Multi-Omics Analysis of Lung Tissue Demonstrates Changes to Lipid Metabolism during Allergic Sensitization in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
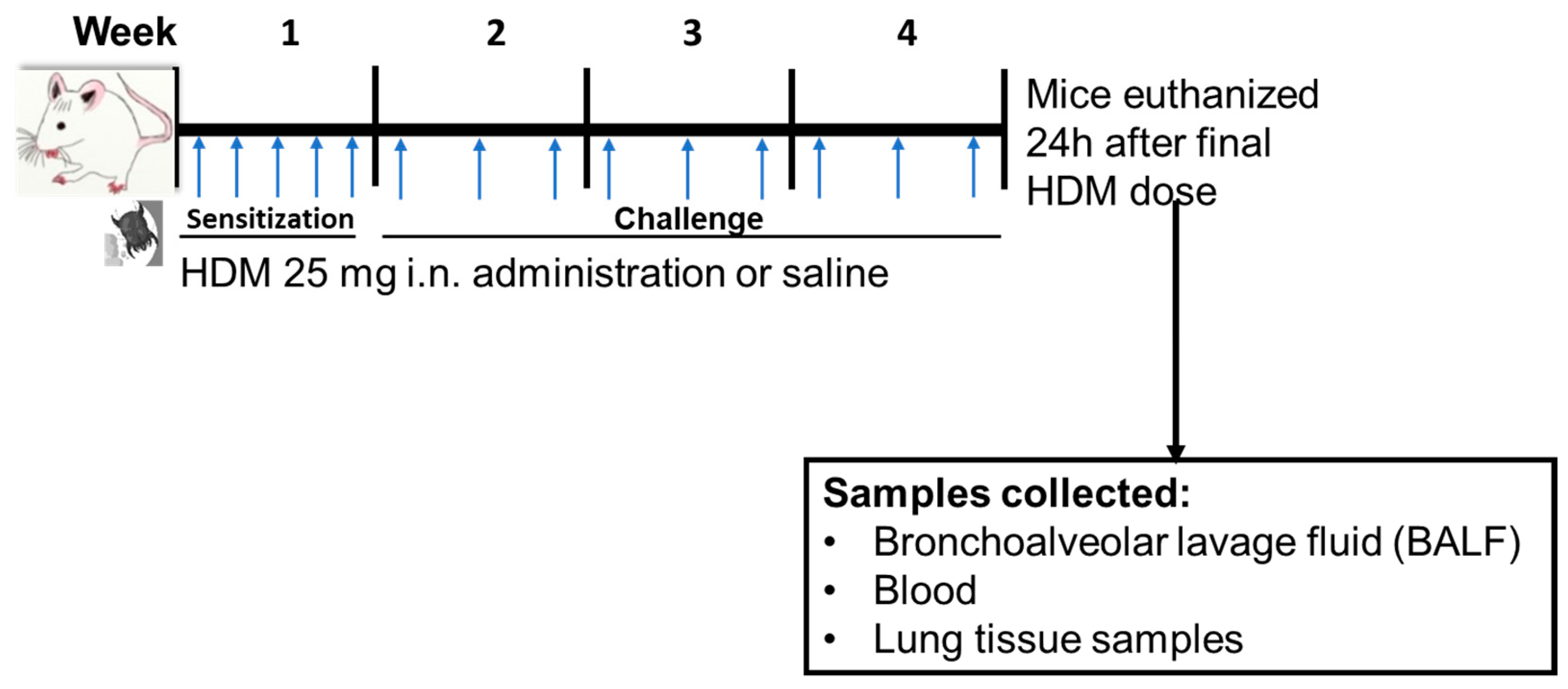
2.2. HDM Sensitization
2.3. Differential Cells Counts and Histology
2.4. Untargeted Metabolomics:
2.5. Quantitative Targeted Analysis of Oxylipins (Lipidomics)
2.6. Global Gene Expression
2.7. Statistical Analysis
3. Results
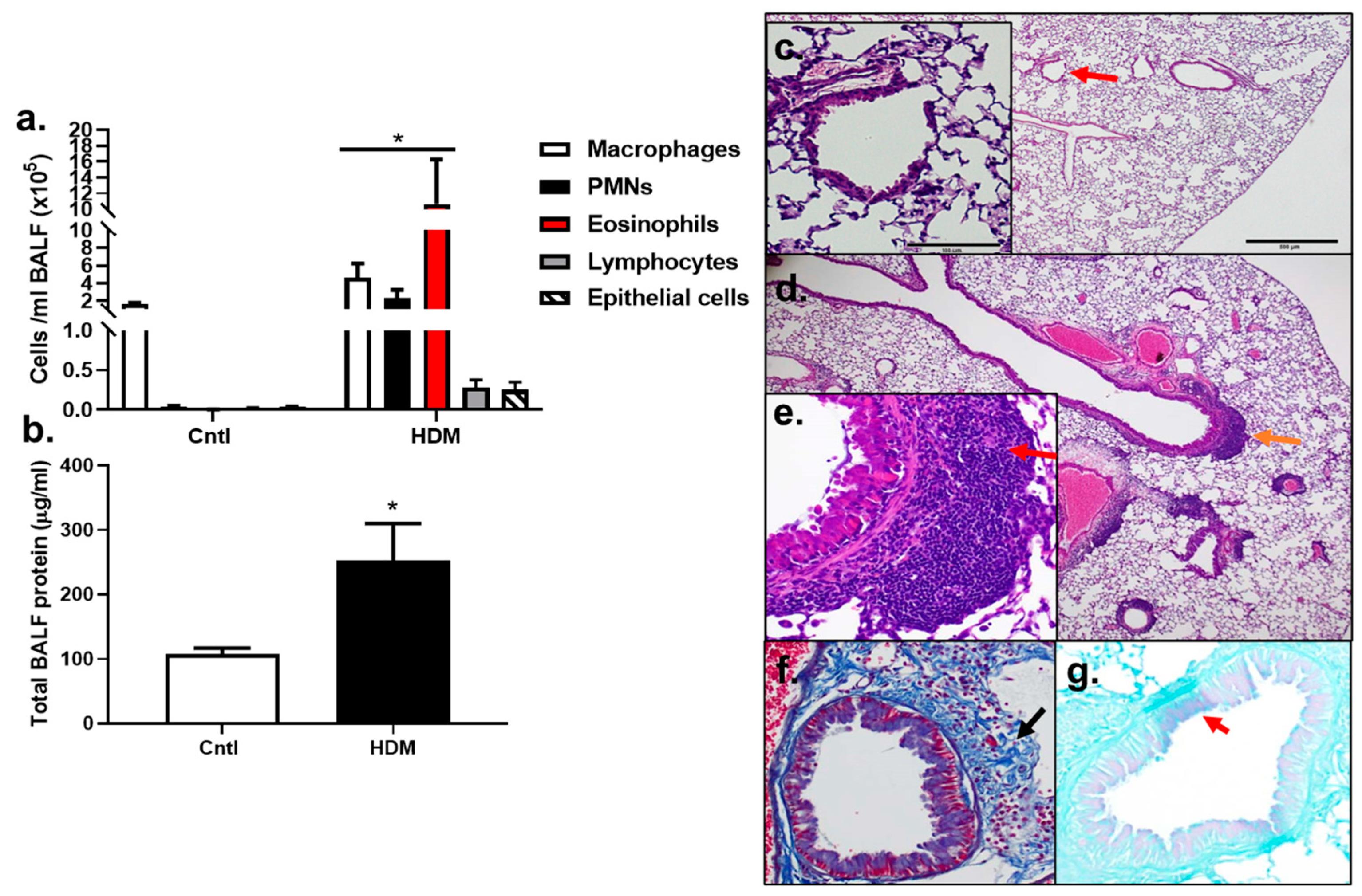
3.1. Inflammatory Cells and Histopathology Changes in HDM-Sensitized Mice
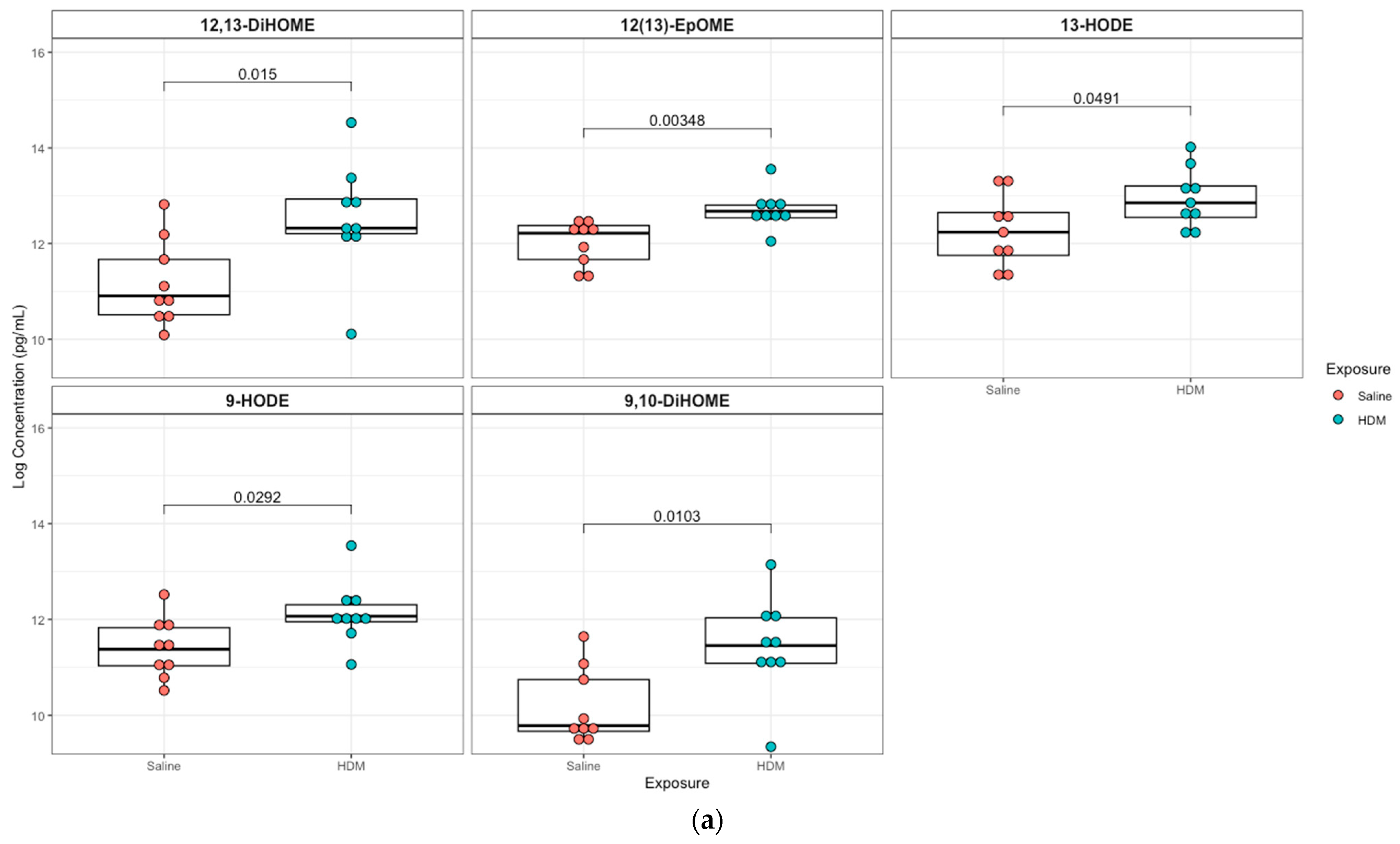
3.2. Differentially Regulated Compouds (Untargeted Metabolomics) in HDM-Sensitized Mice
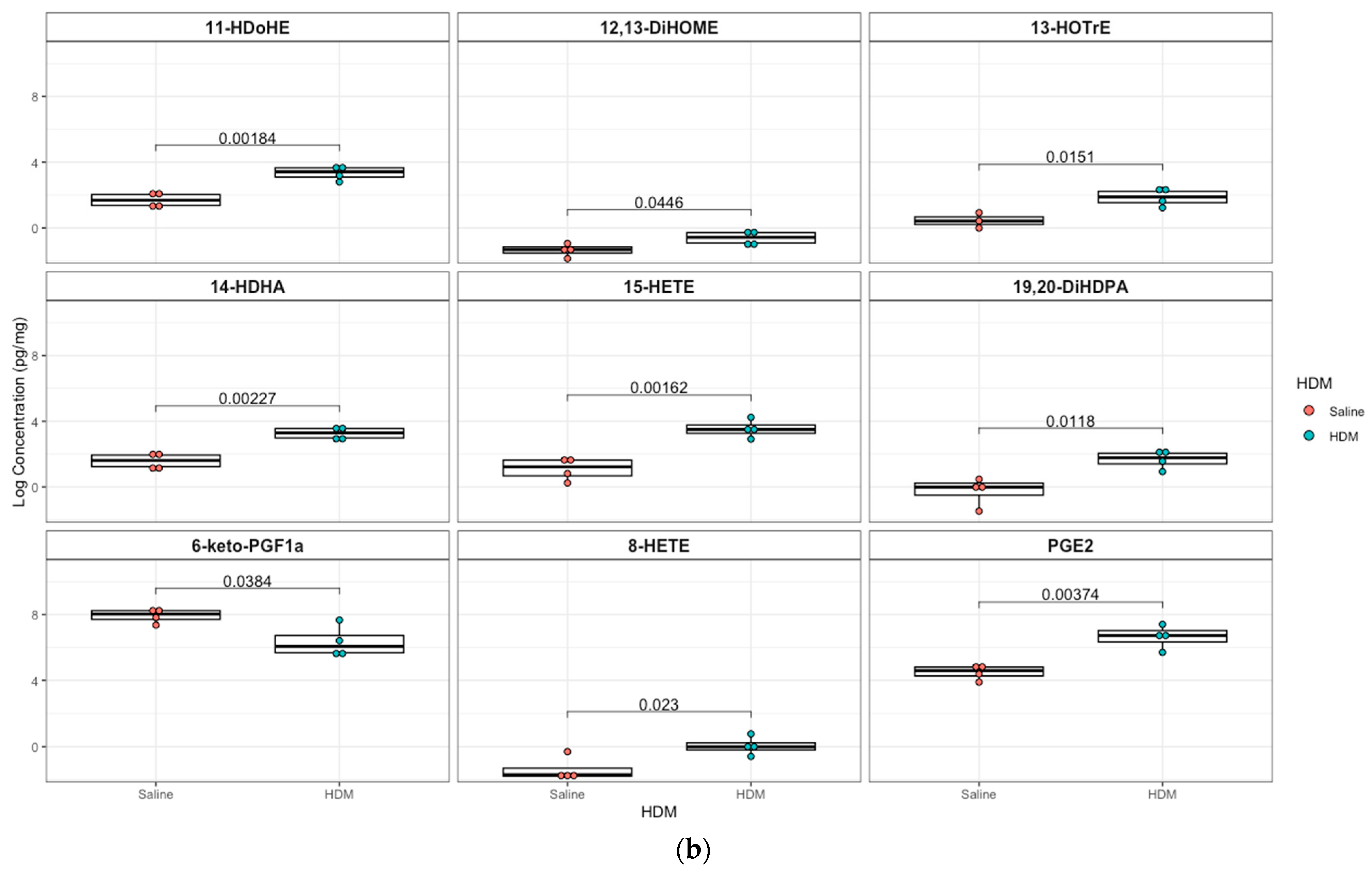
3.3. Differentially Regulated Oxylipins (Targeted Lipidomics) in HDM-Sensitized Mice
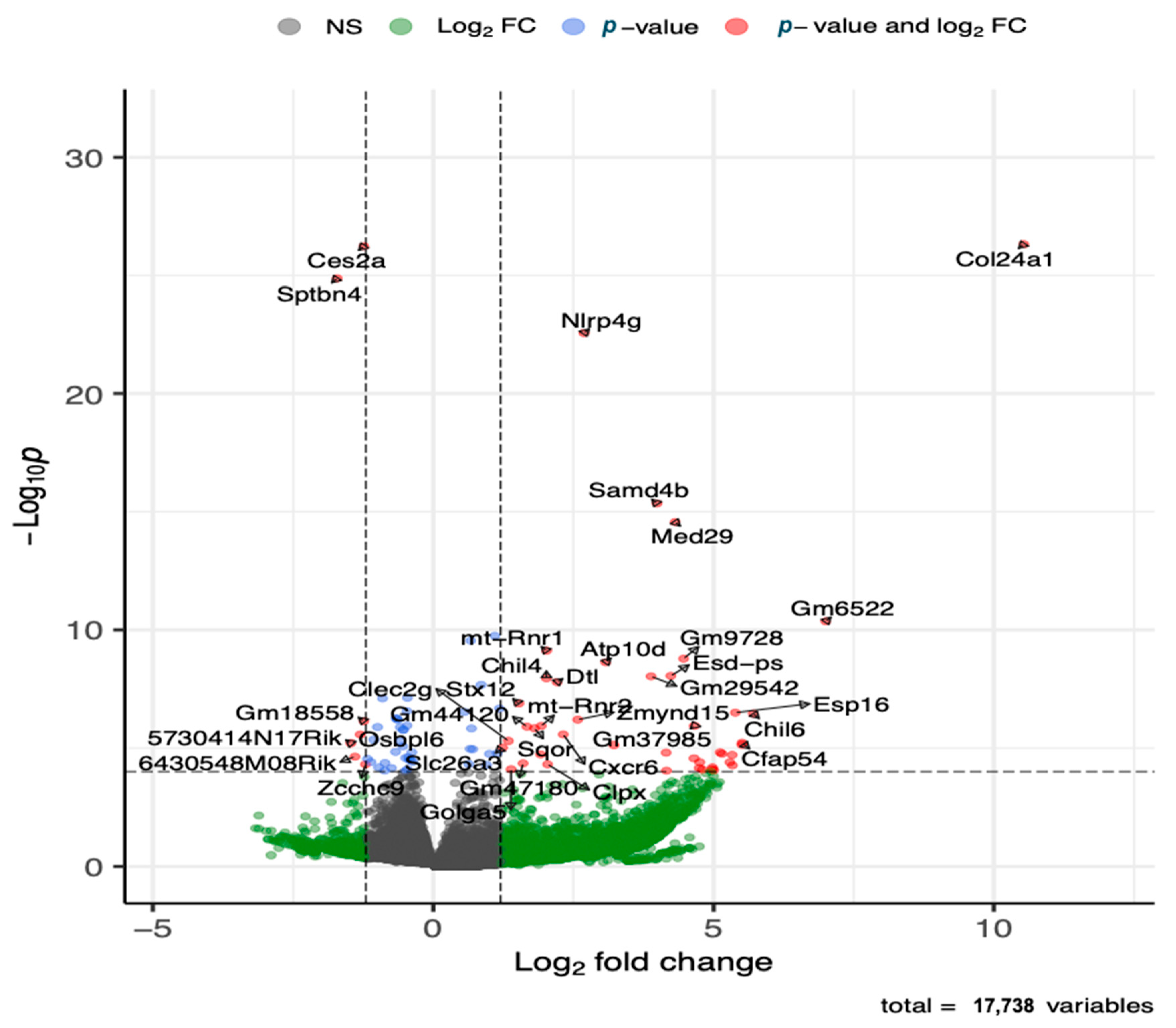
3.4. Differentially Expressed Genes (Global Gene Expression) in HDM-Sensitized Mice
3.5. Joint Pathways of Differentially Regulated Metabolic Compounds and Differentially Expressed Genes in HDM-Sensitized Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Endo, Y.; Hirahara, K.; Yagi, R.; Tumes, D.J.; Nakayama, T. Pathogenic memory type Th2 cells in allergic inflammation. Trends Immunol. 2014, 35, 69–78. [Google Scholar] [CrossRef]
- Athari, S.S. Targeting cell signaling in allergic asthma. Signal Transduct. Target. Ther. 2019, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Comhair, S.A.; McDunn, J.; Bennett, C.; Fettig, J.; Erzurum, S.C.; Kalhan, S.C. Metabolomic Endotype of Asthma. J. Immunol. 2015, 195, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Kleniewska, P.; Pawliczak, R. The participation of oxidative stress in the pathogenesis of bronchial asthma. Biomed. Pharmacother. 2017, 94, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.J.; Karmaus, W.J.; Yang, C.C. Polycyclic aromatic hydrocarbons exposure, oxidative stress, and asthma in children. Int. Arch. Occup. Environ. Health 2017, 90, 297–303. [Google Scholar] [CrossRef]
- Ple, C.; Fan, Y.; Ait Yahia, S.; Vorng, H.; Everaere, L.; Chenivesse, C.; Balsamelli, J.; Azzaoui, I.; de Nadai, P.; Wallaert, B.; et al. Polycyclic aromatic hydrocarbons reciprocally regulate IL-22 and IL-17 cytokines in peripheral blood mononuclear cells from both healthy and asthmatic subjects. PLoS ONE 2015, 10, e0122372. [Google Scholar] [CrossRef]
- Shin, Y.S.; Takeda, K.; Gelfand, E.W. Understanding asthma using animal models. Allergy Asthma Immunol. Res. 2009, 1, 10–18. [Google Scholar] [CrossRef]
- Turi, K.N.; Romick-Rosendale, L.; Ryckman, K.K.; Hartert, T.V. A review of metabolomics approaches and their application in identifying causal pathways of childhood asthma. J. Allergy Clin. Immunol. 2018, 141, 1191–1201. [Google Scholar] [CrossRef]
- Pite, H.; Aguiar, L.; Morello, J.; Monteiro, E.C.; Alves, A.C.; Bourbon, M.; Morais-Almeida, M. Metabolic Dysfunction and Asthma: Current Perspectives. J. Asthma Allergy 2020, 13, 237–247. [Google Scholar] [CrossRef]
- Reisdorph, N.; Wechsler, M.E. Utilizing metabolomics to distinguish asthma phenotypes: Strategies and clinical implications. Allergy 2013, 68, 959–962. [Google Scholar] [CrossRef]
- Mahood, T.H.; Pascoe, C.D.; Karakach, T.K.; Jha, A.; Basu, S.; Ezzati, P.; Spicer, V.; Mookherjee, N.; Halayko, A.J. Integrating Proteomes for Lung Tissues and Lavage Reveals Pathways That Link Responses in Allergen-Challenged Mice. Acs Omega 2021, 6, 1171–1189. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.E.; Xu, Y.J.; Xu, F.G.; Cheng, C.; Peh, H.Y.; Tannenbaum, S.R.; Wong, W.S.F.; Ong, C.N. Metabolomics Reveals Altered Metabolic Pathways in Experimental Asthma. Am. J. Respir. Cell Mol. Biol. 2013, 48, 204–211. [Google Scholar] [CrossRef]
