Bark Beetles Utilize Ophiostomatoid Fungi to Circumvent Host Tree Defenses
Abstract
1. Introduction
2. Methods
2.1. Field Phloem Sample Collection
2.2. Laboratory Experiment
2.3. Chemical Analysis
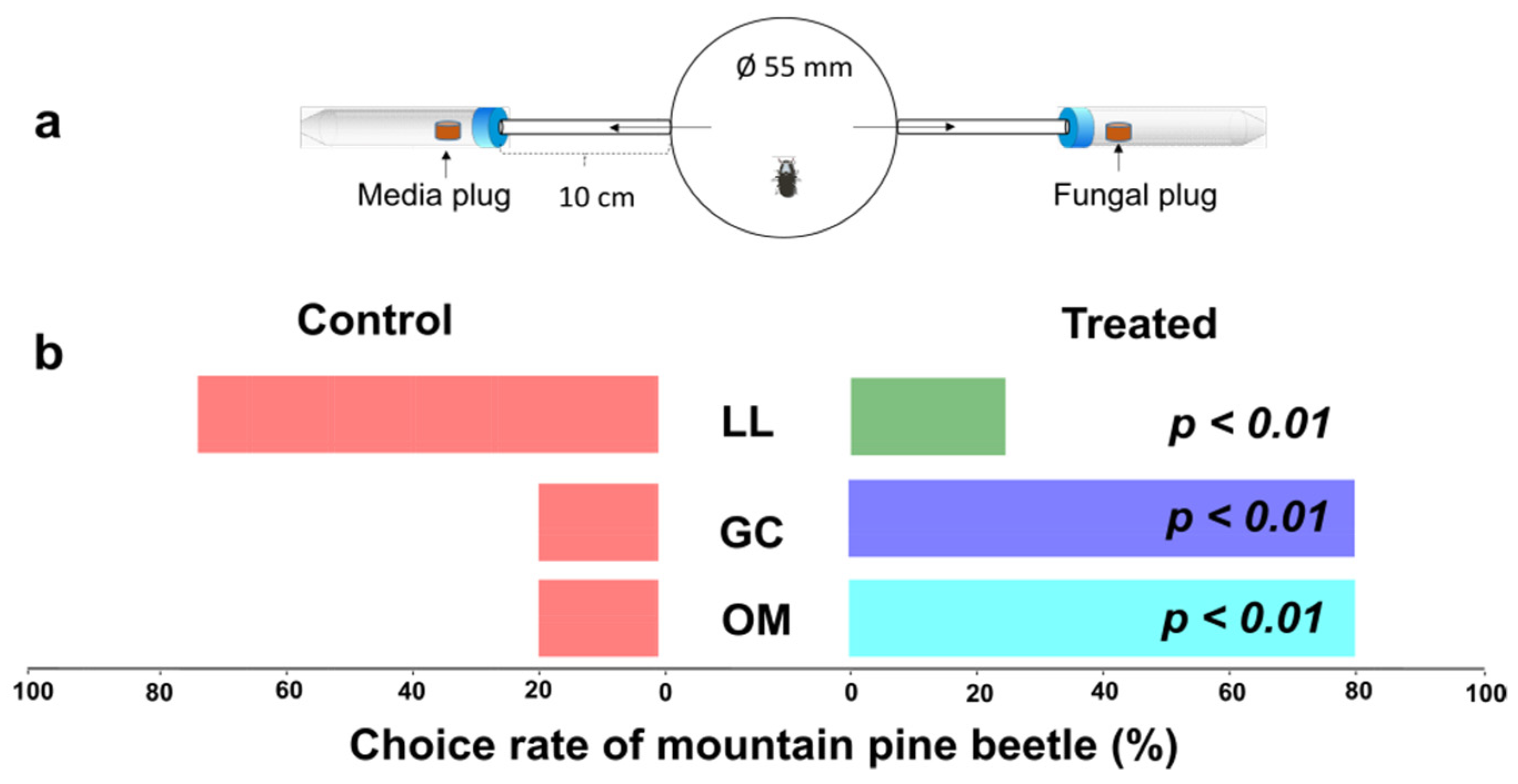
2.4. Two-Choice Olfactometer Test
2.5. Statistical Analysis
3. Results
3.1. Metabolic Profiles of Live P. contorta Trees Inoculated with Symbiotic Fungi
3.2. Kinetic Metabolic Pattern of Potential Biomarkers Following Fungal Infection
3.3. Effect of Fungal Inoculations on Chemotypic Traits of P. contorta Logs
3.4. Symbiotic Fungi Influence the Proportion of Oxygenated Monoterpenes
3.5. Mountain Pine Beetles Were Attracted to Their Symbiotic Fungi
4. Discussion
4.1. Several Monoterpene Biomarkers Are Associated with Tree Responses to Fungal Inoculations
4.2. Conversion of Monoterpenes to Oxygenated Derivatives Appears to Be a Common Strategy to Reduce the Toxicity among Bark Beetle
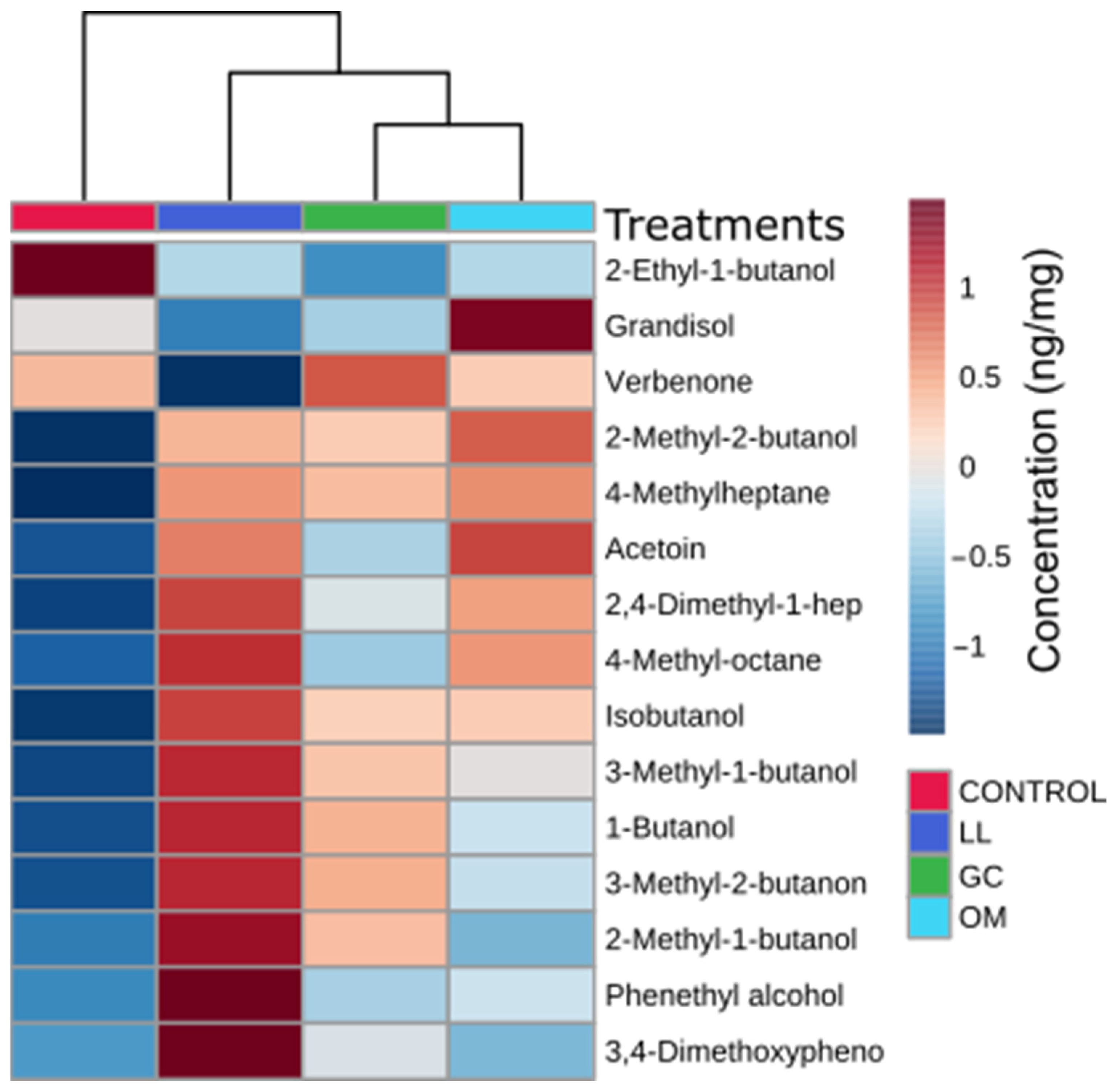
4.3. Fungal-Produced Volatile Organic Compounds Serve as Attractant Cues for Beetles
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frago, E.; Dicke, M.; Godfray, H.C.J. Insect Symbionts as Hidden Players in Insect-Plant Interactions. Trends Ecol. Evol. 2012, 27, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Blomquist, G.J.; Figueroa-Teran, R.; Aw, M.; Song, M.; Gorzalski, A.; Abbott, N.L.; Chang, E.; Tittiger, C. Pheromone Production in Bark Beetles. Insect Biochem. Mol. Biol. 2010, 40, 699–712. [Google Scholar] [CrossRef] [PubMed]
- VitÉ, J.P.; Pitman, G.B. Bark Beetle Aggregation: Effects of Feeding on the Release of Pheromones in Dendroctonus and Ips. Nature 1968, 218, 169–170. [Google Scholar] [CrossRef]
- Zhao, T.; Axelsson, K.; Krokene, P.; Borg-Karlson, A.K. Fungal Symbionts of the Spruce Bark Beetle Synthesize the Beetle Aggregation Pheromone 2-Methyl-3-Buten-2-Ol. J. Chem. Ecol. 2015, 41, 848–852. [Google Scholar] [CrossRef]
- Safranyik, L.; Wilson, B. The Mountain Pine Beetle: A Synthesis of Biology, Management, and Impacts on Lodgepole Pine; Canadian Forest Service: Victoria, BC, Canada, 2007; pp. 67–94.
- Six, D.L. Ecological and Evolutionary Determinants of Bark Beetle—Fungus Symbioses. Insects 2012, 3, 339–366. [Google Scholar] [CrossRef]
- Six, D.L. The Bark Beetle Holobiont: Why Microbes Matter. J. Chem. Ecol. 2013, 39, 989–1002. [Google Scholar] [CrossRef]
- Wadke, N.; Kandasamy, D.; Vogel, H.; Lah, L.; Wingfield, B.D.; Paetz, C.; Wright, L.P.; Gershenzon, J.; Hammerbacher, A. Catechol Dioxygenases Catalyzing the First Step in Norway Spruce Phenolic Degradation Are Key Virulence Factors in the Bark Beetle-Vectored Fungus Endoconidiophora polonica. Plant Physiol. 2016, 171, 914–931. [Google Scholar] [CrossRef]
