Characterization of Peptaibols Produced by a Marine Strain of the Fungus Trichoderma endophyticum via Mass Spectrometry, Genome Mining and Phylogeny-Based Prediction
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Fungal Acquisition
2.2. Fungal Identification
2.3. Phylogenetic Analyses
2.4. Fungal Fermentation and Extraction
2.5. Genome Mining and Prediction of the Peptaibol Modular Assembly
2.6. LC-MS/MS Analysis
2.7. Molecular Networking
3. Results
3.1. Fungal Phylogenetic Identificatio
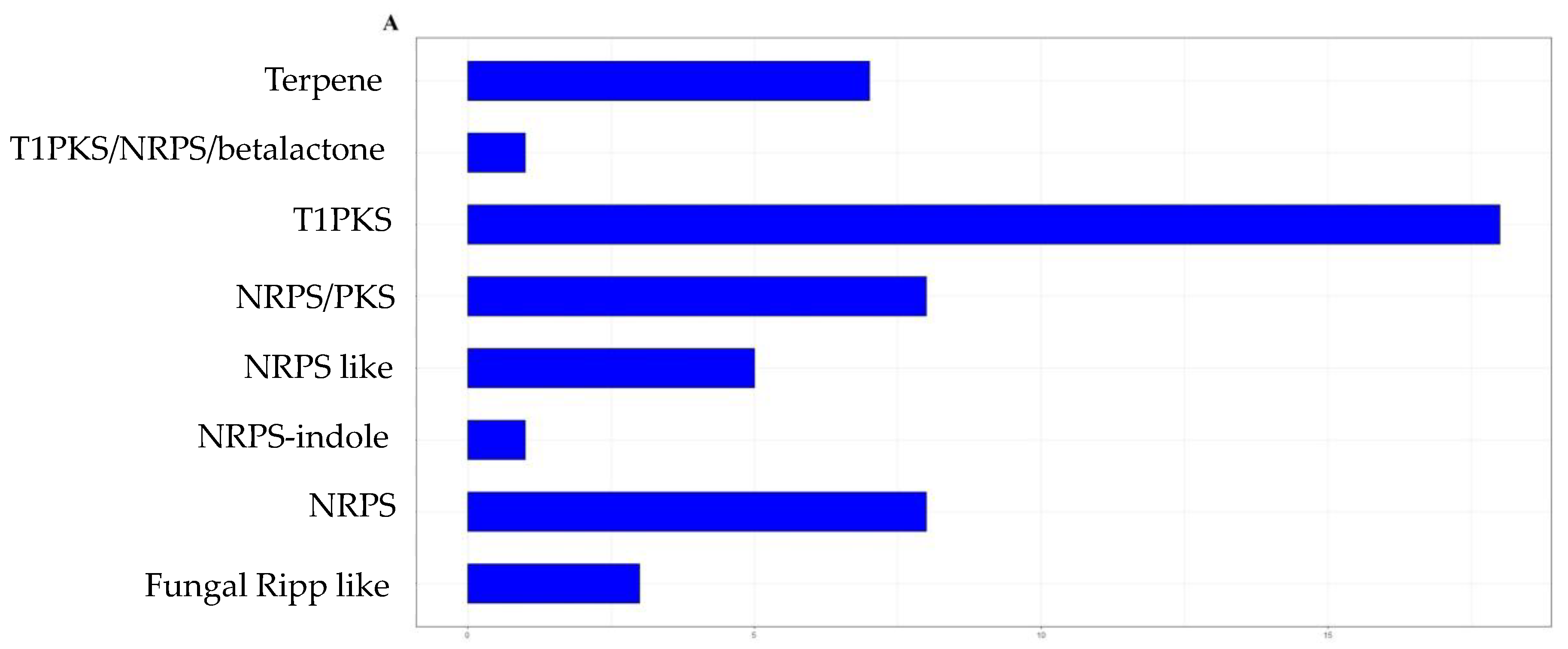
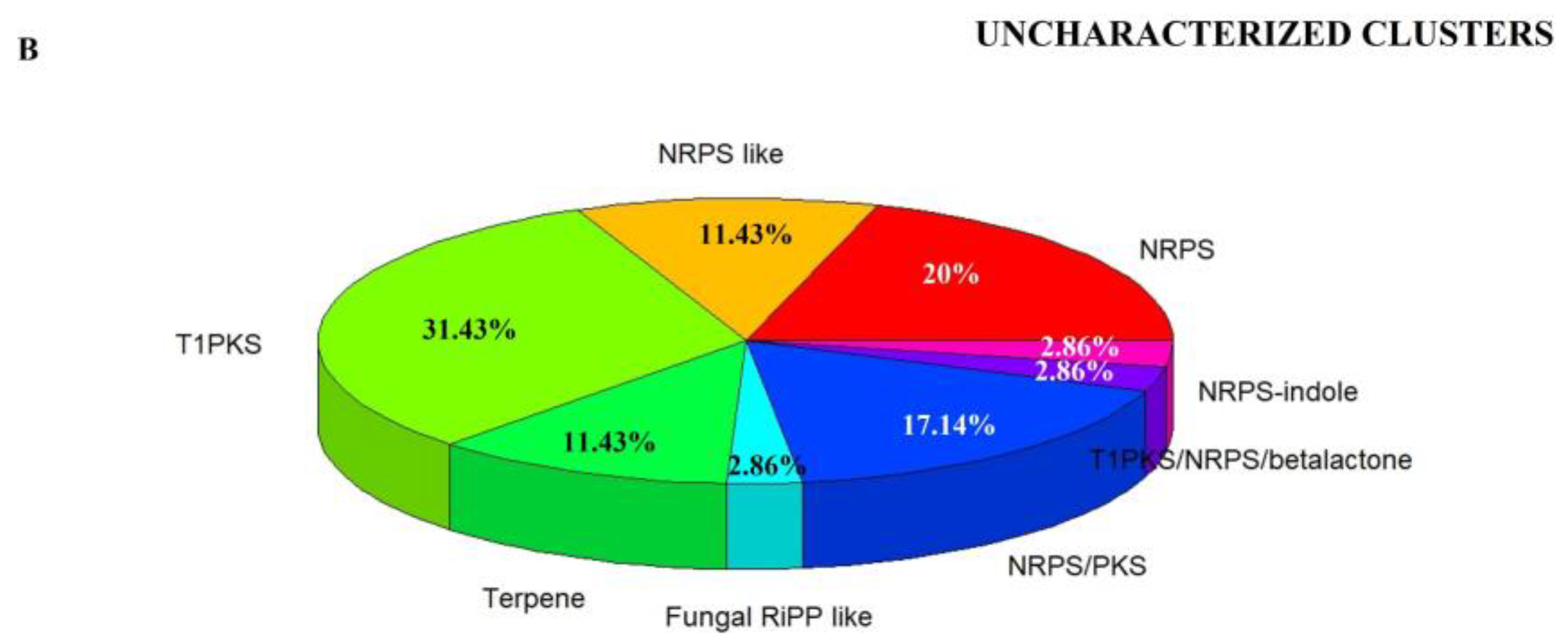
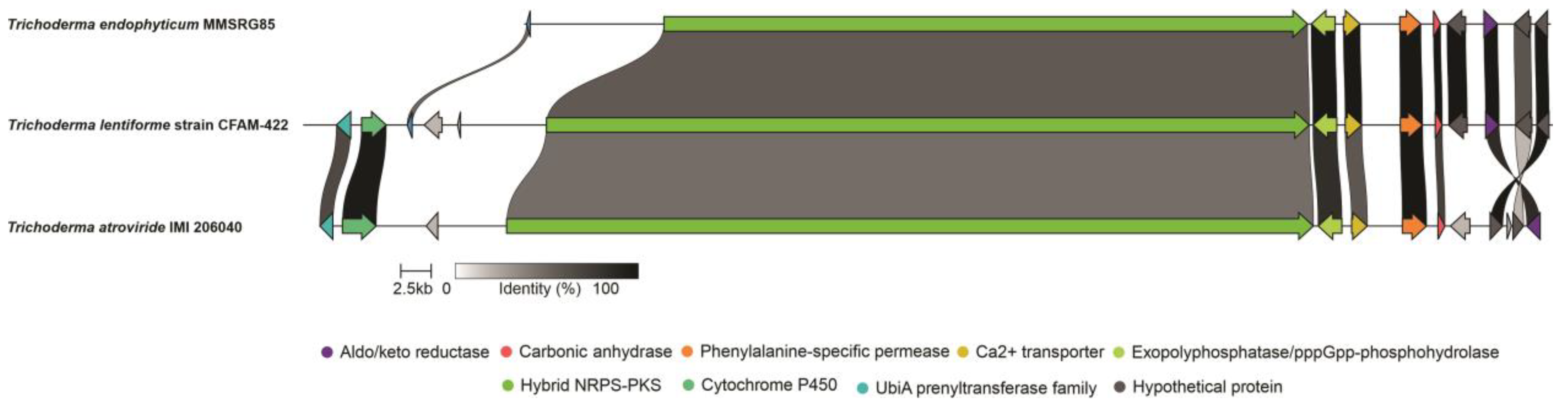
3.2. Genome Mining
3.3. Analysis of 14-Module NRPS and Prediction of Peptaibol Assembly
3.4. Analysis of 15-Module NRPS and Prediction of Peptaibol Assembly
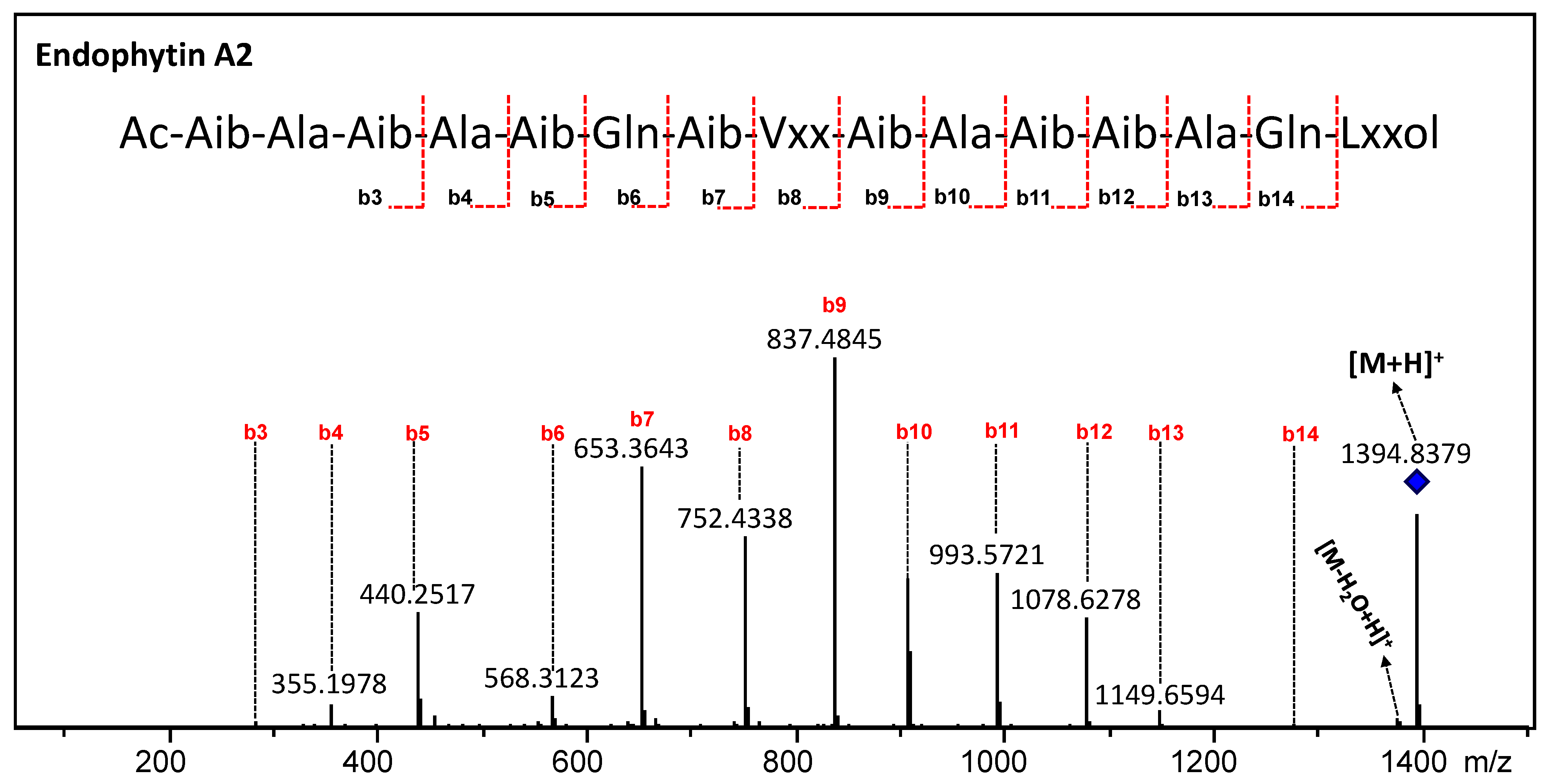
3.5. Annotation of the Peptaibols
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bisby, G.R. Trichoderma viride Pers. ex Fries, and notes on Hypocrea. Trans. Br. Mycol. Soc. 1939, 23, 149–168. [Google Scholar] [CrossRef]
- Cai, F.; Druzhinina, I.S. In honor of John Bissett: Authoritative guidelines on molecular identification of Trichoderma. Fungal Divers. 2021, 107, 1–69. [Google Scholar] [CrossRef]
- Kuhls, K.; Lieckfeldt, E.; Börner, T.; Guého, E. Molecular reidentification of human pathogenic Trichoderma isolates as Trichoderma longibrachiatum and Trichoderma citrinoviride. Med. Mycol. 1999, 37, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Sicher, R.C.; Kim, M.S.; Kim, S.H.; Strem, M.D.; Melnick, R.L.; Bailey, B.A. The beneficial endophyte Trichoderma hamatum isolate DIS 219b promotes growth and delays the onset of the drought response in Theobroma cacao. J. Exp. Bot. 2009, 60, 3279–3295. [Google Scholar] [CrossRef]
- Rodrigues, A.; Passarini, M.R.Z.; Ferro, M.; Nagamoto, N.S.; Forti, L.C., Jr.; Strem, M.B.; Sette, L.D.; Pagnocca, F.C. Fungal communities in the garden chamber soils of leaf-cutting ants. J. Basic Microbiol. 2014, 54, 1186–1196. [Google Scholar] [CrossRef]
- Ruano-Rosa, D.; Prieto, P.; Rincón, A.M.; Gómez-Rodríguez, M.V.; Valderrama, R.; Barroso, J.B.; Mercado-Blanco, J. Fate of Trichoderma harzianum in the olive rhizosphere: Time course of the root colonization process and interaction with the fungal pathogen Verticillium dahliae. BioControl 2016, 61, 269–282. [Google Scholar] [CrossRef]
- Safwan, S.; Wang, S.W.; Hsiao, G.; Hsiao, S.W.; Hsu, S.J.; Lee, T.H.; Lee, C.K. New trichothecenes isolated from the marine algicolous Fungus Trichoderma brevicompactum. Mar. Drugs 2022, 20, 80. [Google Scholar] [CrossRef]
