The Impact of Nutrient Intake and Metabolic Wastes during Pregnancy on Offspring Hypertension: Challenges and Future Opportunities
Abstract
1. Introduction
2. Nutrition and Metabolism during Pregnancy and Fetal Development
2.1. Maternal Malnutrition and Offspring Hypertension in Humans
2.2. Animal Models of Maternal Malnutrition-Induced Programmed Hypertension
2.3. Impact of Metabolic Wastes in Pregnancy on Programmed Hypertension
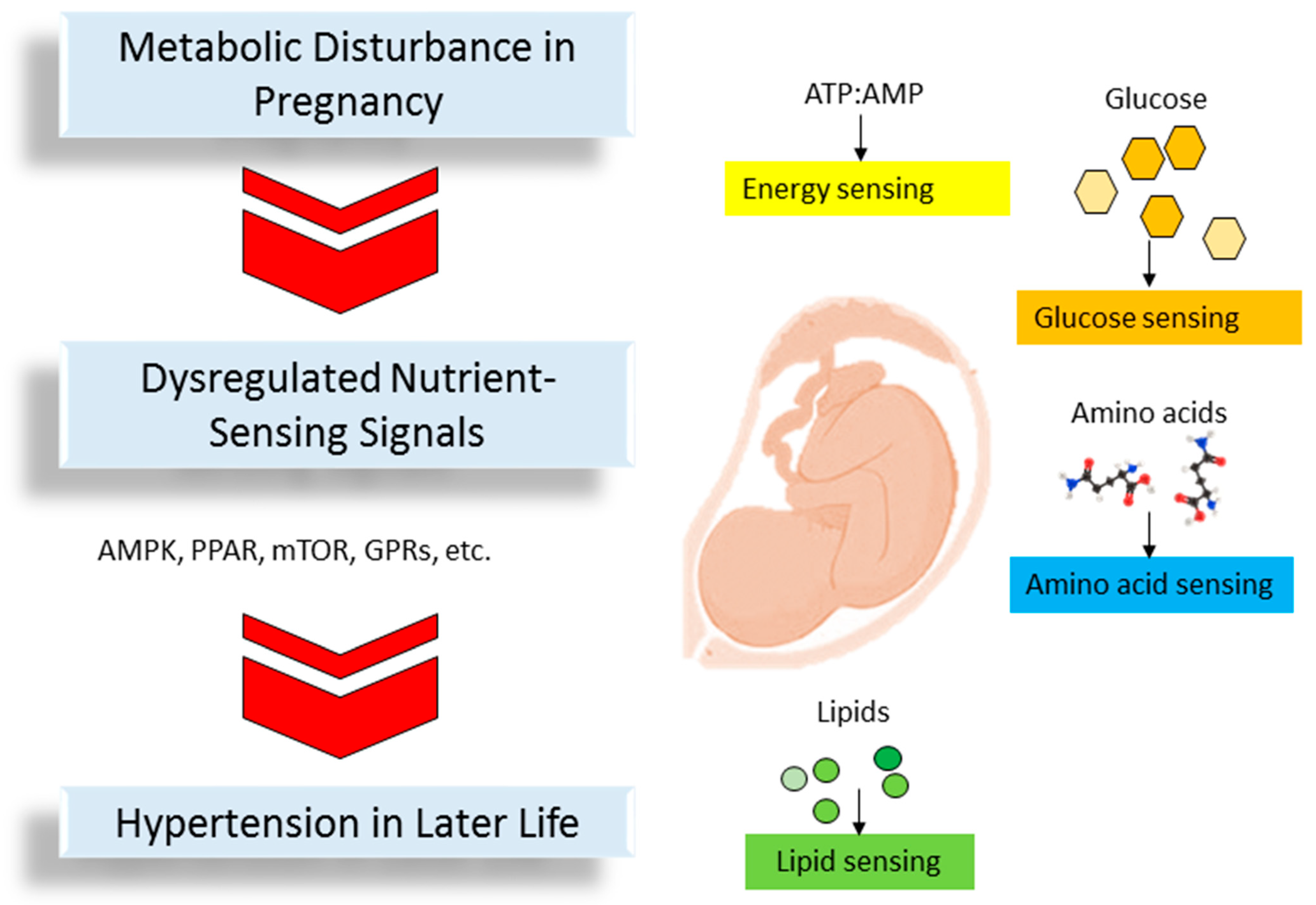
3. The Link between Nutrient-Sensing Signals, Disturbed Metabolism, and Programmed Hypertension
3.1. Energy Sensing
3.2. Glucose Sensing
3.3. Amino Acid Sensing
3.4. Lipid Sensing
3.5. Other Common Mechanisms
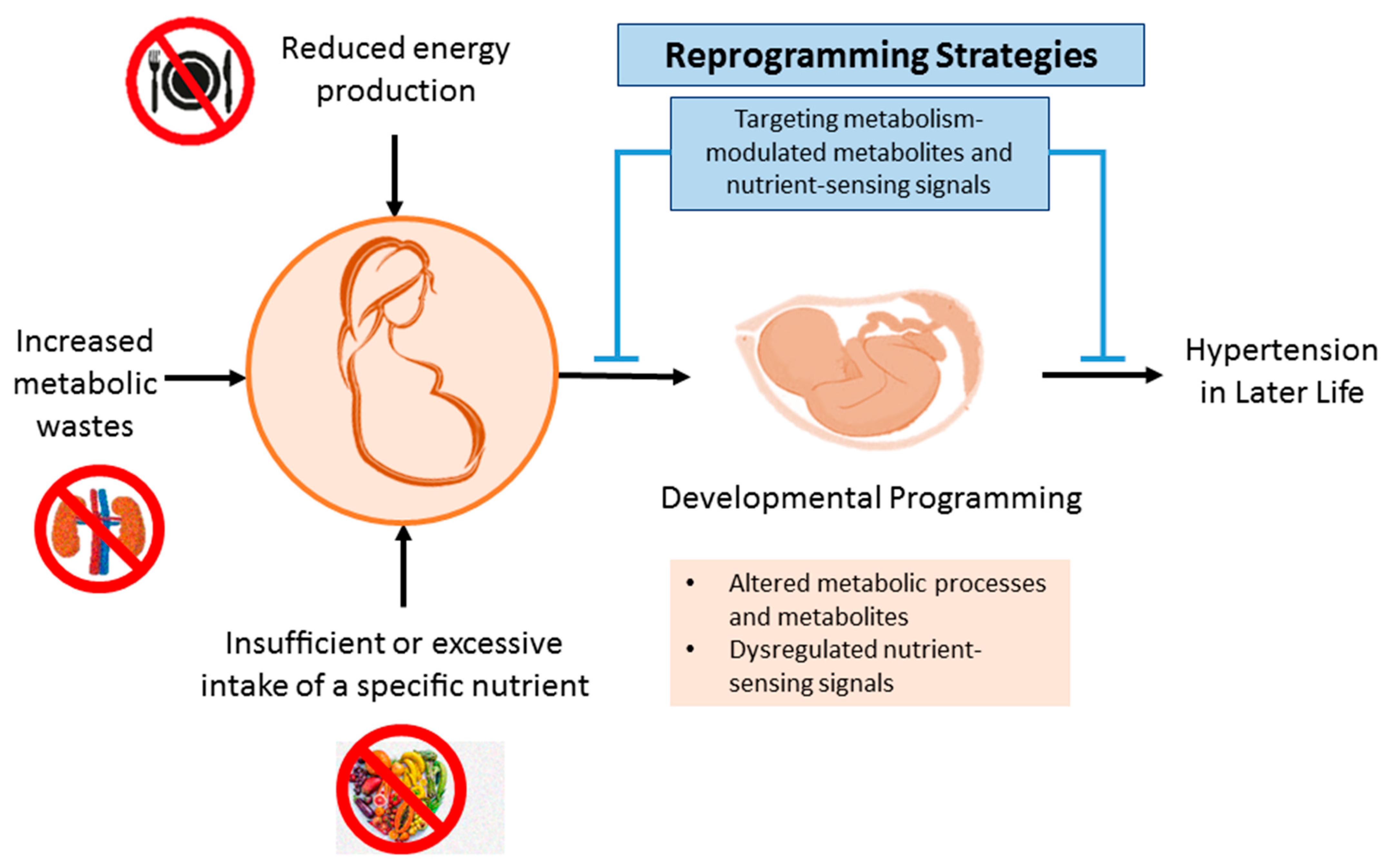
4. Reprogramming Strategy
4.1. Nutritional Intervention
4.1.1. Protein
4.1.2. Lipids
4.1.3. Carbohydrate
4.1.4. Micronutrients
4.1.5. Others
4.2. Targeting Nutrient-Sensing Signal
4.2.1. AMPK
4.2.2. PPAR
4.3. Reduction of Metabolic Wastes
4.3.1. TMAO
4.3.2. ADMA
4.3.3. Tryptophan Metabolites
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar]
- Baker-Smith, C.M.; Flinn, S.K.; Flynn, J.T.; Kaelber, D.C.; Blowey, D.; Carroll, A.E.; Daniels, S.R.; de Ferranti, S.D.; Dionne, J.M.; Falkner, B.; et al. Diagnosis, Evaluation, and Management of High Blood Pressure in Children and Adolescents. Pediatrics 2018, 142, e20182096. [Google Scholar] [CrossRef] [PubMed]
- Oparil, S.; Schmieder, R.E. New approaches in the treatment of hypertension. Circ. Res. 2015, 116, 1074–1095. [Google Scholar] [CrossRef]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef]
- Luyckx, V.A.; Bertram, J.F.; Brenner, B.M.; Fall, C.; Hoy, W.E.; Ozanne, S.E.; Vikse, B.E. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 2013, 382, 273–283. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. Animal Models for DOHaD Research: Focus on Hypertension of Developmental Origins. Biomedicines 2021, 9, 623. [Google Scholar] [CrossRef]
- Hanson, M. The birth and future health of DOHaD. J. Dev. Orig. Health Dis. 2015, 6, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Nijland, M.J.; Ford, S.P.; Nathanielsz, P.W. Prenatal origins of adult disease. Curr. Opin. Obstet. Gynecol. 2008, 20, 132–138. [Google Scholar] [CrossRef] [PubMed]
- McMillen, I.C.; Robinson, J.S. Developmental origins of the metabolic syndrome: Prediction, plasticity, and programming. Physiol. Rev. 2005, 85, 571–633. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Hsu, W.H.; Tain, Y.L. Early-Life Origins of Metabolic Syndrome: Mechanisms and Preventive Aspects. Int. J. Mol. Sci. 2021, 22, 11872. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Itoh, H. Hypertension as a Metabolic Disorder and the Novel Role of the Gut. Curr. Hypertens. Rep. 2019, 21, 63. [Google Scholar] [CrossRef] [PubMed]
- Jahan-Mihan, A.; Luhovyy, B.L.; El Khoury, D.; Anderson, G.H. Dietary proteins as determinants of metabolic and physiologic functions of the gastrointestinal tract. Nutrients 2011, 3, 574–603. [Google Scholar] [CrossRef] [PubMed]
- Felig, P.; Kim, Y.J.; Lynch, V.; Hendler, R. Amino acid metabolism during starvation in human pregnancy. J. Clin. Investig. 1972, 51, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Elango, R.; Ball, R.O. Protein and amino acid requirements during pregnancy. Adv. Nutr. 2016, 7, 839S–844S. [Google Scholar] [CrossRef]
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes: Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids; National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Gouveia, I.F.; Silva, J.R.; Santos, C.; Carvalho, C. Maternal and fetal outcomes of pregnancy in chronic kidney disease: Diagnostic challenges, surveillance and treatment throughout the spectrum of kidney disease. J. Bras. Nefrol. 2021, 43, 88–102. [Google Scholar] [CrossRef]
- Painter, R.C.; Roseboom, T.J.; Bleker, O.P. Prenatal exposure to the Dutch famine and disease in later life: An overview. Reprod. Toxicol. 2005, 20, 345–352. [Google Scholar] [CrossRef]
- Stein, A.D.; Zybert, P.A.; van der Pal-de Bruin, K.; Lumey, L.H. Exposure to famine during gestation, size at birth, and blood pressure at age 59 y: Evidence from the Dutch Famine. Eur. J. Epidemiol. 2006, 21, 759–765. [Google Scholar] [CrossRef]
- Hult, M.; Tornhammar, P.; Ueda, P.; Chima, C.; Bonamy, A.K.; Ozumba, B.; Norman, M. Hypertension, diabetes and overweight: Looming legacies of the Biafran famine. PLoS ONE 2010, 5, e13582. [Google Scholar] [CrossRef]
- Li, C.; Lumey, L.H. Exposure to the Chinese famine of 1959–61 in early life and long-term health conditions: A systematic review and meta-analysis. Int. J. Epidemiol. 2017, 46, 1157–1170. [Google Scholar] [CrossRef]
- Campbell, D.M.; Hall, M.H.; Barker, D.J.; Cross, J.; Shiell, A.W.; Godfrey, K.M. Diet in pregnancy and the offspring’s blood pressure 40 years later. Br. J. Obstet. Gynaecol. 1996, 103, 273–280. [Google Scholar] [CrossRef]
- Shiell, A.W.; Campbell-Brown, M.; Haselden, S.; Robinson, S.; Godfrey, K.M.; Barker, D.J. High-meat, low-carbohydrate diet in pregnancy: Relation to adult blood pressure in the offspring. Hypertension 2001, 38, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Hrolfsdottir, L.; Halldorsson, T.I.; Rytter, D.; Bech, B.H.; Birgisdottir, B.E.; Gunnarsdottir, I.; Granström, C.; Henriksen, T.B.; Olsen, S.F.; Maslova, E. Maternal Macronutrient Intake and Offspring Blood Pressure 20 Years Later. J. Am. Heart Assoc. 2017, 6, e005808. [Google Scholar] [CrossRef] [PubMed]
- Hrudey, E.J.; Reynolds, R.M.; Oostvogels, A.J.; Brouwer, I.A.; Vrijkotte, T.G. The association between maternal 25-hydroxyvitamin D concentration during gestation and early childhood cardio-metabolic outcomes: Is there interaction with pre-pregnancy BMI? PLoS ONE 2015, 10, e0133313. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.M.; Fraser, A.; Fraser, W.D.; Hyppönen, E.; Davey Smith, G.; Deanfield, J.; Hingorani, A.; Sattar, N.; Lawlor, D.A. Associations of maternal 25-hydroxyvitamin D in pregnancy with offspring cardiovascular risk factors in childhood and adolescence: Findings from the Avon Longitudinal Study of Parents and Children. Heart 2013, 99, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Woodall, S.M.; Johnston, B.M.; Breier, B.H.; Gluckman, P.D. Chronic maternal undernutrition in the rat leads to delayed postnatal growth and elevated blood pressure of offspring. Pediatr. Res. 1996, 40, 438–443. [Google Scholar] [CrossRef]