- Zhao, T.; Kandasamy, D.; Krokene, P.; Chen, J.; Gershenzon, J.; Hammerbacher, A. Fungal Associates of the Tree-Killing Bark Beetle, Ips typographus, Vary in Virulence, Ability to Degrade Conifer Phenolics and Influence Bark Beetle Tunneling Behavior. Fungal Ecol. 2019, 38, 71–79. [Google Scholar] [CrossRef]
- Hammerbacher, A.; Schmidt, A.; Wadke, N.; Wright, L.P.; Schneider, B.; Bohlmann, J.; Brand, W.A.; Fenning, T.M.; Gershenzon, J.; Paetz, C. A Common Fungal Associate of the Spruce Bark Beetle Metabolizes the Stilbene Defenses of Norway Spruce. Plant Physiol. 2013, 162, 1324–1336. [Google Scholar] [CrossRef]
- Wang, Y.; Lim, L.; Madilao, L.; Lah, L.; Bohlmann, J.; Breuil, C. Gene Discovery for Enzymes Involved in Limonene Modification or Utilization by the Mountain Pine Beetle-Associated Pathogen Grosmannia clavigera. Appl. Environ. Microbiol. 2014, 80, 4566. [Google Scholar] [CrossRef]
- Lehenberger, M.; Foh, N.; Göttlein, A.; Six, D.; Biedermann, P.H.W. Nutrient-Poor Breeding Substrates of Ambrosia Beetles Are Enriched with Biologically Important Elements. Front. Microbiol. 2021, 12, 664542. [Google Scholar] [CrossRef]
- Cale, J.A.; Collignon, R.M.; Klutsch, J.G.; Kanekar, S.S.; Hussain, A.; Erbilgin, N. Fungal Volatiles Can Act as Carbon Sources and Semiochemicals to Mediate Interspecific Interactions among Bark Beetle-Associated Fungal Symbionts. PLoS ONE 2016, 11, e0162197. [Google Scholar] [CrossRef]
- Cale, J.A.; Ding, R.; Wang, F.; Rajabzadeh, R.; Erbilgin, N. Ophiostomatoid Fungi Can Emit the Bark Beetle Pheromone Verbenone and Other Semiochemicals in Media Amended with Various Pine Chemicals and Beetle-Released Compounds. Fungal Ecol. 2019, 39, 285–295. [Google Scholar] [CrossRef]
- Kandasamy, D.; Gershenzon, J.; Hammerbacher, A. Volatile Organic Compounds Emitted by Fungal Associates of Conifer Bark Beetles and Their Potential in Bark Beetle Control. J. Chem. Ecol. 2016, 42, 952–969. [Google Scholar] [CrossRef]
- Kandasamy, D.; Gershenzon, J.; Andersson, M.N.; Hammerbacher, A. Volatile Organic Compounds Influence the Interaction of the Eurasian Spruce Bark Beetle (Ips typographus) with Its Fungal Symbionts. ISME J. 2019, 13, 1788–1800. [Google Scholar] [CrossRef]
- Pureswaran, D.S.; Gries, R.; Borden, J.H.; Pierce, H.D. Dynamics of Pheromone Production and Communication in the Mountain Pine Beetle, Dendroctonus ponderosae Hopkins, and the Pine Engraver, Ips pini (Say) (Coleoptera: Scolytidae). Chemoecology 2000, 10, 153–168. [Google Scholar] [CrossRef]
