Abstract
Beauveria bassiana is a globally distributed entomopathogenic fungus that produces various secondary metabolites to support its pathogenesis in insects. Two polyketide synthase genes, pks14 and pks15, are highly conserved in entomopathogenic fungi and are important for insect virulence. However, understanding of their mechanisms in insect pathogenicity is still limited. Here, we overexpressed these two genes in B. bassiana and compared the metabolite profiles of pks14 and pks15 overexpression strains to those of their respective knockout strains in culture and in vivo using tandem liquid chromatography-mass spectrometry (LC-MS/MS) with Global Natural Products Social Molecular Networking (GNPS). The pks14 and pks15 clusters exhibited crosstalk with biosynthetic clusters encoding insect-virulent metabolites, including beauvericins, bassianolide, enniatin A, and the intracellular siderophore ferricrocin under certain conditions. These secondary metabolites were upregulated in the pks14-overexpressing strain in culture and the pks15-overexpressing strain in vivo. These data suggest that pks14 and pks15, their proteins or their cluster components might be directly or indirectly associated with key pathways in insect pathogenesis of B. bassiana, particularly those related to secondary metabolism. Information about interactions between the polyketide clusters and other biosynthetic clusters improves scientific understanding about crosstalk among biosynthetic pathways and mechanisms of pathogenesis.
1. Introduction
Beauveria bassiana is an entomopathogenic fungus with a broad host range among insect pests. This fungus has great potential to control the diamondback moth, European corn borer, corn earworm, black cutworm, cabbage worm, and cabbage looper [1]. Major B. bassiana secondary metabolites are polyketides, nonribosomal peptides (NRPs), and hybrid polyketide NRPs, which are widely used in medical and agricultural applications, such as insecticides, antitumor applications, antibiotics, antioxidative stress treatments, and immunosuppression [2,3,4,5]. Well-known NRPs reported to be involved in insect pathogenesis include beauvericin, bassianolide, and enniatin, which are synthesized by NRP synthetases (NRPS), e.g., beauvericin synthetase, bassianolide synthetase, and enniatin synthetase [2,3,6]. These compounds have much potential for use in the management of insects such as Galleria mellonella, Salix exigua, Helicoverpa zea, and Choristoneura fumiferana [6,7,8]. In addition, ferricrocin has been considered a virulence factor in several hosts. It is an intracellular siderophore commonly found in Aspergillus fumigatus, A. nidulans, B. bassiana, Metarhizium robertsii, and M. grisea [9,10,11,12,13,14]. Ferricrocin has a crucial role in conidiation, conidial germination, resistance to oxidative stress, and virulence against insects [10,12,14,15].
In addition to NRPs, polyketides such as stilbenes, spinosyn A, spinosyn, avermectins, ivermectins, and anthraquinones also play important roles in insect virulence. While they are mainly found in bacteria, insecticidal polyketides can be found in fungi as well, and encompass compounds such as dihydroxynaphthalene (DHN)-melanin, dipicolinic acid, neurosporin A, and phomalactone [16]. Noticeably, entomopathogenic fungi have an abundance of polyketide synthases (PKS); for example, there are 24, 13, and 12 PKS genes in M. robertsii (ARSEF 2575), M. acridum, and B. bassiana, respectively [17,18]. However, study of their functions and mechanisms in controlling insect pests is still limited. Of the 12 B. bassiana BCC 2660 PKS genes, two of these, pks14 and pks15, are highly conserved in entomopathogenic fungi and play a crucial role in virulence against insects and in anti-phagocytic activity against insect hemocytes [19,20,21]. Despite these findings, there is still limited knowledge about how B. bassiana polyketides are involved in insect virulence. Moreover, to our knowledge, no study has demonstrated cross-relationships between polyketide clusters and other secondary metabolite clusters such as NRPs with respect to insect pathogenesis.
Mass-spectrometry-based metabolomics is a powerful analytical technique used to explore secondary metabolites in biological samples. Liquid chromatography–mass spectrometry (LC-MS) coupling of liquid chromatography and mass spectrometry are widely used in metabolomic analysis [22]. Integration of tandem mass spectrometry (MS/MS) with Global Natural Products Social Molecular Networking (GNPS) generates molecular networking by grouping similar mass spectra from MS/MS. [23]. Molecular networking not only allows the discovery of novel compounds and evaluation of differences in metabolite profiles from different sources, but also evaluates metabolite production in response to specific interactions [24,25,26].
In this study, we explored the impacts of PKS14 and PKS15 in B. bassiana BCC 2660 on fungal virulence by comparing mass-spectrometry-based metabolite profiles between overexpressing (OEpks) and knockout (∆pks) strains in culture media and in vivo. Molecular networking revealed the influences of PKS14 and PKS15 on the production of well-described insect-virulence NRPs, including beauvericins, enniatin A, bassianolide, and the intracellular siderophore ferricrocin. These PKSs and their metabolites may be associated with certain biosynthetic pathways of insect virulence factors, in turn helping govern the core processes of pathogenesis.
2. Materials and Methods
2.1. Fungal Strains and Culture Conditions
The knockout mutants ∆pks14 and ∆pks15 were previously generated in B. bassiana BCC 2660 and deposited in the Thailand Bioresource Research Center [19,20]. The PKS genes were disrupted by integration of the bialaphos resistance gene bar in the respective genes in B. bassiana BCC2660 using Agrobacterium-mediated transformation. B. bassiana wild type and all derivative strains were maintained on potato dextrose agar (PDA; Difco) at 28 °C for 7 days.
2.2. Generating Overexpressing Strains of pks14 and pks15
To generate strains overexpressing pks14 (sGFP + pks14 + ::barR) and pks15 (sGFP + pks15 + ::barR), the green fluorescence protein gene sGFP fused to full-length pks14 or pks15 under the control of the constitutive promoter toxA from Pyrenophora tritici-repentis [27] was inserted into a vector, as previously described [28]. Briefly, the full-length pks14 was amplified from genomic DNA from the start codon to 300 bp downstream of the stop codon with primers PKS14Eco1F (5′-GTGAATTCATGGAGCCAATCGCCATTGTCGG-3′) and PKS14Nhe7591R (5′-ATTGCTAGCGTCTGTCGAGCCGCGTCAGTG-3′). EcoRI and NheI sites in the primers are underlined, respectively. The pks14 fragment was then inserted into the pToxA vector [29] at EcoRI and AvrII (compatible with NheI) to generate the pTxA-sGFP-PKS14 vector. The full-length pks15 was amplified from genomic DNA from the start codon to 314 bp downstream of the stop codon with the primers PKSIII-start-Mfe: 5′-GGGCAATTGATGCTCATCGACAAAATGGAGACG-3′ and PKSIII-3′-NC-BamHI: 5′-TTTGGATCCCTCCCGAGTCTACCTTGATGC-3′. The MfeI and BamHI sites in the primers are underlined, respectively. The pks15 fragment was inserted into the pToxA vector [30] at EcoRI and BamHI (compatible with MfeI) to generate the pTxA-sGFP-PKS15 vector. The overexpression vectors pTxA-sGFP-PKS14 and pTxA-sGFP-PKS15 were transformed individually into B. bassiana strain BCC 2660 using PEG-protoplast transformation [30].
Transformants were selected on minimal medium (2% w/v dextrose, 0.51% w/v (NH4)2SO4 (Sigma Aldrich), 0.17% w/v yeast nitrogen base without amino acid (Difco), 1.8% w/v agar) supplemented with 200 mg·L−1 glufosinate ammonium (Zhejiang Yongnong Chem, Wenzhou, China) and confirmed by PCR amplification. Sequence integrity of the sGFP-pks14 or sGFP-pks15 fusion constructs was verified by DNA sequencing (Macrogen, Seoul, Republic of Korea. For gene expression analysis of the pks14-overexpressing (OEpks14) strains compared to the wild type, each strain was cultured in PDB at 150 rpm, 28 °C, for 3 days. Total RNA was extracted using the AmbionTM TRIzol (Thermo Fisher Scientific, Waltham, MA, USA), treated with DNase I (Thermo Fisher Scientific, Waltham, MA, USA) and generated cDNA using RevertAid Reverse Transcriptase and random hexamers (Thermo Fisher Scientific, Waltham, MA, USA). Gene expression levels were quantified using reverse transcription—quantitative polymerase chain reaction (RT-qPCR) with specific primers (pks14-F5′-CTT GAT CCT GTC AGC CGA TC-3′ and pks14-R5′-GCA TAC ACG TCT CTG ATG AG-3′). The beta-tubulin gene was used as the reference. Expression levels were calculated using the 2-∆∆Ct method [31].
2.3. Phenotypic Characterization of pks14- and pks15-Overexpressing Strains
Transformants were verified for the integration of sGFP-pks14 or sGFP-pks15 fusion constructs in the genomes of overexpression strains by PCR analysis with specific primers for sGFP (sGFP-550F: 5′-CAG CAG AAC AC C CCC ATC GGC-3′ and sGFP-R: 5′-CTT GTA CAG CTC GTC CAT GCC GTG A-3′), pks14 (PKS14-R: 5′-CTC AAG GTA CCA GAG TAG GCT AC-3′), pks15 (PKS15-480R: 5′-CAA GCT TCC GGT ACG ATA GTC-3′), and the ToxA promoter (pToxA-F: 5′-TGG AAT CCA TGG AGG AGT TCT GTA C -3′).
Conidial yield and radial growth of the overexpression strains were determined by comparison to the wild type. For conidial yield, 100 µL of a conidial suspension of 1 × 107 conidia·mL−1 was spread on PDA for 7 days, and conidial yield was determined using a hemocytomoter. For radial growth, 10 µL of a conidial suspension of 1 × 107 conidia·mL−1 was dropped on PDA, and colony diameter was determined on day 12 after inoculation.
The subcellular localization of PKS15 In the pks15-overexpressing (oEpks15) strains was determined by incubating the strains in diluted PDB (5% (v/v) in water) on a glass slide for 48 h followed by visualization using a confocal laser scanning microscope model FV1000 (Olympus), as previously described [13].
Insect virulence was determined using fourth-instar Spodoptera exigua (beet armyworm, BAW) larvae. For each strain, 20 BAWs were injected with 3 µL of 1 × 104 conidia·mL−1 (30 conidia per larva) using a specialized 33-gauge needle-syringe set (Hamilton), and cumulative insect mortalities were recorded for 7 days.
2.4. Metabolomic Preparation of OEpks14, OEpks15, Δpks14, and Δpks15 from Culture and In Vivo Samples
For the metabolomes from culture, 1 mL of conidia at 1 × 108 conidia·mL−1 of OEpks14, OEpks15, Δpks14, and Δpks15 was inoculated into 2 L PDB for the overexpression strains and 3 L PDB for the knockout strains. Cultures were shaken at 110 rpm and 28 °C for 7 days. Fungal cell extracts were prepared by sonication in methanol for 20 min, followed by overnight incubation. Extraction of culture broth was performed in ethyl acetate (volume ratio 1:1) three times. Crude extracts were obtained after condensation and lyophilization.
For in vivo metabolomes, ten fourth-instar BAW larvae were injected with 3 μL of conidial suspension of OEpks14, OEpks15, Δpks14, or Δpks15 at 1 × 107 conidia·mL−1. Inoculated larvae were collected 3, 5, and 7 days post-inoculation (DPI). Saline-injected larvae were used as controls. Larval extracts were prepared in methanol as described above. Crude extracts were obtained after condensation and lyophilization.