- Quinn, K.D.; Schedel, M.; Nkrumah-Elie, Y.; Joetham, A.; Armstrong, M.; Cruickshank-Quinn, C.; Reisdorph, R.; Gelfand, E.W.; Reisdorph, N. Dysregulation of metabolic pathways in a mouse model of allergic asthma. Allergy 2017, 72, 1327–1337. [Google Scholar] [CrossRef]
- Lee, H.S.; Seo, C.; Hwang, Y.H.; Shin, T.H.; Park, H.J.; Kim, Y.; Ji, M.; Min, J.; Choi, S.; Kim, H.; et al. Metabolomic approaches to polyamines including acetylated derivatives in lung tissue of mice with asthma. Metabolomics 2019, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.P.; Lee, W.J.; Hong, J.Y.; Lee, S.B.; Park, J.H.; Kim, D.; Park, S.; Park, C.S.; Park, S.W.; Kwon, S.W. Novel Approach for Analysis of Bronchoalveolar Lavage Fluid (BALF) Using HPLC-QTOF-MS-Based Lipidomics: Lipid Levels in Asthmatics and Corticosteroid-Treated Asthmatic Patients. J. Proteome Res. 2014, 13, 3919–3929. [Google Scholar] [CrossRef]
- Tian, M.; Chen, M.; Bao, Y.L.; Xu, C.D.; Qin, Q.Z.; Zhang, W.X.; He, Y.T.; Shao, Q. Sputum metabolomic profiling of bronchial asthma based on quadruple time-of-flight mass spectrometry. Int. J. Clin. Exp. Pathol. 2017, 10, 10363–10373. [Google Scholar]
- Johnson, R.K.; Manke, J.; Campbell, M.; Armstrong, M.; Boorgula, M.P.; Pinheiro, G.; Santana, C.V.N.; Mathias, R.A.; Barnes, K.C.; Cruz, A.; et al. Lipid mediators are detectable in the nasal epithelium and differ by asthma status in female subjects. J. Allergy Clin. Immunol. 2022, 150, 965–971.e968. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.K.; Brunetti, T.; Quinn, K.; Doenges, K.; Campbell, M.; Arehart, C.; Taub, M.A.; Mathias, R.A.; Reisdorph, N.; Barnes, K.C.; et al. Discovering metabolite quantitative trait loci in asthma using an isolated population. J. Allergy Clin. Immunol. 2022, 149, 1807–1811.e1816. [Google Scholar] [CrossRef]
- Rabinovitch, N.; Mauger, D.T.; Reisdorph, N.; Covar, R.; Malka, J.; Lemanske, R.F., Jr.; Morgan, W.J.; Guilbert, T.W.; Zeiger, R.S.; Bacharier, L.B.; et al. Predictors of asthma control and lung function responsiveness to step 3 therapy in children with uncontrolled asthma. J. Allergy Clin. Immunol. 2014, 133, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Rabinovitch, N.; Reisdorph, N.; Silveira, L.; Gelfand, E.W. Urinary leukotriene E(4) levels identify children with tobacco smoke exposure at risk for asthma exacerbation. J. Allergy Clin. Immunol. 2011, 128, 323–327. [Google Scholar] [CrossRef]
- Calhoun, W.J.; Reed, H.E.; Moest, D.R.; Stevens, C.A. Enhanced superoxide production by alveolar macrophages and air-space cells, airway inflammation, and alveolar macrophage density changes after segmental antigen bronchoprovocation in allergic subjects. Am. Rev. Respir. Dis. 1992, 145, 317–325. [Google Scholar] [CrossRef]
- Comhair, S.A.; Erzurum, S.C. Redox control of asthma: Molecular mechanisms and therapeutic opportunities. Antioxid. Redox Signal. 2010, 12, 93–124. [Google Scholar] [CrossRef]
- Sahiner, U.M.; Birben, E.; Erzurum, S.; Sackesen, C.; Kalayci, O. Oxidative stress in asthma. World Allergy Organ. J. 2011, 4, 151–158. [Google Scholar] [CrossRef]