- Jirošová, A.; Modlinger, R.; Hradecký, J.; Ramakrishnan, R.; Beránková, K.; Kandasamy, D. Ophiostomatoid Fungi Synergize Attraction of the Eurasian Spruce Bark Beetle, Ips typographus to Its Aggregation Pheromone in Field Traps. Front. Microbiol. 2022, 13, 980251. [Google Scholar] [CrossRef]
- Wermelinger:, B. Ecology and Management of the Spruce Bark Beetle Ips typographus—A Review of Recent Research. For. Ecol. Manag. 2004, 202, 67–82. [Google Scholar] [CrossRef]
- Raffa, K.F.; Aukema, B.H.; Bentz, B.J.; Carroll, A.L.; Hicke, J.A.; Turner, M.G.; Romme, W.H. Cross-Scale Drivers of Natural Disturbances Prone to Anthropogenic Amplification: The Dynamics of Bark Beetle Eruptions. Bioscience 2008, 58, 501–517. [Google Scholar] [CrossRef]
- Bentz, B.J.; Rgnire, J.; Fettig, C.J.; Hansen, E.M.; Hayes, J.L.; Hicke, J.A.; Kelsey, R.G.; Negron, J.F.; Seybold, S.J. Climate Change and Bark Beetles of the Western United States and Canada: Direct and Indirect Effects. Bioscience 2010, 60, 602–613. [Google Scholar] [CrossRef]
- Bohlmann, J.; Gershenzon, J.; Aubourg, S. Biochemical, Molecular, Genetic and Evolutionary Aspects of Defense-Related Terpenoid Metabolism in Conifers. In Evolution of Metabolic Pathways; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Franceschi, V.R.; Krokene, P.; Christiansen, E.; Krekling, T. Anatomical and Chemical Defenses of Conifer Bark against Bark Beetles and Other Pests. New Phytol. 2005, 167, 353–376. [Google Scholar] [CrossRef] [PubMed]
- Keeling, C.I.; Bohlmann, J. Genes, Enzymes and Chemicals of Terpenoid Diversity in the Constitutive and Induced Defence of Conifers against Insects and Pathogens. New Phytol. 2006, 170, 657–675. [Google Scholar] [CrossRef]
- Bohlmann, J. Insect-Induced Terpenoid Defenses in Spruce. In Induced Plant Resistance to Herbivory; Springer Science & Business Media: Berlin, Germany, 2008; ISBN 9781402081828. [Google Scholar]
- Krokene, P. Conifer Defense and Resistance to Bark Beetles. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega Fernando, E., Hofstetter, R.W., Eds.; Academic Press: London, UK, 2015; pp. 177–207. ISBN 9780124171732. [Google Scholar]
- Raffa, K.F.; Berryman, A.A. The Role of Host Plant Resistance in the Colonization Behavior and Ecology of Bark Beetles (Coleoptera: Scolytidae). Ecol. Monogr. 1983, 53, 27–49. [Google Scholar] [CrossRef]
- Klepzig, K.D.; Smalley, E.B.; Raffa, K.F. Combined Chemical Defenses against an Insect-Fungal Complex. J. Chem. Ecol. 1996, 22, 1367–1388. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Klutsch, J.G.; Erbilgin, N. Production of Complementary Defense Metabolites Reflects a Co-Evolutionary Arms Race between a Host Plant and a Mutualistic Bark Beetle-Fungal Complex. Plant Cell Environ. 2021, 44, 3064–3077. [Google Scholar] [CrossRef]
