Dietary Administration of Black Raspberries and Arsenic Exposure: Changes in the Gut Microbiota and Its Functional Metabolites
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Diets
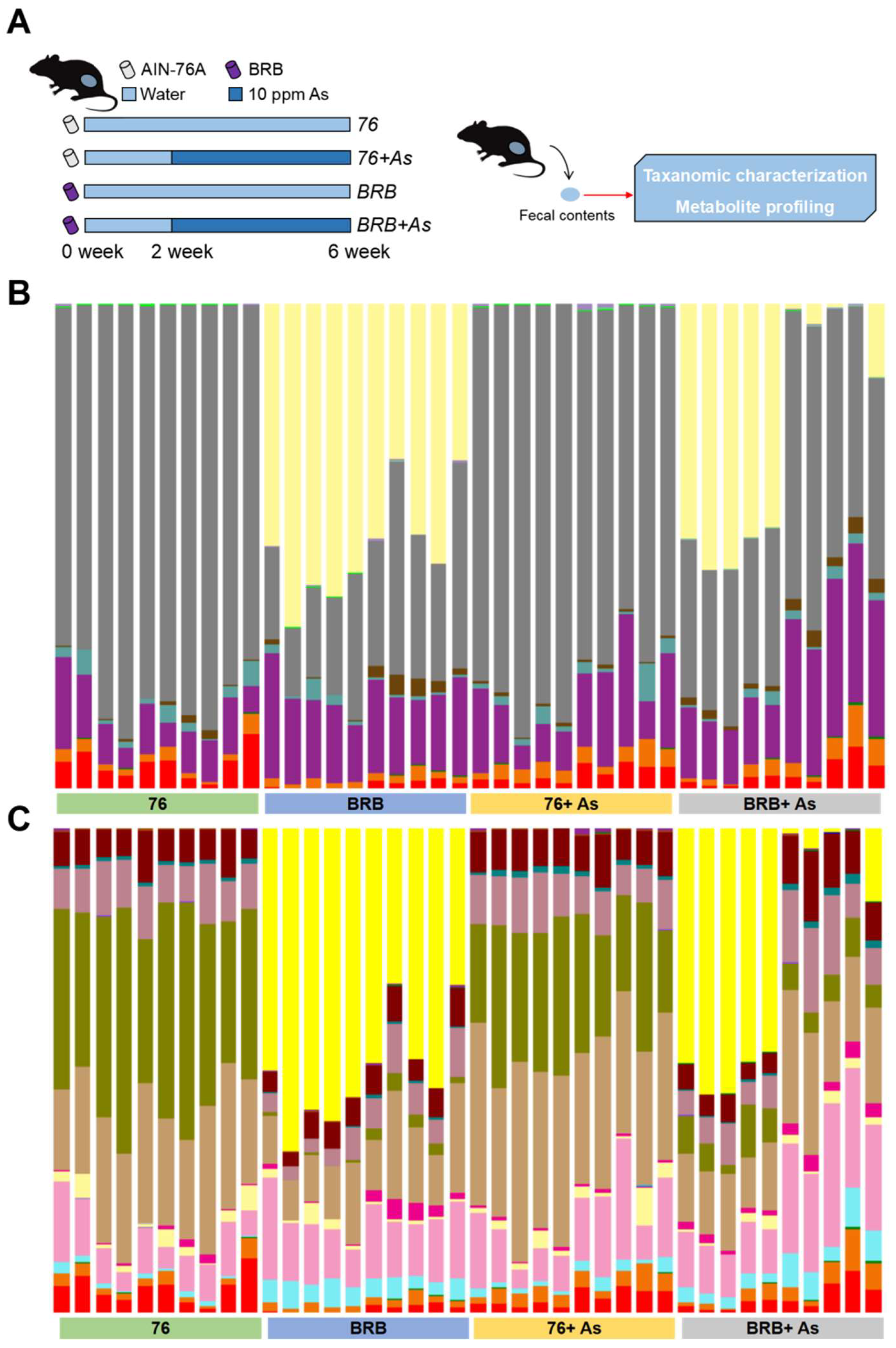
2.2. Workflow to Investigate Functional Alterations of the Gut Microbiome by Dietary Administration of BRBs upon As Exposure
2.3. Animals
2.4. 16S rRNA Gene Sequencing
2.5. Untargeted Metabolomic Analysis
2.6. Statistical Analysis
3. Results
3.1. Gut Microbial Changes at Compositional Level
3.2. Principal Coordinate Analysis (PCoA) and Alpha Rarefaction Analysis
3.3. Comparative Analysis of Metabolite Profiles
3.4. Key Metabolites Associated with Dietary Administration of BRBs upon As Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naujokas, M.F.; Anderson, B.; Ahsan, H.; Aposhian, H.V.; Graziano, J.H.; Thompson, C.; Suk, W.A. The broad scope of health effects from chronic arsenic exposure: Update on a worldwide public health problem. Environ. Health Perspect. 2013, 121, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.F.; Beck, B.D.; Chen, Y.; Lewis, A.S.; Thomas, D.J. Arsenic exposure and toxicology: A historical perspective. Toxicol. Sci. Off. J. Soc. Toxicol. 2011, 123, 305–332. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Abo, R.P.; Schlieper, K.A.; Graffam, M.E.; Levine, S.S.; Wishnok, J.S.; Swenberg, J.A.; Tannenbaum, S.R.; Fox, J.G. Arsenic exposure perturbs the gut microbiome and its metabolic profile in mice: An integrated metagenomics and metabolomics analysis. Environ. Health Perspect. 2014, 122, 284–291. [Google Scholar] [CrossRef]
- Chi, L.; Bian, X.; Gao, B.; Ru, H.; Tu, P.; Lu, K. Sex-Specific Effects of Arsenic Exposure on the Trajectory and Function of the Gut Microbiome. Chem. Res. Toxicol. 2016, 29, 949–951. [Google Scholar] [CrossRef] [PubMed]