2.5. Metabolomic Analysis Using LC-MS and LC-MS/MS
Crude extracts at 10 mg/mL in methanol were separated by C18 (ACQUITY UPLC BEH-C18, 130 Å, 1.7 μm, 2.1 × 100 mm) with the following gradients: 0–6 min at 5–99.5% acetone nitrile (ACN), 6–8 min at 99.5% ACN, 8–8.2 min at 99.5–5% ACN, and 8.2–10 min of 5% ACN with a flow rate 0.4 mL·min−1. Mass data were acquired in triplicate using UPLC-HR-ESIMS (Thermo Orbitrap Elite system) and analyzed in positive and negative-mode ion detection between m/z 50–1500 with 30,000 resolutions. The top five ions with the highest intensities from each full mass scan were selected for collision-induced dissociation (CID) fragmentation for tandem mass data. For CID, the isolation width was 2 Da, and the selected ions were fragmented with a normalized collision energy of 30.0 or 35.0, activation Q of 0.250, activation time of 10.0, and 15,000 resolutions.
2.6. Molecular Networking, Chemical Classification, and Structural Elucidation
2.6.1. LC-MS/MS Data Processing
LC-MS/MS raw data were converted to mzML format using MSConvert software (Part of the ProteoWizard package; ProteoWizard Software Foundation, Palo Alto, USA) before data processing in MZmine software (version 2.53), as previously described [32]. The mass detection noise level was set to 1000 for MS1 and 100 for MS2. Chromatogram building was performed using the Automated Data Analysis Pipeline (ADAP) chromatogram builder with the following settings: minimum group size of scans, 3; group intensity threshold, 1000; minimum highest intensity, 10,000; and m/z tolerance, 0.001 m/z (or 20 ppm). Chromatographic deconvolution was set with S/N threshold, 10; minimum feature height of 50,000 (20,000 for negative-ion data); coefficient/area threshold, 50; peak duration range, 0.05–0.80 min; and retention time (RT) wavelet range, 0.03–0.15 min. The mass range for MS/MS scan pairing was set to 0.02 Da, and the RT range was set to 0.2 min. The isotopic peak grouping was an m/z tolerance of 0.001 m/z (or 20 ppm) and an RT tolerance of 0.2 min. Peak alignment was generated using the join aligner with an m/z tolerance of 0.001 m/z (or 20 ppm), an RT tolerance of 0.2 min, a weight for m/z of 70, and a weight for RT of 30. Gap filling was performed with an intensity tolerance of 30%, an m/z tolerance of 0.001 m/z (or 20 ppm), and an RT tolerance of 0.2 min. Finally, the processed data were exported in .mgf format for MS/MS spectral information and .csv format for a feature list of MS1 m/z, peak retention time, and peak area information.
2.6.2. Molecular Networking
Molecular networking was generated using Global Natural Products Social Molecular Networking (GNPS; https://gnps.ucsd.edu; accessed on 23 May 2022) [33]. The preprocessed data were submitted to a feature-based molecular networking workflow (version 28.2) in GNPS [34]. The precursor-ion mass tolerance and MS/MS fragment ion tolerance were set to 0.02 Da. Edges in the network were created when a cosine score was above 0.7 with at least 6 matched fragment ions. The molecular network data were visualized by Cytoscape (version 3.8.0.). Annotated metabolites were collected, and structures were confirmed with their ion fragmentation from LC-MS/MS using ChemDraw Professional 16.
3. Results
3.1. pks14- and pks15-Overexpressing Strains Exhibit Increased Insect Virulence
To characterize phenotypes of the pks14- and pks15-overexpressing strains OEpks14 and OEpks15, gene expression level, conidial yield, radial growth, insect mortality, and cellular localization were determined with respect to the wild type.
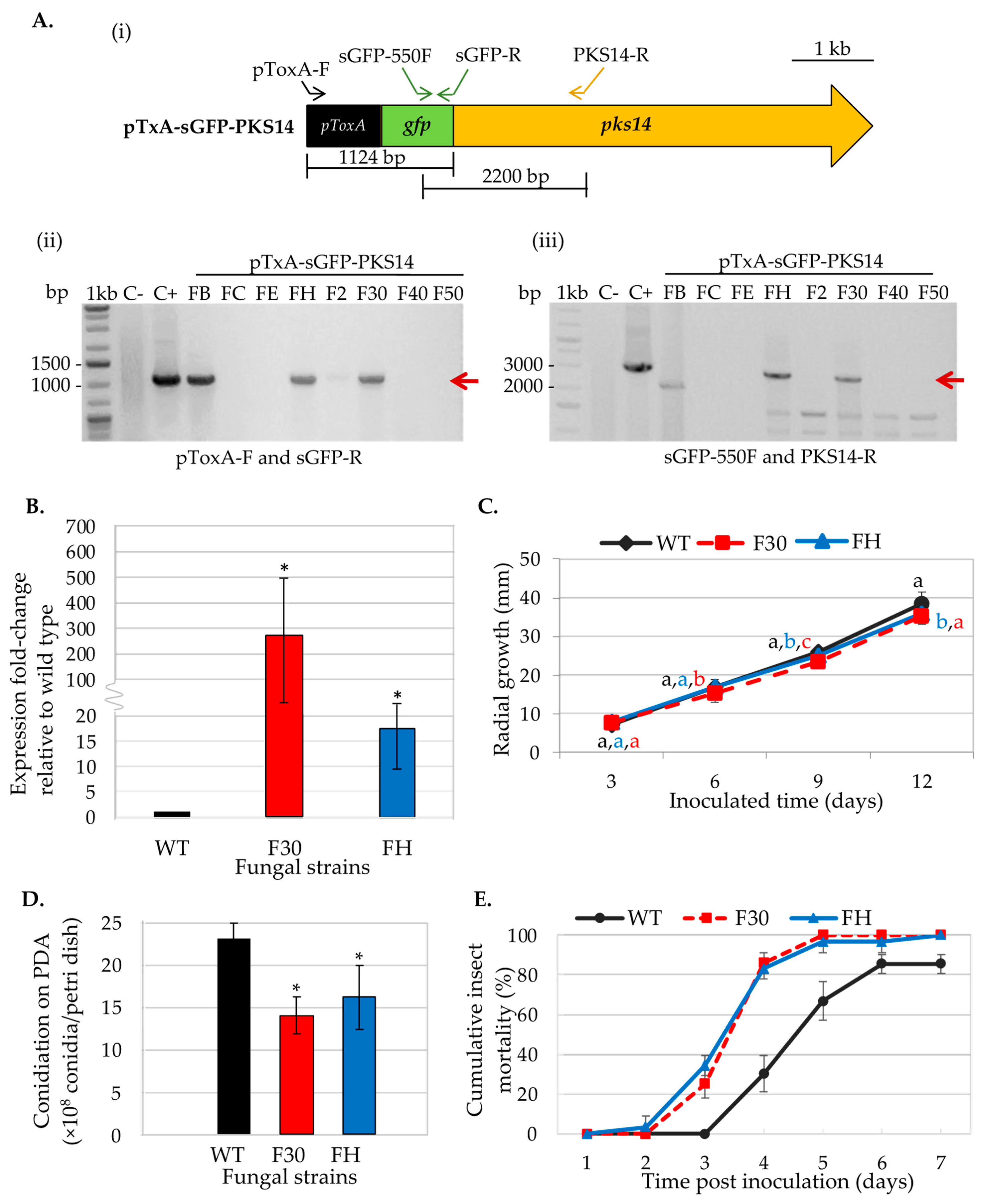
The pTxA-sGFP-PKS14 vector designed for pks14 overexpression was transformed into the B. bassiana wild type. Eight transformants were obtained and examined for ectopic integration of the overexpression cassette by PCR. B. bassiana BCC2660 genomic DNA and the pTxA-sGFP-PKS14 vector were included as negative and positive controls, respectively. The specific primers used for this PCR are shown in Figure 1A. Two OEpks14 strains, FH and F30, showed the expected bands at 1124 bp (using primers pToxA-F and sGFP-R specific for the ToxA promoter and sGFP, respectively) and at 2200 bp (using primers sGFP-550F and PKS14-R specific for sGFP and pks14, respectively) (Figure 1A). Expression levels of pks14 in OEpks14 strains F30 and FH increased by 245-fold and 18-fold, respectively, compared to the B. bassiana wild type (Figure 1B). In addition, these strains exhibited a slight reduction in radial growth on days 9–12 compared to that of the wild type (Figure 1C). The conidial yield of OEpks14 strains also showed a significant reduction by approximately 1.5-fold compared to that of wild type (Figure 1D). However, the reduced conidial yield did not affect the insect virulence of OEpks14 strains. F30 and FH virulence increased significantly by 177–187% and 18% on days 4 and days 7 after inoculation, compared to the wild type (Figure 1E). However, the GFP fluorescence was not detected in either of the OEpks14 strains, probably due to the nature of PKS14 or unknown complexities of this s-GFP-PKS14 fusion (e.g., N- or C-terminus fusion). Therefore, OEpks14 strain F30, which exhibited an elevated pks14 expression level and insect virulence, was used in subsequent analyses.
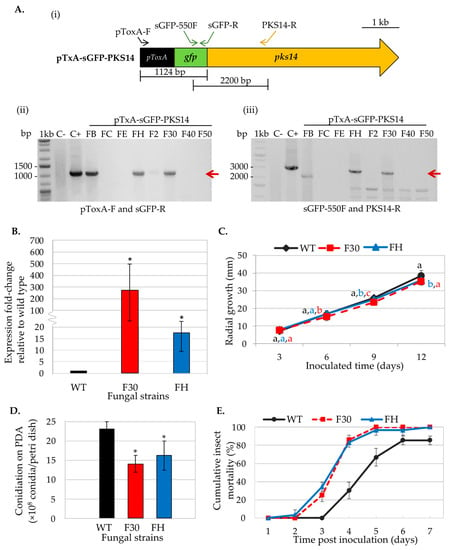
Figure 1.
Phenotype characterization of OEpks14 strains. (A) Ectopic integration of the pks14-overexpression cassette was verified by PCR with specific primers (i). OEpks14 strains FH and F30 showed the expected bands at 1124 bp (red arrow) using primers pToxA-F and sGFP-R, specific for the ToxA promoter and sGFP, respectively (ii), and 2200 bp (red arrow) using primers sGFP-550F and PKS14-R, specific for the sGFP and pks14, respectively (iii). B. bassiana BCC2660 genomic DNA and the vector pTxA-sGFP-PKS14 were used as negative (C−) and positive (C+) controls, respectively. (B) Gene expression level, (C) radial growth, (D) conidiation, and (E) insect mortality were impacted by OEpks14 strains FH and F30. The letters indicate a significant difference (ANOVA, p < 0.05). Asterisks indicate statistical significance (t-test, p < 0.05).
