The Role of Reprogrammed Glucose Metabolism in Cancer
Abstract
1. Introduction
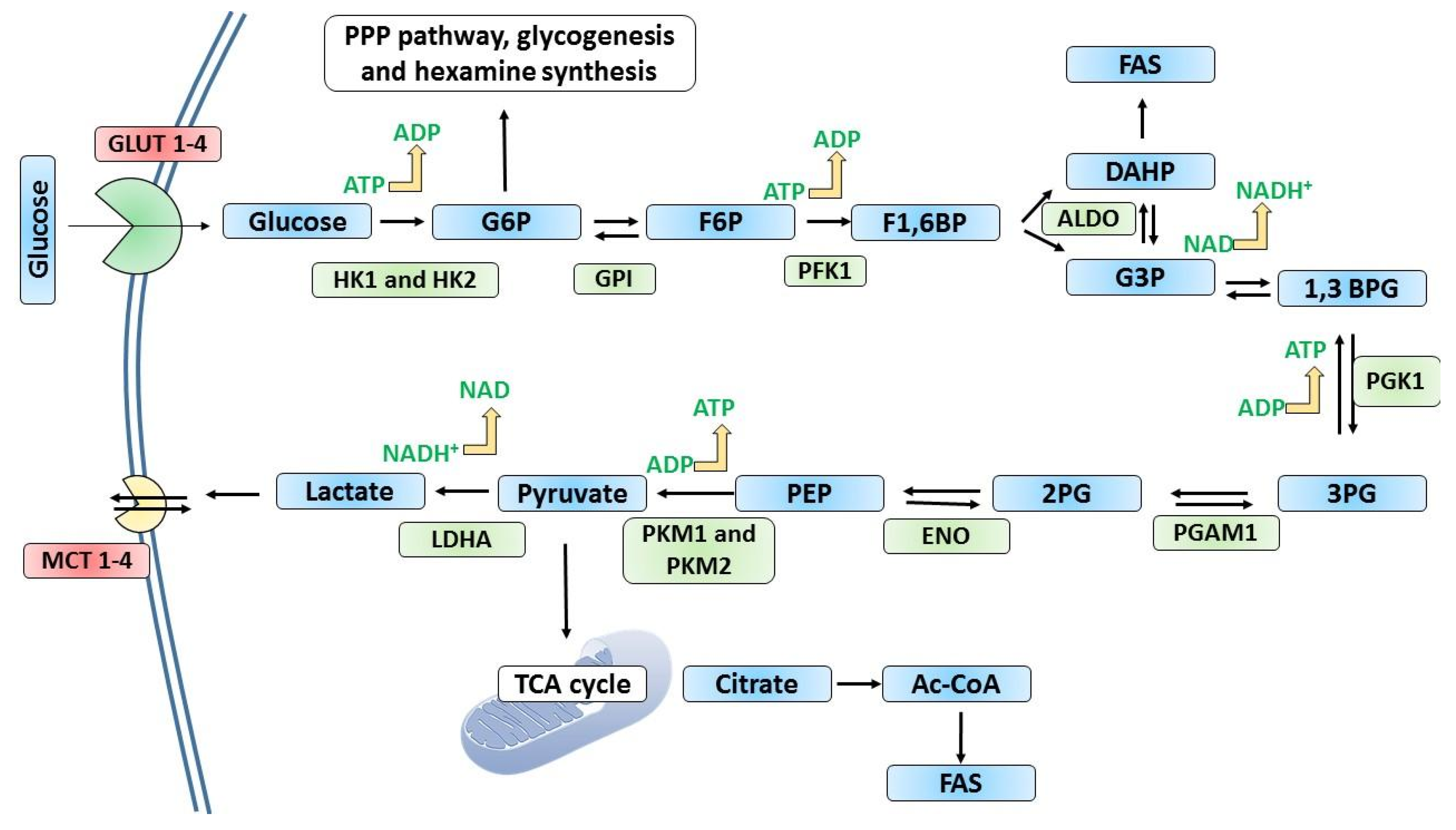
2. Overview of Glycolysis
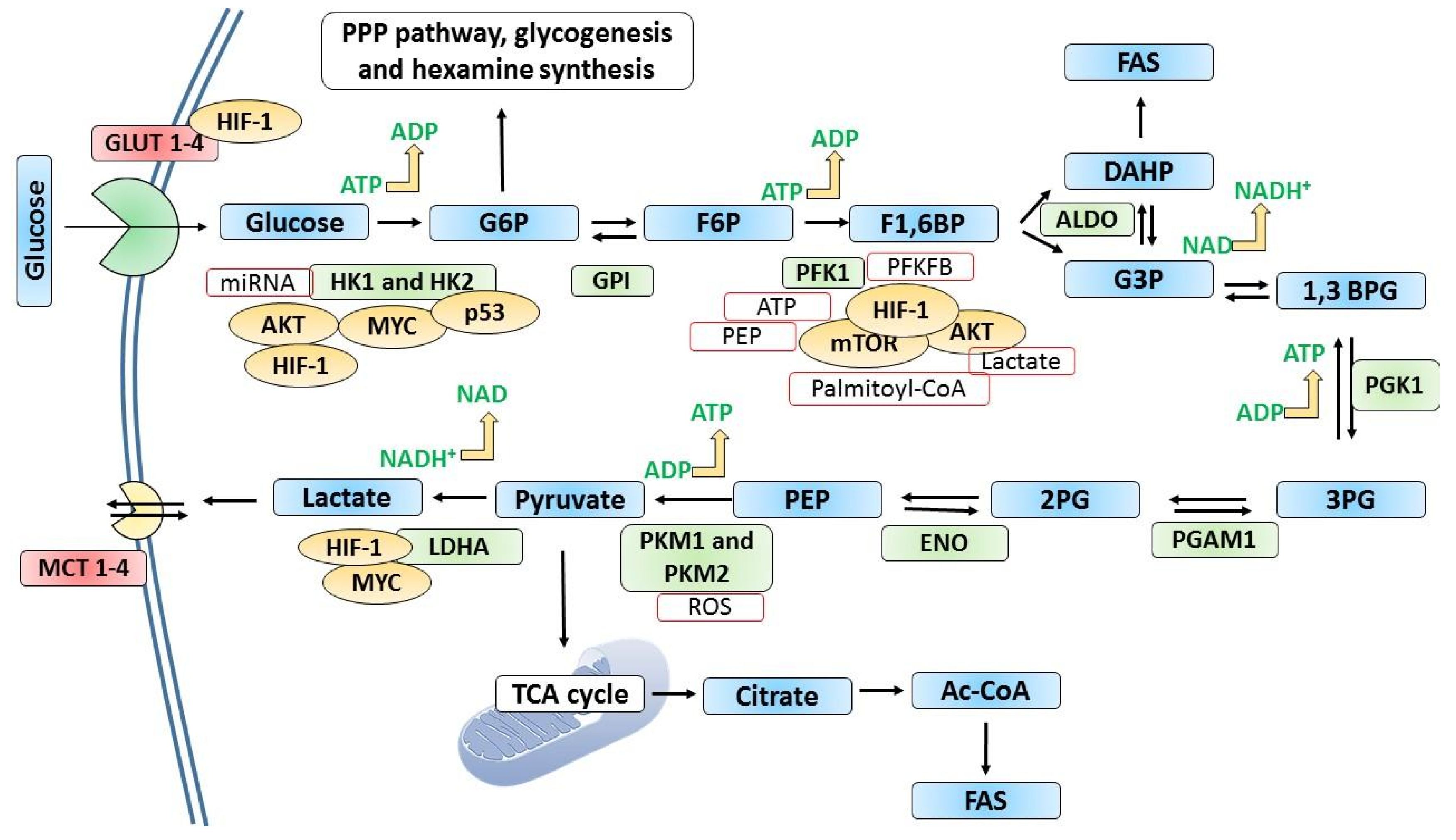
3. Metabolic Reprogramming in Cancer at the Rate-Limiting Steps of Glycolysis
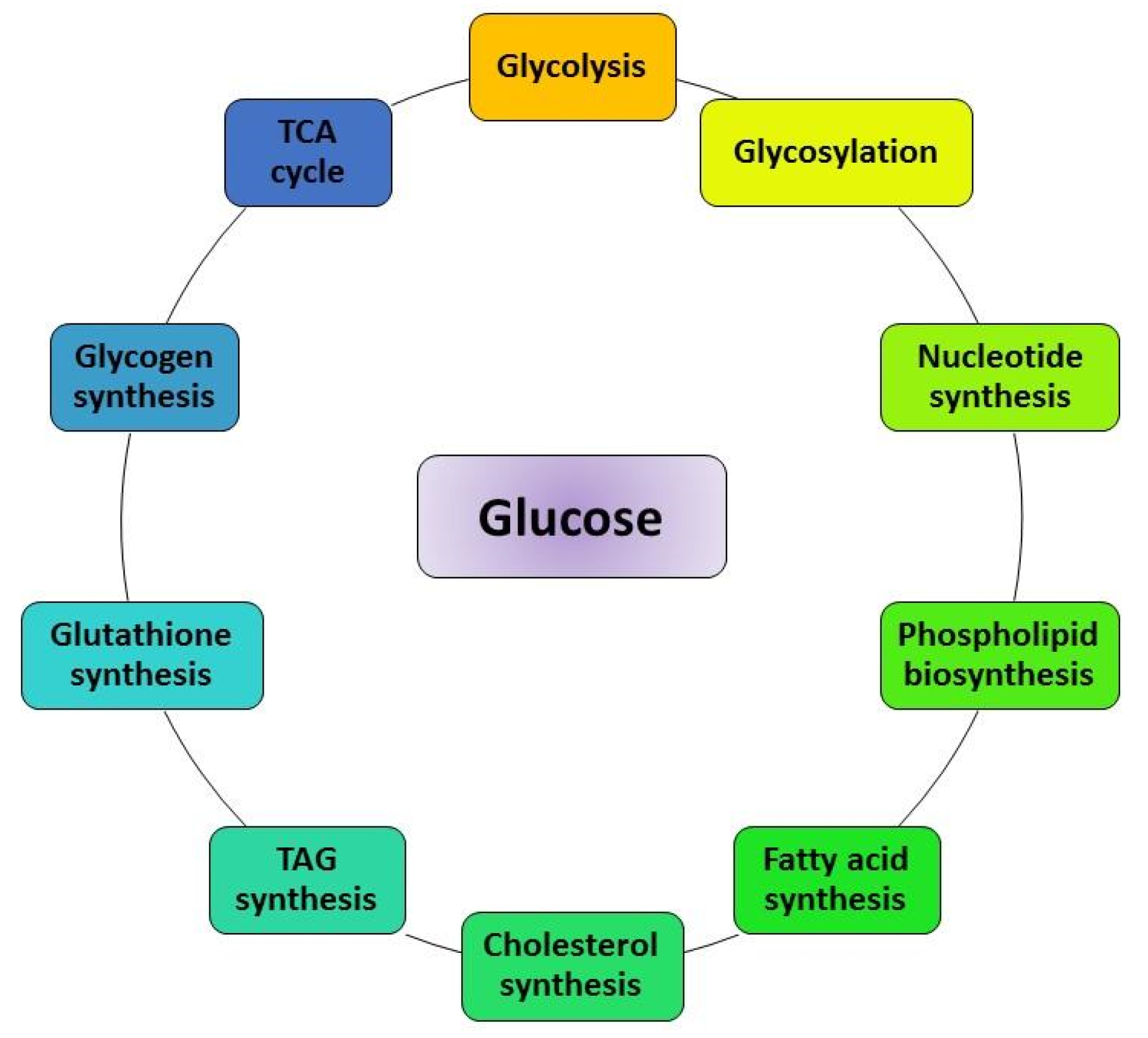
4. Impact of Glucose Metabolism on Cancer Hallmarks
4.1. Sustained Proliferation
4.2. Apoptotic Resistance
4.3. Angiogenesis
4.4. Metastasis
5. Epigenetic Impact on the Glycolytic Events
6. Pharmacological Targets
7. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eagle, H. Nutrition Needs of Mammalian Cells in Tissue Culture. Science 1955, 122, 501–514. [Google Scholar] [CrossRef]
- Zapsalis, C.; Anderle Beck, R. Food Chemistry and Nutritional Biochemistry; John Wiley & Sons: Nashville, TN, USA, 1985. [Google Scholar]
- Millward, D.J. The Hormonal Control of Protein Turnover. Clin. Nutr. 1990, 9, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.; Posener, K.; Negelein, E. The metabolism of cancer cells. Biochem. Z. 1924, 152, 319–344. [Google Scholar] [CrossRef]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondria and Cancer. Nat. Rev. Cancer 2012, 12, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Kierans, S.J.; Taylor, C.T. Regulation of Glycolysis by the Hypoxia-Inducible Factor (HIF): Implications for Cellular Physiology. J. Physiol. 2021, 599, 23–37. [Google Scholar] [CrossRef]
- Al Tameemi, W.; Dale, T.P.; Al-Jumaily, R.M.K.; Forsyth, N.R. Hypoxia-Modified Cancer Cell Metabolism. Front. Cell Dev. Biol. 2019, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Masson, N.; Ratcliffe, P.J. Hypoxia Signaling Pathways in Cancer Metabolism: The Importance of Co-Selecting Interconnected Physiological Pathways. Cancer Metab. 2014, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Tchernyshyov, I.; Semenza, G.L.; Dang, C.V. HIF-1-Mediated Expression of Pyruvate Dehydrogenase Kinase: A Metabolic Switch Required for Cellular Adaptation to Hypoxia. Cell Metab. 2006, 3, 177–185. [Google Scholar] [CrossRef]
- Robey, R.B.; Hay, N. Is Akt the “Warburg Kinase”?-Akt-Energy Metabolism Interactions and Oncogenesis. Semin. Cancer Biol. 2009, 19, 25–31. [Google Scholar] [CrossRef]
- Pfeiffer, T.; Schuster, S.; Bonhoeffer, S. Cooperation and Competition in the Evolution of ATP-Producing Pathways. Science 2001, 292, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Du, W.; Wu, M. Regulation of the Pentose Phosphate Pathway in Cancer. Protein Cell 2014, 5, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Lehninger, A.; Cox, M.M.; Nelson, D.L. Lehninger Principles of Biochemistry & Absolute Ultimate Guide; W.H. Freeman & Company: New York, NY, USA, 2008. [Google Scholar]
- Hay, N. Reprogramming Glucose Metabolism in Cancer: Can It Be Exploited for Cancer Therapy? Nat. Rev. Cancer 2016, 16, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.A. Mammalian hexokinases and their abnormal expression in cancer. Br. J. Biomed. Sci. 2000, 57, 170–178. [Google Scholar] [PubMed]
- Robey, R.B.; Hay, N. Mitochondrial Hexokinases, Novel Mediators of the Antiapoptotic Effects of Growth Factors and Akt. Oncogene 2006, 25, 4683–4696. [Google Scholar] [CrossRef] [PubMed]
- Patra, K.C.; Wang, Q.; Bhaskar, P.T.; Miller, L.; Wang, Z.; Wheaton, W.; Chandel, N.; Laakso, M.; Muller, W.J.; Allen, E.L.; et al. Hexokinase 2 Is Required for Tumor Initiation and Maintenance and Its Systemic Deletion Is Therapeutic in Mouse Models of Cancer. Cancer Cell 2013, 24, 399. [Google Scholar] [CrossRef]
- DeWaal, D.; Nogueira, V.; Terry, A.R.; Patra, K.C.; Jeon, S.-M.; Guzman, G.; Au, J.; Long, C.P.; Antoniewicz, M.R.; Hay, N. Author Correction: Hexokinase-2 Depletion Inhibits Glycolysis and Induces Oxidative Phosphorylation in Hepatocellular Carcinoma and Sensitizes to Metformin. Nat. Commun. 2018, 9, 2539. [Google Scholar] [CrossRef]
- Wolf, A.; Agnihotri, S.; Micallef, J.; Mukherjee, J.; Sabha, N.; Cairns, R.; Hawkins, C.; Guha, A. Hexokinase 2 Is a Key Mediator of Aerobic Glycolysis and Promotes Tumor Growth in Human Glioblastoma Multiforme. J. Exp. Med. 2011, 208, 313–326. [Google Scholar] [CrossRef]
- Li, R.; Mei, S.; Ding, Q.; Wang, Q.; Yu, L.; Zi, F. A Pan-Cancer Analysis of the Role of Hexokinase II (HK2) in Human Tumors. Res. Sq. 2022, 12, 18807. [Google Scholar] [CrossRef]
- Mathupala, S.P.; Heese, C.; Pedersen, P.L. Glucose Catabolism in Cancer Cells: The Type Ii Hexokinase Promoter Contains Functionally Active Response Elements for the Tumor Suppressor P53. J. Biol. Chem. 1997, 272, 22776–22780. [Google Scholar] [CrossRef]
- Mathupala, S.P.; Rempel, A.; Pedersen, P.L. Glucose Catabolism in Cancer Cells: Identification and Characterization of a Marked Activation Response of the Type Ii Hexokinase Gene to Hypoxic Conditions. J. Biol. Chem. 2001, 276, 43407–43412. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Kim, H.; Son, T.; Jeong, Y.; Kim, S.U.; Dong, S.M.; Park, Y.N.; Lee, J.D.; Lee, J.M.; Park, J.H. Regulation of HK2 Expression through Alterations in CpG Methylation of the HK2 Promoter during Progression of Hepatocellular Carcinoma. Oncotarget 2016, 7, 41798–41810. [Google Scholar] [CrossRef]
- Roh, J.-I.; Kim, Y.; Oh, J.; Kim, Y.; Lee, J.; Lee, J.; Chun, K.-H.; Lee, H.-W. Hexokinase 2 Is a Molecular Bridge Linking Telomerase and Autophagy. PLoS ONE 2018, 13, e0193182. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Hou, H.; Zhao, H.; Yu, T.; Hu, Y.; Hu, Y.; Guo, J. HK2 Is a Crucial Downstream Regulator of MiR-148a for the Maintenance of Sphere-Forming Property and Cisplatin Resistance in Cervical Cancer Cells. Front. Oncol. 2021, 11, 794015. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Dai, W.; Ji, J.; Wu, L.; Feng, J.; Li, J.; Zheng, Y.; Li, Y.; Cheng, Z.; Zhang, J.; et al. Sodium butyrate inhibits aerobic glycolysis of hepatocellular carcinoma cells via the c-myc/hexokinase 2 pathway. J. Cell. Mol. Med. 2022, 26, 3031–3045. [Google Scholar] [CrossRef]
- Ma, J.; Fan, Y.; Feng, T.; Chen, F.; Xu, Z.; Li, S.; Lin, Q.; He, X.; Shi, W.; Liu, Y.; et al. Correction: HOTAIR Regulates HK2 Expression by Binding Endogenous MiR-125 and MiR-143 in Oesophageal Squamous Cell Carcinoma Progression. Oncotarget 2019, 10, 2112. [Google Scholar] [CrossRef]
- Miyamoto, S.; Murphy, A.N.; Brown, J.H. Akt Mediates Mitochondrial Protection in Cardiomyocytes through Phosphorylation of Mitochondrial Hexokinase-II. FASEB J. 2008, 22, 1017–1019. [Google Scholar] [CrossRef]
- Roberts, D.J.; Tan-Sah, V.P.; Smith, J.M.; Miyamoto, S. Akt Phosphorylates HK-II at Thr-473 and Increases Mitochondrial HK-II Association to Protect Cardiomyocytes. J. Biol. Chem. 2013, 288, 23798–23806. [Google Scholar] [CrossRef]
- Bryson, J.M.; Coy, P.E.; Gottlob, K.; Hay, N.; Robey, R.B. Increased Hexokinase Activity, of Either Ectopic or Endogenous Origin, Protects Renal Epithelial Cells against Acute Oxidant-Induced Cell Death. J. Biol. Chem. 2002, 277, 11392–11400. [Google Scholar] [CrossRef]
- Ros, S.; Schulze, A. Balancing Glycolytic Flux: The Role of 6-Phosphofructo-2-Kinase/Fructose 2, 6-Bisphosphatases in Cancer Metabolism. Cancer Metab. 2013, 1, 8. [Google Scholar] [CrossRef]
- Evans, P.R.; Farrants, G.W.; Hudson, P.J.; Britton, H.G.; Phillips, D.C.; Blake, C.C.F.; Watson, H.C. Phosphofructokinase: Structure and Control. Philos. Trans. R. Soc. Lond. 1981, 293, 53–62. [Google Scholar] [CrossRef]
- Shi, L.; Pan, H.; Liu, Z.; Xie, J.; Han, W. Roles of PFKFB3 in Cancer. Signal Transduct. Target. Ther. 2017, 2, 17044. [Google Scholar] [CrossRef]
- Lu, L.; Chen, Y.; Zhu, Y. The Molecular Basis of Targeting PFKFB3 as a Therapeutic Strategy against Cancer. Oncotarget 2017, 8, 62793–62802. [Google Scholar] [CrossRef]
- Kotowski, K.; Rosik, J.; Machaj, F.; Supplitt, S.; Wiczew, D.; Jabłońska, K.; Wiechec, E.; Ghavami, S.; Dzięgiel, P. Role of PFKFB3 and PFKFB4 in Cancer: Genetic Basis, Impact on Disease Development/Progression, and Potential as Therapeutic Targets. Cancers 2021, 13, 909. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, A.; Telang, S.; Clem, B.; Chesney, J. Regulation of Glucose Metabolism by 6-Phosphofructo-2-Kinase/Fructose-2,6-Bisphosphatases in Cancer. Exp. Mol. Pathol. 2009, 86, 174–179. [Google Scholar] [CrossRef]
