1. Introduction
Obesity causes various lethal metabolic disorders, such as type 2 diabetes, hypertension, and cardiovascular diseases [
1]. In obesity, adipocytes secrete adipokines, which cause chronic inflammation and associated metabolic disorders [
2]. When insulin resistance is induced in adipocytes, excess fatty acid binding and transport proteins lead to an increase in fatty acid uptake by non-adipocytes [
2]. The accumulation of free fatty acids in the liver causes insulin resistance, and a large amount of glucose begins to liberate [
3]. The accumulation of triglyceride (TG) in hepatocytes causes non-alcoholic fatty liver disease (NAFLD), which results in fat accumulation in hepatocytes and finally causes fibrosis due to the necrosis of hepatocytes [
4]. Thus, a balance in hepatocytes fat decomposition and synthesis provides an important therapeutic target that can prevent the induction of NAFLD and insulin resistance caused by a metabolic syndrome [
5].
A high-fat diet (HFD) can induce severe obesity in rodent mice. In earlier studies, significant insulin resistance, hyperglycemia, hyperlipidemia, mild diabetic nephropathy, and nonalcoholic steatohepatitis (NASH) have been observed in HFD-fed obese mice compared to normal pellet diet (NFD)-fed mice [
6,
7,
8,
9]. The extensively used HFD-fed animal model for studying the efficacy of functional foods causes obesity and hyperglycemia at an appropriate level and prevents metabolic syndromes including NAFLD [
8,
9,
10]. Therefore, in this study, a 45% Kcal HFD-fed mouse model was used to evaluate the dose-dependent pharmacological effects of a new candidate for metabolic syndromes, particularly obesity.
Currently, various treatments for metabolic syndromes have been developed; however, their use is restricted due to the associated adverse effects [
11]. Therefore, the development of alternative medicines from natural sources with few side effects and a higher effectivity is being actively tried [
8,
9,
10]. Recently, the control of oxidative stress, which is a major etiology of diabetes and related complications, along with blood sugar control, has emerged as the most essential method for diabetes treatment [
8,
9,
10,
11]. Therefore, the development of more effective α-glucosidase blockers or antioxidants with few side effects is being actively tried [
8,
9,
12,
13]. Among those, metformin is a representative oral biguanide-based antidiabetic drug and a well-known 5’-AMP-activated protein kinase (AMPK) activator [
14,
15]. It lowers the onset of cardiovascular disease, one of the serious side effects caused by diabetes, and the secretion of enzyme granules from the pancreas, which are directly involved in the digestion and decomposition of fats [
8,
9,
10]. It also regulates the activity of enzymes related to hepatic glucose metabolism [
16,
17]. In the present study, 250 mg/kg of metformin was used as a standard drug based on our earlier drug efficacy studies [
8,
9,
10].
The black cumin (
Nigella sativa L.) belongs to the family Ranunculaceae, and its seeds are commonly used as food spices [
18,
19]. In Islamic culture, they are called “El Habba Saouda (seeds of blessing)” and are known as a medicinal herb traditionally used for all ailments except for death [
19]. Black cumin contains a wide variety of active ingredients, such as polyphenols, flavonoids, saponins, and alkaloids [
20]. Thymoquinone, present in
N. sativa, is an especially important pharmacologically active compound and has been proven to have anti-rheumatoid arthritis, anti-inflammatory, and anti-cancer properties [
21]. Furthermore, it has shown anti-diabetic and anti-obesity effects by increasing phosphorylated Sirtuin 1 (SIRT1) in the liver and muscle and AMPKα in the muscle [
22].
