Effect of Aerobic Exercise on Intestinal Microbiota with Amino Acids and Short-Chain Fatty Acids in Methamphetamine-Induced Mice
Abstract
1. Introduction
2. Methods and Materials
2.1. Experimental Subjects
2.2. Experimental Protocol
2.3. Conditional Place Preference Experiments
2.4. Sequencing of 16SrRNAs
2.5. Amino Acid Identification
2.6. Identification of SCFAs
2.7. Statistical Analysis
3. Results
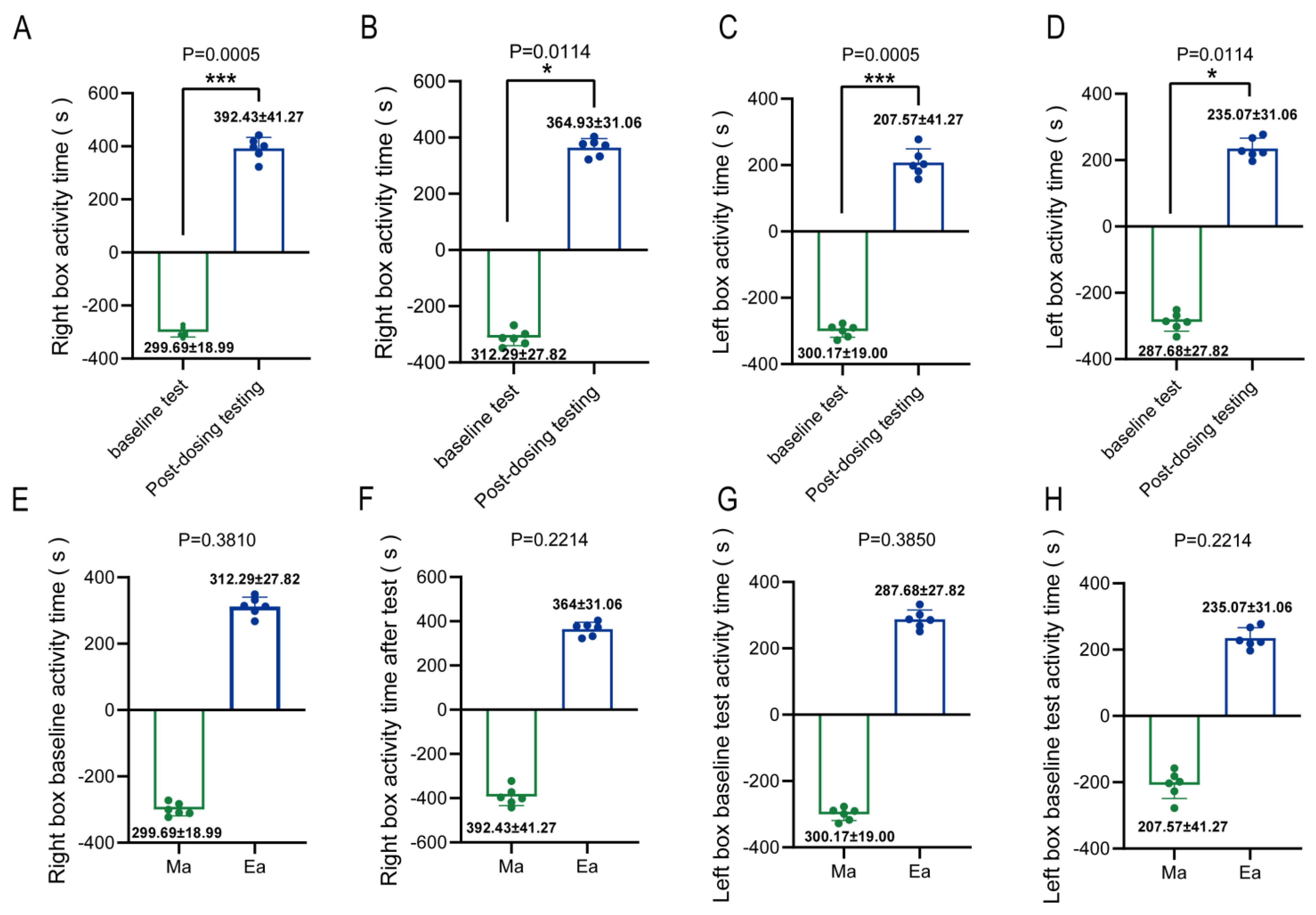
3.1. MA-Induced Conditioned Place Preference in Mice Exhibited Addictive Behavior
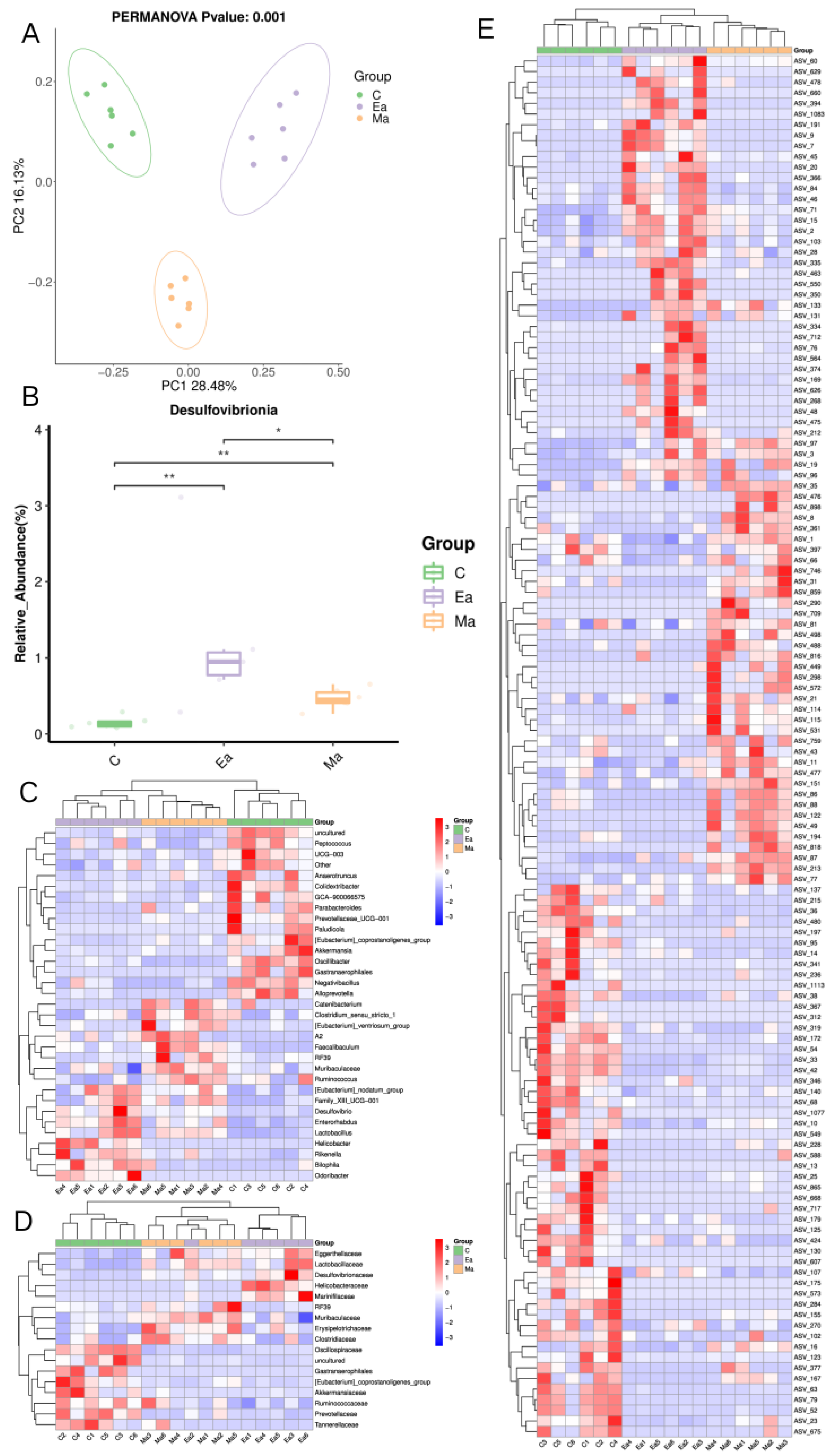
3.2. Disturbance of Intestinal Microbiota in Mice Induced by MA before and after Exercise
3.3. Differential Expression of Amino Acid Levels in MA-Induced Mice before and after Exercise
3.4. Differential Expression of SCFAs Levels in MA-Induced Mice before and after Exercise
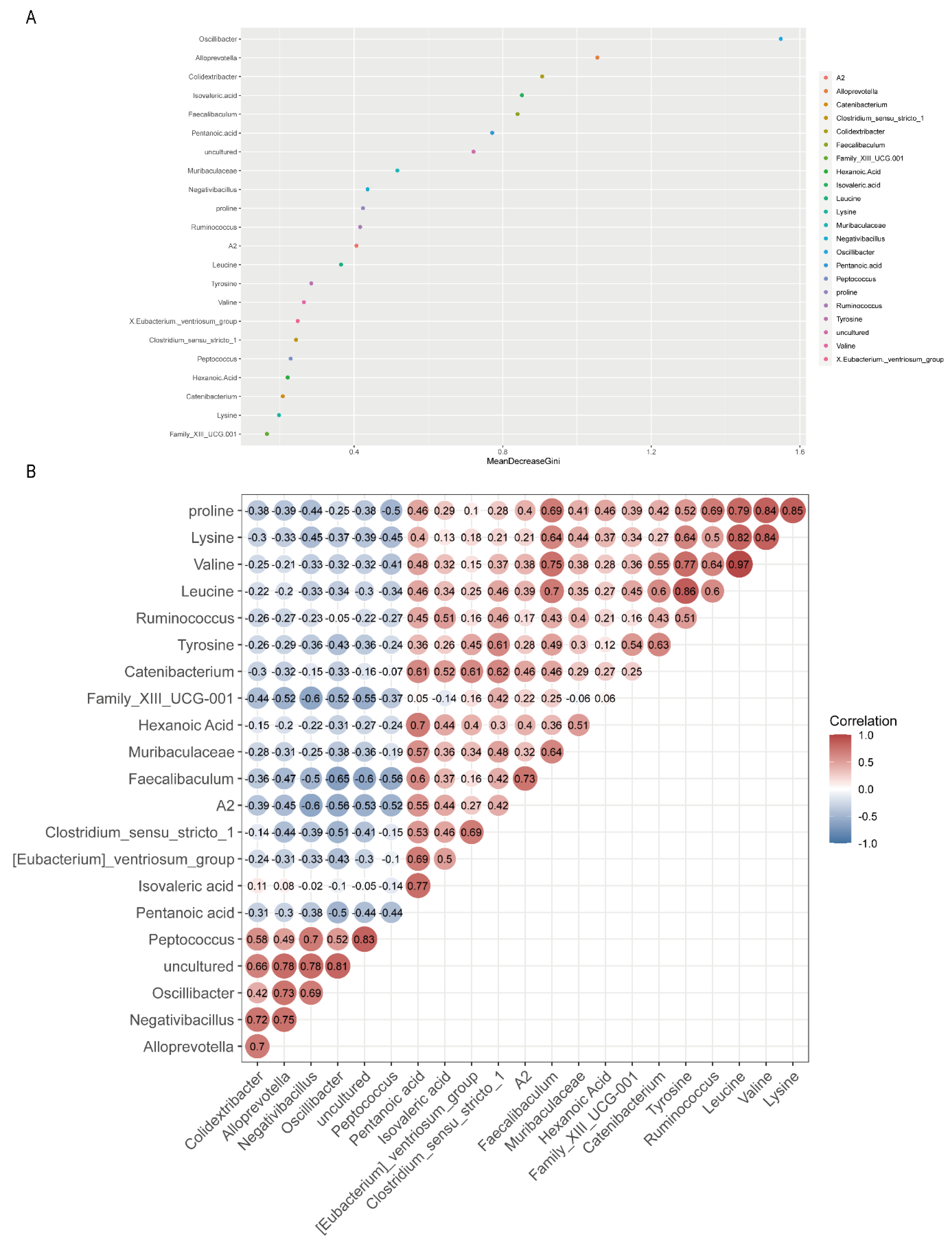
3.5. Relationships of Gut Microbiota with Amino Acids and SCFAs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Ministry of Public Security of the People’s Republic of China. China Poison Situation Report. 2021. Available online: https://www.mps.gov.cn/n2255079/n6865805/n7355741/n7355780/c8553877/content.html (accessed on 20 August 2022).
- Homer, B.D.; Solomon, T.M.; Moeller, R.W.; Mascia, A.; DeRaleau, L.; Halkitis, P.N. Methamphetamine abuse and impairment of social functioning: A review of the underlying neurophysiological causes and behavioral implications. Psychol. Bull. 2008, 134, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Voce, A.; Burns, R.; Castle, D.; Calabria, B.; McKetin, R. A latent class analysis of psychiatric symptom profiles associated with past-month methamphetamine use. Psychiatry Res. 2021, 298, 113760. [Google Scholar] [CrossRef]
- Harada, T.; Tsutomi, H.; Mori, R.; Wilson, D.B. Cognitive-behavioural treatment for amphetamine-type stimulants (ATS)-use disorders. Cochrane Database Syst. Rev. 2018, 12, Cd011315. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Compton, W.M.; Mustaquim, D. Patterns and Characteristics of Methamphetamine Use Among Adults—United States, 2015–2018. Morb. Mortal. Wkly. Rep. 2020, 69, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S.; Nurnberger, J., Jr.; Simmons, S.; Gershon, E.S.; Polinsky, R.; Keiser, H.R. Effects of injected sympathomimetic amines on plasma catecholamines and circulatory variables in man. Life Sci. 1983, 32, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Newton, T.F.; Kalechstein, A.D.; Duran, S.; Vansluis, N.; Ling, W. Methamphetamine abstinence syndrome: Preliminary findings. Am. J. Addict. 2004, 13, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Zweben, J.E.; Cohen, J.B.; Christian, D.; Galloway, G.P.; Salinardi, M.; Parent, D.; Iguchi, M. Psychiatric symptoms in methamphetamine users. Am. J. Addict. 2004, 13, 181–190. [Google Scholar] [CrossRef]