- Mendes-Pereira, T.; Moreira, C.C.; Kloss, T.G.; Fonseca, P.L.C.; Elliot, S.L.; Loreto, R.G. Fungus-insect symbiosis: Diversity and negative ecological role of the hypocrealean fungus Trichoderma harzianum in colonies of neotropical termites (Blattodea: Termitidae). Fungal Ecol. 2022, 57–58, 101152. [Google Scholar] [CrossRef]
- Lorito, M.; Woo, S.L.; Harman, G.E.; Monte, E. Translational research on Trichoderma: From ‘omics to the field. Annu. Rev. Phytopathol. 2010, 48, 395–417. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Wiest, A.; Ruiz, N.; Keightley, A.; Moran-Diez, A.E.; McCluskey, K.; Pouchus, Y.F.; Kenerley, C.M. Two classes of new peptaibols are synthesized by a single non-ribosomal peptide synthetase of Trichoderma virens. J. Biol. Chem. 2011, 286, 4544–4554. [Google Scholar] [CrossRef]
- Ding, G.; Chen, L.; Zhou, C.; Hong-Mei, J.; Liu, Y.-T.; Chang, X.; Song, B.; Liu, X.-Z.; Gu, Y.-C.; Zou, Z.-M. Trichoderamides A and B, a pair of stereoisomers from the plant endophytic fungus Trichoderma gamsii. J. Antibiot. 2015, 68, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Moya, P.; Barrera, V.; Cipollone, J.; Bedoya, C.; Kohan, L.; Toledo, A.; Sisterna, M. New isolates of Trichoderma spp. as biocontrol and plant growth–promoting agents in the pathosystem Pyrenophora teres-barley in Argentina. Biol. Control 2020, 141, 104152. [Google Scholar] [CrossRef]
- Degani, O.; Rabinovitz, O.; Becher, P.; Gordani, A.; Chen, A. Trichoderma longibrachiatum and Trichoderma asperellum confer growth promotion and protection against late wilt disease in the field. J. Fungus 2021, 7, 444. [Google Scholar] [CrossRef]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “secrets” of a multitalented biocontrol agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J. Trichoderma as biocontrol agent against pests: New uses for a mycoparasite. Biol. Control 2021, 159, 104634. [Google Scholar] [CrossRef]
- Ali, S.; Khan, M.J.; Anjum, M.M.; Khan, G.R.; Ali, N. Trichoderma harzianum modulates phosphate and micronutrient solubilization in the rhizosphere. Gesunde Pflanzen 2022, 74, 853–862. [Google Scholar] [CrossRef]
- Zaman, K.A.U.; Wu, X.; Sarotti, A.M.; Cao, S. New and bioactive polyketides from Hawaiian marine-derived fungus Trichoderma sp. FM652. Nat. Prod. Res. 2022, 36, 5984–5990. [Google Scholar] [CrossRef]