- Atsumi, T.; Chesney, J.; Metz, C.; Leng, L.; Donnelly, S.; Makita, Z.; Mitchell, R.; Bucala, R. High Expression of Inducible 6-Phosphofructo-2-Kinase/Fructose-2,6-Bisphosphatase (IPFK-2; PFKFB3) in Human Cancers. Cancer Res. 2002, 62, 5881–5887. [Google Scholar] [PubMed]
- Minchenko, O.; Opentanova, I.; Caro, J. Hypoxic Regulation of the 6-Phosphofructo-2-Kinase/Fructose-2,6-Bisphosphatase Gene Family (PFKFB-1-4) Expression in Vivo. FEBS Lett. 2003, 554, 264–270. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, L. MTOR Up-Regulation of PFKFB3 Is Essential for Acute Myeloid Leukemia Cell Survival. Biochem. Biophys. Res. Commun. 2017, 483, 897–903. [Google Scholar] [CrossRef]
- Ausina, P.; Da Silva, D.; Majerowicz, D.; Zancan, P.; Sola-Penna, M. Insulin Specifically Regulates Expression of Liver and Muscle Phosphofructokinase Isoforms. Biomed. Pharmacother. 2018, 103, 228–233. [Google Scholar] [CrossRef]
- Lang, L.; Chemmalakuzhy, R.; Shay, C.; Teng, Y. PFKP Signaling at a Glance: An Emerging Mediator of Cancer Cell Metabolism. Adv. Exp. Med. Biol. 2019, 1134, 243–258. [Google Scholar] [CrossRef]
- Lee, J.-H.; Liu, R.; Li, J.; Zhang, C.; Wang, Y.; Cai, Q.; Qian, X.; Xia, Y.; Zheng, Y.; Piao, Y.; et al. Stabilization of Phosphofructokinase 1 Platelet Isoform by AKT Promotes Tumorigenesis. Nat. Commun. 2017, 8, 949. [Google Scholar] [CrossRef] [PubMed]
- Christofk, H.R.; Vander Heiden, M.G.; Harris, M.H.; Ramanathan, A.; Gerszten, R.E.; Wei, R.; Fleming, M.D.; Schreiber, S.L.; Cantley, L.C. The M2 Splice Isoform of Pyruvate Kinase Is Important for Cancer Metabolism and Tumour Growth. Nature 2008, 452, 230–233. [Google Scholar] [CrossRef]
- Israelsen, W.J.; Vander Heiden, M.G. Pyruvate Kinase: Function, Regulation and Role in Cancer. Semin. Cell Dev. Biol. 2015, 43, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Bluemlein, K.; Grüning, N.-M.; Feichtinger, R.G.; Lehrach, H.; Kofler, B.; Ralser, M. No Evidence for a Shift in Pyruvate Kinase PKM1 to PKM2 Expression during Tumorigenesis. Oncotarget 2011, 2, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.; Ojo, D.; Yan, J.; Tang, D. PKM2 contributes to cancer metabolism. Cancer Lett. 2015, 356, 184–191. [Google Scholar] [CrossRef]
- Spoden, G.A.; Rostek, U.; Lechner, S.; Mitterberger, M.; Mazurek, S.; Zwerschke, W. Pyruvate Kinase Isoenzyme M2 Is a Glycolytic Sensor Differentially Regulating Cell Proliferation, Cell Size and Apoptotic Cell Death Dependent on Glucose Supply. Exp. Cell Res. 2009, 315, 2765–2774. [Google Scholar] [CrossRef]
- Wang, L.; Yang, L.; Yang, Z.; Tang, Y.; Tao, Y.; Zhan, Q.; Lei, L.; Jing, Y.; Jiang, X.; Jin, H.; et al. Glycolytic Enzyme PKM2 Mediates Autophagic Activation to Promote Cell Survival in NPM1-Mutated Leukemia. Int. J. Biol. Sci. 2019, 15, 882–894. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, L.-M.; Xie, J.-Y.; Han, H.; Zhu, B.-F.; Wang, L.-J.; Wang, W.-J. High Expression of PKM2 Was Associated with the Poor Prognosis of Acute Leukemia. Cancer Manag. Res. 2021, 13, 7851–7858. [Google Scholar] [CrossRef]
- Xia, L.; Jiang, Y.; Zhang, X.-H.; Wang, X.-R.; Wei, R.; Qin, K.; Lu, Y. SUMOylation Disassembles the Tetrameric Pyruvate Kinase M2 to Block Myeloid Differentiation of Leukemia Cells. Cell Death Dis. 2021, 12, 101. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Locasale, J.W.; Swanson, K.D.; Sharfi, H.; Heffron, G.J.; Amador-Noguez, D.; Christofk, H.R.; Wagner, G.; Rabinowitz, J.D.; Asara, J.M.; et al. Evidence for an Alternative Glycolytic Pathway in Rapidly Proliferating Cells. Science 2010, 329, 1492–1499. [Google Scholar] [CrossRef]
- Wong, C.C.-L.; Au, S.L.-K.; Tse, A.P.-W.; Xu, I.M.-J.; Lai, R.K.-H.; Chiu, D.K.-C.; Wei, L.L.; Fan, D.N.-Y.; Tsang, F.H.-C.; Lo, R.C.-L.; et al. Switching of Pyruvate Kinase Isoform L to M2 Promotes Metabolic Reprogramming in Hepatocarcinogenesis. PLoS ONE 2014, 9, e115036. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, D.; Poulogiannis, G.; Asara, J.M.; Boxer, M.B.; Jiang, J.-K.; Shen, M.; Bellinger, G.; Sasaki, A.T.; Locasale, J.W.; Auld, D.S.; et al. Inhibition of Pyruvate Kinase M2 by Reactive Oxygen Species Contributes to Cellular Antioxidant Responses. Science 2011, 334, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Augoff, K.; Hryniewicz-Jankowska, A.; Tabola, R. Lactate Dehydrogenase 5: An Old Friend and a New Hope in the War on Cancer. Cancer Lett. 2015, 358, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Valvona, C.J.; Fillmore, H.L.; Nunn, P.B.; Pilkington, G.J. The Regulation and Function of Lactate Dehydrogenase A: Therapeutic Potential in Brain Tumor: Regulation and Function of Lactate Dehydrogenase A. Brain Pathol. 2016, 26, 3–17. [Google Scholar] [CrossRef]
- Shim, H.; Dolde, C.; Lewis, B.C.; Wu, C.S.; Dang, G.; Jungmann, R.A.; Dalla-Favera, R.; Dang, C.V. C-Myc Transactivation of LDH-A: Implications for Tumor Metabolism and Growth. Proc. Natl. Acad. Sci. USA 1997, 94, 6658–6663. [Google Scholar] [CrossRef]
- Gallo, M.; Sapio, L.; Spina, A.; Naviglio, D.; Calogero, A.; Naviglio, S. Lactic Dehydrogenase and Cancer an Overview. Front. Biosci. 2015, 20, 1234–1249. [Google Scholar] [CrossRef]
- Dhup, S.; Dadhich, R.K.; Porporato, P.E.; Sonveaux, P. Multiple Biological Activities of Lactic Acid in Cancer: Influences on Tumor Growth, Angiogenesis and Metastasis. Curr. Pharm. Des. 2012, 18, 1319–1330. [Google Scholar] [CrossRef]
- Yao, F.; Zhao, T.; Zhong, C.; Zhu, J.; Zhao, H. LDHA Is Necessary for the Tumorigenicity of Esophageal Squamous Cell Carcinoma. Tumour Biol. 2013, 34, 25–31. [Google Scholar] [CrossRef]
- Sheng, S.L.; Liu, J.J.; Dai, Y.H.; Sun, X.G.; Xiong, X.P.; Huang, G. Knockdown of Lactate Dehydrogenase A Suppresses Tumor Growth and Metastasis of Human Hepatocellular Carcinoma. FEBS J. 2012, 279, 3898–3910. [Google Scholar] [CrossRef]
- Feron, O. Pyruvate into Lactate and Back: From the Warburg Effect to Symbiotic Energy Fuel Exchange in Cancer Cells. Radiother. Oncol. 2009, 92, 329–333. [Google Scholar] [CrossRef]
- He, T.L.; Zhang, Y.J.; Jiang, H.; Li, X.H.; Zhu, H.; Zheng, K.L. The c-Myc-LDHA axis positively regulates aerobic glycolysis and promotes tumor progression in pancreatic cancer. Med. Oncol. 2015, 32, 187. [Google Scholar] [CrossRef]
- Jin, X.; Kuang, Y.; Li, L.; Li, H.; Zhao, T.; He, Y.; Di, C.; Kang, J.; Yuan, L.; Yu, B.; et al. A Positive Feedback Circuit Comprising P21 and HIF-1α Aggravates Hypoxia-Induced Radioresistance of Glioblastoma by Promoting Glut1/LDHA-Mediated Glycolysis. FASEB J. 2022, 36, e22229. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, A.S.; Appling, D.R. Compartmentalization of Mammalian Folate-Mediated One-Carbon Metabolism. Annu. Rev. Nutr. 2010, 30, 57–81. [Google Scholar] [CrossRef] [PubMed]
- Locasale, J.W. Serine, Glycine and One-Carbon Units: Cancer Metabolism in Full Circle. Nat. Rev. Cancer 2013, 13, 572–583. [Google Scholar] [CrossRef]
- Possemato, R.; Marks, K.M.; Shaul, Y.D.; Pacold, M.E.; Kim, D.; Birsoy, K.; Sethumadhavan, S.; Woo, H.-K.; Jang, H.G.; Jha, A.K.; et al. Functional Genomics Reveal That the Serine Synthesis Pathway Is Essential in Breast Cancer. Nature 2011, 476, 346–350. [Google Scholar] [CrossRef]
- Locasale, J.W.; Grassian, A.; Beroukhim, R.; Meyerson, M.; Wagner, G.; Asara, J.M.; Brugge, J.S.; Vander Heiden, M.G.; Cantley, L.C. Amplification of Phosphoglycerate Dehydrogenase Diverts Glycolytic Flux and Contributes to Oncogenesis. BMC Proc. 2012, 6, O15. [Google Scholar] [CrossRef]
- Labuschagne, C.F.; van den Broek, N.J.F.; Mackay, G.M.; Vousden, K.H.; Maddocks, O.D.K. Serine, but Not Glycine, Supports One-Carbon Metabolism and Proliferation of Cancer Cells. Cell Rep. 2014, 7, 1248–1258. [Google Scholar] [CrossRef]
- Rose, M.L.; Cattley, R.C.; Dunn, C.; Wong, V.; Li, X.; Thurman, R.G. Dietary Glycine Prevents the Development of Liver Tumors Caused by the Peroxisome Proliferator WY-14,643. Carcinogenesis 1999, 20, 2075–2081. [Google Scholar] [CrossRef]
- Pacold, M.E.; Brimacombe, K.R.; Chan, S.H.; Rohde, J.M.; Lewis, C.A.; Swier, L.J.Y.M.; Possemato, R.; Chen, W.W.; Sullivan, L.B.; Fiske, B.P.; et al. A PHGDH Inhibitor Reveals Coordination of Serine Synthesis and One-Carbon Unit Fate. Nat. Chem. Biol. 2016, 12, 452–458. [Google Scholar] [CrossRef]
- Paone, A.; Marani, M.; Fiascarelli, A.; Rinaldo, S.; Giardina, G.; Contestabile, R.; Paiardini, A.; Cutruzzolà, F. SHMT1 Knockdown Induces Apoptosis in Lung Cancer Cells by Causing Uracil Misincorporation. Cell Death Dis. 2014, 5, e1525. [Google Scholar] [CrossRef]
