Current Understanding of Flavonoids in Cancer Therapy and Prevention
Abstract
1. Introduction
1.1. Aim of the Study
1.2. Source of the Data
2. Overview of Dietary Flavonoids
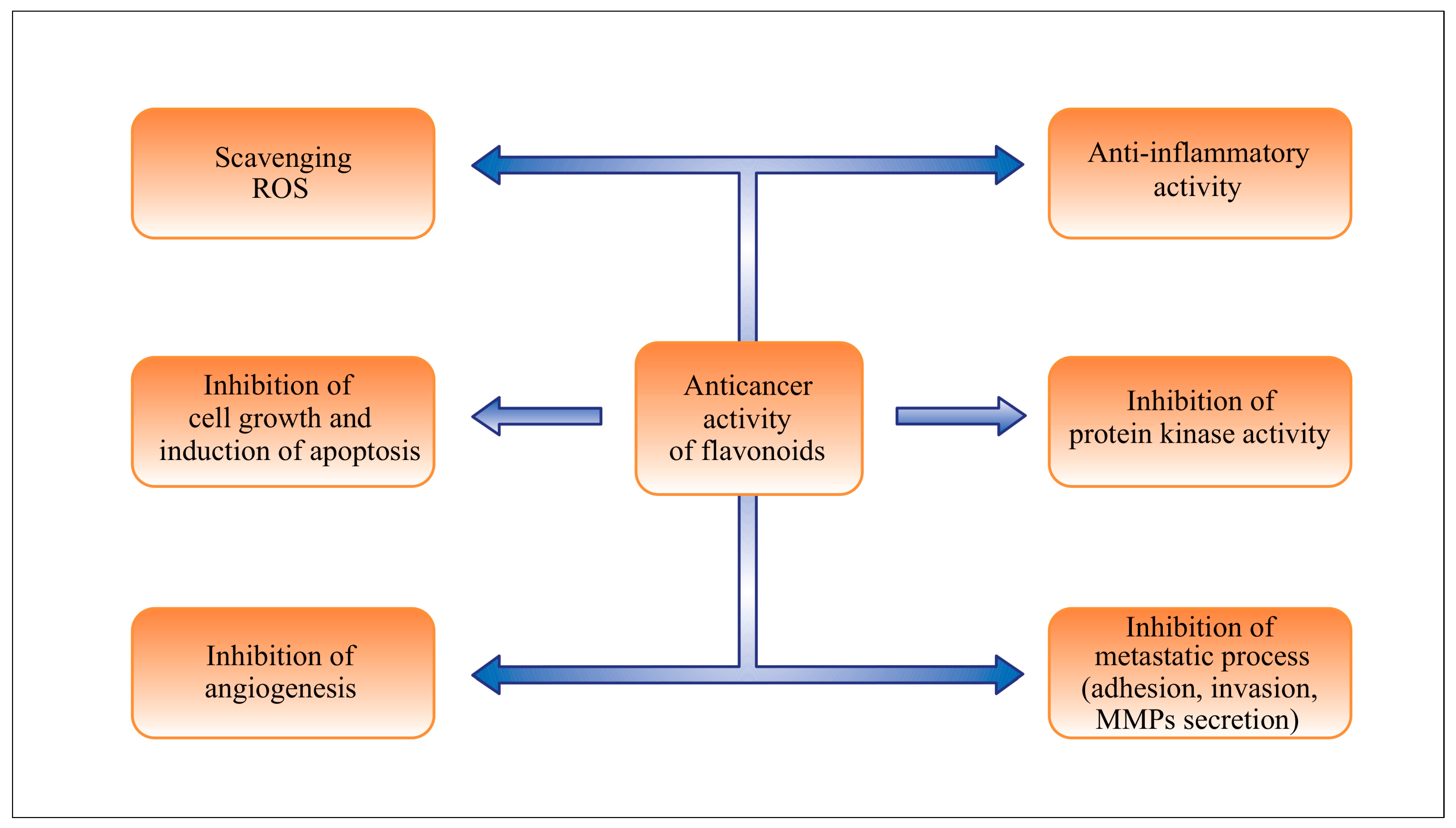
3. Effects of Flavonoids in Chemoprevention and Chemotherapy
3.1. Role of Flavonoids in Inflammation
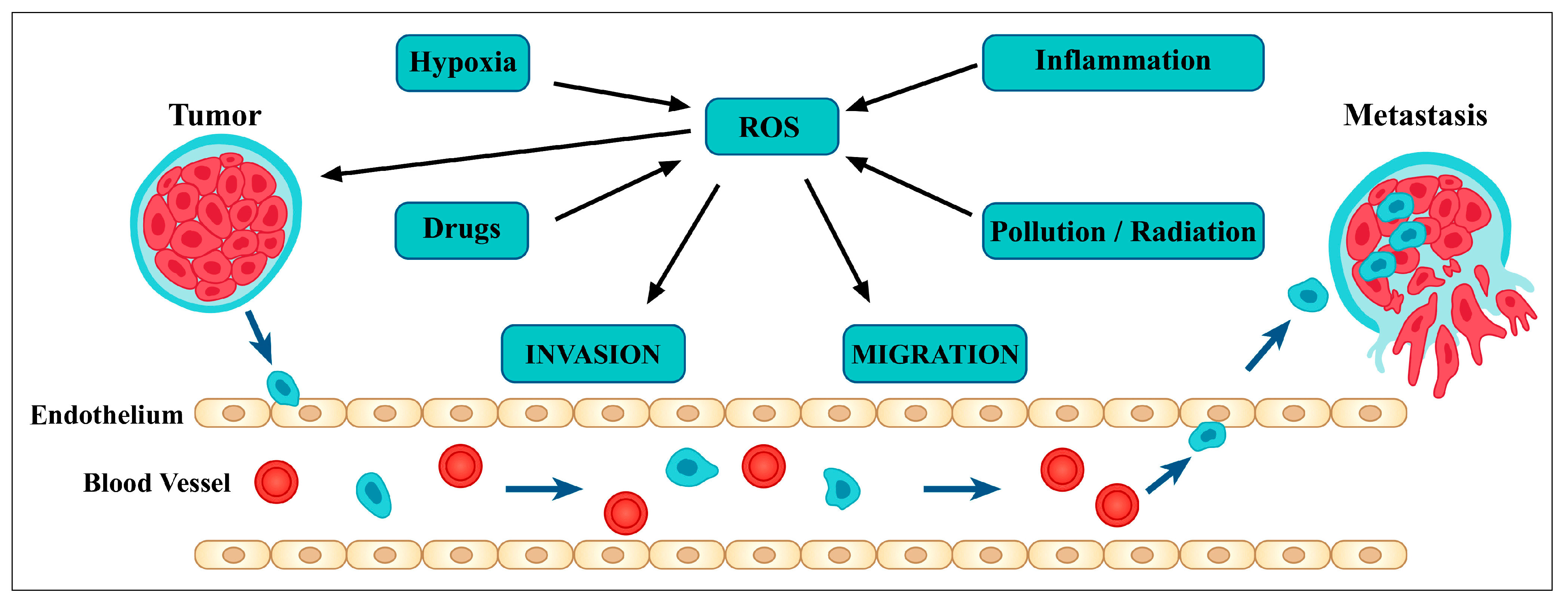
3.2. Relationship between Flavonoids and Oxidative Stress
3.3. The Role of Flavonoids in Apoptosis and Autophagy
3.4. Relationship between Flavonoids and Cancer Stem Cells
3.5. Flavonoids Inhibit Angiogenesis and Metastasis
3.6. The Role of Flavonoids in Cancer Cell Differentiation
3.7. Immunomodulatory Effect of Flavonoids
3.8. Incorporating Flavonoids with Chemotherapy
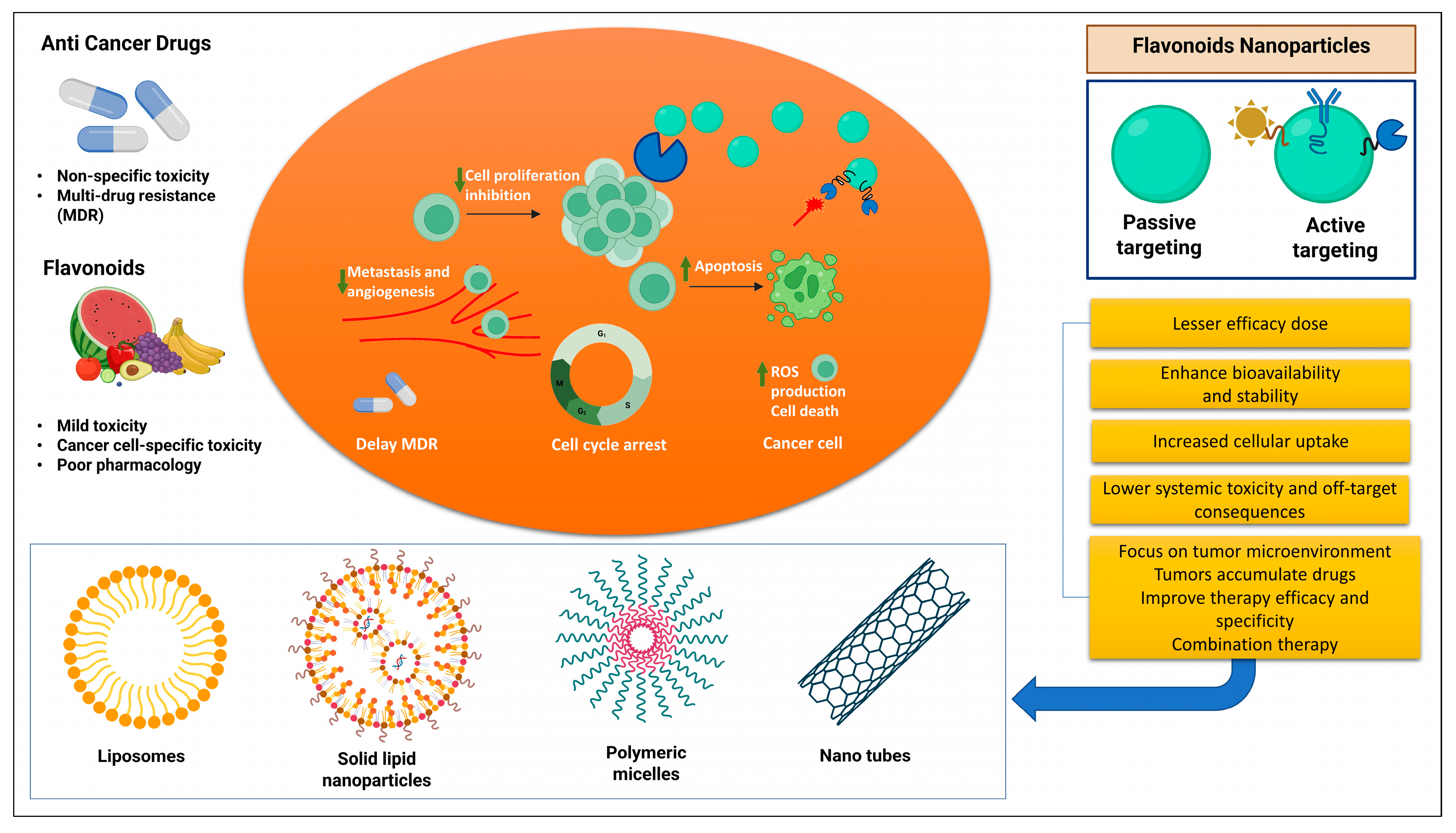
3.9. Flavonoids Nanoformulations in Cancer Therapy
3.10. Flavonoids in Clinical Trials
4. Conclusions
Funding
Conflicts of Interest
References
- Steck, S.E.; Murphy, E.A. Dietary patterns and cancer risk. Nat. Rev. Cancer 2020, 20, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J.J. Dietary Flavonoids as Cancer Chemopreventive Agents: An Updated Review of Human Studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Cao, C.; Cao, J.; Chen, W.; Zhang, Y.; Wang, C.; Wang, J.; Zhang, X.; Zhao, X. Dietary flavonol and flavone intakes and their major food sources in Chinese adults. Nutr. Cancer 2010, 62, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wang, H.; Wang, D.; Chen, Y.; Zhao, Y.; Xia, W. Using an FFQ to assess intakes of dietary flavonols and flavones among female adolescents in the Suihua area of northern China. Public Health Nutr. 2015, 18, 632–639. [Google Scholar] [CrossRef]
- Kim, Y.J.; Park, M.Y.; Chang, N.; Kwon, O. Intake and major sources of dietary flavonoid in Korean adults: Korean National Health and Nutrition Examination Survey 2010–2012. Asia Pac. J. Clin. Nutr. 2015, 24, 456–463. [Google Scholar]
- Jun, S.; Shin, S.; Joung, H. Estimation of dietary flavonoid intake and major food sources of Korean adults. Br. J. Nutr. 2016, 115, 480–489. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Agudo, A.; Lujan-Barroso, L.; Romieu, I.; Ferrari, P.; Knaze, V.; Bueno-de-Mesquita, H.B.; Leenders, M.; Travis, R.C.; Navarro, C.; et al. Dietary flavonoid and lignan intake and gastric adenocarcinoma risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Am. J. Clin. Nutr. 2012, 96, 1398–1408. [Google Scholar] [CrossRef]
- Popiolek-Kalisz, J. The impact of dietary flavonols on central obesity parameters in polish adults. Nutrients 2022, 14, 5051. [Google Scholar] [CrossRef]
- Gallardo-Fernandez, M.; Gonzalez-Ramirez, M.; Cerezo, A.B.; Troncoso, A.M.; Garcia-Parrilla, M.C. Hydroxytyrosol in foods: Analysis, food sources, eu dietary intake, and potential uses. Foods 2022, 11, 2355. [Google Scholar] [CrossRef] [PubMed]
- Almanza-Aguilera, E.; Ceballos-Sanchez, D.; Achaintre, D.; Rothwell, J.A.; Laouali, N.; Severi, G.; Katzke, V.; Johnson, T.; Schulze, M.B.; Palli, D.; et al. Urinary concentrations of (+)-catechin and (−)-epicatechin as biomarkers of dietary intake of flavan-3-ols in the european prospective investigation into cancer and nutrition (epic) study. Nutrients 2021, 13, 4157. [Google Scholar] [CrossRef]
- Vogiatzoglou, A.; Mulligan, A.A.; Lentjes, M.A.; Luben, R.N.; Spencer, J.P.; Schroeter, H.; Khaw, K.T.; Kuhnle, G.G. Flavonoid intake in European adults (18 to 64 years). PLoS ONE 2015, 10, e0128132. [Google Scholar] [CrossRef]
- Murphy, K.J.; Walker, K.M.; Dyer, K.A.; Bryan, J. Estimation of daily intake of flavonoids and major food sources in middle-aged australian men and women. Nutr. Res. 2019, 61, 64–81. [Google Scholar] [CrossRef] [PubMed]
- Somerset, S.M.; Johannot, L. Dietary flavonoid sources in Australian adults. Nutr. Cancer 2008, 60, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Kent, K.; Charlton, K.; O'Sullivan, T.; Oddy, W.H. Estimated intake and major food sources of flavonoids among australian adolescents. Eur. J. Nutr. 2020, 59, 3841–3856. [Google Scholar] [CrossRef]
- Grotto, D.; Zied, E. The Standard American Diet and its relationship to the health status of Americans. Nutr. Clin. Pract. 2010, 25, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Bertoia, M.L.; Rimm, E.B.; Mukamal, K.J.; Hu, F.B.; Willett, W.C.; Cassidy, A. Dietary flavonoid intake and weight maintenance: Three prospective cohorts of 124,086 US men and women followed for up to 24 years. BMJ 2016, 352, i17. [Google Scholar] [CrossRef]
- Chun, O.K.; Song, W.O.; Chung, S.J. Estimated dietary flavonoid intake and major food sources of U.S. adults. J. Nutr. 2007, 137, 1244–1252. [Google Scholar] [CrossRef]
- Kim, K.; Vance, T.M.; Chun, O.K. Estimated intake and major food sources of flavonoids among US adults: Changes between 1999–2002 and 2007–2010 in NHANES. Eur. J. Nutr. 2016, 55, 833–843. [Google Scholar] [CrossRef]
- Arif, H.; Rehmani, N.; Farhan, M.; Ahmad, A.; Hadi, S.M. Mobilization of Copper ions by Flavonoids in Human Peripheral Lymphocytes Leads to Oxidative DNA Breakage: A Structure Activity Study. Int. J. Mol. Sci. 2015, 16, 26754–26769. [Google Scholar] [CrossRef] [PubMed]
- Hazafa, A.; Iqbal, M.O.; Javaid, U.; Tareen, M.B.K.; Amna, D.; Ramzan, A.; Piracha, S.; Naeem, M. Inhibitory effect of polyphenols (phenolic acids, lignans, and stilbenes) on cancer by regulating signal transduction pathways: A review. Clin. Transl. Oncol. 2022, 24, 432–445. [Google Scholar] [CrossRef] [PubMed]
- Alhasawi, M.A.I.; Aatif, M.; Muteeb, G.; Alam, M.W.; Oirdi, M.E.; Farhan, M. Curcumin and its derivatives induce apoptosis in human cancer cells by mobilizing and redox cycling genomic copper ions. Molecules 2022, 27, 7410. [Google Scholar] [CrossRef] [PubMed]
- Duo, J.; Ying, G.G.; Wang, G.W.; Zhang, L. Quercetin inhibits human breast cancer cell proliferation and induces apoptosis via bcl-2 and bax regulation. Mol. Med. Rep. 2012, 5, 1453–1456. [Google Scholar] [PubMed]
- Wattel, A.; Kamel, S.; Mentaverri, R.; Lorget, F.; Prouillet, C.; Petit, J.P.; Fardelonne, P.; Brazier, M. Potent inhibitory effect of naturally occurring flavonoids quercetin and kaempferol on in vitro osteoclastic bone resorption. Biochem. Pharmacol. 2003, 65, 35–42. [Google Scholar] [CrossRef]
- Zaid, S.S.M.; Ruslee, S.S.; Mokhtar, M.H. Protective Roles of Honey in Reproductive Health: A Review. Molecules 2021, 26, 3322. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M.; Shamim, U.; Hadi, S. Green Tea Polyphenols: A putative mechanism for cytotoxic action against cancer cells. In Nutraceuticals and Natural Product Derivatives: Disease Prevention & Drug Discovery; Wiley: Hoboken, NY, USA, 2019; pp. 305–332. [Google Scholar]
- Farhan, M.; Rizvi, A.; Ahmad, A.; Aatif, M.; Alam, M.W.; Hadi, S.M. Structure of Some Green Tea Catechins and the Availability of Intracellular Copper Influence Their Ability to Cause Selective Oxidative DNA Damage in Malignant Cells. Biomedicines 2022, 10, 664. [Google Scholar] [CrossRef]
- Farhan, M. Naringin’s Prooxidant Effect on Tumor Cells: Copper’s Role and Therapeutic Implications. Pharmaceuticals 2022, 15, 1431. [Google Scholar] [CrossRef]
- Bendokas, V.; Stanys, V.; Mažeikienė, I.; Trumbeckaite, S.; Baniene, R.; Liobikas, J. Anthocyanins: From the Field to the Antioxidants in the Body. Antioxidants 2020, 9, 819. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef]
- Majdan, M.; Bobrowska-Korczak, B. Active Compounds in Fruits and Inflammation in the Body. Nutrients 2022, 14, 2496. [Google Scholar] [CrossRef] [PubMed]
- Desmawati, D.; Sulastri, D. Phytoestrogens and Their Health Effect. Open Access Maced. J. Med. Sci. 2019, 14, 495–499. [Google Scholar] [CrossRef]
- Hammerstone, F.J.; Lazarus, S.A.; Schmitz, H.H. Procyanidin Content and Variation in Some Commonly Consumed Foods. J. Nutr. 2020, 130, 2086S–2092S. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Moreira, I.; Arnaez, E.; Quesada, S.; Azofeifa, G.; Alvarado, D.; Monagas, M.J. Proanthocyanidin Characterization, Antioxidant and Cytotoxic Activities of Three Plants Commonly Used in Traditional Medicine in Costa Rica: Petiveria alliaceae L., Phyllanthus niruri L. and Senna reticulata Willd. Plants 2017, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhang, P.; Warner, R.D.; Fang, Z. 3-Deoxyanthocyanidin Colorant: Nature, Health, Synthesis, and Food Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1533–1549. [Google Scholar] [CrossRef]
