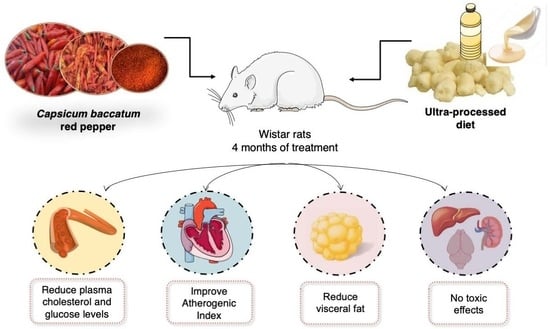
Capsicum baccatum Red Pepper Prevents Cardiometabolic Risk in Rats Fed with an Ultra-Processed Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction
2.2. Animals and Experimental Design
2.3. Blood Sampling, Organs, and Tissues Collections
2.4. Glucose Tolerance Test and Glycosylated Hemoglobin
2.5. Lipid Profile and Atherogenic Index
2.6. Biochemical and Hematological Analysis
2.7. Behavioral Tasks
2.7.1. Open Field Task
2.7.2. Light–Dark Exploration Task
2.7.3. Elevated Plus-Maze Task
2.8. Statistical Analysis
3. Results
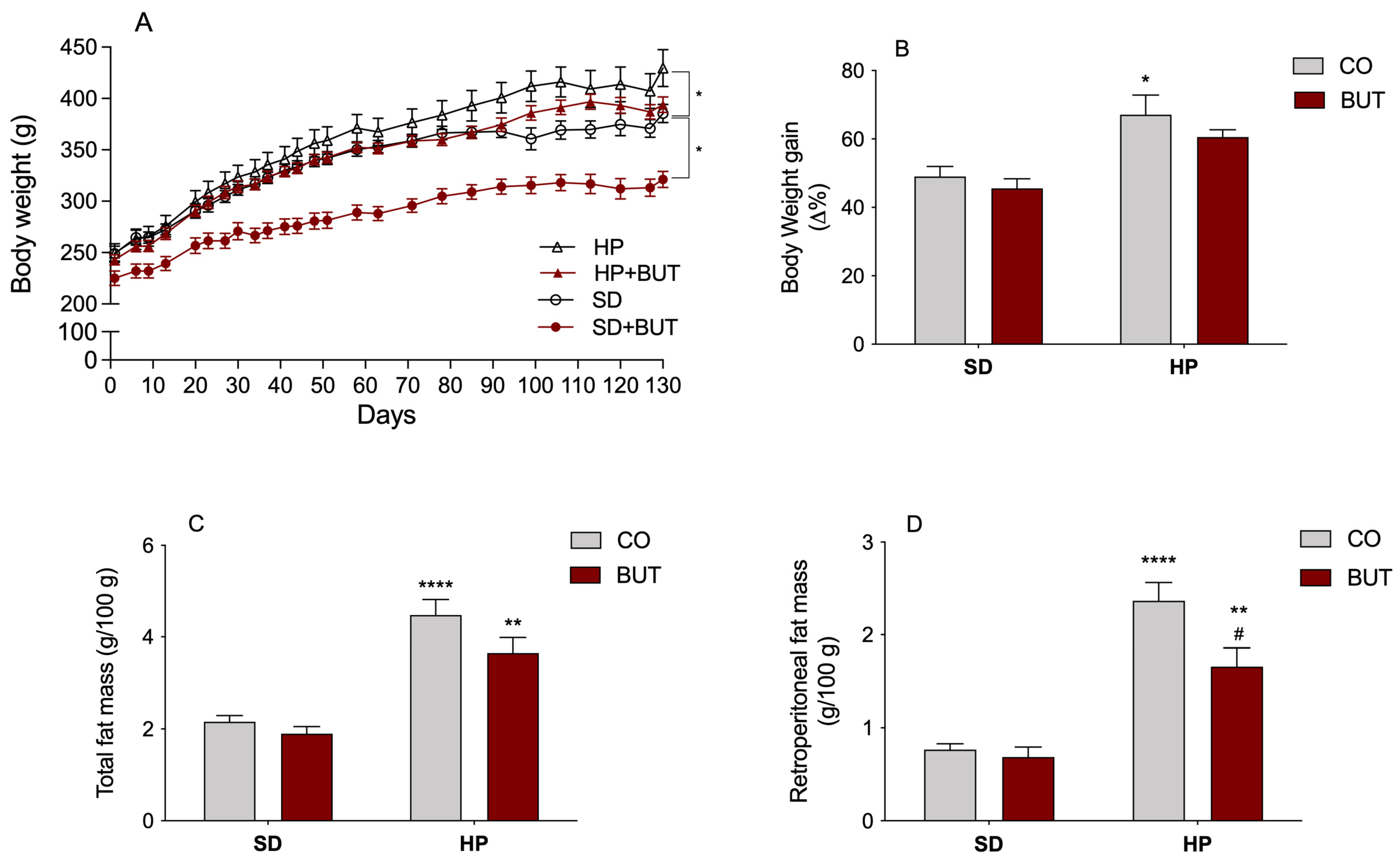
3.1. Body Weight, Adipose Tissue Weights
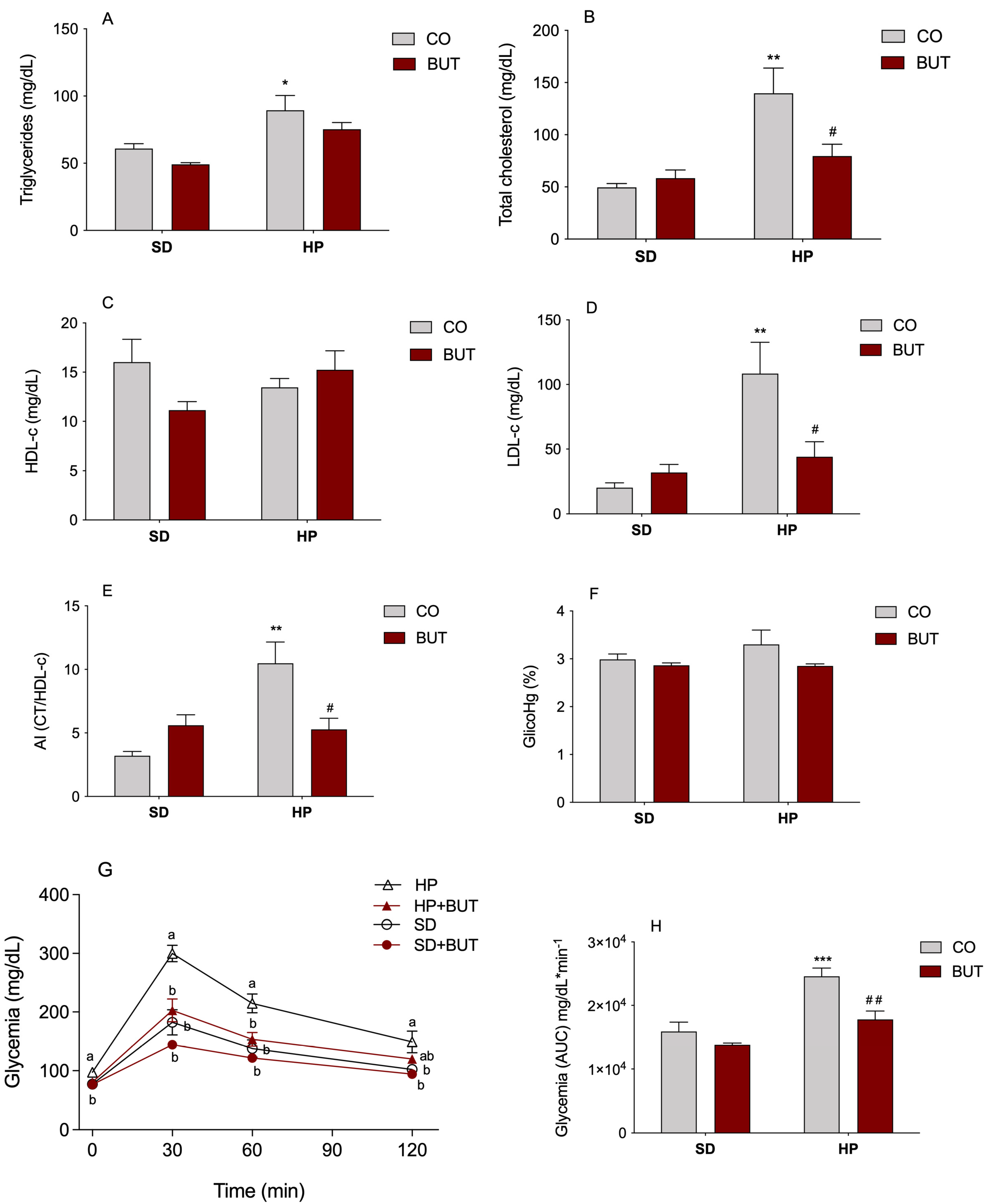
3.2. Lipid and Glycemic Profiles
3.3. Examination of the Organs, Biochemical, and Hematological Analysis
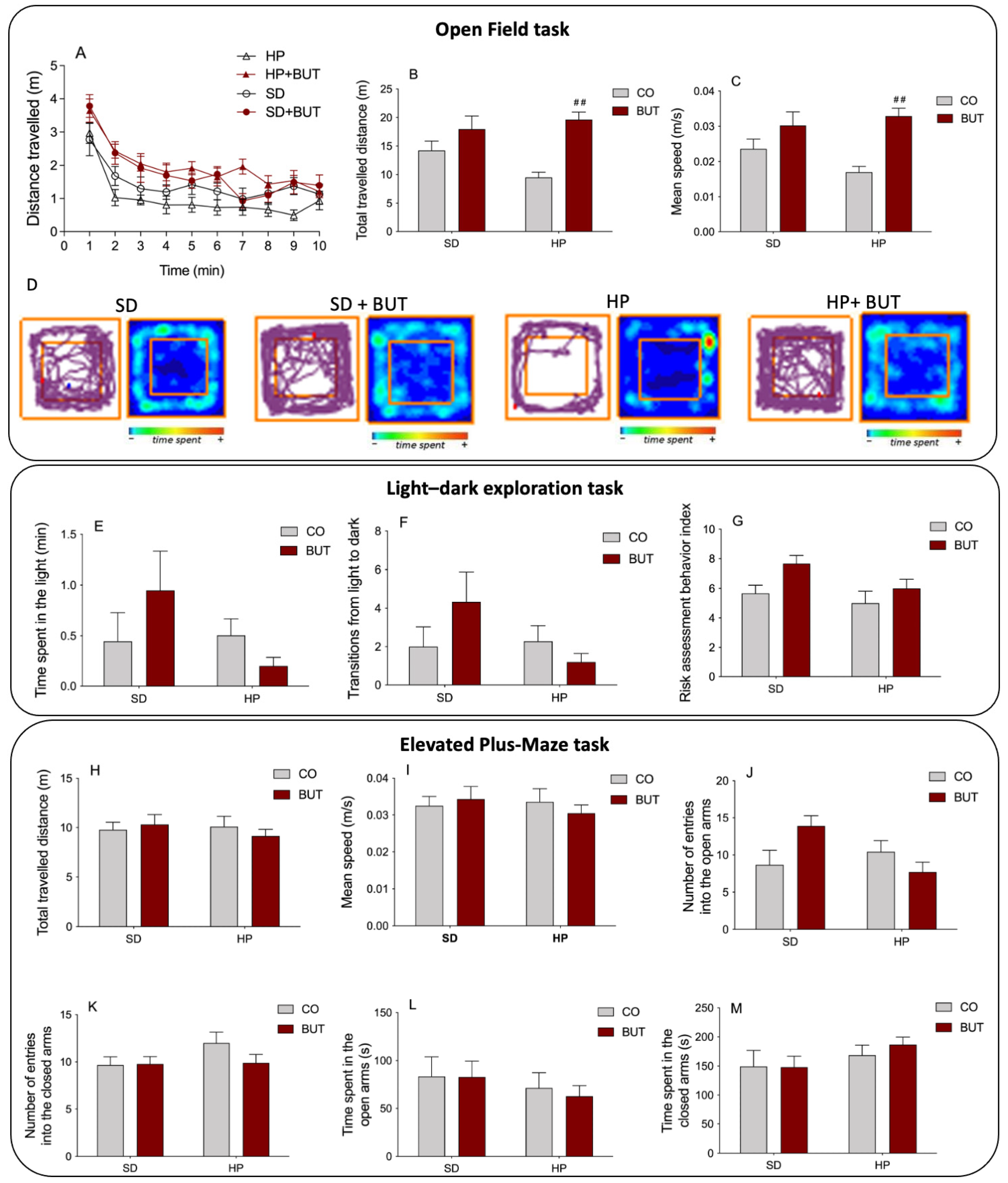
3.4. Behavioral Tasks
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Rossi, J.L.S.; Barbalho, S.M.; de Araujo, R.R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diab. Metabol. Res. Rev. 2022, 38, e3502. [Google Scholar] [CrossRef]
- O'Neill, S.; O'Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Balkau, B.; Valensi, P.; Eschwège, E.; Slama, G. A review of the metabolic syndrome. Diabetes Metab. 2007, 33, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Mills, K.T.; Yao, L.; Demanelis, K.; Eloustaz, M.; Yancy, W.S., Jr.; Kelly, T.N.; He, J.; Bazzano, L.A. Effects of Low-Carbohydrate Diets versus Low-Fat Diets on Metabolic Risk Factors: A Meta-Analysis of Randomized Controlled Clinical Trials. Am. J. Epidemiol. 2012, 176, S44–S54. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Dahiya, V.; Vasudeva, N.; Sharma, S.; Kumar, A.; Rowley, D. Lead Anti-Obesity Compounds from Nature. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1637–1653. [Google Scholar] [CrossRef]
