Biochar-Mediated Control of Metabolites and Other Physiological Responses in Water-Stressed Leptocohloa fusca
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Setup
2.2. Soil Preparation and Analysis
2.3. Morphological Characteristics
2.4. Nutrient Uptake
2.5. Physiological Parameters
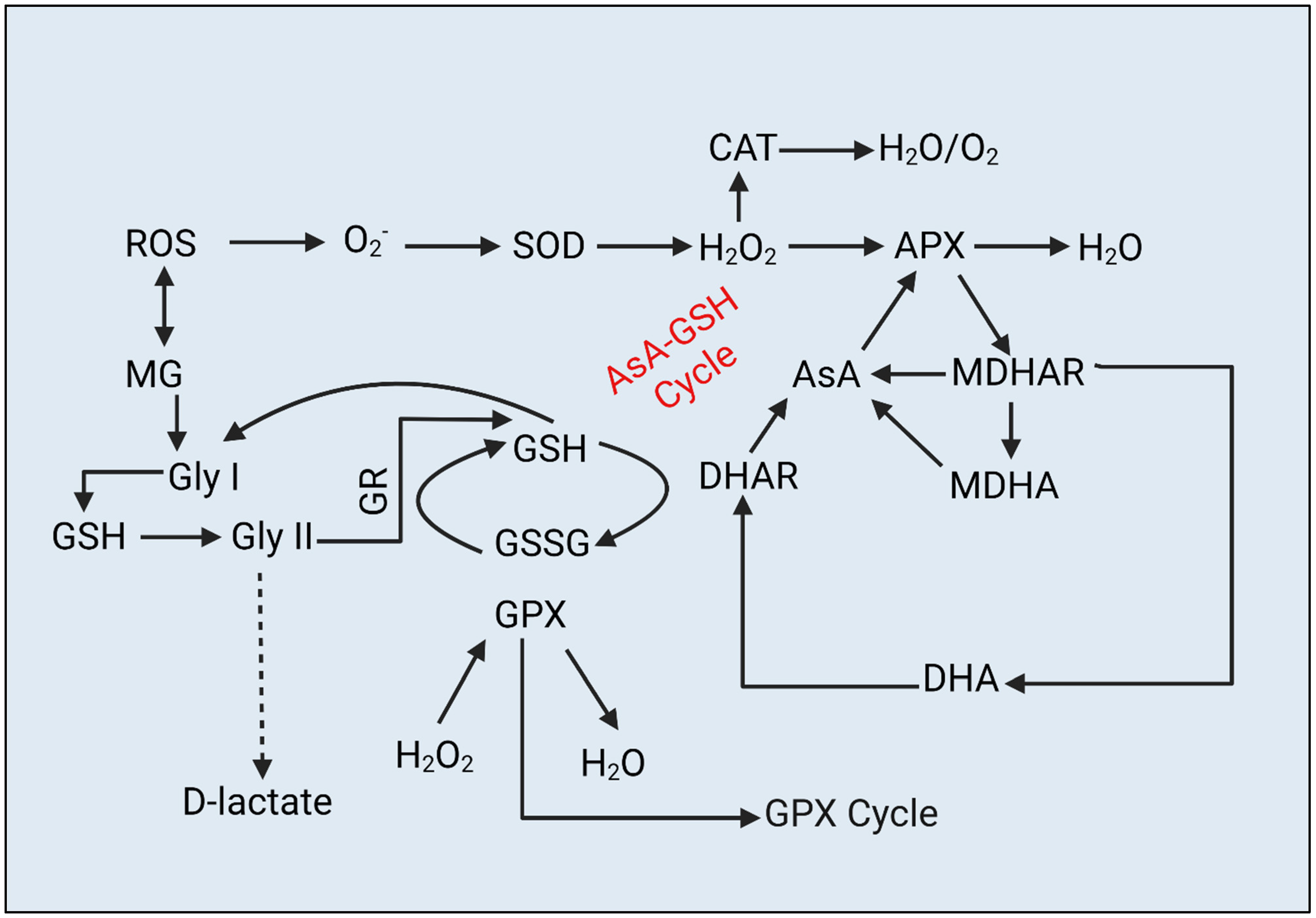
2.6. ROS and MG Content
2.7. Antioxidant Activity
2.8. Metabolite Extraction
2.9. Statistical Analysis
3. Results
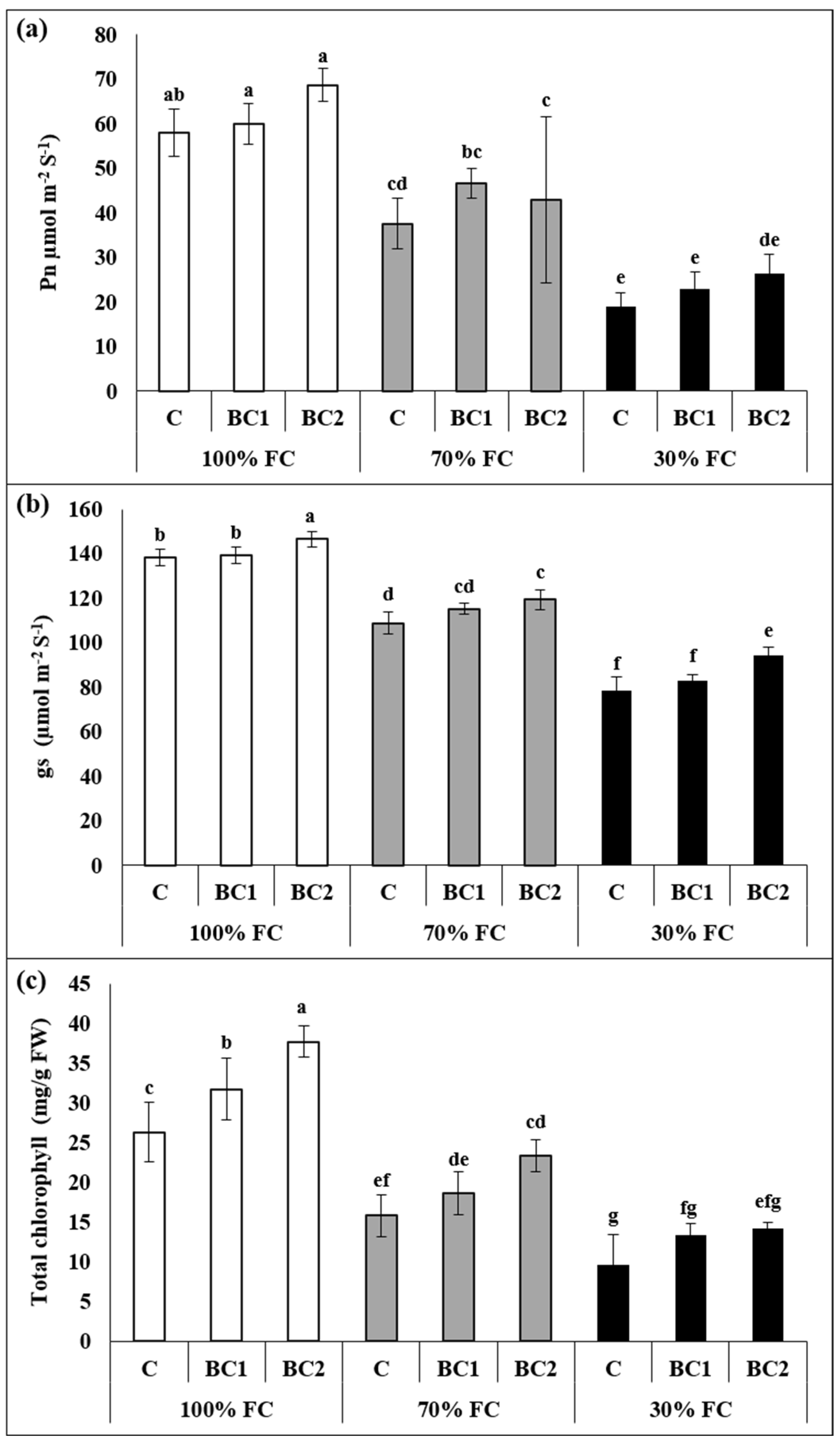
3.1. Interactive Effect of Drought and Biochar on Morphological and Physiological Attributes of L. fusca
3.2. Alleviation of Oxidative-Stress Biomarkers by Biochar under Drought Stress
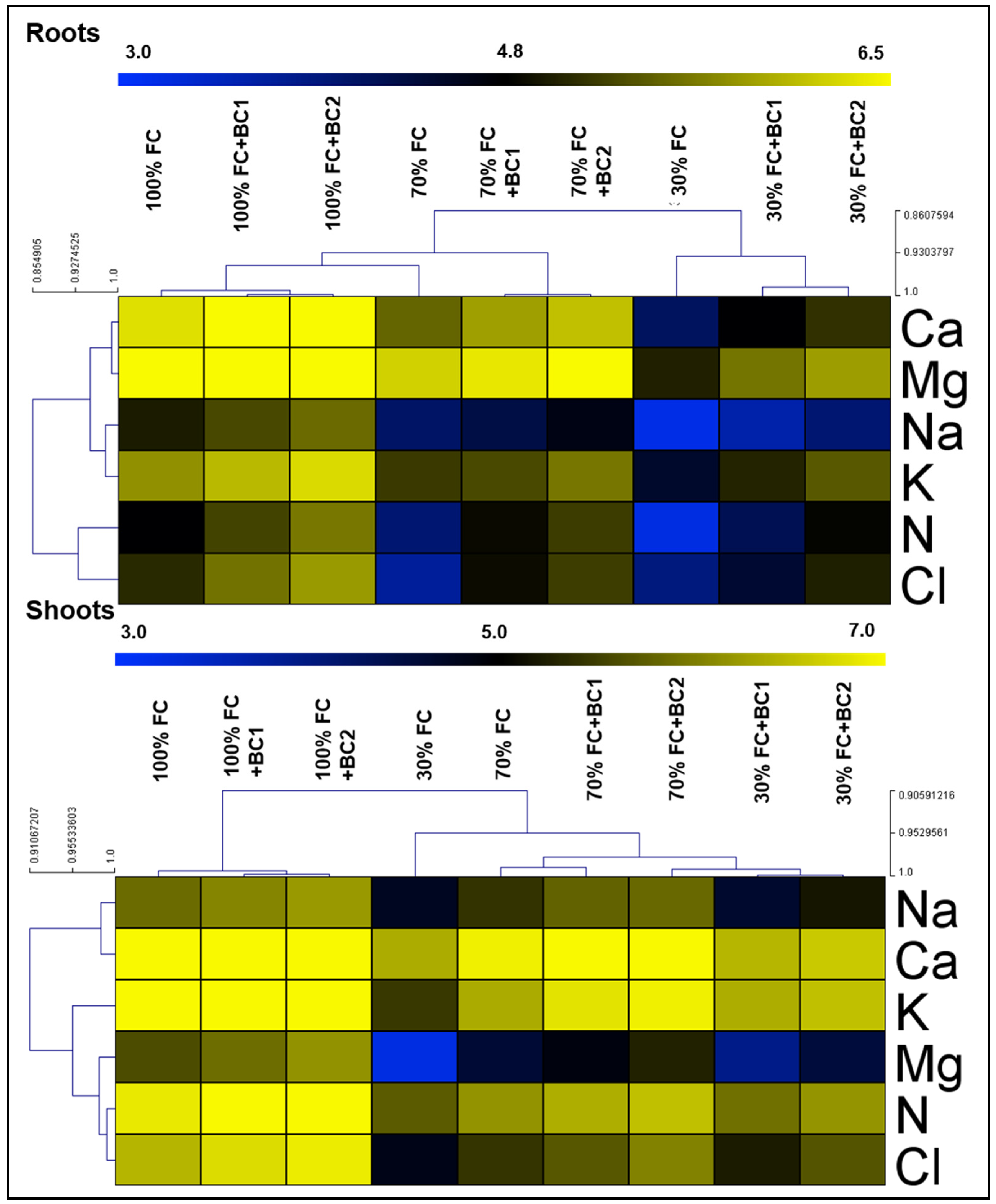
3.3. Enhancement in Nutrient Uptake by Biochar under Drought Stress
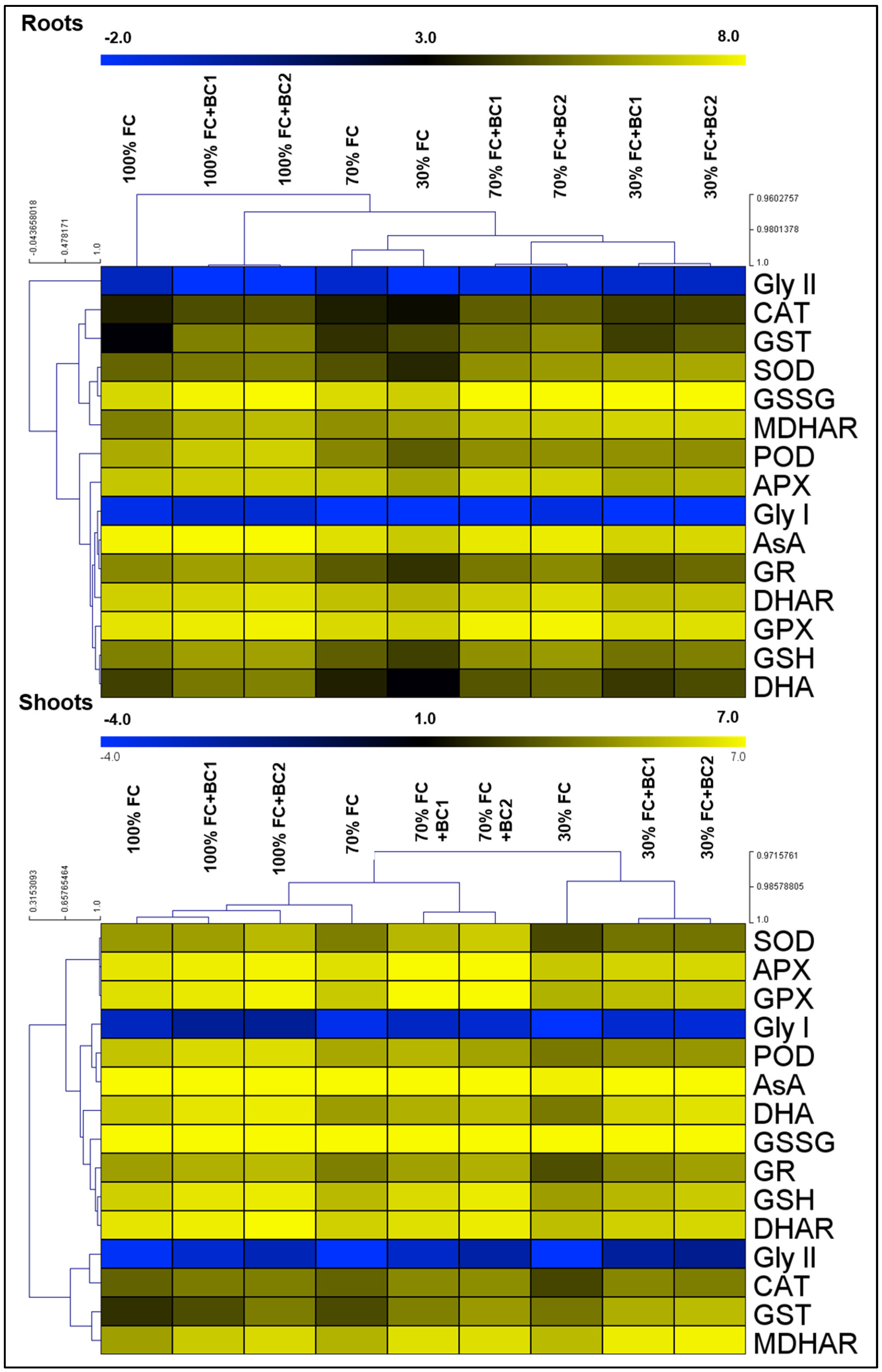
3.4. Biochar-Dependent Changes in Antioxidant Activities under Drought Stress
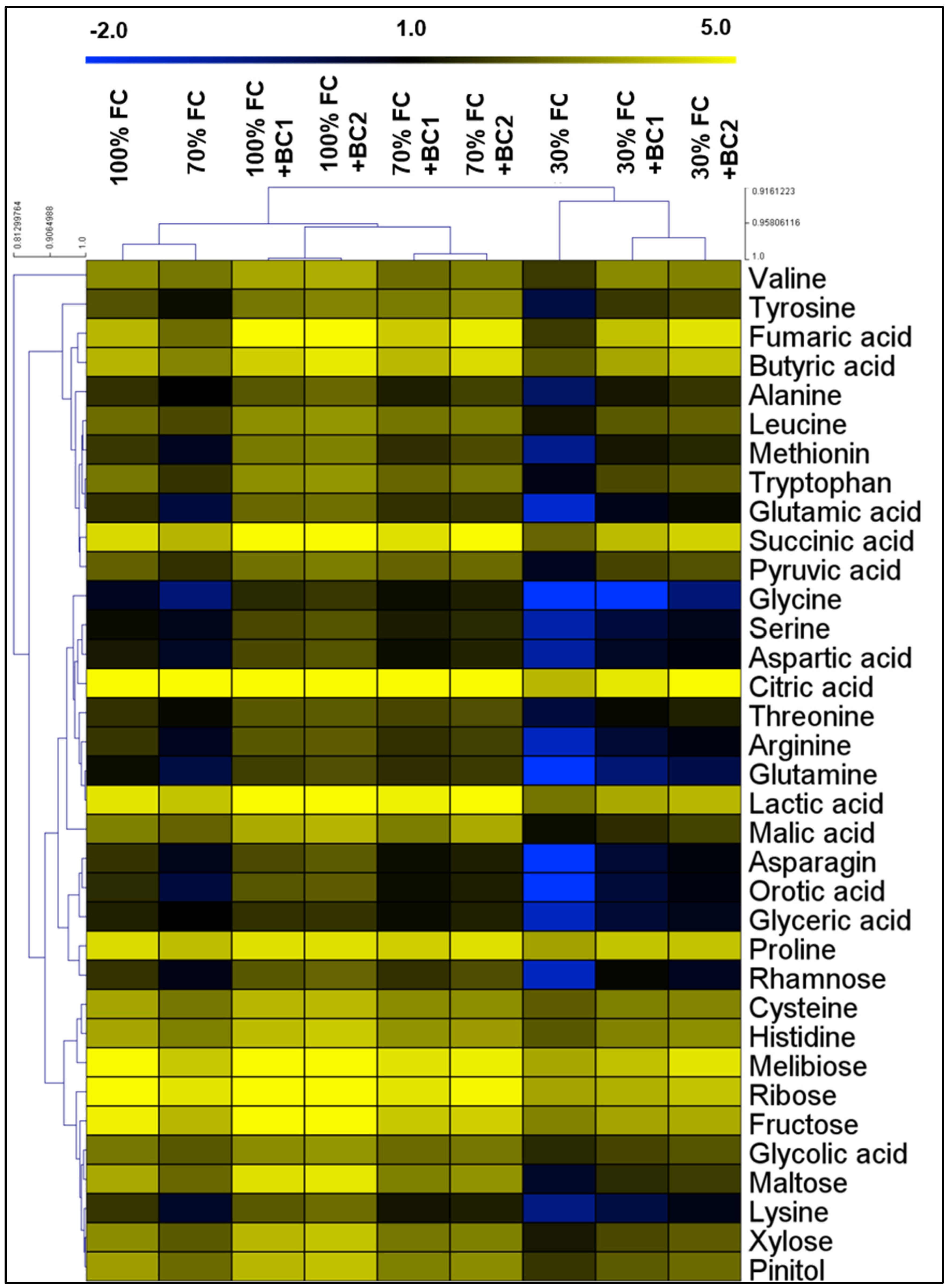
3.5. Modifications in Metabolite Levels by Biochar under Drought Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Ali, S.; Zandi, P.; Mehmood, A.; Ullah, S.; Ikram, M.; Ismail, I.; Shahid, M.A.; Babar, M.A. Role of Sugars, Amino Acids and Organic Acids in Improving Plant Abiotic Stress Tolerance. Pak. J. Bot. 2020, 52, 355–363. [Google Scholar] [CrossRef]
- Asghar, M.A.; Ahmad, B.; Raza, A.; Adil, B.; Hassan Javed, H.; Farooq, M.U.; Ghafoor, A.; Iftikhar Hussain, M.; Shafiq, I.; Karim, H.; et al. Shade and Microbes Enhance Drought Stress Tolerance in Plants by Inducing Phytohormones at Molecular Levels: A Review. J. Plant Ecol. 2022, 15, 1107–1117. [Google Scholar] [CrossRef]
- Asghar, M.A.; Li, Y.; Jiang, H.; Sun, X.; Ahmad, B.; Imran, S.; Yu, L.; Liu, C.; Yang, W.; Du, J. Crosstalk between Abscisic Acid and Auxin under Osmotic Stress. Agron. J. 2019, 111, 2157–2162. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Castro, B.; Citterico, M.; Kimura, S.; Stevens, D.M.; Wrzaczek, M.; Coaker, G. Stress-Induced Reactive Oxygen Species Compartmentalization, Perception and Signalling. Nat. Plants 2021, 7, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Hatami, M.; Hadian, J.; Ghorbanpour, M. Mechanisms Underlying Toxicity and Stimulatory Role of Single-Walled Carbon Nanotubes in Hyoscyamus Niger during Drought Stress Simulated by Polyethylene Glycol. J. Hazard. Mater. 2017, 324, 306–320. [Google Scholar] [CrossRef]
- Verma, G.; Srivastava, D.; Tiwari, P.; Chakrabarty, D. ROS Modulation in Crop Plants under Drought Stress. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 311–336. [Google Scholar]
- Hussain, S.; Rao, M.J.; Anjum, M.A.; Ejaz, S.; Zakir, I.; Ali, M.A.; Ahmad, N.; Ahmad, S. Oxidative Stress and Antioxidant Defense in Plants under Drought Conditions. In Plant Abiotic Stress Tolerance; Springer: Berlin, Germany, 2019; pp. 207–219. [Google Scholar]
- Ramu, V.S.; Preethi, V.; Nisarga, K.N.; Srivastava, K.R.; Sheshshayee, M.S.; Mysore, K.S.; Udayakumar, M. Carbonyl Cytotoxicity Affects Plant Cellular Processes and Detoxifying Enzymes Scavenge These Compounds to Improve Stress Tolerance. J. Agric. Food Chem. 2020, 68, 6237–6247. [Google Scholar] [CrossRef]
- Yalcinkaya, T.; Uzilday, B.; Ozgur, R.; Turkan, I.; Mano, J. Lipid Peroxidation-Derived Reactive Carbonyl Species (RCS): Their Interaction with ROS and Cellular Redox during Environmental Stresses. Environ. Exp. Bot. 2019, 165, 139–149. [Google Scholar] [CrossRef]
- Mano, J.; Biswas, M.; Sugimoto, K. Reactive Carbonyl Species: A Missing Link in ROS Signaling. Plants 2019, 8, 391. [Google Scholar] [CrossRef]
- Muhammad, A.A.; Jiang, H.; Shui, Z.; Cao, X.; Huang, X.; Imran, S.; Ahmad, B.; Zhang, H.; Yang, Y.; Shang, J.; et al. Interactive Effect of Shade and PEG-Induced Osmotic Stress on Physiological Responses of Soybean Seedlings. J. Integr. Agric. 2021, 20, 2382–2394. [Google Scholar] [CrossRef]
