Usefulness of Oral Fluid for Measurement of Methylone and Its Metabolites: Correlation with Plasma Drug Concentrations and the Effect of Oral Fluid pH
Abstract
1. Introduction
- To investigate the concentrations of methylone and its metabolites’ MDC and HMMC in OF following controlled administration of different doses to healthy volunteers.
- To assess the eventual correlation between OF and methylone plasma concentrations and to determine the effect of the pH of OF on the methylone OF-to-plasma ratio (OF/P).
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Chemicals
2.3. Oral Fluid Samples Collection
2.4. Sample Preparation
2.5. pH Measurements of Oral Fluid Samples
2.6. Pharmacokinetics and Statistical Analysis
3. Results
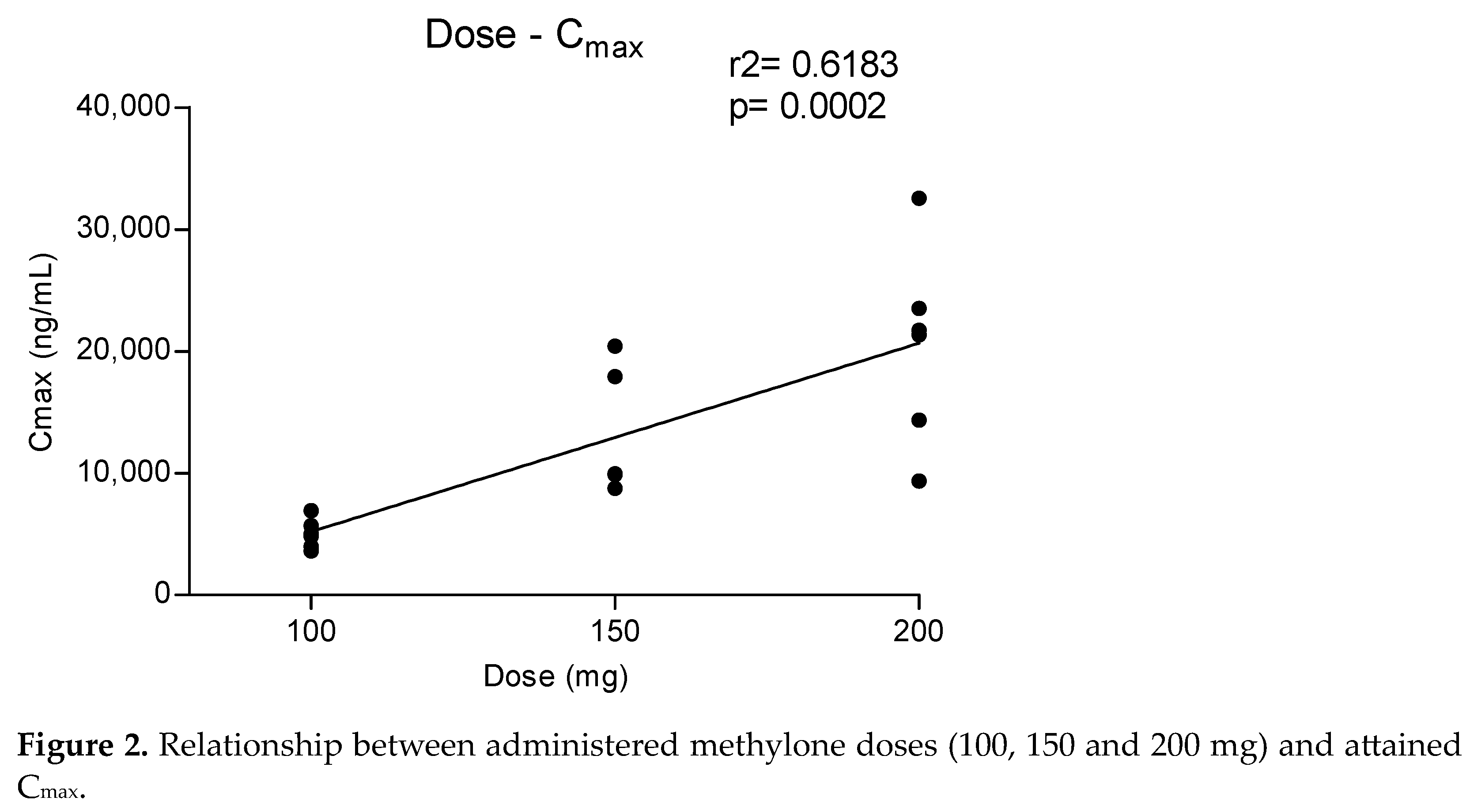
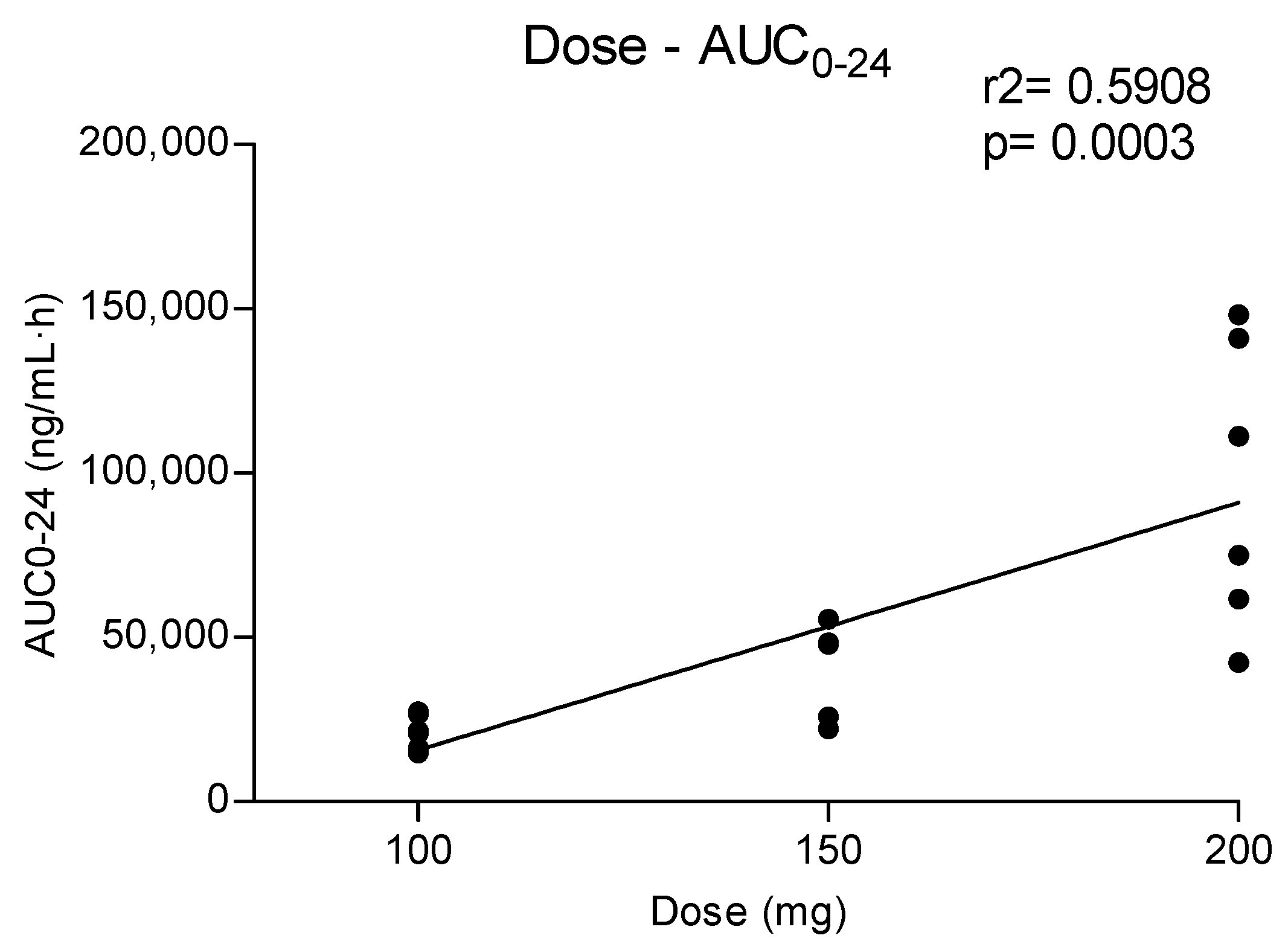
