Abstract
The mechanisms underlying selective serotonin reuptake inhibitor (SSRI) use during pregnancy as a major autism risk factor are unclear. Here, brain neurochemical changes following fluoxetine exposure and in an autism model were compared to determine the effects on autism risk. The study was performed on neonatal male western albino rats which were divided into Groups one (control), two (propionic acid [PPA]-induced autism model), and three (prenatal SSRI-exposed newborn rats whose mothers were exposed to 5 mg/kg of fluoxetine over gestation days 10–20). SSRI (fluoxetine) induced significant neurochemical abnormalities in the rat brain by increasing lipid peroxide (MDA), Interferon-gamma (IFN-γ), and caspase-3 levels and by depleting Glutathione (GSH), Glutathione S-transferases (GST), Catalase, potassium (K+), and Creatine kinase (CK) levels, similarly to what has been discovered in the PPA model of autism when compared with control. Prenatal fluoxetine exposure plays a significant role in asset brain damage in newborns; further investigation of fluoxetine as an autism risk factor is thus warranted.
1. Introduction
Serotonin (5-hydroxytryptamine; 5-HT) is a monoamine neurotransmitter that controls various neuronal interactions through specific transporters, receptors, and intracellular signaling pathways [1]. It is one of the first neurotransmitters to emerge in the mammalian brain. Serotonergic neurons start at five weeks old in human embryo and proliferate until ten weeks of gestation, increase for the first 2 years after birth, and decrease to reach adult levels at five years of age. Serotonergic activity can shape the develop neuronal circuitry and is involved in plasticity during the perinatal period [2]. Serotonin signaling pathways are important for normal brain functions in sensory processing, regulating emotion and motor activity in addition to being involved in morphogenetic activities during brain development including neural cell proliferation, migration, and differentiation. It reduces neural plasticity and acts as a nervous tissue growth factor in the developing brain [3]. It is also involved in several developmental processes, such as cell division, neural migration, cell differentiation, synaptogenesis, and early cortex development [4]. Reduction in central serotonin levels can result in various psychiatric disorders such as depression, anxiety, aggression, childhood hyperactivity, and some behavioral disorders [5]. Thus, most of the antidepressants prescribed these days are among the selective serotonin reuptake inhibitors (SSRIs), which can increase the levels of extracellular serotonin in the brain by blocking its reabsorption. Some members of the SSRIs group of drugs not only decrease the uptake of serotonin but are reported to inhibit the uptake of some other neurotransmitters as well. Sertraline inhibits dopamine reuptake; fluoxetine inhibits noradrenaline and dopamine; imipramine inhibits both noradrenaline and serotonin reuptake [6]. Although SSRIs are repeatedly used during pregnancy, they can be teratogenic and increase the risk of premature delivery, low birth weight, neonatal cardiovascular abnormalities, and offspring metabolic disorders [7]. Impaired serotonin-dependent shaping of neural circuitry in SSRIs-exposed babies might increase the risk of neurodevelopmental disorders. Magnetic resonance imaging has reported hyper-connectivity in auditory resting-state networks in infants exposed prenatally to SSRIs in comparison to healthy control infants [8].
Many studies have reported the associations of prenatal depression with the brain development of infants. Depression during pregnancy is a major health issue not only for mothers but also for newborns. Children of depressed females during pregnancy are four times more likely to be diagnosed with depression in their teenage years than those unexposed to antenatal depression. Maternal anxiety in pregnancy is associated with lower mental development scores, hyperactivity, and behavioral and emotional problems in children. Most women use antidepressants during pregnancy as they experience depression and anxiety disorders in their reproductive years [9]. SSRIs are among the most prescribed medications for maternal depression as it mainly targets the serotoninergic system [10]. SSRIs can pass the human placenta to reach the fetus, and they can also be consumed by neonates through nursing because their active metabolites are excreted into human milk [11]. Many studies have reported neurological issues and other health problems in children exposed to SSRIs before birth. Recently, Zengeler et al. [12] reported a strong inflammatory reaction at the maternal–fetal interface due to SSRI treatment during pregnancy. Babies that are exposed to inflammation develop behavioral changes such as diminished communication and decreased interest in social interactions. An immune challenge during pregnancy can lead to permanent brain changes [12]. Many epidemiological reports have linked maternal immune activation with neurodevelopmental disorders, psychiatric conditions, and other neurologic disorders in children [13,14]. Low immunity promotes infections and autoimmune conditions and elevates the chance of mental conditions in offspring [15]. Some animal-based studies reported that exposure to SSRIs during pregnancy can interrupt brain development resulting in neurodevelopmental abnormalities [16,17]. Altered pain response and subtle delays in motor development in SSRI-exposed infants have been reported by Oberlander [18]. Moreover, Croen et al. [19] showed that prenatal SSRI exposure can double the risk of autism in newborns.