The antioxidant [
23], anti-inflammatory [
24], immunomodulatory [
25], anticancer [
26], antibacterial [
27], antifungal [
28], and hypersensitivity inhibition properties [
29] of black cumin have been experimentally proven. As black cumin is also known for its glycemic control activity through its glycated hemoglobin inhibitory effect [
30], its potential as a preventive and therapeutic agent for diabetes is attracting attention [
31,
32]. In a recent study, Esmail et al. [
33] investigated the anti-diabetic potential of
N. sativa, where they focused only on blood lipid- and liver-related markers and liver biopsy. Although the bio-efficiency of thymoquinone or various organic solvent extracts of black cumin has been extensively reported, the efficacy study of supercritical water extract is not available. Thus, in the present study, we focus on the overall efficacy of black cumin seeds supercritical water extract (BCS extract) for anti-obesity, anti-diabetes, and related complications to validate the previously available effects of
N. sativa. As part of the development of a natural product-derived functional food material for improving obesity and diabetes-related complications, the dose-dependent effects of BCS aqueous extract on improving obesity and diabetes-related complications were evaluated using an HFD-fed mice model, which is a commonly used experimental animal model for mild type 2 diabetes and obesity [
7,
8,
9].
4. Discussion
The incidence of metabolic syndrome and NAFLD is rapidly increasing worldwide [
52,
53]. Hepatic intracellular fat accumulation during NAFLD causes inflammation, liver damage, and fibrosis, which lead to more severe hepatocellular carcinoma, liver cirrhosis, and NASH [
54,
55]. However, there are no conclusive drugs for completely treating a metabolic syndrome [
55,
56]. Nevertheless, there are some drugs that can control metabolic syndrome, but their use is limited due to various side effects [
57]. Therefore, changes in lifestyle, such as exercise therapy, are usually recommended for treating a metabolic syndrome [
55]. Hence, an alternative drug with few adverse effects that can suppress the overall metabolic syndrome and can be used for a prolonged period of time needs to be developed. As black cumin is known for its glycemic control activity through its glycated pigment inhibitory effect [
30], its potential as a preventive and therapeutic agent for diabetes is attracting attention [
31,
32]. Therefore, in the present study, we analyzed the pharmacological potential of BCS extract.
Fat accumulation is the most common phenomenon caused by obesity [
7,
8,
9,
10]. Changes in adipokine secretion by adipose tissue lead to complex diseases, such as obesity and insulin resistance [
8,
9,
58]. All three doses of BCS extract (400, 200, and 100 mg/kg) significantly and dose-dependently inhibited the fat accumulation and adipocyte hypertrophy. Particularly, BCS extract (200 mg/kg) administration significantly inhibited the HFD-induced fat accumulation and adipocyte hypertrophy as compared to the metformin (250 mg/kg)-administered group. These results show that, at least under the studied circumstances, the oral administration of BCS extract (400, 200, and 100 mg/kg) showed a dose-dependent improvement trend in obesity as compared to the metformin (250 mg/kg). Similar to earlier studies [
7,
8,
9,
10], the feed intake in the HFD control group was significantly decreased as compared to the NFD control group. Nevertheless, from a caloric point of view, the effect on the induction of obesity was considered not significant, as the calorific value of HFD used in this study was 4.73 kcal/g, and that of NFD was 4.00 kcal/g, which is slightly less than the calorie value of HFD. Furthermore, no significant differences in feed intake were observed in any of the experimental groups. Thus, it is judged that the pharmacological properties of BCS extract observed in this study are not simply due to a reduction in feed intake.
The enzymes present in zymogen granules digest proteins and fats [
59]. The pancreas causes a significant decrease in these zymogen granules, along with the accumulation of fat during obesity [
7,
8,
9,
10]. The HFD control group showed the promoted secretion of pancreatic enzymes involved in fat digestion, as a significant decrease in accumulated zymogen granules was observed histopathologically compared to the NFD control group. Accordingly, obesity was confirmed due to increased fat absorption. Furthermore, in histopathological examination, the proportion of zymogen granules in the pancreas was significantly inhibited by all three doses of BCS extract (400, 200, and 100 mg/kg). Particularly, BCS extract (200 mg/kg) administration inhibited the reduction in the HFD-induced zymogen granules ratio, comparable to that in the metformin (250 mg/kg)-administered group. These results show a dose-dependent improvement trend in HFD mice through BCS extract-inhibited fat absorption by regulating the secretion of enzymes involved in pancreatic fat digestion.
