Unexpected Value of Honey Color for Prediction of a Non-Enzymatic H2O2 Production and Honey Antibacterial Activity: A Perspective
Abstract
1. Introduction
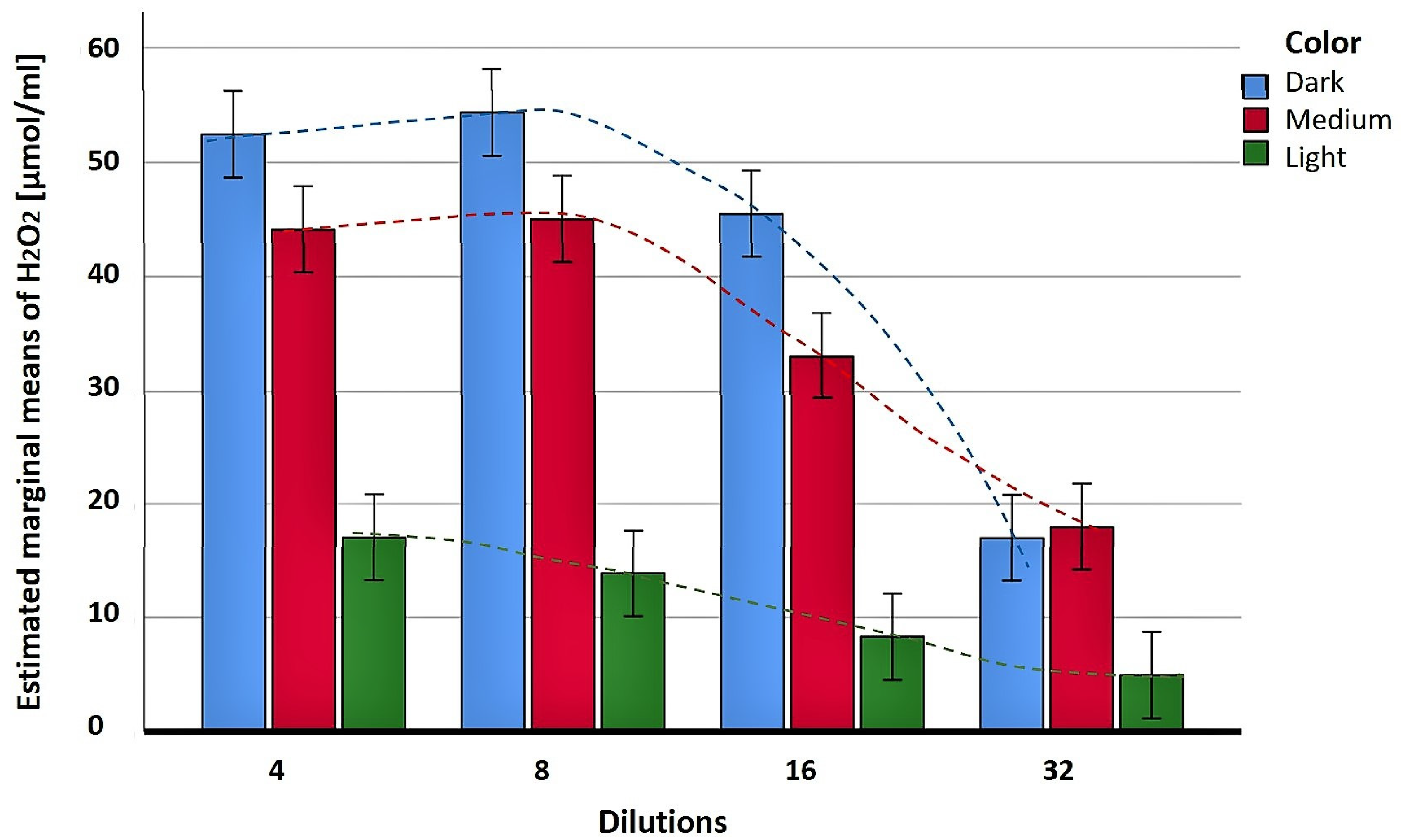
2. Correlation between H2O2 Production and Color of Honey Variety as a First Indication of the Pro-Oxidant Activity
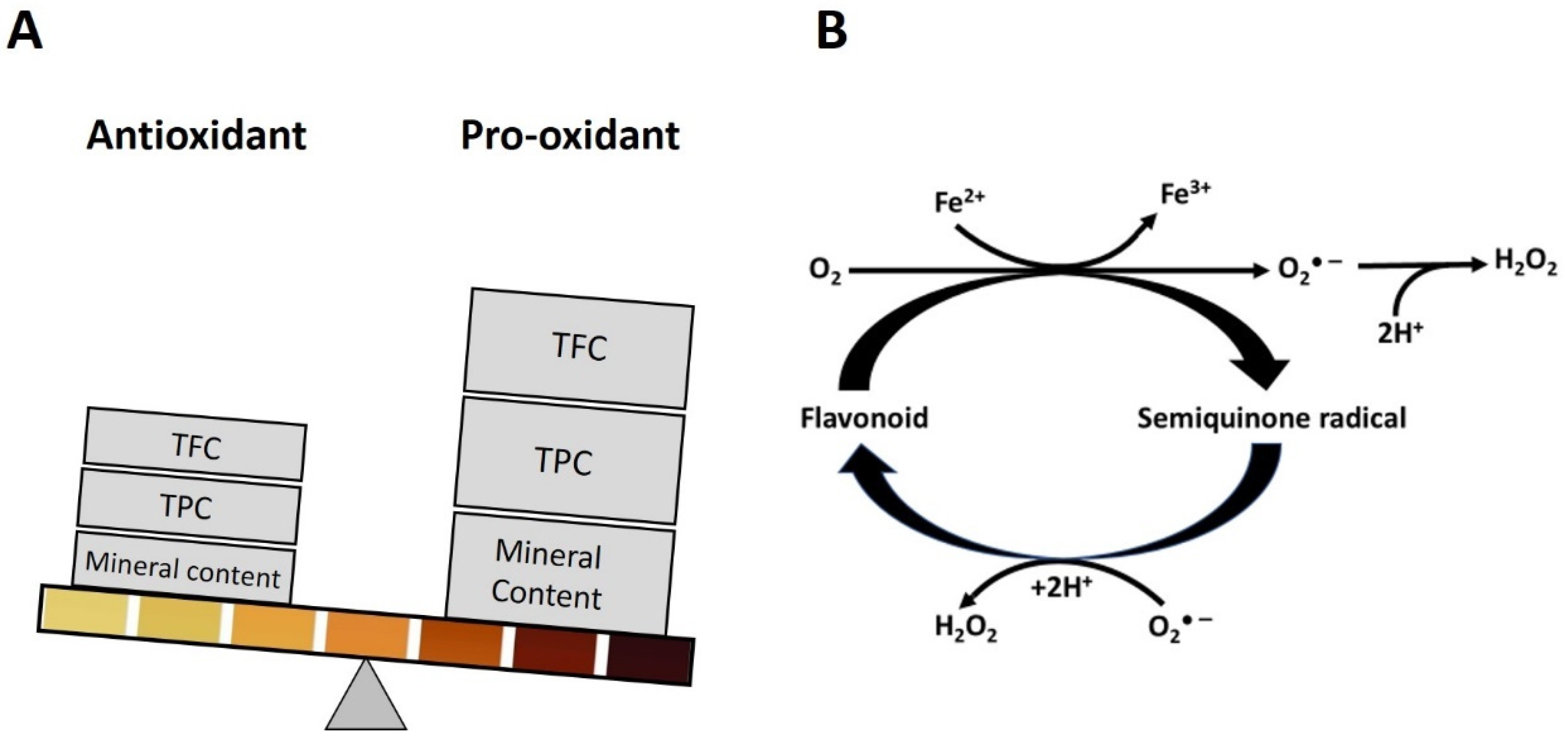
3. The Interrelationship between the Content of Pigmented Polyphenols, Honey Color, and H2O2 Production
3.1. Honey Color Appeared as a Dominant Parameter in Honey Classifications in Multivariate Analysis
3.2. Correlation between Honey Color, Polyphenol Content and H2O2 Production
4. Basic Concept of H2O2 Production via Polyphenols Autoxidation
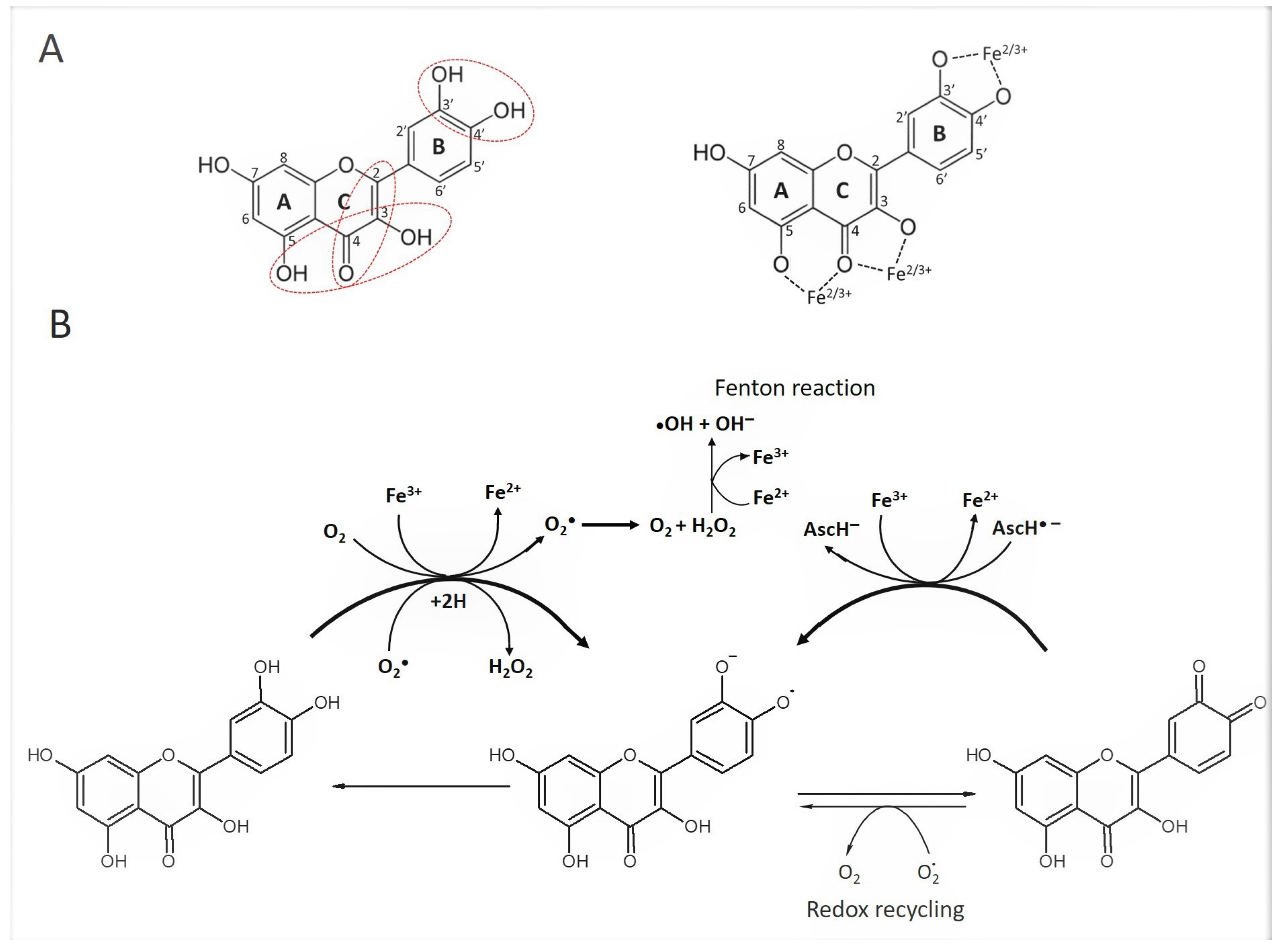
5. Presence of Phenolic Acids and Flavonoids of Required Structures for Pro-Oxidant Activity in Honey
6. Presence of Transition Metals
7. Association between Transition Metals and Honey Color
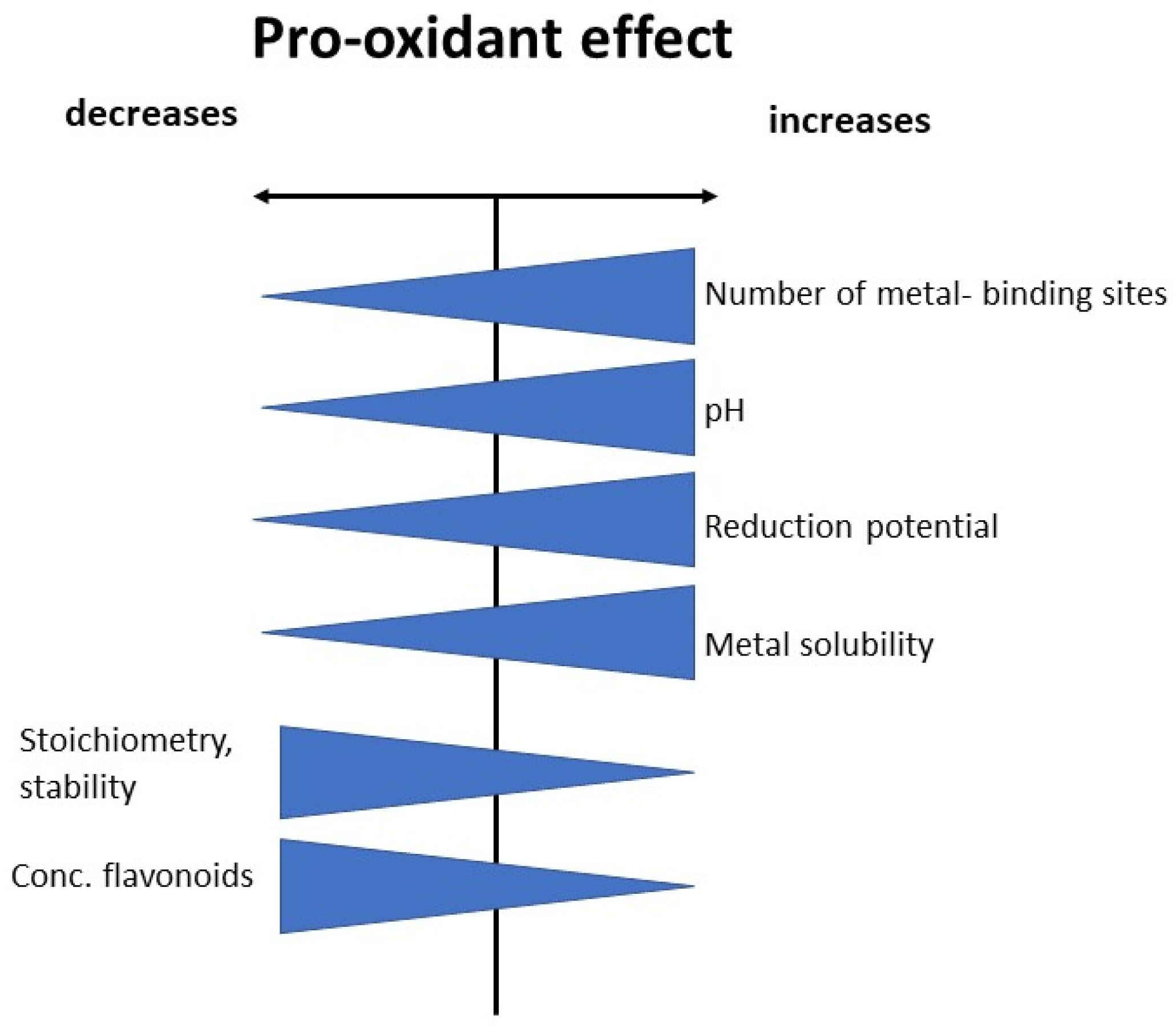
8. Conditions Favoring the Switch from Antioxidant to Pro-Oxidant Activities
8.1. Pro-Oxidation Depends on the Number and Position of Hydroxyl Group in Polyphenol Structure and Type of Transition Metal
8.2. Pro-Oxidation Effect Increases in the Presence of Strong Chelators
8.3. Effect of pH
8.4. Stoichiometry
8.5. Solubility
8.6. Formation of Metal-Phenolic Networks
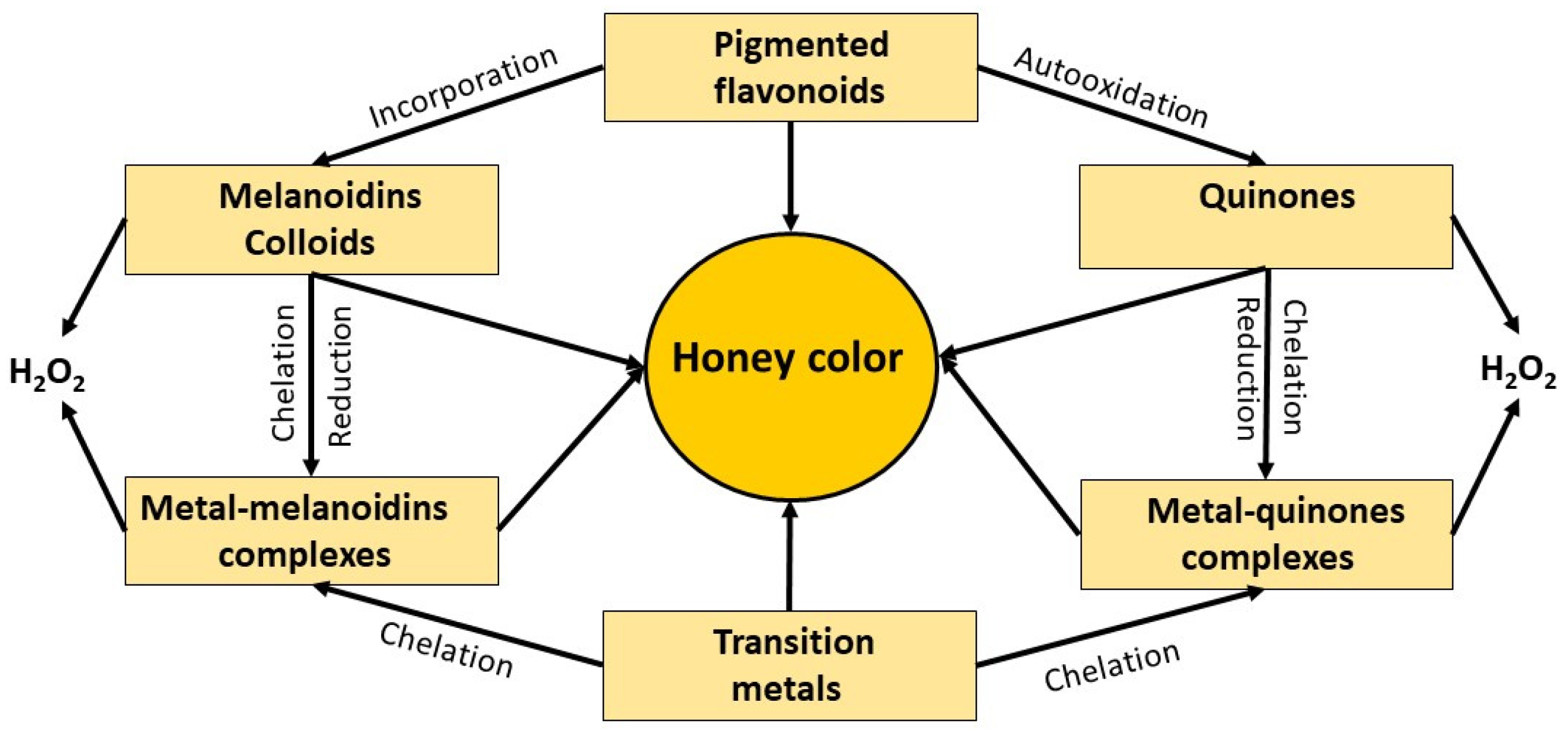
9. Association between Polyphenols Autooxidation, Formation of Higher-Order Structures and H2O2 Production
9.1. Association between Melanoidin Formation and H2O2 Production
9.2. Association between the Formation of Colloidal Particles and H2O2 Production
10. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Dold, H.; Du, H.; Dziao, S.T. Detection of antibacterial, heat- and light-sensitive inhibitors (inhibins) in natural honey (blossom honey). Z. Hyg. InfektKr. 1937, 120, 155–600. [Google Scholar] [CrossRef]
- White, J.W., Jr.; Subers, M.H.; Schepartz, A.I. The identification of inhibine, antibacterial factor in the honey, as hydrogen peroxide, and its origin in a honey glucose oxidase. Biochem. Biophys. Acta 1963, 73, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.L.; Molan, P.C.; Reid, G.M. A Survey of the Antibacterial Activity of Some New Zealand Honeys. J. Pharm. Pharmacol. 1991, 43, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Molan, P.C. The Antibacterial Activity of Honey. 2. Variation in the potency of the antibacterial activity. Bee World 1992, 73, 59–76. [Google Scholar] [CrossRef]
- Taormina, P.I.; Niemira, B.A.; Beuchat, L.R. Inhibitory activity of honey against foodborne pathogens as influenced by the presence of hydrogen peroxide and level of antioxidant power. Int. J. Food Microbiol. 2001, 69, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K. Effect of hydrogen peroxide on antibacterial activities of Canadian honeys. Can. J. Microbiol. 2006, 52, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K.; Abubaker, K.; St. Martin, L.; Castle, A. Re-examining the role of hydrogen peroxide in bacteriostatic and bactericidal activities of honey. Front. Microbiol. 2011, 2, 213. [Google Scholar] [CrossRef] [PubMed]
- Kwakman, P.H.S.; Zaat, S.A.J. Antibacterial Components of Honey. IUBMB Life 2012, 64, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Sowa, P.; Grabek-Lejko, D.; Wesołowska, M.; Swacha, S.; Dżugan, M. Hydrogen peroxide-dependent antibacterial action of Melilotus albus honey. Lett. Appl. Microbiol. 2017, 65, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Bucekova, M.; Buriova, M.; Pekarik, L.; Majtan, V.; Majtan, J. Phytochemicals-mediated production of hydrogen peroxide is crucial for high antibacterial activity of honeydew honey. Sci. Rep. 2018, 8, 9061. [Google Scholar] [CrossRef] [PubMed]