- Franco Mdo, C.; Ponzio, B.F.; Gomes, G.N.; Gil, F.Z.; Tostes, R.; Carvalho, M.H.; Fortes, Z.B. Micronutrient prenatal supplementation prevents the development of hypertension and vascular endothelial damage induced by intrauterine malnutrition. Life Sci. 2009, 85, 327–333. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsieh, C.S.; Lin, I.C.; Chen, C.C.; Sheen, J.M.; Huang, L.T. Effects of maternal L-citrulline supplementation on renal function and blood pressure in offspring exposed to maternal caloric restriction: The impact of nitric oxide pathway. Nitric Oxide 2010, 23, 34–41. [Google Scholar] [CrossRef]
- Ozaki, T.; Nishina, H.; Hanson, M.A.; Poston, L. Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. J. Physiol. 2001, 530, 141–152. [Google Scholar] [CrossRef]
- Sathishkumar, K.; Elkins, R.; Yallampalli, U.; Yallampalli, C. Protein restriction during pregnancy induces hypertension and impairs endothelium-dependent vascular function in adult female offspring. J. Vasc. Res. 2009, 46, 229–239. [Google Scholar] [CrossRef]
- Woods, L.L.; Ingelfinger, J.R.; Nyengaard, J.R.; Rasch, R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr. Res. 2001, 49, 460–467. [Google Scholar] [CrossRef]
- Cambonie, G.; Comte, B.; Yzydorczyk, C.; Ntimbane, T.; Germain, N.; Lê, N.L.; Pladys, P.; Gauthier, C.; Lahaie, I.; Abran, D.; et al. Antenatal antioxidant prevents adult hypertension, vascular dysfunction, and microvascular rarefaction associated with in utero exposure to a low-protein diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1236–R1245. [Google Scholar] [CrossRef]
- Bai, S.Y.; Briggs, D.I.; Vickers, M.H. Increased systolic blood pressure in rat offspring following a maternal low-protein diet is normalized by maternal dietary choline supplementation. J. Dev. Orig. Health Dis. 2012, 3, 342–349. [Google Scholar] [CrossRef]
- Tain, Y.L.; Chan, J.Y.H.; Lee, C.T.; Hsu, C.N. Maternal melatonin therapy attenuates methyl-donor diet-induced programmed hypertension in male adult rat offspring. Nutrients 2018, 10, 1407. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Yu, H.R.; Lin, I.C.; Tiao, M.M.; Huang, L.T.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Sodium butyrate modulates blood pressure and gut microbiota in maternal tryptophan-free diet-induced hypertension rat offspring. J. Nutr. Biochem. 2022, 108, 109090. [Google Scholar] [CrossRef] [PubMed]
- Koleganova, N.; Piecha, G.; Ritz, E.; Becker, L.E.; Müller, A.; Weckbach, M.; Nyengaard, J.R.; Schirmacher, P.; Gross-Weissmann, M.L. Both high and low maternal salt intake in pregnancy alter kidney development in the offspring. Am. J. Physiol. Renal Physiol. 2011, 301, F344–F354. [Google Scholar] [CrossRef]
- Bergel, E.; Belizán, J.M. A deficient maternal calcium intake during pregnancy increases blood pressure of the offspring in adult rats. BJOG 2002, 109, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.M.; Petry, C.J.; Ozanne, S.E.; Hales, C.N. Effects of maternal iron restriction in the rat on blood pressure, glucose tolerance, and serum lipids in the 3-month-old offspring. Metabolism 2001, 50, 562–567. [Google Scholar] [CrossRef]
- Tare, M.; Emmett, S.J.; Coleman, H.A.; Skordilis, C.; Eyles, D.W.; Morley, R.; Parkington, H.C. Vitamin D insufficiency is associated with impaired vascular endothelial and smooth muscle function and hypertension in young rats. J. Physiol. 2011, 589, 4777–4786. [Google Scholar] [CrossRef]
- Tomat, A.; Elesgaray, R.; Zago, V.; Fasoli, H.; Fellet, A.; Balaszczuk, A.M.; Schreier, L.; Costa, M.A.; Arranz, C. Exposure to zinc deficiency in fetal and postnatal life determines nitric oxide system activity and arterial blood pressure levels in adult rats. Br. J. Nutr. 2010, 104, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Shi, A.; Zhu, D.; Bo, L.; Zhong, Y.; Wang, J.; Xu, Z.; Mao, C. High sucrose intake during gestation increases angiotensinII type 1 receptor-mediated vascular contractility associated with epigenetic alterations in aged offspring rats. Peptides 2016, 86, 133–144. [Google Scholar] [CrossRef]
- Gray, C.; Gardiner, S.M.; Elmes, M.; Gardner, D.S. Excess maternal salt or fructose intake programmes sex-specific, stress- and fructose-sensitive hypertension in the offspring. Br. J. Nutr. 2016, 115, 594–604. [Google Scholar] [CrossRef]
- Seong, H.Y.; Cho, H.M.; Kim, M.; Kim, I. Maternal High-Fructose Intake Induces Multigenerational Activation of the Renin-Angiotensin-Aldosterone System. Hypertension 2019, 74, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Wu, K.L.; Lee, W.C.; Leu, S.; Chan, J.Y. Maternal fructose-intake-induced renal programming in adult male offspring. J. Nutr. Biochem. 2015, 26, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Lin, Y.J.; Hou, C.Y.; Tain, Y.L. Maternal Administration of Probiotic or Prebiotic Prevents Male Adult Rat Offspring against Developmental Programming of Hypertension Induced by High Fructose Consumption in Pregnancy and Lactation. Nutrients 2018, 10, 1229. [Google Scholar] [CrossRef]
- Chao, Y.M.; Tain, Y.L.; Lee, W.C.; Wu, K.L.H.; Yu, H.R.; Chan, J.Y.H. Protection by –Biotics against Hypertension Programmed by Maternal High Fructose Diet: Rectification of Dysregulated Expression of Short-Chain Fatty Acid Receptors in the Hypothalamic Paraventricular Nucleus of Adult Offspring. Nutrients 2022, 14, 4306. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lee, W.C.; Wu, K.L.H.; Leu, S.; Chan, J.Y.H. Resveratrol Prevents the Development of Hypertension Programmed by Maternal Plus Post-Weaning High-Fructose Consumption through Modulation of Oxidative Stress, Nutrient-Sensing Signals, and Gut Microbiota. Mol. Nutr. Food Res. 2018, 62, e1800066. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Lee, W.C.; Wu, K.L.H.; Leu, S.; Chan, J.Y.H. Maternal High Fructose Intake Increases the Vulnerability to Post-Weaning High-Fat Diet-Induced Programmed Hypertension in Male Offspring. Nutrients 2018, 10, 56. [Google Scholar] [CrossRef]