- Hao, M.J.; Chen, P.N.; Li, H.J.; Wu, F.; Zhang, G.Y.; Shao, Z.Z.; Liu, X.P.; Ma, W.Z.; XU, J.; Mahmud, T.; et al. β-Carboline alkaloids from the deep-sea fungus Trichoderma sp. MCCC 3A01244 as a new type of anti-pulmonary fibrosis agent that inhibits TGF-β/Smad Signaling Pathway. Front. Microbiol. 2022, 13, 947226. [Google Scholar] [CrossRef]
- Vicente, I.; Baroncelli, R.; Morán-Diez, M.E.; Bernardi, R.; Puntoni, G.; Hermosa, R.; Monte, E.; Vannacci, G.; Sarrocco, S. Combined comparative genomics and gene expression analyses provide insights into the terpene synthases inventory in Trichoderma. Microorganisms 2020, 8, 1603. [Google Scholar] [CrossRef]
- Daniel, J.F.D.S.; Rodrigues Filho, E. Peptaibols of Trichoderma. Nat. Prod. Rep. 2007, 24, 1128–1141. [Google Scholar] [CrossRef]
- Krause, C.; Kirschbaum, J.; Jung, G.; Brückner, H. Sequence diversity of the peptaibol antibiotic suzukacillin-A from the mold Trichoderma viride. J. Pept. Sci. 2005, 12, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Sun, R.; Feng, Y.; Zhang, R.; Zhu, T.; Che, Q.; Zhan, G.; Li, D. Peptaibols: Diversity, bioactivity, and biosynthesis. Eng. Microbiol. 2022, 2, 100026. [Google Scholar] [CrossRef]
- Degenkolb, T.; Gräfenhan, T.; Berg, A.; Nirenberg, H.I.; Gams, W.; Brückner, H. Peptaibiomics: Screening for polypeptide antibiotics (peptaibiotics) from plant-protective Trichoderma species. Chem. Biodivers. 2006, 3, 593–610. [Google Scholar] [CrossRef] [PubMed]
- Gavryushina, I.A.; Georgieva, M.L.; Kuvarina, A.E.; Sadykova, V.S. Peptaibols as potential antifungal and anticancer antibiotics: Current and foreseeable development. Appl. Biochem. Microbiol. 2021, 57, 556–563. [Google Scholar] [CrossRef]
- Lee, J.W.; Collins, J.E.; Wendt, K.L.; Chakrabarti, D.; Cichewicz, R.H. Leveraging peptaibol biosynthetic promiscuity for next generation antiplasmodial therapeutics. J. Nat. Prod. 2021, 84, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, N.; Borghese, C.; Gabbatore, L.; Morbiato, L.; Zotti, M.D.; Aldinucci, D. Analogs of a natural peptaibol exert anticancer activity in both cisplatin-and doxorubicin-resistant cells and in multicellular tumor spheroids. Int. J. Mol. Sci. 2021, 22, 8362. [Google Scholar] [CrossRef]
- Weber, T.; Marahiel, M.A. Exploring the domain structure of modular nonribosomal peptide synthetases. Structure 2001, 9, R3–R9. [Google Scholar] [CrossRef]
- Lott, J.S.; Lee, T.V. Revealing the inter-module interactions of multi-modular nonribosomal peptide synthetases. Structure 2017, 25, 693–695. [Google Scholar] [CrossRef]
