | 1.45 (1.05–1.99) | 0.760 | 1.317 | |
| Teeth brushing once a day or more | −0.195 | 0.180 | 0.279 | 0.82 (0.58–1.17) | 0.738 | 1.355 | |
| Hypertension | 0.358 | 0.202 | 0.077 | 1.43 (0.96–2.13) | 0.910 | 1.099 | |
| Hyperlipidemia | 0.971 | 0.512 | 0.058 | 2.63 (0.96–7.19) | 0.958 | 1.044 | |
| Diabetes Mellitus | 0.112 | 0.393 | 0.776 | 1.12 (0.52–2.42) | 0.948 | 1.054 | |
| Obesity | 1.133 | 0.181 | <0.001 | 3.10 (2.18–4.42) | 0.690 | 1.449 | |
| Cardiovascular disease | 0.005 | 0.213 | 0.983 | 1.00 (0.66–1.53) | 0.932 | 1.073 | |
| Non-alcoholic fatty liver disease (NAFLD) | 0.091 | 0.266 | 0.733 | 1.09 (0.65–1.84) | 0.905 | 1.105 | |
| Stroke | 0.029 | 1.054 | 0.978 | 1.03 (0.13–8.13) | 0.939 | 1.065 | |
| Transient ischemic attack (TIA) | 0.548 | 1.047 | 0.601 | 1.73 (0.22–13.5) | 0.937 | 1.067 | |
| Total number of dental appointments | 0.003 | 0.005 | 0.618 | 1.00 (0.99–1.01) | 0.468 | 2.136 | |
| Non-attendance to scheduled dental appointments | 0.013 | 0.023 | 0.558 | 1.01 (0.96–1.06) | 0.526 | 1.901 | |
| Periodontal Disease | 0.699 | 0.189 | <0.001 | 2.01 (1.38–2.91) | 0.967 | 1.034 | |
| Missing teeth | −0.057 | 0.053 | 0.282 | 0.94 (0.85–1.05) | 0.800 | 1.250 | |
| Delivered dental fillings | −0.062 | 0.037 | 0.100 | 0.94 (0.87–1.01) | 0.929 | 1.077 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ytzhaik, N.; Zur, D.; Goldstein, C.; Almoznino, G. Obstructive Sleep Apnea, Metabolic Dysfunction, and Periodontitis—Machine Learning and Statistical Analyses of the Dental, Oral, Medical Epidemiological (DOME) Big Data Study. Metabolites 2023, 13, 595. https://doi.org/10.3390/metabo13050595
Ytzhaik N, Zur D, Goldstein C, Almoznino G. Obstructive Sleep Apnea, Metabolic Dysfunction, and Periodontitis—Machine Learning and Statistical Analyses of the Dental, Oral, Medical Epidemiological (DOME) Big Data Study. Metabolites. 2023; 13(5):595. https://doi.org/10.3390/metabo13050595
Chicago/Turabian StyleYtzhaik, Noya, Dorit Zur, Chen Goldstein, and Galit Almoznino. 2023. "Obstructive Sleep Apnea, Metabolic Dysfunction, and Periodontitis—Machine Learning and Statistical Analyses of the Dental, Oral, Medical Epidemiological (DOME) Big Data Study" Metabolites 13, no. 5: 595. https://doi.org/10.3390/metabo13050595
APA StyleYtzhaik, N., Zur, D., Goldstein, C., & Almoznino, G. (2023). Obstructive Sleep Apnea, Metabolic Dysfunction, and Periodontitis—Machine Learning and Statistical Analyses of the Dental, Oral, Medical Epidemiological (DOME) Big Data Study. Metabolites, 13(5), 595. https://doi.org/10.3390/metabo13050595