- Ungurianu, A.; Şeremet, O.; Gagniuc, E.; Olaru, O.T.; Guţu, C.; Grǎdinaru, D.; Ionescu-Tȋrgovişte, C.; Marginǎ, D.; Dǎnciulescu-Miulescu, R. Preclinical and clinical results regarding the effects of a plant-based antidiabetic formulation versus well established antidiabetic molecules. Pharmacol. Res. 2019, 150, 104522. [Google Scholar] [CrossRef]
- Escalante-Araiza, F.; Rivera-Monroy, G.; E Loza-López, C.; Gutiérrez-Salmeán, G. The effect of plant-based diets on meta-inflammation and associated cardiometabolic disorders: A review. Nutr. Rev. 2022, 80, 2017–2028. [Google Scholar] [CrossRef] [PubMed]
- Mullins, A.; Arjmandi, B. Health Benefits of Plant-Based Nutrition: Focus on Beans in Cardiometabolic Diseases. Nutrients 2021, 13, 519. [Google Scholar] [CrossRef]
- Sirtori, C.R.; Galli, C.; Anderson, J.W.; Sirtori, E.; Arnoldi, A. Functional foods for dyslipidaemia and cardiovascular risk prevention. Nutr. Res. Rev. 2009, 22, 244–261. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, K.D.; Robertson, I.K.; Geraghty, D.P.; Ball, M.J. The effect of 4-week chilli supplementation on metabolic and arterial function in humans. Eur. J. Clin. Nutr. 2007, 61, 326–333. [Google Scholar] [CrossRef]
- Aizawa, K.; Inakuma, T. Dietary capsanthin, the main carotenoid in paprika (Capsicum annuum), alters plasma high-density lipoprotein-cholesterol levels and hepatic gene expression in rats. Br. J. Nutr. 2009, 102, 1760–1766. [Google Scholar] [CrossRef] [PubMed]
- Binshtok, A.M.; Bean, B.P.; Woolf, C.J. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature 2007, 449, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Nair, M.G. Capsaicinoids in the Hottest Pepper Bhut Jolokia and its Antioxidant and Antiinflammatory Activities. Nat. Prod. Commun. 2010, 5, 91–94. [Google Scholar] [CrossRef]
- Srinivasan, K. Dietary spices as beneficial modulators of lipid profile in conditions of metabolic disorders and diseases. Food Funct. 2013, 4, 503–521. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K. Spices as influencers of body metabolism: An overview of three decades of research. Food Res. Int. 2005, 38, 77–86. [Google Scholar] [CrossRef]