- Ono, E.; Dutile, S.; Kazani, S.; Wechsler, M.E.; Yang, J.; Hammock, B.D.; Douda, D.N.; Tabet, Y.; Khaddaj-Mallat, R.; Sirois, M.; et al. Lipoxin generation is related to soluble epoxide hydrolase activity in severe asthma. Am. J. Respir. Crit. Care Med. 2014, 190, 886–897. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.D.; Clish, C.B.; Schmidt, B.; Gronert, K.; Serhan, C.N. Lipid mediator class switching during acute inflammation: Signals in resolution. Nat. Immunol. 2001, 2, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Basil, M.C.; Levy, B.D. Specialized pro-resolving mediators: Endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 2016, 16, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Polinski, K.J.; Armstrong, M.; Manke, J.; Seifert, J.; Crume, T.; Yang, F.; Clare-Salzler, M.; Holers, V.M.; Reisdorph, N.; Norris, J.M. Collection and Storage of Human Plasma for Measurement of Oxylipins. Metabolites 2021, 11, 137. [Google Scholar] [CrossRef] [PubMed]
- Larsson, N.; Lundström, S.L.; Pinto, R.; Rankin, G.; Karimpour, M.; Blomberg, A.; Sandström, T.; Pourazar, J.; Trygg, J.; Behndig, A.F.; et al. Lipid mediator profiles differ between lung compartments in asthmatic and healthy humans. Eur. Respir. J. 2014, 43, 453–463. [Google Scholar] [CrossRef]
- Gieger, C.; Geistlinger, L.; Altmaier, E.; de Angelis, M.H.; Kronenberg, F.; Meitinger, T.; Mewes, H.W.; Wichmann, H.E.; Weinberger, K.M.; Adamski, J.; et al. Genetics Meets Metabolomics: A Genome-Wide Association Study of Metabolite Profiles in Human Serum. PLoS Genet. 2008, 4, e1000282. [Google Scholar] [CrossRef]
- Romero, R.; Espinoza, J.; Gotsch, F.; Kusanovic, J.P.; Friel, L.A.; Erex, O.; Mazaki-Tovi, S.; Than, N.G.; Hassan, S.; Tromp, G. The use of high-dimensional biology (genomics, transcriptomics, proteomics, and metabolomics) to understand the preterm parturition syndrome. BJOG Int. J. Obstet. Gynaecol. 2006, 113 (Suppl. S3), 118–135. [Google Scholar] [CrossRef]
- Valcarcel, B.; Ebbels, T.M.D.; Kangas, A.J.; Soininen, P.; Elliot, P.; Ala-Korpela, M.; Jarvelin, M.R.; de Iorio, M. Genome metabolome integrated network analysis to uncover connections between genetic variants and complex traits: An application to obesity. J. R. Soc. Interface 2014, 11, 20130908. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, A.R.; Pinkerton, K.E. Investigating the Effects of Particulate Matter on House Dust Mite and Ovalbumin Allergic Airway Inflammation in Mice. Curr. Protoc. Toxicol. 2016, 68, 18.18.1–18.18.18. [Google Scholar] [CrossRef]
- Acciani, T.H.; Brandt, E.B.; Hershey, G.K.K.; Le Cras, T.D. Diesel exhaust particle exposure increases severity of allergic asthma in young mice. Clin. Exp. Allergy 2013, 43, 1406–1418. [Google Scholar] [CrossRef]
- Bauer, A.K.; Fostel, J.; Degraff, L.M.; Rondini, E.A.; Walker, C.; Grissom, S.F.; Foley, J.; Kleeberger, S.R. Transcriptomic analysis of pathways regulated by toll-like receptor 4 in a murine model of chronic pulmonary inflammation and carcinogenesis. Mol. Cancer 2009, 8, 107. [Google Scholar] [CrossRef]
- Bauer, A.K.; Rondini, E.A.; Hummel, K.A.; Degraff, L.M.; Walker, C.; Jedlicka, A.E.; Kleeberger, S.R. Identification of candidate genes downstream of TLR4 signaling after ozone exposure in mice: A role for heat-shock protein 70. Environ. Health Perspect. 2011, 119, 1091–1097. [Google Scholar] [CrossRef]
- Woo, L.N.; Guo, W.Y.; Wang, X.; Young, A.; Salehi, S.; Hin, A.; Zhang, Y.; Scott, J.A.; Chow, C.W. A 4-Week Model of House Dust Mite (HDM) Induced Allergic Airways Inflammation with Airway Remodeling. Sci. Rep. 2018, 8, 6925. [Google Scholar] [CrossRef]
- Bauer, A.K.; Umer, M.; Richardson, V.L.; Cumpian, A.M.; Harder, A.Q.; Khosravi, N.; Azzegagh, Z.; Hara, N.M.; Ehre, C.; Mohebnasab, M.; et al. Requirement for MUC5AC in KRAS-dependent lung carcinogenesis. JCI Insight 2018, 3, e120941. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Morgan, D.L.; Bauer, A.K.; Kleeberger, S.R. Signal transduction pathways of tumor necrosis factor—Mediated lung injury induced by ozone in mice. Am. J. Respir. Crit. Care Med. 2007, 175, 829–839. [Google Scholar] [CrossRef]