3.1. Concentration–Time Profile and Pharmacokinetic of Methylone, MDC and HMMC in Oral Fluid
3.2. Measurement of pH in Oral Fluid Samples
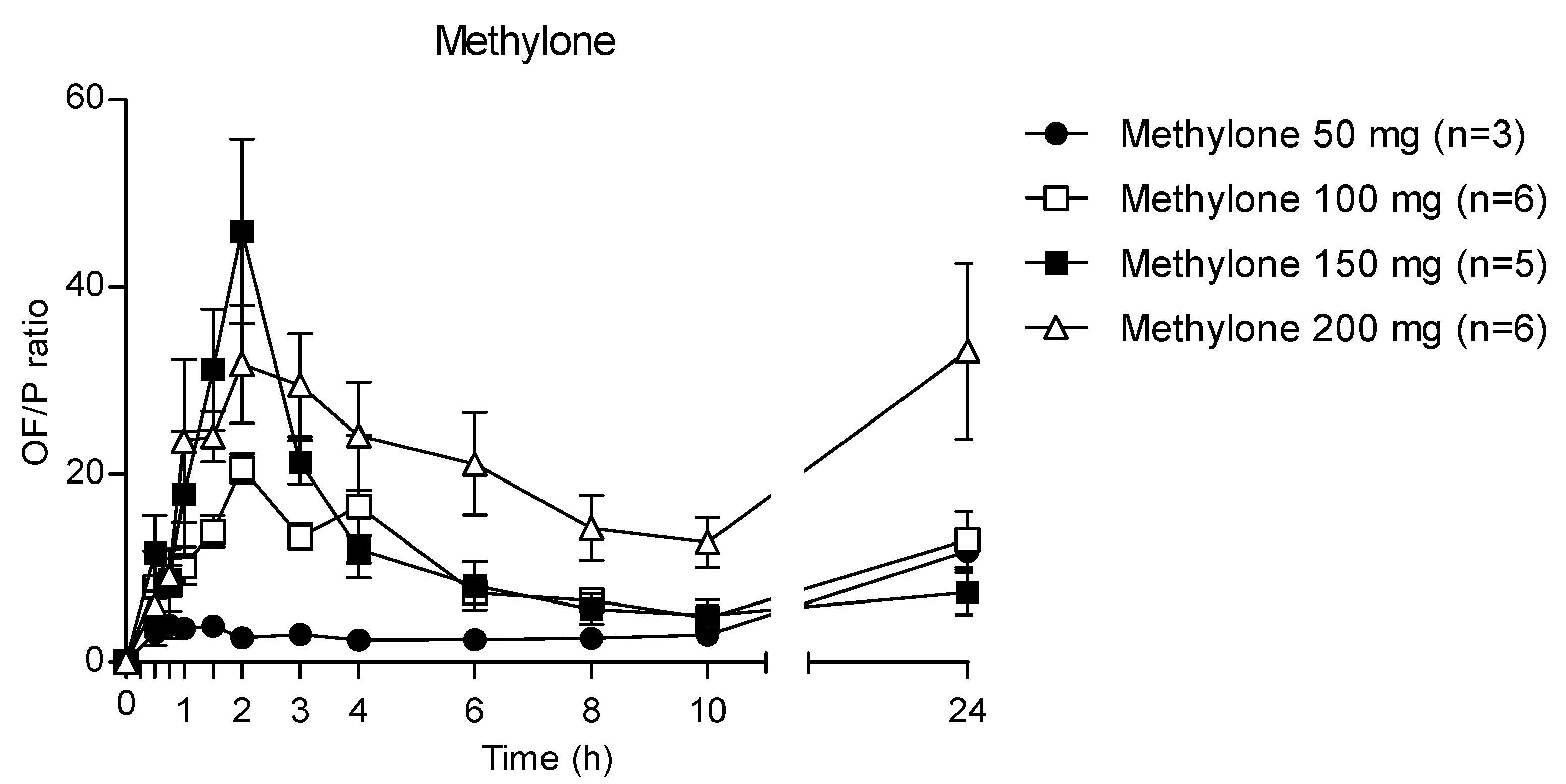
3.3. OF/plasma Ratio for Methylone
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WDR 2022_Booklet 4. Available online: www.unodc.org/unodc/en/data-and-analysis/wdr-2022_booklet-4.html (accessed on 13 February 2023).