- Süssmuth, R.D.; Mainz, A. Nicht-ribosomale peptidsynthese–prinzipien und perspektiven. Angew. Chem. 2017, 129, 3824–3878. [Google Scholar] [CrossRef]
- Skinnider, M.A.; Dejong, C.A.; Rees, P.N.; Johnston, C.W.; Li, H.; Webster, A.L.H.; Wyatt, M.A.; Magarvey, N.A. Genomes to natural products prediction informatics for secondary metabolomes (PRISM). Nucleic Acids Res. 2015, 43, 9645–9662. [Google Scholar] [CrossRef]
- Blin, K.; Kim, H.U.; Medema, M.H.; Weber, T. Recent development of antiSMASH and other computational approaches to mine secondary metabolite biosynthetic gene clusters. Brief Bioinform. 2019, 20, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.T.; Chen, H.; Keating, T.A.; Hubbard, B.K.; Losey, H.C.; Luo, L.; Marshall, C.G.; Miller, D.A.; Patel, H.M. Tailoring enzymes that modify nonribosomal peptides during and after chain elongation on NRPS assembly lines. Curr. Opin. Chem. Biol. 2001, 5, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Behsaz, B.; Bode, E.; Gurevich, A.; Shi, Y.N.; Grundmann, F.; Acharya, D.; Caraballo-Rodríguez, A.M.; Bouslimani, A.; Panitchpakdi, M.; Linck, A.; et al. Integrating genomics and metabolomics for scalable non-ribosomal peptide discovery. Nat. Commun. 2021, 12, 3225. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.; Azeredo, A.R.; Azeredo, R.P.; Azeredo, C.A.F. Avaliação do efeito e dosagens do Trichoderma endophyticum no crescimento e produtividade da cultura da soja (Glycine max): Evaluation of the effect and dosage of Trichoderma endophyticum on the growth and productivity of soybean (Glycine max) cultivation. Braz. J. Dev. 2022, 8, 59168–59188. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.J.W.T.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: Cambridge, MA, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef]
- Okonechnikov, K.; Golosova, O.; Fursov, M. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Nylander, J.A.A.; Ronquist, F.; Huelsenbeck, J.P.; Nieves-Aldrey, J.L. Bayesian phylogenetic analysis of combined data. Syst. Biol. 2004, 53, 47–67. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Lubis, M.D.S.; Sinaga, H.F.R.U.; Batubara, A.D.; Anggraini, E.M.; Saragih, F.S. Analisis desain grafis menggunakan teknologi komputer berbasis software CorelDraw. JTIK 2020, 4, 89–99. [Google Scholar]