- Monsur, M.B.; Datta, J.; Rohman, M.; Hasanuzzaman, M.; Hossain, A.; Islam, M.S.; Bukhari, M.A.; Jabeen, T.; Mubeen, M.; Nasim, W.; et al. Saline Toxicity and Antioxidant Response in Oryza Sativa: An Updated Review. In Managing Plant Production Under Changing Environment; Hasanuzzaman, M., Ahammed, G.J., Nahar, K., Eds.; Springer: Singapore, 2022; pp. 79–102. ISBN 9789811650581. [Google Scholar]
- Ahmed, T.; Noman, M.; Manzoor, N.; Shahid, M.; Abdullah, M.; Ali, L.; Wang, G.; Hashem, A.; Al-Arjani, A.-B.F.; Alqarawi, A.A. Nanoparticle-Based Amelioration of Drought Stress and Cadmium Toxicity in Rice via Triggering the Stress Responsive Genetic Mechanisms and Nutrient Acquisition. Ecotoxicol. Environ. Saf. 2021, 209, 111829. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zheng, X.; Song, Y.; Zhu, L.; Yu, Z.; Gan, L.; Zhou, S.; Liu, H.; Wen, F.; Zhu, C. NtLTP4, a Lipid Transfer Protein That Enhances Salt and Drought Stresses Tolerance in Nicotiana Tabacum. Sci. Rep. 2018, 8, 8873. [Google Scholar] [CrossRef]
- Wang, L.; Feng, C.; Zheng, X.; Guo, Y.; Zhou, F.; Shan, D.; Liu, X.; Kong, J. Plant Mitochondria Synthesize Melatonin and Enhance the Tolerance of Plants to Drought Stress. J. Pineal Res. 2017, 63, e12429. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Exogenous Silicon Attenuates Cadmium-Induced Oxidative Stress in Brassica Napus L. by Modulating AsA-GSH Pathway and Glyoxalase System. Front. Plant Sci. 2017, 8, 1061. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in Plants: Biosynthesis and Physiological Role in Environmental Stress Tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef]
- Burnett, A.C.; Serbin, S.P.; Davidson, K.J.; Ely, K.S.; Rogers, A. Detection of the Metabolic Response to Drought Stress Using Hyperspectral Reflectance. J. Exp. Bot. 2021, 72, 6474–6489. [Google Scholar] [CrossRef]
- Shanker, A.K.; Maheswari, M.; Yadav, S.K.; Desai, S.; Bhanu, D.; Attal, N.B.; Venkateswarlu, B. Drought Stress Responses in Crops. Funct. Integr. Genom. 2014, 14, 11–22. [Google Scholar] [CrossRef]
- de Oliveira Sousa, A.R.; de Andrade Silva, E.M.; Coelho Filho, M.A.; Costa, M.G.C.; dos Santos Soares Filho, W.; Micheli, F.; Maserti, B.; da Silva Gesteira, A. Metabolic Responses to Drought Stress and Rehydration in Leaves and Roots of Three Citrus Scion/Rootstock Combinations. Sci. Hortic. 2022, 292, 110490. [Google Scholar] [CrossRef]
- Guo, R.; Shi, L.; Jiao, Y.; Li, M.; Zhong, X.; Gu, F.; Liu, Q.; Xia, X.; Li, H. Metabolic Responses to Drought Stress in the Tissues of Drought-Tolerant and Drought-Sensitive Wheat Genotype Seedlings. AoB Plants 2018, 10, ply016. [Google Scholar] [CrossRef]
- Fàbregas, N.; Fernie, A.R. The Metabolic Response to Drought. J. Exp. Bot. 2019, 70, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Wang, B.; Sun, H.; Gao, S.; Li, H. H2S Induces NO in the Regulation of AsA-GSH Cycle in Wheat Seedlings by Water Stress. Protoplasma 2020, 257, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Asghar, M.A.; Du, J.; Jiang, H.; Li, Y.; Sun, X.; Shang, J.; Liu, J.; Liu, W.; Imran, S.; Iqbal, N.; et al. Shade Pretreatment Enhanced Drought Resistance of Soybean. Environ. Exp. Bot. 2020, 171, 103952. [Google Scholar] [CrossRef]
- Kaya, C. Nitrate Reductase Is Required for Salicylic Acid-Induced Water Stress Tolerance of Pepper by Upraising the AsA-GSH Pathway and Glyoxalase System. Physiol. Plant. 2021, 172, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Zhang, S.; Ou, X. The Roles of H2S and H2O2 in Regulating AsA-GSH Cycle in the Leaves of Wheat Seedlings under Drought Stress. Protoplasma 2018, 255, 1257–1262. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhu, H.; Zhu, H.; Tao, Y.; Liu, C.; Liu, J.; Yang, F.; Li, M. Exogenous ABA Enhances the Antioxidant Defense System of Maize by Regulating the AsA-GSH Cycle under Drought Stress. Sustainability 2022, 14, 3071. [Google Scholar] [CrossRef]