- Naji, L.; Dennis, B.; Rosic, T.; Wiercioch, W.; Paul, J.; Worster, A.; Thabane, L.; Samaan, Z. Mirtazapine for the treatment of amphetamine and methamphetamine use disorder: A systematic review and meta-analysis. Drug Alcohol Depend. 2022, 232, 109295. [Google Scholar] [CrossRef]
- Kohno, M.; Dennis, L.E.; McCready, H.; Schwartz, D.L.; Hoffman, W.F.; Korthuis, P.T. A preliminary randomized clinical trial of naltrexone reduces striatal resting state functional connectivity in people with methamphetamine use disorder. Drug Alcohol Depend. 2018, 192, 186–192. [Google Scholar] [CrossRef]
- Simmons, S.J.; Martorana, R.; Philogene-Khalid, H.; Tran, F.H.; Gentile, T.A.; Xu, X.; Su, S.; Rawls, S.M.; Muschamp, J.W. Role of hypocretin/orexin receptor blockade on drug-taking and ultrasonic vocalizations (USVs) associated with low-effort self-administration of cathinone-derived 3,4-methylenedioxypyrovalerone (MDPV) in rats. Psychopharmacology 2017, 234, 3207–3215. [Google Scholar] [CrossRef]
- Li, K.X.; Loshak, H. CADTH Rapid Response Reports. In Treatment for Methamphetamine Addiction: A Review of Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2019. [Google Scholar]
- Morais, A.P.D.; Pita, I.R.; Fontes-Ribeiro, C.A.; Pereira, F.C. The neurobiological mechanisms of physical exercise in methamphetamine addiction. CNS Neurosci. Ther. 2018, 24, 85–97. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Li, X.; Wang, J.; Zhou, Y.; Zhou, C. Moderate-Intensity Aerobic Exercise Restores Appetite and Prefrontal Brain Activity to Images of Food Among Persons Dependent on Methamphetamine: A Functional Near-Infrared Spectroscopy Study. Front. Hum. Neurosci. 2019, 13, 400. [Google Scholar] [CrossRef] [PubMed]
- Carrico, A.W.; Gόmez, W.; Jain, J.; Shoptaw, S.; Discepola, M.V.; Olem, D.; Lagana-Jackson, J.; Andrews, R.; Neilands, T.B.; Dilworth, S.E.; et al. Randomized controlled trial of a positive affect intervention for methamphetamine users. Drug Alcohol Depend. 2018, 192, 8–15. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Q.; Jiang, H.; Du, J.; Zhou, C.; Yu, S.; Hashimoto, K.; Zhao, M. Impact of aerobic exercise on cognitive impairment and oxidative stress markers in methamphetamine-dependent patients. Psychiatry Res. 2018, 266, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.U.; Finlayson, G.; Liu, X.; Zhou, Q.; Liu, T.; Zhou, C. Effects of Acute Dance and Aerobic Exercise on Drug Craving and Food Reward in Women with Methamphetamine Dependence. Med. Sci. Sport. Exerc. 2021, 53, 2245–2253. [Google Scholar] [CrossRef] [PubMed]