- Tain, Y.L.; Leu, S.; Lee, W.C.; Wu, K.L.H.; Chan, J.Y.H. Maternal Melatonin Therapy Attenuated Maternal High-Fructose Combined with Post-Weaning High-Salt Diets-Induced Hypertension in Adult Male Rat Offspring. Molecules 2018, 23, 886. [Google Scholar] [CrossRef]
- Resende, A.C.; Emiliano, A.F.; Cordeiro, V.S.; de Bem, G.F.; de Cavalho, L.C.; de Oliveira, P.R.; Neto, M.L.; Costa, C.A.; Boaventura, G.T.; deMoura, R.S. Grape skin extract protects against programmed changes in the adult rat offspring caused by maternal high-fat diet during lactation. J. Nutr. Biochem. 2013, 24, 2119–2126. [Google Scholar] [CrossRef]
- Khan, I.Y.; Taylor, P.D.; Dekou, V.; Seed, P.T.; Lakasing, L.; Graham, D.; Dominiczak, A.F.; Hanson, M.A.; Poston, L. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension 2003, 41, 168–175. [Google Scholar] [CrossRef]
- Torrens, C.; Ethirajan, P.; Bruce, K.D.; Cagampang, F.R.; Siow, R.C.; Hanson, M.A.; Byrne, C.D.; Mann, G.E.; Clough, G.F. Interaction between maternal and offspring diet to impair vascular function and oxidative balance in high fat fed male mice. PLoS ONE 2012, 7, e50671. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Lee, C.T.; Chan, J.Y.H.; Tain, Y.L. The Interplay between Maternal and Post-Weaning High-Fat Diet and Gut Microbiota in the Developmental Programming of Hypertension. Nutrients 2019, 11, 1982. [Google Scholar] [CrossRef]
- Gray, C.; Harrison, C.J.; Segovia, S.A.; Reynolds, C.M.; Vickers, M.H. Maternal salt and fat intake causes hypertension and sustained endothelial dysfunction in fetal, weanling and adult male resistance vessels. Sci. Rep. 2015, 5, 9753. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Hou, C.Y.; Chan, J.Y.H.; Lee, C.T.; Tain, Y.L. Hypertension Programmed by Perinatal High-Fat Diet: Effect of Maternal Gut Microbiota-Targeted Therapy. Nutrients 2019, 11, E2908. [Google Scholar] [CrossRef]
- Thone-Reineke, C.; Kalk, P.; Dorn, M.; Klaus, S.; Simon, K.; Pfab, T.; Godes, M.; Persson, P.; Unger, T.; Hocher, B. High-protein nutrition during pregnancy and lactation programs blood pressure, food efficiency, and body weight of the offspring in a sex-dependent manner. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1025–R1030. [Google Scholar] [CrossRef]
- Sengupta, P. The laboratory rat: Relating its age with human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar] [PubMed]
- Hsu, C.N.; Tain, Y.L. The Good, the Bad, and the Ugly of Pregnancy Nutrients and Developmental Programming of Adult Disease. Nutrients 2019, 11, 894. [Google Scholar] [CrossRef]
- Lee, W.C.; Wu, K.L.; Leu, S.; Tain, Y.L. Translational insights on developmental origins of metabolic syndrome: Focus on fructose consumption. Biomed. J. 2018, 41, 96–101. [Google Scholar] [CrossRef]
- Buettner, R.; Schölmerich, J.; Bollheimer, L.C. High-fat diets: Modeling the metabolic disorders of human obesity in rodents. Obesity 2007, 15, 798–808. [Google Scholar] [CrossRef]
- Williams, L.; Seki, Y.; Vuguin, P.M.; Charron, M.J. Animal models of in utero exposure to a high fat diet: A review. Biochim. Biophys. Acta 2014, 1842, 507–519. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Maternal High-Fat Diet and Offspring Hypertension. Int. J. Mol. Sci. 2022, 23, 8179. [Google Scholar] [CrossRef] [PubMed]
- Hladunewich, M.A. Chronic Kidney Disease and Pregnancy. Semin. Nephrol. 2017, 37, 337–346. [Google Scholar] [CrossRef]
- Munkhaugen, J.; Lydersen, S.; Romundstad, P.R.; Widerøe, T.-E.; Vikse, B.E.; Hallan, S. Kidney function and future risk for adverse pregnancy outcomes: A population-based study from HUNT II, Norway. Nephrol. Dial. Transplant. 2009, 24, 3744–3750. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Yang, H.W.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Maternal Adenine-Induced Chronic Kidney Disease Programs Hypertension in Adult Male Rat Offspring: Implications of Nitric Oxide and Gut Microbiome Derived Metabolites. Int. J. Mol. Sci. 2020, 21, 7237. [Google Scholar] [CrossRef]
- Tain, Y.L.; Chang-Chien, G.P.; Lin, S.; Hou, C.Y.; Hsu, C.N. Iodomethylcholine Inhibits Trimethylamine-N-Oxide Production and Averts Maternal Chronic Kidney Disease Programmed Offspring Hypertension. Int. J. Mol. Sci. 2023, 24, 1284. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Hsu, C.N. Perinatal Garlic Oil Supplementation Averts Rat Offspring Hypertension Programmed by Maternal Chronic Kidney Disease. Nutrients 2022, 14, 4624. [Google Scholar] [CrossRef]
- Efeyan, A.; Comb, W.C.; Sabatini, D.M. Nutrient-sensing mechanisms and pathways. Nature 2015, 517, 302–310. [Google Scholar] [CrossRef]
- Jansson, T.; Powell, T.L. Role of placental nutrient sensing in developmental programming. Clin. Obstet. Gynecol. 2013, 56, 591–601. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Interplay between oxidative stress and nutrient sensing signaling in the developmental origins of cardiovascular disease. Int. J. Mol. Sci. 2017, 18, 841. [Google Scholar] [CrossRef]
- Grahame Hardie, D. AMP-activated protein kinase: A key regulator of energy balance with many roles in human disease. J. Intern. Med. 2014, 276, 543–559. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. AMP-Activated Protein Kinase as a Reprogramming Strategy for Hypertension and Kidney Disease of Developmental Origin. Int. J. Mol. Sci. 2018, 19, 1744. [Google Scholar] [CrossRef]
- Ford, R.J.; Teschke, S.R.; Reid, E.B.; Durham, K.K.; Kroetsch, J.T.; Rush, J.W. AMP-activated protein kinase activator AICAR acutely lowers blood pressure and relaxes isolated resistance arteries of hypertensive rats. J. Hypertens. 2012, 30, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.M.; Kuo, H.C.; Hsu, C.N.; Huang, L.T.; Tain, Y.L. Metformin reduces asymmetric dimethylarginine and prevents hypertension in spontaneously hypertensive rats. Transl. Res. 2014, 164, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Care, A.S.; Sung, M.M.; Panahi, S.; Gragasin, F.S.; Dyck, J.R.; Davidge, S.T.; Bourque, S.L. Perinatal Resveratrol Supplementation to Spontaneously Hypertensive Rat Dams Mitigates the Development of Hypertension in Adult Offspring. Hypertension 2016, 67, 1038–1044. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lin, Y.J.; Sheen, J.M.; Lin, I.C.; Yu, H.R.; Huang, L.T.; Hsu, C.N. Resveratrol prevents the combined maternal plus postweaning high-fat-diets-induced hypertension in male offspring. J. Nutr. Biochem. 2017, 48, 120–127. [Google Scholar] [CrossRef]