- Kim, H.K.; Jeong, J.; Kang, E.Y.; Go, G. Red Pepper (Capsicum annuum L.) Seed Extract Improves Glycemic Control by Inhibiting Hepatic Gluconeogenesis via Phosphorylation of FOXO1 and AMPK in Obese Diabetic db/db Mice. Nutrients 2020, 12, 2546. [Google Scholar] [CrossRef]
- Semwal, D.K.; Ankit, K.; Sonali, S.; Ashutosh, C.; Ruchi Badoni, S. Protective and therapeutic effects of natural products against diabetes mellitus via regenerating pancreatic beta-cells and restoring their dysfunction. Phytother. Res. 2021, 35, 1218–1229. [Google Scholar] [CrossRef]
- Jang, H.-H.; Lee, J.; Lee, S.-H.; Lee, Y.-M. Effects of Capsicum annuum supplementation on the components of metabolic syndrome: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 2091. [Google Scholar] [CrossRef]
- Liu, L.; Ding, C.; Tian, M.; Yi, D.; Wang, J.; Zhao, J.; Hu, Y.; Wang, C. Fermentation improves the potentiality of capsicum in decreasing high-fat diet-induced obesity in C57BL/6 mice by modulating lipid metabolism and hormone response. Food Res. Int. 2019, 124, 49–60. [Google Scholar] [CrossRef]
- Zimmer, A.R.; Leonardi, B.; Miron, D.; Schapoval, E.; de Oliveira, J.R.; Gosmann, G. Antioxidant and anti-inflammatory properties of Capsicum baccatum: From traditional use to scientific approach. J. Ethnopharmacol. 2012, 139, 228–233. [Google Scholar] [CrossRef]
- Kappel, V.D.; Costa, G.M.; Scola, G.; Silva, F.A.; Landell, M.F.; Valente, P.; Souza, D.G.; Vanz, D.C.; Reginatto, F.H.; Moreira, J.C.F. Phenolic content and antioxidant and antimicrobial properties of fruits of Capsicum baccatum L. var. pendulum at different maturity stages. J. Med. Food 2008, 11, 267–274. [Google Scholar] [CrossRef]