- Poli, J.-P.; Guinoiseau, E.; Luciani, A.; Yang, Y.; Battesti, M.-J.; Paolini, J.; Costa, J.; Quilichini, Y.; Berti, L.; Lorenzi, V. Key role of hydrogen peroxide in antimicrobial activity of spring Honeydew maquis and chestnut grove Corsican honeys on Pseudomonas aeruginosa DNA. Lett. Appl. Microbiol. 2018, 66, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Masoura, M.; Passaretti, P.; Overton, T.W.; Lund, P.A.; Gkatzionis, K. Use of a model to understand the synergies underlying the antibacterial mechanism of H2O2-producing honeys. Sci. Rep. 2020, 10, 17692. [Google Scholar] [CrossRef]
- Brudzynski, K. Honey as an Ecological Reservoir of Antibacterial Compounds Produced by Antagonistic Microbial Interactions in Plant Nectars, Honey and Honey Bee. Antibiotics 2021, 10, 551. [Google Scholar] [CrossRef] [PubMed]
- Weston, R.J. The contribution of catalase and other natural products to the antibacterial activity of honey: A review. Food Chem. 2000, 71, 235–239. [Google Scholar] [CrossRef]
- Albaridi, N.A. Antibacterial Potency of Honey. Int. J. Microbiol. 2019, 2, 2464507. [Google Scholar] [CrossRef] [PubMed]
- Strelec, I.; Crevar, B.; Kovac, T.; Rajs, B.B.; Primorac, L.; Flanjak, I. Glucose oxidase activity and hydrogen peroxide accumulation in Croatian honeys. CJFST 2018, 10, 33–41. [Google Scholar] [CrossRef]
- Brudzynski, K. A current perspective on hydrogen peroxide production in honey. A review. Food Chem. 2020, 332, 127229. [Google Scholar] [CrossRef]
- Carter, C.; Thornburg, R.W. Tobacco Nectarin V Is a Flavin-Containing Berberine Bridge Enzyme-Like Protein with Glucose Oxidase Activity. Plant Physiol. 2004, 134, 460–469. [Google Scholar] [CrossRef]
- Long, L.H.; Lan, A.N.; Hsuan, F.T.; Halliwell, B. Generation of hydrogen peroxide by “antioxidant” beverages and the effect of milk addition. Is cocoa the best beverage? Free Radic. Res. 1999, 31, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.A.; Ferreira, A.C.S.; De Freitas, V.; Silva, A.M.S. Oxidation mechanisms occurring in wines. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- Grzesik, M.; Bartosz, G.; Stefaniuk, I.; Pichla, M.; Namieśnik, J.; Sadowska-Bartosz, I. Dietary antioxidants as a source of hydrogen peroxide. Food Chem. 2019, 25, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Akagawa, M.; Shigemitsu, T.; Suyama, K. Production of hydrogen peroxide bypolyphenols and polyphenol-rich beverages under quasi-physiological conditions. Biosci. Biotechnol. Biochem. 2003, 67, 2632–2640. [Google Scholar] [CrossRef] [PubMed]
- Bang, L.M.; Buntting, C.; Molan, P.C. The effect of dilution on the rate of hydrogen peroxide production in honey and its implications for wound healing. J. Altern. Complement. Med. 2003, 9, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K.; Miotto, D.; Kim, L.; Sjaarda, C.; Maldonado-Alvarez, L.; Fukś, H. Active macromolecules of honey form colloidal particles essential for honey antibacterial activity and hydrogen peroxide production. Sci. Rep. 2017, 7, 7637. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K.; Miotto, D. The relationship between the content of Maillard-reaction- like products and bioactivity of Canadian honeys. Food Chem. 2011, 124, 867–874. [Google Scholar] [CrossRef]
- Kuś, P.; Congiu, F.; Teper, D.; Sroka, Z.; Jerković, I.; Tuberoso, C.I.G. Antioxidant activity, color characteristics, total phenol content and general HPLC fingerprints of six Polish unifloral honey types. LWT-Food Sci. Technol. 2014, 55, 124–130. [Google Scholar] [CrossRef]