- Prjibelski, A.; Antipov, D.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using SPAdes de novo assembler. Curr. Protoc. Bioinform. 2020, 70, e102. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, C.L.; Chooi, Y.H. Clinker & clustermap. js: Automatic generation of gene cluster comparison figures. Bioinformatics 2021, 37, 2473–2475. [Google Scholar] [CrossRef]
- Garg, N.; Kapono, C.A.; Lim, Y.W.; Koyama, N.; Vermeij, M.J.; Conrad, D.; Rohwer, F.; Dorrestein, P.C. Mass spectral similarity for untargeted metabolomics data analysis of complex mixtures. IJMS 2015, 377, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.C.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with global natural products social molecular networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Adusumilli, R.; Mallick, P. Conversão de dados com ProteoWizard msConvert. In Proteomics. Methods in Molecular Biology; Comai, L., Katz, J., Mallick, P., Eds.; Humana Press: New York, NY, USA, 2017; Volume 1550. [Google Scholar] [CrossRef]
- Kohl, M.; Wiese, S.; Warscheid, B. Cytoscape: Software for visualization and analysis of biological networks. In Data Mining in Proteomics. Methods in Molecular Biology; Hamacher, M., Eisenacher, M., Stephan, C., Eds.; Humana Press: New York, NY, USA, 2011; Volume 696. [Google Scholar] [CrossRef]
- Chaverri, P.; Branco-Rocha, F.; Gazis, R.; Degenkolb, T.; Samuels, G.J. Systematics of the Trichoderma harzianum species complex and the re-identification of commercial biocontrol strains. Mycologia 2015, 107, 558–590. [Google Scholar] [CrossRef]
- Barrera, V.A.; Iannone, L.; Romero, A.I.; Chaverri, P. Expanding the Trichoderma harzianum species complex: Three new species from Argentine natural and cultivated ecosystems. Mycologia 2021, 113, 1136–1155. [Google Scholar] [CrossRef]
- Armando, N.G.; Marfetán, J.A.; Folgarait, P.J. Trichoderma species associated with acromyrmex ant nests from Argentina and first report of Trichoderma lentiforme for the country. Darwiniana Nueva Serie 2017, 5, 72–82. [Google Scholar] [CrossRef]
- Kautsar, S.A.; Blin, K.; Shaw, S.; Navarro-Muñoz, J.C.; Terlouw, B.R.; Van Der Hooft, J.J.; Santen, J.A.V.; Tracanna, V.; Duran, H.G.S.; Andreu, V.P.; et al. MIBiG 2.0: A repository for biosynthetic gene clusters of known function. Nucleic Acids Res. 2020, 48, D454–D458. [Google Scholar] [CrossRef]
- Chen, M.; Liu, Q.; Gao, S.S.; Young, A.E.; Jacobsen, S.E.; Tang, Y. Genome mining and biosynthesis of a polyketide from a biofertilizer fungus that can facilitate reductive iron assimilation in plant. Proc. Natl. Acad. Sci. USA 2019, 116, 5499–5504. [Google Scholar] [CrossRef] [PubMed]