- Shafi, A.; Berry, A.J.; Sumnall, H.; Wood, D.M.; Tracy, D.K. New Psychoactive Substances: A Review and Updates. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320967197. [Google Scholar] [CrossRef] [PubMed]
- Zaami, S.; Giorgetti, R.; Pichini, S.; Pantano, F.; Marinelli, E.; Busardò, F.P. Synthetic Cathinones Related Fatalities: An Update. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Poyatos, L.; Lo Faro, A.F.; Berardinelli, D.; Sprega, G.; Malaca, S.; Pichini, S.; Huestis, M.A.; Papaseit, E.; Pérez-Mañá, C.; Busardò, F.P.; et al. Methylone and MDMA Pharmacokinetics Following Controlled Administration in Humans. Int. J. Mol. Sci. 2022, 23, 14636. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, N.V.; Sievert, M.K.; Shulgin, A.T.; Jacob, P.; Ruoho, A.E. Inhibition of Plasma Membrane Monoamine Transporters by Beta-Ketoamphetamines. Eur. J. Pharmacol. 1999, 381, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.H.; Ayestas, M.A.; Partilla, J.S.; Sink, J.R.; Shulgin, A.T.; Daley, P.F.; Brandt, S.D.; Rothman, R.B.; Ruoho, A.E.; Cozzi, N.V. The Designer Methcathinone Analogs, Mephedrone and Methylone, Are Substrates for Monoamine Transporters in Brain Tissue. Neuropsychopharmacology 2012, 37, 1192–1203. [Google Scholar] [CrossRef]
- Eshleman, A.J.; Wolfrum, K.M.; Hatfield, M.G.; Johnson, R.A.; Murphy, K.V.; Janowsky, A. Substituted Methcathinones Differ in Transporter and Receptor Interactions. Biochem. Pharmacol. 2013, 85, 1803–1815. [Google Scholar] [CrossRef]
- Centazzo, N.; Chojnacki, M.R.; Elmore, J.S.; Rodriguez, R.; Acosta, T.; Suzuki, M.; Rice, K.C.; Baumann, M.H.; Concheiro, M. Brain Concentrations of Methylone and Its Metabolites after Systemic Methylone Administration: Relationship to Pharmacodynamic Effects. J. Pharmacol. Exp. Ther. 2021, 377, 398–406. [Google Scholar] [CrossRef]
- Elmore, J.S.; Dillon-Carter, O.; Partilla, J.S.; Ellefsen, K.N.; Concheiro, M.; Suzuki, M.; Rice, K.C.; Huestis, M.A.; Baumann, M.H. Pharmacokinetic Profiles and Pharmacodynamic Effects for Methylone and Its Metabolites in Rats. Neuropsychopharmacology 2017, 42, 649–660. [Google Scholar] [CrossRef]
- Poyatos, L.; Pérez-Mañá, C.; Hladun, A.; de la Rosa, G.; Martín, S.; Barriocanal, A.; Carabias, L.; Kelmendi, B.; Taoussi, O.; Busardò, F.P.; et al. Pharmacological Effects of Methylone and MDMA in Humans. Front. Pharmacol. 2023, 14, 1122861. [Google Scholar] [CrossRef]
- Uralets, V.; Rana, S.; Morgan, S.; Ross, W. Testing for Designer Stimulants: Metabolic Profiles of 16 Synthetic Cathinones Excreted Free in Human Urine. J. Anal. Toxicol. 2014, 38, 233–241. [Google Scholar] [CrossRef] [PubMed]