- Gasmi, A.; Mujawdiya, P.K.; Noor, S.; Lysiuk, R.; Darmohray, R.; Piscopo, S.; Lenchyk, L.; Antonyak, H.; Dehtiarova, K.; Shanaida, M.; et al. Polyphenols in Metabolic Diseases. Molecules 2022, 27, 6280. [Google Scholar] [CrossRef]
- Arfaoui, L. Dietary Plant Polyphenols: Effects of Food Processing on Their Content and Bioavailability. Molecules 2021, 26, 2959. [Google Scholar] [CrossRef]
- Thilakarathna, S.H.; Rupasinghe, H.P. Flavonoid Bioavailability and Attempts for Bioavailability Enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Kardum, N.; Glibetic, M. Polyphenols and Their Interactions with Other Dietary Compounds: Implications for Human Health. Adv. Food Nutr. Res. 2018, 84, 103–144. [Google Scholar]
- Liu, J.; Wang, X.; Yong, H.; Kan, J.; Jin, C. Recent advances in flavonoid-grafted polysaccharides: Synthesis, structural characterization, bioactivities and potential applications. Int. J. Biol. Macromol. 2018, 116, 1011–1025. [Google Scholar] [CrossRef]
- Rienks, J.; Barbaresko, J.; Nothlings, U. Association of isoflavone biomarkers with risk of chronic disease and mortality: A systematic review and meta-analysis of observational studies. Nutr. Rev. 2017, 75, 616–641. [Google Scholar] [PubMed]
- Thanushree, M.P.; Sudha, M.L.; Asha, M.; Vanitha, T.; Crassina, K. Enhancing the nutritional and quality profiles of buckwheat noodles: Studies on the effects of methods of milling and improvers. LWT—Food Sci. Technol. 2022, 160, 113286. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Cienfuegos-Jovellanos, E.; Marin, A.; Muguerza, B.; Gil-Izquierdo, A.; Cerda, B.; Zafrilla, P.; Morillas, J.; Mulero, J.; Ibarra, A.; et al. A new process to develop a cocoa powder with higher flavonoid monomer content and enhanced bioavailability in healthy humans. J. Agric. Food Chem. 2007, 55, 3926–3935. [Google Scholar] [CrossRef]
- Jaćimović, S.; Popović-Djordjević, J.; Sarić, B.; Krstić, A.; Mickovski-Stefanović, V.; Pantelić, N.Đ. Antioxidant Activity and Multi-Elemental Analysis of Dark Chocolate. Foods 2022, 11, 1445. [Google Scholar] [CrossRef] [PubMed]
- Uysal, U.D.; Aturki, Z.; Raggi, M.A.; Fanali, S. Separation of catechins and methylxanthines in tea samples by capillary electrochromatography. J. Sep. Sci. 2009, 32, 1002–1010. [Google Scholar] [CrossRef]
- Awad, A.M.; Kumar, P.; Ismail-Fitry, M.R.; Jusoh, S.; Ab Aziz, M.F.; Sazili, A.Q. Green Extraction of Bioactive Compounds from Plant Biomass and Their Application in Meat as Natural Antioxidant. Antioxidants 2021, 10, 1465. [Google Scholar] [PubMed]
- Farhan, M.; Khan, H.Y.; Oves, M.; Al-Harrasi, A.; Rehmani, N.; Arif, H.; Hadi, S.M.; Ahmad, A. Cancer Therapy by Catechins Involves Redox Cycling of Copper Ions and Generation of Reactive Oxygen Species. Toxins 2016, 8, 37. [Google Scholar] [PubMed]
- Guo, J.; Yue, T.; Yuan, Y.; Wang, Y. Chemometric classification of apple juices according to variety and geographical origin based on polyphenolic profiles. J. Agric. Food Chem. 2013, 61, 6949–6963. [Google Scholar] [CrossRef]
- Mazzotti, F.; Bartella, L.; Talarico, I.R.; Napoli, A.; Di Donna, L. High-throughput determination of flavanone-o-glycosides in citrus beverages by paper spray tandem mass spectrometry. Food Chem. 2021, 360, 130060. [Google Scholar] [CrossRef]
- Addi, M.; Elbouzidi, A.; Abid, M.; Tungmunnithum, D.; Elamrani, A.; Hano, C. An Overview of Bioactive Flavonoids from Citrus Fruits. Appl. Sci. 2022, 12, 29. [Google Scholar] [CrossRef]
- Medina-Remón, A.; Manach, C.; Knox, C.; Wishart, D.S.; Perez-Jimenez, J.; Rothwell, J.A.; M’Hiri, N.; García-Lobato, P.; Eisner, R.; Neveu, V.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, N.P.; Bondonno, C.P.; Ward, N.C.; Hodgson, J.M.; Croft, K.D. The cardiovascular health benefits of apples: Whole fruit vs. isolated compounds. Trends Food Sci. Technol. 2017, 69, 243–256. [Google Scholar]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 16, 27–41. [Google Scholar]
- Na, B.K.; Pak, J.H.; Hong, S.J. Clonorchis sinensis and clonorchiasis. Acta Trop. 2020, 203, 105309. [Google Scholar] [CrossRef]
- Baj, J.; Korona-Głowniak, I.; Forma, A.; Maani, A.; Sitarz, E.; Rahnama-Hezavah, M.; Radzikowska, E.; Portincasa, P. Mechanisms of the Epithelial–Mesenchymal Transition and Tumor Microenvironment in Helicobacter Pylori-Induced Gastric Cancer. Cells 2020, 9, 1055. [Google Scholar] [CrossRef]
- Mak, L.Y.; Cruz-Ramòn, V.; Chinchilla-López, P.; Torres, H.A.; Lo Conte, N.K.; Rice, J.P.; Foxhall, L.E.; Sturgis, E.M.; Merrill, J.K.; Bailey, H.H.; et al. Global Epidemiology, Prevention, and Management of Hepatocellular Carcinoma. Am. Soc. Clin. Oncol. Educ. Book 2018, 23, 62–279. [Google Scholar] [CrossRef]
- Mesri, E.A.; Cavallin, L.E.; Ashlock, B.M.; Leung, H.J.; Ma, Q.; Goldschmidt-Clermont, P.J. Molecular studies and therapeutic targeting of kaposi’s sarcoma herpesvirus (kshv/hhv-8) oncogenesis. Immunol. Res. 2013, 57, 159–165. [Google Scholar] [CrossRef]
- Francescone, R.; Hou, V.; Grivennikov, S.I. Microbiome, inflammation, and cancer. Cancer J. 2014, 20, 181–189. [Google Scholar] [CrossRef]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef]
- Que, J.; Garman, K.S.; Souza, R.F.; Spechler, S.J. Pathogenesis and Cells of Origin of Barrett’s Esophagus. Gastroenterology 2019, 157, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, G.; Gupta, A.K.; Das, S.; Gohil, A.J.; Lamba, S. Marjolin Ulcer: An Observational Epidemiological Study from a Tertiary Care Centre in India. Ann. Plast. Surg. 2019, 83, 518–522. [Google Scholar] [CrossRef]
- Klebe, S.; Leigh, J.; Henderson, D.W.; Nurminen, M. Asbestos, Smoking and Lung Cancer: An Update. Int. J. Environ. Res. Public Health 2019, 17, 258. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.L.; Liu, J.; Zhang, L.X.; Wu, C.M.; Chu, A.J.; Wen, B.L.; Ma, C.; Yan, X.Y.; Zhang, X.; Wang, D.M.; et al. Asthma and the risk of lung cancer: A meta-analysis. Oncotarget 2017, 8, 11614–11620. [Google Scholar] [CrossRef] [PubMed]
- Haenen, C.C.P.; Buurma, A.A.J.; Genders, R.E.; Quint, K.D. Squamous cell carcinoma arising in hypertrophic lichen planus. BMJ Case Rep. 2018, 2018, bcr2017224044. [Google Scholar] [CrossRef] [PubMed]
- Hakenberg, O.W.; Dräger, D.L.; Erbersdobler, A.; Naumann, C.M.; Jünemann, K.P.; Protzel, C. The Diagnosis and Treatment of Penile Cancer. Dtsch. Arztebl. Int. 2018, 115, 646–652. [Google Scholar] [CrossRef]
- Savant, S.S.; Sriramkumar, S.; O’Hagan, H.M. The Role of Inflammation and Inflammatory Mediators in the Development, Progression, Metastasis, and Chemoresistance of Epithelial Ovarian Cancer. Cancers 2018, 10, 251. [Google Scholar] [CrossRef]
- Sfanos, K.S.; Yegnasubramanian, S.; Nelson, W.G.; De Marzo, A.M. The inflammatory microenvironment and microbiome in prostate cancer development. Nat. Rev. Urol. 2018, 15, 11–24. [Google Scholar] [CrossRef]
- Rizvi, A.; Farhan, M.; Nabi, F.; Khan, R.H.; Adil, M.; Ahmad, A. Transcriptional control of the oxidative stress response and implications of using plant derived molecules for therapeutic interventions in cancer. Curr. Med. Chem. 2021, 28, 8480–8495. [Google Scholar] [CrossRef]
- Islam, J.; Shree, A.; Vafa, A.; Afzal, S.M.; Sultana, S. Taxifolin ameliorates Benzo [a] pyrene-induced lung injury possibly via stimulating the Nrf2 signalling pathway. Int. Immunopharmacol. 2021, 96, 107566. [Google Scholar] [CrossRef]
- Chen, M.; Li, J.; Liu, X.; Song, Z.; Han, S.; Shi, R.; Zhang, X. Chrysin prevents lipopolysaccharide-induced acute lung injury in mice by suppressing the IRE1alpha/TXNIP/NLRP3 pathway. Pulm. Pharmacol. Ther. 2021, 68, 102018. [Google Scholar] [CrossRef] [PubMed]
- Yuvaraj, S.; Ramprasath, T.; Saravanan, B.; Vasudevan, V.; Sasikumar, S.; Selvam, G.S. Chrysin attenuates high-fat-diet-induced myocardial oxidative stress via upregulating eNOS and Nrf2 target genes in rats. Mol. Cell. Biochem. 2021, 476, 2719–2727. [Google Scholar] [CrossRef] [PubMed]
- Raina, R.; Afroze, N.; Kedhari, M.S.; Haque, S.; Bajbouj, K.; Hamad, M.; Hussain, A. Chrysin inhibits propagation of HeLa cells by attenuating cell survival and inducing apoptotic pathways. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2206–2220. [Google Scholar]
- Zhang, Z.; Shi, J.; Yang, T.; Liu, T.; Zhang, K. Management of aggressive fibromatosis. Oncol Lett. 2021, 21, 43. [Google Scholar]
- Kontomanolis, E.N.; Koutras, A.; Syllaios, A.; Schizas, D.; Mastoraki, A.; Garmpis, N.; Diakosavvas, M.; Angelou, K.; Tsatsaris, G.; Pagkalos, A.; et al. Role of Oncogenes and Tumor-suppressor Genes in Carcinogenesis: A Review. Anticancer Res. 2020, 40, 6009–6015. [Google Scholar] [CrossRef]