- Spiller, F.; Alves, M.K.; Vieira, S.M.; Carvalho, T.A.; Leite, C.E.; Lunardelli, A.; Poloni, J.A.; Cunha, F.Q.; de Oliveira, J.R. Anti-inflammatory effects of red pepper (Capsicum baccatum) on carrageenan- and antigen-induced inflammation. J. Pharm. Pharmacol. 2008, 60, 473–478. [Google Scholar] [CrossRef]
- Zimmer, A.R.; Leonardi, B.; Zimmer, E.R.; Kalinine, E.; de Souza, D.O.; Portela, L.V.; Gosmann, G. Long-Term Oral Administration of Capsicum baccatum Extracts Does Not Alter Behavioral, Hematological, and Metabolic Parameters in CF1 Mice. Evid. Based Complement. Alternat. Med. 2012, 2012, 196358. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Ohsuzu, F. The Roles of Cytokines, Inflammation and Immunity in Vascular Diseases. J. Atheroscler. Thromb. 2004, 11, 313–321. [Google Scholar] [CrossRef][Green Version]
- Tabas, I.; Glass, C.K. Anti-Inflammatory Therapy in Chronic Disease: Challenges and Opportunities. Science 2013, 339, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Portela, L.V.; Brochier, A.W.; Haas, C.B.; de Carvalho, A.K.; Gnoato, J.A.; Zimmer, E.R.; Kalinine, E.; Pellerin, L.; Muller, A.P. Hyperpalatable Diet and Physical Exercise Modulate the Expression of the Glial Monocarboxylate Transporters MCT1 and Mol. Neurobiology 2017, 54, 5807–5814. [Google Scholar] [CrossRef]
- Battú, C.E.; Rieger, D.; Loureiro, S.; Furtado, G.V.; Bock, H.; Saraiva-Pereira, M.-L.; Pessoa-Pureur, R.; Gonçalves, C.-A.; Perry, M.-L.S. Alterations of PI3K and Akt signaling pathways in the hippocampus and hypothalamus of Wistar rats treated with highly palatable food. Nutr. Neurosci. 2012, 15, 10–17. [Google Scholar] [CrossRef]
- Leonardi, B.F.; Gosmann, G.; Zimmer, A.R. Modeling Diet-Induced Metabolic Syndrome in Rodents. Mol. Nutr. Food Res. 2020, 64, 2000249. [Google Scholar] [CrossRef] [PubMed]
- Parekh, P.I.; Petro, A.E.; Tiller, J.M.; Feinglos, M.N.; Surwit, R.S. Reversal of diet-induced obesity and diabetes in C57BL/6J mice. Metabolism 1998, 47, 1089–1096. [Google Scholar] [CrossRef]