- Menglu, S.; Suyong, Y.; Xiaoyan, W.; Schöllhorn, W.I.; Dong, Z. Cognitive effectiveness of high-intensity interval training for individuals with methamphetamine dependence: A study protocol for randomised controlled trial. Trials 2021, 22, 650. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Kim, Y.; Kim, M.; Seo, M.; Kim, S.; Kim, S.; Lee, S. Exercise Pills for Drug Addiction: Forced Moderate Endurance Exercise Inhibits Methamphetamine-Induced Hyperactivity through the Striatal Glutamatergic Signaling Pathway in Male Sprague Dawley Rats. Int. J. Mol. Sci. 2021, 22, 8203. [Google Scholar] [CrossRef]
- Aburahma, A.; Pachhain, S.; Choudhury, S.R.; Rana, S.; Phuntumart, V.; Larsen, R.; Sprague, J.E. Potential Contribution of the Intestinal Microbiome to Phenethylamine-Induced Hyperthermia. Brain Behav. Evol. 2020, 95, 256–271. [Google Scholar] [CrossRef]
- Tran, S.M.; Mohajeri, M.H. The Role of Gut Bacterial Metabolites in Brain Development, Aging and Disease. Nutrients 2021, 13, 732. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, X.; Liu, X.; Liu, G.; Zeng, K.; Wang, G. Altered fecal microbiota composition in individuals who abuse methamphetamine. Sci. Rep. 2021, 11, 18178. [Google Scholar] [CrossRef]
- Angoa-Pérez, M.; Zagorac, B.; Winters, A.D.; Greenberg, J.M.; Ahmad, M.; Theis, K.R.; Kuhn, D.M. Differential effects of synthetic psychoactive cathinones and amphetamine stimulants on the gut microbiome in mice. PloS ONE 2020, 15, e0227774. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Zhi, X.; Zhang, K.K.; Wang, L.B.; Li, J.H.; Liu, J.L.; Xu, L.L.; Yoshida, J.S.; Xie, X.L.; Wang, Q. Escalating dose-multiple binge methamphetamine treatment elicits neurotoxicity, altering gut microbiota and fecal metabolites in mice. Food Chem. Toxicol. 2021, 148, 111946. [Google Scholar] [CrossRef] [PubMed]
- Forouzan, S.; Hoffman, K.L.; Kosten, T.A. Methamphetamine exposure and its cessation alter gut microbiota and induce depressive-like behavioral effects on rats. Psychopharmacology 2021, 238, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Hu, J.; Wan, Y.; Cai, M.; Wang, Z.; Peng, Z.; Liao, Y.; Li, D.; Yao, P.; Liu, L.; et al. Narrative review on potential role of gut microbiota in certain substance addiction. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 106, 110093. [Google Scholar] [CrossRef]
- Bonomo, R.R.; Cook, T.M.; Gavini, C.K.; White, C.R.; Jones, J.R.; Bovo, E.; Zima, A.V.; Brown, I.A.; Dugas, L.R.; Zakharian, E.; et al. Fecal transplantation and butyrate improve neuropathic pain, modify immune cell profile, and gene expression in the PNS of obese mice. Proc. Natl. Acad. Sci. USA 2020, 117, 26482–26493. [Google Scholar] [CrossRef]
- Liu, Q.; Li, H.; Yang, J.; Niu, X.; Zhao, C.; Zhao, L.; Wang, Z. Valproic acid attenuates inflammation of optic nerve and apoptosis of retinal ganglion cells in a rat model of optic neuritis. Biomed. Pharmacother. 2017, 96, 1363–1370. [Google Scholar] [CrossRef]