- Fan, J.; Ye, J.; Kamphorst, J.J.; Shlomi, T.; Thompson, C.B.; Rabinowitz, J.D. Quantitative Flux Analysis Reveals Folate-Dependent NADPH Production. Nature 2014, 510, 298–302. [Google Scholar] [CrossRef]
- Xiu, Y.; Field, M.S. The Roles of Mitochondrial Folate Metabolism in Supporting Mitochondrial DNA Synthesis, Oxidative Phosphorylation, and Cellular Function. Curr. Dev. Nutr. 2020, 4, nzaa153. [Google Scholar] [CrossRef]
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of Glutathione in Cancer Progression and Chemoresistance. Oxid. Med. Cell. Longev. 2013, 2013, 972913. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Zhang, J.; Wu, X.; Du, W.; Zhu, Y.; Liu, X.; Liu, Z.; Meng, B.; Guo, J.; Yang, Q.; et al. Blocking Glycine Utilization Inhibits Multiple Myeloma Progression by Disrupting Glutathione Balance. Nat. Commun. 2022, 13, 4007. [Google Scholar] [CrossRef]
- Yu, X.; Li, S. Non-Metabolic Functions of Glycolytic Enzymes in Tumorigenesis. Oncogene 2017, 36, 2629–2636. [Google Scholar] [CrossRef]
- Sun, X.; Peng, Y.; Zhao, J.; Xie, Z.; Lei, X.; Tang, G. Discovery and development of tumor glycolysis rate-limiting enzyme inhibitors. Bioorg. Chem. 2021, 112, 104891. [Google Scholar] [CrossRef]
- Kassahn, D.; Kolb, C.; Solomon, S.; Bochtler, P.; Illges, H. Few Human Autoimmune Sera Detect GPI. Nat. Immunol. 2002, 3, 411–412. [Google Scholar] [CrossRef]
- Wu, S.-T.; Liu, B.; Ai, Z.-Z.; Hong, Z.-C.; You, P.-T.; Wu, H.-Z.; Yang, Y.-F. Esculetin Inhibits Cancer Cell Glycolysis by Binding Tumor PGK2, GPD2, and GPI. Front. Pharmacol. 2020, 11, 379. [Google Scholar] [CrossRef]
- Van Veen, M.; Matas-Rico, E.; van de Wetering, K.; Leyton-Puig, D.; Kedziora, K.M.; De Lorenzi, V.; Stijf-Bultsma, Y.; van den Broek, B.; Jalink, K.; Sidenius, N.; et al. Negative Regulation of Urokinase Receptor Activity by a GPI-Specific Phospholipase C in Breast Cancer Cells. Elife 2017, 6, e23649. [Google Scholar] [CrossRef]
- Gallardo-Pérez, J.C.; Adán-Ladrón de Guevara, A.; Marín-Hernández, A.; Moreno-Sánchez, R.; Rodríguez-Enríquez, S. HPI/AMF Inhibition Halts the Development of the Aggressive Phenotype of Breast Cancer Stem Cells. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1679–1690. [Google Scholar] [CrossRef]
- Das, M.R.; Bag, A.K.; Saha, S.; Ghosh, A.; Dey, S.K.; Das, P.; Mandal, C.; Ray, S.; Chakrabarti, S.; Ray, M.; et al. Molecular Association of Glucose-6-Phosphate Isomerase and Pyruvate Kinase M2 with Glyceraldehyde-3-Phosphate Dehydrogenase in Cancer Cells. BMC Cancer 2016, 16, 152. [Google Scholar] [CrossRef]
- Feitelson, M.A.; Arzumanyan, A.; Kulathinal, R.J.; Blain, S.W.; Holcombe, R.F.; Mahajna, J.; Marino, M.; Martinez-Chantar, M.L.; Nawroth, R.; Sanchez-Garcia, I.; et al. Sustained proliferation in cancer: Mechanisms and novel therapeutic targets. Semin. Cancer Biol. 2015, 35, S25–S54. [Google Scholar]
- Wu, H.; Ding, Z.; Hu, D.; Sun, F.; Dai, C.; Xie, J.; Hu, X. Central role of lactic acidosis in cancer cell resistance to glucose deprivation-induced cell death. J. Pathol. 2012, 227, 189–199. [Google Scholar] [CrossRef]
- Joly, J.H.; Delfarah, A.; Phung, P.S.; Parrish, S.; Graham, N.A. A synthetic lethal drug combination mimics glucose deprivation-induced cancer cell death in the presence of glucose. J. Biol. Chem. 2020, 295, 1350–1365. [Google Scholar] [CrossRef]
- Graham, N.A.; Tahmasian, M.; Kohli, B.; Komisopoulou, E.; Zhu, M.; Vivanco, I.; Teitell, M.A.; Wu, H.; Ribas, A.; Lo, R.S.; et al. Glucose deprivation activates a metabolic and signaling amplification loop leading to cell death. Mol. Syst. Biol. 2012, 8, 589. [Google Scholar] [CrossRef]
- Mao, D.; Lau, E.S.H.; Wu, H.; Yang, A.; Shi, M.; Fan, B.; Tam, C.H.T.; Chow, E.; Kong, A.P.S.; Ma, R.C.W.; et al. Risk Associations of Long-Term HbA1c Variability and Obesity on Cancer Events and Cancer-Specific Death in 15,286 Patients with Diabetes—A Prospective Cohort Study. Lancet Reg. Health West. Pac. 2022, 18, 100315. [Google Scholar] [CrossRef]
- Lambe, M.; Wigertz, A.; Garmo, H.; Walldius, G.; Jungner, I.; Hammar, N. Impaired Glucose Metabolism and Diabetes and the Risk of Breast, Endometrial, and Ovarian Cancer. Cancer Causes Control. 2011, 22, 1163–1171. [Google Scholar] [CrossRef]
- Pandey, A.; Forte, V.; Abdallah, M.; Alickaj, A.; Mahmud, S.; Asad, S.; McFarlane, S.I. Diabetes Mellitus and the Risk of Cancer. Minerva Endocrinol. 2011, 36, 187–209. [Google Scholar]
- Zakaraia, S.; Takkem, A.; Redwan, H.; Barakat, C. Efficacy of Glucose Starvation of Cancer Cells in the Progress of Oral Squamous Cell Carcinoma Induced in Hamster. Asian Pac. J. Cancer Prev. 2022, 23, 2857–2862. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.D.; Gui, Q.; Wang, X.D.; Zhu, Y.X. Glucagon-Induced Angiogenesis and Tumor Growth through the HIF-1-VEGF-Dependent Pathway in Hyperglycemic Nude Mice. Genet. Mol. Res. 2014, 13, 7173–7183. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Cai, Y.; Peng, S.; Wang, J.; Xiao, Z.; Wang, Y.; Tao, Y.; Li, J.; Leng, Q.; et al. Hyperglycaemia-Induced MiR-301a Promotes Cell Proliferation by Repressing P21 and Smad4 in Prostate Cancer. Cancer Lett. 2018, 418, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Lopez, R.; Arumugam, A.; Joseph, R.; Monga, K.; Boopalan, T.; Agullo, P.; Gutierrez, C.; Nandy, S.; Subramani, R.; de la Rosa, J.M.; et al. Hyperglycemia Enhances the Proliferation of Non-Tumorigenic and Malignant Mammary Epithelial Cells through Increased Leptin/IGF1R Signaling and Activation of AKT/MTOR. PLoS ONE 2013, 8, e79708. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhu, H.; Chen, H.; Xia, J.; Zhang, F.; Xu, R.; Lin, Q. Glucose Promotes Epithelial-Mesenchymal Transitions in Bladder Cancer by Regulating the Functions of YAP1 and TAZ. J. Cell. Mol. Med. 2020, 24, 10391–10401. [Google Scholar] [CrossRef]
- Santos, J.M.; Hussain, F. Higher Glucose Enhances Breast Cancer Cell Aggressiveness. Nutr. Cancer 2020, 72, 734–746. [Google Scholar] [CrossRef]
- Sun, L.; Yin, Y.; Clark, L.H.; Sun, W.; Sullivan, S.A.; Tran, A.-Q.; Han, J.; Zhang, L.; Guo, H.; Madugu, E.; et al. Dual Inhibition of Glycolysis and Glutaminolysis as a Therapeutic Strategy in the Treatment of Ovarian Cancer. Oncotarget 2017, 8, 63551–63561. [Google Scholar] [CrossRef]
- Han, J.; Zhang, L.; Guo, H.; Wysham, W.Z.; Roque, D.R.; Willson, A.K.; Sheng, X.; Zhou, C.; Bae-Jump, V.L. Glucose Promotes Cell Proliferation, Glucose Uptake and Invasion in Endometrial Cancer Cells via AMPK/MTOR/S6 and MAPK Signaling. Gynecol. Oncol. 2015, 138, 668–675. [Google Scholar] [CrossRef]
- Sun, S.; Sun, Y.; Rong, X.; Bai, L. High Glucose Promotes Breast Cancer Proliferation and Metastasis by Impairing Angiotensinogen Expression. Biosci. Rep. 2019, 39, BSR20190436. [Google Scholar] [CrossRef]
- Fischer, O.M.; Hart, S.; Gschwind, A.; Ullrich, A. EGFR Signal Transactivation in Cancer Cells. Biochem. Soc. Trans. 2003, 31 Pt 6, 1203–1208. [Google Scholar] [CrossRef]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging Functions of the EGFR in Cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef]
- Hall, A. Rho Family GTPases. Biochem. Soc. Trans. 2012, 40, 1378–1382. [Google Scholar] [CrossRef]
- Hou, Y.; Zhou, M.; Xie, J.; Chao, P.; Feng, Q.; Wu, J. High Glucose Levels Promote the Proliferation of Breast Cancer Cells through GTPases. Breast Cancer 2017, 9, 429–436. [Google Scholar] [CrossRef]
- Yang, J.; Virostko, J.; Hormuth, D.A., 2nd; Liu, J.; Brock, A.; Kowalski, J.; Yankeelov, T.E. An Experimental-Mathematical Approach to Predict Tumor Cell Growth as a Function of Glucose Availability in Breast Cancer Cell Lines. PLoS ONE 2021, 16, e0240765. [Google Scholar] [CrossRef]
- Zhao, M.; Xia, L.; Chen, G.-Q. Protein Kinase Cδ in Apoptosis: A Brief Overview. Arch. Immunol. Ther. Exp. 2012, 60, 361–372. [Google Scholar] [CrossRef]
- Mason, E.F.; Zhao, Y.; Goraksha-Hicks, P.; Coloff, J.L.; Gannon, H.; Jones, S.N.; Rathmell, J.C. Aerobic Glycolysis Suppresses P53 Activity to Provide Selective Protection from Apoptosis upon Loss of Growth Signals or Inhibition of BCR-Abl. Cancer Res. 2010, 70, 8066–8076. [Google Scholar] [CrossRef]
- Fernald, K.; Kurokawa, M. Evading apoptosis in cancer. Trends Cell Biol. 2013, 23, 620–633. [Google Scholar] [CrossRef]