- Bijlsma, J.; de Bruijn, W.J.C.; Hageman, J.A.; Goos, P.; Velikov, K.P.; Vincken, J.-P. Revealing the main factors and two-way interactions contributing to food discolouration caused by iron-catechol complexation. Sci. Rep. 2020, 10, 8288. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K.; Sjaarda, C.; Maldonado-Alvarez, L. A new look on protein-polyphenol complexation during honey storage. Is this a random or organized event with the help of dirigent-like proteins? PLoS ONE 2013, 8, e72897. [Google Scholar] [CrossRef]
- Pourcel, L.; Jean-Marc Routaboul, J.-M.; Cheynier, V.; Lepiniec, L.; Debeaujon, I. Flavonoid oxidation in plants: From biochemical properties to physiological functions. Trends Plant Sci. 2006, 12, 29–36. [Google Scholar] [CrossRef]
- Tan, J.; de Bruijn, W.J.C.; van Zadelhoff, A.; Lin, Z.; Vincken, J.-P. Browning of epicatechin (EC) and epigallocatechin (EGC) by auto-oxidation. J. Agric. Food Chem. 2020, 68, 13879–13887. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhang, C.; Li, C.; Huang, Z.Y.; Miao, X. Pathway of 5-hydroxymethyl-2-furaldehyde formation in honey. J. Food Sci. Technol. 2019, 56, 2417–2425. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K.; Miotto, D. Honey melanoidins. Analysis of a composition of the high molecular weight melanoidin fractions exhibiting radical scavenging capacity. Food Chem. 2011, 127, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Are polyphenols antioxidants or pro-oxidants? What we learn from cells culture and in vivo studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Mehta, A.; Berenbaum, M.; Zangeri, A.R.; Engeseth, N.J. Honey from different floral sources as inhibitors of enzymatic browning of fruit and vegetable homogenates. J. Agric. Food Chem. 2000, 48, 4997–5000. [Google Scholar] [CrossRef] [PubMed]
- Gheldof, N.; Wang, X.H.; Engeseth, N.J. Identification and quantification of antioxidant components of honeys from various floral sources. J. Agric. Food Chem. 2002, 50, 5870–5877. [Google Scholar] [CrossRef]
- Gheldof, N.; Engeseth, N.J. Antioxidant capacity of honeys from various floral sources based on the determination of oxygen radical absorbance capacity and inhibition of in vitro lipoprotein oxidation in human serum samples. J. Agric. Food Chem. 2002, 50, 3050–3055. [Google Scholar] [CrossRef] [PubMed]
- Bertoncejl, J.; Dobersek, U.; Jamnik, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007, 105, 822–828. [Google Scholar] [CrossRef]
- Vela, L.; de Lorenzo, C.; Perez, R.A. Antioxidant capacity of Spanish honeys and its correlation with polyphenol content and other physicochemical properties. J. Sci. Food Agric. 2007, 87, 1069–1075. [Google Scholar] [CrossRef]
- Socha, R.; Juszczak, L.; Pietrzyk, S.; Gałkowska, D.; Fortuna, T.; Witczak, T. Phenolic profile and antioxidant properties of Polish honeys. Int. J. Food Sci. Technol. 2011, 46, 528–534. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Aires, E.; Barreira, J.C.M.; Estevinho, L.M. Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar] [CrossRef]
- Beretta, G.; Granata, P.; Ferrero, M.; Orioli, M.; Maffei Facino, R. Standardization of antioxidant properties of honey by combination of spectrophotometric/fluorimetric assays and chemometrics. Anal. Chim. Acta 2005, 533, 185–191. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Maruška, A.; Kornyšova, O.; Charczun, N.; Ligor, M.; Buszewski, B. Quantitative and qualitative determination of phenolic compounds in honey. Chem. Technol. 2009, 3, 74–80. [Google Scholar]