- Yang, Y.H.; Cruickshank, C.; Armstrong, M.; Mahaffey, S.; Reisdorph, R.; Reisdorph, N. New sample preparation approach for mass spectrometry-based profiling of plasma results in improved coverage of metabolome. J. Chromatogr. A 2013, 1300, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Cruickshank-Quinn, C.; Quinn, K.D.; Powell, R.; Yang, Y.H.; Armstrong, M.; Mahaffey, S.; Reisdorph, R.; Reisdorph, N. Multi-step Preparation Technique to Recover Multiple Metabolite Compound Classes for In-depth and Informative Metabolomic Analysis. J. Vis. Exp. 2014, 11, e51670. [Google Scholar] [CrossRef]
- Hughes, G.; Cruickshank-Quinn, C.; Reisdorph, R.; Lutz, S.; Petrache, I.; Reisdorph, N.; Bowler, R.; Kechris, K. MSPrep-Summarization, normalization and diagnostics for processing of mass spectrometry-based metabolomic data. Bioinformatics 2014, 30, 133–134. [Google Scholar] [CrossRef]
- Depner, C.M.; Cogswell, D.T.; Bisesi, P.J.; Markwald, R.R.; Cruickshank-Quinn, C.; Quinn, K.; Melanson, E.L.; Reisdorph, N.; Wright, K.P., Jr. Developing preliminary blood metabolomics-based biomarkers of insufficient sleep in humans. Sleep 2020, 43, zsz321. [Google Scholar] [CrossRef]
- Armstrong, M.; Liu, A.H.; Harbeck, R.; Reisdorph, R.; Rabinovitch, N.; Reisdorph, N. Leukotriene-E4 in human urine: Comparison of on-line purification and liquid chromatography-tandem mass spectrometry to affinity purification followed by enzyme immunoassay. J. Chromatogr. B 2009, 877, 3169–3174. [Google Scholar] [CrossRef]
- Kosaraju, R.; Guesdon, W.; Crouch, M.J.; Teague, H.L.; Sullivan, E.M.; Karlsson, E.A.; Schultz-Cherry, S.; Gowdy, K.; Bridges, L.C.; Reese, L.R.; et al. B Cell Activity Is Impaired in Human and Mouse Obesity and Is Responsive to an Essential Fatty Acid upon Murine Influenza Infection. J. Immunol. 2017, 198, 4738–4752. [Google Scholar] [CrossRef]
- Armstrong, M.; Manke, J.; Nkrumah-Elie, Y.; Shaikh, S.R.; Reisdorph, N. Improved quantification of lipid mediators in plasma and tissues by liquid chromatography tandem mass spectrometry demonstrates mouse strain specific differences. Prostaglandins Other Lipid Mediat. 2020, 151, 106483. [Google Scholar] [CrossRef]
- Ward, C.M.; To, T.H.; Pederson, S.M. ngsReports: A Bioconductor package for managing FastQC reports and other NGS related log files. Bioinformatics 2020, 36, 2587–2588. [Google Scholar] [CrossRef]
- Brown, J.; Pirrung, M.; McCue, L.A. FQC Dashboard: Integrates FastQC results into a web-based, interactive, and extensible FASTQ quality control tool. Bioinformatics 2017, 33, 3137–3139. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads. Nucleic Acids Res. 2019, 47, e47. [Google Scholar] [CrossRef]
- Tiffany, C.R.; Bäumler, A.J. omu, a Metabolomics Count Data Analysis Tool for Intuitive Figures and Convenient Metadata Collection. Microbiol. Resour. Announc. 2019, 8, e00129-19. [Google Scholar] [CrossRef]
- Gaud, C.; Sousa, B.C.; Nguyen, A.; Fedorova, M.; Ni, Z.; O’Donnell, V.B.; Wakelam, M.J.; Andrews, S.; Lopez-Clavijo, A.F. BioPAN: A web-based tool to explore mammalian lipidome metabolic pathways on LIPID MAPS. F1000Res 2021, 10, 4. [Google Scholar] [CrossRef]
- Picart-Armada, S.; Fernandez-Albert, F.; Vinaixa, M.; Yanes, O.; Perera-Lluna, A. FELLA: An R package to enrich metabolomics data. BMC Bioinform. 2018, 19, 538. [Google Scholar] [CrossRef]