- Ortega, H.E.; Torres-Mendoza, D.; Cubilla-Rios, L. Patents on Endophytic Fungi for Agriculture and Bio- and Phytoremediation Applications. Microorganisms 2020, 8, 1237. [Google Scholar] [CrossRef]
- Silambarasan, S.; Logeswari, P.; Vangnai, A.S.; Kamaraj, B.; Cornejo, P. Plant Growth-Promoting Actinobacterial Inoculant Assisted Phytoremediation Increases Cadmium Uptake in Sorghum Bicolor under Drought and Heat Stresses. Environ. Pollut. 2022, 307, 119489. [Google Scholar] [CrossRef]
- Imran, S.; Sarker, P.; Hoque, N.; Paul, N.C.; Mahamud, A.; Chakrobortty, J.; Tahjib-Ul-Arif, M.; Latef, A.A.H.A.; Hasanuzzaman, M.; Rhaman, M.S. Biochar Actions for the Mitigation of Plant Abiotic Stress. Crop. Pasture Sci. 2022, 74, 6–20. [Google Scholar] [CrossRef]
- Mansoor, S.; Kour, N.; Manhas, S.; Zahid, S.; Wani, O.A.; Sharma, V.; Wijaya, L.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A. Biochar as a Tool for Effective Management of Drought and Heavy Metal Toxicity. Chemosphere 2021, 271, 129458. [Google Scholar] [CrossRef]
- Kang, X.; Geng, N.; Li, X.; Yu, J.; Wang, H.; Pan, H.; Yang, Q.; Zhuge, Y.; Lou, Y. Biochar Alleviates Phytotoxicity by Minimizing Bioavailability and Oxidative Stress in Foxtail Millet (Setaria italica L.) Cultivated in Cd-and Zn-Contaminated Soil. Front. Plant Sci. 2022, 13, 782963. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, M.; Bouček, J.; Bímová, K.B.; Kraus, K.; Haisel, D.; Kulhánek, M.; Omara-Ojungu, C.; Seyedsadr, S.; Beesley, L.; Soudek, P.; et al. Biochar in Manure Can Suppress Water Stress of Sugar Beet (Beta vulgaris) and Increase Sucrose Content in Tubers. Sci. Total Environ. 2022, 814, 152772. [Google Scholar] [CrossRef] [PubMed]
- Tayyab, M.; Islam, W.; Khalil, F.; Ziqin, P.; Caifang, Z.; Arafat, Y.; Hui, L.; Rizwan, M.; Ahmad, K.; Waheed, S. Biochar: An Efficient Way to Manage Low Water Availability in Plants. Appl. Ecol. Environ. Res. 2018, 16, 2565–2583. [Google Scholar] [CrossRef]
- Ola, H.A.E.; Reham, E.F.; Eisa, S.S.; Habib, S.A. Morpho-Anatomical Changes in Salt Stressed Kallar Grass (Leptochloa fusca L. Kunth). Res. J. Agric. Biol. Sci. 2012, 8, 158–166. [Google Scholar]
- Rahman, A.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Calcium Supplementation Improves Na+/K+ Ratio, Antioxidant Defense and Glyoxalase Systems in Salt-Stressed Rice Seedlings. Front. Plant Sci. 2016, 7, 609. [Google Scholar] [CrossRef]
- Parvin, K.; Nahar, K.; Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Mohsin, S.M.; Fujita, M. Exogenous Vanillic Acid Enhances Salt Tolerance of Tomato: Insight into Plant Antioxidant Defense and Glyoxalase Systems. Plant Physiol. Biochem. 2020, 150, 109–120. [Google Scholar] [CrossRef]
- Bartoli, C.G.; Simontacchi, M.; Tambussi, E.; Beltrano, J.; Montaldi, E.; Puntarulo, S. Drought and Watering-Dependent Oxidative Stress: Effect on Antioxidant Content in Triticum aestivum L. Leaves. J. Exp. Bot. 1999, 50, 375–383. [Google Scholar] [CrossRef]
- Porra, R.J. The Chequered History of the Development and Use of Simultaneous Equations for the Accurate Determination of Chlorophylls a and b. Photosynth. Res. 2002, 73, 149–156. [Google Scholar] [CrossRef]
- Okuda, T.; Matsuda, Y.; Yamanaka, A.; Sagisaka, S. Abrupt Increase in the Level of Hydrogen Peroxide in Leaves of Winter Wheat Is Caused by Cold Treatment. Plant Physiol. 1991, 97, 1265–1267. [Google Scholar] [CrossRef]