Several studies based on neuroimaging and genetics have linked serotonin to the pathophysiology of autism. Autism patients usually have disturbed serotonin levels and altered serotonin end-metabolites such as 5-hydroxy indole acetic acid in their body and cerebrospinal fluid (CSF), respectively [20,21]. Moreover, nearly one third of autistic children have hyperserotonemia [22]. Autism is a neurodevelopmental ailment, characterized mainly by impaired social interaction and repetitive behaviors [23]. The incidence of autism in low to middle-income countries is not defined but internationally, a single child in 160 children is suffering from this disease [24]. Recently, in the US, the Centers for Disease Control and Prevention (CDC) and Autism and Developmental Disabilities Monitoring (ADDM) Network reported that 1 in 44 children is autistic [25]. Autism is more prevalent in boys with co-occurring disorders with symptoms including anxiety, epilepsy, depression, disturbed sleep, and self-harm [26]. A lot of research on the etiological factors of autism acknowledged both genetic and environmental factors responsible for the pathogenesis of this disease [27]. Many previous studies have linked genetic factors related to depression and the use of SSRIs with the risk of ASD [28]. SSRI exposure to fetal through maternal blood can affect the developing brain by damaging serotonin receptors through negative feedback known as the developmental hyperserotonemia model of autism [29]. This may be the reason for high levels of serotonin in blood with the low binding potential of the same in various brain sites in autistic patients [30]. Hyperserotonemia is reported as the first biomarker identified in autism and is present in more than 25% of autistic children. Positron emission tomography-based studies for normal healthy children showed elevated serotonin synthesis between two and six years of age followed by a decline at puberty but not in autistic children.
Propionic acid (PPA), a by-product of enteric fatty acid bacterial fermentation, can exert profound effects on the stomach, brain, and behavior. The brain tissue of PPA-treated rats exhibits a wide range of neurochemical abnormalities, including neuroinflammation, glutamate excitotoxicity, oxidative stress, GSH depletion, and altered membrane phospholipids, all of which are present in ASD patients [31,32]. Orally administered PPA (250 mg/kg/body wt.) for 3 days in the rodent model of ASD was repeatedly used by our research team as a valid model that offers the same pathophysiology and the symptoms of ASD behavior [32,33]. All of these findings lead us to consider fluoxetine as an autism risk factor. The neurotoxicity of newborn rats exposed to fluoxetine during pregnancy was compared using a rodent model of autism generated by propionic acid as a vehicle.
2. Materials and Methods
2.1. Animals
The experiment was started with 10 female western albino rats (180 to 200 g) which were obtained from the animal house of the Pharmacy College of King Saud University. Healthy female western albino rats were mated overnight with normal male rats in the proportion of 1:1, and the day spermatozoa were detected in the vaginal smear was designated the first day of gestation. Each pregnant female rat was individually kept in a cage. Pregnant female rats were randomly divided into two groups. Set I was grown in a normal condition and Set II was treated with 5 mg/kg/body wt. of fluoxetine from pregnancy day 10 to day 20 [34,35]. Male neonatal rats from both sets were differentiated by observing the urinary papilla and genital opening on day four of birth. Twenty male neonatal rats that were born from Set I were further divided into two groups (with ten neonatal rats in each group). Group one received phosphate-buffered saline throughout the experiment and served as the control group; Group two was treated with oral neurotoxic doses of PA (250 mg/kg/body wt.) for 3 days to induce the autistic features [22]. Ten newborn male rats from Set II were assigned to Group three. As a result, the three groups—each consisting of 10 neonatal rats—were set up as follows:
Group one (control): Male pups from Set I were administered an oral dose of 1 mL of normal saline daily.
Group two (positive control/PA rodent model): Male pups from Set I were administered oral neurotoxic doses of PA (250 mg/kg/body wt.) for 3 days at 19 days after birth.
Group three (fluoxetine-treated mothers): Male pups from Set II were administered an oral dose of 1 mL of normal saline daily. On the 22nd day after birth, all groups were killed by decapitation.
Animals were raised under standard conditions at a temperature of 23 °C, humidity of 37%, with light for 12 h. All animal work was conducted in the animal house in the zoology department at the College of Science at King Saud University.
A schematic presentation of the designed groups is summarized in Figure 1.