- Hadi, S.M.; Bhat, S.H.; Azmi, A.S.; Hanif, S.; Shamim, U.; Ullah, M.F. Oxidative breakage of cellular DNA by plant polyphenols: A putative mechanism for anticancer properties. Semin. Cancer Biol. 2007, 17, 370–376. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef]
- Kanno, S.; Tomizawa, A.; Hiura, T.; Osanai, Y.; Shouji, A.; Ujibe, M.; Ohtake, T.; Kimura, K.; Ishikawa, M. Inhibitory effects of naringenin on tumor growth in human cancer cell lines and sarcoma S-180-implanted mice. Biol. Pharm. Bull. 2005, 28, 527–530. [Google Scholar] [CrossRef]
- Arul, D.; Subramanian, P. Naringenin (Citrus Flavonone) induces growth inhibition, cell cycle arrest and apoptosis in human hepatocellular carcinoma cells. Pathol. Oncol. Res. 2013, 19, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.R.; Liu, C.J.; Yeh, C.C. Naringenin suppresses TPA-induced tumor invasion by suppressing multiple signal transduction pathways in human hepatocellular carcinoma cells. Chem. Biol. Interact. 2015, 235, 1–9. [Google Scholar] [CrossRef]
- Bao, L.; Liu, F.; Guo, H.B.; Li, Y.; Tan, B.B.; Zhang, W.X.; Peng, Y.H. Naringenin inhibits proliferation, migration, and invasion as well as induces apoptosis of gastric cancer SGC7901 cell line by downregulation of AKT pathway. Tumour. Biol. 2016, 37, 11365–11374. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, P.; Elia, A.C.; Magara, G.; Feriotto, G.; Forni, C.; Borromeo, I.; De Martino, A.; Tabolacci, C.; Mischiati, C.; Beninati, S. Reduction of oxidative stress and ornithine decarboxylase expression in a human prostate cancer cell line PC-3 by a combined treatment with alpha-tocopherol and naringenin. Amino Acids 2021, 53, 63–72. [Google Scholar] [CrossRef]
- Rebello, C.J.; Beyl, R.A.; Lertora, J.J.L.; Greenway, F.L.; Ravussin, E.; Ribnicky, D.M.; Poulev, A.; Kennedy, B.J.; Castro, H.F.; Campagna, S.R.; et al. Safety and pharmacokinetics of naringenin: A randomized, controlled, single-ascending-dose clinical trial. Diabetes Obes. Metab. 2020, 22, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Fleming, R.E.; Ponka, P. Iron overload in human disease. N. Engl. J. Med. 2012, 366, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Molina-Sanchez, P.; Lujambio, A. Iron overload and liver cancer. J. Exp. Med. 2019, 216, 723–724. [Google Scholar] [CrossRef]
- Franke, G.N.; Kubasch, A.S.; Cross, M.; Vucinic, V.; Platzbecker, U. Iron overload and its impact on outcome of patients with hematological diseases. Mol. Asp. Med. 2020, 75, 100868. [Google Scholar] [CrossRef]
- Zhai, Z.; Zou, P.; Liu, F.; Xia, Z.; Li, J. Ferroptosis is a potential novel diagnostic and therapeutic target for patients with cardiomyopathy. Front. Cell Dev. Biol. 2021, 9, 649045. [Google Scholar] [CrossRef]
- Chen, X.; Comish, P.B.; Tang, D.; Kang, R. Characteristics and biomarkers of ferroptosis. Front. Cell Dev. Biol. 2021, 9, 637162. [Google Scholar] [CrossRef]
- Mao, L.; Zhao, T.; Song, Y.; Lin, L.; Fan, X.; Cui, B.; Feng, H.; Wang, X.; Yu, Q.; Zhang, J.; et al. The emerging role of ferroptosis in non-cancer liver diseases: Hype or increasing hope? Cell Death Dis. 2020, 11, 518. [Google Scholar] [CrossRef]
- Lesjak, M.; Srai, S.K.S. Role of dietary flavonoids in iron homeostasis. Pharmaceuticals 2019, 12, 119. [Google Scholar] [CrossRef]
- Farhan, M.; Rizvi, A.; Naseem, I.; Hadi, S.M.; Ahmad, A. Targeting increased copper levels in diethylnitrosamine induced hepatocellular carcinoma cells in rats by epigallocatechin-3-gallate. Tumor Biol. 2015, 36, 8861–8867. [Google Scholar] [CrossRef] [PubMed]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Wong, S.K.; Chan, H.T. Casticin from Vitex species: A short review on its anticancer and anti-inflammatory properties. J. Integr. Med. 2018, 16, 147–152. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Q.; Liu, H.; Luo, S. Vitexin induces apoptosis through mitochondrial pathway and PI3K/Akt/mTOR signaling in human non-small cell lung cancer A549 cells. Biol. Res. 2019, 52, 7. [Google Scholar] [CrossRef] [PubMed]
- Teekaraman, D.; Elayapillai, S.P.; Viswanathan, M.P.; Jagadeesan, A. Quercetin inhibits human metastatic ovarian cancer cell growth and modulates components of the intrinsic apoptotic pathway in PA-1 cell line. Chem. Biol. Interact. 2019, 300, 91–100. [Google Scholar] [CrossRef]
- Perez-Montoyo, H. Therapeutic Potential of Autophagy Modulation in Cholangiocarcinoma. Cells 2020, 9, 614. [Google Scholar] [CrossRef]
- Zhang, L.; Shamaladevi, N.; Jayaprakasha, G.K.; Patil, B.S.; Lokeshwar, B.L. Polyphenol-rich extract of Pimenta dioica berries (Allspice) kills breast cancer cells by autophagy and delays growth of triple negative breast cancer in athymic mice. Oncotarget 2015, 6, 16379–16395. [Google Scholar] [CrossRef]
- Han, B.; Yu, Y.Q.; Yang, Q.L.; Shen, C.Y.; Wang, X.J. Kaempferol induces autophagic cell death of hepatocellular carcinoma cells via activating AMPK signaling. Oncotarget 2017, 8, 86227–86239. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Wang, S.F.; Wu, M.Y.; Cai, C.Z.; Li, M.; Lu, J.H. Autophagy modulators from traditional chinese medicine: Mechanisms and therapeutic potentials for cancer and neurodegenerative diseases. J. Ethnopharmacol. 2016, 194, 861–876. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.X.; Wu, Y.; Chen, R.J.; Liu, Y.; Huang, L.S.; Lou, L.G.; Zheng, Z.H.; Chen, Y.Z.; Xu, J.H. Curcumin derivative c817 inhibits proliferation of imatinib-resistant chronic myeloid leukemia cells with wild-type or mutant bcr-abl in vitro. Acta Pharmacol. Sin. 2014, 35, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Huang, Y.; Han, N.; He, F.; Li, M.; Bian, Z.; Liu, J.; Sun, T.; Zhu, L. Quercetin suppresses nlrp3 inflammasome activation and attenuates histopathology in a rat model of spinal cord injury. Spinal Cord 2016, 54, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.W.; Hu, J.J.; Fu, R.Q.; Liu, X.; Zhang, Y.H.; Li, J.; Liu, L.; Li, Y.N.; Deng, Q.; Luo, Q.S.; et al. Flavonoids inhibit cell proliferation and induce apoptosis and autophagy through downregulation of pi3kgamma mediated pi3k/akt/mtor/p70s6k/ulk signaling pathway in human breast cancer cells. Sci. Rep. 2018, 8, 11255. [Google Scholar] [CrossRef]
- Farhan, M. Green Tea Catechins: Nature’s Way of Preventing and Treating Cancer. Int. J. Mol. Sci. 2022, 23, 10713. [Google Scholar] [CrossRef] [PubMed]
- Moharil, R.B.; Dive, A.; Khandekar, S.; Bodhade, A. Cancer stem cells: An insight. J. Oral. Maxillofac. Pathol. 2017, 21, 463. [Google Scholar] [CrossRef] [PubMed]
- Cianciosi, D.; Varela-Lopez, A.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Afrin, S.; Reboredo-Rodriguez, P.; Zhang, J.; Quiles, J.L.; Nabavi, S.F.; Battino, M.; et al. Targeting molecular pathways in cancer stem cells by natural bioactive compounds. Pharmacol. Res. 2018, 135, 150–165. [Google Scholar] [CrossRef]
- Hermawan, A.; Ikawati, M.; Jenie, R.I.; Khumaira, A.; Putri, H.; Nurhayati, I.P.; Angraini, S.M.; Muflikhasari, H.A. Identification of potential therapeutic target of naringenin in breast cancer stem cells inhibition by bioinformatics and in vitro studies. Saudi Pharm. J. 2021, 29, 12–26. [Google Scholar] [CrossRef]
- Kim, B.; Jung, N.; Lee, S.; Sohng, J.K.; Jung, H.J. Apigenin Inhibits Cancer Stem Cell-Like Phenotypes in Human Glioblastoma Cells via Suppression of c-Met Signaling. Phytother. Res. 2016, 30, 1833–1840. [Google Scholar] [CrossRef]
- Li, Y.W.; Xu, J.; Zhu, G.Y.; Huang, Z.J.; Lu, Y.; Li, X.Q.; Wang, N.; Zhang, F.X. Apigenin suppresses the stem cell-like properties of triple-negative breast cancer cells by inhibiting YAP/TAZ activity. Cell Death Discov. 2018, 4, 105. [Google Scholar] [CrossRef]
- Erdogan, S.; Turkekul, K.; Serttas, R.; Erdogan, Z. The natural flavonoid apigenin sensitizes human CD44(+) prostate cancer stem cells to cisplatin therapy. Biomed. Pharmacother. 2017, 88, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Tu, D.G.; Lin, W.T.; Yu, C.C.; Lee, S.S.; Peng, C.Y.; Lin, T.; Yu, C.H. Chemotherapeutic effects of luteolin on radio-sensitivity enhancement and interleukin-6/signal transducer and activator of transcription 3 signaling repression of oral cancer stem cells. J. Formos. Med. Assoc. 2016, 115, 1032–1038. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Khan, I.A.; Ur-Rehman, M.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer potential of quercetin: A comprehensive review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Kallifatidis, G.; Baumann, B.; Rausch, V.; Mattern, J.; Gladkich, J.; Giese, N.; Moldenhauer, G.; Wirth, T.; Büchler, M.W.; et al. Dietary polyphenol quercetin targets pancreatic cancer stem cells. Int. J. Oncol. 2010, 37, 551–561. [Google Scholar] [PubMed]