- Raffa, K.F.; Andersson, M.N.; Schlyter, F. Host Selection by Bark Beetles: Playing the Odds in a High-Stakes Game. Adv. Insect Phys. 2016, 50, 1–74. [Google Scholar] [CrossRef]
- Erbilgin, N.; Mori, S.R.; Sun, J.H.; Stein, J.D.; Owen, D.R.; Merrill, L.D.; Bolaños, R.C.; Raffa, K.F.; Montiel, T.M.; Wood, D.L.; et al. Response to Host Volatiles by Native and Introduced Populations of Dendroctonus valens (Coleoptera: Curculionidae, Scolytinae) in North America and China. J. Chem. Ecol. 2007, 33, 131–146. [Google Scholar] [CrossRef]
- Klimetzek, D.; Francke, W. Relationship between the Enantiomeric Composition of α-Pinene in Host Trees and the Production of Verbenols in Ips Species. Experientia 1980, 36, 1343–1345. [Google Scholar] [CrossRef]
- Lanne, B.S.; Ivarsson, P.; Johnsson, P.; Bergström, G.; Wassgren, A.B. Biosynthesis of 2-Methyl-3-Buten-2-Ol, a Pheromone Component of Ips typographus (Coleoptera: Scolytidae). Insect Biochem. 1989, 19, 163–167. [Google Scholar] [CrossRef]
- Bleiker, K.P.; Potter, S.E.; Lauzon, C.R.; Six, D.L. Transport of Fungal Symbionts by Mountain Pine Beetles. Can. Entomol. 2009, 141, 503–514. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.J.; Breuil, C. Diversity of Fungi Associated with the Mountain Pine Beetle, Dendroctonus ponderosae and Infested Lodgepole Pines in British Columbia. Fungal Divers. 2006, 22, 91–105. [Google Scholar]
- Roe, A.D.; James, P.M.A.; Rice, A.V.; Cooke, J.E.K.; Sperling, F.A.H. Spatial Community Structure of Mountain Pine Beetle Fungal Symbionts Across a Latitudinal Gradient. Microb. Ecol. 2011, 62, 347–360. [Google Scholar] [CrossRef]
- Erbilgin, N. Phytochemicals as Mediators for Host Range Expansion of a Native Invasive Forest Insect Herbivore. New Phytol. 2019, 221, 1268–1278. [Google Scholar] [CrossRef]
- Kandasamy, D.; Zaman, R.; Nakamura, Y.; Zhao, T.; Hartmann, H.; Andersson, M.N.; Hammerbacher, A.; Gershenzon, J. Bark Beetles Locate Fungal Symbionts by Detecting Volatile Fungal Metabolites of Host Tree Resin Monoterpenes. bioRxiv 2021. [Google Scholar] [CrossRef]
- Hulcr, J.; Mann, R.; Stelinski, L.L. The Scent of a Partner: Ambrosia Beetles Are Attracted to Volatiles from Their Fungal Symbionts. J. Chem. Ecol. 2011, 37, 1374–1377. [Google Scholar] [CrossRef]
- Wang, F.; Cale, J.A.; Hussain, A.; Erbilgin, N. Exposure to Fungal Volatiles Can Influence Volatile Emissions from Other Ophiostomatoid Fungi. Front. Microbiol. 2020, 11, 567462. [Google Scholar] [CrossRef]