- Lacher, S.K.; Mayer, R.; Sichardt, K.; Nieber, K.; Müller, C.E. Interaction of valerian extracts of different polarity with adenosine receptors: Identification of isovaltrate as an inverse agonist at A1 receptors. Biochem. Pharmacol. 2007, 73, 248–258. [Google Scholar] [CrossRef]
- Manville, R.W.; Abbott, G.W. Ancient and modern anticonvulsants act synergistically in a KCNQ potassium channel binding pocket. Nat. Commun. 2018, 9, 3845. [Google Scholar] [CrossRef]
- González, B.; Torres, O.V.; Jayanthi, S.; Gomez, N.; Sosa, M.H.; Bernardi, A.; Urbano, F.J.; García-Rill, E.; Cadet, J.L.; Bisagno, V. The effects of single-dose injections of modafinil and methamphetamine on epigenetic and functional markers in the mouse medial prefrontal cortex: Potential role of dopamine receptors. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 88, 222–234. [Google Scholar] [CrossRef]
- Jayanthi, S.; McCoy, M.T.; Chen, B.; Britt, J.P.; Kourrich, S.; Yau, H.J.; Ladenheim, B.; Krasnova, I.N.; Bonci, A.; Cadet, J.L. Methamphetamine downregulates striatal glutamate receptors via diverse epigenetic mechanisms. Biol. Psychiatry 2014, 76, 47–56. [Google Scholar] [CrossRef]
- Lai, S.; Wang, J.; Wang, B.; Wang, R.; Li, G.; Jia, Y.; Chen, T.; Chen, Y. Alterations in gut microbiota affect behavioral and inflammatory responses to methamphetamine in mice. Psychopharmacology 2022, 239, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gubert, C.; Kong, G.; Renoir, T.; Hannan, A.J. Exercise, diet and stress as modulators of gut microbiota: Implications for neurodegenerative diseases. Neurobiol. Dis. 2020, 134, 104621. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, P.; Zhou, D.; Huang, L.; Zhang, L.; Gao, X.; Maitiabula, G.; Wang, S.; Wang, X. Multi-Omics Analyses Characterize the Gut Microbiome and Metabolome Signatures of Soldiers Under Sustained Military Training. Front. Microbiol. 2022, 13, 827071. [Google Scholar] [CrossRef] [PubMed]
- Coqueiro, A.Y.; Rogero, M.M.; Tirapegui, J. Glutamine as an Anti-Fatigue Amino Acid in Sports Nutrition. Nutrients 2019, 11, 863. [Google Scholar] [CrossRef]
- Lai, Z.; Shan, W.; Li, J.; Min, J.; Zeng, X.; Zuo, Z. Appropriate exercise level attenuates gut dysbiosis and valeric acid increase to improve neuroplasticity and cognitive function after surgery in mice. Mol. Psychiatry 2021, 26, 7167–7187. [Google Scholar] [CrossRef]
- Chen, H.; Shen, L.; Liu, Y.; Ma, X.; Long, L.; Ma, X.; Ma, L.; Chen, Z.; Lin, X.; Si, L.; et al. Strength Exercise Confers Protection in Central Nervous System Autoimmunity by Altering the Gut Microbiota. Front. Immunol. 2021, 12, 628629. [Google Scholar] [CrossRef]