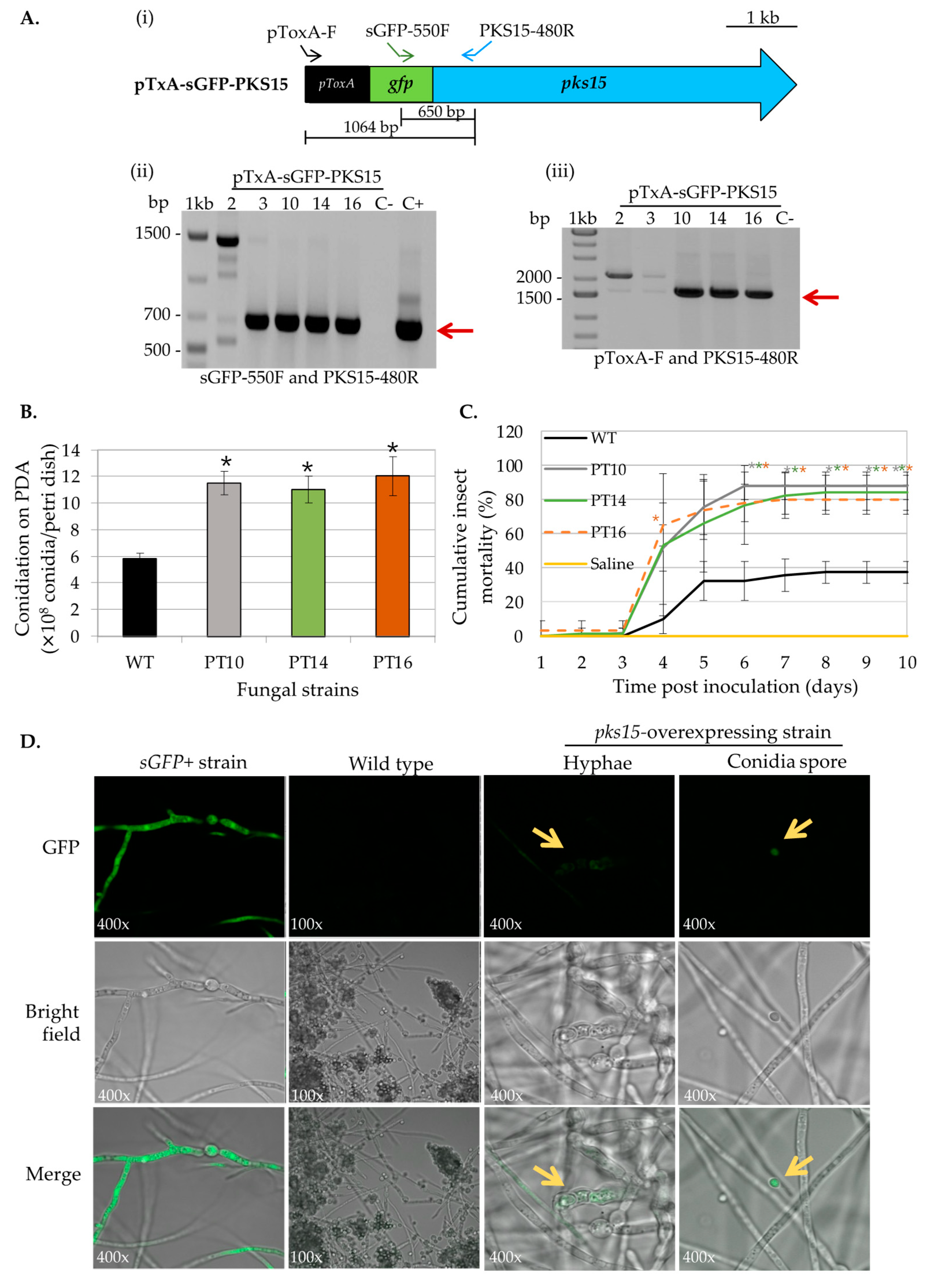
For pks15 overexpression, five transformants were obtained from the fungal transformation. Transformants 2, 3, 10, 14, and 16 were randomly selected for detection of ectopic integration of the overexpression cassette by PCR. The specific primers targeting the pTxA-SGFP-PKS15 vector are shown in Figure 2A. Three transformants, 10, 14, and 16, showed the expected bands at 650 bp (using primers sGFP-550F and PKS15-480R specific for sGFP and pks15, respectively) and at 1604 bp (using primers pToxA-F and PKS15-480R specific for the ToxA promoter and pks15, respectively) (Figure 2A). In addition, these three strains also had two-fold increases in conidial yield compared to wild type (Figure 2B), and their radial growths were not different (data not shown). Moreover, OEpks15 strains 10, 14, and 16 exhibited increased insect virulence by 150%, 134%, and 128%, respectively, on day 7 compared to the wild type (Figure 2C).

Figure 2.
Phenotype characterization of OEpks15 strains. (A) Ectopic integration of the pks15-overexpression cassette was verified by PCR with specific primers (i). The OEpks15 strains 10, 14, and 16 showed the expected bands at 650 bp (red arrow) using primers sGFP-550F and PKS15-480R, specific for sGFP and pks15, respectively (ii), and 1604 bp (red arrow) using primers pToxA-F and PKS15-480R, specific for the ToxA promoter and pks15, respectively (iii). B. bassiana BCC2660 genomic DNA and the vector pTxA-sGFP-PKS15 were used as negative (C−) and positive (C+) controls, respectively. (B) Conidiation and (C) insect mortality were increased in OEpks15 strains 10, 14, and 16. (D) Subcellular localization of the PKS15 in Oepks15 strain 16 exhibited the unique pattern of green fluorescence (GFP) in pseudohyphae-like cells and conidia (yellow arrow) compared to the negative control, B. bassiana wild type, and the positive control, the B. bassiana sGFP+ strain (sGFP alone and having GFP fluorescence homogenously in the cytoplasm). Asterisks indicate statistical significance (t-test, p < 0.05).
To determine the location of PKS15 in the fungal cell, cellular localization of the sGFP-PKS15 fusion protein was investigated by assessing GFP fluorescence compared to wild type and sGFP+ (expressing sGFP alone) strains. As expected, the B. bassiana sGFP+ strain showed uniform GFP fluorescence in the cytoplasm of both hyphae and conidia. By contrast, OEpks15 strain 16 had a unique pattern of green fluorescence in pseudohyphae-like cells and conidia (Figure 2D). Therefore, this strain, with its evelated conidial yield and insect virulence and visible expression of the s-GFP-PKS15 fusion protein, was selected for use in subsequent analyses.
3.2. pks14 and pks15 Strains Differentially Express a Number of Metabolites, Including Insect Virulence Factors
Metabolite profiles of pks14 and pks15 overexpression and knockout strains were explored by LC-MS and LC-MS/MS. For culture medium investigation, 1 × 108 conidia of each strain were inoculated in potato dextrose broth (PDB) and mass spectrometry data were collected at 3 days post-inoculation (DPI). For in vivo analyses, a suspension of 1 × 107 conidia·mL−1 per strain was injected into beet armyworm larvae (BAW). Saline-injected BAWs were used as controls. Mass spectrometry data were collected at early-stage infection (3 DPI) when live insect larvae were being colonized by the fungus, at mid-stage infection (5 DPI) from dead larvae, and at late-stage infection (7 DPI) from cadavers covered with fungal hyphae.
To annotate fungal metabolites from these complex samples, LC-MS/MS data of crude extracts were processed by using MZmine, and molecular networking was generated on GNPS from positive and negative-ion data. Similar MS/MS spectra were grouped into the same molecular network with the precursor-ion mass tolerance of 0.02 Da, MS/MS fragment ion tolerance of 0.02 Da, a cosine score > 0.7, and peak matching > 6 peaks. The sources for each node were visualized by different colors: metabolites produced in overexpression strains in red, those from knockout strains in blue, and those from saline-injected BAWs in gray. Chromatographic peak-area proportions are represented by node sizes and pie chart distributions. Based on compound identification matching to the spectral library and MS/MS annotation, molecular families (MFs) and singletons from culture and in vivo were identified for various classes of metabolites. In the positive-ion data, 2038 nodes and 1117 MFs were obtained from cultures of pks14 strains, whereas 2874 nodes and 1620 MFs were found in vivo. In addition, 241 nodes and 84 MFs were obtained from cultures of pks15 strains, and 6801 nodes and 2509 MFs were found in vivo.
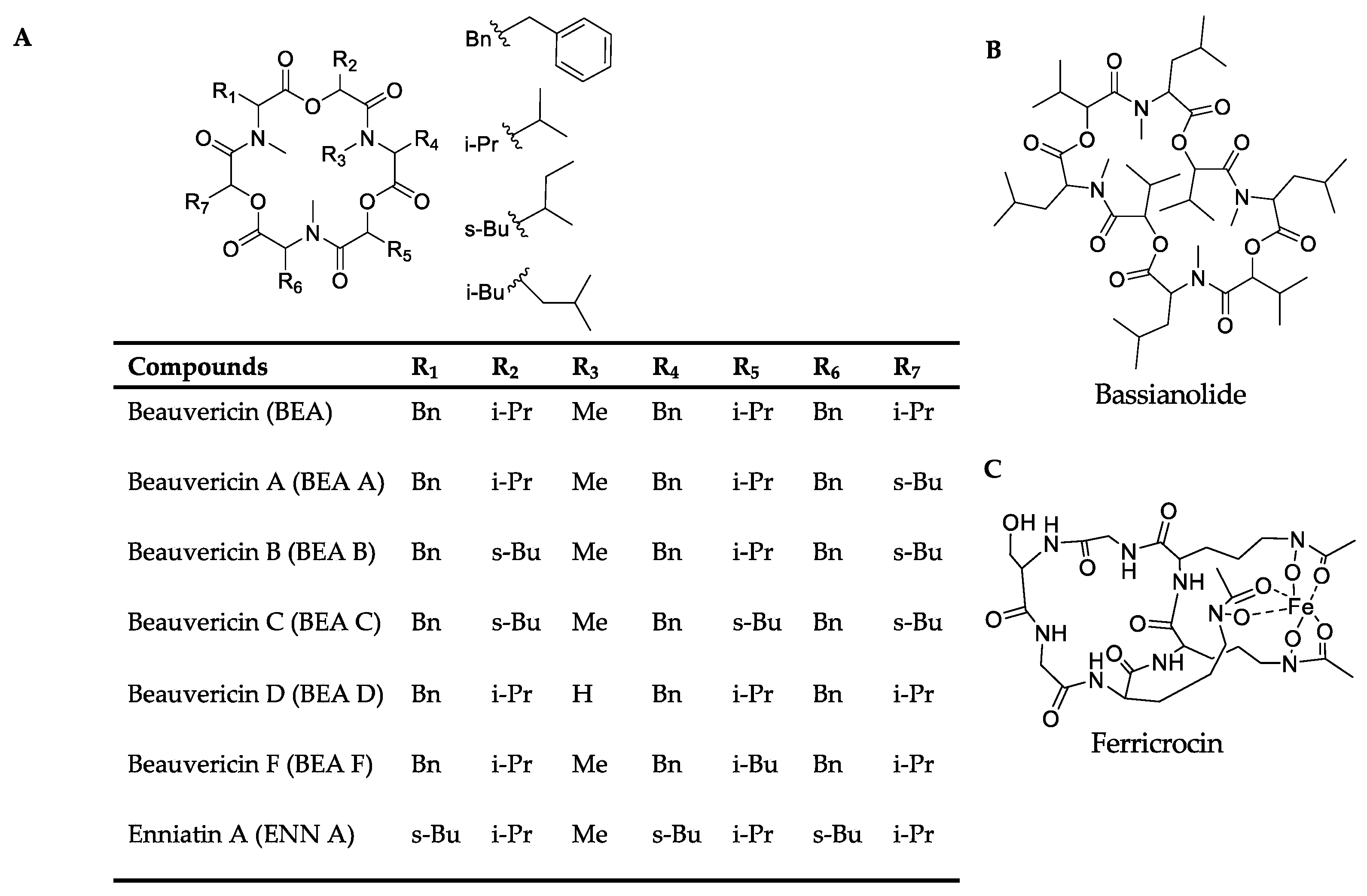
Among the positive-ion data, mass spectrometry data of culture samples revealed classified compounds such as aryl phosphotriester, lactone, and terpenoid exclusively from pks14 strains, and purine nucleosides from pks15 strains (Figures S1 and S3). For the in vivo mass spectrometry data, chemical compounds such as glycerophosphocholines, glycosides, phenolic acid, and phenylpropanes were observed exclusively for the pks14 strains (Figure S2); and pigment was observed for the pks15 strains (Figure S4). Moreover, compounds found in both culture and in vivo for pks14 and pks15 strains were amino acids, depsipeptides, dipeptides, fatty acids, fatty amides, flavins, glycerophospholipids, monoacylglycerols, pantothenic acid, and intracellular siderophores; and organophosphate was detected for the pks14 strains (Figures S1–S4). Intriguingly, major metabolites in the depsipeptide group related to fungal virulence were identified as beauvericins (BEAs), bassianolide (BAS), enniatin A (ENN A), and ferricrocin (FER). They were detected from both the pks14 and pks15 strains in positive-ion data but not negative-ion data (data not shown). The major insecticides identified in this study are shown in Figure 3.