- Guevara-Rozo, S.; Hussain, A.; Cale, J.A.; Klutsch, J.G.; Rajabzadeh, R.; Erbilgin, N. Nitrogen and Ergosterol Concentrations Varied in Live Jack Pine Phloem following Inoculations with Fungal Associates of Mountain Pine Beetle. Front. Microbiol. 2020, 11, 1703. [Google Scholar] [CrossRef]
- Liu, Y.; Anastacio, G.R.; Ishangulyyeva, G.; Rodriguez-Ramos, J.C.; Erbilgin, N. Mutualistic Ophiostomatoid Fungi Equally Benefit from Both a Bark Beetle Pheromone and Host Tree Volatiles as Nutrient Sources. Microb. Ecol. 2021, 81, 1106–1110. [Google Scholar] [CrossRef]
- Agbulu, V.; Zaman, R.; Ishangulyyeva, G.; Cahill, J.F.; Erbilgin, N. Host Defense Metabolites Alter the Interactions between a Bark Beetle and Its Symbiotic Fungi. Microb. Ecol. 2021, 84, 834–843. [Google Scholar] [CrossRef]
- Nones, S.; Sousa, E.; Holighaus, G. Symbiotic Fungi of an Ambrosia Beetle Alter the Volatile Bouquet of Cork Oak Seedlings. Phytopathology 2022, 112, 1965–1978. [Google Scholar] [CrossRef]
- Westrick, N.M.; Smith, D.L.; Kabbage, M. Disarming the Host: Detoxification of Plant Defense Compounds During Fungal Necrotrophy. Front. Plant Sci. 2021, 12, 684. [Google Scholar] [CrossRef] [PubMed]
- Erbilgin, N.; Ma, C.; Whitehouse, C.; Shan, B.; Najar, A.; Evenden, M. Chemical Similarity between Historical and Novel Host Plants Promotes Range and Host Expansion of the Mountain Pine Beetle in a Naïve Host Ecosystem. New Phytol. 2014, 201, 940–950. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Cardona, A. Fiji: An Open-Source Platform for Biological-Image Analysis. Nature Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A Web Server for Metabolomic Data Analysis and Interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, Y.; Hu, C.; Sun, X.; Li, Y.; Xu, N. Dynamic Metabolic Profiles of the Marine Macroalga Ulva Prolifera during Fragmentation-Induced Proliferation. PLoS ONE 2019, 14, e0214491. [Google Scholar] [CrossRef]
- Lieutier, F.; Yart, A.; Salle, A. Stimulation of Tree Defenses by Ophiostomatoid Fungi Can Explain Attack Success of Bark Beetles on Conifers. Ann. For. Sci 2009, 66, 801. [Google Scholar] [CrossRef]
- Kim, J.; Plattner, A.; Lim, Y.; Breuil, C. Comparison of Two Methods to Assess the Virulence of the Mountain Pine Beetle Associate, Grosmannia clavigera, to Pinus contorta. Scand. J. For. Res. 2008, 23, 98–104. [Google Scholar] [CrossRef]
- Plattner, A.; Kim, J.-J.; Diguistini, S.; Breuil, C. Variation in Pathogenicity of a Mountain Pine Beetle-Associated Blue-Stain Fungus, Grosmannia clavigera, on Young Lodgepole Pine in British Columbia. Can. J. Plant Pathol. 2008, 30, 457–466. [Google Scholar] [CrossRef]