- Tain, Y.L.; Wu, K.L.H.; Lee, W.C.; Leu, S.; Chan, J.Y.H. Prenatal Metformin Therapy Attenuates Hypertension of Developmental Origin in Male Adult Offspring Exposed to Maternal High-Fructose and Post-Weaning High-Fat Diets. Int. J. Mol. Sci. 2018, 19, 1066. [Google Scholar] [CrossRef]
- Tsai, W.L.; Hsu, C.N.; Tain, Y.L. Whether AICAR in Pregnancy or Lactation Prevents Hypertension Programmed by High Saturated Fat Diet: A Pilot Study. Nutrients 2020, 12, 448. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Chan, J.Y.; Hsu, C.N. Maternal Fructose Intake Affects Transcriptome Changes and Programmed Hypertension in Offspring in Later Life. Nutrients 2016, 8, 757. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.M.; Tain, Y.L.; Leu, S.; Wu, K.L.; Lee, W.C.; Chan, J.Y. Developmental programming of the metabolic syndrome: Next-generation sequencing analysis of transcriptome expression in a rat model of maternal high fructose intake. Sheng Li Xue Bao 2016, 68, 557–567. [Google Scholar]
- Tain, Y.L.; Hsu, C.N.; Chan, J.Y.; Huang, L.T. Renal Transcriptome Analysis of Programmed Hypertension Induced by Maternal Nutritional Insults. Int. J. Mol. Sci. 2015, 16, 17826–17837. [Google Scholar] [CrossRef]
- Battaglia, F.C.; Regnault, T.R. Placental transport and metabolism of amino acids. Placenta 2001, 22, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Cleal, J.K.; Lewis, R.M. The mechanisms and regulation of placental amino acid transport to the human foetus. J. Neuroendocrinol. 2008, 20, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Tain, Y.L. Amino Acids and Developmental Origins of Hypertension. Nutrients 2020, 12, 1763. [Google Scholar] [CrossRef]
- Pinho, M.J.; Serrao, M.P.; Gomes, P.; Hopfer, U.; Jose, P.A.; Soares-da-Silva, P. Over-expression of renal LAT1 and LAT2 and enhanced L-DOPA uptake in SHR immortalized renal proximal tubular cells. Kidney Int. 2004, 66, 216–226. [Google Scholar] [CrossRef]
- Kakoki, M.; Wang, W.; Mattson, D.L. Cationic amino acid transport in the renal medulla and blood pressure regulation. Hypertension 2002, 39, 287–292. [Google Scholar] [CrossRef]
- Goberdhan, D.C.; Wilson, C.; Harris, A.L. Amino acid sensing by mTORC1: Intracellular transporters mark the spot. Cell Metab. 2016, 23, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Sarbassov, D.D.; Ali, S.M.; King, J.E.; Latek, R.R.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 2002, 110, 163–175. [Google Scholar] [CrossRef]
- Roos, S.; Kanai, Y.; Prasad, P.D.; Powell, T.L.; Jansson, T. Regulation of placental amino acid transporter activity by mammalian target of rapamycin. Am. J. Physiol. Cell Physiol. 2009, 296, C142–C150. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Alexander, B.T.; Morrison, J.L.; South, A.M. Fetal Growth Restriction and Hypertension in the Offspring: Mechanistic Links and Therapeutic Directions. J. Pediatr. 2020, 224, 115–123. [Google Scholar] [CrossRef]
- Ohanian, J.; Heagerty, A.M. The phosphoinositide signaling system and hypertension. Curr. Opin. Nephrol. Hypertens. 1992, 1, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Alaynick, W.A. Nuclear receptors, mitochondria and lipid metabolism. Mitochondrion 2008, 8, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Sugden, M.C.; Caton, P.W.; Holness, M.J. PPAR control: It’s SIRTainly as easy as PGC. J. Endocrinol. 2010, 204, 93–104. [Google Scholar] [CrossRef]
- Blanchard, P.G.; Festuccia, W.T.; Houde, V.P.; St-Pierre, P.; Brûlé, S.; Turcotte, V.; Côté, M.; Bellmann, K.; Marette, A.; Deshaies, Y. Major involvement of mTOR in the PPARγ-induced stimulation of adipose tissue lipid uptake and fat accretion. J. Lipid Res. 2012, 53, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N.; Chan, J.Y.H. PPARs Link Early Life Nutritional Insults to Later Programmed Hypertension and Metabolic Syndrome. Int. J. Mol. Sci. 2016, 17, 20. [Google Scholar] [CrossRef]
- Hsu, C.N.; Lee, C.T.; Huang, L.T.; Tain, Y.L. Aliskiren in early postnatal life prevents hypertension and reduces asymmetric dimethylarginine in offspring exposed to maternal caloric restriction. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Pluznick, J.L. Microbial short-chain fatty acids and blood pressure regulation. Curr. Hypertens. Rep. 2017, 19, 25. [Google Scholar] [CrossRef]
- Ziętek, M.; Celewicz, Z.; Szczuko, M. Short-Chain Fatty Acids, Maternal Microbiota and Metabolism in Pregnancy. Nutrients 2021, 13, 1244. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Hou, C.Y.; Lee, C.T.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Maternal 3,3-Dimethyl-1-Butanol Therapy Protects Adult Male Rat Offspring against Hypertension Programmed by Perinatal TCDD Exposure. Nutrients 2021, 13, 3041. [Google Scholar] [CrossRef]
- Bordoni, A.; Di Nunzio, M.; Danesi, F.; Biagi, P.L. Polyunsaturated fatty acids: From diet to binding to ppars and other nuclear receptors. Genes Nutr. 2006, 1, 95–106. [Google Scholar] [CrossRef]
- Bryant, J.; Hanson, M.; Peebles, C.; Davies, L.; Inskip, H.; Robinson, S.; Calder, P.C.; Cooper, C.; Godfrey, K.M. Higher oily fish consumption in late pregnancy is associated with reduced aortic stiffness in the child at age 9 years. Circ. Res. 2015, 116, 1202–1205. [Google Scholar] [CrossRef]
- Mozos, I.; Jianu, D.; Stoian, D.; Mozos, C.; Gug, C.; Pricop, M.; Marginean, O.; Luca, C.T. The Relationship between Dietary Choices and Health and Premature Vascular Ageing. Heart Lung Circ. 2021, 30, 1647–1657. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Tain, Y.L. Regulation of Nitric Oxide Production in the Developmental Programming of Hypertension and Kidney Disease. Int. J. Mol. Sci. 2019, 20, 681. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Tain, Y.L. Targeting the Renin-Angiotensin-Aldosterone System to Prevent Hypertension and Kidney Disease of Developmental Origins. Int. J. Mol. Sci. 2021, 22, 2298. [Google Scholar] [CrossRef]