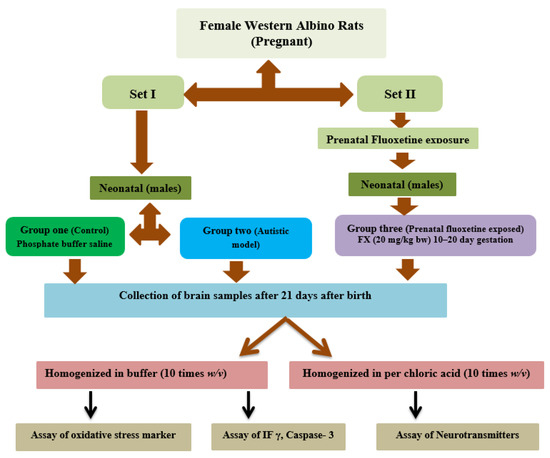
Figure 1.
Schematic representation of the different groups with treatment involved in the study.
The protocols used in the present study were approved by the Ethics Committee at King Saud University (Approval Reference no SE-19-97).
2.2. Brain Tissue Collection
The whole brain was collected and washed with cold normal saline and then homogenized in 1:10 weight/volume in double distilled water followed by centrifugation at 3500 rpm for 15 min. The supernatant was collected and used for various biochemical analyses.
2.3. Biochemical Analyses
The biochemicals mentioned below were measured in the brain homogenate.
2.3.1. Lipid Peroxidation
The method described by Ruiz-Larrea et al. [36] was used to measure lipid oxidation based on the formation of thiobarbituric acid reactive substances (TBARS), namely malondialdehyde (MDA). Brain tissue samples were boiled with TBA to form a pink chromogen; the absorbance was measured at 532 nm and the concentration was calculated as μmoles/mL using the extinction coefficient of MDA.
2.3.2. Glutathione
The method of Beutler et al. [37] was used to measure glutathione using 5,5′-dithiobis (2-nitrobenzoic acid) and sulfhydryl compounds to produce a relatively stable yellow color that was red at 412 nm. A standard glutathione solution was used for calculating the concentration of glutathione.
2.3.3. Glutathione-S-Transferase
An assay kit from BioVision, USA, using glutathione S-transferase (GST)-catalyzed reaction between glutathione and 1-chloro-2,4-dinitrobenzene was used to measure GST activity which was expressed as nmol/mL [38].
2.3.4. Catalase
Catalase activity was measured using the method described by Chance [39] by monitoring the rate of dissociation of hydrogen peroxide per minute at 240 nm.
2.3.5. Potassium
Potassium levels were measured by producing a colloidal suspension by reaction with sodium tetraphenyl boron which was red at 620 nm [40].
2.3.6. Neurotransmitter
The concentrations of noradrenaline, dopamine, and serotonin were determined in brain homogenates using high-performance liquid chromatography with electrochemical detection [41]. Briefly, Brain tissue was homogenized with 0.1 M perchloric acid and then centrifuged at 10,000× g at 4 °C for 30 min. The supernatant was injected into the HPLC instrument with an analytical column of ODS 2 C18, 4.6 × 250 mm protected by a guard column with a particle size of 5 μm. The electrochemical detector model HP 1049 A was used to detect three neurotransmitters and expressed as ng/100 mg.
2.3.7. Creatine Kinase
Creatine kinase was determined using the CU NSC kit from NSC Human, Germany, and the activity is expressed in U/L at 340 nm.
2.3.8. Caspase-3
Caspase-3 was measured using an enzyme-linked immunosorbent assay (ELISA) kit (Cusabio, Wuhan, China) with a detection limit of 0.312 ng/mL–20 ng/mL.
2.3.9. Interferon Gamma
Interferon Gamma was measured using an ELISA kit (Thermo Fisher Scientific, Rockford, IL, USA) with a detection limit of 25.6–1000 pg/mL.
2.4. Statistical Analysis
Results were presented as mean ± standard deviation using SPSS and evaluated using one-way analysis of variance test between all groups and with Dunnett’s test. P values of ≤0.05 were considered significant. Receiver operating characteristic (ROC) analysis was performed to measure the effectiveness of the studied parameters in terms of either the neurotoxicity of PA and fluoxetine.
3. Results
Table 1 presents the levels and percentage changes relative to control for all the measured parameters in brain homogenates of all three groups under study. Table 1 clearly shows that both PPA and fluoxetine induce a significant increase (p ≤ 0.001) in lipid peroxide, IFN-γ, and caspase-3 in rat pup brains compared with the control group. Glutathione, GST, Catalase, K+, and CK were significantly (p ≤ 0.001) decreased in both PPA and fluoxetine groups compared with the control group. In addition, all measured neurotransmitters, including noradrenaline, dopamine, and serotonin, were significantly (p ≤ 0.001) decreased in groups treated with PA and fluoxetine. Figure 2 represents the matrix correlations between all parameters which have a significant correlation at 0.05 level. Table 2 demonstrates the multiple regression using a stepwise method for all the measured parameters as a dependent variable. Table 3 presents the results of ROC analysis with the area under the curve, specificity, and sensitivity of all measured parameters.