- Przulj, N. Cytoscape: A Tool for Analyzing and Visualizing Network Data. In Analyzing Network Data in Biology and Medicine; Cambridge University Press: Cambridge, UK, 2019; pp. 533–592. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- van Iterson, M.; van Zwet, E.W.; Heijmans, B.T.; BIOS Consortium. Controlling bias and inflation in epigenome- and transcriptome-wide association studies using the empirical null distribution. Genome Biol. 2017, 18. [Google Scholar] [CrossRef]
- Smedley, D.; Haider, S.; Ballester, B.; Holland, R.; London, D.; Thorisson, G.; Kasprzyk, A. BioMart—Biological queries made easy. BMC Genom. 2009, 10, 22. [Google Scholar] [CrossRef]
- Chang, A.B.; Gibson, P.G.; Masters, I.B.; Dash, P.; Hills, B.A. The relationship between inflammation and dipalmitoyl phosphatidycholine in induced sputum of children with asthma. J. Asthma 2003, 40, 63–70. [Google Scholar] [CrossRef]
- Voelker, D.R. Phosphatidylserine Functions as the Major Precursor of Phosphatidylethanolamine in Cultured Bhk-21-Cells. Proc. Natl. Acad. Sci. USA 1984, 81, 2669–2673. [Google Scholar] [CrossRef]
- Yu, M.; Jia, H.M.; Cui, F.X.; Yang, Y.; Zhao, Y.; Yang, M.H.; Zou, Z.M. The Effect of Chinese Herbal Medicine Formula mKG on Allergic Asthma by Regulating Lung and Plasma Metabolic Alternations. Int. J. Mol. Sci. 2017, 18, 602. [Google Scholar] [CrossRef]
- Ono, J.G.; Kim, B.I.; Zhao, Y.Z.; Christos, P.J.; Tesfaigzi, Y.; Worgall, T.S.; Worgall, S. Decreased sphingolipid synthesis in children with 17q21 asthma risk genotypes. J. Clin. Investig. 2020, 130, 921–926. [Google Scholar] [CrossRef]
- Wang, S.S.; Tang, K.; Lu, Y.J.; Tian, Z.; Huang, Z.L.; Wang, M.J.; Zhao, J.P.; Xie, J.G. Revealing the role of glycerophospholipid metabolism in asthma through plasma lipidomics. Clin. Chim. Acta 2021, 513, 34–42. [Google Scholar] [CrossRef]
- Szondy, Z.; Sarang, Z.; Kiss, B.; Garabuczi, É.; Köröskényi, K. Anti-inflammatory Mechanisms Triggered by Apoptotic Cells during Their Clearance. Front. Immunol. 2017, 8, 909. [Google Scholar] [CrossRef]
- Klein, M.E.; Rieckmann, M.; Lucas, H.; Meister, A.; Loppnow, H.; Mäder, K. Phosphatidylserine (PS) and phosphatidylglycerol (PG) enriched mixed micelles (MM): A new nano-drug delivery system with anti-inflammatory potential? Eur. J. Pharm. Sci. 2020, 152, 105451. [Google Scholar] [CrossRef]
- Tokes, T.; Tuboly, E.; Varga, G.; Major, L.; Ghyczy, M.; Kaszaki, J.; Boros, M. Protective effects of L-alpha-glycerylphosphorylcholine on ischaemia-reperfusion-induced inflammatory reactions. Eur. J. Nutr. 2015, 54, 109–118. [Google Scholar] [CrossRef]
- Miki, H.; Nakahashi-Oda, C.; Sumida, T.; Shibuya, A. Involvement of CD300a Phosphatidylserine Immunoreceptor in Aluminum Salt Adjuvant-Induced Th2 Responses. J. Immunol. 2015, 194, 5069–5076. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef]
- Shimizu, T. Lipid Mediators in Health and Disease: Enzymes and Receptors as Therapeutic Targets for the Regulation of Immunity and Inflammation. Annu. Rev. Pharmacol. 2009, 49, 123–150. [Google Scholar] [CrossRef]
- Vangaveti, V.; Baune, B.T.; Kennedy, R.L. Hydroxyoctadecadienoic acids: Novel regulators of macrophage differentiation and atherogenesis. Ther. Adv. Endocrinol. Metab. 2010, 1, 51–60. [Google Scholar] [CrossRef]
- Piomelli, D.; Sasso, O. Peripheral gating of pain signals by endogenous lipid mediators. Nat. Neurosci. 2014, 17, 164–174. [Google Scholar] [CrossRef]
- Cho, J.S.; Han, I.H.; Lee, H.R.; Lee, H.M. Prostaglandin E2 Induces IL-6 and IL-8 Production by the EP Receptors/Akt/NF-kappaB Pathways in Nasal Polyp-Derived Fibroblasts. Allergy Asthma Immunol. Res 2014, 6, 449–457. [Google Scholar] [CrossRef]
- Patrono, C.; Rotella, C.M.; Toccafondi, R.S.; Aterini, S.; Pinca, E.; Tanini, A.; Zonefrati, R. Prostacyclin stimulates the adenylate cyclase system of human thyroid tissue. Prostaglandins 1981, 22, 105–115. [Google Scholar] [CrossRef]