- Muller, A.P.; Tort, A.H.; Gnoatto, J.; Moreira, J.D.; Vinadé, E.R.; Perry, M.L.; Souza, D.O.; Lara, D.R.; Portela, L.V. Metabolic and behavioral effects of chronic olanzapine treatment and cafeteria diet in rats. Behav. Pharmacol. 2010, 21, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Brant, N.M.F.; Gasparotto, F.M.; Araújo, V.D.O.; Maraschin, J.C.; Ribeiro, R.D.C.L.; Lourenço, E.L.B.; Junior, E.C.; Junior, A.G. Cardiovascular protective effects of Casearia sylvestris Swartz in Swiss and C57BL/6 LDLr-null mice undergoing high fat diet. J. Ethnopharmacol. 2014, 154, 419–427. [Google Scholar] [CrossRef]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Crawley, J.; Goodwin, F.K. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol. Biochem. Behav. 1980, 13, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open: Closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Magkos, F.; Hjorth, M.F.; Astrup, A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 545–555. [Google Scholar] [CrossRef]
- Srinivasan, K. Plant foods in the management of diabetes mellitus: Spices as beneficial antidiabetic food adjuncts. Int. J. Food Sci. Nutr. 2005, 56, 399–414. [Google Scholar] [CrossRef]
- Shi, Z.; Riley, M.; Taylor, A.W.; Page, A. Chilli consumption and the incidence of overweight and obesity in a Chinese adult population. Int. J. Obes. 2017, 41, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Antonious, G.F.; Kochhar, T.S.; Jarret, R.L.; Snyder, J.C. Antioxidants in Hot Pepper: Variation Among Accessions. J. Environ. Sci. Heal. Part B 2006, 41, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- de Sá Mendes, N.; Santos, M.C.P.; Cameron, L.C.; Ferreira, M.S.L.; Gonçalves, É.C.B.A. Characterization of pepper (Capsicum baccatum)–A potential functional ingredient. LWT Food Sci. Technol. 2019, 112, 1–9. [Google Scholar] [CrossRef]