- Lingham, R.B.; Silverman, K.C.; Jayasuriya, H.; Kim, B.M.; Amo, S.E.; Wilson, F.R.; Rew, D.J.; Schaber, M.D.; Bergstrom, J.D.; Koblan, K.S.; et al. Clavaric acid and steroidal analogues as Ras-and FPP-directed inhibitors of human farnesyl-protein transferase. J. Nat. Prod. 1998, 41, 4492–4501. [Google Scholar] [CrossRef] [PubMed]
- Kubicek, C.P.; Komoń-Zelazowska, M.; Sándor, E.; Druzhinina, I.S. Facts and challenges in the understanding of the biosynthesis of peptaibols by Trichoderma. Chem. Biodivers. 2007, 4, 1068–1082. [Google Scholar] [CrossRef]
- Marik, T.; Tyagi, C.; Balázs, D.; Urbán, P.; Szepesi, Á.; Bakacsy, L.; Endre, G.; Rakk, D.; Szekeres, A.; Andersson, M.A.; et al. Structural diversity and bioactivities of peptaibol compounds from the Longibrachiatum clade of the filamentous fungal genus Trichoderma. Front. Microbiol. 2019, 10, 1434. [Google Scholar] [CrossRef] [PubMed]
- Van Bohemen, A.I.; Ruiz, N.; Zalouk-Vergnoux, A.; Michaud, A.; Robiou du Pont, T.; Druzhinina, I.; Atanasova, L.; Prado, S.; Bodo, B.; Meslet-Cladiere, L.; et al. Pentadecaibins I–V: 15-residue peptaibols produced by a marine-derived Trichoderma sp. of the Harzianum clade. J. Nat. Prod. 2021, 84, 1271–1282. [Google Scholar] [CrossRef]
- Degenkolb, T.; Aghcheh, R.K.; Dieckmann, R.; Neuhof, T.; Baker, S.E.; Druzhinina, I.S.; Kubicek, C.P.; Brückner, H.; Döhren, H.V. The production of multiple small peptaibol families by single 14-module peptide synthetases in Trichoderma/Hypocrea. Chem. Biodivers. 2012, 9, 499–535. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Horwitz, B.A.; Herrera-Estrella, A.; Schmoll, M.; Kenerley, C.M. Trichoderma research in the genome era. Annu. Rev. Phytopathol. 2013, 51, 105–129. [Google Scholar] [CrossRef]
- Rawa, M.S.A.; Nogawa, T.; Okano, A.; Futamura, Y.; Wahab, H.A.; Osada, H. Zealpeptaibolin, an 11-mer cytotoxic peptaibol group with 3 Aib-Pro motifs isolated from Trichoderma sp. RK10-F026. J. Antibiot. 2021, 74, 485–495. [Google Scholar] [CrossRef]
- Neuhof, T.; Dieckmann, R.; Druzhinina, I.S.; Kubicek, C.P.; Döhren, H.V. Intact-cell MALDI-TOF mass spectrometry analysis of peptaibol formation by the genus Trichoderma/Hypocrea: Can molecular phylogeny of species predict peptaibol structures? Microbiology 2007, 153, 3417–3437. [Google Scholar] [CrossRef]
- Zeilinger, S.; Gruber, S.; Bansal, R.; Mukherjee, P.K. Secondary metabolism in Trichoderma—Chemistry meets genomics. Fungal Biol. Rev. 2016, 30, 74–90. [Google Scholar] [CrossRef]