- Aruoma, O.I. Deoxyribose Assay for Detecting Hydroxyl Radicals. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1994; Volume 233, pp. 57–66. [Google Scholar]
- Benov, L.; Sztejnberg, L.; Fridovich, I. Critical Evaluation of the Use of Hydroethidine as a Measure of Superoxide Anion Radical. Free Radic. Biol. Med. 1998, 25, 826–831. [Google Scholar] [CrossRef]
- Matsui, K.; Sugimoto, K.; Kakumyan, P.; Khorobrykh, S.A.; Mano, J. Volatile Oxylipins and Related Compounds Formed under Stress in Plants. In Lipidomics; Springer: Berlin, Germany, 2009; pp. 17–28. [Google Scholar]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Maehly, A.C. Assay of Catalases and Peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Marshall, M.J.; Worsfold, M. Superoxide Dismutase: A Direct, Continuous Linear Assay Using the Oxygen Electrode. Anal. Biochem. 1978, 86, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. Radical Production and Scavenging in the Chloroplasts. In Photosynthesis and the Environment; Springer: Berlin, Germany, 1996; pp. 123–150. [Google Scholar]
- Flohé, L.; Günzler, W.A. Assays of Glutathione Peroxidase. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 114–120. [Google Scholar]
- Romero-Puertas, M.C.; Corpas, F.J.; Sandalio, L.M.; Leterrier, M.; Rodriguez-Serrano, M.; Rio, L.A.; Palma, J.M. Glutathione Reductase from Pea Leaves: Response to Abiotic Stress and Characterization of the Peroxisomal Isozyme. New Phytol. 2006, 170, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Nitric Oxide Modulates Antioxidant Defense and the Methylglyoxal Detoxification System and Reduces Salinity-Induced Damage of Wheat Seedlings. Plant Biotechnol. Rep. 2011, 5, 353–365. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Suzuki, T.; Fujita, M. Polyamine and Nitric Oxide Crosstalk: Antagonistic Effects on Cadmium Toxicity in Mung Bean Plants through Upregulating the Metal Detoxification, Antioxidant Defense and Methylglyoxal Detoxification Systems. Ecotoxicol. Environ. Saf. 2016, 126, 245–255. [Google Scholar] [CrossRef]
- Principato, G.B.; Rosi, G.; Talesa, V.; Giovanni, E.; Uotila, L. Purification and Characterization of Two Forms of Glyoxalase II from the Liver and Brain of Wistar Rats. Biochim. Et Biophys. Acta (BBA)—Protein Struct. Mol. Enzymol. 1987, 911, 349–355. [Google Scholar] [CrossRef]
- Smith, I.K. Stimulation of Glutathione Synthesis in Photorespiring Plants by Catalase Inhibitors. Plant Physiol. 1985, 79, 1044–1047. [Google Scholar] [CrossRef]
- Müller, J.; Gödde, V.; Niehaus, K.; Zörb, C. Metabolic Adaptations of White Lupin Roots and Shoots under Phosphorus Deficiency. Front. Plant Sci. 2015, 6, 1014. [Google Scholar] [CrossRef]
- Kopka, J.; Schauer, N.; Krueger, S.; Birkemeyer, C.; Usadel, B.; Bergmuller, E.; Dormann, P.; Weckwerth, W.; Gibon, Y.; Stitt, M.; et al. GMD@CSB.DB: The Golm Metabolome Database. Bioinformatics 2005, 21, 1635–1638. [Google Scholar] [CrossRef]
- Fiehn, O.; Kopka, J.; Dörmann, P.; Altmann, T.; Trethewey, R.N.; Willmitzer, L. Metabolite Profiling for Plant Functional Genomics. Nat. Biotechnol. 2000, 18, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.C.; Nogueira, R.; da Silva, M.A.; de Albuquerque, M.B. Drought Stress and Plant Nutrition. Plant Stress 2011, 5, 32–41. [Google Scholar]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Salehi-Lisar, S.Y.; Bakhshayeshan-Agdam, H. Drought Stress in Plants: Causes, Consequences, and Tolerance. In Drought Stress Tolerance in Plants, Vol 1; Springer: Berlin, Germany, 2016; pp. 1–16. [Google Scholar]
- EL Sabagh, A.; Hossain, A.; Islam, M.; Barutcular, C.; Ratnasekera, D.; Gormus, O.; Amanet, K.; Mubeen, M.; Nasim, W.; Fahad, S. Drought and Heat Stress in Cotton (Gossypium hirsutum L.): Consequences and Their Possible Mitigation Strategies. Agron. Crops 2020, 3, 613–634. [Google Scholar]