- Wang, R.; Yang, L.; Li, S.; Ye, D.; Yang, L.; Liu, Q.; Zhao, Z.; Cai, Q.; Tan, J.; Li, X. Quercetin Inhibits Breast Cancer Stem Cells via Downregulation of Aldehyde Dehydrogenase 1A1 (ALDH1A1), Chemokine Receptor Type 4 (CXCR4), Mucin 1 (MUC1), and Epithelial Cell Adhesion Molecule (EpCAM). Med. Sci. Monit. 2018, 24, 412–420. [Google Scholar] [CrossRef]
- Shen, X.; Si, Y.; Wang, Z.; Wang, J.; Guo, Y.; Zhang, X. Quercetin inhibits the growth of human gastric cancer stem cells by inducing mitochondrial-dependent apoptosis through the inhibition of PI3K/Akt signaling. Int. J. Mol. Med. 2016, 38, 619–626. [Google Scholar] [CrossRef]
- Viallard, C.; Larrivée, B. Tumor angiogenesis and vascular normalization: Alternative therapeutic targets. Angiogenesis 2017, 20, 409–426. [Google Scholar] [CrossRef]
- Ramjiawan, R.R.; Griffioen, A.W.; Duda, D.G. Anti-angiogenesis for cancer revisited: Is there a role for combinations with immunotherapy? Angiogenesis 2017, 20, 185–204. [Google Scholar] [CrossRef]
- Suvarna, V.; Murahari, M.; Khan, T.; Chaubey, P.; Sangave, P. Phytochemicals and PI3K Inhibitors in Cancer—An Insight. Front. Pharmacol. 2017, 8, 916. [Google Scholar] [CrossRef]
- Mirossay, L.; Varinská, L.; Mojžiš, J. Antiangiogenic Effect of Flavonoids and Chalcones: An Update. Int. J. Mol. Sci. 2017, 19, 27. [Google Scholar] [CrossRef]
- Chin, H.K.; Horng, C.T.; Liu, Y.S.; Lu, C.C.; Su, C.Y.; Chen, P.S.; Chiu, H.Y.; Tsai, F.J.; Shieh, P.C.; Yang, J.S. Kaempferol inhibits angiogenic ability by targeting VEGF receptor-2 and downregulating the PI3K/AKT, MEK and ERK pathways in VEGF-stimulated human umbilical vein endothelial cells. Oncol. Rep. 2018, 39, 2351–2357. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, H.; Yuan, B.; Hu, X.; Okazaki, M. Chemopreventive and anticancer activity of flavonoids and its possibility for clinical use by combining with conventional chemotherapeutic agents. Am. J. Cancer Res. 2019, 9, 1517–1535. [Google Scholar] [PubMed]
- Li, H.; Chen, C. Quercetin Has Antimetastatic Effects on Gastric Cancer Cells via the Interruption of uPA/uPAR Function by Modulating NF-kappab, PKC-delta, ERK1/2, and AMPKalpha. Integr. Cancer Ther. 2018, 17, 511–523. [Google Scholar] [CrossRef]
- Yao, X.; Jiang, W.; Yu, D.; Yan, Z. Luteolin inhibits proliferation and induces apoptosis of human melanoma cells in vivo and in vitro by suppressing MMP-2 and MMP-9 through the PI3K/AKT pathway. Food Funct. 2019, 10, 703–712. [Google Scholar] [CrossRef]
- Du, G.; Lin, H.; Wang, M.; Zhang, S.; Wu, X.; Lu, L.; Ji, L.; Yu, L. Quercetin greatly improved therapeutic index of doxorubicin against 4t1 breast cancer by its opposing effects on hif-1alpha in tumor and normal cells. Cancer Chemother. Pharmacol. 2010, 65, 277–287. [Google Scholar] [CrossRef]
- Provenzano, B.; Lentini, A.; Tatti, R.; De Martino, A.; Borromeo, I.; Mischiati, C.; Feriotto, G.; Forni, C.; Tabolacci, C.; Beninati, S. Evaluation of polyamines as marker of melanoma cell proliferation and differentiation by an improved high-performance liquid chromatographic method. Amino Acids 2019, 51, 1623–1631. [Google Scholar] [CrossRef] [PubMed]
- Forni, C.; Braglia, R.; Lentini, A.; Nuccetelli, M.; Provenzano, B.; Tabolacci, C.; Beninati, S. Role of transglutaminase 2 in quercetin-induced differentiation of B16-F10 murine melanoma cells. Amino Acids 2009, 36, 731–738. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Grandits, A.M.; Purton, L.E.; Sill, H.; Wieser, R. All-trans retinoic acid in non-promyelocytic acute myeloid leukemia: Driver lesion dependent effects on leukemic stem cells. Cell Cycle 2020, 19, 2573–2588. [Google Scholar] [CrossRef] [PubMed]
- Moradzadeh, M.; Roustazadeh, A.; Tabarraei, A.; Erfanian, S.; Sahebkar, A. Epigallocatechin-3-gallate enhances differentiation of acute promyelocytic leukemia cells via inhibition of PML-RARalpha and HDAC1. Phytother. Res. 2018, 32, 471–479. [Google Scholar] [CrossRef]
- Yang, H.; Hui, H.; Wang, Q.; Li, H.; Zhao, K.; Zhou, Y.; Zhu, Y.; Wang, X.; You, Q.; Guo, Q.; et al. Wogonin induces cell cycle arrest and erythroid differentiation in imatinib-resistant K562 cells and primary CML cells. Oncotarget 2020, 11, 300–301. [Google Scholar] [CrossRef]
- Tomko, A.M.; Whynot, E.G.; Ellis, L.D.; Dupré, D.J. Anti-Cancer Potential of Cannabinoids, Terpenes, and Flavonoids Present in Cannabis. Cancers 2020, 12, 1985. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Sun, H.; Dang, Y.; Li, Z. Isoliquiritigenin inhibits the proliferation and induces the differentiation of human glioma stem cells. Oncol. Rep. 2018, 39, 687–694. [Google Scholar] [CrossRef]
- He, M.H.; Zhang, Q.; Shu, G.; Lin, J.C.; Zhao, L.; Liang, X.X.; Yin, L.; Shi, F.; Fu, H.L.; Yuan, Z.X. Dihydromyricetin sensitizes human acute myeloid leukemia cells to retinoic acid-induced myeloid differentiation by activating STAT1. Biochem. Biophys. Res. Commun. 2018, 495, 1702–1707. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Fu, Q.; Deng, C.; Luo, L.; Xiang, T.; Zhao, H. Immunomodulatory potential of flavonoids for the treatment of autoimmune diseases and tumour. Scand. J. Immunol. 2022, 95, 16–34. [Google Scholar] [CrossRef]
- Hosseinzade, A.; Sadeghi, O.; Naghdipour Biregani, A.; Soukhtehzari, S.; Brandt, G.S.; Esmaillzadeh, A. Immunomodulatory effects of flavonoids: Possible induction of t cd4+ regulatory cells through suppression of mtor pathway signaling activity. Front. Immunol. 2019, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Ahn-Jarvis, J.H.; Parihar, A.; Doseff, A.I. Dietary flavonoids for immunoregulation and cancer: Food design for targeting disease. Antioxidants 2019, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Fereidouni, M.; Pirro, M.; Bianconi, V.; Sahebkar, A. Modulation of regulatory t cells by natural products in cancer. Cancer Lett. 2019, 459, 72–85. [Google Scholar] [CrossRef]
- Chen, X.; Du, Y.; Lin, X.; Qian, Y.; Zhou, T.; Huang, Z. Cd4+cd25+ regulatory t cells in tumor immunity. Int. Immunopharmacol. 2016, 34, 244–249. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Tian, K.; Chen, X.; Zhang, R.; Mu, X.; Wu, Y.; Wang, D.; Wang, S.; Liu, F.; et al. Apigenin suppresses pd-l1 expression in melanoma and host dendritic cells to elicit synergistic therapeutic effects. J. Exp. Clin. Cancer Res. 2018, 37, 261. [Google Scholar] [CrossRef]
- Casey, S.C.; Amedei, A.; Aquilano, K.; Azmi, A.S.; Benencia, F.; Bhakta, D.; Bilsland, A.E.; Boosani, C.S.; Chen, S.; Ciriolo, M.R.; et al. Cancer prevention and therapy through the modulation of the tumor microenvironment. Semin. Cancer Biol. 2015, 35, S199–S223. [Google Scholar] [CrossRef]
- Martinez, G.; Mijares, M.R.; De Sanctis, J.B. Effects of flavonoids and its derivatives on immune cell responses. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 84–104. [Google Scholar] [CrossRef]
- Ke, M.; Zhang, Z.; Xu, B.; Zhao, S.; Ding, Y.; Wu, X.; Wu, R.; Lv, Y.; Dong, J. Baicalein and baicalin promote antitumor immunity by suppressing pd-l1 expression in hepatocellular carcinoma cells. Int. Immunopharmacol. 2019, 75, 105824. [Google Scholar] [CrossRef] [PubMed]
- Coombs, M.R.P.; Harrison, M.E.; Hoskin, D.W. Apigenin inhibits the inducible expression of programmed death ligand 1 by human and mouse mammary carcinoma cells. Cancer Lett. 2016, 380, 424–433. [Google Scholar] [CrossRef]
- Lamy, S.; Blanchette, M.; Michaud-Levesque, J.; Lafleur, R.; Durocher, Y.; Moghrabi, A.; Barrette, S.; Gingras, D.; Béliveau, R. Delphinidin, a dietary anthocyanidin, inhibits vascular endothelial growth factor receptor-2 phosphorylation. Carcinogenesis 2005, 27, 989–996. [Google Scholar] [CrossRef]
- Mace, T.A.; King, S.A.; Ameen, Z.; Elnaggar, O.; Young, G.; Riedl, K.M.; Schwartz, S.J.; Clinton, S.K.; Knobloch, T.J.; Weghorst, C.M.; et al. Bioactive compounds or metabolites from black raspberries modulate T lymphocyte proliferation, myeloid cell differentiation and Jak/STAT signaling. Cancer Immunol. Immunother. 2014, 63, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Ting, H.; Deep, G.; Kumar, S.; Jain, A.K.; Agarwal, C.; Agarwal, R. Beneficial effects of the naturally occurring flavonoid silibinin on the prostate cancer microenvironment: Role of monocyte chemotactic protein-1 and immune cell recruitment. Carcinogenesis 2016, 37, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Oghumu, S.; Casto, B.C.; Ahn-Jarvis, J.; Weghorst, L.C.; Maloney, J.; Geuy, P.; Horvath, K.Z.; Bollinger, C.E.; Warner, B.M.; Summersgill, K.F.; et al. Inhibition of pro-inflammatory and anti-apoptotic biomarkers during experimental oral cancer chemoprevention by dietary black raspberries. Front. Immunol. 2017, 8, 1325. [Google Scholar] [CrossRef] [PubMed]