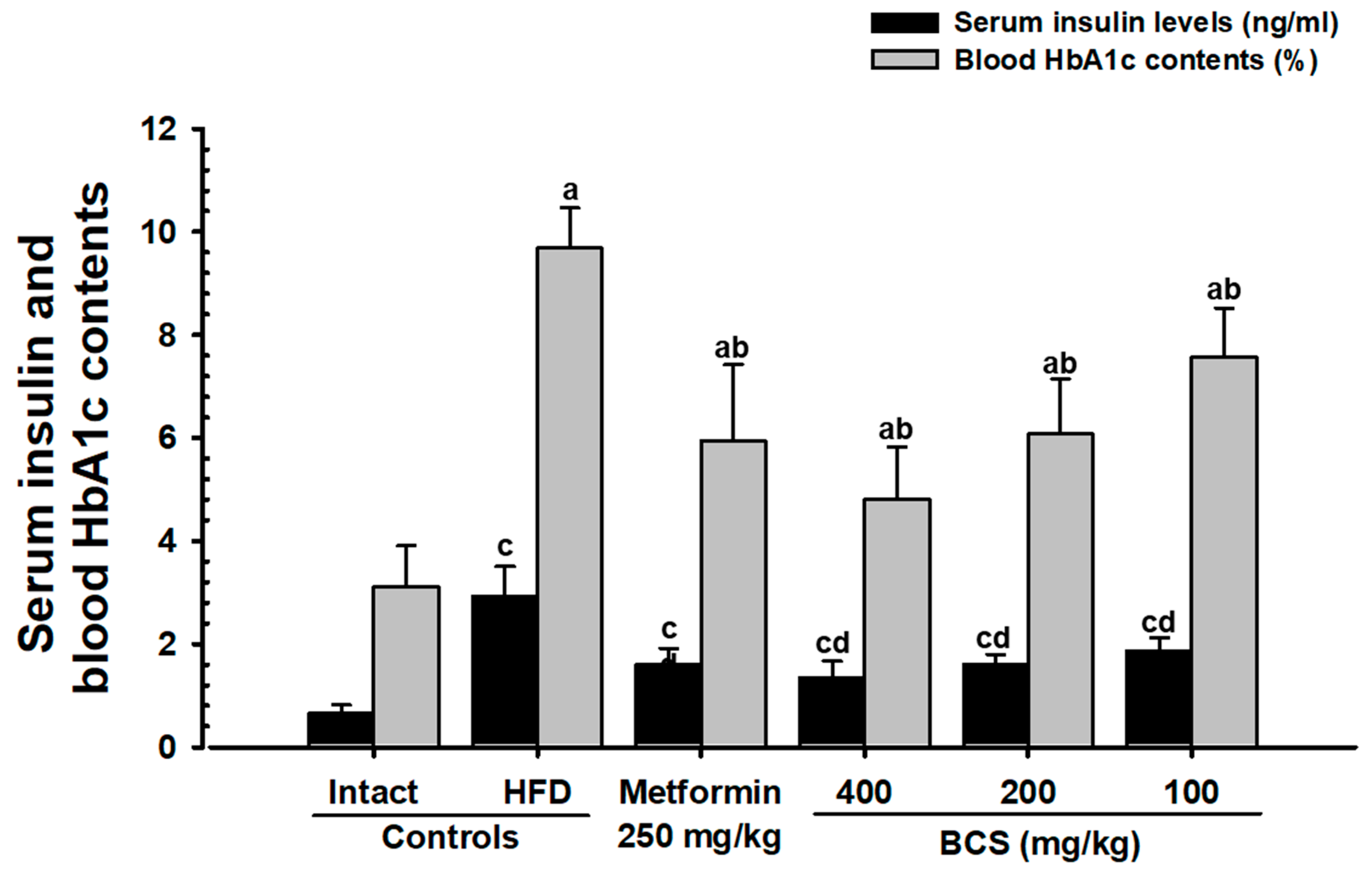
HbA1c is a key indicator for long-term hyperglycemia [
60,
61]. In type 2 diabetes, an increase in the blood insulin and HbA1c content and long-term hyperglycemia is generally observed [
7,
8,
9,
10,
62]. In addition, a long-term supply of HFD in mice supports the homeostasis of blood sugar, allows for growth and the expansion of the pancreatic islet, and increases insulin- and glucagon-producing cells [
63,
64]. Similar to previous studies (64,65), the HFD control group in this study showed an increase in the number and expansion of pancreatic islets, along with significant increases in blood glucose, blood insulin, and HbA1c contents and increases in the glucagon, insulin, and insulin/glucagon ratio, as confirmed histopathologically. This shows the successful induction of typical insulin-resistant type 2 diabetes mellitus, whereas BCS extract (400, 200, and 100 mg/kg)-administered groups dose-dependently suppressed the blood sugar, blood insulin, and HbA1c content and histological and immunohistological changes in the pancreatic endocrine region. In particular, the inhibitory effect of HFD-induced insulin-resistant type 2 diabetes in BCS extract (200 mg/kg) was comparable to that of the metformin (250 mg/kg)-administered group. These results are judged as clear evidence showing a dose-dependent improvement effect on blood glucose through the control of pancreatic endocrine function by the oral administration of BCS extract.
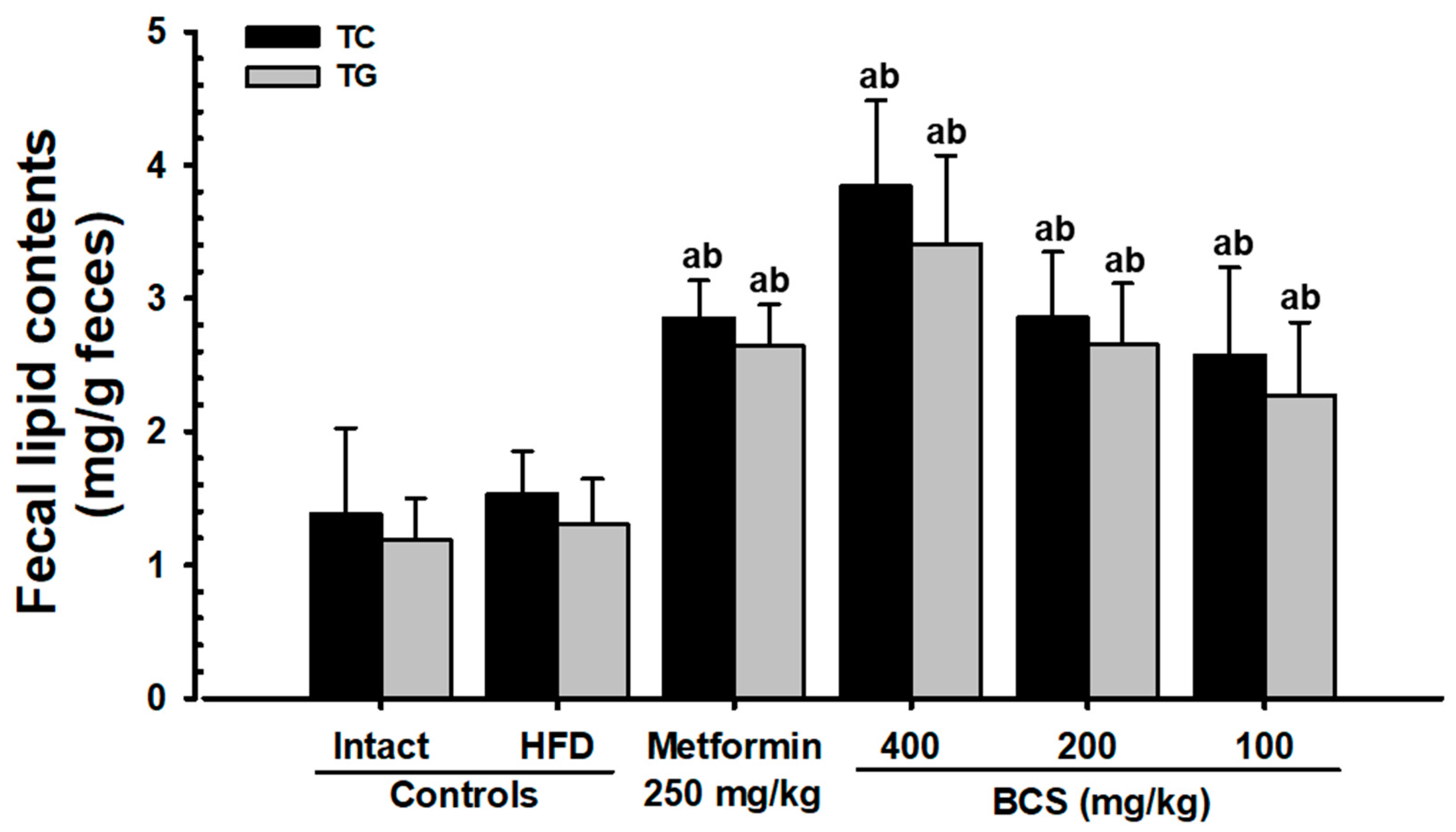