- Whittle, B.J.; Moncada, S. Prostacyclin and its analogues for the therapy of thromboembolic disorders. Adv. Exp. Med. Biol. 1984, 164, 193–209. [Google Scholar] [CrossRef]
- Hata, A.N.; Breyer, R.M. Pharmacology and signaling of prostaglandin receptors: Multiple roles in inflammation and immune modulation. Pharmacol. Ther. 2004, 103, 147–166. [Google Scholar] [CrossRef]
- Kern, K.; Schafer, S.M.G.; Cohnen, J.; Pierre, S.; Osthues, T.; Tarighi, N.; Hohmann, S.; Ferreiros, N.; Brune, B.; Weigert, A.; et al. The G2A Receptor Controls Polarization of Macrophage by Determining Their Localization Within the Inflamed Tissue. Front. Immunol. 2018, 9, 2261. [Google Scholar] [CrossRef]
- Fujimura, K.E.; Sitarik, A.R.; Haystad, S.; Lin, D.L.; Levan, S.; Fadrosh, D.; Panzer, A.R.; LaMere, B.; Rackaityte, E.; Lukacs, N.W.; et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 2016, 22, 1187–1191. [Google Scholar] [CrossRef]
- Levan, S.R.; Stamnes, K.A.; Lin, D.L.; Panzer, A.R.; Fukui, E.; McCauley, K.; Fujimura, K.E.; McKean, M.; Ownby, D.R.; Zoratti, E.M.; et al. Elevated faecal 12,13-diHOME concentration in neonates at high risk for asthma is produced by gut bacteria and impedes immune tolerance. Nat. Microbiol. 2019, 4, 1851–1861. [Google Scholar] [CrossRef]
- Hildreth, K.; Kodani, S.D.; Hammock, B.D.; Zhao, L. Cytochrome P450-derived linoleic acid metabolites EpOMEs and DiHOMEs: A review of recent studies. J. Nutr. Biochem. 2020, 86, 108484. [Google Scholar] [CrossRef]
- Mabalirajan, U.; Rehman, R.; Ahmad, T.; Kumar, S.; Singh, S.; Leishangthem, G.D.; Aich, J.; Kumar, M.; Khanna, K.; Singh, V.P.; et al. Linoleic acid metabolite drives severe asthma by causing airway epithelial injury. Sci. Rep. 2013, 3, 1349. [Google Scholar] [CrossRef]
- Kumar, N.; Gupta, G.; Anilkumar, K.; Fatima, N.; Karnati, R.; Reddy, G.V.; Giri, P.V.; Reddanna, P. 15-Lipoxygenase metabolites of alpha-linolenic acid, [13-(S)-HPOTrE and 13-(S)-HOTrE], mediate anti-inflammatory effects by inactivating NLRP3 inflammasome. Sci. Rep. 2016, 6, 31649. [Google Scholar] [CrossRef]
- Pauls, S.D.; Rodway, L.A.; Winter, T.; Taylor, C.G.; Zahradka, P.; Aukema, H.M. Anti-inflammatory effects of alpha-linolenic acid in M1-like macrophages are associated with enhanced production of oxylipins from alpha-linolenic and linoleic acid. J. Nutr. Biochem. 2018, 57, 121–129. [Google Scholar] [CrossRef]
- Walker, R.E.; Savinova, O.V.; Pedersen, T.L.; Newman, J.W.; Shearer, G.C. Effects of inflammation and soluble epoxide hydrolase inhibition on oxylipin composition of very low-density lipoproteins in isolated perfused rat livers. Physiol. Rep. 2021, 9, e14480. [Google Scholar] [CrossRef]
- Cucchi, D.; Camacho-Munoz, D.; Certo, M.; Niven, J.; Smith, J.; Nicolaou, A.; Mauro, C. Omega-3 polyunsaturated fatty acids impinge on CD4(+) T cell motility and adipose tissue distribution via direct and lipid mediator-dependent effects. Cardiovasc. Res. 2020, 116, 1006–1020. [Google Scholar] [CrossRef]
- Xu, S.Z.; Muraki, K.; Zeng, F.; Li, J.; Sukumar, P.; Shah, S.; Dedman, A.M.; Flemming, P.K.; McHugh, D.; Naylor, J.; et al. A sphingosine-1-phosphate-activated calcium channel controlling vascular smooth muscle cell motility. Circ. Res. 2006, 98, 1381–1389. [Google Scholar] [CrossRef]
- Sanderson, M.J.; Delmotte, P.; Bai, Y.; Perez-Zogbhi, J.F. Regulation of airway smooth muscle cell contractility by Ca2+ signaling and sensitivity. Proc. Am. Thorac. Soc. 2008, 5, 23–31. [Google Scholar] [CrossRef]