Table 1.
Mean ± SD of all the measured parameters in brain homogenate of all treated groups compared with the control.
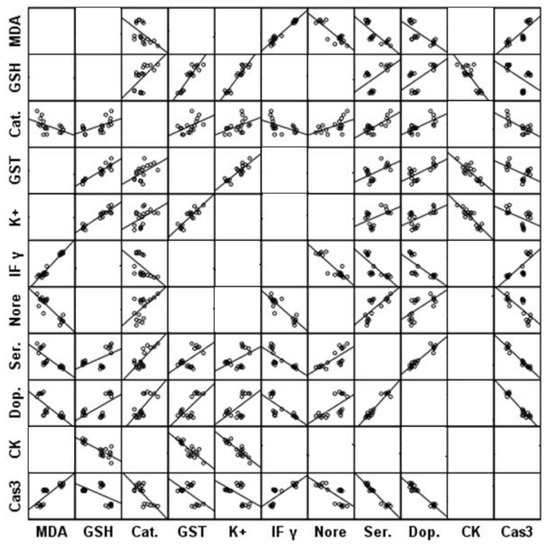
Figure 2.
Matrix correlations between all parameters which have significant correlation at 0.05 level.

Table 2.
Multiple regression using the stepwise method for measured parameters as a dependent variable.

Table 3.
ROC curve of all parameters in all groups.
4. Discussion
Autism is diagnosed at the age of 3–4 years and persists throughout the life of a patient with symptoms such as shortfalls of social interaction and communication, negligible eye contact, expression of emotions, etc. To date, no effective therapy to treat or prevent autism is available, hence identification of environmental risk factors is extremely needed as a public health goal. The environment may possibly act as one of the major risk factors for various neurodevelopmental disorders including autism. Prenatal SSRI exposure has been linked to an increased risk of autism in babies in a number of recent population-based studies; however, the underlying processes are not fully understood [42,43,44,45]. Due to the lack of human brain tissue samples, animal modeling is the most effective method for understanding the neurochemical alterations in individuals with brain diseases [46]. Thus, we relate the neurochemical changes in the brain caused by exposure to fluoxetine with the PPA-induced autism model to determine whether fluoxetine is a risk factor for autism (Table 1). Our findings revealed that prenatal exposure to fluoxetine could result in the deterioration of brain neurochemistry in almost the same trend as observed in the PPA model of autism.
Concerns about the dangers of prenatal exposure to environmental toxins have resulted in a mother bias in epidemiological studies examining trends in ASD onset [47,48]. Many risk factors related to the development of autism in children are directly linked to oxidative stress [49]. Many studies have linked autism with high oxidative stress mainly due to abnormal metabolism and accumulation of toxins resulting in reactive oxygen species (ROS). Autistic patients have decreased antioxidant capacity, elevated lipid peroxidation, and reduced glutathione in both plasma and primary immune cells. The current results discovered a significant increase in MDA and CK; in addition to a significant decrease in GSH, GST, catalase, and K+ levels in the brain tissue of neonatal pups after intrauterine exposure to fluoxetine, which can be connected with the development of oxidative stress related to autism, many studies have shown that increase in oxidative stress due to maternal exposure to environmental toxins or some drugs during pregnancy have been associated with the development of autism [48,50].
Significant decrease in noradrenaline, dopamine, and serotonin levels was observed in the brain tissues of prenatal fluoxetine-treated rat pups. These neurotransmitters play a major role in the normal brain development and regulate memory, motor activities, and behaviour [51]. Prenatal exposure to fluoxetine has affected the brain neurotransmitter levels of neonates as this drug can cross the placenta and can reach the embryos during the developmental stage. We also observed that the brain tissues of PA-induced rodent model of autism showed abnormal neurotransmitter levels. Our results are in good agreement with multiple studies which reported that drugs provided to pregnant women could disrupt neurotransmitter systems, increasing the likelihood of having a child with autism [43,45,52].