- Vargo, M.A.; Voss, O.H.; Poustka, F.; Cardounel, A.J.; Grotewold, E.; Doseff, A.I. Apigenin-induced-apoptosis is mediated by the activation of PKCδ and caspases in leukemia cells. Biochem. Pharmacol. 2006, 72, 681–692. [Google Scholar] [CrossRef]
- Nicholas, C.; Batra, S.; Vargo, M.A.; Voss, O.H.; Gavrilin, M.A.; Wewers, M.D.; Guttridge, D.C.; Grotewold, E.; Doseff, A.I. Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-κB through the suppression of p65 phosphorylation. J. Immunol. 2007, 179, 7121–7127. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Barcellos-Hoff, M.H. It takes a tissue to make a tumor: Epigenetics, cancer and the microenvironment. J. Mammary Gland Biol. Neoplasia 2001, 6, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Tlsty, T.D. Stromal cells can contribute oncogenic signals. Semin. Cancer Biol. 2001, 11, 97–104. [Google Scholar] [CrossRef]
- Pupa, S.M.; Ménard, S.; Forti, S.; Tagliabue, E. New insights into the role of extracellular matrix during tumor onset and progression. J. Cell. Physiol. 2002, 192, 259–267. [Google Scholar] [CrossRef] [PubMed]
- De Wever, O.; Mareel, M. Role of tissue stroma in cancer cell invasion. J. Pathol. 2003, 200, 429–447. [Google Scholar] [CrossRef]
- Lyssiotis, C.A.; Kimmelman, A.C. Metabolic interactions in the tumor microenvironment. Trends Cell Biol. 2017, 27, 863–875. [Google Scholar] [CrossRef]
- Chambers, A.M.; Wang, J.; Lupo, K.B.; Yu, H.; Lanman, N.M.A.; Matosevic, S. Adenosinergic signaling alters natural killer cell functional responses. Front. Immunol. 2018, 9, 2533. [Google Scholar] [CrossRef]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef]
- Murray, S.; Lundqvist, A. Targeting the tumor microenvironment to improve natural killer cell-based immunotherapies: On being in the right place at the right time, with resilience. Hum. Vaccin. Immunother. 2016, 12, 607–611. [Google Scholar] [CrossRef]
- Ichihara, F.; Kono, K.; Takahashi, A.; Kawaida, H.; Sugai, H.; Fujii, H. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin. Cancer Res. 2003, 9, 4404–4408. [Google Scholar]
- Kono, K.; Kawaida, H.; Takahashi, A.; Sugai, H.; Mimura, K.; Miyagawa, N.; Omata, H.; Fujii, H. CD4 (+) CD25 high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol. Immunother. 2006, 55, 1064–1071. [Google Scholar] [CrossRef]
- Diaz-Montero, C.M.; Salem, M.L.; Nishimura, M.I.; Garrett-Mayer, E.; Cole, D.J.; Montero, A.J. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin–cyclophosphamide chemotherapy. Cancer Immunol. Immunother. 2009, 58, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Porembka, M.R.; Mitchem, J.B.; Belt, B.A.; Hsieh, C.S.; Lee, H.M.; Herndon, J.; Gillanders, W.E.; Linehan, D.C.; Goedegebuure, P. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer Immunol. Immunother. 2012, 61, 1373–1385. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dabrosin, C.; Yin, X.; Fuster, M.M.; Arreola, A.; Rathmell, W.K.; Generali, D.; Nagaraju, G.P.; El-Rayes, B.; Ribatti, D.; et al. Broad targeting of angiogenesis for cancer prevention and therapy. Semin. Cancer Biol. 2015, 35, S224–S243. [Google Scholar] [CrossRef] [PubMed]
- Jassar, A.S.; Suzuki, E.; Kapoor, V.; Sun, J.; Silverberg, M.B.; Cheung, L.; Burdick, M.D.; Strieter, R.M.; Ching, L.M.; Kaiser, L.R.; et al. Activation of tumor-associated macrophages by the vascular disrupting agent 5, 6-dimethylxanthenone-4-acetic acid induces an effective CD8+ T-cell–mediated antitumor immune response in murine models of lung cancer and mesothelioma. Cancer Res. 2005, 65, 11752–11761. [Google Scholar] [CrossRef]
- Genard, G.; Lucas, S.; Michiels, C. Reprogramming of tumor-associated macrophages with anticancer therapies: Radiotherapy versus chemo-and immunotherapies. Front. Immunol. 2017, 8, 828. [Google Scholar] [CrossRef]
- Tan, H.Y.; Wang, N.; Man, K.; Tsao, S.W.; Che, C.M.; Feng, Y. Autophagy-induced RelB/p52 activation mediates tumour-associated macrophage repolarisation and suppression of hepatocellular carcinoma by natural compound baicalin. Cell Death Dis. 2015, 6, e1942. [Google Scholar] [CrossRef]
- Choi, H.J.; Choi, H.J.; Chung, T.W.; Ha, K.T. Luteolin inhibits recruitment of monocytes and migration of Lewis lung carcinoma cells by suppressing chemokine (C–C motif) ligand 2 expression in tumor-associated macrophage. Biochem. Biophys. Res. Commun. 2016, 470, 101–106. [Google Scholar] [CrossRef]
- Uchino, R.; Madhyastha, R.; Madhyastha, H.; Dhungana, S.; Nakajima, Y.; Omura, S.; Maruyama, M. NFκB-dependent regulation of urokinase plasminogen activator by proanthocyanidin-rich grape seed extract: Effect on invasion by prostate cancer cells. Blood Coagul. Fibrinolysis 2010, 21, 528–533. [Google Scholar] [CrossRef]
- Katiyar, S.K. Grape seed proanthocyanidines and skin cancer prevention: Inhibition of oxidative stress and protection of immune system. Mol. Nutr Food Res. 2008, 52, S71–S76. [Google Scholar] [CrossRef]
- Feriotto, G.; Tagliati, F.; Giriolo, R.; Casciano, F.; Tabolacci, C.; Beninati, S.; Khan, M.T.H.; Mischiati, C. Caffeic Acid Enhances the Anti-Leukemic Effect of Imatinib on Chronic Myeloid Leukemia Cells and Triggers Apoptosis in Cells Sensitive and Resistant to Imatinib. Int. J. Mol. Sci. 2021, 22, 1644. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Okusumi, S.; Yoshino, Y.; Moriyama, C.; Tanaka, S.; Hirano, T.; Takagi, N.; Toyoda, H. Delphinidin induces cytotoxicity and potentiates cytocidal effect in combination with arsenite in an acute promyelocytic leukemia NB4 cell line. Oncol. Rep. 2015, 34, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Sang, D.P.; Li, R.J.; Lan, Q. Quercetin sensitizes human glioblastoma cells to temozolomide in vitro via inhibition of Hsp27. Acta Pharmacol. Sin. 2014, 35, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Desai, V.; Jain, A.; Shaghaghi, H.; Summer, R.; Lai, J.C.K.; Bhushan, A. Combination of Biochanin A and Temozolomide Impairs Tumor Growth by Modulating Cell Metabolism in Glioblastoma Multiforme. Anticancer. Res. 2019, 39, 57–66. [Google Scholar] [CrossRef]
- Bieg, D.; Sypniewski, D.; Nowak, E.; Bednarek, I. Morin decreases galectin-3 expression and sensitizes ovarian cancer cells to cisplatin. Arch. Gynecol. Obstet. 2018, 298, 1181–1194. [Google Scholar] [CrossRef]
- Singh, M.P.; Cho, H.J.; Kim, J.T.; Baek, K.E.; Lee, H.G.; Kang, S.C. Morin hydrate reverses cisplatin resistance by impairing parp1/hmgb1-dependent autophagy in hepatocellular carcinoma. Cancers 2019, 11, 986. [Google Scholar] [CrossRef]
- Riahi-Chebbi, I.; Souid, S.; Othman, H.; Haoues, M.; Karoui, H.; Morel, A.; Srairi-Abid, N.; Essafi, M.; Essafi-Benkhadir, K. The phenolic compound kaempferol overcomes 5-fluorouracil resistance in human resistant ls174 colon cancer cells. Sci. Rep. 2019, 9, 195. [Google Scholar] [CrossRef]
- Scagliarini, A.; Mathey, A.; Aires, V.; Delmas, D. Xanthohumol, a prenylated flavonoid from hops, induces DNA damages in colorectal cancer cells and sensitizes sw480 cells to the sn38 chemotherapeutic agent. Cells 2020, 9, 932. [Google Scholar] [CrossRef]
- Hua, R.; Pei, Y.; Gu, H.; Sun, Y.; He, Y. Antitumor effects of flavokawain-b flavonoid in gemcitabine-resistant lung cancer cells are mediated via mitochondrial-mediated apoptosis, ros production, cell migration and cell invasion inhibition and blocking of pi3k/akt signaling pathway. J. Balk. Union Oncol. 2021, 26, 645. [Google Scholar]
- Karin, M. Nuclear factor-kappab in cancer development and progression. Nature 2006, 441, 431–436. [Google Scholar] [CrossRef]
- Holley, A.K.; Xu, Y.; St Clair, D.K.; St Clair, W.H. Relb regulates manganese superoxide dismutase gene and resistance to ionizing radiation of prostate cancer cells. Ann. N. Y. Acad. Sci. 2010, 1201, 129–136. [Google Scholar] [CrossRef]
- Hellweg, C.E.; Spitta, L.F.; Henschenmacher, B.; Diegeler, S.; Baumstark-Khan, C. Transcription factors in the cellular response to charged particle exposure. Front. Oncol. 2016, 6, 61. [Google Scholar] [CrossRef]
- Fan, M.; Ahmed, K.M.; Coleman, M.C.; Spitz, D.R.; Li, J.J. Nuclear factor-kappab and manganese superoxide dismutase mediate adaptive radioresistance in low-dose irradiated mouse skin epithelial cells. Cancer Res. 2007, 67, 3220–3228. [Google Scholar] [CrossRef]