- Dennis, E.A.; Cao, J.; Hsu, Y.H.; Magrioti, V.; Kokotos, G. Phospholipase A(2) Enzymes: Physical Structure, Biological Function, Disease Implication, Chemical Inhibition, and Therapeutic Intervention. Chem. Rev. 2011, 111, 6130–6185. [Google Scholar] [CrossRef]
- Fink, E.L.; Clark, R.S.B.; Kochanek, P.M. Chapter 62—Hypoxic-Ischemic Encephalopathy: Pathobiology and Therapy of the Post-Resuscitation Syndrome in Children. In Pediatric Critical Care, 4th ed.; Fuhrman, B.P., Zimmerman, J.J., Eds.; Mosby: Saint Louis, MO, USA, 2011; pp. 871–892. [Google Scholar]
- So, S.Y.; Ip, M.; Lam, W.K. Calcium-Channel Blockers and Asthma. Lung 1986, 164, 1–16. [Google Scholar] [CrossRef]
- Chiu, K.Y.; Li, J.G.; Lin, Y. Calcium channel blockers for lung function improvement in asthma A systematic review and meta-analysis. Ann. Allergy Asthma Immunol. 2017, 119, 518–523. [Google Scholar] [CrossRef]



| Term | Odds Ratio | Genes | Regulation |
|---|---|---|---|
| Positive regulation of vesicle fusion | 142.41 | Akt2, Doc2b | Down |
| Regulation of vesicle fusion | 101.71 | Akt2, Doc2b | Down |
| Central nervous system neuron axonogenesis | 64.71 | Chrnb2, Sptbn4 | Down |
| Central nervous system projection neuron axonogenesis | 64.71 | Chrnb2, Sptbn4 | Down |
| Regulation of glycogen biosynthetic process | 29.64 | Akt2, Pask | Down |
| Positive regulation of organelle organization | 22.94 | Akt2, Doc2b | Down |
| Regulation of TORC1 signaling | 21.55 | Atm, Gpr137c | Down |
| Regulation of B cell proliferation | 16.15 | Chrnb2, Atm | Down |
| Organic hydroxy compound biosynthetic process | 14.80 | Osbpl6, Hsd17b1 | Down |
| Regulation of calcium ion transmembrane transport via high voltage-gated calcium channel | 52.66 | Camk2d, Cacna2d1 | Up |
| Regulation of cardiac muscle contraction by regulation of the release of sequestered calcium ion | 31.59 | Ryr2, Camk2d | Up |
| Cardiac muscle cell contraction | 27.87 | Camk2d, Cacna2d1 | Up |
| Regulation of cardiac muscle contraction by calcium ion signaling | 24.93 | Ryr2, Camk2d | Up |
| Calcium ion transport into cytosol | 24.93 | Ryr2, Cacna2d1 | Up |
| Calcium-mediated signaling using intracellular calcium source | 24.93 | Ryr2, Stimate | Up |
| Regulation of release of sequestered calcium ion into cytosol by sarcoplasmic reticulum | 22.55 | Ryr2, Camk2d | Up |
| Regulation of calcium ion transmembrane transport | 22.55 | Camk2d, Cacna2d1 | Up |
| Regulation of cardiac muscle cell action potential | 20.59 | Ryr2, Camk2d | Up |
| Regulation of cardiac muscle cell contraction | 18.94 | Ryr2, Camk2d | Up |
| Cytosolic calcium ion transport | 18.21 | Ryr2, Cacna2d1 | Up |
| Ion homeostasis | 16.91 | Slc4a8, Camk2d | Up |
| Cardiac muscle cell action potential involved in contraction | 16.33 | Ryr2, Cacna2d1 | Up |
| Cardiac muscle contraction | 14.79 | Ryr2, Camk2d | Up |
| Metal ion transport | 8.43 | Ryr2, Cacna2d1, Cdh23 | Up |
| Collagen fibril organization | 8.33 | Col24a1, Col11a2, Col19a1 | Up |
| Calcium-mediated signaling | 7.23 | Ryr2, Stimate, Cxcr6 | Up |
| Term | Odds.Ratio | Genes | Regulation |
|---|---|---|---|
| Testosterone dehydrogenase [NAD(P)] activity | 87.45 | Hsd17b1 | Down |
| Chromatin insulator sequence binding | 87.45 | Repin1 | Down |
| RNA strand annealing activity | 87.45 | Eif4b | Down |
| Neuroligin family protein binding | 87.45 | Nrxn2 | Down |
| CCR5 chemokine receptor binding | 87.45 | Cnih4 | Down |
| Phosphatidylinositol-3,5-bisphosphate 3-phosphatase activity | 87.45 | Mtm1 | Down |
| Annealing activity | 87.45 | Eif4b | Down |
| Oncostatin M receptor activity | 69.95 | Lifr | Down |
| Leukemia inhibitory factor receptor activity | 69.95 | Lifr | Down |