- Bogdarina, I.; Welham, S.; King, P.J.; Burns, S.P.; Clark, A.J. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ. Res. 2007, 100, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Kett, M.M.; Denton, K.M. Renal programming: Cause for concern? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R791–R803. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.S.; Nijland, M.J. Sex differences in the developmental origins of hypertension and cardiorenal disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1941–R1952. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Hypertension of Developmental Origins: Consideration of Gut Microbiome in Animal Models. Biomedicines 2022, 10, 875. [Google Scholar] [CrossRef]
- Ojeda, N.B.; Grigore, D.; Alexander, B.T. Developmental programming of hypertension: Insight from animal models of nutritional manipulation. Hypertension 2008, 52, 44–50. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Lu, Q.; Ren, D.; Sun, X.; Rousselle, T.; Li, J.; Leng, J. AMPK: A balancer of the renin-angiotensin system. Biosci. Rep. 2019, 39, BSR20181994. [Google Scholar] [CrossRef]
- Lal, M.K.; Sharma, E.; Tiwari, R.K.; Devi, R.; Mishra, U.N.; Thakur, R.; Gupta, R.; Dey, A.; Lal, P.; Kumar, A.; et al. Nutrient-Mediated Perception and Signalling in Human Metabolism: A Perspective of Nutrigenomics. Int. J. Mol. Sci. 2022, 23, 11305. [Google Scholar] [CrossRef]
- Duca, F.A.; Lam, T.K. Gut microbiota, nutrient sensing and energy balance. Diabetes Obes. Metab. 2014, 16, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 442–456. [Google Scholar] [CrossRef] [PubMed]
- Paauw, N.D.; van Rijn, B.B.; Lely, A.T.; Joles, J.A. Pregnancy as a critical window for blood pressure regulation in mother and child: Programming and reprogramming. Acta Physiol. 2017, 219, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Tain, Y.L. The double-edged sword effects of maternal nutrition in the developmental programming of hypertension. Nutrients 2018, 10, 1917. [Google Scholar] [CrossRef]
- Roysommuti, S.; Lerdweeraphon, W.; Malila, P.; Jirakulsomchok, D.; Wyss, J.M. Perinatal taurine alters arterial pressure control and renal function in adult offspring. Adv. Exp. Med. Biol. 2009, 643, 145–156. [Google Scholar]
- Thaeomor, A.; Teangphuck, P.; Chaisakul, J.; Seanthaweesuk, S.; Somparn, N.; Roysommuti, S. Perinatal Taurine Supplementation Prevents Metabolic and Cardiovascular Effects of Maternal Diabetes in Adult Rat Offspring. Adv. Exp. Med. Biol. 2017, 975, 295–305. [Google Scholar] [PubMed]
- Tain, Y.L.; Sheen, J.M.; Chen, C.C.; Yu, H.R.; Tiao, M.M.; Kuo, H.C.; Huang, L.T. Maternal citrulline supplementation prevents prenatal dexamethasone-induced programmed hypertension. Free Radic. Res. 2014, 48, 580–586. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lee, W.C.; Hsu, C.N.; Lee, W.C.; Huang, L.T.; Lee, C.T.; Lin, C.Y. Asymmetric dimethylarginine is associated with developmental programming of adult kidney disease and hypertension in offspring of streptozotocin-treated mothers. PLoS ONE 2013, 8, e55420. [Google Scholar] [CrossRef]
- Tain, Y.L.; Huang, L.T.; Lee, C.T.; Chan, J.Y.; Hsu, C.N. Maternal citrulline supplementation prevents prenatal NG-nitro-l-arginine-methyl ester (L-NAME)-induced programmed hypertension in rats. Biol. Reprod. 2015, 92, 7. [Google Scholar] [CrossRef]
- Jackson, A.A.; Dunn, R.L.; Marchand, M.C.; Langley-Evans, S.C. Increased systolic blood pressure in rats induced by a maternal low-protein diet is reversed by dietary supplementation with glycine. Clin. Sci. 2002, 103, 633–639. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Dietary Supplementation with Cysteine during Pregnancy Rescues Maternal Chronic Kidney Disease-Induced Hypertension in Male Rat Offspring: The Impact of Hydrogen Sulfide and Microbiota-Derived Tryptophan Metabolites. Antioxidants 2022, 11, 483. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Lin, I.C.; Yu, H.R.; Huang, L.T.; Tiao, M.M.; Tain, Y.L. Maternal Tryptophan Supplementation Protects Adult Rat Offspring against Hypertension Programmed by Maternal Chronic Kidney Disease: Implication of Tryptophan-Metabolizing Microbiome and Aryl Hydrocarbon Receptor. Int. J. Mol. Sci. 2020, 21, 4552. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Yura, S.; Tatsumi, K.; Kondoh, E.; Mogami, H.; Fujita, K.; Kakui, K.; Aoe, S.; Itoh, H.; Sagawa, N.; et al. Branched-chain amino acid supplemented diet during maternal food restriction prevents developmental hypertension in adult rat offspring. J. Dev. Orig. Health Dis. 2011, 2, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.; Vickers, M.H.; Segovia, S.A.; Zhang, X.D.; Reynolds, C.M. A maternal high fat diet programmes endothelial function and cardiovascular status in adult male offspring independent of body weight, which is reversed by maternal conjugated linoleic acid (CLA) supplementation. PLoS ONE 2015, 10, e0115994. [Google Scholar]
- Gregório, B.M.; Souza-Mello, V.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Maternal fish oil supplementation benefits programmed offspring from rat dams fed low-protein diet. Am. J. Obstet. Gynecol. 2008, 199, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Chang-Chien, G.P.; Lin, S.; Hou, C.Y.; Tain, Y.L. Targeting on gut microbial metabolite trimethylamine-N-Oxide and short-chain fatty acid to prevent maternal high-fructose-diet-induced developmental programming of hypertension in adult male offspring. Mol. Nutr. Food Res. 2019, 63, e1900073. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Yu, H.R.; Chan, J.Y.H.; Lee, W.C.; Lin, I.C.; Wu, K.L.H.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Maternal Acetate Supplementation Reverses Blood Pressure Increase in Male Offspring Induced by Exposure to Minocycline during Pregnancy and Lactation. Int. J. Mol. Sci. 2022, 23, 7924. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.F.; Hsu, C.N. Perinatal Propionate Supplementation Protects Adult Male Offspring from Maternal Chronic Kidney Disease-Induced Hypertension. Nutrients 2022, 14, 3435. [Google Scholar] [CrossRef]