- La Maida, N.; Mannocchi, G.; Pichini, S.; Basile, G.; Di Giorgi, A.; Busardò, F.P.; Marchei, E. Targeted Screening and Quantification of Synthetic Cathinones and Metabolites in Hair by UHPLC-HRMS. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 5033–5042. [Google Scholar] [CrossRef] [PubMed]
- Mucklow, J.C.; Bending, M.R.; Kahn, G.C.; Dollery, C.T. Drug Concentration in Saliva. Clin. Pharmacol. Ther. 1978, 24, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Gorodischer, R.; Koren, G. Salivary Excretion of Drugs in Children: Theoretical and Practical Issues in Therapeutic Drug Monitoring. Dev. Pharmacol. Ther. 1992, 19, 161–177. [Google Scholar] [CrossRef]
- Schramm, W.; Smith, R.H.; Craig, P.A.; Kidwell, D.A. Drugs of Abuse in Saliva: A Review. J. Anal. Toxicol. 1992, 16, 1–9. [Google Scholar] [CrossRef]
- Drobitch, R.K.; Svensson, C.K. Therapeutic Drug Monitoring in Saliva. An Update. Clin. Pharmacokinet. 1992, 23, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Desrosiers, N.A.; Huestis, M.A. Oral Fluid Drug Testing: Analytical Approaches, Issues and Interpretation of Results. J. Anal. Toxicol. 2019, 43, 415–443. [Google Scholar] [CrossRef]
- Barnes, A.J.; Scheidweiler, K.B.; Kolbrich-Spargo, E.A.; Gorelick, D.A.; Goodwin, R.S.; Huestis, M.A. MDMA and Metabolite Disposition in Expectorated Oral Fluid after Controlled Oral MDMA Administration. Ther. Drug Monit. 2011, 33, 602–608. [Google Scholar] [CrossRef]
- Navarro, M.; Pichini, S.; Farré, M.; Ortuño, J.; Roset, P.N.; Segura, J.; de la Torre, R. Usefulness of Saliva for Measurement of 3,4-Methylenedioxymethamphetamine and Its Metabolites: Correlation with Plasma Drug Concentrations and Effect of Salivary PH. Clin. Chem. 2001, 47, 1788–1795. [Google Scholar] [CrossRef]
- De Castro, A.; Lendoiro, E.; Fernández-Vega, H.; Steinmeyer, S.; López-Rivadulla, M.; Cruz, A. Liquid Chromatography Tandem Mass Spectrometry Determination of Selected Synthetic Cathinones and Two Piperazines in Oral Fluid. Cross Reactivity Study with an on-Site Immunoassay Device. J. Chromatogr. A 2014, 1374, 93–101. [Google Scholar] [CrossRef]
- Mercolini, L.; Protti, M.; Catapano, M.C.; Rudge, J.; Sberna, A.E. LC-MS/MS and Volumetric Absorptive Microsampling for Quantitative Bioanalysis of Cathinone Analogues in Dried Urine, Plasma and Oral Fluid Samples. J. Pharm. Biomed. Anal. 2016, 123, 186–194. [Google Scholar] [CrossRef]