- Moley, K.H.; Mueckler, M.M. Glucose transport and apoptosis. Apoptosis 2000, 5, 99–105. [Google Scholar] [CrossRef]
- Andersen, J.L.; Kornbluth, S. The tangled circuitry of metabolism and apoptosis. Mol. Cell 2013, 49, 399–410. [Google Scholar] [CrossRef]
- Elstrom, R.L.; Bauer, D.E.; Buzzai, M.; Karnauskas, R.; Harris, M.H.; Plas, D.R.; Zhuang, H.; Cinalli, R.M.; Alavi, A.; Rudin, C.M.; et al. Akt Stimulates Aerobic Glycolysis in Cancer Cells. Cancer Res. 2004, 64, 3892–3899. [Google Scholar] [CrossRef]
- Barthel, A.; Okino, S.T.; Liao, J.; Nakatani, K.; Li, J.; Whitlock, J.P., Jr.; Roth, R.A. Regulation of GLUT1 Gene Transcription by the Serine/Threonine Kinase Akt1. J. Biol. Chem. 1999, 274, 20281–20286. [Google Scholar] [CrossRef]
- Datta, S.R.; Brunet, A.; Greenberg, M.E. Cellular Survival: A Play in Three Akts. Genes Dev. 1999, 13, 2905–2927. [Google Scholar] [CrossRef]
- Kennedy, S.G.; Kandel, E.S.; Cross, T.K.; Hay, N. Akt/Protein Kinase B Inhibits Cell Death by Preventing the Release of Cytochrome c from Mitochondria. Mol. Cell. Biol. 1999, 19, 5800–5810. [Google Scholar] [CrossRef]
- Gottlob, K.; Majewski, N.; Kennedy, S.; Kandel, E.; Robey, R.B.; Hay, N. Inhibition of Early Apoptotic Events by Akt/PKB Is Dependent on the First Committed Step of Glycolysis and Mitochondrial Hexokinase. Genes Dev. 2001, 15, 1406–1418. [Google Scholar] [CrossRef]
- Rathmell, J.C.; Fox, C.J.; Plas, D.R.; Hammerman, P.S.; Cinalli, R.M.; Thompson, C.B. Akt-Directed Glucose Metabolism Can Prevent Bax Conformation Change and Promote Growth Factor-Independent Survival. Mol. Cell. Biol. 2003, 23, 7315–7328. [Google Scholar] [CrossRef]
- Birnbaum, M.J. On the InterAktion between Hexokinase and the Mitochondrion. Dev. Cell 2004, 7, 781–782. [Google Scholar] [CrossRef]
- Haloi, N.; Wen, P.-C.; Cheng, Q.; Yang, M.; Natarajan, G.; Camara, A.K.S.; Kwok, W.-M.; Tajkhorshid, E. Structural Basis of Complex Formation between Mitochondrial Anion Channel VDAC1 and Hexokinase-II. Commun. Biol. 2021, 4, 667. [Google Scholar] [CrossRef]
- Majewski, N.; Nogueira, V.; Bhaskar, P.; Coy, P.E.; Skeen, J.E.; Gottlob, K.; Chandel, N.S.; Thompson, C.B.; Robey, R.B.; Hay, N. Hexokinase-Mitochondria Interaction Mediated by Akt Is Required to Inhibit Apoptosis in the Presence or Absence of Bax and Bak. Mol. Cell 2004, 16, 819–830. [Google Scholar] [CrossRef]
- Chiara, F.; Castellaro, D.; Marin, O.; Petronilli, V.; Brusilow, W.S.; Juhaszova, M.; Sollott, S.J.; Forte, M.; Bernardi, P.; Rasola, A. Hexokinase II Detachment from Mitochondria Triggers Apoptosis through the Permeability Transition Pore Independent of Voltage-Dependent Anion Channels. PLoS ONE 2008, 3, e1852. [Google Scholar] [CrossRef]
- Shulga, N.; Wilson-Smith, R.; Pastorino, J.G. Hexokinase II Detachment from the Mitochondria Potentiates Cisplatin Induced Cytotoxicity through a Caspase-2 Dependent Mechanism. Cell Cycle 2009, 8, 3355–3364. [Google Scholar] [CrossRef]
- Atlante, A.; Bobba, A.; de Bari, L.; Fontana, F.; Calissano, P.; Marra, E.; Passarella, S. Caspase-Dependent Alteration of the ADP/ATP Translocator Triggers the Mitochondrial Permeability Transition Which Is Not Required for the Low-Potassium-Dependent Apoptosis of Cerebellar Granule Cells. J. Neurochem. 2006, 97, 1166–1181. [Google Scholar] [CrossRef]
- Ferrari, D.; Stepczynska, A.; Los, M.; Wesselborg, S.; Schulze-Osthoff, K. Differential Regulation and ATP Requirement for Caspase-8 and Caspase-3 Activation during CD95- and Anticancer Drug-Induced Apoptosis. J. Exp. Med. 1998, 188, 979–984. [Google Scholar] [CrossRef]
- Pradelli, L.A.; Villa, E.; Zunino, B.; Marchetti, S.; Ricci, J.-E. Glucose Metabolism Is Inhibited by Caspases upon the Induction of Apoptosis. Cell Death Dis. 2014, 5, e1406. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Coloff, J.L.; Ferguson, E.C.; Jacobs, S.R.; Cui, K.; Rathmell, J.C. Glucose Metabolism Attenuates P53 and Puma-Dependent Cell Death upon Growth Factor Deprivation. J. Biol. Chem. 2008, 283, 36344–36353. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Bonni, A.; Zigmond, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. Akt Promotes Cell Survival by Phosphorylating and Inhibiting a Forkhead Transcription Factor. Cell 1999, 96, 857–868. [Google Scholar] [CrossRef]
- Datta, S.R.; Dudek, H.; Tao, X.; Masters, S.; Fu, H.; Gotoh, Y.; Greenberg, M.E. Akt Phosphorylation of BAD Couples Survival Signals to the Cell-Intrinsic Death Machinery. Cell 1997, 91, 231–241. [Google Scholar] [CrossRef]
- Wu, X.; Luo, Q.; Liu, Z. Ubiquitination and Deubiquitination of MCL1 in Cancer: Deciphering Chemoresistance Mechanisms and Providing Potential Therapeutic Options. Cell Death Dis. 2020, 11, 556. [Google Scholar] [CrossRef]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting MCL-1 in Cancer: Current Status and Perspectives. J. Hematol. Oncol. 2021, 14, 67. [Google Scholar] [CrossRef]
- Liang, Y.-Y.; Niu, F.-Y.; Xu, A.-A.; Jiang, L.-L.; Liu, C.-S.; Liang, H.-P.; Huang, Y.-F.; Shao, X.-F.; Mo, Z.-W.; Yuan, Y.-W. Increased MCL-1 Synthesis Promotes Irradiation-Induced Nasopharyngeal Carcinoma Radioresistance via Regulation of the ROS/AKT Loop. Cell Death Dis. 2022, 13, 131. [Google Scholar] [CrossRef] [PubMed]
- Yecies, D.; Carlson, N.E.; Deng, J.; Letai, A. Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood. J. Am. Soc. Hematol. 2010, 115, 3304–3313. [Google Scholar]
- Alves, N.L.; Derks, I.A.M.; Berk, E.; Spijker, R.; van Lier, R.A.W.; Eldering, E. The Noxa/Mcl-1 Axis Regulates Susceptibility to Apoptosis under Glucose Limitation in Dividing T Cells. Immunity 2006, 24, 703–716. [Google Scholar] [CrossRef]
- Ariës, I.M.; Hansen, B.R.; Koch, T.; van den Dungen, R.; Evans, W.E.; Pieters, R.; den Boer, M.L. The Synergism of MCL1 and Glycolysis on Pediatric Acute Lymphoblastic Leukemia Cell Survival and Prednisolone Resistance. Haematologica 2013, 98, 1905–1911. [Google Scholar] [CrossRef] [PubMed]
- Prew, M.S.; Adhikary, U.; Choi, D.W.; Portero, E.P.; Paulo, J.A.; Gowda, P.; Budhraja, A.; Opferman, J.T.; Gygi, S.P.; Danial, N.N.; et al. MCL-1 Is a Master Regulator of Cancer Dependency on Fatty Acid Oxidation. Cell Rep. 2022, 41, 111445. [Google Scholar] [CrossRef] [PubMed]
- Coloff, J.L.; Macintyre, A.; Nichols, A.G.; Liu, T.; Gallo, C.A.; Plas, D.R.; Rathmell, J.C. Akt-Dependent Glucose Metabolism Promotes Mcl-1 Synthesis to Maintain Cell Survival and Resistance to Bcl-2 Inhibition. Cancer Res. 2011, 71, 5204–5213. [Google Scholar] [CrossRef] [PubMed]
- Wright, N.; Gong, Z.; Kittles, R.; Natarajan, R.; Jovanovic-Talisman, T.; Rida, P.; LaBarge, M.; Seewaldt, V. Kinesin Family Member C1 (KIFC1/HSET): A Potential Actionable Biomarker of Early Stage Breast Tumorigenesis and Progression of High-Risk Lesions. J. Pers. Med. 2021, 11, 1361. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Lin, J.; Dai, M.; He, Y.; Xu, J.; Lin, Q. KIFC1 Promotes Aerobic Glycolysis in Endometrial Cancer Cells by Regulating the C-Myc Pathway. J. Bioenerg. Biomembr. 2021, 53, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Canfield, K.; Feng, W.; Kurokawa, M. Metabolic regulation of apoptosis in cancer. Int. Rev. Cell Mol. Biol. 2016, 327, 43–87. [Google Scholar] [PubMed]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- García-Caballero, M.; Sokol, L.; Cuypers, A.; Carmeliet, P. Metabolic Reprogramming in Tumor Endothelial Cells. Int. J. Mol. Sci. 2022, 23, 11052. [Google Scholar] [CrossRef]
- Talks, K.L.; Turley, H.; Gatter, K.C.; Maxwell, P.H.; Pugh, C.W.; Ratcliffe, P.J.; Harris, A.L. The Expression and Distribution of the Hypoxia-Inducible Factors HIF-1α and HIF-2α in Normal Human Tissues, Cancers, and Tumor-Associated Macrophages. Am. J. Pathol. 2000, 157, 411–421. [Google Scholar] [CrossRef]
- Bos, R.; Zhong, H.; Hanrahan, C.F.; Mommers, E.C.; Semenza, G.L.; Pinedo, H.M.; Abeloff, M.D.; Simons, J.W.; van Diest, P.J.; van der Wall, E. Levels of hypoxia-inducible factor-1 alpha during breast carcinogenesis. J. Natl. Cancer Inst. 2001, 93, 309–314. [Google Scholar] [CrossRef]
- Ravi, R.; Mookerjee, B.; Bhujwalla, Z.M.; Sutter, C.H.; Artemov, D.; Zeng, Q.; Dillehay, L.E.; Madan, A.; Semenza, G.L.; Bedi, A. Regulation of Tumor Angiogenesis by P53-Induced Degradation of Hypoxia-Inducible Factor 1α. Genes Dev. 2000, 14, 34–44. [Google Scholar] [CrossRef]