- Torrens, C.; Brawley, L.; Anthony, F.W.; Dance, C.S.; Dunn, R.; Jackson, A.A.; Poston, L.; Hanson, M.A. Folate supplementation during pregnancy improves offspring cardiovascular dysfunction induced by protein restriction. Hypertension 2006, 47, 982–987. [Google Scholar] [CrossRef]
- Takemoto, Y. Amino acids that centrally influence blood pressure and regional blood flow in conscious rats. J. Amino Acids 2012, 2012, 831759. [Google Scholar] [CrossRef] [PubMed]
- Militante, J.D.; Lombardini, J.B. Treatment of hypertension with oral taurine: Experimental and clinical studies. Amino Acids 2002, 23, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Cynober, L.; Moinard, C.; De Bandt, J.P. The 2009 ESPEN Sir David Cuthbertson. Citrulline: A new major signaling molecule or just another player in the pharmaconutrition game? Clin. Nutr. 2010, 29, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Tain, Y.L. Hydrogen Sulfide in Hypertension and Kidney Disease of Developmental Origins. Int. J. Mol. Sci. 2018, 19, 1438. [Google Scholar] [CrossRef]
- Filippou, C.; Tatakis, F.; Polyzos, D.; Manta, E.; Thomopoulos, C.; Nihoyannopoulos, P.; Tousoulis, D.; Tsioufis, K. Overview of salt restriction in the Dietary Approaches to Stop Hypertension (DASH) and the Mediterranean diet for blood pressure reduction. Rev. Cardiovasc. Med. 2022, 23, 36. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Ogura, Y.; Monno, I.; Koya, D. The impact of dietary protein intake on longevity and metabolic health. EBioMedicine 2019, 43, 632–640. [Google Scholar] [CrossRef]
- Accardi, G.; Aiello, A.; Gambino, C.M.; Virruso, C.; Caruso, C.; Candore, G. Mediterranean nutraceutical foods: Strategy to improve vascular ageing. Mech. Ageing Dev. 2016, 159, 63–70. [Google Scholar] [CrossRef]
- Achón, M.; Úbeda, N.; García-González, Á.; Partearroyo, T.; Varela-Moreiras, G. Effects of Milk and Dairy Product Consumption on Pregnancy and Lactation Outcomes: A Systematic Review. Adv. Nutr. 2019, 10, S74–S87. [Google Scholar] [CrossRef]
- Bhupathi, V.; Mazariegos, M.; Cruz Rodriguez, J.B.; Deoker, A. Dairy Intake and Risk of Cardiovascular Disease. Curr. Cardiol. Rep. 2020, 22, 11. [Google Scholar] [CrossRef]
- Barengolts, E. Gut microbiota, prebiotics, probiotics, and synbiotics in management of obesity and prediabetes: Review of randomized controlled trials. Endocr. Pract. 2016, 22, 1224–1234. [Google Scholar] [CrossRef]
- Mozos, I.; Stoian, D.; Luca, C.T. Crosstalk between Vitamins A, B12, D, K, C, and E Status and Arterial Stiffness. Dis. Markers 2017, 2017, 8784971. [Google Scholar] [CrossRef]
- Aaron, K.J.; Sanders, P.W. Role of dietary salt and potassium intake in cardiovascular health and disease: A review of the evidence. Mayo Clin. Proc. 2013, 88, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Anjum, F.M.; Sohaib, M.; Sameen, A. Tackling metabolic syndrome by functional foods. Rev. Endocr. Metab. Disord. 2013, 14, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.Y.C.; Lim, X.Y.; Yeo, J.H.H.; Lee, S.W.H.; Lai, N.M. The Health Effects of Chocolate and Cocoa: A Systematic Review. Nutrients 2021, 13, 2909. [Google Scholar] [CrossRef]
- Pereira, T.; Maldonado, J.; Laranjeiro, M.; Coutinho, R.; Cardoso, E.; Andrade, I.; Conde, J. Central arterial hemodynamic effects of dark chocolate ingestion in young healthy people: A randomized and controlled trial. Cardiol. Res. Pract. 2014, 2014, 945951. [Google Scholar] [CrossRef]
- Latif, R. Maternal and fetal effects of chocolate consumption during pregnancy: A systematic review. J. Matern. Fetal Neonatal Med. 2019, 32, 2915–2927. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Novel Insights on Dietary Polyphenols for Prevention in Early-Life Origins of Hypertension: A Review Focusing on Preclinical Animal Models. Int. J. Mol. Sci. 2022, 23, 6620. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Oest, M.E.; Prater, M.R. Intrauterine exposure to high saturated fat diet elevates risk of adult-onset chronic diseases in C57BL/6 mice. Birth Defects Res. B Dev. Reprod. Toxicol. 2009, 86, 377–384. [Google Scholar] [CrossRef]
- Kim, J.; Yang, G.; Kim, Y.; Kim, J.; Ha, J. AMPK activators: Mechanisms of action and physiological activities. Exp. Mol. Med. 2016, 48, e224. [Google Scholar] [CrossRef] [PubMed]
- Lamothe, J.; Khurana, S.; Tharmalingam, S.; Williamson, C.; Byrne, C.J.; Lees, S.J.; Khaper, N.; Kumar, A.; Tai, T.C. Oxidative Stress Mediates the Fetal Programming of Hypertension by Glucocorticoids. Antioxidants 2021, 10, 531. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Maternal Garlic Oil Supplementation Prevents High-Fat Diet-Induced Hypertension in Adult Rat Offspring: Implications of H2S-Generating Pathway in the Gut and Kidneys. Mol. Nutr. Food Res. 2021, 65, e2001116. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Tain, Y.L. Preventive Aspects of Early Resveratrol Supplementation in Cardiovascular and Kidney Disease of Developmental Origins. Int. J. Mol. Sci. 2021, 22, 4210. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Developmental Programming of the Metabolic Syndrome: Can We Reprogram with Resveratrol? Int. J. Mol. Sci. 2018, 19, 2584. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Gerevini, G.T.; Repossi, G.; Dain, A.; Tarres, M.C.; Das, U.N.; Eynard, A.R. Beneficial action of resveratrol: How and why? Nutrition 2016, 32, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.E.; Lin, Y.J.; Lin, I.C.; Yu, H.R.; Sheen, J.M.; Tsai, C.C.; Huang, L.T.; Tain, Y.L. Resveratrol prevents combined prenatal NG-nitro-L-arginine-methyl ester (L-NAME) treatment plus postnatal high-fat diet induced programmed hypertension in adult rat offspring: Interplay between nutrient-sensing signals, oxidative stress and gut microbiota. J. Nutr. Biochem. 2019, 70, 28–37. [Google Scholar] [CrossRef]
- Azhar, S. Peroxisome proliferator-activated receptors, metabolic syndrome and cardiovascular disease. Future Cardiol. 2010, 6, 657–691. [Google Scholar] [CrossRef] [PubMed]
- Monsalve, F.A.; Pyarasani, R.D.; Delgado-Lopez, F.; Moore-Carrasco, R. Peroxisome proliferator-activated receptor targets for the treatment of metabolic diseases. Mediat. Inflamm. 2013, 2013, 549627. [Google Scholar] [CrossRef]