In a persistent hyperglycemic state, hyperlipidemia results in several complications [
11], such as a decrease in HDL along with an increase in LDL, TC, and TG content [
7,
8,
9,
10]. Therefore, the antihyperlipidemic effect of candidate substances is important to study [
7,
8,
9,
10]. All three doses of BCS extract (400, 200, and 100 mg/kg) showed significant decreases in the blood TG, TC, and LDL content and increases in the HDL content. In particular, the inhibitory effect of HFD-induced hyperlipidemia in the BCS extract (200 mg/kg)-administered group was comparable to that of the metformin (250 mg/kg)-administered group. In addition to the hyperlipidemia improvement effects, increases in the fecal TC and TG content and increases in the pancreatic zymogen content were observed histopathologically. Similar results were observed in the metformin (250 mg/kg)-supplied group. These improvement effects are judged to be due to the increase in lipid excretion caused by the suppression of lipids digestion by regulating the secretion of pancreatic digestive enzymes and a decrease in absorption.
ALT, AST, GGT, LDH, and ALP are the most common blood chemistry values for measuring liver damage [
65]. Hepatic fat degradation and accumulation in the liver causes an increase in the blood ALT, AST, GGT, LDH, and ALP content [
7,
8,
9,
10]. HFD supply causes liver damage, accompanied by hyperlipidemia, an increase in the liver weight, and a consequent increase in the blood AST, ALT, ALP, LDH, and GGT content, thereby inducing NAFLD [
7,