- Shakeel, A.A.; Xiao-yu, X.; Long-chang, W.; Muhammad, F.S.; Chen, M.; Wang, L. Morphological, Physiological and Biochemical Responses of Plants to Drought Stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response Mechanism of Plants to Drought Stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Toscano, S.; Ferrante, A.; Romano, D. Response of Mediterranean Ornamental Plants to Drought Stress. Horticulturae 2019, 5, 6. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Farhangi-Abriz, S. Biochar Alleviates Fluoride Toxicity and Oxidative Stress in Safflower (Carthamus tinctorius L.) Seedlings. Chemosphere 2019, 223, 406–415. [Google Scholar] [CrossRef]
- Jones, D.L.; Rousk, J.; Edwards-Jones, G.; DeLuca, T.H.; Murphy, D.V. Biochar-Mediated Changes in Soil Quality and Plant Growth in a Three Year Field Trial. Soil Biol. Biochem. 2012, 45, 113–124. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Parvin, K.; Bardhan, K.; Nahar, K.; Anee, T.I.; Masud, A.A.C.; Fotopoulos, V. Biostimulants for the Regulation of Reactive Oxygen Species Metabolism in Plants under Abiotic Stress. Cells 2021, 10, 2537. [Google Scholar] [CrossRef]
- Saleem, K.; Asghar, M.A.; Saleem, M.H.; Raza, A.; Kocsy, G.; Iqbal, N.; Ali, B.; Albeshr, M.F.; Bhat, E.A. Chrysotile-Asbestos-Induced Damage in Panicum Virgatum and Phleum Pretense Species and Its Alleviation by Organic-Soil Amendment. Sustainability 2022, 14, 10824. [Google Scholar] [CrossRef]
- Mansour, E.; Desoky, E.-S.M.; Ali, M.M.; Abdul-Hamid, M.I.; Ullah, H.; Attia, A.; Datta, A. Identifying Drought-Tolerant Genotypes of Faba Bean and Their Agro-Physiological Responses to Different Water Regimes in an Arid Mediterranean Environment. Agric. Water Manag. 2021, 247, 106754. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Han, A.R.; Han, A.; Kim, H.S. Responses to Drought Stress in Prunus Sargentii and Larix Kaempferi Seedlings Using Morphological and Physiological Parameters. For. Ecol. Manag. 2020, 465, 118099. [Google Scholar] [CrossRef]
- Haider, I.; Raza, M.A.S.; Iqbal, R.; Aslam, M.U.; Habib-ur-Rahman, M.; Raja, S.; Khan, M.T.; Aslam, M.M.; Waqas, M.; Ahmad, S. Potential Effects of Biochar Application on Mitigating the Drought Stress Implications on Wheat (Triticum aestivum L.) under Various Growth Stages. J. Saudi Chem. Soc. 2020, 24, 974–981. [Google Scholar] [CrossRef]
- Ali, L.; Manzoor, N.; Li, X.; Naveed, M.; Nadeem, S.M.; Waqas, M.R.; Khalid, M.; Abbas, A.; Ahmed, T.; Li, B.; et al. Impact of Corn Cob-Derived Biochar in Altering Soil Quality, Biochemical Status and Improving Maize Growth under Drought Stress. Agronomy 2021, 11, 2300. [Google Scholar] [CrossRef]
- Hussain, H.A.; Men, S.; Hussain, S.; Chen, Y.; Ali, S.; Zhang, S.; Zhang, K.; Li, Y.; Xu, Q.; Liao, C.; et al. Interactive Effects of Drought and Heat Stresses on Morpho-Physiological Attributes, Yield, Nutrient Uptake and Oxidative Status in Maize Hybrids. Sci. Rep. 2019, 9, 3890. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Schwarz, D.; Franken, P.; Colla, G. Effects of Drought on Nutrient Uptake and Assimilation in Vegetable Crops. In Plant Responses to Drought Stress; Springer: Berlin, Germany, 2012; pp. 171–195. [Google Scholar]
- Al-Karaki, G.N.; Al-Raddad, A. Effects of Arbuscular Mycorrhizal Fungi and Drought Stress on Growth and Nutrient Uptake of Two Wheat Genotypes Differing in Drought Resistance. Mycorrhiza 1997, 7, 83–88. [Google Scholar] [CrossRef]