IFN-γ is a T-helper type 1 cytokine, which is expressed in neurons during cell-mediated immune responses, whereas caspase-3 is determined to be essential for normal brain development and is also involved in the programmed cell death of neurons. A significant increase in the IFN-γ and caspase-3 levels was observed in the brain tissues of rat pups exposed to fluoxetine during pregnancy. High levels of IFN-γ in fetal tissues can affect brain development and synapse formation, which can result in neurodevelopmental disorders [53]. Studies have shown that prenatal IFN-γ imbalances can be linked to autism [54]. This could help to prove the role of the immune abnormality with special emphasis on the impact of immunological variables connected to the maternal influence on autism development, comorbidities and severity [55]. The significant increase in CK and decrease in K+ in PPA and fluoxetine-treated rats demonstrate its role in energy impairment as a known etiological mechanism in ASD [56].
These significant positive and negative correlations shown in Figure 2 can collectively prove the contribution of oxidative stress, neuroinflammation, altered neurochemistry, apoptosis, and impaired energy metabolism as etiological mechanisms related to brain neurotoxicity in ASD.
The results of multilevel regression shown in Table 2 demonstrate that neuroinflammation (IF-γ) serves as a predictor variable for 95% of the increase in MDA, a sign of oxidative stress. This could support the idea that both pathways had a part in the etiology of ASD. Furthermore, oxidative stress and poor energy metabolism (K+, catalase, GST) are connected to 92.2% of the CK rise as predictive variables. Serotonin, dopamine, MDA, and K+, markers of neurochemistry, oxidative stress, and energy metabolism, account for 98.4% of the significant rise in caspase-3 as a proapoptotic marker.
ROC analysis presented in Table 3 shows the AUC together with the cut-off values of all test markers of the PA and fluoxetine group’s parameters; the total AUC was determined as a measure of the performance of PA or prenatal fluoxetine treatment. It is clearly seen that almost all the test markers under study can be used as excellent markers of both groups with AUC values equal to 1.
5. Conclusions
Fluoxetine affects the mother’s brain similarly to the way PA affects the brain of a newborn through increased oxidative stress, neuroinflammation and neurochemical alterations in the brain. We reported significant increase in MDA, IFN-γ, and caspase-3 with the significant decrease in GSH, GST, Catalase, K+, and CK and all measured neurotransmitters in both PPA and fluoxetine groups when compared with control group. Therefore, because of its developmental neurotoxicity, fluoxetine may be one of the causes of autism.
Author Contributions
Conceptualization, R.S.B. and A.E.-A.; methodology, R.S.B.; software S.A.-D. and M.A.; validation, A.E.-A. and R.S.B.; formal analysis, R.S.B. and S.A.-D.; investigation, A.E.-A.; resources, A.E.-A.; data curation R.S.B. and S.A.-D.; writing—original draft preparation, R.S.B.; writing—review and editing, A.E.-A. and R.S.B.; visualization, A.E.-A.; supervision, A.E.-A.; project administration A.E.-A.; funding acquisition M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded through Researchers’ Supporting Project number (RSP-2023/237), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
King Saud University approved all the animal experiments conducted in this study (Ethics Approval Reference no as SE-19-97).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in the current manuscript.
Acknowledgments
The authors extend their appreciation to the researchers’ Supporting Project number (RSP-2023/237), King Saud University, Riyadh, Saudi Arabia, for funding this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Israelyan, N.; Margolis, K.G. Serotonin as a link between the gut-brain-microbiome axis in autism spectrum disorders. Pharmacol. Res. 2018, 132, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Courtiol, E.; Castellanos, F.X.; Teixeira, C.M. Abnormal Serotonin Levels During Perinatal Development Lead to Behavioral Deficits in Adulthood. Front. Behav. Neurosci. 2018, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Lesch, K.-P.; Waider, J. Serotonin in the Modulation of Neural Plasticity and Networks: Implications for Neurodevelopmental Disorders. Neuron 2012, 76, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Azmitia, E.C. Modern views on an ancient chemical: Serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res. Bull. 2001, 56, 413–424. [Google Scholar] [CrossRef]