- Cataldi, S.; Poli, L.; Şahin, F.N.; Patti, A.; Santacroce, L.; Bianco, A.; Greco, G.; Ghinassi, B.; Di Baldassarre, A.; Fischetti, F. The Effects of Physical Activity on the Gut Microbiota and the Gut-Brain Axis in Preclinical and Human Models: A Narrative Review. Nutrients 2022, 14, 3293. [Google Scholar] [CrossRef]
- McGarry, T.; Biniecka, M.; Veale, D.J.; Fearon, U. Hypoxia, oxidative stress and inflammation. Free. Radic. Biol. Med. 2018, 125, 15–24. [Google Scholar] [CrossRef]
- Torre-Villalvazo, I.; Alemán-Escondrillas, G.; Valle-Ríos, R.; Noriega, L.G. Protein intake and amino acid supplementation regulate exercise recovery and performance through the modulation of mTOR, AMPK, FGF21, and immunity. Nutr. Res. 2019, 72, 1–17. [Google Scholar] [CrossRef]
- Arazi, H.; Eghbali, E.; Suzuki, K. Creatine Supplementation, Physical Exercise and Oxidative Stress Markers: A Review of the Mechanisms and Effectiveness. Nutrients 2021, 13, 869. [Google Scholar] [CrossRef]
- Barton, W.; Penney, N.C.; Cronin, O.; Garcia-Perez, I.; Molloy, M.G.; Holmes, E.; Shanahan, F.; Cotter, P.D.; O’Sullivan, O. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut 2018, 67, 625–633. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Guo, L.K.; Han, X.; Song, R.; Dong, G.M.; Ma, C.M.; Wu, N.; Li, J. Naltrexone attenuates methamphetamine-induced behavioral sensitization and conditioned place preference in mice. Behav. Brain Res. 2021, 399, 112971. [Google Scholar] [CrossRef] [PubMed]
- Kesby, J.P.; Chang, A.; Markou, A.; Semenova, S. Modeling human methamphetamine use patterns in mice: Chronic and binge methamphetamine exposure, reward function and neurochemistry. Addict. Biol. 2018, 23, 206–218. [Google Scholar] [CrossRef]
- Brecht, M.L.; O’Brien, A.; von Mayrhauser, C.; Anglin, M.D. Methamphetamine use behaviors and gender differences. Addict. Behav. 2004, 29, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Huang, X.D.; Hu, X.F.; Wang, S.Q.; Chen, K.; Wei, J.A.; Yan, L.; So, K.F.; Yuan, T.F.; Zhang, L. Physical exercise rescues cocaine-evoked synaptic deficits in motor cortex. Mol. Psychiatry 2021, 26, 6187–6197. [Google Scholar] [CrossRef] [PubMed]
- Re, G.F.; Li, H.; Yang, J.Q.; Li, Y.; Zhang, Z.; Wu, X.; Zhou, R.; Kong, D.; Luo, H.; Kuang, Y.Q.; et al. Exercise modulates central and peripheral inflammatory responses and ameliorates methamphetamine-induced anxiety-like symptoms in mice. Front. Mol. Neurosci. 2022, 15, 955799. [Google Scholar] [CrossRef]
- Thorn, D.A.; Winter, J.C.; Li, J.X. Agmatine attenuates methamphetamine-induced conditioned place preference in rats. Eur. J. Pharmacol. 2012, 680, 69–72. [Google Scholar] [CrossRef]
- Oebiotech. D2 Clustering, Pearson Correlation Calculations. 2022. Available online: https://hiplot-academic.com/basic/cor-heatmaphttps://hiplot-academic.com/basic/cor-heatmap (accessed on 25 September 2022).