- Bista, D.R.; Heckathorn, S.A.; Jayawardena, D.M.; Mishra, S.; Boldt, J.K. Effects of Drought on Nutrient Uptake and the Levels of Nutrient-Uptake Proteins in Roots of Drought-Sensitive and-Tolerant Grasses. Plants 2018, 7, 28. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; Belal, E.E.; Rady, M.O.; Abd El-Mageed, S.A.; Mansour, E.; Awad, M.F.; Semida, W.M. Acidified Biochar as a Soil Amendment to Drought Stressed (Vicia faba L.) Plants: Influences on Growth and Productivity, Nutrient Status, and Water Use Efficiency. Agronomy 2021, 11, 1290. [Google Scholar] [CrossRef]
- Atkinson, C.J. How Good Is the Evidence That Soil-Applied Biochar Improves Water-Holding Capacity? Soil Use Manag. 2018, 34, 177–186. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Rahman, M.; Ansary, M.U.; Keya, S.S.; Abdelrahman, M.; Miah, G.; Phan Tran, L.-S. Silicon in Mitigation of Abiotic Stress-Induced Oxidative Damage in Plants. Crit. Rev. Biotechnol. 2021, 41, 918–934. [Google Scholar] [CrossRef] [PubMed]
- Abideen, Z.; Waqif, H.; Munir, N.; El-Keblawy, A.; Hasnain, M.; Radicetti, E.; Mancinelli, R.; Nielsen, B.L.; Haider, G. Algal-Mediated Nanoparticles, Phycochar, and Biofertilizers for Mitigating Abiotic Stresses in Plants: A Review. Agronomy 2022, 12, 1788. [Google Scholar] [CrossRef]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Ashraful Alam, M.; Syed, M.A.; Hossain, J.; Sarkar, S.; Saha, S.; Bhadra, P. Consequences and Mitigation Strategies of Abiotic Stresses in Wheat (Triticum aestivum L.) under the Changing Climate. Agronomy 2021, 11, 241. [Google Scholar] [CrossRef]
- Singh, S.; Husen, A. Role of Nanomaterials in the Mitigation of Abiotic Stress in Plants. In Nanomaterials and Plant Potential; Springer: Berlin, Germany, 2019; pp. 441–471. [Google Scholar]
- Sultana, S.; Yamamoto, S.; Biswas, S.; Sakurai, C.; Isoai, H.; Mano, J. Histidine-Containing Dipeptides Mitigate Salt Stress in Plants by Scavenging Reactive Carbonyl Species. J. Agric. Food Chem. 2022, 70, 11169–11178. [Google Scholar] [CrossRef]
- Tao, Z.; Li, C.; Xu, X.; Pan, Y. Scavenging Activity and Mechanism Study of Ferulic Acid against Reactive Carbonyl Species Acrolein. J. Zhejiang Univ.-Sci. B 2019, 20, 868–876. [Google Scholar] [CrossRef]
- Mano, J.; Biswas, M.; Sugimoto, K.; Murata, Y. Determination of Reactive Carbonyl Species, Which Mediate Reactive Oxygen Species Signals in Plant Cells. In Reactive Oxygen Species in Plants; Springer: Berlin, Germany, 2022; pp. 201–213. [Google Scholar]
- Aziz, H.; Murtaza, G.; Saleem, M.H.; Ali, S.; Rizwan, M.; Riaz, U.; Niaz, A.; Abualreesh, M.H.; Alatawi, A. Alleviation of Chlorpyrifos Toxicity in Maize (Zea mays L.) by Reducing Its Uptake and Oxidative Stress in Response to Soil-Applied Compost and Biochar Amendments. Plants 2021, 10, 2170. [Google Scholar] [CrossRef]
- Kamran, M.; Malik, Z.; Parveen, A.; Zong, Y.; Abbasi, G.H.; Rafiq, M.T.; Shaaban, M.; Mustafa, A.; Bashir, S.; Rafay, M. Biochar Alleviates Cd Phytotoxicity by Minimizing Bioavailability and Oxidative Stress in Pak Choi (Brassica chinensis L.) Cultivated in Cd-Polluted Soil. J. Environ. Manag. 2019, 250, 109500. [Google Scholar] [CrossRef]
- Shi, Z.; Yan, J.; Ren, X.; Wen, M.; Zhao, Y.; Wang, C. Effects of Biochar and Thermally Treated Biochar on Eisenia Fetida Survival, Growth, Lysosomal Membrane Stability and Oxidative Stress. Sci. Total Environ. 2021, 770, 144778. [Google Scholar] [CrossRef]
- Demidchik, V. Mechanisms of Oxidative Stress in Plants: From Classical Chemistry to Cell Biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Chepsergon, J.; Mwamburi, L.; Kassim, M.K. Mechanism of Drought Tolerance in Plants Using Trichoderma spp. Int. J. Sci. Res. 2014, 3, 1592–1595. [Google Scholar]
- Sheng, Q.; Yi, L.; Zhong, B.; Wu, X.; Liu, L.; Zhang, B. Shikimic Acid Biosynthesis in Microorganisms: Current Status and Future Direction. Biotechnol. Adv. 2023, 62, 108073. [Google Scholar] [CrossRef]