- Catz, S.D.; Johnson, J.L. Transcriptional regulation of bcl-2 by nuclear factor kappa b and its significance in prostate cancer. Oncogene 2001, 20, 7342–7351. [Google Scholar] [CrossRef] [PubMed]
- Galeaz, C.; Totis, C.; Bisio, A. Radiation resistance: A matter of transcription factors. Front. Oncol. 2021, 11, 662840. [Google Scholar] [CrossRef] [PubMed]
- Minnelli, C.; Cianfruglia, L.; Laudadio, E.; Mobbili, G.; Galeazzi, R.; Armeni, T. Effect of Epigallocatechin-3-Gallate on EGFR Signaling and Migration in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2021, 22, 11833. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Abuawad, A.; Thiab, S.; Alshweiat, A.; Mahmod, A.I. Flavonoid-based nanomedicines to target tumor microenvironment. OpenNano 2022, 8, 100081. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, G.; Liu, S.; Su, H.; Wang, Y.; Li, J.; Luo, C. Remodeling the tumor microenvironment with emerging nanotherapeutics. Trends Pharmacol. Sci. 2018, 39, 59–74. [Google Scholar] [CrossRef]
- Luo, C.; Sun, J.; Sun, B.; He, Z. Prodrug-based nanoparticulate drug delivery strategies for cancer therapy. Trends Pharmacol. Sci. 2014, 35, 556–566. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Mendes, R.; Baptista, P.V.; Fernandes, A.R. Targeting tumor microenvironment for cancer therapy. Int. J. Mol. Sci. 2019, 20, 840. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Reviews. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef]
- Feng, L.; Cheng, L.; Dong, Z.; Tao, D.; Barnhart, T.E.; Cai, W.; Chen, M.; Liu, Z. Theranostic liposomes with hypoxia-activated prodrug to effectively destruct hypoxic tumors post-photodynamic therapy. ACS Nano 2017, 11, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Bu, W.; Cheng, C.; Zuo, C.; Xiao, Q.; Sun, Y.; Ni, D.; Zhang, C.; Liu, J.; et al. Hypoxia induced by upconversion-based photodynamic therapy: Towards highly effective synergistic bioreductive therapy in tumors. Angew. Chem. 2015, 54, 8105–8109. [Google Scholar] [CrossRef]
- Hsieh, D.S.; Wang, H.; Tan, S.W.; Huang, Y.H.; Tsai, C.Y.; Yeh, M.K.; Wu, C.J. The treatment of bladder cancer in a mouse model by epigallocatechin-3-gallate-gold nanoparticles. Biomaterials 2011, 32, 7633–7640. [Google Scholar] [CrossRef]
- Shukla, R.; Chanda, N.; Zambre, A.; Upendran, A.; Katti, K.; Kulkarni, R.R.; Nune, S.K.; Casteel, S.W.; Smith, C.J.; Vimal, J.; et al. Laminin receptor specific therapeutic gold nanoparticles (198aunp-egcg) show efficacy in treating prostate cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 12426–12431. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Hsieh, D.S.; Huang, K.J.; Chan, Y.L.; Hong, P.D.; Yeh, M.K.; Wu, C.J. Improving anticancer efficacy of (-)-epigallocatechin-3-gallate gold nanoparticles in murine b16f10 melanoma cells. Drug Des. Dev. Ther. 2014, 8, 459–474. [Google Scholar]
- Wang, W.; Tang, Q.; Yu, T.; Li, X.; Gao, Y.; Li, J.; Liu, Y.; Rong, L.; Wang, Z.; Sun, H.; et al. Surfactant-free preparation of au@resveratrol hollow nanoparticles with photothermal performance and antioxidant activity. ACS Appl. Mater. Interfaces 2017, 9, 3376–3387. [Google Scholar] [CrossRef]
- Park, S.Y.; Chae, S.Y.; Park, J.O.; Lee, K.J.; Park, G. Gold-conjugated resveratrol nanoparticles attenuate the invasion and mmp-9 and cox-2 expression in breast cancer cells. Oncol. Rep. 2016, 35, 3248–3256. [Google Scholar] [CrossRef]
- Kamal, R.; Chadha, V.D.; Dhawan, D.K. Physiological uptake and retention of radiolabeled resveratrol loaded gold nanoparticles ((99m)tc-res-aunp) in colon cancer tissue. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1059–1071. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, J.; Zeng, J.; Li, Z.; Zuo, H.; Huang, C.; Zhao, X. Nano-gold loaded with resveratrol enhance the anti-hepatoma effect of resveratrol in vitro and in vivo. J. Biomed. Nanotechnol. 2019, 15, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Thipe, V.C.; Panjtan Amiri, K.; Bloebaum, P.; Raphael Karikachery, A.; Khoobchandani, M.; Katti, K.K.; Jurisson, S.S.; Katti, K.V. Development of resveratrol-conjugated gold nanoparticles: Interrelationship of increased resveratrol corona on anti-tumor efficacy against breast, pancreatic and prostate cancers. Int. J. Nanomed. 2019, 14, 4413–4428. [Google Scholar] [CrossRef]
- Kasthuri, J.; Veerapandian, S.; Rajendiran, N. Biological synthesis of silver and gold nanoparticles using apiin as reducing agent. Colloids Surf. B Biointerfaces 2009, 68, 55–60. [Google Scholar] [CrossRef]
- Satyavani, K.; Gurudeeban, S.; Ramanathan, T.; Balasubramanian, T. Biomedical potential of silver nanoparticles synthesized from calli cells of citrullus colocynthis (l.) schrad. J. Nanobiotechnology 2011, 9, 43. [Google Scholar]
- Stolarczyk, E.U.; Stolarczyk, K.; Laszcz, M.; Kubiszewski, M.; Maruszak, W.; Olejarz, W.; Bryk, D. Synthesis and characterization of genistein conjugated with gold nanoparticles and the study of their cytotoxic properties. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2017, 96, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Govindaraju, S.; Roshini, A.; Lee, M.H.; Yun, K. Kaempferol conjugated gold nanoclusters enabled efficient for anticancer therapeutics to a549 lung cancer cells. Int. J. Nanomed. 2019, 14, 5147–5157. [Google Scholar] [CrossRef] [PubMed]
- Binu, N.M.; Prema, D.; Prakash, J.; Balagangadharan, K.; Balashanmugam, P.; Selvamurugan, N.; Venkatasubbu, G.D. Folic acid decorated pH sensitive polydopamine coated honeycomb structured nickel oxide nanoparticles for targeted delivery of quercetin to triple negative breast cancer cells. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127609. [Google Scholar] [CrossRef]
- Guo, D.; Wu, C.; Li, J.; Guo, A.; Li, Q.; Jiang, H.; Chen, B.; Wang, X. Synergistic effect of functionalized nickel nanoparticles and quercetin on inhibition of the smmc-7721 cells proliferation. Nanoscale Res. Lett. 2009, 4, 1395–1402. [Google Scholar] [CrossRef]
- Sivakumar, P.; Lee, M.; Kim, Y.S.; Shim, M.S. Photo-triggered antibacterial and anticancer activities of zinc oxide nanoparticles. J. Mater. Chem. B 2018, 6, 4852–4871. [Google Scholar] [CrossRef] [PubMed]
- DeLong, R.K.; Comer, J.; Mathew, E.N.; Jaberi-Douraki, M. Comparative molecular immunological activity of physiological metal oxide nanoparticle and its anticancer peptide and rna complexes. Nanomaterials 2019, 9, 1670. [Google Scholar] [CrossRef]
- Sadhukhan, P.; Kundu, M.; Chatterjee, S.; Ghosh, N.; Manna, P.; Das, J.; Sil, P.C. Targeted delivery of quercetin via ph-responsive zinc oxide nanoparticles for breast cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Kundu, M.; Sadhukhan, P.; Ghosh, N.; Chatterjee, S.; Manna, P.; Das, J.; Sil, P.C. Ph-responsive and targeted delivery of curcumin via phenylboronic acid-functionalized zno nanoparticles for breast cancer therapy. J. Adv. Res. 2019, 18, 161–172. [Google Scholar] [CrossRef] [PubMed]
- George, D.; Maheswari, P.U.; Begum, K. Chitosan-cellulose hydrogel conjugated with l-histidine and zinc oxide nanoparticles for sustained drug delivery: Kinetics and in-vitro biological studies. Carbohydr. Polym. 2020, 236, 116101. [Google Scholar] [CrossRef] [PubMed]
- Perera, W.; Dissanayake, R.K.; Ranatunga, U.I.; Hettiarachchi, N.M.; Perera, K.D.C.; Unagolla, J.M.; De Silva, R.T.; Pahalagedara, L.R. Curcumin loaded zinc oxide nanoparticles for activity-enhanced antibacterial and anticancer applications. RSC Adv. 2020, 10, 30785–30795. [Google Scholar] [CrossRef]
- Sawant, V.J.; Bamane, S.R. PEG-Beta-Cyclodextrin Functionalized Zinc Oxide Nanoparticles Show Cell Imaging with High Drug Payload and Sustained pH Responsive Delivery of Curcumin in to MCF-7 cells. J. Drug Deliv. Sci. Technol. 2018, 43, 397–408. [Google Scholar] [CrossRef]
- Cordani, M.; Strippoli, R.; Somoza, Á. Nanomaterials as Inhibitors of Epithelial Mesenchymal Transition in Cancer Treatment. Cancers 2020, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Arvizo, R.; Bhattacharya, R.; Mukherjee, P. Gold nanoparticles: Opportunities and challenges in nanomedicine. Expert Opin. Drug Deliv. 2010, 7, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Arvizo, R.R.; Saha, S.; Wang, E.; Robertson, J.D.; Bhattacharya, R.; Mukherjee, P. Inhibition of tumor growth and metastasis by a self-therapeutic nanoparticle. Proc. Natl. Acad. Sci. USA 2013, 110, 6700–6705. [Google Scholar] [CrossRef]