- Ngamukote, S.; Mäkynen, K.; Thilawech, T.; Adisakwattana, S. Cholesterol-Lowering Activity of the Major Polyphenols in Grape Seed. Molecules 2011, 16, 5054–5061. [Google Scholar] [CrossRef]
- Assini, J.M.; Mulvihill, E.E.; Huff, M.W. Citrus flavonoids and lipid metabolism. Curr. Opin. Infect. Dis. 2013, 24, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.; Markus, M.A.; Morris, B.J. Resveratrol: Cellular actions of a potent natural chemical that confers a diversity of health benefits. Int. J. Biochem. Cell Biol. 2009, 41, 2125–2128. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Sang, S.; Lambert, J.D.; Lee, M.-J. Bioavailability issues in studying the health effects of plant polyphenolic compounds. Mol. Nutr. Food Res. 2008, 52, S139–S151. [Google Scholar] [CrossRef]
- Izzi, V.; Masuelli, L.; Tresoldi, I.; Sacchetti, P.; Modesti, A.; Galvano, F.; Bei, R. The effects of dietary flavonoids on the regulation of redox inflammatory networks. Front. Biosci. Landmark Ed. 2012, 17, 2396–2418. [Google Scholar] [CrossRef]
- Gil-Lespinard, M.; Castañeda, J.; Almanza-Aguilera, E.; Gómez, J.H.; Tjønneland, A.; Kyrø, C.; Overvad, K.; Katzke, V.; Schulze, M.B.; Masala, G.; et al. Dietary Intake of 91 Individual Polyphenols and 5-Year Body Weight Change in the EPIC-PANACEA Cohort. Antioxidants 2022, 11, 2425. [Google Scholar] [CrossRef]
- Yang, Y.; Trevethan, M.; Wang, S.; Zhao, L. Beneficial effects of citrus flavanones naringin and naringenin and their food sources on lipid metabolism: An update on bioavailability, pharmacokinetics, and mechanisms. J. Nutr. Biochem. 2022, 104, 108967. [Google Scholar] [CrossRef]
- Moghaddam, R.H.; Samimi, Z.; Moradi, S.Z.; Little, P.J.; Xu, S.; Farzaei, M.H. Naringenin and naringin in cardiovascular disease prevention: A preclinical review. Eur. J. Pharmacol. 2020, 887, 173535. [Google Scholar] [CrossRef] [PubMed]
- Gotto, A.M.; Moon, J.E. Management of Cardiovascular Risk: The Importance of Meeting Lipid Targets. Am. J. Cardiol. 2012, 110, 3A–14A. [Google Scholar] [CrossRef] [PubMed]