- Blasa, M.; Candiracci, M.; Accorsi, A.; Piacentini, M.P.; Albertini, M.C.; Piatti, E. Raw Millefiori honey is packed full of antioxidants. Food Chem. 2006, 97, 217–222. [Google Scholar] [CrossRef]
- Flanjak, I.; Kenjeric, D.; Bubalo, D.; Primorac, L. Characterisation of selected Croatian honey types based on the combination of antioxidant capacity, quality parameters, and chemometrics. Eur. Food Res. Technol. 2016, 242, 467–475. [Google Scholar] [CrossRef]
- Gašić, U.; Kečkeš, S.; Dabić, D.; Trifković, J.; Milojković-Opsenica, D.; Natić, M.; Tešić, Z. Phenolic profile and antioxidant activity of Serbian polyfloral honeys. Food Chem. 2014, 145, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Dżugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant Activity as Biomarker of Honey Variety. Molecules 2018, 23, 2069. [Google Scholar] [CrossRef] [PubMed]
- Pontis, A.; Costa, L.A.; da Silva, S.J.; Flach, A. Color, phenolic and flavonoid content, and antioxidant activity of honey from Roraima, Brazil. Food Sci. Technol. Camp. 2014, 34, 69–73. [Google Scholar] [CrossRef]
- Yayinie, M.; Atlabachew, M.; Tesfaye, A.; Hilluf, W.; Reta, C.; Alemneh, T. Polyphenols, flavonoids, and antioxidant content of honey coupled with chemometric method: Geographical origin classification from Amhara region, Ethiopia. Int. J. Food Prop. 2022, 25, 76–92. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Muzolf-Panek, M.; Tomaszewska-Gras, J.; Konieczny, P. Predicting the Botanical Origin of Honeys with Chemometric Analysis According to Their Antioxidant and Physicochemical Properties. Pol. J. Food Nutr. Sci. 2019, 69, 191–201. [Google Scholar] [CrossRef]
- Sant’Ana, L.D.; Sousa, J.P.L.M.; Salgueiro, F.B.; Lorenzon, M.C.A.; Castro, R.N. Characterization of monofloral honeys with multivariate analysis of their chemical profile and antioxidant activity. J. Food. Sci. 2012, 71, C135–C140. [Google Scholar] [CrossRef] [PubMed]
- González-Miret, M.L.; Terab, A.; Hernanz, D.; Fernández-Recamales, M.Á.; Heredia, F.J. Multivariate correlation between color and mineral composition of honeys and by their botanical origin. J. Agric. Food Chem. 2005, 53, 2574–2580. [Google Scholar] [CrossRef] [PubMed]
- Kedzierska-Matysek, M.; Stryjecka, M.; Teter, A.; Skałecki, P.; Domaradzki, P.; Florek, M. Relationships between the Content of Phenolic Compounds and the Antioxidant Activity of Polish Honey Varieties as a Tool for Botanical Discrimination. Molecules 2021, 26, 1810. [Google Scholar] [CrossRef] [PubMed]
- Halagarda, M.; Groth, S.; Popek, S.; Rohn, S.; Pedan, V. Antioxidant Activity and Phenolic Profile of Selected Organic and Conventional Honeys from Poland. Antioxidants 2020, 9, 44. [Google Scholar] [CrossRef]
- Rodopoulou, M.A.; Tananaki, C.; Kanelis, D.; Liolios, V.; Dimou, M.; Thrasyvoulou, A. A chemometric approach for the differentiation of 15 monofloral honeys based on physicochemical parameters. J. Sci. Food Agric. 2022, 102, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, J.N.; Paganga, C. Structure-antioxidant activity relationship of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef] [PubMed]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Radical chemistry of flavonoid antioxidants. In Antioxidants in Therapy and Preventive Medicine; Springer: Berlin/Heidelberg, Germany, 1990; pp. 165–170. [Google Scholar]
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical Scavenging Mechanisms of Phenolic Compounds: A Quantitative Structure-Property Relationship (QSPR) Study. Front Nutr. 2022, 9, 882458. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Nakayama, T.; Un, B. Importance of Proton-Coupled Electron Transfer from Natural Phenolic Compounds in Superoxide Scavenging. Chem. Pharm. Bull. 2015, 63, 967–973. [Google Scholar] [CrossRef]