- Oebiotech. Random Forest Algorithms. 2022. Available online: https://cloud.oebiotech.com/task/detail/randomforest/ (accessed on 25 September 2022).
- Toborek, M.; Seelbach, M.J.; Rashid, C.S.; András, I.E.; Chen, L.; Park, M.; Esser, K.A. Voluntary exercise protects against methamphetamine-induced oxidative stress in brain microvasculature and disruption of the blood-brain barrier. Mol. Neurodegener. 2013, 8, 22. [Google Scholar] [CrossRef]
- Park, M.; Levine, H.; Toborek, M. Exercise protects against methamphetamine-induced aberrant neurogenesis. Sci. Rep. 2016, 6, 34111. [Google Scholar] [CrossRef]
- Mudd, A.T.; Berding, K.; Wang, M.; Donovan, S.M.; Dilger, R.N. Serum cortisol mediates the relationship between fecal Ruminococcus and brain N-acetylaspartate in the young pig. Gut Microbes 2017, 8, 589–600. [Google Scholar] [CrossRef]
- Crocker, C.E.; Bernier, D.C.; Hanstock, C.C.; Lakusta, B.; Purdon, S.E.; Seres, P.; Tibbo, P.G. Prefrontal glutamate levels differentiate early phase schizophrenia and methamphetamine addiction: A (1)H MRS study at 3Tesla. Schizophr. Res. 2014, 157, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, T.C.; Mon, A.; Gazdzinski, S.; Meyerhoff, D.J. Chronic cigarette smoking in alcohol dependence: Associations with cortical thickness and N-acetylaspartate levels in the extended brain reward system. Addict. biology 2013, 18, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Yücel, M.; Lubman, D.I.; Harrison, B.J.; Fornito, A.; Allen, N.B.; Wellard, R.M.; Roffel, K.; Clarke, K.; Wood, S.J.; Forman, S.D.; et al. A combined spectroscopic and functional MRI investigation of the dorsal anterior cingulate region in opiate addiction. Mol. Psychiatry 2007, 12, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Wegierska, A.E.; Charitos, I.A.; Topi, S.; Potenza, M.A.; Montagnani, M.; Santacroce, L. The Connection Between Physical Exercise and Gut Microbiota: Implications for Competitive Sports Athletes. Sport. Med. 2022, 52, 2355–2369. [Google Scholar] [CrossRef]
- Cook, M.D.; Allen, J.M.; Pence, B.D.; Wallig, M.A.; Gaskins, H.R.; White, B.A.; Woods, J.A. Exercise and gut immune function: Evidence of alterations in colon immune cell homeostasis and microbiome characteristics with exercise training. Immunol. Cell Biol. 2016, 94, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Yang, J.; Jian, Y.; Lei, Y.; Yao, S.; Hu, Z.; Liu, X.; Tang, C.; Liu, W. Treadmill Exercise Modulates Intestinal Microbes and Suppresses LPS Displacement to Alleviate Neuroinflammation in the Brains of APP/PS1 Mice. Nutrients 2022, 14, 4134. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Huang, Y.; Tan, X.; Chai, T.; Wu, J.; Zhang, H.; Li, Y.; Hu, X.; Zheng, P.; Ji, P.; et al. Characterization of gut microbiome in mice model of depression with divergent response to escitalopram treatment. Transl. Psychiatry 2021, 11, 303. [Google Scholar] [CrossRef]
- Verhaar, B.J.H.; Hendriksen, H.M.A.; de Leeuw, F.A.; Doorduijn, A.S.; van Leeuwenstijn, M.; Teunissen, C.E.; Barkhof, F.; Scheltens, P.; Kraaij, R.; van Duijn, C.M.; et al. Gut Microbiota Composition Is Related to AD Pathology. Front. Immunol. 2021, 12, 794519. [Google Scholar] [CrossRef]
- D’Amato, A.; Di Cesare Mannelli, L.; Lucarini, E.; Man, A.L.; Le Gall, G.; Branca, J.J.V.; Ghelardini, C.; Amedei, A.; Bertelli, E.; Regoli, M.; et al. Faecal microbiota transplant from aged donor mice affects spatial learning and memory via modulating hippocampal synaptic plasticity- and neurotransmission-related proteins in young recipients. Microbiome 2020, 8, 140. [Google Scholar] [CrossRef]