- Torres, T.S.; D’Oliveira Silva, G.; Aguila, M.B.; de Carvalho, J.J.; Mandarim-De-Lacerda, C.A. Effects of rosiglitazone (a peroxysome proliferator-activated receptor γ agonist) on the blood pressure and aortic structure in metabolically programmed (perinatal low protein) rats. Hypertens. Res. 2008, 31, 965–975. [Google Scholar] [CrossRef]
- Koeners, M.P.; Wesseling, S.; Sánchez, M.; Braam, B.; Joles, J.A. Perinatal Inhibition of NF-κB has long-term antihypertensive and renoprotective effects in fawn-hooded hypertensive rats. Am. J. Hypertens. 2016, 29, 123–131. [Google Scholar] [CrossRef]
- Michalik, L.; Auwerx, J.; Berger, J.P.; Chatterjee, V.K.; Glass, C.K.; Gonzalez, F.J.; Grimaldi, P.A.; Kadowaki, T.; Lazar, M.A.; O’Rahilly, S.; et al. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol. Rev. 2006, 58, 726–741. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Park, J.; Choi, J.H. Revisiting PPARγ as a target for the treatment of metabolic disorders. BMB Rep. 2014, 47, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Zixin, Y.; Lulu, C.; Xiangchang, Z.; Qing, F.; Binjie, Z.; Chunyang, L.; Tai, R.; Dongsheng, O. TMAO as a potential biomarker and therapeutic target for chronic kidney disease: A review. Front. Pharmacol. 2022, 13, 929262. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Targeting on asymmetric dimethylarginine related nitric oxide-reactive oxygen species imbalance to reprogram the development of hypertension. Int. J. Mol. Sci. 2016, 17, 2020. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. Developmental programming and reprogramming of hypertension and kidney disease: Impact of tryptophan metabolism. Int. J. Mol. Sci. 2020, 21, 8705. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. Chronic Kidney Disease and Gut Microbiota: What Is Their Connection in Early Life? Int. J. Mol. Sci. 2022, 23, 3954. [Google Scholar] [CrossRef] [PubMed]
- Schiattarella, G.G.; Sannino, A.; Toscano, E.; Giugliano, G.; Gargiulo, G.; Franzone, A.; Trimarco, B.; Esposito, G.; Perrino, C. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: A systematic review and dose-response meta-analysis. Eur. Heart J. 2017, 38, 2948–2956. [Google Scholar] [CrossRef]
- Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. Trimethylamine N-Oxide: The good, the bad and the unknown. Toxins 2016, 8, 326. [Google Scholar] [CrossRef]
- Organ, C.L.; Li, Z.; Sharp, T.E.; Polhemus, D.J.; Guptam, N.; Goodchild, T.T.; Tang, W.H.W.; Hazen, S.L.; Lefer, D.J. Nonlethal Inhibition of Gut Microbial Trimethylamine N-oxide Production Improves Cardiac Function and Remodeling in a Murine Model of Heart Failure. J. Am. Heart Assoc. 2020, 9, e016223. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Chan, J.Y.H.; Lee, C.T.; Tain, Y.L. Maternal resveratrol therapy protected adult rat offspring against hypertension programmed by combined exposures to asymmetric dimethylarginine and trimethylamine-N oxide. J. Nutr. Biochem. 2021, 93, 108630. [Google Scholar] [CrossRef]
- Beltowski, J.; Kedra, A. Asymmetric dimethylarginine (ADMA) as a target for pharmacotherapy. Pharmacol. Rep. 2006, 58, 159–178. [Google Scholar]
- Tain, Y.L.; Hsu, C.N. Toxic Dimethylarginines: Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA). Toxins 2017, 9, E92. [Google Scholar] [CrossRef]
- Hsu, C.N.; Lin, Y.J.; Lu, P.C.; Tain, Y.L. Maternal Resveratrol Therapy Protects Male Rat Offspring against Programmed Hypertension Induced by TCDD and Dexamethasone Exposures: Is It Relevant to Aryl Hydrocarbon Receptor? Int. J. Mol. Sci. 2018, 19, E2459. [Google Scholar] [CrossRef] [PubMed]
- Tai, I.H.; Sheen, J.M.; Lin, Y.J.; Yu, H.R.; Tiao, M.M.; Chen, C.C.; Huang, L.T.; Tain, Y.L. Maternal N-acetylcysteine therapy regulates hydrogen sulfide-generating pathway and prevents programmed hypertension in male offspring exposed to prenatal dexamethasone and postnatal high-fat diet. Nitric Oxide 2016, 53, 6–12. [Google Scholar] [CrossRef]
- Addi, T.; Dou, L.; Burtey, S. Tryptophan-Derived Uremic Toxins and Thrombosis in Chronic Kidney Disease. Toxins 2018, 10, 412. [Google Scholar] [CrossRef]
- Sallée, M.; Dou, L.; Cerini, C.; Poitevin, S.; Brunet, P.; Burtey, S. The aryl hydrocarbon receptor-activating effect of uremic toxins from tryptophan metabolism: A new concept to understand cardiovascular complications of chronic kidney disease. Toxins 2014, 6, 934–949. [Google Scholar] [CrossRef]
- Niwa, T. Targeting protein-bound uremic toxins in chronic kidney disease. Expert Opin. Ther. Targets 2013, 17, 1287–1301. [Google Scholar] [CrossRef]
- Sanaka, T.; Sugino, N.; Teraoka, S.; Ota, K. Therapeutic effects of oral sorbent in undialyzed uremia. Am. J. Kidney Dis. 1988, 12, 97–103. [Google Scholar] [CrossRef]
- Toyoda, S.; Hashimoto, R.; Tezuka, T.; Sakuma, M.; Abe, S.; Ishikawa, T.; Taguchi, I.; Inoue, T. Antioxidative effect of an oral adsorbent, AST-120, and long-term outcomes in chronic kidney disease patients with cardiovascular disease. Hypertens. Res. 2020, 43, 1128–1131. [Google Scholar] [CrossRef]
- Savouret, J.F.; Berdeaux, A.; Casper, R.F. The aryl hydrocarbon receptor and its xenobiotic ligands: A fundamental trigger for cardiovascular diseases. Nutr. Metab. Cardiovasc. Dis. 2003, 13, 104–113. [Google Scholar] [CrossRef]

| Maternal Malnutrition | Cohort Study/Country | Age at Measure (Year) | Case Number | References |
|---|---|---|---|---|
| Undernutrition | Dutch famine study/Netherlands | 50 | 741 | Painter et al., 2005 [17] |
| Undernutrition | Dutch famine study/Netherlands | 59 | 359 | Stein et al., 2006 [18] |
| Undernutrition | Biafran famine study/Nigeria | 40 | 1339 | Hult et al., 2010 [19] |
| Undernutrition | China great leap forward famine study/China | 55 | 1029 | Li et al., 2017 [20] |
| High-protein, low-carbohydrate diet | Aberdeen maternity hospital study/Scotland | 40 | 253 | Campbell et al., 1996 [21] |
| High-protein, low-carbohydrate diet | Motherwell study/Scotland | 30 | 626 | Shiell et al., 2001 [22] |
| High-protein intake | DaFO88/Denmark | 20 | 434 | Hrolfsdottir et al., 2017 [23] |
| Animal Models | Intervention Periods | Age at Measure | Species/Gender | References |
|---|---|---|---|---|
| Under-nutrition | ||||
| Caloric restriction, 30% | Gestation | 54 weeks | Wistar rat/M+F | [26] |