- Quintero-Saumeth, J.; Rincón, D.A.; Doerr, M.; Daza, M.C. Concerted double proton-transfer electron-transfer between catechol and superoxide radical anion. Phys. Chem. Chem. Phys. 2017, 19, 26179–26190. [Google Scholar] [CrossRef] [PubMed]
- Trautvetter, S.; Koelling-Speer, I.; Speer, K. Confirmation of phenolic acids and flavonoids in honeys by UPLC-MS. Apidologie 2009, 40, 140–150. [Google Scholar] [CrossRef]
- Kečkeš, S.; Gašić, U.; Veličković, T.Ć.; Milojković-Opsenica, D.; Natić, M.; Tešić, Ž. The determination of phenolic profiles of Serbian unifloral honeys using ultra-high-performance liquid chromatography/high resolution accurate mass spectrometry. Food Chem. 2013, 138, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Sarikaya, A.O.; Ulusoy, E.; Öztürk, N.; Tunçezl, M.; Kolayli, S. Antioxidant activity and phenolic acid constituents of chestnut (Castania sativa Mill.) honey and propolis. J. Food Biochem. 2009, 33, 470–481. [Google Scholar] [CrossRef]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef]
- Nešović, M.; Gašić, U.; Tosti, T.; Horvacki, N.; Šikoparija, B.; Nedić, N.; Blagojević, S.; Ignjatović, L.; Tešić, Ž. Polyphenol profile of buckwheat honey, nectar and pollen. R. Soc. Open Sci. 2020, 7, 201576. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Andrade, P.; Tomás-Barberán, F.A. Flavonoids from Portuguese heather honey. Z. Lebensm. Unters Forch. 1994, 199, 32–37. [Google Scholar] [CrossRef]
- Güneş, M.E.; Şahin, S.; Demir, C.; Borum, E.; Tosunoğlu, A. Determination of phenolic compounds profile in chestnut and floral honeys and their antioxidant and antimicrobial activities. J. Food Biochem. 2016, 41, e12345. [Google Scholar] [CrossRef]
- Furusawa, M.; Tanaka, T.; Ito, T.; Nishikawa, A.; Yamazaki, N.; Nakaya, K.; Matsuura, N.; Tsuchiya, H.; Nagayama, M.; Iinuma, M. Antioxidant Activity of Hydroxyflavonoids. J. Health Sci. 2005, 51, 376–378. [Google Scholar] [CrossRef]
- Goslinski, M.; Nowak, D.; Szwengiel, A. Multidimensional Comparative Analysis of Bioactive Phenolic Compounds of Honeys of Various Origin. Antioxidants 2021, 10, 530. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, M.; Koziara, Z.; Suliborska, K.; Chrzanowski, W.; Wormstone, M.; Namieśnik, J.; Bartoszek, A. Interactions between polyphenolic antioxidants quercetin and naringenin dictate the distinctive redox-related chemical and biological behaviour of their mixtures. Sci. Rep. 2021, 11, 12282. [Google Scholar] [CrossRef]
- Chobot, V.; Hadacek, F. Exploration of pro-oxidant and antioxidant activities of the flavonoid myricetin. Redox Rep. 2011, 16, 242–247, Erratum in Redox Rep. 2012, 17, 180. [Google Scholar] [CrossRef] [PubMed]
- Pohl, P.; Stecka, H.; Greda, K.; Jamroz, P. Bioaccessibility of Ca, Cu, Fe, Mg, Mn and Zn from commercial bee honeys. Food Chem. 2012, 134, 392–396. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Louppis, A.P.; Kontakos, S.; Papastephanou, C.; Kontominas, M.G. Characterization and geographical discrimination of Greek pine and thyme honeys based on their mineral content, using chemometrics. Eur. Food Res. Technol. 2017, 243, 101–113. [Google Scholar] [CrossRef]
- Bogdanov, S.; Haldimann, M.; Luginbühl, W.; Gllmann, P. Minerals in honey: Environmental, geographical and botanical aspects. J. Apic. Res. Bee World 2007, 46, 269–275. [Google Scholar] [CrossRef]