- Vignolle, G.A.; Mach, R.L.; Mach-Aigner, A.R.; Derntl, C. Novel approach in whole genome mining and transcriptome analysis reveal conserved RiPPs in Trichoderma spp. BMC Genom. 2020, 21, 258. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, J.; Mou, P.; Yan, Y.; Chen, M.; Tang, Y. Genome mining of cryptic tetronate natural products from a PKS-NRPS encoding gene cluster in Trichoderma harzianum t-22. Org. Biomol. Chem. 2021, 19, 1985–1990. [Google Scholar] [CrossRef]
- Quandt, C.A.; Bushley, K.E.; Spatafora, J.W. The genome of the truffle-parasite Tolypocladium ophioglossoides and the evolution of antifungal peptaibiotics. BMC Genom. 2015, 16, 553. [Google Scholar] [CrossRef] [PubMed]
- Tehan, R.M.; Blount, R.R.; Goold, R.L.; Mattos, D.R.; Spatafora, N.R.; Tabima, J.F.; Gazis, R.; Wang, C.; Ishmael, J.E.; Spatafora, J.W.; et al. Tolypocladamide H and the proposed Tolypocladamide NRPS in Tolypocladium species. J. Nat. Prod. 2022, 85, 1363–1373. [Google Scholar] [CrossRef] [PubMed]



| Ac-Aib-Ala-Aib-Ala-AA1-Gln-AA2-AA3-Aib-Ala-Aib-Aib-AA4-AA5-AA6ol | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Peptaibols | Compound a | Exp. m/z Δm/z (in ppm) | Chemical Formula | AA1 | AA2 | AA3 | AA4 | AA5 | AA6 |
| Endophytins Series A | |||||||||
| Endophytin A1 | 1 | 1394.8370 (−0.07) | C63H111N17O18 | Ala | Aib | Lxx | Ala | Gln | Lxxol |
| Endophytin A2 | 2 | 1394.8322 (−3.51) | C63H111N17O18 | Aib | Aib | Vxx | Ala | Gln | Lxxol |
| Endophytin A3 | 5 | 1408.8505 (1.56) | C64H113N17O18 | Aib | Aib | Vxx | Aib | Gln | Lxxol |
| Endophytin A4 | 7 | 1408.8424 (1.06) | C64H113N17O18 | Aib | Vxx | Vxx | Ala | Gln | Lxxol |
| Endophytin A5 | 8 | 1422.8657 (1.89) | C65H115N17O18 | Aib | Aib | Lxx | Aib | Gln | Lxxol |
| Endophytin A6 | 12 | 1422.8665 (1.56) | C64H112N16O19 | Vxx | Aib | Vxx | Ala | Gln | Lxxol |
| Endophytin | 11 | 1409.8345 (1.33) | C65H115N17O18 | Aib | Aib | Vxx | Aib | Glu | Lxxol |
| Endophytin A8 | 14 | 1436.8805 (2.43) | C66H117N17O18 | Aib | Vxx | Lxx | Aib | Gln | Lxxol |
| Endophytin A9 | 22 | 1436.8827 (8.28) | C65H114N16O19 | Vxx | Vxx | Lxx | Ala | Gln | Lxxol |
| Endophytin A10 | 15 | 1423.8642 (0.07) | C65H114N16O19 | Vxx | Vxx | Vxx | Ala | Glu | Lxxol |
| Endophytin A11 | 19 | 1423.8525 (0.90) | C66H117N17O18 | Aib | Vxx | Vxx | Aib | Glu | Lxxol |
| Endophytin A12 | 23 | 1450.8867 (−1.51) | C67H119N17O18 | Vxx | Vxx | Lxx | Aib | Gln | Lxxol |
| Endophytin A13 | 24 | 1437.8672 (0.55) | C66H116N16O19 | Vxx | Aib | Lxx | Aib | Glu | Lxxol |