- Rice, A.V.; Thormann, M.N.; Langor, D.W. Mountain Pine Beetle Associated Blue-Stain Fungi Cause Lesions on Jack Pine, Lodgepole Pine, and Lodgepole x Jack Pine Hybrids in Alberta. Can. J. Bot. 2007, 85, 307–315. [Google Scholar] [CrossRef]
- Zhao, T.; Krokene, P.; Hu, J.; Christiansen, E.; Björklund, N.; Långström, B.; Solheim, H.; Borg-Karlson, A.K. Induced Terpene Accumulation in Norway Spruce Inhibits Bark Beetle Colonization in a Dose-Dependent Manner. PLoS ONE 2011, 6, e26649. [Google Scholar] [CrossRef]
- Ben Jamaa, M.L.; Lieutier, F.; Yart, A.; Jerraya, A.; Khouja, M.L. The Virulence of Phytopathogenic Fungi Associated with the Bark Beetles Tomicus piniperda and Orthotomicus erosus in Tunisia. For. Pathol. 2007, 37, 51–63. [Google Scholar] [CrossRef]
- Brignolas, F.; Lieutier, F.; Sauvard, D.; Yart, A.; Drouet, A.; Claudot, A.C. Changes in Soluble-phenol Content of Norway-spruce (Picea abies) Phloem in Response to Wounding and Inoculation with Ophiostoma polonicum. Eur. J. For. Pathol. 1995, 25, 253–265. [Google Scholar] [CrossRef]
- Cale, J.A.; Klutsch, J.G.; Dykstra, C.B.; Peters, B.; Erbilgin, N. Pathophysiological Responses of Pine Defensive Metabolites Largely Lack Differences between Pine Species but Vary with Eliciting Ophiostomatoid Fungal Species. Tree Physiol. 2019, 39, 1121–1135. [Google Scholar] [CrossRef]
- Chiu, C.C.; Keeling, C.I.; Bohlmann, J. Toxicity of Pine Monoterpenes to Mountain Pine Beetle. Sci. Rep. 2017, 7, 6–13. [Google Scholar] [CrossRef]
- Cheng, S.S.; Chung, M.J.; Lin, C.Y.; Wang, Y.N.; Chang, S.T. Phytochemicals from Cunninghamia konishii Hayata Act as Antifungal Agents. J. Agric. Food Chem. 2012, 60, 124–128. [Google Scholar] [CrossRef]
- Everaerts, C.; Grégoire, J.-C.; Merlin, J. The Toxicity of Norway Spruce Monoterpenes to Two Bark Beetle Species and Their Associates. In Mechanisms of Woody Plant Defenses Against Insects; Springer: New York, NY, USA, 1988; pp. 335–344. [Google Scholar]
- Reid, M.L.; Sekhon, J.K.; LaFramboise, L.M. Toxicity of Monoterpene Structure, Diversity and Concentration to Mountain Pine Beetles, Dendroctonus ponderosae: Beetle Traits Matter More. J. Chem. Ecol. 2017, 43, 351–361. [Google Scholar] [CrossRef]
- Coats, J.R.; Karr, L.L.; Drewes, C.D. Toxicity and Neurotoxic Effects of Monoterpenoids. Am. Chem. Soc. Symp. Ser. 1991, 449, 305–316. [Google Scholar] [CrossRef]
- Werner, R.A. Toxicity of Repellency of 4-Allylanisole and Monoterpenes from White Spruce and Tamarack to the Spruce Beetle and Eastern Larch Beetle (Coleoptera: Scolytidae). Environ. Entomol. 1995, 24, 372–389. [Google Scholar] [CrossRef]