- Fernández-Torres, R.; Pérez-Bernal, J.L.; Bello-López, M.A.; Callejón-Mochón, M.; Jiménez-Sánchez, J.C.; Guiraúm-Pérez, A. Mineral content and botanical origin of Spanish honeys. Talanta 2005, 65, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.; Simonetti, A.; Intaglietta, I.; Sofo, A.; Gambacorta, E. Metal content of southern Italy honey of different botanical origins and its correlation with polyphenol content and antioxidant activity. Int. J. Food Sci. Technol. 2012, 47, 1909–1917. [Google Scholar] [CrossRef]
- Anklam, E. A review of the analytical methods to determine the geographical and botanical origin of honey. Food Chem. 1998, 63, 549–562. [Google Scholar] [CrossRef]
- Kędzierska-Matysek, M.; Florek, M.; Wolanciuk, A.; Barłowska, J.; Litwińczuk, Z. Concentration of Minerals in Nectar Honeys from Direct Sale and Retail in Poland. Biol. Trace Elem. Res. 2018, 186, 579–588. [Google Scholar] [CrossRef]
- Kędzierska-Matysek, M.; Teter, A.; Stryjecka, M.; Skałecki, P.; Domaradzki, P.; Rudaś, M.; Florek, M. Relationships Linking the Colour and Elemental Concentrations of Blossom Honeys with Their Antioxidant Activity: A Chemometric Approach. Agriculture 2021, 11, 702. [Google Scholar] [CrossRef]
- Dżugan, M.; Zaguła, G.; Wesołowska, M.; Sowa, P.; Puchalski, C. Levels of Toxic And Essential Metals in Varietal Honeys from Podkarpacie. J. Elem. 2017, 22, 1039–1048. [Google Scholar] [CrossRef]
- Bijlsma, J.; de Bruijn, W.J.C.; Velikov, K.P.; Vincken, J.P. Unravelling discolouration caused by iron-flavonoid interactions: Complexation, oxidation, and formation of networks. Food Chem. 2022, 370, 131292. [Google Scholar] [CrossRef] [PubMed]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Jomova, L.; Hudecova, L.; Lauro, P.; Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Valko, M. A Switch between Antioxidant and Prooxidant Properties of the Phenolic Compounds Myricetin, Morin, 3′, 4′-Dihydroxyflavone, Taxifolin and 4-Hydroxy-Coumarin in the Presence of Copper (II) Ions: A Spectroscopic, Absorption Titration and DNA Damage Study. Molecules 2019, 23, 4335. [Google Scholar] [CrossRef]
- De Graft-Johnson, J.; Kolodziejczyk, K.; Krol, M.; Nowak, P.; Krol, B.; Nowak, D. Ferric-reducing ability power of selected plant polyphenols and their metabolites: Implications for clinical studies on the antioxidant effects of fruits and vegetable consumption. Basic Clin. Pharmacol. Toxicol. 2007, 100, 345–352. [Google Scholar] [CrossRef]
- Mira, L.; Fernandez, M.T.; Santos, M.; Rocha, R.; Florencio, M.; Jennings, K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radic. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef]
- Firuzi, O.; Lacanna, A.; Petrucci, R.; Marrosu, G.; Saso, L. Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochim. Biophys. Acta 2005, 1721, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, M.; Murakami, K. Interaction of Iron with Polyphenolic Compounds: Application to Antioxidant Characterization. Anal. Biochem. 1998, 257, 40–44. [Google Scholar] [CrossRef]
- Zheng, L.-F.; Dai, F.; Zhou, B.; Yang, L.; Liu, Z.-L. Prooxidant activity of hydroxycinnamic acids on DNA damage in the presence of Cu (II) ions: Mechanism and structure–activity relationship. Food Chem. Toxicol. 2008, 46, 149–156. [Google Scholar] [CrossRef]
- Jomova, K.; Lawson, M.; Drostinova, L.; Lauro, P.; Poprac, P.; Brezova, V.; Michalik, M.; Lukes, V.; Valko, M. Protective role of quercetin against copper (II)-induced oxidative stress: A spectroscopic, theoretical and DNA damage study. Food Chem. Toxicol. 2017, 110, 340–350. [Google Scholar] [CrossRef]