- Xiong, X.; Arvizo, R.R.; Saha, S.; Robertson, D.J.; McMeekin, S.; Bhattacharya, R.; Mukherjee, P. Sensitization of ovarian cancer cells to cisplatin by gold nanoparticles. Oncotarget 2014, 5, 6453–6465. [Google Scholar] [CrossRef]
- Huai, Y.; Zhang, Y.; Xiong, X.; Das, S.; Bhattacharya, R.; Mukherjee, P. Gold nanoparticles sensitize pancreatic cancer cells to gemcitabine. Cell Stress 2019, 3, 267–279. [Google Scholar] [CrossRef]
- Paolini, A.; Curti, V.; Pasi, F.; Mazzini, G.; Nano, R.; Capelli, E. Gallic acid exerts a protective or an anti-proliferative effect on glioma T98G cells via dose-dependent epigenetic regulation mediated by miRNAs. Int. J. Oncol. 2015, 46, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.-H.; Chang, C.-S.; Ho, W.-C.; Liao, S.-Y.; Wu, C.-H.; Wang, C.-J. Anti-metastasis effects of gallic acid on gastric cancer cells involves inhibition of NF-κB activity and downregulation of PI3K/AKT/small GTPase signals. Food Chem. Toxicol. 2010, 48, 2508–2516. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Wu, Y.C.; Chia, Y.C.; Chang, F.R.; Hsu, H.K.; Hsieh, Y.C.; Chen, C.C.; Yuan, S.S. Gallic acid, a major component of Toona sinensis leaf extracts, contains a ROS-mediated anti-cancer activity in human prostate cancer cells. Cancer Lett. 2009, 286, 161–171. [Google Scholar] [CrossRef]
- Jin, L.; Piao, Z.H.; Sun, S.; Liu, B.; Ryu, Y.; Choi, S.Y.; Kim, G.R.; Kim, H.S.; Kee, H.J.; Jeong, M.H. Gallic acid attenuates pulmonary fibrosis in a mouse model of transverse aortic contraction-induced heart failure. Vasc. Pharm. 2017, 99, 74–82. [Google Scholar] [CrossRef]
- Cordani, M.; Somoza, Á. Targeting autophagy using metallic nanoparticles: A promising strategy for cancer treatment. Cell. Mol. Life Sci. 2019, 76, 1215–1242. [Google Scholar] [CrossRef]
- Sunil Gowda, S.N.; Rajasowmiya, S.; Vadivel, V.; Banu Devi, S.; Celestin Jerald, A.; Marimuthu, S.; Devipriya, N. Gallic acid-coated sliver nanoparticle alters the expression of radiation-induced epithelial-mesenchymal transition in non-small lung cancer cells. Toxicol. In Vitro 2018, 52, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Deng, J.; Man, Y.; Qu, Y. Green tea extracts epigallocatechin-3-gallate for different treatments. Biomed Res. Int. 2017, 2017, 5615647. [Google Scholar] [CrossRef]
- Li, T.; Zhao, N.; Lu, J.; Zhu, Q.; Liu, X.; Hao, F.; Jiao, X. Epigallocatechin gallate (EGCG) suppresses epithelial-Mesenchymal transition (EMT) and invasion in anaplastic thyroid carcinoma cells through blocking of TGF-β1/Smad signaling pathways. Bioengineered 2019, 10, 282–291. [Google Scholar] [CrossRef]
- Kanlaya, R.; Khamchun, S.; Kapincharanon, C.; Thongboonkerd, V. Protective effect of epigallocatechin-3-gallate (EGCG) via Nrf2 pathway against oxalate-induced epithelial mesenchymal transition (EMT) of renal tubular cells. Sci. Rep. 2016, 6, 30233. [Google Scholar] [CrossRef]
- Lu, H.; Meng, X.; Yang, C.S. Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (−)-epigallocatechin gallate. Drug Metab. Dispos. 2003, 31, 572–579. [Google Scholar] [CrossRef]
- Huo, C.; Wan, S.B.; Lam, W.H.; Li, L.; Wang, Z.; Landis-Piwowar, K.R.; Chen, D.; Dou, Q.P.; Chan, T.H. The challenge of developing green tea polyphenols as therapeutic agents. Inflammopharmacology 2008, 16, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.K.; Sharma, P.; Zia, A.; Siwan, D.; Nandave, D.; Nandave, M.; Gautam, R.K. Metabolomics and EMT Markers of Breast Cancer: A Crosstalk and Future Perspective. Pathophysiology 2022, 29, 200–222. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jiang, W.; Xie, M. Flavonoids: Recent advances as anticancer drugs. Recent Pat. Anti-Cancer Drug Discov. 2010, 5, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Ye, T.; Xiang, Y.; Shi, Z.; Zhang, J.; Lou, B.; Zhang, F.; Chen, B.; Zhou, M. Quercetin inhibits epithelial–mesenchymal transition, decreases invasiveness and metastasis, and reverses IL-6 induced epithelial–mesenchymal transition, expression of MMP by inhibiting STAT3 signaling in pancreatic cancer cells. Onco Targets Ther. 2017, 10, 4719–4729. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Sharma, N. Inhibitory effect of quercetin on epithelial to mesenchymal transition in SK-MEL-28 human melanoma cells defined by in vitro analysis on 3D collagen gels. Onco Targets Ther. 2016, 9, 6445–6459. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Bhat, F.A.; Raja Singh, P.; Mukherjee, S.; Elumalai, P.; Das, S.; Patra, C.R.; Arunakaran, J. Gold nanoparticle–conjugated quercetin inhibits epithelial–mesenchymal transition, angiogenesis and invasiveness via EGFR/VEGFR-2-mediated pathway in breast cancer. Cell Prolif. 2016, 49, 678–697. [Google Scholar] [CrossRef]
- Hosta-Rigau, L.; Schattling, P.; Teo, B.M.; Lynge, M.E.; Städler, B. Recent progress of liposomes in nanomedicine. J. Mater. Chem. B 2014, 2, 6686–6691. [Google Scholar] [CrossRef]
- Fan, J.X.; Zheng, D.W.; Rong, L.; Zhu, J.Y.; Hong, S.; Li, C.; Xu, Z.S.; Cheng, S.X.; Zhang, X.Z. Targeting epithelial-mesenchymal transition: Metal organic network nano-complexes for preventing tumor metastasis. Biomaterials 2017, 139, 116–126. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Y.Y.; Li, Z.Y.; Ning, S.Q. Evaluation of the efficacy of paclitaxel with curcumin combination in ovarian cancer cells. Oncol. Lett. 2016, 12, 3944–3948. [Google Scholar] [CrossRef]
- Paramita, P.; Wardhani, B.W.K.; Wanandi, S.I.; Louisa, M. Curcumin for the prevention of epithelial-mesenchymal transition in endoxifen-treated MCF-7 breast cancer cells. Asian Pac. J. Cancer Prev. 2018, 19, 1243–1249. [Google Scholar]
- Jiao, D.; Wang, J.; Lu, W.; Tang, X.; Chen, J.; Mou, H.; Chen, Q. Curcumin inhibited HGF-induced EMT and angiogenesis through regulating c-Met dependent PI3K/Akt/mTOR signaling pathways in lung cancer. Mol. Ther. Oncolytics 2016, 3, 16018. [Google Scholar] [CrossRef]
- Zhao, J.L.; Guo, M.Z.; Zhu, J.J.; Zhang, T.; Min, D.Y. Curcumin suppresses epithelial-to-mesenchymal transition of peritoneal mesothelial cells (HMrSV5) through regulation of transforming growth factor-activated kinase 1 (TAK1). Cell. Mol. Biol. Lett. 2019, 24, 32. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Jaggi, M.C.; Chauhan, S. Curcumin nanomedicine: A road to cancer therapeutics. Curr. Pharm. Des. 2013, 19, 1994–2010. [Google Scholar]
- Kong, L.; Yuan, Q.; Zhu, H.; Li, Y.; Guo, Q.; Wang, Q.; Bi, X.; Gao, X. The suppression of prostate LNCaP cancer cells growth by Selenium nanoparticles through Akt/Mdm2/AR controlled apoptosis. Biomaterials 2011, 32, 6515–6522. [Google Scholar] [CrossRef]
- Luo, H.; Wang, F.; Bai, Y.; Chen, T.; Zheng, W. Selenium nanoparticles inhibit the growth of HeLa and MDA-MB-231 cells through induction of S phase arrest. Colloids Surf. B Biointerfaces 2012, 94, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Li, X.; Zhang, Y.; Xie, Q.; Wong, Y.S.; Zheng, W.; Chen, T. PEG-nanolized ultrasmall selenium nanoparticles overcome drug resistance in hepatocellular carcinoma HepG2 cells through induction of mitochondria dysfunction. Int. J. Nanomed. 2012, 7, 3939–3949. [Google Scholar]
- Kumari, M.; Ray, L.; Purohit, M.P.; Patnaik, S.; Pant, A.B.; Shukla, Y.; Kumar, P.; Gupta, K.C. Curcumin loading potentiates the chemotherapeutic efficacy of selenium nanoparticles in HCT116 cells and Ehrlich’s ascites carcinoma bearing mice. Eur. J. Pharm. Biopharm. 2017, 117, 346–362. [Google Scholar] [CrossRef]
- Kumari, M.; Purohit, M.P.; Patnaik, S.; Shukla, Y.; Kumar, P.; Gupta, K.C. Curcumin loaded selenium nanoparticles synergize the anticancer potential of doxorubicin contained in self-assembled, cell receptor targeted nanoparticles. Eur. J. Pharm. Biopharm. 2018, 130, 185–199. [Google Scholar] [CrossRef]
- Byers, L.A.; Diao, L.; Wang, J.; Saintigny, P.; Girard, L.; Peyton, M.; Shen, L.; Fan, Y.; Giri, U.; Tumula, P.K.; et al. An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin. Cancer Res. 2013, 19, 279–290. [Google Scholar] [CrossRef]
- Li, X.; Xing, L.; Zhang, Y.; Xie, P.; Zhu, W.; Meng, X.; Wang, Y.; Kong, L.; Zhao, H.; Yu, J. Phase ii trial of epigallocatechin-3-gallate in acute radiation-induced esophagitis for esophagus cancer. J. Med. Food 2020, 23, 43–49. [Google Scholar] [CrossRef]