- Scalerandi, E.; Flores, G.A.; Palacio, M.; Defagó, M.T.; Carpinella, M.C.; Valladares, G.; Bertoni, A.; Palacios, S.M. Understanding Synergistic Toxicity of Terpenes as Insecticides: Contribution of Metabolic Detoxification in Musca domestica. Front. Plant Sci. 2018, 871, 1579. [Google Scholar] [CrossRef]
- Regnault-Roger, C.; Hamraoui, A. Fumigant Toxic Activity and Reproductive Inhibition Induced by Monoterpenes on Acanthoscelides obtectus (Say) (Coleoptera), a Bruchid of Kidney Bean (Phaseolus vulgaris L.). J. Stored Prod. Res. 1995, 31, 291–299. [Google Scholar] [CrossRef]
- Seth Davis, T.; Stewardship, R. Toxicity of Two Engelmann Spruce (Pinaceae) Monoterpene Chemotypes from the Southern Rocky Mountains to North American Spruce Beetle (Coleoptera: Scolytidae). Can. Entomol. 2020, 152, 790–796. [Google Scholar] [CrossRef]
- Saerens, S.M.G.; Delvaux, F.R.; Verstrepen, K.J.; Thevelein, J.M. Production and Biological Function of Volatile Esters in Saccharomyces Cerevisiae. Microb. Biotechnol. 2010, 3, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, J.F.; Franco, L.M.; Cools, T.L.; de Meester, L.; Michiels, J.; Wenseleers, T.; Hassan, B.A.; Yaksi, E.; Verstrepen, K.J. The Fungal Aroma Gene ATF1 Promotes Dispersal of Yeast Cells through Insect Vectors. Cell Rep. 2014, 9, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Luna, E.; Cranshaw, W.; Tisserat, N. Attraction of Walnut Twig Beetle Pityophthorus juglandis (Coleoptera: Curculionidae) to the Fungus Geosmithia morbida. Plant Heal. Prog. 2014, 15, 135–140. [Google Scholar] [CrossRef]
- Brand, J.M.; Schultz, J.; Barras, S.J.; Edson, L.J.; Payne, T.L.; Hedden, R.L. Bark-Beetle Pheromones Enhancement of Dendroctonus frontalis (Coleoptera: Scolytidae) Aggregation Pheromone by Yeast Metabolites in Laboratory Bioassays. Chem. Ecol. 1977, 3, 657–666. [Google Scholar] [CrossRef]
- Davis, T.S.; Crippen, T.L.; Hofstetter, R.W.; Tomberlin, J.K. Microbial Volatile Emissions as Insect Semiochemicals. J. Chem. Ecol. 2013, 39, 840–859. [Google Scholar] [CrossRef]


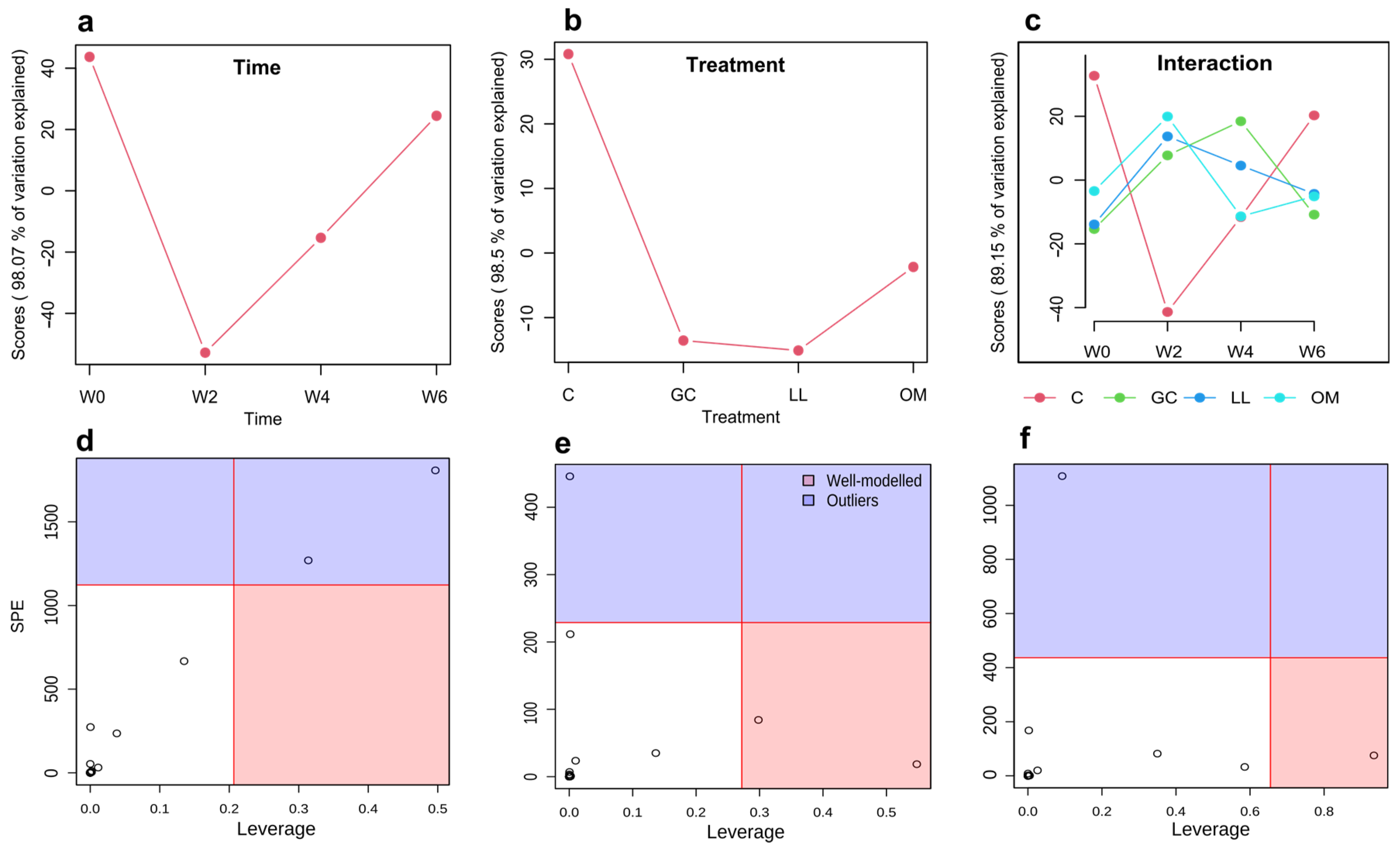
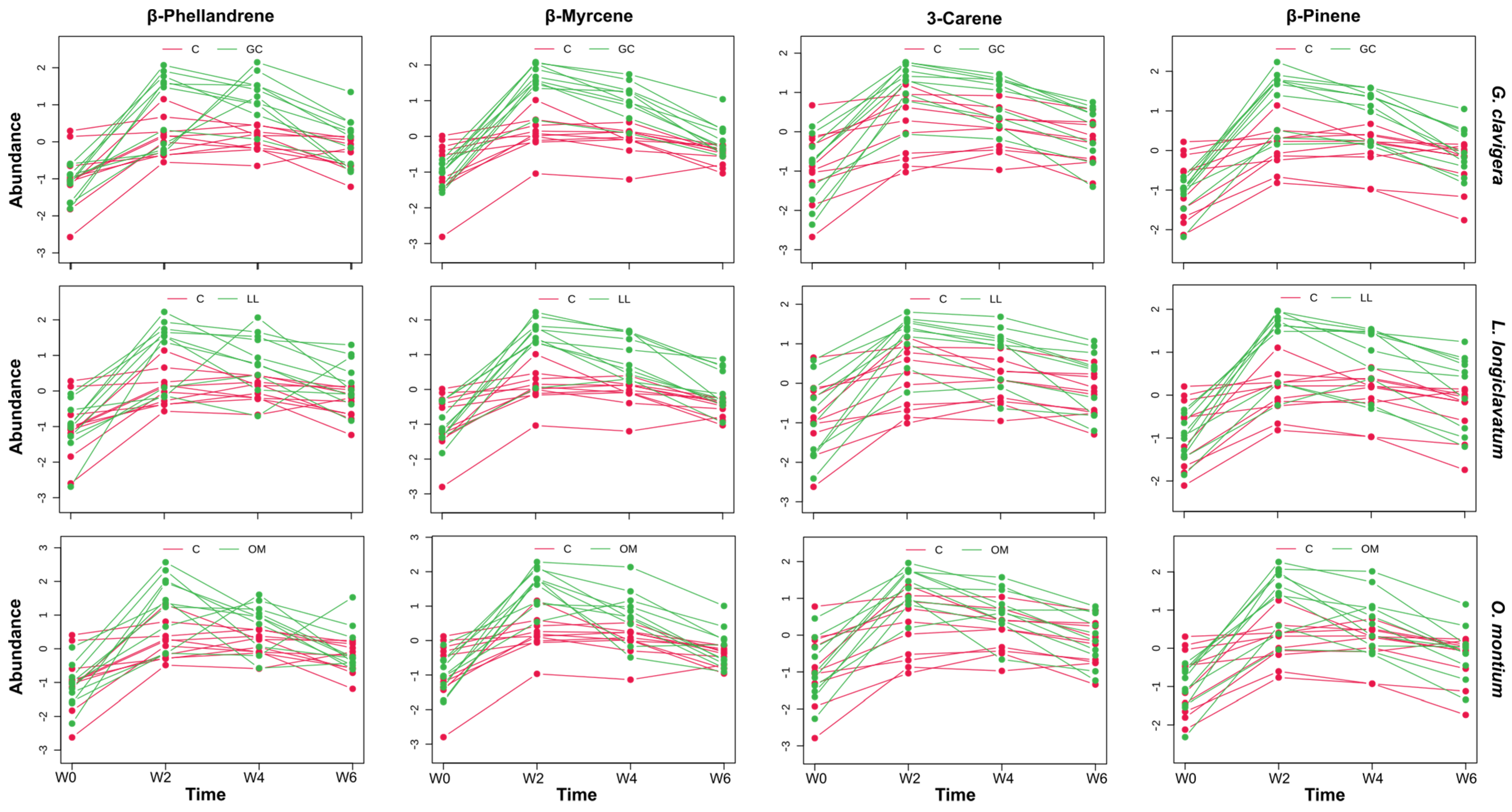
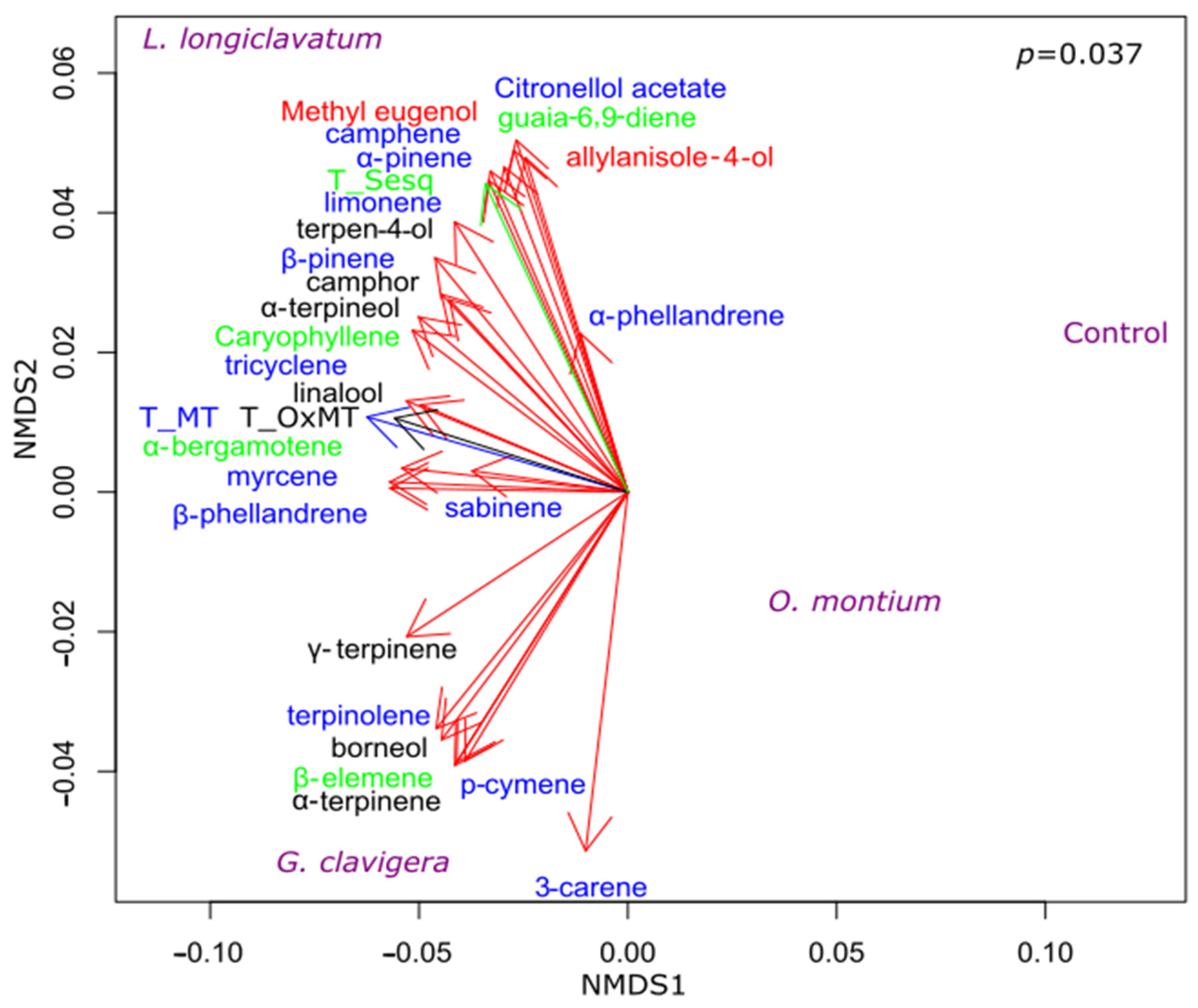
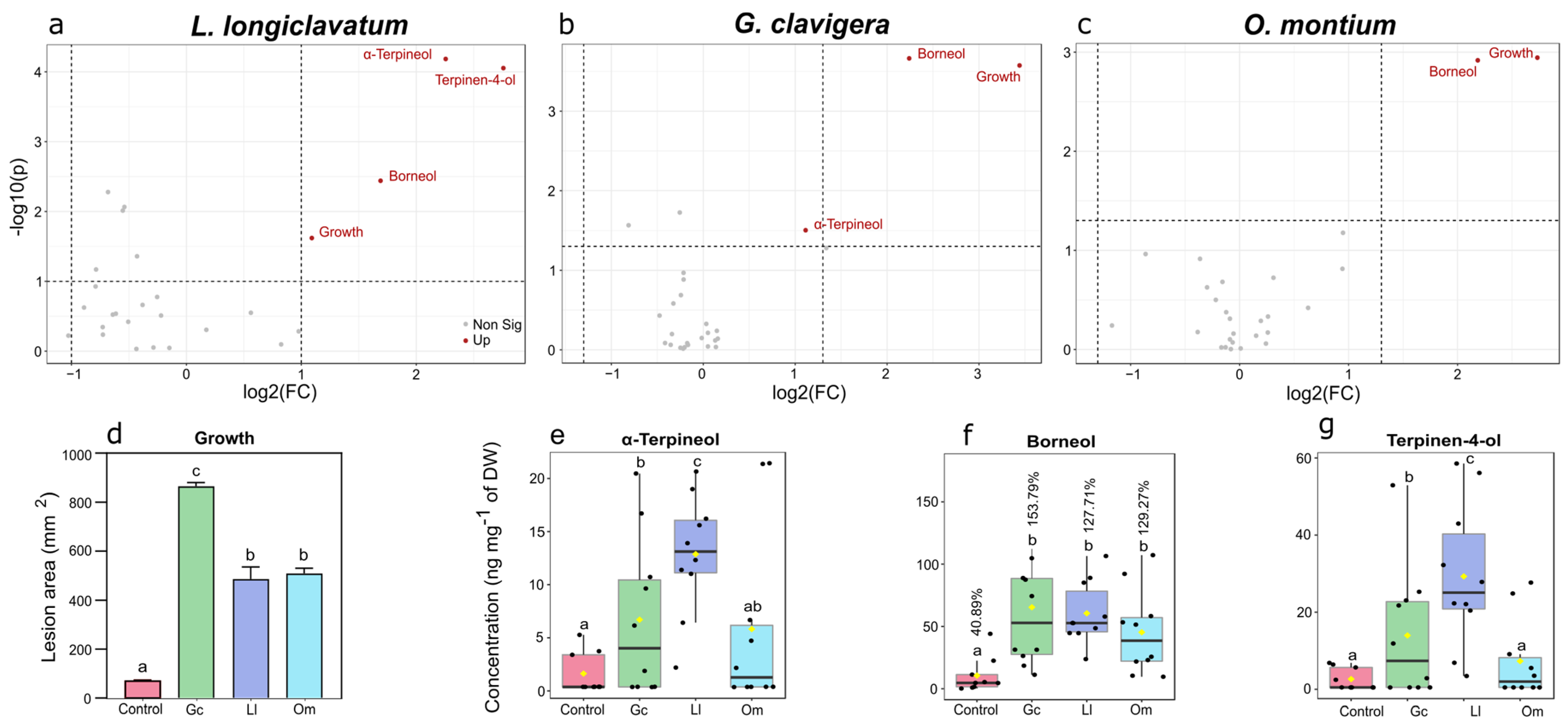
| Treatment | Time | Interaction | Hotelling-T2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabolites | F | p* | p^ | F | p* | p^ | F | p* | p^ | G | Ll | Om |
| β-Myrcene | 6.333 | 0.001 | 0.026 | 80.864 | 1.69 × 10−27 | 3.39 × 10−26 | 6.085 | 6.93 × 10−7 | 1.39 × 10−5 | 27.102 | 17.5 | 15.052 |
| β-Pinene | 5.311 | 0.004 | 0.026 | 43.314 | 1.86 × 10−18 | 5.31 × 10−18 | 4.387 | 6.72 × 10−5 | 0.001 | 23.415 | 21.095 | 13.926 |
| Camphene | 5.077 | 0.005 | 0.026 | 71.673 | 1.39 × 10−25 | 1.39 × 10−24 | 4.9301 | 1.52 × 10−5 | 0.000 | 8.676 | 5.779 | 5.2164 |