- Kasprzak, M.M.; Erxleben, A.; Ochocki, J. Properties and applications of flavonoid metal complexes. RSC Adv. 2015, 5, 45853–45877. [Google Scholar] [CrossRef]
- Samsonowicz, M.; Regulska, E. Spectroscopic study of molecular structure, antioxidant activity and biological effects of metal hydroxyflavonol complexes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 173, 757–771. [Google Scholar] [CrossRef] [PubMed]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Messele, S.A.; Bengoa, C.; Stüber, F.E.; Giralt, J.; Fortuny, A.; Fabregat, A.; Font, J. Enhanced Degradation of Phenol by a Fenton-Like System (Fe/EDTA/H2O2) at Circumneutral pH. Catalysts 2019, 9, 474. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, T.; Zhao, J.; Xu, B. Interactive Enhancements of Ascorbic Acid and Iron in Hydroxyl Radical Generation in Quinone Redox Cycling. Environ. Sci. Technol. 2012, 46, 10302–10309. [Google Scholar] [CrossRef]
- Nakayama, T.; Ichiba, M.; Kuwabara, M.; Kujiya, K.; Kumazawa, S. Mechanisms and structural specificity of hydrogen peroxide formation during oxidation of catechins. Food Sci. Technol. Res. 2002, 8, 261–267. [Google Scholar] [CrossRef]
- Fernandez, M.; Mira, M.; Florêncio, M.; Jennings, K.R. Iron and copper chelation by flavonoids: An electrospray mass spectrometry study. J. Inorg. Biochem. 2002, 92, 105–111. [Google Scholar] [CrossRef]
- Kejík, Z.; Kaplánek, R.; Masařík, M.; Babula, P.; Matkowski, A.; Filipenský, P.; Veselá, K.; Gburek, J.; Sýkora, D.; Martásek, P.; et al. Iron Complexes of Flavonoids-Antioxidant Capacity and Beyond. Int. J. Mol. Sci. 2021, 22, 646. [Google Scholar] [CrossRef]
- Hider, R.C.; Liu, Z.D.; Khodr, H.H. Metal chelation of polyphenols. Meth. Enzymol. 2001, 335, 190–203. [Google Scholar]
- Kim, J.Y.; Yi, B.; Lee, C.; Gim, S.-Y.; Kim, M.-J.; Lee, J. Effects of pH on the rates of lipid oxidation in oil–water system. Appl. Biol. Chem. 2016, 59, 157–161. [Google Scholar] [CrossRef]
- Brudzynski, K.; Sjaarda, C. Colloidal structure of honey and its influence on antibacterial activity. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2063–2080. [Google Scholar] [CrossRef] [PubMed]
- Kiokias, S.; Oreopoulou, V. Review on the Antioxidant Activity of Phenolics in o/w Emulsions along with the Impact of a Few Important Factors on Their Interfacial Behaviour. Colloids Interfaces 2022, 6, 79. [Google Scholar] [CrossRef]
- Pierpoint, W.S. The enzymic oxidation of chlorogenic acid and some reactions of the quinone produced. Biochem. J. 1966, 98, 567–580. [Google Scholar] [CrossRef]
- Charlton, A.J.; Baxter, N.J.; Lokman Khan, M.; Moir, A.J.G.; Haslam, E.; Davies, A.P.; Williamson, M.P. Polyphenol/peptide binding and precipitation. J. Agric. Food Chem. 2002, 50, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Lund, M. Reactions of plant polyphenols in foods: Impact of molecular structure. Trends Food Sci. Technol. 2021, 112, 241–251. [Google Scholar] [CrossRef]
- Cilliers, J.J.L.; Singleton, V.L. Characterization of the products of nonenzymic autoxidative phenolic reactions in a caffeic acid model system. J. Agric. Food Chem. 1991, 39, 1298–1303. [Google Scholar] [CrossRef]
- Hotta, H.; Sakamoto, H.; Nagano, S.; Osakai, T.; Tsujino, Y. Unusually large numbers of electrons for the oxidation of polyphenolic antioxidants. Biochim. Biophys. Acta 2001, 1526, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Bors, W.; Michel, C.; Stettmaier, K.; Lu, Y.; Foo, Y. Antioxidant Mechanisms of Polyphenolic Caffeic Acid Oligomers, Constituents of Salvia officinalis. Biol. Res. 2004, 37, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K.; Maldonado-Alvarez, L. Polyphenol-protein complexes and their consequences for the redox activity, structure and function of honey. A current view and new hypotheses—A Review. Pol. J. Food Nutr. Sci. 2015, 65, 71–80. [Google Scholar] [CrossRef]