- Han, B.; Compton, W.M.; Jones, C.M.; Einstein, E.B.; Volkow, N.D. Methamphetamine Use, Methamphetamine Use Disorder, and Associated Overdose Deaths Among US Adults. JAMA Psychiatry 2021, 78, 1329–1342. [Google Scholar] [CrossRef]
- Mizoguchi, H.; Yamada, K. Methamphetamine use causes cognitive impairment and altered decision-making. Neurochem. Int. 2019, 124, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Paulus, M.P.; Stewart, J.L. Neurobiology, Clinical Presentation, and Treatment of Methamphetamine Use Disorder: A Review. JAMA Psychiatry 2020, 77, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Xiao, Q.; Tang, J.; Liang, X.; Wang, J.; Hu, M.; Jiang, Y.; Liu, L.; Qin, L.; Zhou, M.; et al. Positive effects of running exercise on astrocytes in the medial prefrontal cortex in an animal model of depression. J. Comp. Neurol. 2022, 530, 3056–3071. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liang, X.F.; He, S.; Zhang, Y.P.; Li, J.; Huang, K.; Shi, L.J.; Ren, P. Valine acts as a nutritional signal in brain to activate TORC1 and attenuate postprandial ammonia-N excretion in Chinese perch (Siniperca chuatsi). Fish Physiol. Biochem. 2020, 46, 2015–2025. [Google Scholar] [CrossRef]
- Son, S.M.; Park, S.J.; Lee, H.; Siddiqi, F.; Lee, J.E.; Menzies, F.M.; Rubinsztein, D.C. Leucine Signals to mTORC1 via Its Metabolite Acetyl-Coenzyme A. Cell Metab. 2019, 29, 192–201.e197. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Wu, W.R.; Lee, L.M.; Huang, P.R.; Chen, J.C. mTOR signaling in the nucleus accumbens mediates behavioral sensitization to methamphetamine. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 86, 331–339. [Google Scholar] [CrossRef] [PubMed]
- D’Hulst, G.; Masschelein, E.; De Bock, K. Resistance exercise enhances long-term mTORC1 sensitivity to leucine. Mol. Metab. 2022, 66, 101615. [Google Scholar] [CrossRef]
- Margolis, L.M.; Karl, J.P.; Wilson, M.A.; Coleman, J.L.; Whitney, C.C.; Pasiakos, S.M. Serum Branched-Chain Amino Acid Metabolites Increase in Males When Aerobic Exercise Is Initiated with Low Muscle Glycogen. Metabolites 2021, 11, 828. [Google Scholar] [CrossRef]
- Watford, M.; Wu, G. Protein. Adv. Nutr. 2018, 9, 651–653. [Google Scholar] [CrossRef]
- Li, Y.; Peng, Y.; Ma, P.; Yang, H.; Xiong, H.; Wang, M.; Peng, C.; Tu, P.; Li, X. Antidepressant-Like Effects of Cistanche tubulosa Extract on Chronic Unpredictable Stress Rats Through Restoration of Gut Microbiota Homeostasis. Front. Pharmacol. 2018, 9, 967. [Google Scholar] [CrossRef]
- Schülke, S. Induction of Interleukin-10 Producing Dendritic Cells As a Tool to Suppress Allergen-Specific T Helper 2 Responses. Front. Immunol. 2018, 9, 455. [Google Scholar] [CrossRef] [PubMed]
- Canedo, T.; Portugal, C.C.; Socodato, R.; Almeida, T.O.; Terceiro, A.F.; Bravo, J.; Silva, A.I.; Magalhães, J.D.; Guerra-Gomes, S.; Oliveira, J.F.; et al. Astrocyte-derived TNF and glutamate critically modulate microglia activation by methamphetamine. Neuropsychopharmacology 2021, 46, 2358–2370. [Google Scholar] [CrossRef] [PubMed]
- Magzal, F.; Shochat, T.; Haimov, I.; Tamir, S.; Asraf, K.; Tuchner-Arieli, M.; Even, C.; Agmon, M. Increased physical activity improves gut microbiota composition and reduces short-chain fatty acid concentrations in older adults with insomnia. Sci. Rep. 2022, 12, 2265. [Google Scholar] [CrossRef] [PubMed]
- Estaki, M.; Pither, J.; Baumeister, P.; Little, J.P.; Gill, S.K.; Ghosh, S.; Ahmadi-Vand, Z.; Marsden, K.R.; Gibson, D.L. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome 2016, 4, 42. [Google Scholar] [CrossRef]


| After MA Administration (Ma/C) | After Exercise Intervention (Ea/Ma) | |||