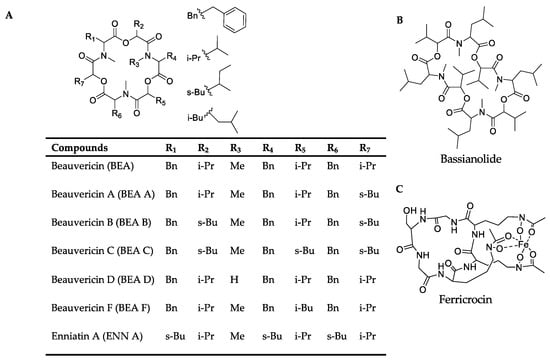
Figure 3.
Chemical structures of (A) the beauvericins analyzed in this study (BEAs), enniatin A (ENN A), (B) bassianolide (BAS), and (C) ferricrocin (FER).
3.3. Insect Virulence Factors Were Found Mainly for OEpks14 in Culture
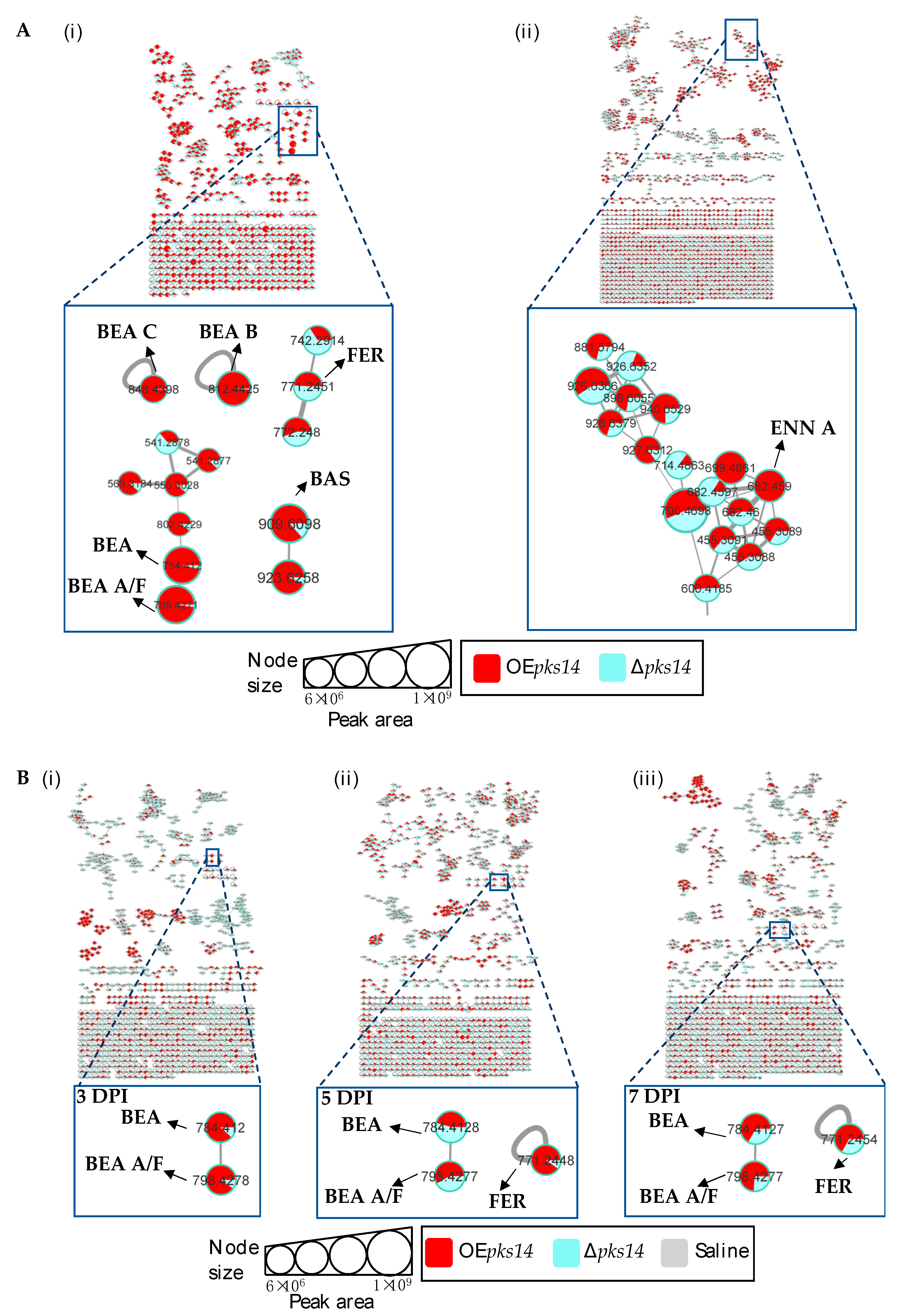
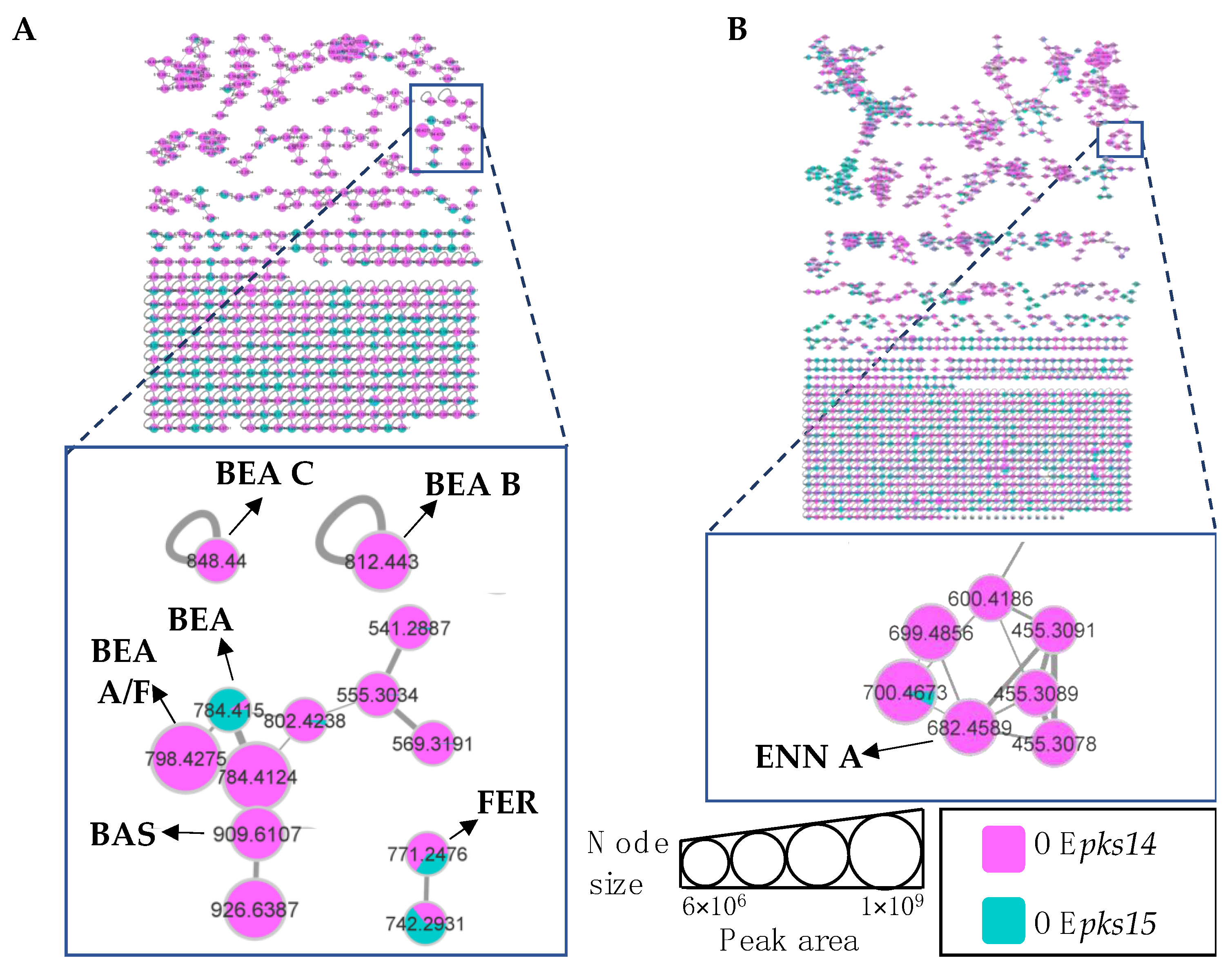
Positive ion data of the OEpks14 and ∆pks14 strains in culture media were compared to assess differences in metabolite profiles. Six insect-virulence compounds and one siderophore were mainly produced in OEpks14, as opposed to ∆pks14. The three MFs of insect-virulence compounds were an MF of BEA (m/z 784.4120, calcd. For C45H57N3O9H+, Δ−6.75 ppm) and BEA A/F (m/z 798.4277, calcd. For C46H59N3O9H+, Δ−6.89 ppm), an MF of BAS (m/z 909.6098, calcd. For C48H84N4O12H+, Δ−7.26 ppm), and an MF of ENN A (m/z 682.4590, calcd. For C36H63N3O9H+, Δ−7.76 ppm) (Figure 4A). BEA A and BEA F have similar chemical formulas and molecular weights but are different in structure. BEA A contains a secondary butyl (s-Bu) group of isoleucine (Ile) at R7 instead of the isopropyl (i-Pr) group of valine (Val) in BEA F (Figure 3). Ile and Val have similar molecular weights, and their MS/MS spectra cannot be distinguished; therefore, m/z 798.4277 [M+H]+ could be BEA A or BEA F. Two singletons of insect-virulence compounds represented BEA B (m/z 812.4425, calcd. for C47H61N3O9H+, Δ−7.50 ppm) and BEA C (m/z 848.4398, calcd. for C48H63N3O9Na+, Δ−7.54 ppm). One siderophore MF was FER (m/z 771.2446, calcd. for C28H44FeN9O13H+, Δ−5.19 ppm) (Figure 4A). ENN A was uniquely found in OEpks14 and not in ∆pks14. BEA, BEA A, BEA B, BEA C, and BAS were increased in OEpks14 compared to ∆pks14, and there was no difference for FER (Figure 4A and Figure S5). MS/MS spectrum annotation of classified metabolites was performed based on their fragmentation pattern (Figure S6). MF and singleton metabolites of interest are summarized in Table 1.