8,
9,
10]. The BCS extract (400, 200, and 100 mg/kg) significantly and dose-dependently inhibited the HFD-induced increases in the absolute liver weight and blood ALT, AST, GGT, LDH, and ALP content. The histopathological examination showed a significant suppression of changes in hepatic fat and hepatocyte hypertrophy. In particular, the HFD-induced NAFLD improvement effect in BCS extract (200 mg/kg)-administered mice was similar to that of the metformin (250 mg/kg)-administered mice. It is judged as clear evidence that the BCS extract at a dose of 200 mg/kg shows improvement in the HFD-induced NAFLD, similar to that of the metformin (250 mg/kg)-administered group.
The blood creatinine and BUN contents are some of the most important blood chemistry values indicating the condition of kidneys [
65]. The HFD supply in the control group showed significant increases in the blood creatinine and BUN content and the absolute kidney weights. Histopathological examinations confirmed tubular vacuolization, which is characterized by the infiltration of fat droplets. These findings of diabetic nephropathy were significantly inhibited by all three doses of BCS extract (400, 200, and 100 mg/kg). In particular, the administration of BCS extract (200 mg/kg) showed metformin (250 mg/kg)-comparable improvement effects for the HFD-induced diabetic nephropathy. These results show that the oral administration of BCS extract (400, 200, and 100 mg/kg) improves HFD-induced diabetic nephropathy as compared to that of metformin (250 mg/kg).