- Duarte Lau, F.; Giugliano, R.P. Lipoprotein(a) and its Significance in Cardiovascular Disease: A Review. JAMA Cardiol. 2022, 7, 760–769. [Google Scholar] [CrossRef]
- Dandona, P.; Aljada, A.; Chaudhuri, A.; Mohanty, P.; Garg, R. Metabolic syndrome: A comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation 2005, 111, 1448–1454. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [PubMed]
- Pladevall, M.; Singal, B.; Williams, L.K.; Brotons, C.; Guyer, H.; Sadurni, J.; Falces, C.; Serrano-Rios, M.; Gabriel, R.; Shaw, J.E.; et al. A single factor underlies the metabolic syndrome: A confirmatory factor analysis. Diabetes Care 2006, 29, 113–122. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kahn, C.R.; Wang, G.; Lee, K.Y. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J. Clin. Investig. 2019, 129, 3990–4000. [Google Scholar] [CrossRef] [PubMed]
- Wajchenberg, B.L. Subcutaneous and visceral adipose tissue: Their relation to the metabolic syndrome. Endocr. Rev. 2000, 21, 697–738. [Google Scholar] [CrossRef]
| Parameters | SD | SD + BUT | HP | HP + BUT |
|---|---|---|---|---|
| Brain (g/100 g b.w.) | 0.523 ± 0.021 | 0.586 ± 0.068 | 0.485 ± 0.050 | 0.517 ± 0.032 |
| Heart (g/100 g b.w.) | 0.291 ± 0.019 | 0.311 ± 0.027 | 0.284 ± 0.018 | 0.274 ± 0.024 |
| Kidney (g/100 g b.w.) | 0.645 ± 0.061 | 0.665 ± 0.055 | 0.627 ± 0.064 | 0.569 ± 0.064 |
| Liver (g/100 g b.w.) | 2.998 ± 0.134 | 3.080 ± 0.173 | 2.670 ± 0.233 | 2.795 ± 0.288 |
| ALT (IU/L) | 21.1 ± 2.3 | 23.2 ± 11.1 | 21.3 ± 16.8 | 14.7 ± 9.8 |
| ALP (IU/L) | 133.3 ± 35.8 | 123.9 ± 39.7 | 110.8 ± 13.9 | 98.1 ± 49.1 |
| GGT (IU/L) | 4.8 ± 2.1 | 6.0 ± 2.0 | 5.3 ± 2.0 | 9.4 ± 8.4 |
| Albumin (g/dL) | 4.0 ± 0.3 | 4.0 ± 0.2 | 4.0 ± 0.1 | 4.2 ± 0.3 |
| Urea (mg/dL) | 36.9 ± 5.6 | 35.8 ± 8.5 | 33.1 ± 8.0 | 43.6 ± 12.2 |
| Creatinine (mg/dL) | 0.37 ± 0.1 | 0.35 ± 0.1 | 0.51 ± 0.1 | 0.33 ± 0.1 |