| Caloric restriction, 50% | Gestation | 16 weeks | Wistar rat/M+F | [27] |
| Caloric restriction, 50% | Gestation and lactation | 12 weeks | SD rat/M | [28] |
| Caloric restriction, 70% | Gestation days 0–18 | 28 weeks | Wistar rat/M+F | [29] |
| Protein restriction, 6% | Gestation | 52 weeks | SD rat/F | [30] |
| Protein restriction, 8.5% | Gestation | 20 weeks | SD rat/M | [31] |
| Protein restriction, 9% | Gestation | 12 weeks | Wistar rat/M | [32] |
| Protein restriction, 9% | Gestation | 22 weeks | Wistar rat/M+F | [33] |
| Methyl-deficient diet | Gestation and lactation | 12 weeks | SD rat/M | [34] |
| Tryptophan-free diet | Gestation and lactation | 16 weeks | SD rat/M | [35] |
| Low-salt diet, 0.07% | Gestation and lactation | 21 weeks | SD rat/M | [36] |
| Calcium-deficient die | Gestation | 52 weeks | Wistar-Kyoto rat/M+F | [37] |
| Iron restriction | 4 weeks before conception and throughout pregnancy | 3 months | Wistar rat/M+F | [38] |
| Vitamin D restriction | 6 weeks before conception and throughout pregnancy and lactation | 8 weeks | SD rat/M+F | [39] |
| Zinc-deficient diet | Gestation and lactation | 12 weeks | Wistar rat/M | [40] |
| Over-nutrition | ||||
| High-sucrose solution, 20% | Gestation | 22 months | SD rat/M | [41] |
| High fructose/salt solution, 10%/4% | 4 weeks before conception and throughout pregnancy and lactation | 9 weeks | SD rat/M | [42] |
| High-fructose solution, 20% | Gestation and lactation | 8 months | C57BL6J mice/M+F | [43] |
| High-fructose diet, 60% | Gestation and lactation | 12 weeks | SD rat/M | [44,45,46] |
| Maternal and post-weaning high-fructose diet | Gestation and lactation | 12 weeks | SD rat/M | [47] |
| Maternal high-fructose diet plus post-weaning high-fat diet | Gestation and lactation | 12 weeks | SD rat/M | [48] |
| Maternal high-fructose diet plus post-weaning high-salt diet | Gestation and lactation | 12 weeks | SD rat/M | [49] |
| High-fat diet, 24% | Lactation | 22 weeks | Wistar rat/M | [50] |
| High-fat diet, 25.7% | Lactation | 22 weeks | SD rat/F | [51] |
| High-fat diet, 45% | Gestation and lactation | 30 weeks | C57BL6J mice/M | [52] |
| High-fat diet, 58% | 5 weeks before the delivery and throughout pregnancy and lactation | 16 weeks | SD rat/M | [53] |
| High fat plus high-salt diet, 45%/4% | 3 weeks before conception and throughout pregnancy and lactation | 19 weeks | SD rat/M | [54] |
| Maternal and post-weaning high-fat diet, 58% | Gestation and lactation | 16 weeks | SD rat/M | [55] |
| High-protein diet | Gestation and lactation | 22 weeks | Wistar rat/M | [56] |
| High methyl-donor diet | Gestation and lactation | 12 weeks | SD rat/M | [34] |
| High-salt diet, 4% | Gestation and lactation | 21 weeks | SD rat/M | [36] |
| Intervention | Periods | Animal Models | Age at Measure | Species/Gender | References |
|---|---|---|---|---|---|
| Protein | |||||
| 3% taurine | Gestation and lactation | Maternal high-sugar diet | 8 weeks | SD rat/F | [116] |
| 3% taurine | Gestation and lactation | Streptozotocin-induced diabetes | 16 weeks | Wistar rat/M+F | [117] |
| 0.25% citrulline | Gestation and lactation | Maternal caloric restriction | 12 weeks | SD rat/M | [28] |
| 0.25% citrulline | Gestation and lactation | Prenatal dexamethasone administration | 12 weeks | SD rat/M | [118] |
| 0.25% citrulline | Gestation and lactation | Streptozotocin-induced diabetes | 12 weeks | SD rat/M | [119] |
| 0.25% citrulline | Gestation and lactation | Maternal L-NAME administration | 12 weeks | SD rat/M | [120] |
| 3% glycine | Gestation and lactation | Maternal low protein diet | 4 weeks | Wistar rat/F | [121] |
| Oral gavage of D- or L-cysteine 8 mmol/kg/day | Gestation | Maternal CKD | 12 weeks | SD rat/M | [122] |
| Oral gavage of tryptophan 200 mg/kg/day | Gestation and lactation | Maternal CKD | 12 weeks | SD rat/M | [123] |
| BCAA-supplemented diet | Gestation | Maternal caloric restriction | 16 weeks | SD rat/M | [124] |
| Lipid | |||||
| Conjugated linoleic acid | Gestation and lactation | Maternal high-fat diet | 18 weeks | SD rat/M | [125] |
| Omega-3 polyunsaturated fatty acids | Gestation and lactation | Maternal low protein diet | 6 months | Wistar rat/M+F | [126] |
| Magnesium acetate 200 mmol/L | Gestation and lactation | Maternal high-fructose diet | 12 weeks | SD rat/M | [127] |
| Magnesium acetate 200 mmol/L | Gestation and lactation | Maternal minocycline exposure | 12 weeks | SD rat/M | [128] |
| Sodium butyrate 400 mmol/L | Gestation and lactation | Maternal tryptophan-free diet | 12 weeks | SD rat/M | [35] |
| Propionate 200 mmol/L | Gestation and lactation | Maternal CKD | 12 weeks | SD rat/M | [129] |
| Carbohydrate | |||||
| 5% w/w long chain inulin | Gestation and lactation | Maternal high-fructose diet | 12 weeks | SD rat/M | [45] |
| 5% w/w long chain inulin | Gestation and lactation | Maternal high-fat diet | 16 weeks | SD rat/M | [55] |
| Micronutrients | |||||
| Folic acid, vitamin C, E, and selenium | Gestation | Maternal caloric restriction | 16 weeks | Wistar rat/M+F | [27] |
| Folic acid 5 mg/kg/day | Gestation | Maternal low protein diet | 15 weeks | Wistar rat/M | [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tain, Y.-L.; Hsu, C.-N. The Impact of Nutrient Intake and Metabolic Wastes during Pregnancy on Offspring Hypertension: Challenges and Future Opportunities. Metabolites 2023, 13, 418. https://doi.org/10.3390/metabo13030418
Tain Y-L, Hsu C-N. The Impact of Nutrient Intake and Metabolic Wastes during Pregnancy on Offspring Hypertension: Challenges and Future Opportunities. Metabolites. 2023; 13(3):418. https://doi.org/10.3390/metabo13030418
Chicago/Turabian StyleTain, You-Lin, and Chien-Ning Hsu. 2023. "The Impact of Nutrient Intake and Metabolic Wastes during Pregnancy on Offspring Hypertension: Challenges and Future Opportunities" Metabolites 13, no. 3: 418. https://doi.org/10.3390/metabo13030418
APA StyleTain, Y.-L., & Hsu, C.-N. (2023). The Impact of Nutrient Intake and Metabolic Wastes during Pregnancy on Offspring Hypertension: Challenges and Future Opportunities. Metabolites, 13(3), 418. https://doi.org/10.3390/metabo13030418