- Ye, H.; Folz, J.; Li, C.; Zhang, Y.; Hou, Z.; Zhang, L.; Su, S. Response of Metabolic and Lipid Synthesis Gene Expression Changes in Camellia Oleifera to Mulched Ecological Mat under Drought Conditions. Sci. Total Environ. 2021, 795, 148856. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Bano, A.; Zandi, P. Effects of Exogenously Applied Plant Growth Regulators in Combination with PGPR on the Physiology and Root Growth of Chickpea (Cicer arietinum) and Their Role in Drought Tolerance. J. Plant Interact. 2018, 13, 239–247. [Google Scholar] [CrossRef]
- Suguiyama, V.F.; Silva, E.A.; Meirelles, S.T.; Centeno, D.C.; Braga, M.R. Leaf Metabolite Profile of the Brazilian Resurrection Plant Barbacenia Purpurea Hook. (Velloziaceae) Shows Two Time-Dependent Responses during Desiccation and Recovering. Front. Plant Sci. 2014, 5, 96. [Google Scholar] [CrossRef] [PubMed]
- Witt, S.; Galicia, L.; Lisec, J.; Cairns, J.; Tiessen, A.; Araus, J.L.; Palacios-Rojas, N.; Fernie, A.R. Metabolic and Phenotypic Responses of Greenhouse-Grown Maize Hybrids to Experimentally Controlled Drought Stress. Mol. Plant 2012, 5, 401–417. [Google Scholar] [CrossRef]
- Obata, T.; Witt, S.; Lisec, J.; Palacios-Rojas, N.; Florez-Sarasa, I.; Araus, J.L.; Cairns, J.E.; Yousfi, S.; Fernie, A.R. Metabolite Profiles of Maize Leaves in Drought, Heat and Combined Stress Field Trials Reveal the Relationship between Metabolism and Grain Yield. Plant Physiol. 2015, 169, 2665–2683. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Rahman, M.A.; Rathinasabapathi, B.; Babar, M.A. UPLC-HRMS-Based Untargeted Metabolic Profiling Reveals Changes in Chickpea (Cicer arietinum) Metabolome Following Long-Term Drought Stress. Plant Cell Environ. 2019, 42, 115–132. [Google Scholar] [CrossRef]
- Furukawa, Y.; Chikaraishi, Y.; Ohkouchi, N.; Ogawa, N.O.; Glavin, D.P.; Dworkin, J.P.; Abe, C.; Nakamura, T. Extraterrestrial Ribose and Other Sugars in Primitive Meteorites. Proc. Natl. Acad. Sci. USA 2019, 116, 24440–24445. [Google Scholar] [CrossRef]
- Ajisaka, K.; Agawa, S.; Nagumo, S.; Kurato, K.; Yokoyama, T.; Arai, K.; Miyazaki, T. Evaluation and Comparison of the Antioxidative Potency of Various Carbohydrates Using Different Methods. J. Agric. Food Chem. 2009, 57, 3102–3107. [Google Scholar] [CrossRef]
- Morelli, R.; Russo-Volpe, S.; Bruno, N.; Lo Scalzo, R. Fenton-Dependent Damage to Carbohydrates: Free Radical Scavenging Activity of Some Simple Sugars. J. Agric. Food Chem. 2003, 51, 7418–7425. [Google Scholar] [CrossRef]
- Xie, H.; Bai, G.; Lu, P.; Li, H.; Fei, M.; Xiao, B.-G.; Chen, X.-J.; Tong, Z.-J.; Wang, Z.-Y.; Yang, D.-H. Exogenous Citric Acid Enhances Drought Tolerance in Tobacco (Nicotiana tabacum). Plant Biol. J. 2022, 24, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Ullah, N.; Ditta, A.; Imtiaz, M.; Li, X.; Jan, A.U.; Mehmood, S.; Rizwan, M.S.; Rizwan, M. Appraisal for Organic Amendments and Plant Growth-promoting Rhizobacteria to Enhance Crop Productivity under Drought Stress: A Review. J. Agron. Crop. Sci. 2021, 207, 783–802. [Google Scholar] [CrossRef]
- Gao, Q.-H.; Wu, C.-S.; Wang, M.; Xu, B.-N.; Du, L.-J. Effect of Drying of Jujubes (Ziziphus jujuba Mill.) on the Contents of Sugars, Organic Acids, α-Tocopherol, β-Carotene, and Phenolic Compounds. J. Agric. Food Chem. 2012, 60, 9642–9648. [Google Scholar] [CrossRef] [PubMed]
- Colinet, H.; Larvor, V.; Laparie, M.; Renault, D. Exploring the Plastic Response to Cold Acclimation through Metabolomics: Metabolomics of Cold Acclimation Response. Funct. Ecol. 2012, 26, 711–722. [Google Scholar] [CrossRef]