Figure 4.
(A) Molecular networking of classified metabolites from OEpks14 (red) and Δpks14 (light blue) strains in culture identified beauvericin (BEA), beauvericin A/F (BEA A/F), beauvericin B (BEA B), beauvericin C (BEA C), bassianolide (BAS), and ferricrocin (FER) in cells (i) and enniatin A (ENN A) in culture broth (ii). (B) Molecular networking of classified compounds from OEpks14 (red) and Δpks14 (light blue) strains in vivo at early-stage infection (3 DPI) for live larvae (i), mid-stage infection (5 DPI) for dead larvae (ii), and late-stage infection (7 DPI) for cadavers covered with fungal hyphae (iii) identified beauvericin (BEA), beauvericin A/F (BEA A/F), and ferricrocin (FER). Saline-injected BAWs were used as controls (gray). Node sizes represent the sums of chromatographic peak areas, and pie charts indicate chromatographic peak-area proportions for the detected insect virulence factors.

Table 1.
Summary of classified insect-virulence metabolites and a siderophore identified from OEpks14 and ∆pks14 strains in culture and in vivo.
For in vivo data, MFs from comparative positive-ion data for BAWs injected with OEpks14 or ∆pks14 were generated for samples from 3, 5, and 7 DPI. The results identified one MF of insect virulence factors, namely, BEA (m/z 784.4120, calcd. for C45H57N3O9H+, Δ−6.75 ppm) and BEA A/F (m/z 798.4278, calcd. for C46H59N3O9H+, Δ−6.76 ppm), and one singleton of the siderophore FER (m/z 771.2459, calcd. for C28H44FeN9O13H+, Δ−3.50 ppm). BEA and BEA A were detected at higher levels in BAWs injected with OEpks14 than with ∆pks14 throughout the infection and colonization periods (3, 5, and 7 DPI). FER was found at higher levels from OEpks14 samples at mid- (5 DPI) and late-stage (7 DPI) infections compared to ∆pks14 samples (Figure 4B and Figure S7). MS/MS spectrum annotation of classified metabolites was determined using their fragmentation pattern (Figure S8). Annotated metabolites of interest are summarized in Table 1, and their chromatographic peak areas summarized in Table S1.
Although insect-virulence metabolites were mainly produced by OEpks14 both in culture and in vivo, ENN A, BEA B, BEA C, and BAS were exclusively found in cultured OEpks14.
3.4. Beauvericins and Ferricrocin Were Upregulated in OEpks15 In Vivo
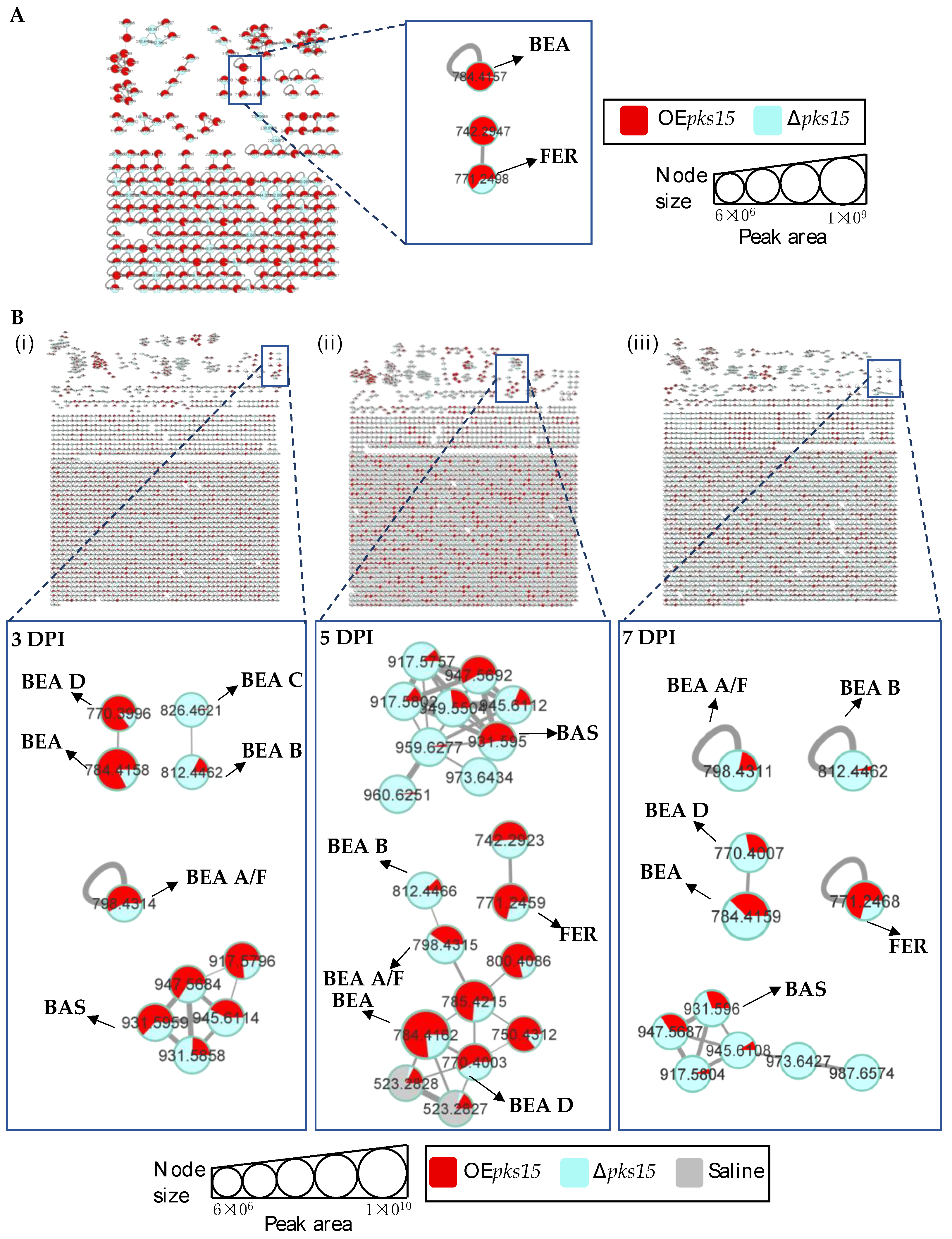
Next, insect-virulence metabolite profiles were compared for OEpks15 and ∆pks15 strains in culture. MS/MS spectral annotation of these compounds was determined using their fragmentation patterns (Figure S10). Positive ion data revealed increased levels of one singleton, BEA (m/z 784.4157, calcd. for C45H57N3O9H+, Δ−2.04 ppm), and one MF, FER (m/z 771.2498, calcd. for C28H44FeN9O13H+, Δ−1.56 ppm) for OEpks15 compared to ∆pks15 (Figure 5A and Figure S9).
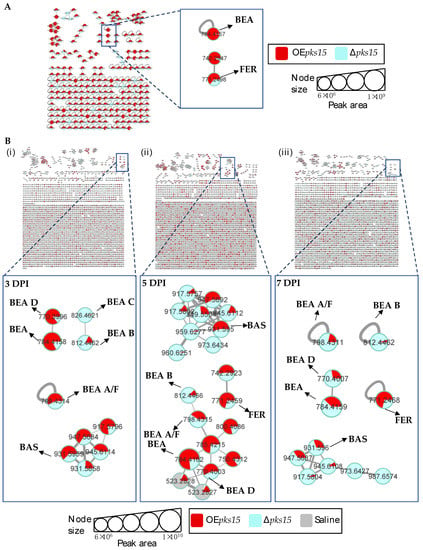
Figure 5.
(A) Molecular networking of classified insect-virulence metabolites and a siderophore from OEpks15 (red) and Δpks15 mutant (light blue) strains in culture identified beauvericin (BEA) and ferricrocin (FER). (B) Molecular networking of classified compounds from OEpks15 (red) and Δpks15 mutant (light blue) strains in vivo at early-stage infection (3 DPI) for live larvae (i), mid-stage infection (5 DPI) for dead larvae (ii), and late-stage infection (7 DPI) for cadavers covered with fungal hyphae (iii) identified beauvericin (BEA), beauvericin A/F (BEA A/F), beauvericin B (BEA B), beauvericin C (BEA C), beauvericin D (BEA D), bassianolide (BAS), and ferricrocin (FER). Saline-injected BAWs were used as controls (gray). Node sizes represent the sums of chromatographic peak areas, and pie charts indicate chromatographic peak-area proportions for the detected insect virulence factors.
In vivo comparative metabolite profiles for OEpks15 and ∆pks15 strains were also analyzed. The results identified seven insect-virulence metabolites, including BEA, BEA A, BEA B, BEA C, BEA D, ENN A, and BAS and the siderophore FER from the positive-ion data.
BEA (m/z 784.4157, calcd. for C45H57N3O9H+, Δ−2.04 ppm), BEA A/F (m/z 798.4305, calcd. for C46H59N3O9H+, Δ−3.38 ppm), BEA D (m/z 770.4002, calcd. for C44H55N3O9H+, Δ−1.94 ppm), and BAS (m/z 931.5959, calcd. for C48H84N4O12Na+, Δ−2.58 ppm) were upregulated in samples from BAWs injected with OEpks15 at 3 and 5 DPI, except for BEA A/F, which was decreased at 5 DPI. All these insect-virulence compounds were subsequently reduced at 7 DPI in cadavers covered with hyphae (Figure 5B and Figure S11).
In BAWs injected with OEpks15, BEA B (m/z 812.4464, calcd. for C47H61N3O9H+, Δ−2.70 ppm) decreased throughout the experimental period, whereas BEA C (m/z 826.4626, calcd. for C48H63N3O9H+, Δ−2.05 ppm) was exclusively detected at 3 DPI (Figure 5B and Figure S11). FER (m/z 771.2498, calcd. for C28H44FeN9O13H+, Δ1.55 ppm) increased at 5 and 7 DPI (Figure 5B and Figure S11). MS/MS spectral annotation of these compounds was performed using their fragmentation patterns (Figure S12). Annotated metabolites in the MF and singletons, their MS/MS spectra, and chromatographic peak areas are summarized in Table 2 and Table S2. In summary, OEpks15 mainly produced insect-virulence metabolites and ferricrocin in vivo rather than in culture.

Table 2.
Summary of classified insect-virulence metabolites and a siderophore identified from OEpks15 and ∆pks15 strains in culture media and in vivo.