- Dhanasekaran, R.; Deutzmann, A.; Mahauad-Fernandez, W.D.; Hansen, A.S.; Gouw, A.M.; Felsher, D.W. The MYC oncogene—The grand orchestrator of cancer growth and immune evasion. Nat. Rev. Clin. Oncol. 2022, 19, 23–36. [Google Scholar] [CrossRef]
- Kim, J.W.; Gao, P.; Liu, Y.C.; Semenza, G.L.; Dang, C.V. Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively induce vascular endothelial growth factor and metabolic switches hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol. Cell. Biol. 2007, 27, 7381–7393. [Google Scholar] [CrossRef]
- Yasuda, S.; Arii, S.; Mori, A.; Isobe, N.; Yang, W.; Oe, H.; Fujimoto, A.; Yonenaga, Y.; Sakashita, H.; Imamura, M. Hexokinase II and VEGF Expression in Liver Tumors: Correlation with Hypoxia-Inducible Factor-1α and Its Significance. J. Hepatol. 2004, 40, 117–123. [Google Scholar] [CrossRef]
- Giatromanolaki, A.I.; Koukourakis, M.; Sivridis, E.; Turley, H.; Talks, K.; Pezzella, F.; Gatter, K.C.; Harris, A.L. Relation of hypoxia inducible factor 1α and 2α in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br. J. Cancer 2001, 85, 881–890. [Google Scholar] [CrossRef]
- Karth, J.; Ferrer, F.A.; Perlman, E.; Hanrahan, C.; Simons, J.W.; Gearhart, J.P.; Rodriguez, R. Coexpression of Hypoxia-Inducible Factor 1-Alpha and Vascular Endothelial Growth Factor in Wilms’ Tumor. J. Pediatr. Surg. 2000, 35, 1749–1753. [Google Scholar] [CrossRef]
- Ryan, H.E.; Lo, J.; Johnson, R.S. HIF-1α is required for solid tumor formation and embryonic vascularization. EMBO J. 1998, 17, 3005–3015. [Google Scholar] [CrossRef]
- 149 Lu, C.; Qiao, P.; Sun, Y.; Ren, C.; Yu, Z. Positive regulation of PFKFB3 by PIM2 promotes glycolysis and paclitaxel resistance in breast cancer. Clin. Transl. Med. 2021, 11, e400. [Google Scholar]
- Schoors, S.; De Bock, K.; Cantelmo, A.R.; Georgiadou, M.; Ghesquière, B.; Cauwenberghs, S.; Kuchnio, A.; Wong, B.W.; Quaegebeur, A.; Goveia, J.; et al. Partial and Transient Reduction of Glycolysis by PFKFB3 Blockade Reduces Pathological Angiogenesis. Cell Metab. 2014, 19, 37–48. [Google Scholar] [CrossRef]
- Xu, Y.; An, X.; Guo, X.; Habtetsion, T.G.; Wang, Y.; Xu, X.; Kandala, S.; Li, Q.; Li, H.; Zhang, C.; et al. Endothelial PFKFB3 Plays a Critical Role in Angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1231–1239. [Google Scholar] [CrossRef]
- Kim, S.; Yazici, Y.D.; Calzada, G.; Wang, Z.-Y.; Younes, M.N.; Jasser, S.A.; El-Naggar, A.K.; Myers, J.N. Sorafenib Inhibits the Angiogenesis and Growth of Orthotopic Anaplastic Thyroid Carcinoma Xenografts in Nude Mice. Mol. Cancer Ther. 2007, 6, 1785–1792. [Google Scholar] [CrossRef]
- Yoo, J.-J.; Yu, S.J.; Na, J.; Kim, K.; Cho, Y.Y.; Lee, Y.B.; Cho, E.J.; Lee, J.-H.; Kim, Y.J.; Youn, H.; et al. Hexokinase-II Inhibition Synergistically Augments the Anti-Tumor Efficacy of Sorafenib in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2019, 20, 1292. [Google Scholar] [CrossRef]
- Bergers, G.; Song, S. The Role of Pericytes in Blood-Vessel Formation and Maintenance. Neuro. Oncol. 2005, 7, 452–464. [Google Scholar] [CrossRef]
- Sun, R.; Kong, X.; Qiu, X.; Huang, C.; Wong, P.-P. The Emerging Roles of Pericytes in Modulating Tumor Microenvironment. Front. Cell Dev. Biol. 2021, 9, 676342. [Google Scholar] [CrossRef]
- Meng, Y.-M.; Jiang, X.; Zhao, X.; Meng, Q.; Wu, S.; Chen, Y.; Kong, X.; Qiu, X.; Su, L.; Huang, C.; et al. Hexokinase 2-Driven Glycolysis in Pericytes Activates Their Contractility Leading to Tumor Blood Vessel Abnormalities. Nat. Commun. 2021, 12, 6011. [Google Scholar] [CrossRef]
- Lu, J.; Liang, X.; Gao, Y.; Fu, G.; Shen, Q. Hexokinase2 Controls Angiogenesis in Melanoma by Promoting Aerobic Glycolysis and Activating the P38-MAPK Signaling. J. Cell. Biochem. 2019, 120, 19721–19729. [Google Scholar] [CrossRef]
- Watanabe, M.M.; Laurindo, F.R.M.; Fernandes, D.C. Methods of Measuring Protein Disulfide Isomerase Activity: A Critical Overview. Front. Chem. 2014, 2, 73. [Google Scholar] [CrossRef]
- Powell, L.E.; Foster, P.A. Protein Disulphide Isomerase Inhibition as a Potential Cancer Therapeutic Strategy. Cancer Med. 2021, 10, 2812–2825. [Google Scholar] [CrossRef]
- Yu, S.J.; Yoon, J.-H.; Yang, J.-I.; Cho, E.J.; Kwak, M.S.; Jang, E.S.; Lee, J.-H.; Kim, Y.J.; Lee, H.-S.; Kim, C.Y. Enhancement of Hexokinase II Inhibitor-Induced Apoptosis in Hepatocellular Carcinoma Cells via Augmenting ER Stress and Anti-Angiogenesis by Protein Disulfide Isomerase Inhibition. J. Bioenerg. Biomembr. 2012, 44, 101–115. [Google Scholar] [CrossRef]
- De la Cruz-López, K.G.; Castro-Muñoz, L.J.; Reyes-Hernández, D.O.; García-Carrancá, A.; Manzo-Merino, J. Lactate in the Regulation of Tumor Microenvironment and Therapeutic Approaches. Front. Oncol. 2019, 9, 1143. [Google Scholar] [CrossRef]
- Sonveaux, P.; Vegran, F.; Schroeder, T.; Wergin, M.; Verrax, J.; Rabbani, Z.N.; De Saedeleer, C.J.; Kennedy, K.M.; Diepart, C.; Jordan, B.F.; et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Investig. 2008, 118, 3930–3942. [Google Scholar] [CrossRef]
- Végran, F.; Boidot, R.; Michiels, C.; Sonveaux, P.; Feron, O. Lactate Influx through the Endothelial Cell Monocarboxylate Transporter MCT1 Supports an NF-ΚB/IL-8 Pathway That Drives Tumor Angiogenesis. Cancer Res. 2011, 71, 2550–2560. [Google Scholar] [CrossRef] [PubMed]
- Arundhathi, J.R.D.; Mathur, S.R.; Gogia, A.; Deo, S.V.S.; Mohapatra, P.; Prasad, C.P. Metabolic changes in triple negative breast cancer-focus on aerobic glycolysis. Mol Biol Rep. 2021, 48, 4733–4745. [Google Scholar] [CrossRef]
- Young, C.D.; Anderson, S.M. Sugar and fat—That’s where it’s at: Metabolic changes in tumors. Breast Cancer Res. 2008, 10, 202. [Google Scholar] [CrossRef]
- Zhong, H.; De Marzo, A.M.; Laughner, E.; Lim, M.; Hilton, D.A.; Zagzag, D.; Buechler, P.; Isaacs, W.B.; Semenza, G.L.; Simons, J.W. Overexpression of Hypoxia-Inducible Factor 1α in Common Human Cancers and Their Metastases. Cancer Res. 1999, 59, 5830–5835. [Google Scholar] [PubMed]
- Zhang, W.; Shi, X.; Peng, Y.; Wu, M.; Zhang, P.; Xie, R.; Wu, Y.; Yan, Q.; Liu, S.; Wang, J. HIF-1α Promotes Epithelial-Mesenchymal Transition and Metastasis through Direct Regulation of ZEB1 in Colorectal Cancer. PLoS ONE 2015, 10, e0129603. [Google Scholar] [CrossRef]
- Hu, X.; Lin, J.; Jiang, M.; He, X.; Wang, K.; Wang, W.; Hu, C.; Shen, Z.; He, Z.; Lin, H.; et al. HIF-1α Promotes the Metastasis of Esophageal Squamous Cell Carcinoma by Targeting SP1. J. Cancer 2020, 11, 229–240. [Google Scholar] [CrossRef]
- Yang, M.-H.; Wu, M.-Z.; Chiou, S.-H.; Chen, P.-M.; Chang, S.-Y.; Liu, C.-J.; Teng, S.-C.; Wu, K.-J. Direct Regulation of TWIST by HIF-1alpha Promotes Metastasis. Nat. Cell Biol. 2008, 10, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Guo, X.; Chen, M.; Xie, C.; Jiang, J. HIF-3α Promotes Metastatic Phenotypes in Pancreatic Cancer by Transcriptional Regulation of the RhoC-ROCK1 Signaling Pathway. Mol. Cancer Res. 2018, 16, 124–134. [Google Scholar] [CrossRef]
- Zhang, H.; Wong, C.C.L.; Wei, H.; Gilkes, D.M.; Korangath, P.; Chaturvedi, P.; Schito, L.; Chen, J.; Krishnamachary, B.; Winnard, P.T., Jr.; et al. HIF-1-Dependent Expression of Angiopoietin-like 4 and L1CAM Mediates Vascular Metastasis of Hypoxic Breast Cancer Cells to the Lungs. Oncogene 2012, 31, 1757–1770. [Google Scholar] [CrossRef]
- Anderson, M.; Marayati, R.; Moffitt, R.; Yeh, J.J. Hexokinase 2 Promotes Tumor Growth and Metastasis by Regulating Lactate Production in Pancreatic Cancer. Oncotarget 2017, 8, 56081–56094. [Google Scholar] [CrossRef]
- Blaha, C.S.; Ramakrishnan, G.; Jeon, S.-M.; Nogueira, V.; Rho, H.; Kang, S.; Bhaskar, P.; Terry, A.R.; Aissa, A.F.; Frolov, M.V.; et al. A Non-Catalytic Scaffolding Activity of Hexokinase 2 Contributes to EMT and Metastasis. Nat. Commun. 2022, 13, 899. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Capetillo, O.; Celeste, A.; Nussenzweig, A. Focusing on Foci: H2AX and the Recruitment of DNA-Damage Response Factors. Cell Cycle 2003, 2, 426–427. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, H.; Wilson, C.N.; Bai, H.J.; Boufraqech, M.; Weyemi, U. Histone H2AX Promotes Metastatic Progression by Preserving Glycolysis via Hexokinase-2. Sci. Rep. 2022, 12, 3758. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Huang, Q.; Ma, Y.; Wang, L.; Rivera, G.O.; Ouyang, Y.; Whitaker, R.; Gibson, R.A.; Kontos, C.D.; Berchuck, A.; et al. G6PD Inhibition Sensitizes Ovarian Cancer Cells to Oxidative Stress in the Metastatic Omental Microenvironment. Cell Rep. 2022, 39, 111012. [Google Scholar] [CrossRef] [PubMed]