- Saldanha, D.; Kumar, N.; Ryali, V.; Srivastava, K.; Pawar, A. Serum Serotonin Abnormality in Depression. Med. J. Armed Forces India 2009, 65, 108–112. [Google Scholar] [CrossRef]
- Abdel-Salam, O.M.; Morsy, S.M.Y.; Sleem, A.A. The effect of different antidepressant drugs on oxidative stress after lipopolysaccharide administration in mice. EXCLI J. 2011, 10, 290–302. [Google Scholar] [PubMed] [PubMed Central]
- Ornoy, A.; Koren, G. SSRIs and SNRIs (SRI) in Pregnancy: Effects on the Course of Pregnancy and the Offspring: How Far Are We from Having All the Answers? Int. J. Mol. Sci. 2019, 20, 2370. [Google Scholar] [CrossRef]
- Rotem-Kohavi, N.; Williams, L.J.; Virji-Babul, N.; Bjornson, B.; Brain, U.; Werker, J.F.; Grunau, R.E.; Miller, S.; Oberlander, T.F. Alterations in Resting-State Networks Following In Utero Selective Serotonin Reuptake Inhibitor Exposure in the Neonatal Brain. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging 2018, 4, 39–49. [Google Scholar] [CrossRef]
- Yonkers, K.A.; Blackwell, K.A.; Glover, J.; Forray, A. Antidepressant Use in Pregnant and Postpartum Women. Annu. Rev. Clin. Psychol. 2014, 10, 369–392. [Google Scholar] [CrossRef]
- Pei, S.; Liu, L.; Zhong, Z.; Wang, H.; Lin, S.; Shang, J. Risk of prenatal depression and stress treatment: Alteration on serotonin system of offspring through exposure to Fluoxetine. Sci. Rep. 2016, 6, 33822. [Google Scholar] [CrossRef]
- Kristensen, J.H.; Ilett, K.F.; Hackett, L.P.; Yapp, P.; Paech, M.; Begg, E.J. Distribution and excretion of fluoxetine and norfluoxetine in human milk. Br. J. Clin. Pharmacol. 1999, 48, 521–527. [Google Scholar] [CrossRef]
- Zengeler, K.E.; Shapiro, D.A.; Bruch, K.R.; Lammert, C.R.; Ennerfelt, H.; Lukens, J.R. SSRI treatment modifies the effects of maternal inflammation on in utero physiology and offspring neurobiology. Brain, Behav. Immun. 2023, 108, 80–97. [Google Scholar] [CrossRef]
- Estes, M.L.; McAllister, A.K. Maternal immune activation: Implications for neuropsychiatric disorders. Science 2016, 353, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Han, V.X.; Patel, S.; Jones, H.F.; Dale, R.C. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat. Rev. Neurol. 2021, 17, 564–579. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S.; Derkits, E.J. Prenatal Infection and Schizophrenia: A Review of Epidemiologic and Translational Studies. Am. J. Psychiatry 2010, 167, 261–280. [Google Scholar] [CrossRef]
- Casper, R.C.; Fleisher, B.E.; Lee-Ancajas, J.C.; Gilles, A.; Gaylor, E.; DeBattista, A.; Hoyme, H. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J. Pediatr. 2003, 142, 402–408. [Google Scholar] [CrossRef]
- Pedersen, L.H.; Henriksen, T.B.; Olsen, J. Fetal exposure to antidepressantand normal milestone development at 6 and 19 months of age. Pediatrics 2010, 125, e600–e608. [Google Scholar] [CrossRef] [PubMed]
- Oberlander, T.F.; Grunau, R.E.; Fitzgerald, C.; Papsdorf, M.; Rurak, D.; Riggs, W. Pain reactivity in 2-month-old infants after prenatal and postnatal selectiveserotonin reuptake inhibitor medication exposure. Pediatrics 2005, 115, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Croen, L.A.; Grether, J.K.; Yoshida, C.K.; Odouli, R.; Hendrick, V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry 2011, 68, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Anacker, A.; Veenstra-VanderWeele, J. The serotonin system in autism spectrum disorder: From biomarker to animal models. Neuroscience 2016, 321, 24–41. [Google Scholar] [CrossRef]