3.5. pks14 Overexpression in Culture and pks15 Overexpression In Vivo Strongly Stimulated Insect-Virulence Metabolite Production
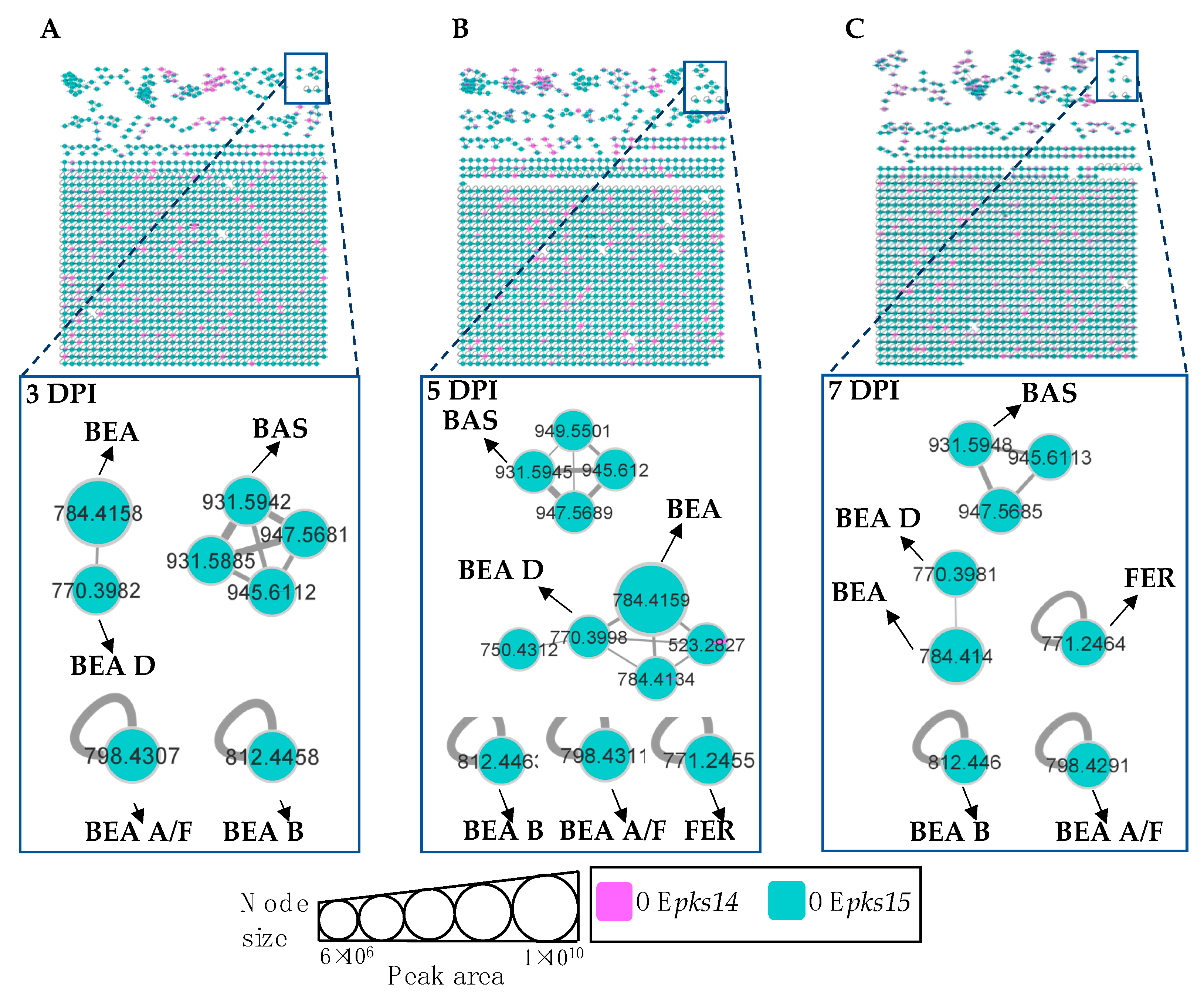
Molecular networking comparisons between OEpks14 and OEpks15 strains in culture and in vivo were subsequently performed. In culture, expression of insect-virulence metabolites, including BEA A/F (m/z 798.4275, calcd. for C46H59N3O9H+, Δ−7.14 ppm), BEA B (m/z 812.4430, calcd. for C47H61N3O9H+, Δ−6.89 ppm), BEA C (m/z 848.4400, calcd. for C48H63N3O9Na+, Δ−7.31 ppm), BAS (m/z 909.6107, calcd. for C48H84N4O12H+, Δ−6.27 ppm), and ENN A (m/z 682.4598, calcd. for C36H63N3O9H+, Δ−6.59 ppm) were observed only for OEpks14, and FER levels were higher for OEpks14 compared OEpks15 (Figure 6). In contrast, in vivo comparison showed that several insect-virulence compounds were only detected from OEpks15-injected BAWs throughout the experimental period (3, 5, and 7 DPI). These compounds were BEA (m/z 784.4158, calcd. for C45H57N3O9H+, Δ−1.91 ppm), BEA A/F (m/z 798.4307, calcd. for C46H59N3O9H+, Δ−3.13 ppm), BEA B (m/z 812.4458, calcd. for C47H61N3O9H+, Δ−3.45 ppm), BEA D (m/z 770.3982, calcd. for C44H55N3O9H+, Δ−4.54 ppm), FER (m/z 771.2464, calcd. for C28H44FeN9O13H+, Δ−2.85 ppm), and BAS (m/z 931.5942, calcd. for C48H84N4O12Na+, Δ−4.40 ppm) (Figure 7).
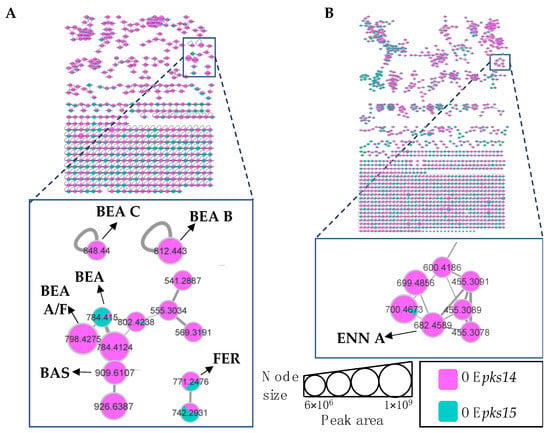
Figure 6.
Molecular networking of insect-virulence metabolites and a siderophore from OEpks14 (pink) and OEpks15 (green) strains in culture identified beauvericin (BEA) and ferricrocin (FER) from both strains. (A) Beauvericin A/F (BEA A/F), beauvericin B (BEA B), beauvericin C (BEA C), and bassianolide (BAS) were found exclusively from OEpks14 cells. (B) Enniatin A (ENN A) was found exclusively in OEpks14 culture broth. Node sizes represent the sums of chromatographic peak areas, and pie charts indicate chromatographic peak-area proportions for the detected insect virulence factors.

Figure 7.
Molecular networking of insect-virulence metabolites and a siderophore for OEpks14 (pink) and OEpks15 (green) strains in vivo at (A) early-stage infection (3 DPI) for live larvae, (B) mid-stage infection (5 DPI) for dead larvae, and (C) late-stage infection (7 DPI) for cadavers covered with fungal hyphae identified beauvericin (BEA), beauvericin A/F (BEA A/F), beauvericin B (BEA B), beauvericin D (BEA D), bassianolide (BAS), and ferricrocin (FER) exclusively from OEpks15. Node sizes represent the sums of chromatographic peak areas, and pie charts indicate chromatographic peak area-proportions for the detected insect virulence factors.
Together, these results clearly demonstrated that the expression of insect-virulence compounds, such as BEA, ENN A, and BAS, was induced in pks-overexpressing strains. These compounds were detected exclusively from OEpks14 in culture and from OEpks15 in vivo. These findings suggest that PKS14 and PKS15 and their metabolites play roles in the production of insect-virulence compounds under specific conditions.
3.6. pks14 and pks15 Promoters Share Motifs with Beauvericin and Bassianolide Gene Cluster Promoters
Given the induction of insect virulence factors in the presence of overexpressed PKS14 and PSK15, we performed a comparative analysis of the promoter sequences. Interestingly, some surprising similarities were observed between the pks14 and pks15 promoters and those of genes in the BEA and BAS biosynthetic clusters. The promoter of pks14, the main synthase gene in cluster 22 of the B. bassiana BCC 2660 genome, shares motifs with the promoters of BEA synthetase (CTcgcCAAcACaGGa.CTTGAaCA; uppecase letters indicate high (>90%) similarity, and lowercase letter indicate low (≤20%) similarity), a gene for a hypothetical protein in the BEA biosynthetic cluster (TCTCTTCaAGacgGaCC) (GenBank accession number XM_008604818.1), and BAS synthetase (CTaC..CGTC.GaGTGC..C) (Figure S13A).
Similarly, the pks15 promoter shares motifs with the promoters of BEA synthetase (TcGCAgAcCAa.aTCaTTCacTcagCcacaCATTCaTTCaTACaTa.CAaaCATaA), a gene for a hypothetical protein in BEA gene cluster (TTcCAcATCac.cAaCacTCATaCATcCaTTaaaTCAa.cCATaC) (GenBank accession number XM_008604825), BAS synthetase (TCATCAaTC.TaCATC.AgTCaT.CaTTcaTaCA), and a gene for a hypothetical protein in the BAS biosynthetic cluster (TATcaTCAcCaTgC.TTCaTCaaTTCAcTcaTTCaTacagACA) (GenBank accession number XM_008597724.1) (Figure S13B). These data suggest that PKS14 and PKS15 can engage in crosstalk with the BEA and BAS biosynthetic clusters.
4. Discussion
In the event of fungal infection and colonization of insect hosts, numerous fungal secondary metabolites and enzymes coordinate to attack hosts. Thus, for secondary metabolite biosynthesis, crosstalk between secondary metabolite clusters will be needed; however, such crosstalk has rarely been found in fungi due to their complexity. Here, we show that the two B. bassiana insect-virulence factors, PKS14 and PKS15 [19,20,21], were involved in fungal growth and development and secondary metabolite production. PKS14 and PKS15 affected conidial yield and insect virulence, which has also been shown in previous studies [19,20,21]. Moreover, PKS15 expression was found in pseudohyphae-like cells and conidia, a cell type previously observed to be affected by knocking out pks15 [21]. The pseudohyphae form is important for host infection for several fungal species, mainly yeasts such as Candida lusitaniae [35] and Saccharomyces cerevisiae clinical isolates [36]. These results emphasize that PKS14, PKS15, and their products are associated with the pathogenicity of B. bassiana. In addition, these two insect-virulence factors orchestrated the production of various NRPs required for insect pathogenesis. Our data demonstrate that the pks14 and pks15 clusters or PKS14 and PKS15 themselves support and enhance the production of some compounds implicated in insect pathogenesis, including an intracellular siderophore, in B. bassiana. We used integrated metabolomic approaches of LC-MS, LC-MS/MS, and GNPS molecular networking. Metabolomic profiles from culture and in vivo sampling of OEpks14, ∆pks14, OEpks15, and ∆pks15 were determined and compared. Notable detected compounds included some widely recognized insect-virulence metabolites such as BEAs, BAS, and ENN A, and the intracellular siderophore FER. For OEpks14, their production was induced in culture rather than in vivo. In contrast, OEpks15 exhibited enhanced production in vivo throughout the experimental period rather than in culture.
Differentially enhanced production of insect-virulence metabolites under certain conditions, namely, by OEpks14 in culture and OEpks15 in vivo, could be the result of stereoisomers. A difference between culture and in vivo results has been described previously for cytochrome P450 2D6 (CYP2D6) inhibition by bupropion [37,38]. Bupropion is a strong inhibitor of CYP2D6 in vivo, but a weak inhibitor of CYP2D6 in vitro. Bupropion is a mixture of R- and S-bupropion which has stereoselectivity in pharmacology. The difference in inhibitor activity between in vitro and in vivo was found to be due to differences in stereoselectivity. In vivo stereoselectivity for R-bupropion was higher than that for S-bupropion. Moreover, stereoselective downregulation of CYP2D6 expression was also found to be higher in vivo than in vitro [39]. Since PKS14 and PKS15 each exhibited a specific induction condition for metabolite production, in culture or in vivo, respectively, this induction may be due to the stereoisomers of inducers.