- Ghergurovich, J.M.; Esposito, M.; Chen, Z.; Wang, J.Z.; Bhatt, V.; Lan, T.; White, E.; Kang, Y.; Guo, J.Y.; Rabinowitz, J.D. Glucose-6-Phosphate Dehydrogenase Is Not Essential for K-Ras–Driven Tumor Growth or Metastasis. Cancer Res. 2020, 80, 3820–3829. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Lu, W.; Ghergurovich, J.M.; Guo, L.; Blair, I.A.; Rabinowitz, J.D.; Yang, X. Upregulation of Antioxidant Capacity and Nucleotide Precursor Availability Suffices for Oncogenic Transformation. Cell Metab. 2021, 33, 94–109. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Lu, W.; Li, J.; Yu, S.; Brown, E.J.; Stanger, B.Z.; Rabinowitz, J.D.; Yang, X. G6PD-Mediated Increase in de Novo NADP+ Biosynthesis Promotes Antioxidant Defense and Tumor Metastasis. Sci. Adv. 2022, 8, eabo0404. [Google Scholar] [CrossRef]
- Liu, W.-R.; Tian, M.-X.; Yang, L.-X.; Lin, Y.-L.; Jin, L.; Ding, Z.-B.; Shen, Y.-H.; Peng, Y.-F.; Gao, D.-M.; Zhou, J.; et al. PKM2 Promotes Metastasis by Recruiting Myeloid-Derived Suppressor Cells and Indicates Poor Prognosis for Hepatocellular Carcinoma. Oncotarget 2015, 6, 846–861. [Google Scholar] [CrossRef]
- Jin, L.; Chun, J.; Pan, C.; Alesi, G.N.; Li, D.; Magliocca, K.R.; Kang, Y.; Chen, Z.G.; Shin, D.M.; Khuri, F.R.; et al. Phosphorylation-mediated activation of LDHA promotes cancer cell invasion and tumour metastasis. Oncogene 2017, 36, 3797–3806. [Google Scholar] [CrossRef]
- Ediriweera, M.K.; Cho, S.K. Targeting MiRNAs by Histone Deacetylase Inhibitors (HDACi): Rationalizing Epigenetics-Based Therapies for Breast Cancer. Pharmacol. Ther. 2020, 206, 107437. [Google Scholar] [CrossRef]
- Esteller, M. Epigenetics in Cancer. N. Engl. J. Med. 2008, 358, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.; Warmoes, M.O.; Shen, X.; Locasale, J.W. Epigenetics and cancer metabolism. Cancer Lett. 2015, 356, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, K.; Nazim, U.M.; Kumar, V.; Kang, J.; Kim, J.; Huh, S.-O.; Sadra, A. Reversing the HDAC-Inhibitor Mediated Metabolic Escape in MYCN-Amplified Neuroblastoma. Biomed. Pharmacother. 2022, 150, 113032. [Google Scholar] [CrossRef] [PubMed]
- Lei, I.; Tian, S.; Gao, W.; Liu, L.; Guo, Y.; Tang, P.; Chen, E.; Wang, Z. Acetyl-CoA Production by Specific Metabolites Promotes Cardiac Repair after Myocardial Infarction via Histone Acetylation. Elife 2021, 10, e60311. [Google Scholar] [CrossRef]
- Bradshaw, P.C. Acetyl-CoA Metabolism and Histone Acetylation in the Regulation of Aging and Lifespan. Antioxidants 2021, 10, 572. [Google Scholar] [CrossRef]
- Lee, J.V.; Carrer, A.; Shah, S.; Snyder, N.W.; Wei, S.; Venneti, S.; Worth, A.J.; Yuan, Z.-F.; Lim, H.-W.; Liu, S.; et al. Akt-Dependent Metabolic Reprogramming Regulates Tumor Cell Histone Acetylation. Cell Metab. 2014, 20, 306–319. [Google Scholar] [CrossRef]
- Michealraj, K.A.; Kumar, S.A.; Kim, L.J.Y.; Cavalli, F.M.G.; Przelicki, D.; Wojcik, J.B.; Delaidelli, A.; Bajic, A.; Saulnier, O.; MacLeod, G.; et al. Metabolic Regulation of the Epigenome Drives Lethal Infantile Ependymoma. Cell 2020, 181, 1329–1345. [Google Scholar] [CrossRef]
- Mardis, E.R.; Ding, L.; Dooling, D.J.; Larson, D.E.; McLellan, M.D.; Chen, K.; Koboldt, D.C.; Fulton, R.S.; Delehaunty, K.D.; McGrath, S.D.; et al. Recurring Mutations Found by Sequencing an Acute Myeloid Leukemia Genome. N. Engl. J. Med. 2009, 361, 1058–1066. [Google Scholar] [CrossRef]
- Yan, H.; Parsons, D.W.; Jin, G.; McLendon, R.; Rasheed, B.A.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.J.; et al. IDH1 and IDH2 Mutations in Gliomas. N. Engl. J. Med. 2009, 360, 765–773. [Google Scholar] [CrossRef]
- Xu, W.; Yang, H.; Liu, Y.; Yang, Y.; Wang, P.; Kim, S.-H.; Ito, S.; Yang, C.; Wang, P.; Xiao, M.-T.; et al. Oncometabolite 2-Hydroxyglutarate Is a Competitive Inhibitor of α-Ketoglutarate-Dependent Dioxygenases. Cancer Cell 2011, 19, 17–30. [Google Scholar] [CrossRef]
- Tsukada, Y.-I.; Fang, J.; Erdjument-Bromage, H.; Warren, M.E.; Borchers, C.H.; Tempst, P.; Zhang, Y. Histone Demethylation by a Family of JmjC Domain-Containing Proteins. Nature 2006, 439, 811–816. [Google Scholar] [CrossRef]
- Ascherio, A.; Munger, K.L. Epstein-barr virus infection and multiple sclerosis: A review. J. Neuroimmune Pharmacol. 2010, 5, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sham, J.S.; Ng, M.H.; Tsao, S.W.; Zhang, D.; Lowe, S.W.; Cao, L. LMP1 of Epstein-Barr Virus Induces Proliferation of Primary Mouse Embryonic Fibroblasts and Cooperatively Transforms the Cells with a P16-Insensitive CDK4 Oncogene. J. Virol. 2000, 74, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Hu, Z.-Y.; Dong, X.; Tan, Z.; Li, W.; Tang, M.; Chen, L.; Yang, L.; Tao, Y.; Jiang, Y.; et al. Targeting Epstein-Barr Virus Oncoprotein LMP1-Mediated Glycolysis Sensitizes Nasopharyngeal Carcinoma to Radiation Therapy. Oncogene 2014, 33, 4568–4578. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Hong, L.; Cheng, C.; Li, N.; Zhao, X.; Shi, F.; Liu, J.; Fan, J.; Zhou, J.; Bode, A.M.; et al. DNMT1 Mediates Metabolic Reprogramming Induced by Epstein-Barr Virus Latent Membrane Protein 1 and Reversed by Grifolin in Nasopharyngeal Carcinoma. Cell Death Dis. 2018, 9, 619. [Google Scholar] [CrossRef]
- Dowling, C.M.; Hollinshead, K.E.R.; Di Grande, A.; Pritchard, J.; Zhang, H.; Dillon, E.T.; Haley, K.; Papadopoulos, E.; Mehta, A.K.; Bleach, R.; et al. Multiple Screening Approaches Reveal HDAC6 as a Novel Regulator of Glycolytic Metabolism in Triple-Negative Breast Cancer. Sci. Adv. 2021, 7, eabc4897. [Google Scholar] [CrossRef]
- Bao, F.; Yang, K.; Wu, C.; Gao, S.; Wang, P.; Chen, L.; Li, H. New natural inhibitors of hexokinase 2 (HK2): Steroids from Ganoderma sinense. Fitoterapia 2018, 125, 123–129. [Google Scholar] [CrossRef]
- Kim, W.; Yoon, J.-H.; Jeong, J.-M.; Cheon, G.-J.; Lee, T.-S.; Yang, J.-I.; Park, S.-C.; Lee, H.-S. Apoptosis-Inducing Antitumor Efficacy of Hexokinase II Inhibitor in Hepatocellular Carcinoma. Mol. Cancer Ther. 2007, 6, 2554–2562. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Zhang, P.; Chao, Z.; Xia, F.; Jiang, C.; Zhang, X.; Jiang, Z.; Liu, H. Hexokinase II inhibitor, 3-BrPA induced autophagy by stimulating ROS formation in human breast cancer cells. Genes Cancer 2014, 5, 100. [Google Scholar] [CrossRef]
- Pajak, B.; Siwiak, E.; Sołtyka, M.; Priebe, A.; Zieliński, R.; Fokt, I.; Ziemniak, M.; Jaśkiewicz, A.; Borowski, R.; Domoradzki, T.; et al. 2-Deoxy-d-Glucose and Its Analogs: From Diagnostic to Therapeutic Agents. Int. J. Mol. Sci. 2019, 21, 234. [Google Scholar] [CrossRef]
- Li, W.; Zheng, M.; Wu, S.; Gao, S.; Yang, M.; Li, Z.; Min, Q.; Sun, W.; Chen, L.; Xiang, G.; et al. Benserazide, a Dopadecarboxylase Inhibitor, Suppresses Tumor Growth by Targeting Hexokinase 2. J. Exp. Clin. Cancer Res. 2017, 36, 58. [Google Scholar] [CrossRef]
- Zheng, M.; Wu, C.; Yang, K.; Yang, Y.; Liu, Y.; Gao, S.; Wang, Q.; Li, C.; Chen, L.; Li, H. Novel Selective Hexokinase 2 Inhibitor Benitrobenrazide Blocks Cancer Cells Growth by Targeting Glycolysis. Pharmacol. Res. 2021, 164, 105367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-N.; Yang, L.; Ling, J.-Y.; Czajkowsky, D.M.; Wang, J.-F.; Zhang, X.-W.; Zhou, Y.-M.; Ge, F.; Yang, M.-K.; Xiong, Q.; et al. Systematic Identification of Arsenic-Binding Proteins Reveals That Hexokinase-2 Is Inhibited by Arsenic. Proc. Natl. Acad. Sci. USA 2015, 112, 15084–15089. [Google Scholar] [CrossRef] [PubMed]
- Al-Ziaydi, A.G.; Al-Shammari, A.M.; Hamzah, M.I.; Kadhim, H.S.; Jabir, M.S. Hexokinase Inhibition Using D-Mannoheptulose Enhances Oncolytic Newcastle Disease Virus-Mediated Killing of Breast Cancer Cells. Cancer Cell Int. 2020, 20, 420. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Li, C.; Sang, F.; Cao, W.; Qin, Z.; Zhang, P. Natural products targeting glycolytic signaling pathways-an updated review on anti-cancer therapy. Front. Pharmacol. 2022, 13, 1035882. [Google Scholar] [CrossRef]
- Garcia, S.N.; Guedes, R.C.; Marques, M.M. Unlocking the Potential of HK2 in Cancer Metabolism and Therapeutics. Curr. Med. Chem. 2019, 26, 7285–7322. [Google Scholar] [CrossRef]