- Charlton, A.J.; Donarski, J.A.; Harrison, M.; Jones, S.A.; Godward, J.; Oehlschlager, S.; Arques, J.L.; Ambrose, M.; Chinoy, C.; Mullineaux, P.M. Responses of the Pea (Pisum sativum L.) Leaf Metabolome to Drought Stress Assessed by Nuclear Magnetic Resonance Spectroscopy. Metabolomics 2008, 4, 312–327. [Google Scholar] [CrossRef]
- Shewry, P.R.; Piironen, V.; Lampi, A.-M.; Edelmann, M.; Kariluoto, S.; Nurmi, T.; Fernandez-Orozco, R.; Ravel, C.; Charmet, G.; Andersson, A.A. The Healthgrain Wheat Diversity Screen: Effects of Genotype and Environment on Phytochemicals and Dietary Fiber Components. J. Agric. Food Chem. 2010, 58, 9291–9298. [Google Scholar] [CrossRef]
- Sun, C.; Wang, D.; Shen, X.; Li, C.; Liu, J.; Lan, T.; Wang, W.; Xie, H.; Zhang, Y. Effects of Biochar, Compost and Straw Input on Root Exudation of Maize (Zea mays L.): From Function to Morphology. Agric. Ecosyst. Environ. 2020, 297, 106952. [Google Scholar] [CrossRef]
- Sun, C.X.; Hao, L.; Wang, D.; Li, C.; Zhang, C.; Chen, X.; Fu, J.; Zhang, Y.L. Nitrogen Utilisation and Metabolism in Maize (Zea mays L.) Plants under Different Rates of Biochar Addition and Nitrogen Input Conditions. Plant Biol. 2019, 21, 882–890. [Google Scholar] [CrossRef]
- Haghighi, T.M.; Saharkhiz, M.J.; Kavoosi, G.; Jowkar, A. Monitoring Amino Acid Profile and Protein Quality of Licorice (Glycyrrhiza glabra L.) under Drought Stress, Silicon Nutrition and Mycorrhiza Inoculation. Sci. Hortic. 2022, 295, 110808. [Google Scholar] [CrossRef]


| Parameters | Garden Soil | Biochar |
|---|---|---|
| pH | 5.78 | 8.24 |
| EC (mS/cm) | 87.79 | 5.31 |
| Organic matter (mg/kg) | 54.93 | 135.47 |
| Total soluble solids (TSS) (mg/kg) | 49.65 | 178.95 |
| Total carbon (mg/kg) | 8.68 | 287.46 |
| Total nitrogen (mg/kg) | 7.94 | 9.56 |
| NaCl content (mg/kg) | 32.06 | 65.78 |
| Treatments | SFW (g) | RFW (g) | SDW (g) | RDW (g) |
|---|---|---|---|---|
| 100% FC | 43.2 4b | 12.16 a | 19.42 b | 5.16 c |
| 100% FC + BC1 | 46.51 b | 11.89 a | 21.61 b | 6.65 b |
| 100% FC + BC2 | 52.21 a | 12.49 a | 26.64 a | 7.77 a |
| 70% FC | 14.28 d | 6.97 b | 6.18 e | 2.93 ef |
| 70% FC + BC1 | 17.88 cd | 8.13 b | 8.97 cde | 4.21 cd |
| 70% FC + BC2 | 20.77 c | 8.84 b | 11.16 cd | 3.9 de |
| 30% FC | 8.84 e | 2.83 c | 1.81 f | 0.74 h |
| 30% FC + BC1 | 16.28 cd | 3.81 c | 8.27 de | 1.78 g |
| 30% FC + BC2 | 19.5 c | 2.9 c | 12.43 c | 2.21 fg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, K.; Asghar, M.A.; Raza, A.; Javed, H.H.; Farooq, T.H.; Ahmad, M.A.; Rahman, A.; Ullah, A.; Song, B.; Du, J.; et al. Biochar-Mediated Control of Metabolites and Other Physiological Responses in Water-Stressed Leptocohloa fusca. Metabolites 2023, 13, 511. https://doi.org/10.3390/metabo13040511
Saleem K, Asghar MA, Raza A, Javed HH, Farooq TH, Ahmad MA, Rahman A, Ullah A, Song B, Du J, et al. Biochar-Mediated Control of Metabolites and Other Physiological Responses in Water-Stressed Leptocohloa fusca. Metabolites. 2023; 13(4):511. https://doi.org/10.3390/metabo13040511
Chicago/Turabian StyleSaleem, Khansa, Muhammad Ahsan Asghar, Ali Raza, Hafiz Hassan Javed, Taimoor Hassan Farooq, Muhammad Arslan Ahmad, Altafur Rahman, Abd Ullah, Baiquan Song, Junbo Du, and et al. 2023. "Biochar-Mediated Control of Metabolites and Other Physiological Responses in Water-Stressed Leptocohloa fusca" Metabolites 13, no. 4: 511. https://doi.org/10.3390/metabo13040511
APA StyleSaleem, K., Asghar, M. A., Raza, A., Javed, H. H., Farooq, T. H., Ahmad, M. A., Rahman, A., Ullah, A., Song, B., Du, J., Xu, F., Riaz, A., & Yong, J. W. H. (2023). Biochar-Mediated Control of Metabolites and Other Physiological Responses in Water-Stressed Leptocohloa fusca. Metabolites, 13(4), 511. https://doi.org/10.3390/metabo13040511