- Williams, M.; Martin, J.; Galettis, P. A Validated Method for the Detection of 32 Bath Salts in Oral Fluid. J. Anal. Toxicol. 2017, 41, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Denia, A.; Esteve-Turrillas, F.A.; Armenta, S. Analysis of Drugs Including Illicit and New Psychoactive Substances in Oral Fluids by Gas Chromatography-Drift Tube Ion Mobility Spectrometry. Talanta 2022, 238, 122966. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.; Kim, J.; Concheiro, M. Stability of Synthetic Cathinones in Oral Fluid Samples. Forensic Sci. Int. 2017, 274, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Vallersnes, O.M.; Persett, P.S.; Øiestad, E.L.; Karinen, R.; Heyerdahl, F.; Hovda, K.E. Underestimated Impact of Novel Psychoactive Substances: Laboratory Confirmation of Recreational Drug Toxicity in Oslo, Norway. Clin. Toxicol. 2017, 55, 636–644. [Google Scholar] [CrossRef]
- Krotulski, A.J.; Mohr, A.L.A.; Fogarty, M.F.; Logan, B.K. The Detection of Novel Stimulants in Oral Fluid from Users Reporting Ecstasy, Molly and MDMA Ingestion. J. Anal. Toxicol. 2018, 42, 544–553. [Google Scholar] [CrossRef]
- da Cunha, K.F.; Oliveira, K.D.; Cardoso, M.S.; Arantes, A.C.F.; Coser, P.H.P.; Lima, L.D.N.; Maluf, A.C.S.; Comis, M.A.D.C.; Huestis, M.A.; Costa, J.L. Prevalence of New Psychoactive Substances (NPS) in Brazil Based on Oral Fluid Analysis of Samples Collected at Electronic Music Festivals and Parties. Drug Alcohol. Depend. 2021, 227, 108962. [Google Scholar] [CrossRef]
- Axelsson, M.A.B.; Lövgren, H.; Kronstrand, R.; Green, H.; Bergström, M.A. Retrospective Identification of New Psychoactive Substances in Patient Samples Submitted for Clinical Drug Analysis. Basic Clin. Pharmacol. Toxicol. 2022, 131, 420–434. [Google Scholar] [CrossRef]
- O’Neal, C.L.; Crouch, D.J.; Rollins, D.E.; Fatah, A.; Cheever, M.L. Correlation of Saliva Codeine Concentrations with Plasma Concentrations after Oral Codeine Administration. J. Anal. Toxicol. 1999, 23, 452–459. [Google Scholar] [CrossRef]
- Kidwell, D.A.; Holland, J.C.; Athanaselis, S. Testing for Drugs of Abuse in Saliva and Sweat. J. Chromatogr. B Biomed. Sci. Appl. 1998, 713, 111–135. [Google Scholar] [CrossRef]
- Mas, M.; Farré, M.; de la Torre, R.; Roset, P.N.; Ortuño, J.; Segura, J.; Camí, J. Cardiovascular and Neuroendocrine Effects and Pharmacokinetics of 3, 4-Methylenedioxymethamphetamine in Humans. J. Pharmacol. Exp. Ther. 1999, 290, 136–145. [Google Scholar]
- Poyatos, L.; Papaseit, E.; Olesti, E.; Pérez-Mañá, C.; Ventura, M.; Carbón, X.; Grifell, M.; Fonseca, F.; Torrens, M.; de la Torre, R.; et al. A Comparison of Acute Pharmacological Effects of Methylone and MDMA Administration in Humans and Oral Fluid Concentrations as Biomarkers of Exposure. Biology 2021, 10, 788. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.E.; Jeffcoat, A.R.; Hill, J.M.; Pugh, D.E.; Patetta, P.K.; Sadler, B.M.; White, W.R.; Perez-Reyes, M. Pharmacokinetics of Methamphetamine Self-Administered to Human Subjects by Smoking S-(+)-Methamphetamine Hydrochloride. Drug Metab. Dispos. 1993, 21, 717–723. [Google Scholar] [PubMed]