- Gomez, L.S.; Zancan, P.; Marcondes, M.C.; Ramos-Santos, L.; Meyer-Fernandes, J.R.; Sola-Penna, M.; Da Silva, D. Resveratrol Decreases Breast Cancer Cell Viability and Glucose Metabolism by Inhibiting 6-Phosphofructo-1-Kinase. Biochimie 2013, 95, 1336–1343. [Google Scholar] [CrossRef]
- Spitz, G.A.; Furtado, C.M.; Sola-Penna, M.; Zancan, P. Acetylsalicylic Acid and Salicylic Acid Decrease Tumor Cell Viability and Glucose Metabolism Modulating 6-Phosphofructo-1-Kinase Structure and Activity. Biochem. Pharmacol. 2009, 77, 46–53. [Google Scholar] [CrossRef]
- Zancan, P.; Rosas, A.O.; Marcondes, M.C.; Marinho-Carvalho, M.M.; Sola-Penna, M. Clotrimazole Inhibits and Modulates Heterologous Association of the Key Glycolytic Enzyme 6-Phosphofructo-1-Kinase. Biochem. Pharmacol. 2007, 73, 1520–1527. [Google Scholar] [CrossRef]
- Clem, B.F.; O’Neal, J.; Tapolsky, G.; Clem, A.L.; Imbert-Fernandez, Y.; Kerr, D.A., 2nd; Klarer, A.C.; Redman, R.; Miller, D.M.; Trent, J.O.; et al. Targeting 6-Phosphofructo-2-Kinase (PFKFB3) as a Therapeutic Strategy against Cancer. Mol. Cancer Ther. 2013, 12, 1461–1470. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Y.; Zhang, E.; Yan, H.; Lv, N.; Cai, Z. The Synergistic Effect of PFK15 with Metformin Exerts Anti-Myeloma Activity via PFKFB3. Biochem. Biophys. Res. Commun. 2019, 515, 332–338. [Google Scholar] [CrossRef]
- Zheng, J.B.; Wong, C.W.; Liu, J.; Luo, X.-J.; Zhou, W.-Y.; Chen, Y.-X.; Luo, H.-Y.; Zeng, Z.-L.; Ren, C.; Xie, X.-M.; et al. Glucose Metabolism Inhibitor PFK-015 Combined with Immune Checkpoint Inhibitor Is an Effective Treatment Regimen in Cancer. Oncoimmunology 2022, 11, 2079182. [Google Scholar] [CrossRef]
- Mondal, S.; Roy, D.; Sarkar Bhattacharya, S.; Jin, L.; Jung, D.; Zhang, S.; Kalogera, E.; Staub, J.; Wang, Y.; Xuyang, W.; et al. Therapeutic Targeting of PFKFB3 with a Novel Glycolytic Inhibitor PFK158 Promotes Lipophagy and Chemosensitivity in Gynecologic Cancers: Therapeutic Targeting of PFKFB3. Int. J. Cancer 2019, 144, 178–189. [Google Scholar] [CrossRef] [PubMed]
- De Maria, S.; Scognamiglio, I.; Lombardi, A.; Amodio, N.; Caraglia, M.; Cartenì, M.; Ravagnan, G.; Stiuso, P. Polydatin, a natural precursor of resveratrol, induces cell cycle arrest and differentiation of human colorectal Caco-2 cell. J. Transl. Med. 2013, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Mele, L.; Paino, F.; Papaccio, F.; Regad, T.; Boocock, D.; Stiuso, P.; Lombardi, A.; Liccardo, D.; Aquino, G.; Barbieri, A.; et al. A New Inhibitor of Glucose-6-Phosphate Dehydrogenase Blocks Pentose Phosphate Pathway and Suppresses Malignant Proliferation and Metastasis in Vivo. Cell Death Dis. 2018, 9, 572. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Cai, P.; Xu, C.; Cao, D.; Yu, W.; Zhao, Z.; Huang, M.; Jin, J. Inhibition of Glucose-6-Phosphate Dehydrogenase Reverses Cisplatin Resistance in Lung Cancer Cells via the Redox System. Front. Pharmacol. 2018, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Ai, G.; Dachineni, R.; Kumar, D.R.; Alfonso, L.F.; Marimuthu, S.; Bhat, G.J. Aspirin Inhibits Glucose-6-phosphate Dehydrogenase Activity in HCT 116 Cells through Acetylation: Identification of Aspirin-Acetylated Sites. Mol. Med. Rep. 2016, 14, 1726–1732. [Google Scholar] [CrossRef]
- Fang, Z.; Jiang, C.; Feng, Y.; Chen, R.; Lin, X.; Zhang, Z.; Han, L.; Chen, X.; Li, H.; Guo, Y.; et al. Effects of G6PD Activity Inhibition on the Viability, ROS Generation and Mechanical Properties of Cervical Cancer Cells. Biochim. Biophys. Acta 2016, 1863, 2245–2254. [Google Scholar] [CrossRef]
- Oronsky, B.; Scicinski, J.; Reid, T.; Oronsky, A.; Carter, C.; Oronsky, N.; Cabrales, P. RRx-001, a Novel Clinical-Stage Chemosensitizer, Radiosensitizer, and Immunosensitizer, Inhibits Glucose 6-Phosphate Dehydrogenase in Human Tumor Cells. Discov. Med. 2016, 21, 251–265. [Google Scholar] [PubMed]
- Zara, R.; Rasul, A.; Sultana, T.; Jabeen, F.; Selamoglu, Z. Identification of Macrolepiota Procera Extract as a Novel G6PD Inhibitor for the Treatment of Lung Cancer. Saudi J. Biol. Sci. 2022, 29, 3372–3379. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, L.; Ali, S.; Rasul, A.; Tahir, H.M. Smilax China Root Extract as a Novel Glucose- 6-Phosphate Dehydrogenase Inhibitor for the Treatment of Hepatocellular Carcinoma. Saudi J. Biol. Sci. 2022, 29, 103400. [Google Scholar] [CrossRef]
- Le, A.; Cooper, C.R.; Gouw, A.M.; Dinavahi, R.; Maitra, A.; Deck, L.M.; Royer, R.E.; Vander Jagt, D.L.; Semenza, G.L.; Dang, C.V. Inhibition of Lactate Dehydrogenase A Induces Oxidative Stress and Inhibits Tumor Progression. Proc. Natl. Acad. Sci. USA. 2010, 107, 2037–2042. [Google Scholar] [CrossRef]
- Maftouh, M.; Avan, A.; Sciarrillo, R.; Granchi, C.; Leon, L.G.; Rani, R.; Funel, N.; Smid, K.; Honeywell, R.; Boggi, U.; et al. Synergistic Interaction of Novel Lactate Dehydrogenase Inhibitors with Gemcitabine against Pancreatic Cancer Cells in Hypoxia. Br. J. Cancer 2014, 110, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-Y.; Chung, T.-W.; Han, C.W.; Park, S.Y.; Park, K.H.; Jang, S.B.; Ha, K.-T. A Novel Lactate Dehydrogenase Inhibitor, 1-(Phenylseleno)-4-(Trifluoromethyl) Benzene, Suppresses Tumor Growth through Apoptotic Cell Death. Sci. Rep. 2019, 9, 3969. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Sheng, X.; Jones, H.M.; Jackson, A.L.; Kilgore, J.; Stine, J.E.; Schointuch, M.N.; Zhou, C.; Bae-Jump, V.L. Evaluation of the Anti-Tumor Effects of Lactate Dehydrogenase Inhibitor Galloflavin in Endometrial Cancer Cells. J. Hematol. Oncol. 2015, 8, 2. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, P.; Wang, M.; Xu, P.; Lu, W.; Lei, P.; You, Q. Development of Novel Human Lactate Dehydrogenase A Inhibitors: High-Throughput Screening, Synthesis, and Biological Evaluations. Eur. J. Med. Chem. 2019, 177, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Halestrap, A.P.; Wilson, M.C. The Monocarboxylate Transporter Family--Role and Regulation. IUBMB Life 2012, 64, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Payen, V.L.; Mina, E.; Van Hée, V.F.; Porporato, P.E.; Sonveaux, P. Monocarboxylate Transporters in Cancer. Mol. Metab. 2020, 33, 48–66. [Google Scholar] [CrossRef]
- Puri, S.; Juvale, K. Monocarboxylate Transporter 1 and 4 Inhibitors as Potential Therapeutics for Treating Solid Tumours: A Review with Structure-Activity Relationship Insights. Eur. J. Med. Chem. 2020, 199, 112393. [Google Scholar] [CrossRef]
- Chen, W.; Tan, Q.; Guo, M.; Liao, T.; Li, Y.; Yin, Z.; Zhou, E.; Deng, J.; Li, M.; Yang, Z.; et al. Tumor Cell-Derived Microparticles Packaging Monocarboxylate Transporter4 Inhibitor Fluvastatin Suppress Lung Adenocarcinoma via Tumor Microenvironment Remodeling and Improve Chemotherapy. Chem. Eng. J. 2023, 451, 138972. [Google Scholar] [CrossRef]
- Macheda, M.L.; Rogers, S.; Best, J.D. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J. Cell Physiol. 2005, 202, 654–662. [Google Scholar] [CrossRef]
- Hussein, Y.R.; Bandyopadhyay, S.; Semaan, A.; Ahmed, Q.; Albashiti, B.; Jazaerly, T.; Nahleh, Z.; Ali-Fehmi, R. Glut-1 Expression Correlates with Basal-like Breast Cancer. Transl. Oncol. 2011, 4, 321–327. [Google Scholar] [CrossRef]
- Gunnink, L.K.; Alabi, O.D.; Kuiper, B.D.; Gunnink, S.M.; Schuiteman, S.J.; Strohbehn, L.E.; Hamilton, K.E.; Wrobel, K.E.; Louters, L.L. Curcumin directly inhibits the transport activity of GLUT1. Biochimie 2016, 125, 179–185. [Google Scholar] [CrossRef]
- Reckzeh, E.S.; Waldmann, H. Development of Glucose Transporter (GLUT) Inhibitors: Development of Glucose Transporter (GLUT) Inhibitors. Eur. J. Org. Chem. 2020, 2020, 2321–2329. [Google Scholar] [CrossRef]
- Pérez, A.; Ojeda, P.; Ojeda, L.; Salas, M.; Rivas, C.I.; Vera, J.C.; Reyes, A.M. Hexose Transporter GLUT1 Harbors Several Distinct Regulatory Binding Sites for Flavones and Tyrphostins. Biochemistry 2011, 50, 8834–8845. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, A.; Molt, M.; Uribe, E.; Salas, M. Glut 1 in Cancer Cells and the Inhibitory Action of Resveratrol as A Potential Therapeutic Strategy. Int. J. Mol. Sci. 2019, 20, 3374. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, Y.; He, C.; Gao, G.; Li, J.; Qiu, L.; Wang, X.; Gao, Y.; Qi, Y.; Sun, K.; et al. Identification of a Novel GLUT1 Inhibitor with in Vitro and in Vivo Anti-Tumor Activity. Int. J. Biol. Macromol. 2022, 216, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Siebeneicher, H.; Bauser, M.; Buchmann, B.; Heisler, I.; Müller, T.; Neuhaus, R.; Rehwinkel, H.; Telser, J.; Zorn, L. Identification of Novel GLUT Inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 1732–1737. [Google Scholar] [CrossRef]