|---|---|---|---|---|
| Taxon | Trend | Taxon | Trend | p-Value |
| Oscillibacter | ↓ | Oscillibacter | ↑ | 0.0000021 |
| uncultured | ↓ | uncultured | ↑ | 0.0000055 |
| Negativibacillus | ↓ | Negativibacillus | ↑ | 0.0000478 |
| Alloprevotella | ↓ | Alloprevotella | ↑ | 0.0002397 |
| Faecalibaculum | ↑ | Faecalibaculum | ↓ | 0.0008663 |
| Enterorhabdus | ↑ | Enterorhabdus | ↑ | 0.0008799 |
| Colidextribacter | ↓ | Colidextribacter | ↑ | 0.0028403 |
| Gastranaerophilales | ↓ | 0.0029036 | ||
| Bilophila | ↑ | Bilophila | ↑ | 0.0039085 |
| Odoribacter | ↑ | Odoribacter | ↑ | 0.0044914 |
| Clostridium_sensu_stricto_1 | ↑ | Clostridium_sensu_stricto_1 | ↓ | 0.005689 |
| GCA-900066575 | ↓ | GCA-900066575 | ↓ | 0.0066066 |
| Lactobacillus | ↑ | Lactobacillus | ↑ | 0.0070059 |
| Catenibacterium | ↑ | Catenibacterium | ↓ | 0.0073016 |
| Muribaculaceae | ↑ | Muribaculaceae | ↓ | 0.0102365 |
| Peptococcus | ↓ | Peptococcus | ↑ | 0.0108253 |
| Ruminococcus | ↑ | Ruminococcus | ↓ | 0.013363 |
| {Eubacterium}_ventriosum_group | ↑ | {Eubacterium}_ventriosum_group | ↓ | 0.0135516 |
| {Eubacterium}_nodatum_group | ↑ | {Eubacterium}_nodatum_group | ↑ | 0.0150138 |
| A2 | ↑ | A2 | ↓ | 0.0151286 |
| Desulfovibrio | ↑ | Desulfovibrio | ↑ | 0.0269891 |
| Paludicola | ↓ | 0.0353126 | ||
| Family_XIII_UCG-001 | ↑ | Family_XIII_UCG-001 | ↓ | 0.0452011 |
| After MA Administration (Ma/C) | After Exercise Intervention (Ea/Ma) | ||||
|---|---|---|---|---|---|
| Amino Acid | Trend | p-Value (Ma/C) | Amino Acid | Trend | p-Value (Ea/Ma) |
| Asparagine | ↑ | 0.03 | Alanine | ↓ | 0.01 |
| Glutamine | ↑ | 0.04 | Glycine | ↓ | 0.02 |
| Leucine | ↑ | 0.04 | Isoleucine | ↓ | 0.03 |
| Lysine | ↑ | 0.04 | Leucine | ↓ | 0.02 |
| proline | ↑ | 0.02 | Lysine | ↓ | 0.04 |
| Tyrosine | ↑ | 0.04 | Methionine | ↓ | 0.05 |
| Valine | ↑ | 0.04 | Phenylalanine | ↓ | 0.02 |
| - | - | - | proline | ↓ | 0.01 |
| - | - | - | Tyrosine | ↓ | 0.05 |
| - | - | - | Valine | ↓ | 0.02 |
| After MA Administration (Ma/C) | After Exercise Intervention (Ea/Ma) | ||||
|---|---|---|---|---|---|
| SCFAs | Trend | p-Value (Ma/C) | SCFAs | Trend | p-Value (Ea/Ma) |
| Acetic Acid | ↑ | 0.00 | Acetic Acid | ↑ | 0.00 |
| Hexanoic Acid | ↑ | 0.0117 | Butyric Acid | ↓ | 0.0444 |
| Isovaleric acid | ↑ | 0.0010 | Hexanoic Acid | ↓ | 0.0296 |
| Pentanoic acid | ↑ | 0.0007 | Isobutyric acid | ↓ | 0.0013 |
| - | - | - | Isovaleric acid | ↓ | 0.0032 |
| - | - | - | Pentanoic acid | ↓ | 0.0004 |
| - | - | - | Propionic acid | ↓ | 0.0299 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Li, X.; Jin, Y.; Wang, Y.; Wei, C.; Zhu, Z. Effect of Aerobic Exercise on Intestinal Microbiota with Amino Acids and Short-Chain Fatty Acids in Methamphetamine-Induced Mice. Metabolites 2023, 13, 361. https://doi.org/10.3390/metabo13030361
Liang X, Li X, Jin Y, Wang Y, Wei C, Zhu Z. Effect of Aerobic Exercise on Intestinal Microbiota with Amino Acids and Short-Chain Fatty Acids in Methamphetamine-Induced Mice. Metabolites. 2023; 13(3):361. https://doi.org/10.3390/metabo13030361
Chicago/Turabian StyleLiang, Xin, Xue Li, Yu Jin, Yi Wang, Changling Wei, and Zhicheng Zhu. 2023. "Effect of Aerobic Exercise on Intestinal Microbiota with Amino Acids and Short-Chain Fatty Acids in Methamphetamine-Induced Mice" Metabolites 13, no. 3: 361. https://doi.org/10.3390/metabo13030361
APA StyleLiang, X., Li, X., Jin, Y., Wang, Y., Wei, C., & Zhu, Z. (2023). Effect of Aerobic Exercise on Intestinal Microbiota with Amino Acids and Short-Chain Fatty Acids in Methamphetamine-Induced Mice. Metabolites, 13(3), 361. https://doi.org/10.3390/metabo13030361