| β-Phellandrene | 5.043 | 0.005 | 0.026 | 30.098 | 3.20 × 10−14 | 6.41 × 10−14 | 2.994 | 0.003 | 0.010 | 61.257 | 25.006 | 44.444 |
| γ-Terpinene | 3.172 | 0.036 | 0.143 | 57.656 | 2.49 × 10−22 | 1.14 × 10−21 | 3.653 | 0.001 | 0.003 | 1.309 | 0.8404 | 0.616 |
| 3-Carene | 2.831 | 0.052 | 0.173 | 57.416 | 2.86 × 10−22 | 1.14 × 10−21 | 3.348 | 0.001 | 0.005 | 23.153 | 18.273 | 13.56 |
| Terpinolene | 2.654 | 0.063 | 0.181 | 49.326 | 3.69 × 10−20 | 1.23 × 10−19 | 2.975 | 0.003 | 0.010 | 16.078 | 11.555 | 9.421 |
| Terpinen-4-ol | 2.083 | 0.120 | 0.299 | 34.55 | 9.82 × 10−16 | 2.46 × 10−15 | 2.196 | 0.028 | 0.055 | 0.039 | 0.025 | 0.019 |
| Limonene | 1.924 | 0.143 | 0.318 | 22.144 | 2.99 × 10−11 | 5.43 × 10−11 | 2.533 | 0.011 | 0.025 | 10.265 | 8.910 | 9.106 |
| Borneol | 1.769 | 0.171 | 0.341 | 34.235 | 1.25 × 10−15 | 2.77 × 10−15 | 2.675 | 0.008 | 0.019 | 0.014 | 0.014 | 0.009 |
| p-Cymene | 1.349 | 0.274 | 0.456 | 68.208 | 8.04 × 10−25 | 5.36 × 10−24 | 1.217 | 0.292 | 0.450 | 0.021 | 0.103 | 0.021 |
| α-Pinene | 1.206 | 0.321 | 0.494 | 15.264 | 2.39 × 10−8 | 3.67 × 10−8 | 1.842 | 0.069 | 0.125 | 11.495 | 8.122 | 4.828 |
| α-Terpineol | 1.036 | 0.388 | 0.537 | 18.548 | 8.90 × 10−10 | 1.48 × 10−9 | 1.040 | 0.413 | 0.551 | 0.180 | 0.078 | 0.063 |
| Camphor | 0.959 | 0.423 | 0.537 | 14.371 | 6.05 × 10−8 | 8.64 × 10−8 | 1.130 | 0.348 | 0.498 | 0.001 | 0.001 | 0.001 |
| epi-13-Manool | 0.944 | 0.430 | 0.537 | 3.202 | 0.026 | 0.031 | 1.359 | 0.221 | 0.368 | 3.295 | 4.982 | 1.629 |
| Germacrene-D-4-ol | 0.284 | 0.837 | 0.877 | 5.457 | 0.002 | 0.002 | 0.581 | 0.811 | 0.897 | 3.313 | 1.627 | 1.228 |
| Bornyl acetate | 0.227 | 0.877 | 0.877 | 4.914 | 0.003 | 0.004 | 0.528 | 0.852 | 0.897 | 1.369 | 1.187 | 0.709 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaman, R.; May, C.; Ullah, A.; Erbilgin, N. Bark Beetles Utilize Ophiostomatoid Fungi to Circumvent Host Tree Defenses. Metabolites 2023, 13, 239. https://doi.org/10.3390/metabo13020239
Zaman R, May C, Ullah A, Erbilgin N. Bark Beetles Utilize Ophiostomatoid Fungi to Circumvent Host Tree Defenses. Metabolites. 2023; 13(2):239. https://doi.org/10.3390/metabo13020239
Chicago/Turabian StyleZaman, Rashaduz, Courtney May, Aziz Ullah, and Nadir Erbilgin. 2023. "Bark Beetles Utilize Ophiostomatoid Fungi to Circumvent Host Tree Defenses" Metabolites 13, no. 2: 239. https://doi.org/10.3390/metabo13020239
APA StyleZaman, R., May, C., Ullah, A., & Erbilgin, N. (2023). Bark Beetles Utilize Ophiostomatoid Fungi to Circumvent Host Tree Defenses. Metabolites, 13(2), 239. https://doi.org/10.3390/metabo13020239