- Kumar, N.B.; Pow-Sang, J.; Egan, K.M.; Spiess, P.E.; Dickinson, S.; Salup, R.; Helal, M.; McLarty, J.; Williams, C.R.; Schreiber, F.; et al. Randomized, placebo-controlled trial of green tea catechins for prostate cancer prevention. Cancer Prev. Res. 2015, 8, 879–887. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Call, T.G.; Zent, C.S.; LaPlant, B.; Bowen, D.A.; Roos, M.; Secreto, C.R.; Ghosh, A.K.; Kabat, B.F.; Lee, M.J.; et al. Phase i trial of daily oral polyphenon e in patients with asymptomatic rai stage 0 to ii chronic lymphocytic leukemia. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 3808–3814. [Google Scholar] [CrossRef] [PubMed]
- Farsad-Naeimi, A.; Alizadeh, M.; Esfahani, A.; Darvish Aminabad, E. Effect of fisetin supplementation on inflammatory factors and matrix metalloproteinase enzymes in colorectal cancer patients. Food Funct. 2018, 9, 2025–2031. [Google Scholar] [CrossRef]
- McLarty, J.; Bigelow, R.L.; Smith, M.; Elmajian, D.; Ankem, M.; Cardelli, J.A. Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in vitro. Cancer Prev. Res. 2009, 2, 673–682. [Google Scholar]
- Boots, A.W.; Drent, M.; de Boer, V.C.; Bast, A.; Haenen, G.R. Quercetin reduces markers of oxidative stress and inflammation in sarcoidosis. Clin. Nutr. 2011, 30, 506–512. [Google Scholar] [CrossRef]
- Domingo, D.S.; Camouse, M.M.; Hsia, A.H.; Matsui, M.; Maes, D.; Ward, N.L.; Cooper, K.D.; Baron, E.D. Anti-angiogenic effects of epigallocatechin-3-gallate in human skin. Int. J. Clin. Exp. Pathol. 2010, 3, 705–709. [Google Scholar] [PubMed]
- Farhan, M.; Rizvi, A. Understanding the prooxidant action of plant polyphenols in the cellular microenvironment of malignant cells: Role of copper and therapeutic implications. Front. Pharmacol. 2022, 13, 929853. [Google Scholar] [CrossRef] [PubMed]
- Ponte, L.G.S.; Pavan, I.C.B.; Mancini, M.C.S.; da Silva, L.G.S.; Morelli, A.P.; Severino, M.B.; Bezerra, R.M.N.; Simabuco, F.M. The Hallmarks of Flavonoids in Cancer. Molecules 2021, 26, 2029. [Google Scholar] [CrossRef]
- Messing, E.; Gee, J.R.; Saltzstein, D.R.; Kim, K.; DiSant’Agnese, A.; Kolesar, J.; Harris, L.; Faerber, A.; Havighurst, T.; Young, J.M.; et al. A Phase 2 Cancer Chemoprevention Biomarker Trial of Isoflavone G-2535 (Genistein) in Presurgical Bladder Cancer Patients. Cancer Prev. Res. 2012, 5, 621–630. [Google Scholar] [CrossRef]
- Pintova, S.; Dharmupari, S.; Moshier, E.; Zubizarreta, N.; Ang, C.; Holcombe, R.F. Genistein combined with FOLFOX or FOLFOX–Bevacizumab for the treatment of metastatic colorectal cancer: Phase I/II pilot study. Cancer Chemother. Pharmacol. 2019, 84, 591–598. [Google Scholar] [CrossRef]
- Citrin, D.E.; Prasanna, P.G.S.; Walker, A.J.; Freeman, M.L.; Eke, I.; Barcellos-Hoff, M.H.; Arankalayil, M.J.; Cohen, E.P.; Wilkins, R.C.; Ahmed, M.M.; et al. Radiation-Induced Fibrosis: Mechanisms and Opportunities to Mitigate. Report of an NCI Workshop, September 19, 2016. Radiat. Res. 2017, 188, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Gálvez, M.Á.; García-Villalba, R.; Martínez-Díaz, F.; Ocaña-Castillo, B.; Monedero-Saiz, T.; Torrecillas-Sánchez, A.; Abellán, B.; González-Sarrías, A.; Espín, J.C. Metabolic Profiling of Dietary Polyphenols and Methylxanthines in Normal and Malignant Mammary Tissues from Breast Cancer Patients. Mol. Nutr. Food Res. 2019, 63, 1801239. [Google Scholar] [CrossRef]
- Nguyen, M.M.; Ahmann, F.R.; Nagle, R.B.; Hsu, C.-H.; Tangrea, J.A.; Parnes, H.L.; Sokoloff, M.H.; Gretzer, M.B.; Chow, H.-H.S. Randomized, double-blind, placebo-controlled trial of polyphenon E in prostate cancer patients before prostatectomy: Evaluation of potential chemopreventive activities. Cancer Prev. Res. 2012, 5, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Samavat, H.; Wu, A.H.; Ursin, G.; Torkelson, C.J.; Wang, R.; Yu, M.C.; Yee, D.; Kurzer, M.S.; Yuan, J.-M. Green Tea Catechin Extract Supplementation Does Not Influence Circulating Sex Hormones and Insulin-Like Growth Factor Axis Proteins in a Randomized Controlled Trial of Postmenopausal Women at High Risk of Breast Cancer. J. Nutr. 2019, 149, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Garcia, F.A.R.; Cornelison, T.; Nuño, T.; Greenspan, D.L.; Byron, J.W.; Hsu, C.-H.; Alberts, D.S.; Chow, H.-H.S. Results of a phase II randomized, double-blind, placebo-controlled trial of Polyphenon E in women with persistent high-risk HPV infection and low-grade cervical intraepithelial neoplasia. Gynecol. Oncol. 2014, 132, 377–382. [Google Scholar] [CrossRef]
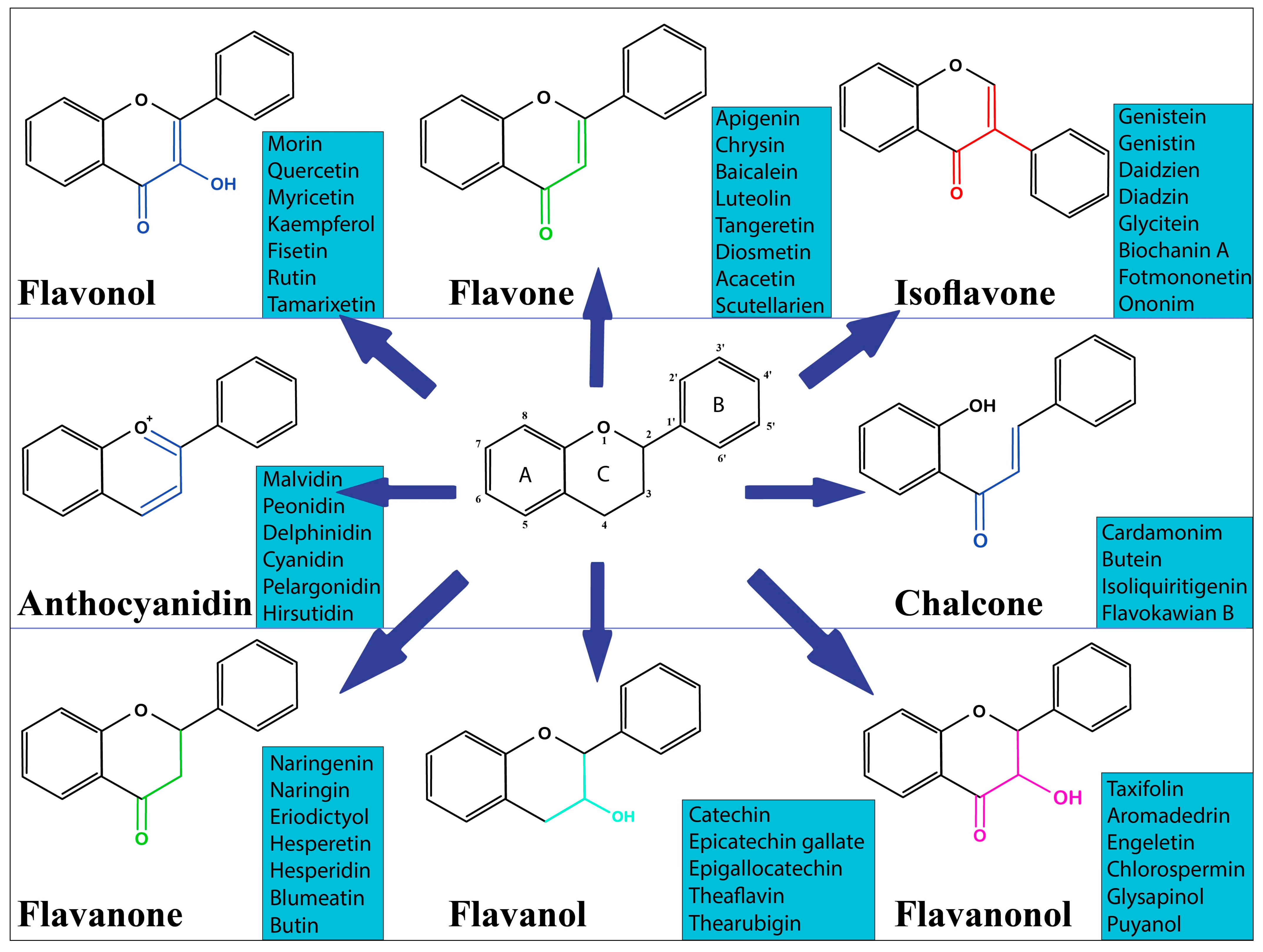
| Classes | Representative Flavonoids | Food Sources | References |
|---|---|---|---|
| Flavanol | Catechin, epicatechin, epigallocatechin-3-gallate | Tea | [27,28] |
| Flavanone | Naringin, naringenin, herperidin | Citrus fruits, oranges, grapefruits, lemons | [29] |
| Anthocyanins | Cyanidin, peonidin | Blackberry, blueberry, cherry, strawberry | [30] |
| Flavones | Chrysin, apigenin, luteolin | Celery, green peppers, parsley, peppermint | [31] |
| Flavonols | Kaempferol, quercetin, myricetin, rutin | Blueberries, apple, cabbage, broccoli, cherries, garlic, onion, tea, red wine | [32] |
| Isoflavonoids | Daidzein, genistein, glycitein | Legumes, soy | [33] |
| Tannins (condensed form) | Proanthocyanidins | Cocoa beans, apples, red wine | [34,35] |
| Deoxyanthocyanidins | Apigeninidin, luteolinidin | Sorghum, purple corn | [36] |
| Source of Flavonoid | Total Flavonoid Content (mg/100 g of Food/or Drink) | Major Flavonoid Subclass Present |
|---|---|---|
| Black elderberry | 1358.66 | Anthocyanin |
| Black chokeberry | 1012.98 | Anthocyanin |
| Blackcurrant | 608.43 | Anthocyanin |
| Cocoa powder | 511.62 | Flavan-3-ols |
| Soybean roasted | 253.11 | Isoflavonoids |
| Chocolate dark | 237.36 | Flavan-3-ols |
| Blackberry | 203.33 | Anthocyanin |
| Broad bean pod | 189.54 | Flavan-3-ols |
| Sweet cherry | 185.05 | Anthocyanin |
| Black olive | 159.83 | Anthocyanin |
| Soy tempeh | 147.74 | Isoflavonoids |
| Red onion | 131.51 | Flavonols |
| Spinach | 119.27 | Flavonols |
| Shallot | 112.22 | Flavonols |
| Plum | 101.67 | Anthocyanin |
| American cranberry | 93.73 | Anthocyanin |
| Black tea | 83.35 | Flavonols |
| Aestivalis grape | 81.44 | Anthocyanin |
| Green tea | 77.44 | Flavonols |
| Common wheat, whole grain flour | 77.4 | Flavones |
| Apple | 56.35 | Flavan-3-ols |
| Apple juice | 54.99 | Flavonols |
| Broad bean seed whole | 49.37 | Flavan-3-ols |
| Orange juice | 48.02 | Flavanones |
| Grapefruit juice | 47.12 | Flavanones |
| Lemon juice | 37.43 | Flavanones |
| Buckwheat, whole grain flour | 37.04 | Flavonols |
| Barley, whole grain flour | 35.2 | Flavan-3-ols |
| Plum juice | 30.55 | Flavonols |
| Broccoli | 27.8 | Flavonols |
| Red lettuce | 22.78 | Flavonols |
| Pistachio | 7.193 | Flavan-3-ols |
| Flavonoid | Cancer Type | Participant Count | FDA Certification Status | Trial Stage | References |
|---|---|---|---|---|---|
| Daidzein | Prostate Cancer | 43 | Phase II | Finished | [259] |
| Genistein | Prostate Cancer | 24 | Phase II | On hold | [259] |
| Bladder Cancer | 60 | Phase II | Ongoing | [260] | |
| Colon and Rectal Cancer | 13 | Phase II | Finished | [261] | |
| Non-small Cell Lung Cancer | 21 | Phase II | Ongoing | [262] | |
| Quercetin | Prostate Cancer | 31 | Phase I | Ongoing | [259] |
| Squamous Cell Carcinoma | 55 | Phase II | Ongoing | [259] | |
| Apigenin | Colorectal Cancer | 382 | Phase II | On hold | [259] |
| Hesperidin | Breast Cancer | 40 | N/A | Finished | [263] |
| Catechins | Prostate Cancer | 50 | Phase I | Finished | [264] |
| Breast Cancer | 1075 | Phase II | Finished | [265] | |
| Lung Cancer | 53 | Phase II | Finished | [259] | |
| Esophageal Cancer | 55 | Phase I | Finished | [259] | |
| Cervical Cancer | 98 | Phase II | Finished | [266] | |
| Cyanidin | Myelodysplastic Syndrome/Myeloproliferative Neoplasm | 21 | Phase II | Ongoing | [259] |
| Oral Cancer | 58 | N/A | Suspended | [259] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farhan, M.; Rizvi, A.; Aatif, M.; Ahmad, A. Current Understanding of Flavonoids in Cancer Therapy and Prevention. Metabolites 2023, 13, 481. https://doi.org/10.3390/metabo13040481
Farhan M, Rizvi A, Aatif M, Ahmad A. Current Understanding of Flavonoids in Cancer Therapy and Prevention. Metabolites. 2023; 13(4):481. https://doi.org/10.3390/metabo13040481
Chicago/Turabian StyleFarhan, Mohd, Asim Rizvi, Mohammad Aatif, and Aamir Ahmad. 2023. "Current Understanding of Flavonoids in Cancer Therapy and Prevention" Metabolites 13, no. 4: 481. https://doi.org/10.3390/metabo13040481
APA StyleFarhan, M., Rizvi, A., Aatif, M., & Ahmad, A. (2023). Current Understanding of Flavonoids in Cancer Therapy and Prevention. Metabolites, 13(4), 481. https://doi.org/10.3390/metabo13040481