- Pedersen, A.J.; Petersen, T.H.; Linnet, K. In Vitro Metabolism and Pharmacokinetic Studies on Methylone. Drug Metab. Dispos. 2013, 41, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Hole, K.; Arnestad, M.; Molden, E.; Haslemo, T. Dose-Dependent Inhibition of CYP2D6 by Bupropion in Patients With Depression. J. Clin. Psychopharmacol. 2021, 41, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Giessmann, T.; Modess, C.; Hecker, U.; Zschiesche, M.; Dazert, P.; Kunert-Keil, C.; Warzok, R.; Engel, G.; Weitschies, W.; Cascorbi, I.; et al. CYP2D6 Genotype and Induction of Intestinal Drug Transporters by Rifampin Predict Presystemic Clearance of Carvedilol in Healthy Subjects. Clin. Pharmacol. Ther. 2004, 75, 213–222. [Google Scholar] [CrossRef]
- Czerwinska, J.; Parkin, M.C.; George, C.; Kicman, A.T.; Dargan, P.I.; Abbate, V. Pharmacokinetics of Mephedrone and Its Metabolites in Whole Blood and Plasma after Controlled Intranasal Administration to Healthy Human Volunteers. J. Anal. Toxicol. 2021, 45, 730–738. [Google Scholar] [CrossRef]




| Methylone 50 mg (n = 3) | Methylone 100 mg (n = 6) | Methylone 150 mg (n = 5) | Methylone 200 mg (n = 6) | Placebo (n = 12) | |
|---|---|---|---|---|---|
| Age (years) | 22.3 ± 0.6 (22–23) | 22.7 ± 0.8 (22–24) | 23.4 ± 0.9 (22–24) | 24.0 ± 0.0 (24–24) | 23.3 ± 0.9 (22–24) |
| Weight (kg) | 69.7 ± 14.2 (60.4–86.0) | 71.0 ± 12.3 (60.4–87.0) | 71.9 ± 10.0 (61.9–87.0) | 70.0 ± 3.9 (66.7–76.6) | 70.2 ± 8.7 (60.4–87.0) |
| Height (cm) | 178.4 ± 3.4 (175.0–181.8) | 177.0 ± 2.8 (181.8–174.2) | 176.9 ± 4.8 (172.8–185.0) | 183.6 ± 9.8 (172.8–193.5) | 180.4 ± 7.2 (172.8–193.5) |
| BMI (kg/m2) | 21.9 ± 4.5 (18.3–27.0) | 22.7 ± 3.9 (18.3–27.9) | 23.1 ± 3.7 (19.4–27.9) | 21.0 ± 3.3 (18.0–25.7) | 21.7 ± 3.5 (18.0–27.9) |
| Methylone | HMMC | MDC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 50 mg (Mean ± SD, CV%) (n = 3) | 100 mg (Mean ± SD, CV%) (n = 6) | 150 mg (Mean ± SD, CV%) (n = 5) | 200 mg (Mean ± SD, CV%) (n = 6) | 100 mg (Mean ± SD, CV%) | 150 mg (Mean ± SD, CV%) | 200 mg (Mean ± SD, CV%) | 100 mg (Mean ± SD, CV%) | 150 mg (Mean ± SD, CV%) | 200 mg (Mean ± SD, CV%) | |
| AUC0–10 (ng/mL × h) | 2009.3 ± 425.2, 21% | 16,724.1 ± 4179.4, 25% | 34,454.6 ± 12,481.2, 36% | 72,247.7 ± 33,659.2, 46% | 778.5 ± 159.1, 20% | 1118.0 ± 86.7, 8% | 5179.0 ± 583.5, 11% | 898.8 ± 95.0, 11% | 2735.4 ± 441.3, 16% | 6283.7 ± 1491.2, 24% |