The free radicals play a significant role in the induction of diabetes and diabetic complications [
66]. When diabetes develops, more free radicals are formed due to the increase in oxidative stress, along with the inhibition of endogenous antioxidants [
67]. The free radicals formed in this way are the main cause of diabetic complications [
67,
68]. Similar to earlier studies [
62,
69], the HFD control group showed a decrease in the endogenous antioxidant (GSH) content, a decrease in the endogenous antioxidant enzymes activity (SOD and CAT), and an increase in the MDA content in the liver parenchyma by lipid peroxidation. All three doses of BCS extract (400, 200, and 100 mg/kg) significantly inhibited the disturbances in the antioxidant defense system and related lipid peroxidation. In particular, the antioxidant effects of the BCS extract (200 mg/kg) were comparable to those of the metformin (250 mg/kg). These results provide clear evidence that the oral administration of BCS extract (400, 200, and 100 mg/kg) shows significant antioxidant effects in HFD-fed mice as compared to the metformin (250 mg/kg).
Increases in PEPCK and G6pase enzyme activity and decreases in GK enzyme activity in the HFD-supplied experimental animal models have been associated with hyperglycemia [
7,
8,
9,
10,
62]. Similar to previous studies [
7,
8,
9,
10], the HFD control group in this study showed significant decreases in GK enzyme activity and increases in the PEPCK and G6pase enzyme activities in the liver, compared to the NFD control group. However, changes in the GK, G6pase, and PEPCK activities, which are enzymes related to glucose metabolism in the liver tissue, were significantly inhibited by all three doses of BCS (400, 200, and 100 mg/kg). In particular, the oral administration of 200 mg/kg of BCS extract showed improvement effects for HFD-induced GK, G6pase, and PEPCK activities, comparable to those of the metformin (250 mg/kg). These findings show that supplying BCS extract (400, 200, and 100 mg/kg) to mice dose-dependently modulated the enzymes related to glucose metabolism, showing considerable effects at a dose of 200 mg/kg in HFD-fed mice.
To understand the mechanism of action of candidate substances regarding related complications including NAFLD, obesity, and diabetes, the mRNA expression of genes linked with the fat metabolism in the liver and adipose tissues was recorded. AMPK activity in the adipose and liver is considered one of the most important cell signaling pathways that regulate fat metabolism and blood sugar [
70,
71]. An increase in insulin sensitivity and fat oxidation by adipocytes-derived adiponectin occurs in an AMPK-dependent manner [
8,
9,
14,
72,
73]. Therefore, the mRNA expressions of genes involved in the AMPK signaling pathway in the liver and adipose tissue were studied. The results in the HFD control group were directly related to the NAFLD pathogenesis. Decreased AMPKα1 and AMPKα2 mRNA expression and increased ACC1 mRNA expression were observed in liver tissue. Increased mRNA expression for C/EBPa, C/EBPβ, FAS, PPARγ, SREBP1c, and leptin and decreased mRNA expression for UCP2, adiponectin, and PPARα were observed in adipose tissue, whereas the HFD-induced changes in the AMPK and lipid metabolism-related gene expression were significantly inhibited by all three doses of BCS extract (400, 200, and 100 mg/kg). These results suggest that, at least under the circumstances of the present study, the oral supply of BCS extract (400, 200, and 100 mg/kg) can consistently improve lipid metabolism in a dose-dependent manner through the inhibition of lipid synthesis mediated by the regulation of AMPK expression and increased fatty acid oxidation in HFD-fed mice as compared to the metformin (250 mg/kg).
Even though the BCS extract showed a dose-dependent tendency for improving metabolic disorders, further studies checking the dose-dependent significance between different drug groups are required to verify the findings of this study. Rather than a purified extract, a crude BCS aqueous extract was used in this study. Thus, further studies could focus on composition analyses of the BCS super critical aqueous extract to identify the active substances and to study the efficacy relationship with thymoquinone, a known component in back cumin. In addition, there may be differences in efficacy depending on species specificity between humans and mice. Thus, the possibility of unexpected side effects cannot be ruled out.