- Rufián-Henares, J.A.; de la Cueva, S.P. Antimicrobial Activity of Coffee Melanoidins. A Study of Their Metal-Chelating Properties. J. Agric. Food Chem. 2009, 57, 432–438. [Google Scholar] [CrossRef] [PubMed]
| Honey Sample. | Plant Source | Color * (A560–720 nm) | Total Phenolic * (ug GAE/g of Honey) | ORAC * (uM TE/g of Honey) | H2O2 Production ** (Fluorescence) |
|---|---|---|---|---|---|
| Dark | |||||
| H77 | Buckwheat | 1.27 | 513.25 | 19.13 | 1319.3 |
| H76 | Buckwheat | 0.72 | 318.74 | 19.77 | 1208 |
| H226 | Buckwheat | 0.62 | 362.86 | 15.45 | 1152 |
| H23 | Buckwheat | 1.03 | 410 | 15.28 | 1210 |
| H149 | Buckwheat | 0.88 | 408.03 | 12.75 | 1470 |
| Medium | |||||
| H221 | Buckwheat (light) | 0.38 | 144 | 5.31 | 886 |
| H208 | Buckwheat | 0.37 | 143.55 | 4.86 | 680 |
| H11 | Wildflower/clover | 0.13 | 106.97 | 5.37 | 1280 |
| Light | |||||
| H210 | Wildflower | 0.3 | 120.2 | 4.74 | 560 |
| H20 | Sweet clover/buckwheat | 0.24 | 83.75 | 4.49 | 1220 |
| 114 | Sunflower | 0.12 | 80.82 | 2.75 | 424 |
| H62 | Borage | 0.05 | 66.45 | 2.84 | 529 |
| Honey Color | TPC | TFC | MRP | Antioxidant | |||||
|---|---|---|---|---|---|---|---|---|---|
| ABTS | FRAP | DPPH | ORAC | ||||||
| Honey color | Average | 1.00 | 0.89 | 0.93 | 0.98 | 0.90 | 0.86 | 0.83 | |
| Range | 0.68–0.99 | 0.89–0.92 | 0.84–0.88 | 0.73–0.93 | |||||
| TPC | Average | 0.88 | 1.00 | 0.86 | 0.95 | 0.97 | 0.89 | 0.86 | |
| Range | 0.68–0.99 | 0.71–0.98 | 0.72–0.96 | 0.87–0.92 | |||||
| TFC | Average | 0.87 | 0.97 | 1.00 | |||||
| Range | 0.77–0.98 | 0.73–0.98 | |||||||
| MRP | Average | 0.98 | 0.95 | 1.00 | 0.94 | ||||
| Range | |||||||||
| Anti- oxidant: ABTS | Average | 0.85 | 1.00 | ||||||
| Range | 0.72–0.96 | ||||||||
| Anti- oxidant: FRAP | Average | 0.92 | 0.91 | 0.80 | 1.00 | ||||
| Range | 0.95–0.88 | 0.72–0.89 | |||||||
| Anti- oxidant: PPH | Average | 0.88 | 0.90 | 0.89 | 1.00 | ||||
| Range | 0.89–0.92 | ||||||||
| Anti- oxidant: ORAC | Average | 0.83 | 0.86 | 0.94 | 0.82 | 1.00 | |||
| Range | 0.93–0.73 | 0.86–0.87 | |||||||
| Variable | Color | Total Phenolic | ORAC | H2O2 Production |
|---|---|---|---|---|
| Color | 1.000 | 0.9721 p < 0.0001 | 0.8892 p < 0.0001 | 0.6512 p = 0.0218 |
| Total phenolic | 0.9721 p < 0.0001 | 1.000 | 0.9259 p < 0.0001 | 0.6826 p = 0.0144 |
| ORAC | 0.8892 p < 0.0001 | 0.9259 p < 0.0001 | 1.000 | 0.6512 |
| H2O2 production | 0.6512 p = 0.0218 | 0.6826 p = 0.0144 | 0.6823 p = 0.0145 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brudzynski, K. Unexpected Value of Honey Color for Prediction of a Non-Enzymatic H2O2 Production and Honey Antibacterial Activity: A Perspective. Metabolites 2023, 13, 526. https://doi.org/10.3390/metabo13040526
Brudzynski K. Unexpected Value of Honey Color for Prediction of a Non-Enzymatic H2O2 Production and Honey Antibacterial Activity: A Perspective. Metabolites. 2023; 13(4):526. https://doi.org/10.3390/metabo13040526
Chicago/Turabian StyleBrudzynski, Katrina. 2023. "Unexpected Value of Honey Color for Prediction of a Non-Enzymatic H2O2 Production and Honey Antibacterial Activity: A Perspective" Metabolites 13, no. 4: 526. https://doi.org/10.3390/metabo13040526
APA StyleBrudzynski, K. (2023). Unexpected Value of Honey Color for Prediction of a Non-Enzymatic H2O2 Production and Honey Antibacterial Activity: A Perspective. Metabolites, 13(4), 526. https://doi.org/10.3390/metabo13040526