| AUC0–24 (ng/mL × h) | 3350.0 ± 378.7, 11% | 21,353.9 ± 5079.8, 24% | 39,993.2 ± 14,995.3, 37% | 96,628.3 ± 43,532.8, 45% | 1023.1 ± 198.2, 19% | 1307.3 ± 91.7, 7% | 6821.7 ± 727.1, 11% | 1017.5 ± 117.6, 12% | 4086.4 ± 1104.4, 27% | 7652.2 ± 1611.1, 21% |
| tmax (h) | 1.5 | 2.0 | 2.0 | 1.5 | 1.75 | 2.0 | 2.0 | 2.0 | 4.0 | 4.0 |
| Cmax (ng/mL) | 547.8 ± 84.8, 15% | 5002.3 ± 1192.7, 24% | 13,383.6 ± 5379.8, 40% | 20,464.9 ± 7979.5, 39% | 236.8 ± 45.3, 19% | 473.3 ± 56.4, 11% | 1370.6 ± 420.6, 31% | 226.2 ± 3.6, 2% | 474.4 ± 55.9, 12% | 1430.5 ± 383.2, 27% |
| Kd (h−1) | 0.053 ± 0.022, 42% | 0.116 ± 0.02, 17% | 0.147 ± 0.01, 7% | 0.108 ± 0.02, 18% | 0.115 ± 0.01, 9% | 0.142 ± 0.01, 7% | 0.106 ± 0.02, 19% | 0.156 ± 0.02, 13% | 0.115 ± 0.05, 43% | 0.17 ± 0.03, 18% |
| t1/2d (h) | 14.6 ± 6.2, 43% | 6.1 ± 1.0, 16% | 4.7 ± 0.4, 8% | 6.6 ± 1.5, 22% | 6.1 ± 0.5, 8% | 4.9 ± 0.4, 9% | 6.7 ± 1.0, 15% | 4.5 ± 0.6, 13% | 7.6 ± 4.6, 60% | 4.3 ± 0.9, 22% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sprega, G.; Di Giorgi, A.; Poyatos, L.; Papaseit, E.; Pérez-Mañá, C.; Tini, A.; Pichini, S.; Busardò, F.P.; Lo Faro, A.F.; Farré, M. Usefulness of Oral Fluid for Measurement of Methylone and Its Metabolites: Correlation with Plasma Drug Concentrations and the Effect of Oral Fluid pH. Metabolites 2023, 13, 468. https://doi.org/10.3390/metabo13040468
Sprega G, Di Giorgi A, Poyatos L, Papaseit E, Pérez-Mañá C, Tini A, Pichini S, Busardò FP, Lo Faro AF, Farré M. Usefulness of Oral Fluid for Measurement of Methylone and Its Metabolites: Correlation with Plasma Drug Concentrations and the Effect of Oral Fluid pH. Metabolites. 2023; 13(4):468. https://doi.org/10.3390/metabo13040468
Chicago/Turabian StyleSprega, Giorgia, Alessandro Di Giorgi, Lourdes Poyatos, Esther Papaseit, Clara Pérez-Mañá, Anastasio Tini, Simona Pichini, Francesco Paolo Busardò, Alfredo Fabrizio Lo Faro, and Magí Farré. 2023. "Usefulness of Oral Fluid for Measurement of Methylone and Its Metabolites: Correlation with Plasma Drug Concentrations and the Effect of Oral Fluid pH" Metabolites 13, no. 4: 468. https://doi.org/10.3390/metabo13040468
APA StyleSprega, G., Di Giorgi, A., Poyatos, L., Papaseit, E., Pérez-Mañá, C., Tini, A., Pichini, S., Busardò, F. P., Lo Faro, A. F., & Farré, M. (2023). Usefulness of Oral Fluid for Measurement of Methylone and Its Metabolites: Correlation with Plasma Drug Concentrations and the Effect of Oral Fluid pH. Metabolites, 13(4), 468. https://doi.org/10.3390/metabo13040468