| RBC count (106/µL) | 6.4 ± 0.8 | 6.5 ± 0.9 | 5.8 ± 0.7 | 6.7 ± 0.7 |
| Hb (g/dL) | 14.2 ± 0.9 | 13.9 ± 0.5 | 13.4 ± 0.6 | 13.6 ± 0.4 |
| HCT (%) | 33.6 ± 4.8 | 34.2 ± 5.1 | 31.5 ± 3.1 | 35.0 ± 3.9 |
| MCV (µm3) | 52.7 ± 1.5 | 52.6 ± 1.0 | 54.8 ± 1.3 | 52.5 ± 0.8 |
| MCH (pg) | 22.6 ± 2.2 | 21.8 ± 2.5 | 23.3 ± 2.1 | 20.6 ± 2.4 |
| MCHC (g/dl) | 42.6 ± 4.7 | 41.4 ± 5.1 | 42.7 ± 3.1 | 39.2 ± 4.6 |
| RDW (%) | 13.2 ± 0.3 | 12.9 ± 0.5 | 13.6 ± 0.7 | 12.9 ± 0.5 |
| Platelets (103/mm3) | 670.5 ± 326.4 | 732.7 ± 170.4 | 693.8 ± 107.3 | 775.0 ± 108.0 |
| WBC count (103/µL) | 7.6 ± 2.5 | 5.9 ± 0.7 | 6.37 ± 1.4 | 6.07 ± 1.7 |
| Lymphocytes (%) | 77.5 ± 4.7 | 80.2 ± 4.5 | 77.5 ± 6.0 | 79.0 ± 4.6 |
| Neutrophils (%) | 16.5 ± 5.6 | 16.2 ± 4.1 | 16.0 ± 5.9 | 14.2 ± 3.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmer, A.R.; Leonardi, B.F.; Zimmer, E.R.; Muller, A.P.; Gosmann, G.; Valmor Cruz Portela, L. Capsicum baccatum Red Pepper Prevents Cardiometabolic Risk in Rats Fed with an Ultra-Processed Diet. Metabolites 2023, 13, 385. https://doi.org/10.3390/metabo13030385
Zimmer AR, Leonardi BF, Zimmer ER, Muller AP, Gosmann G, Valmor Cruz Portela L. Capsicum baccatum Red Pepper Prevents Cardiometabolic Risk in Rats Fed with an Ultra-Processed Diet. Metabolites. 2023; 13(3):385. https://doi.org/10.3390/metabo13030385
Chicago/Turabian StyleZimmer, Aline Rigon, Bianca Franco Leonardi, Eduardo Rigon Zimmer, Alexandre Pastoris Muller, Grace Gosmann, and Luis Valmor Cruz Portela. 2023. "Capsicum baccatum Red Pepper Prevents Cardiometabolic Risk in Rats Fed with an Ultra-Processed Diet" Metabolites 13, no. 3: 385. https://doi.org/10.3390/metabo13030385
APA StyleZimmer, A. R., Leonardi, B. F., Zimmer, E. R., Muller, A. P., Gosmann, G., & Valmor Cruz Portela, L. (2023). Capsicum baccatum Red Pepper Prevents Cardiometabolic Risk in Rats Fed with an Ultra-Processed Diet. Metabolites, 13(3), 385. https://doi.org/10.3390/metabo13030385