- De Grandis, E.; Serrano, M.; Pérez-Dueñas, B.; Ormazábal, A.; Montero, R.; Veneselli, E.; Pineda, M.; González, V.; Sanmartí, F.; Fons, C.; et al. Cerebrospinal fluid alterations of the serotonin product, 5-hydroxyindolacetic acid, in neurological disorders. J. Inherit. Metab. Dis. 2010, 33, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Mulder, E.J.; Anderson, G.M.; Kema, I.P.; De Bildt, A.; Van Lang, N.D.; Den Boer, J.A.; Minderaa, R.B. Platelet serotonin levels in pervasive developmental disorders and mental retardation: Diagnostic group differences, within-group distribution, and behavioral correlates. J. Am. Acad. Child Adolesc. Psychiatry 2004, 43, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Hodges, H.; Fealko, C.; Soares, N. Autism spectrum disorder: Definition, epidemiology, causes, and clinical evaluation. Transl. Pediatr. 2020, 9, S55. [Google Scholar] [CrossRef] [PubMed]
- Malwane, M.I.; Nguyen, E.B.; Trejo, S.; Kim, E.Y.; Cucalón-Calderón, J.R. A Delayed Diagnosis of Autism Spectrum Disorder in the Setting of Complex Attention Deficit Hyperactivity Disorder. Cureus 2022, 14, e25825. [Google Scholar] [CrossRef]
- Maenner, M.J.; Shaw, K.A.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Esler, A.; Furnier, S.M.; Hallas, L.; Hall-Lande, J.; Hudson, A.; et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2018. MMWR Surveill Summ. 2021, 70, 1–16. [Google Scholar] [CrossRef]
- Yang, T.; Chen, L.; Dai, Y.; Jia, F.; Hao, Y.; Li, L.; Zhang, J.; Wu, L.; Ke, X.; Yi, M.; et al. Vitamin A Status Is More CommonlyAssociated with Symptoms and Neurodevelopment in Boys with Autism Spectrum Disorders-A Multicenter Study in China. Front. Nutr. 2022, 9, 851980. [Google Scholar] [CrossRef]
- Wang, L.; Wang, B.; Wu, C.; Wang, J.; Sun, M. Autism Spectrum Disorder: Neurodevelopmental Risk Factors, Biological Mechanism, and Precision Therapy. Int. J. Mol. Sci. 2023, 24, 1819. [Google Scholar] [CrossRef]
- Kapra, O.; Rotem, R.; Gross, R. The Association Between Prenatal Exposure to Antidepressants and Autism: Some Research and Public Health Aspects. Front. Psychiatry 2020, 11, 55740. [Google Scholar] [CrossRef]
- Mathew, S.; Bichenapally, S.; Khachatryan, V.; Muazzam, A.; Hamal, C.; Velugoti, L.S.D.R.; Tabowei, G.; Gaddipati, G.N.; Mukhtar, M.; Alzubaidee, M.J.; et al. Role of Serotoninergic Antidepressants in the Development of Autism Spectrum Disorders: A Systematic Review. Cureus 2022, 14, e28505. [Google Scholar] [CrossRef]
- Sujan, A.C.; Öberg, A.S.; Quinn, P.D.; D’Onofrio, B.M. Annual Research Review: Maternal antidepressant use during pregnancy and offspring neurodevelopmental problems—A critical review and recommendations for future research. J. Child Psychol. Psychiatry 2018, 60, 356–376. [Google Scholar] [CrossRef]
- El-Ansary, A.K.; Ben Bacha, A.; Kotb, M. Etiology of autistic features: The persisting neurotoxic effects of propionic acid. J. Neuroinflammation 2012, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Jiji, K.N.; Muralidharan, P. Evaluation of the protective effect of Clitoria ternatea L. against propionic acid induced autistic spectrum disorders in rat model. Bull. Natl. Res. Cent. 2022, 46, 71. [Google Scholar] [CrossRef]
- Abuaish, S.; Al-Otaibi, N.M.; Abujamel, T.S.; Alzahrani, S.A.; Alotaibi, S.M.; AlShawakir, Y.A.; Aabed, K.; El-Ansary, A. Fecal Transplant and Bifidobacterium Treatments Modulate Gut Clostridium Bacteria and Rescue Social Impairment and Hippocampal BDNF Expression in a Rodent Model of Autism. Brain Sci. 2021, 11, 1038. [Google Scholar] [CrossRef]
- Pawluski, J.L.; Van Donkelaar, E.; Abrams, Z.; Houbart, V.; Fillet, M.; Steinbusch, H.W.M.; Charlier, T.D. Fluoxetine Dose and Administration Method Differentially Affect Hippocampal Plasticity in Adult Female Rats. Neural Plast. 2014, 2014, 123026. [Google Scholar] [CrossRef]
- Bhat, R.S.; GezeerY, A.E.; Bachan, A.B.; AlonazI, M.A.; Alsuhaibani, L.S.; El-Ansary, A. Prenatal exposure to the fluoride containing psychiatric drug fluoxetine and anti-oxidative alterations in the neonatal rat brain. Biocell 2019, 43, 65–71. [Google Scholar]
- Ruiz-Larrea, M.B.; Leal, A.M.; Liza, M.; Lacort, M.; de Groot, H. Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids 1994, 59, 383–388. [Google Scholar] [CrossRef]