Noticeably, pks14 and pks15 overexpression in this study contributed to the induced or increased production of these insect-virulence compounds. This suggested that PKS14 and PKS15, or their metabolites, might be directly or indirectly associated with key pathways in insect pathogenesis of B. bassiana. The observed associations and co-regulation between secondary metabolites could be the result of crosstalk between biosynthetic clusters, as the promoters of pks14 and pks15 exhibit similarities to promoters of various genes in the BEA and BAS gene clusters. Crosstalk between different clusters of secondary metabolites has also been described for A. nidulans [40]. Overexpression of the regulatory gene scpR in the NRPS gene cluster induced production of the polyketide asperfuranone by ScpR binding to the promoter of afoA, a regulatory gene in the asperfuranone biosynthetic cluster which shares a motif with the promoter of scpR [40]. In another Aspergillus species, A. fumigatus, crosstalk between two adjacent clusters is mediated by two non-regulatory genes. The genes psoF (a putative dual-functional methyltransferase/monooxygenase) and psoG (a hypothetical protein), located in the fumagillin biosynthetic cluster, are crucial for biosynthesis of pseurotin, a secondary metabolite belonging to the adjacent cluster [41].
In addition, some PKSs are notable for stimulating other molecules or biological factors in fungi. Dictyostelium discoideum PKS1 has been shown to be involved in production of a signaling molecule, 4-methyl-5-pentylbenzene-1,3-diol (MPBD) [42]. MPBD induced spore development by triggering the release of phosphopeptide spore differentiation factor 1 and controlling cell aggregation by regulation of the cAMP signaling pathway during Dictyostelium development [42,43]. An A. carbonarius PKS (AcPKS) has demonstrated a contribution to the expression of the global transcription factor LaeA—playing a crucial role in the pathogenicity and regulation of mycotoxin biosynthesis in the fungus [44]. Additionally, loss of AcPKS reduced the laeA transcript level. Nevertheless, in this study, it was not demonstrated how PKS14 and PKS15 or their polyketides are associated with the production of other insect-virulence metabolites, particularly NRPs. Therefore, identification of polyketides synthesized by PKS14 and PKS15, and their mechanistic involvement in biosynthetic pathways of those NRPs, need to be studied. This could lead to better understanding of the crosstalk between PKS clusters and other secondary metabolite clusters.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/metabo13030425/s1. Table S1: Summary of chromatographic peak area of classified insect-virulence metabolites and a siderophore identified from OEpks14 in culture and in vivo. Table S2: Summary of chromatographic peak area of classified insect-virulence metabolites and a siderophore identified from OEpks15 in culture and in vivo. Figure S1: Molecular networking of classified metabolites for OEpks14 (red) and Δpks14 (light blue) strains in (A) culture cells and (B) culture broth. Figure S2: Molecular networking of classified metabolites in OEpks14 (red) and Δpks14 (light blue) strains in vivo at (A) early-stage infection (3 DPI) for live larvae, (B) mid-stage infection (5 DPI) for dead larvae, and (C) late-stage infection (7 DPI) for cadavers covered with fungal hyphae. Saline-injected BAWs were used as controls (gray). Figure S3: Molecular networking of classified metabolites for OEpks15 (red) and Δpks15 (light blue) strains in culture. Figure S4: Molecular networking of classified metabolites for OEpks15 (red) and Δpks15 (light blue) strains in vivo at (A) early-stage infection (3 DPI) for live larvae, (B) mid-stage infection (5 DPI) for dead larvae, and (C) late-stage infection (7 DPI) for cadavers covered with fungal hyphae. Saline-injected BAWs were used as controls (gray). Figure S5: Comparison of full insecticides and siderophore MS profiles between OEpks14 and Δpks14 in culture revealed upregulation of beauvericin (BEA), beauvericin A/F (BEA A/F), beauvericin B (BEA B), beauvericin C (BEA C), bassianolide (BAS), and enniatin A (ENN A) in OEpks14 compared to Δpks14, whereas no difference was seen for ferricrocin (FER). Figure S6: MS/MS spectra of (A) beauvericin (BEA), (B) beauvericin A/F (BEA A/F), (C) beauvericin B (BEA B), (D) beauvericin C (BEA C), (E) enniatin A (ENN A), (F) bassianolide (BAS), and (G) ferricrocin (FER) from OEpks14 and Δpks14 in culture. Hiv = 2-hydroxyisovaleric acid, NMePhe = N-methylphenylalanine, HMVA= 2-hydroxy-3methylvaleric acid, NMeIle= N-methylisoleucine, NMeLeu= N-methylleucine, Gly = glycine, and L-Ser = L-serine. Figure S7: Comparison of full insecticides and siderophore MS profiles between OEpks14 and Δpks14 strains in vivo at early-stage infection (3 DPI) for live larvae, mid-stage infection (5 DPI) for dead larvae, and late-stage infection (7 DPI) for cadavers covered with fungal hyphae. Beauvericin (BEA), beauvericin A/F (BEA A/F), and ferricrocin (FER) were upregulated in OEpks14 compared to Δpks14. Figure S8: MS/MS spectra of (A) beauvericin (BEA), (B) beauvericin A/F (BEA A/F), and (C) ferricrocin (FER) from OEpks14 in vivo at early-stage infection (3 DPI) for live larvae, mid-stage infection (5 DPI) for dead larvae, and late-stage infection (7 DPI) for cadavers covered with fungal hyphae. Hiv = 2-hydroxyisovaleric acid, NMePhe = N-methylphenylalanine, HMVA= 2-hydroxy-3methylvaleric acid, Gly = glycine, and L-Ser = L-serine. Figure S9: Comparison of insecticide and siderophore MS profiles between OEpks15 and Δpks15 in culture revealed upregulation of beauvericin (BEA) and ferricrocin (FER) in OEpks15 compared to Δpks15. Figure S10: MS/MS spectra of (A) beauvericin (BEA) and (B) ferricrocin (FER) from OEpks15 and Δpks15 in culture. Hiv = 2-hydroxyisovaleric acid, NMePhe = N-methylphenylalanine, Gly = glycine, and L-Ser = L-serine. Figure S11: Comparison of insecticide and siderophore MS profiles between OEpks15 and Δpks15 in vivo at early-stage infection (3 DPI) for live larvae, mid-stage infection (5 DPI) for dead larvae, and late-stage infection (7 DPI) for cadavers covered with fungal hyphae. (A) Beauvericin (BEA), beauvericin A/F (BEA A/F), beauvericin B (BEA B), beauvericin C (BEA C), beauvericin D (BEA D), (B) bassianolide (BAS), and (C) ferricrocin (FER) were identified. Figure S12: MS/MS spectra of (A) beauvericin (BEA), (B) beauvericin A/F (BEA A/F), (C) beauvericin B (BEA B), (E) beauvericin D (BEA D), (F) bassianolide (BAS), and (G) ferricrocin (FER) from OEpks15 and (D) beauvericin C (BEA D) from Δpks15 in vivo at early-stage infection (3 DPI) for live larvae, mid-stage infection (5 DPI) for dead larvae, and late-stage infection (7 DPI) for cadavers covered with fungal hyphae. Hiv = 2-hydroxyisovaleric acid, NMePhe = N-methylphenylalanine, HMVA= 2-hydroxy-3methylvaleric acid, Gly = glycine, and L-Ser = L-serine. Figure S13. Similarities between the promoters of genes (A) pks14 or (B) pks15 with those of genes in the beauvericin and bassianolide biosynthetic clusters.
Author Contributions
Conceptualization, A.A., Y.-L.Y., W.T. and M.T.; methodology, W.T., K.P. and C.S.; software, W.T. and K.P.; formal analysis, W.T. and K.P.; investigation, W.T. and K.P.; resources, A.A. and Y.-L.Y.; data curation, A.A., Y.-L.Y., W.T., K.P., W.-C.H. and C.-C.L.; writing—original draft preparation, W.T.; writing—review and editing, A.A., Y.-L.Y. and W.T.; visualization, M.T., A.A. and Y.-L.Y.; supervision, A.A., Y.-L.Y. and M.T; project administration, A.A. and Y.-L.Y.; funding acquisition, A.A. and Y.-L.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the National Science and Technology Council, Taiwan (MOST 106-2320-B-001-006-MY3), and Thailand’s National Center for Genetic Engineering and Biotechnology (BIOTEC)(P1952101) and Research Development Innovation Management for National Strategic and Network Division (RNS). We are grateful for the PhD fellowship awarded to W.T. by the Taiwan International Graduate Program and the Agricultural Biotechnology Research Center (ABRC), Academia Sinica, Taiwan; and the master’s scholarship awarded to K.P. by the National Taiwan University, Taiwan.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data of LC-MS in this study are available on the MassIVE website (https://massive.ucsd.edu; accessed on 3 January 2023) with accession numbers MSV000090990 (pks14-overexpressing strain in the culture medium), MSV000090991 (pks14-knockout strain in the culture medium), MSV000090992 (pks14-overexpressing strain in vivo), MSV000090993 (pks14-knockout strain in vivo), MSV000090994 (saline for PKS14 in vivo), MSV000090995 (pks15-overexpressing strain in the culture medium), MSV000090996 (pks15-knockout strain in the culture medium), MSV000090997 (pks15-overexpressing strain in vivo), MSV000090998 (pks15-knockout strain in vivo), and MSV000090999 (saline for PKS15 in vivo).
Acknowledgments
LC-MS data were collected in the Metabolomics Core Facility, ABRC, Academia Sinica. We appreciate Gong-Min Lin’s kind help with data collection. We appreciate Samaporn Teeravechyan for language editing and proofreading.
Conflicts of Interest
There are no conflicts of interest to declare.