| Endophytins series B | |||||||||
| Endophytin B1 | 25 | 1484.8862 (1.48) | C70H117N17O18 | Vxx | Vxx | Lxx | Aib | Gln | Pheol |
| Endophytin B2 | 26 | 1471.8369 (3.05) | C69H114N16O19 | Vxx | Aib | Lxx | Aib | Glu | Pheol |
| Endophytin B3 | 6 | 1442.8390 (6.72) | C67H111N17O18 | Aib | Aib | Vxx | Aib | Gln | Pheol |
| Endophytin B4 | 10 | 1456.8501 (1.78) | C68H113N17O18 | Aib | Aib | Lxx | Aib | Gln | Pheol |
| Endophytin B5 | 18 | 1456.8505 (2.37) | C69H115N17O18 | Vxx | Vxx | Vxx | Ala | Gln | Pheol |
| Endophytin B6 | 17 | 1470.8676 (−1.51) | C68H113N17O18 | Aib | Vxx | Vxx | Aib | Gln | Pheol |
| Endophytin B7 | 20 | 1470.8673 (0.74) | C69H115N17O18 | Vxx | Vxx | Vxx | Aib | Gln | Pheol |
| Endophytin B8 | 27 | 1485.8639 (−2.75) | C70H116N16O19 | Vxx | Vxx | Lxx | Aib | Glu | Pheol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, G.S.; Sousa, T.F.; da Silva, G.F.; Pedroso, R.C.N.; Menezes, K.S.; Soares, M.A.; Dias, G.M.; Santos, A.O.; Yamagishi, M.E.B.; Faria, J.V.; et al. Characterization of Peptaibols Produced by a Marine Strain of the Fungus Trichoderma endophyticum via Mass Spectrometry, Genome Mining and Phylogeny-Based Prediction. Metabolites 2023, 13, 221. https://doi.org/10.3390/metabo13020221
Castro GS, Sousa TF, da Silva GF, Pedroso RCN, Menezes KS, Soares MA, Dias GM, Santos AO, Yamagishi MEB, Faria JV, et al. Characterization of Peptaibols Produced by a Marine Strain of the Fungus Trichoderma endophyticum via Mass Spectrometry, Genome Mining and Phylogeny-Based Prediction. Metabolites. 2023; 13(2):221. https://doi.org/10.3390/metabo13020221
Chicago/Turabian StyleCastro, Gleucinei S., Thiago F. Sousa, Gilvan F. da Silva, Rita C. N. Pedroso, Kelly S. Menezes, Marcos A. Soares, Gustavo M. Dias, Aline O. Santos, Michel E. B. Yamagishi, Jéssica V. Faria, and et al. 2023. "Characterization of Peptaibols Produced by a Marine Strain of the Fungus Trichoderma endophyticum via Mass Spectrometry, Genome Mining and Phylogeny-Based Prediction" Metabolites 13, no. 2: 221. https://doi.org/10.3390/metabo13020221
APA StyleCastro, G. S., Sousa, T. F., da Silva, G. F., Pedroso, R. C. N., Menezes, K. S., Soares, M. A., Dias, G. M., Santos, A. O., Yamagishi, M. E. B., Faria, J. V., Januário, A. H., & Koolen, H. H. F. (2023). Characterization of Peptaibols Produced by a Marine Strain of the Fungus Trichoderma endophyticum via Mass Spectrometry, Genome Mining and Phylogeny-Based Prediction. Metabolites, 13(2), 221. https://doi.org/10.3390/metabo13020221