| Mannosyl-oligosaccharide 1,2-alpha-mannosidase activity | 58.29 | Man1b1 | Down |
| Mannosyl-oligosaccharide mannosidase activity | 58.29 | Man1b1 | Down |
| Phosphatidylinositol-3,5-bisphosphate phosphatase activity | 58.29 | Mtm1 | Down |
| Estradiol 17-beta-dehydrogenase activity | 49.96 | Hsd17b1 | Down |
| Ciliary neurotrophic factor receptor activity | 49.96 | Lifr | Down |
| Ciliary neurotrophic factor receptor binding | 43.71 | Lifr | Down |
| 1-phosphatidylinositol-3-kinase activity | 38.86 | Atm | Down |
| Acetylcholine-gated cation-selective channel activity | 34.97 | Chrnb2 | Down |
| Phosphatidylinositol 3-kinase activity | 31.79 | Atm | Down |
| Water channel activity | 29.14 | Aqp6 | Down |
| Phosphatidylinositol kinase activity | 24.97 | Atm | Down |
| Water transmembrane transporter activity | 24.97 | Aqp6 | Down |
| Phosphatidylinositol-3-phosphatase activity | 24.97 | Mtm1 | Down |
| Nuclear import signal receptor activity | 23.31 | Ipo4 | Down |
| Ribosomal small subunit binding | 21.85 | Eif4b | Down |
| Phosphatidylinositol monophosphate phosphatase activity | 21.85 | Mtm1 | Down |
| phosphatidylinositol binding | 7.88 | Pask, Mtm1 | Down |
| Benzodiazepine receptor binding | 58.56 | Tspoap1 | Up |
| Voltage-gated calcium channel activity involved in cardiac muscle cell action potential | 58.56 | Cacna2d1 | Up |
| Oncostatin M receptor activity | 46.84 | Prlr | Up |
| Sodium:bicarbonate symporter activity | 46.84 | Slc4a8 | Up |
| Solute:bicarbonate symporter activity | 46.84 | Slc4a8 | Up |
| Alpha-glucosidase activity | 46.84 | Ganab | Up |
| Leukemia inhibitory factor receptor activity | 46.84 | Prlr | Up |
| G protein-coupled serotonin receptor binding | 46.84 | Gna11 | Up |
| Bicarbonate transmembrane transporter activity | 36.45 | Slc4a8, Slc26a3 | Up |
| Chloride transmembrane transporter activity | 33.84 | Slc4a8, Slc26a3 | Up |
| Ciliary neurotrophic factor receptor activity | 33.46 | Prlr | Up |
| Sodium channel inhibitor activity | 33.46 | Camk2d | Up |
| Glucosidase activity | 33.46 | Ganab | Up |
| Ciliary neurotrophic factor receptor binding | 29.27 | Prlr | Up |
| Oxalate transmembrane transporter activity | 29.27 | Slc26a3 | Up |
| Acyl-CoA dehydrogenase activity | 29.27 | Ivd | Up |
| Lys63-specific deubiquitinase activity | 26.02 | Otud4 | Up |
| Intracellular ligand-gated ion channel activity | 23.42 | Ryr2 | Up |
| Small GTPase binding | 4.15 | Unc13b, Dock4, Golga5 | Up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turi, K.N.; Michel, C.R.; Manke, J.; Doenges, K.A.; Reisdorph, N.; Bauer, A.K. Multi-Omics Analysis of Lung Tissue Demonstrates Changes to Lipid Metabolism during Allergic Sensitization in Mice. Metabolites 2023, 13, 406. https://doi.org/10.3390/metabo13030406
Turi KN, Michel CR, Manke J, Doenges KA, Reisdorph N, Bauer AK. Multi-Omics Analysis of Lung Tissue Demonstrates Changes to Lipid Metabolism during Allergic Sensitization in Mice. Metabolites. 2023; 13(3):406. https://doi.org/10.3390/metabo13030406
Chicago/Turabian StyleTuri, Kedir N., Cole R. Michel, Jonathan Manke, Katrina A. Doenges, Nichole Reisdorph, and Alison K. Bauer. 2023. "Multi-Omics Analysis of Lung Tissue Demonstrates Changes to Lipid Metabolism during Allergic Sensitization in Mice" Metabolites 13, no. 3: 406. https://doi.org/10.3390/metabo13030406
APA StyleTuri, K. N., Michel, C. R., Manke, J., Doenges, K. A., Reisdorph, N., & Bauer, A. K. (2023). Multi-Omics Analysis of Lung Tissue Demonstrates Changes to Lipid Metabolism during Allergic Sensitization in Mice. Metabolites, 13(3), 406. https://doi.org/10.3390/metabo13030406