- Nugent, C.; Prins, J.B.; Whitehead, J.P.; Wentworth, J.M.; Chatterjee, V.K.; O’Rahilly, S. Arachidonic acid stimulates glucose uptake in 3T3-L1 adipocytes by increasing GLUT1 and GLUT4 levels at the plasma membrane. Evidence for involvement of lipoxygenase metabolites and peroxisome proliferator-activated receptor gamma. J. Biol. Chem. 2001, 276, 9149–9157. [Google Scholar] [CrossRef]
- Scatena, R.; Bottoni, P.; Pontoglio, A.; Mastrototaro, L.; Giardina, B. Glycolytic enzyme inhibitors in cancer treatment. Expert Opin. Investig. Drugs 2008, 17, 1533–1545. [Google Scholar] [CrossRef]
- Tu, S.-H.; Chang, C.-C.; Chen, C.-S.; Tam, K.-W.; Wang, Y.-J.; Lee, C.-H.; Lin, H.-W.; Cheng, T.-C.; Huang, C.-S.; Chu, J.-S.; et al. Increased Expression of Enolase Alpha in Human Breast Cancer Confers Tamoxifen Resistance in Human Breast Cancer Cells. Breast Cancer Res. Treat. 2010, 121, 539–553. [Google Scholar] [CrossRef]
- Jung, D.-W.; Kim, W.-H.; Park, S.-H.; Lee, J.; Kim, J.; Su, D.; Ha, H.-H.; Chang, Y.-T.; Williams, D.R. A Unique Small Molecule Inhibitor of Enolase Clarifies Its Role in Fundamental Biological Processes. ACS Chem. Biol. 2013, 8, 1271–1282. [Google Scholar] [CrossRef]
- Evans, M.J.; Saghatelian, A.; Sorensen, E.J.; Cravatt, B.F. Target Discovery in Small-Molecule Cell-Based Screens by in Situ Proteome Reactivity Profiling. Nat. Biotechnol. 2005, 23, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tang, S.; Wang, Q.-Q.; Leung, E.L.-H.; Jin, H.; Huang, Y.; Liu, J.; Geng, M.; Huang, M.; Yuan, S.; et al. Identification of Epigallocatechin-3- Gallate as an Inhibitor of Phosphoglycerate Mutase 1. Front. Pharmacol. 2017, 8, 325. [Google Scholar] [CrossRef]
- Huang, K.; Jiang, L.; Liang, R.; Li, H.; Ruan, X.; Shan, C.; Ye, D.; Zhou, L. Synthesis and Biological Evaluation of Anthraquinone Derivatives as Allosteric Phosphoglycerate Mutase 1 Inhibitors for Cancer Treatment. Eur. J. Med. Chem. 2019, 168, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, G.; de Jong, P.R.; James, B.P.; Koh, M.Y.; Lemos, R.; Kingston, J.; Aleshin, A.; Bankston, L.A.; Miller, C.P.; Cho, E.J.; et al. Definition of a Novel Feed-Forward Mechanism for Glycolysis-HIF1α Signaling in Hypoxic Tumors Highlights Aldolase A as a Therapeutic Target. Cancer Res. 2016, 76, 4259–4269. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Chiou, J.; Yang, Y.-F.; Su, C.-Y.; Lin, Y.-F.; Yang, C.-N.; Lu, P.-J.; Huang, M.-S.; Yang, C.-J.; Hsiao, M. Therapeutic Targeting of Aldolase A Interactions Inhibits Lung Cancer Metastasis and Prolongs Survival. Cancer Res. 2019, 79, 4754–4766. [Google Scholar] [CrossRef]
- Funasaka, T.; Hogan, V.; Raz, A. Phosphoglucose Isomerase/Autocrine Motility Factor Mediates Epithelial and Mesenchymal Phenotype Conversions in Breast Cancer. Cancer Res. 2009, 69, 5349–5356. [Google Scholar] [CrossRef]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer: Biological and therapeutic significance. Semin. Cancer Biol. 2019, 59, 147–160. [Google Scholar] [CrossRef]
- Tang, Z.; Xu, Z.; Zhu, X.; Zhang, J. New insights into molecules and pathways of cancer metabolism and therapeutic implications. Cancer Commun. 2020, 41(1), 16–36. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef] [PubMed]
- Morandi, A.; Indraccolo, S. Linking metabolic reprogramming to therapy resistance in cancer. Biochim. Biophys. Acta Bioenerg. 2017, 1868, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, R.B.; Chandel, N.S. Targeting glucose metabolism for cancer therapy. J. Exp. Med. 2012, 209, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, S.; Anderson, N.; Hainz, C.; Eckhardt, S.G.; Serkova, N.J. Imatinib (STI571)-mediated changes in glucose metabolism in human leukemia BCR-ABL-positive cells. Clin. Cancer Res. 2004, 10, 6661–6668. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Y.T.-K.; To, N.B.; Truong, V.N.-P.; Kim, H.Y.; Ediriweera, M.K.; Lim, Y.; Cho, S.K. Impairment of Glucose Metabolism and Suppression of Stemness in MCF−7/SC Human Breast Cancer Stem Cells by Nootkatone. Pharmaceutics 2022, 14, 906. [Google Scholar] [CrossRef] [PubMed]
| Anti-Cancer Mechanism/s Associated | Reference/s | |
|---|---|---|
| Hexokinase 2 inhibitor/s | ||
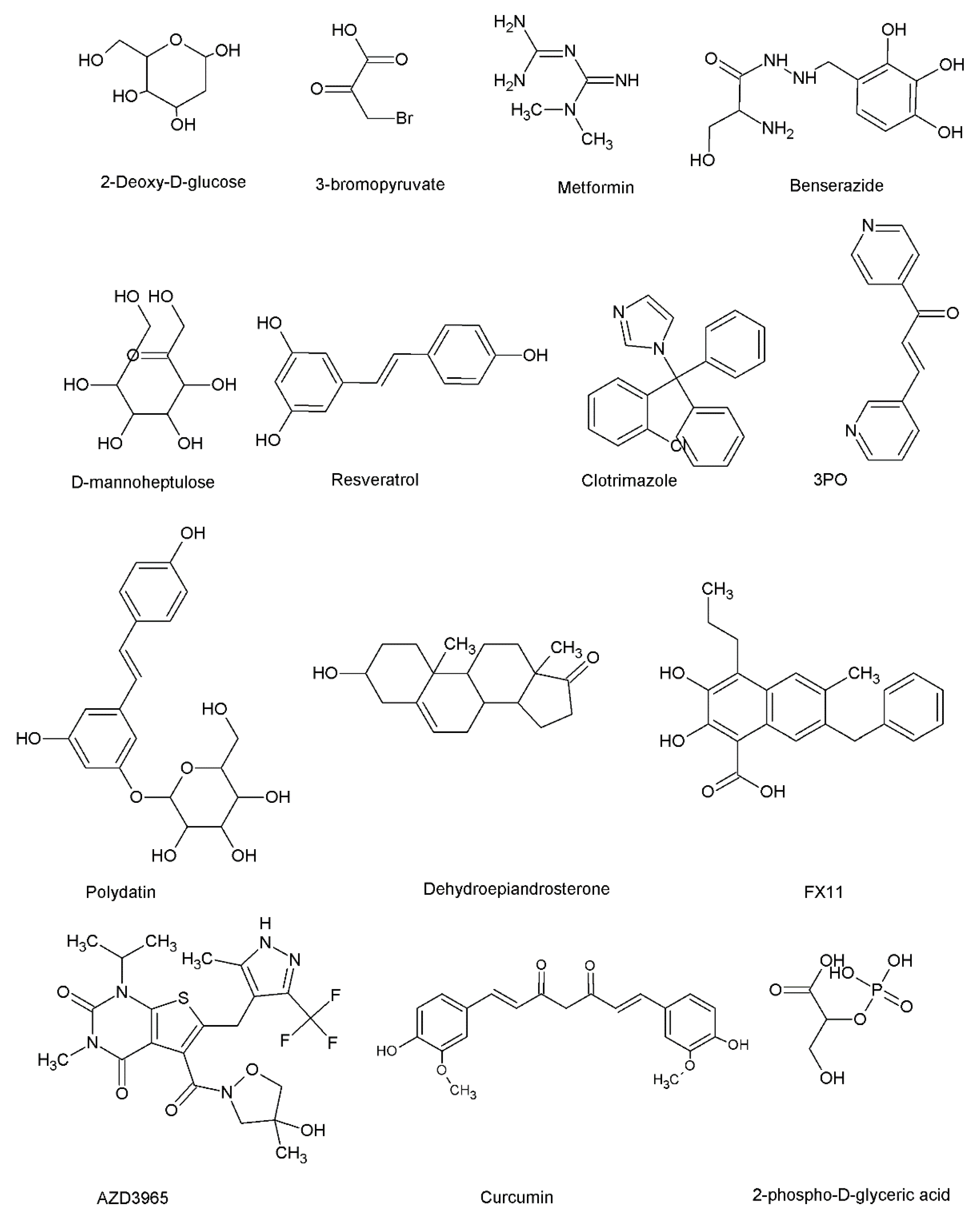
| 2-deoxyglucose, 3-bromopyruvate, and metformin | 2-deoxyglucose, 3-bromopyruvate, and metformin have been reported to exert anti-cancer effects through various molecular mechanisms in a range of cancer cells. | [199,200,201,202] |
| Benserazide | Selectively inhibits HK2 activity. Benserazide inhibited glycolysis, induced apoptosis, demonstrated inhibitory effects on the loss of mitochondrial membrane potential in cancer cells, and inhibited tumor formation in vivo. | [203] |
| Benitrobenrazide | Benitrobenrazide exerted HK2 inhibitory effects at nanomolar concentrations. Induced apoptosis in pancreatic cancer cells and inhibited tumor growth in vivo. | [204] |
| Arsenic | Arsenic directly binds with HK2 and induces apoptosis. | [205] |
| D-mannoheptulose | The combined treatment of Newcastle disease virus (NDV) and D-mannoheptulose showed synergistic anti-cancer effects and induced apoptosis. | [206] |
| 6-phosphofructo-1-kinase inhibitor/s | ||
| Resveratrol | Reduced the viability of breast cancer cells by disrupting glucose metabolism. | [209] |
| PFK1 inhibitor/s | ||
| Acetylsalicylic acid and salicylic | Reduced the glucose consumption of breast cancer cells while exerting anti-proliferative effects in breast cancer cells. | [210] |
| Clotrimazole | Inhibition of mitochondrial-bound glycolytic enzymes, calcium-dependent potassium channel, and calmodulin. | [211] |
| PFKFB3 inhibitor/s | ||
| PFK15 and metformin | Combined treatment of PFK15 and metformin demonstrated anti-myeloma effects through the PFKFB3/MAPKs/STAT signaling pathway | [213] |
| G6PD inhibitor/s | ||
| Polydatin | Improves the accumulation of reactive oxygen species (ROS) and increases endoplasmic reticulum stress. Induces apoptosis and cell cycle block. | [217] |
| Aspirin | Acetylation of G6PD by aspirin contributes to anti-cancer effects. | [219] |
| LDH inhibitor/s | ||
| FX11 | Induces oxidative stress and inhibits tumor formation. | [224] |
| PSTMB | Exerted anti-proliferative effects and induced apoptosis in a range of cancer cells (liver, breast, colorectal, and lung). | [226] |
| MCT inhibitor/s | ||
| AZD3965 | Modulated tumor lactate levels and induced anti-tumor effects in vivo. The combined exposure of AZD3965 and inhibitors of glutaminase resulted in the induction of apoptosis in lymphoma cells. | [231] |
| GLUT1 inhibitor/s | ||
| Genistein, quercetin, resveratrol, and curcumin | Exerts anti-cancer through various molecular mechanisms. | [235,236,237,238] |
| SMI277 | SMI277 inhibited glucose uptake, decreased lactate secretion, induced apoptosis in tumor cells, and enhanced CD8+ T cell response. | [239] |
| Phosphoglycerate mutase inhibitor/s | ||
| MJE3 | Inhibits the proliferation of breast cancer cells | [245] |
| Aldolase inhibitor/s | ||
| TDZD-8 | Exerts anti-proliferative effects and aldolase inhibitory effects by reducing the stability of HIF-1α | [248] |
| Raltegravir | Breaks aldolase A-γ-actin interactions to exert anti-metastasis effects in vitro and in vivo. | [249] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ediriweera, M.K.; Jayasena, S. The Role of Reprogrammed Glucose Metabolism in Cancer. Metabolites 2023, 13, 345. https://doi.org/10.3390/metabo13030345
Ediriweera MK, Jayasena S. The Role of Reprogrammed Glucose Metabolism in Cancer. Metabolites. 2023; 13(3):345. https://doi.org/10.3390/metabo13030345
Chicago/Turabian StyleEdiriweera, Meran Keshawa, and Sharmila Jayasena. 2023. "The Role of Reprogrammed Glucose Metabolism in Cancer" Metabolites 13, no. 3: 345. https://doi.org/10.3390/metabo13030345
APA StyleEdiriweera, M. K., & Jayasena, S. (2023). The Role of Reprogrammed Glucose Metabolism in Cancer. Metabolites, 13(3), 345. https://doi.org/10.3390/metabo13030345