- Beutle, E.; Duran, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Chance, B. Catalases and peroxidess, part II. Special methods. Methods Biochem. Anal. 1954, 1, 408. [Google Scholar]
- Terri, A.E.; Sesin, P.G. Determination of potassium in blood serum. Am. Soc. Clin. Pathol. 1958, 29, 86–89. [Google Scholar] [CrossRef]
- Zagrodzka, J.; Romaniuk, A.; Wieczorek, M.; Boguszewski, P. Bicuculline administration into ventromedial hypothalamus: Effects on fear and regional brainmonoamines and GABA concentrations in rats. Acta Neurobiol. Exp. 2000, 60, 333–343. [Google Scholar]
- Boukhris, T.; Sheehy, O.; Mottron, L.; Bérard, A. Antidepressant use during pregnancy and the risk of autism spectrum disorder in children. JAMA Pediatr. 2016, 170, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.K.; Hussain-Shamsy, N.; Lunsky, Y.; Dennis, C.L.E.; Vigod, S.N. The Association between antenatal exposure to selective serotonin reuptake inhibitors and autism: A systematic review and meta-analysis. J. Clin. Psychiatry 2017, 78, e48. [Google Scholar] [CrossRef] [PubMed]
- Gidaya, N.B.; Lee, B.K.; Burstyn, I.; Yudell, M.; Mortensen, E.L.; Newschaffer, C.J. In utero exposure to selective serotonin reuptake inhibitors and risk for autism spectrum disorder. J. Autism. Dev. Disord. 2014, 44, 2558–2567. [Google Scholar] [CrossRef] [PubMed]
- Rai, D.; Lee, B.K.; Dalman, C.; Golding, J.; Lewis, G.; Magnusson, C. Parental depression, maternalantidepressant use during pregnancy, and risk of autism spectrum disorders:population based case-control study. BMJ 2013, 346, f2059. [Google Scholar] [CrossRef]
- Shen, H.H. News Feature: Better models for brain disease. Proc. Natl. Acad. Sci. USA 2016, 113, 5461–5464. [Google Scholar] [CrossRef]
- Bölte, S.; Girdler, S.; Marschik, P.B. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell. Mol. Life Sci. 2019, 76, 1275–1297. [Google Scholar] [CrossRef]
- Pugsley, K.; Scherer, S.W.; Bellgrove, M.A.; Hawi, Z. Environmental exposures associated with elevated risk for autism spectrum disorder may augment the burden of deleterious de novo mutations among probands. Mol. Psychiatry 2021, 27, 710–730. [Google Scholar] [CrossRef]
- Parker, W.; Hornik, C.D.; Bilbo, S.; Holzknecht, Z.E.; Gentry, L.; Rao, R.; Lin, S.S.; Herbert, M.R.; Nevison, C.D. The role of oxidative stress, inflammation and acetaminophen exposure from birth to early childhood in the induction of autism. J. Int. Med. Res. 2017, 45, 407–438. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Genuis, S.J.; Frye, R.E. Environmental toxicants and autism spectrum disorders: A systematic review. Transl. Psychiatry 2014, 4, e360. [Google Scholar] [CrossRef]
- Quaak, I.; Brouns, M.R.; Van de Bor, M. The dynamics of autism spectrum disorders: How neurotoxic compounds and neurotransmitters interact. Int. J. Environ. Res. Public Health 2013, 10, 3384–3408. [Google Scholar] [CrossRef]
- Janecka, M.; Kodesh, A.; Levine, S.Z.; Lusskin, S.I.; Viktorin, A.; Rahman, R.; Buxbaum, J.D.; Schlessinger, A.; Sandin, S.; Reichenberg, A. Association of Autism Spectrum Disorder With Prenatal Exposure to Medication Affecting Neurotransmitter Systems. JAMA Psychiatry 2018, 75, 1217–1224. [Google Scholar] [CrossRef]
- Goines, P.E.; Ashwood, P. Cytokine dysregulation in autism spectrum disorders (ASD): Possible role of the environment. Neurotoxicology Teratol. 2012, 36, 67–81. [Google Scholar] [CrossRef]
- Goines, P.E.; Croen, L.A.; Braunschweig, D.; Yoshida, C.K.; Grether, J.; Hansen, R.; Kharrazi, M.; Ashwood, P.; Van de Water, J. Increased midgestational IFN-γ, IL-4 and IL-5 in women bearing a child with autism: A case-control study. Mol. Autism 2011, 2, 13. [Google Scholar] [CrossRef]
- Robinson-Agramonte, M.D.L.A.; García, E.N.; Guerra, J.F.; Hurtado, Y.V.; Antonucci, N.; Semprún-Hernández, N.; Schultz, S.; Siniscalco, D. Immune Dysregulation in Autism Spectrum Disorder: What Do We Know about It? Int. J. Mol. Sci. 2022, 23, 3033. [Google Scholar] [CrossRef]
- Frye, R.E. Mitochondrial Dysfunction in Autism Spectrum Disorder: Unique Abnormalities and Targeted Treatments. Semin. Pediatr. Neurol. 2020, 35, 100829. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).