References
- Wraight, S.P.; Ramos, M.E.; Avery, P.B.; Jaronski, S.T.; Vandenberg, J.D. Comparative virulence of Beauveria bassiana isolates against lepidopteran pests of vegetable crops. J. Invertebr. Pathol. 2010, 103, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Xu, F.; Zi, J.; Wang, S.; Gage, D.; Zeng, J.; Zhan, J. Engineered production of fungal anticancer cyclooligomer depsipeptides in Saccharomyces cerevisiae. Metab. Eng. 2013, 18, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, L. Beauvericin, a bioactive compound produced by fungi: A short review. Molecules 2012, 17, 2367–2377. [Google Scholar] [CrossRef] [PubMed]
- Jirakkakul, J.; Cheevadhanarak, S.; Punya, J.; Chutrakul, C.; Senachak, J.; Buajarern, T.; Tanticharoen, M.; Amnuaykanjanasin, A. Tenellin acts as an iron chelator to prevent iron-generated reactive oxygen species toxicity in the entomopathogenic fungus Beauveria bassiana. FEMS Microbiol. Lett. 2015, 362, 1–8. [Google Scholar] [CrossRef]
- Feng, P.; Shang, Y.; Cen, K.; Wang, C. Fungal biosynthesis of the bibenzoquinone oosporein to evade insect immunity. Proc. Natl. Acad. Sci. USA 2015, 112, 11365–11370. [Google Scholar] [CrossRef]
- Sy-Cordero, A.A.; Pearce, C.J.; Oberlies, N.H. Revisiting the enniatins: A review of their isolation, biosynthesis, structure determination and biological activities. J. Antibiot. 2012, 65, 541–549. [Google Scholar] [CrossRef]
- Xu, Y.; Orozco, R.; Wijeratne, E.M.; Gunatilaka, A.A.; Stock, S.P.; Molnar, I. Biosynthesis of the cyclooligomer depsipeptide beauvericin, a virulence factor of the entomopathogenic fungus Beauveria bassiana. Chem. Biol. 2008, 15, 898–907. [Google Scholar] [CrossRef]
- Xu, Y.; Orozco, R.; Kithsiri Wijeratne, E.M.; Espinosa-Artiles, P.; Leslie Gunatilaka, A.A.; Patricia Stock, S.; Molnar, I. Biosynthesis of the cyclooligomer depsipeptide bassianolide, an insecticidal virulence factor of Beauveria bassiana. Fungal Genet. Biol. 2009, 46, 353–364. [Google Scholar] [CrossRef]
- Wallner, A.; Blatzer, M.; Schrettl, M.; Sarg, B.; Lindner, H.; Haas, H. Ferricrocin, a siderophore involved in intra-and transcellular iron distribution in Aspergillus fumigatus. Appl. Environ. Microbiol. 2009, 75, 4194–4196. [Google Scholar] [CrossRef]
- Eisendle, M.; Schrettl, M.; Kragl, C.; Müller, D.; Illmer, P.; Haas, H. The intracellular siderophore ferricrocin is involved in iron storage, oxidative-stress resistance, germination, and sexual development in Aspergillus nidulans. Eukaryot. Cell 2006, 5, 1596–1603. [Google Scholar] [CrossRef]
- Gibson, D.M.; Donzelli, B.G.; Krasnoff, S.B.; Keyhani, N.O. Discovering the secondary metabolite potential encoded within entomopathogenic fungi. Nat. Prod. Rep. 2014, 31, 1287–1305. [Google Scholar] [CrossRef] [PubMed]
- Hof, C.; Eisfeld, K.; Welzel, K.; Antelo, L.; Foster, A.J.; Anke, H. Ferricrocin synthesis in Magnaporthe grisea and its role in pathogenicity in rice. Mol. Plant Pathol. 2007, 8, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Jirakkakul, J.; Wichienchote, N.; Likhitrattanapisal, S.; Ingsriswang, S.; Yoocha, T.; Tangphatsornruang, S.; Wasuwan, R.; Cheevadhanarak, S.; Tanticharoen, M.; Amnuaykanjanasin, A. Iron homeostasis in the absence of ferricrocin and its consequences in fungal development and insect virulence in Beauveria bassiana. Sci. Rep. 2021, 11, 19624. [Google Scholar] [CrossRef]
- Schrettl, M.; Bignell, E.; Kragl, C.; Sabiha, Y.; Loss, O.; Eisendle, M.; Wallner, A.; Arst, H.N., Jr.; Haynes, K.; Haas, H. Distinct roles for intra- and extracellular siderophores during Aspergillus fumigatus infection. PLoS Pathog. 2007, 3, 1195–1207. [Google Scholar] [CrossRef]
- Giuliano Garisto Donzelli, B.; Gibson, D.M.; Krasnoff, S.B. Intracellular siderophore but not extracellular siderophore is required for full virulence in Metarhizium robertsii. Fungal Genet. Biol. 2015, 82, 56–68. [Google Scholar] [CrossRef]
- Toopaang, W.; Bunnak, W.; Srisuksam, C.; Wattananukit, W.; Tanticharoen, M.; Yang, Y.-L.; Amnuaykanjanasin, A. Microbial polyketides and their roles in insect virulence: From genomics to biological functions. Nat. Prod. Rep. 2022, 39, 2008–2029. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Jin, K.; Ying, S.H.; Zhang, Y.; Xiao, G.; Shang, Y.; Duan, Z.; Hu, X.; Xie, X.Q.; Zhou, G.; et al. Genome sequencing and comparative transcriptomics of the model entomopathogenic fungi Metarhizium anisopliae and M. acridum. PLoS Genet. 2011, 7, e1001264. [Google Scholar] [CrossRef]
- Punya, J.; Swangmaneecharern, P.; Pinsupa, S.; Nitistaporn, P.; Phonghanpot, S.; Kunathigan, V.; Cheevadhanarak, S.; Tanticharoen, M.; Amnuaykanjanasin, A. Phylogeny of type I polyketide synthases (PKSs) in fungal entomopathogens and expression analysis of PKS genes in Beauveria bassiana BCC 2660. Fungal Biol. 2015, 119, 538–550. [Google Scholar] [CrossRef]
- Srisuksam, C.; Punya, J.; Wattanachaisaereekul, S.; Toopaang, W.; Cheevadhanarak, S.; Tanticharoen, M.; Amnuaykanjanasin, A. The reducing clade IIb polyketide synthase PKS14 acts as a virulence determinant of the entomopathogenic fungus Beauveria bassiana. FEMS Microbiol. Lett. 2018, 365, 1578–1586. [Google Scholar] [CrossRef]
- Toopaang, W.; Phonghanpot, S.; Punya, J.; Panyasiri, C.; Klamchao, K.; Wasuwan, R.; Srisuksam, C.; Sangsrakru, D.; Sonthirod, C.; Tangphatsornruang, S.; et al. Targeted disruption of the polyketide synthase gene pks15 affects virulence against insects and phagocytic survival in the fungus Beauveria bassiana. Fungal Biol. 2017, 121, 664–675. [Google Scholar] [CrossRef]
- Udompaisarn, S.; Toopaang, W.; Sae-Ueng, U.; Srisuksam, C.; Wichienchote, N.; Wasuwan, R.; Nahar, N.A.S.; Tanticharoen, M.; Amnuaykanjanasin, A. The polyketide synthase PKS15 has a crucial role in cell wall formation in Beauveria bassiana. Sci. Rep. 2020, 10, 12630. [Google Scholar] [CrossRef]
- Zhou, B.; Xiao, J.F.; Tuli, L.; Ressom, H.W. LC-MS-based metabolomics. Mol. Biosyst. 2012, 8, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Watrous, J.; Roach, P.; Alexandrov, T.; Heath, B.S.; Yang, J.Y.; Kersten, R.D.; van der Voort, M.; Pogliano, K.; Gross, H.; Raaijmakers, J.M.; et al. Mass spectral molecular networking of living microbial colonies. Proc. Natl. Acad. Sci. USA 2012, 109, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.; da Graça, J.P.; Porto, C.; Martin do Prado, R.; Hoffmann-Campo, C.B.; Meyer, M.C.; de Oliveira Nunes, E.; Pilau, E.J. Unraveling Asian Soybean Rust metabolomics using mass spectrometry and Molecular Networking approach. Sci. Rep. 2020, 10, 138. [Google Scholar] [CrossRef]
- Purves, K.; Macintyre, L.; Brennan, D.; Hreggviðsson, G.Ó.; Kuttner, E.; Ásgeirsdóttir, M.E.; Young, L.C.; Green, D.H.; Edrada-Ebel, R.; Duncan, K.R. Using molecular networking for microbial secondary metabolite bioprospecting. Metabolites 2016, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- DesRochers, N.; Walsh, J.P.; Renaud, J.B.; Seifert, K.A.; Yeung, K.K.; Sumarah, M.W. Metabolomic Profiling of Fungal Pathogens Responsible for Root Rot in American Ginseng. Metabolites 2020, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Anjard, C.; Su, Y.; Loomis, W.F. The polyketide MPBD initiates the SDF-1 signaling cascade that coordinates terminal differentiation in Dictyostelium. Eukaryot. Cell 2011, 10, 956–963. [Google Scholar] [CrossRef]
- Narita, T.B.; Chen, Z.-h.; Schaap, P.; Saito, T. The hybrid type polyketide synthase SteelyA is required for cAMP signalling in early Dictyostelium development. PLoS ONE 2014, 9, e106634. [Google Scholar] [CrossRef]
- Amnuaykanjanasin, A.; Epstein, L. A class Vb chitin synthase in Colletotrichum graminicola is localized in the growing tips of multiple cell types, in nascent septa, and during septum conversion to an end wall after hyphal breakage. Protoplasma 2006, 227, 155–164. [Google Scholar] [CrossRef]
- Epstein, L.; Lusnak, K.; Kaur, S. Transformation-Mediated Developmental Mutants of Glomerella graminicola (Colletotrichum graminicola). Fungal Genet. Biol. 1998, 23, 189–203. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform 2010, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Nothias, L.-F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M. Feature-based molecular networking in the GNPS analysis environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Silao, F.G.S.; Bigol, U.G.; Bungay, A.A.C.; Nicolas, M.G.; Heitman, J.; Chen, Y.-L. Calcineurin is required for pseudohyphal growth, virulence, and drug resistance in Candida lusitaniae. PLoS ONE 2012, 7, e44192. [Google Scholar] [CrossRef]
- de Llanos, R.; Fernández-Espinar, M.T.; Querol, A. A comparison of clinical and food Saccharomyces cerevisiae isolates on the basis of potential virulence factors. Antonie Van Leeuwenhoek 2006, 90, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Kharasch, E.D.; Mitchell, D.; Coles, R. Stereoselective bupropion hydroxylation as an in vivo phenotypic probe for cytochrome P4502B6 (CYP2B6) activity. J. Clin. Pharmacol. 2008, 48, 464–474. [Google Scholar] [CrossRef]
- Masters, A.R.; Gufford, B.T.; Lu, J.B.L.; Metzger, I.F.; Jones, D.R.; Desta, Z. Chiral plasma pharmacokinetics and urinary excretion of bupropion and metabolites in healthy volunteers. J. Pharmacol. Exp. Ther. 2016, 358, 230–238. [Google Scholar] [CrossRef]
- Sager, J.E.; Tripathy, S.; Price, L.S.; Nath, A.; Chang, J.; Stephenson-Famy, A.; Isoherranen, N. In vitro to in vivo extrapolation of the complex drug-drug interaction of bupropion and its metabolites with CYP2D6; simultaneous reversible inhibition and CYP2D6 downregulation. Biochem. Pharmacol. 2017, 123, 85–96. [Google Scholar] [CrossRef]
- Bergmann, S.; Funk, A.N.; Scherlach, K.; Schroeckh, V.; Shelest, E.; Horn, U.; Hertweck, C.; Brakhage, A.A. Activation of a silent fungal polyketide biosynthesis pathway through regulatory cross talk with a cryptic nonribosomal peptide synthetase gene cluster. Appl. Environ. Microbiol. 2010, 76, 8143–8149. [Google Scholar] [CrossRef]
- Wiemann, P.; Guo, C.-J.; Palmer, J.M.; Sekonyela, R.; Wang, C.C.C.; Keller, N.P. Prototype of an intertwined secondary-metabolite supercluster. Proc. Natl. Acad. Sci. USA 2013, 110, 17065–17070. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Chhabra, A.; Phatale, P.A.; Samrat, S.K.; Sharma, J.; Gosain, A.; Mohanty, D.; Saran, S.; Gokhale, R.S. Dissecting the functional role of polyketide synthases in Dictyostelium discoideum: Biosynthesis of the differentiation regulating factor 4-methyl-5-pentylbenzene-1,3-diol. J. Biol. Chem. 2008, 283, 11348–11354. [Google Scholar] [CrossRef] [PubMed]
- Narita, T.B.; Koide, K.; Morita, N.; Saito, T. Dictyostelium hybrid polyketide synthase, SteelyA, produces 4-methyl-5-pentylbenzene-1, 3-diol and induces spore maturation. FEMS Microbiol. Lett. 2011, 319, 82–87. [Google Scholar] [CrossRef]
- Maor, U.; Barda, O.; Sadhasivam, S.; Bi, Y.; Levin, E.; Zakin, V.; Prusky, D.B.; Sionov, E. Functional roles of LaeA, polyketide synthase, and glucose oxidase in the regulation of ochratoxin A biosynthesis and virulence in Aspergillus carbonarius. Mol. Plant Pathol. 2021, 22, 117–129. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).