- Chi, L.; Bian, X.; Gao, B.; Tu, P.; Ru, H.; Lu, K. The Effects of an Environmentally Relevant Level of Arsenic on the Gut Microbiome and Its Functional Metagenome. Toxicol. Sci. Off. J. Soc. Toxicol. 2017, 160, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Chi, L.; Lai, Y.; Tu, P.; Liu, C.-W.; Xue, J.; Ru, H.; Lu, K. Lipid and Cholesterol Homeostasis after Arsenic Exposure and Antibiotic Treatment in Mice: Potential Role of the Microbiota. Environ. Health Perspect. 2019, 127, 97002. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Li, N.; Duan, X.; Niu, H. Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 2017, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Van de Wiele, T.; Gallawa, C.M.; Kubachk, K.M.; Creed, J.T.; Basta, N.; Dayton, E.A.; Whitacre, S.; Laing, G.D.; Bradham, K. Arsenic metabolism by human gut microbiota upon in vitro digestion of contaminated soils. Environ. Health Perspect. 2010, 118, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Van de Wiele, T.; Vanhaecke, L.; Boeckaert, C.; Peru, K.; Headley, J.; Verstraete, W.; Siciliano, S. Human colon microbiota transform polycyclic aromatic hydrocarbons to estrogenic metabolites. Environ. Health Perspect. 2005, 113, 6–10. [Google Scholar] [CrossRef]
- Choi, J.J.; Eum, S.Y.; Rampersaud, E.; Daunert, S.; Abreu, M.T.; Toborek, M. Exercise attenuates PCB-induced changes in the mouse gut microbiome. Environ. Health Perspect. 2013, 121, 725–730. [Google Scholar] [CrossRef]
- Tu, P.; Chi, L.; Bodnar, W.; Zhang, Z.; Gao, B.; Bian, X.; Stewart, J.; Fry, R.; Lu, K. Gut Microbiome Toxicity: Connecting the Environment and Gut Microbiome-Associated Diseases. Toxics 2020, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Tu, P.; Bian, X.; Chi, L.; Gao, B.; Ru, H.; Knobloch, T.J.; Weghorst, C.M.; Lu, K. Characterization of the Functional Changes in Mouse Gut Microbiome Associated with Increased Population Modulated by Dietary Black Raspberries. ACS Omega 2018, 3, 10927–10937. [Google Scholar] [CrossRef]
- Tu, P.; Bian, X.; Chi, L.; Xue, J.; Gao, B.; Lai, Y.; Ru, H.; Lu, K. Metabolite Profiling of the Gut Microbiome in Mice with Dietary Administration of Black Raspberries. ACS Omega 2020, 5, 1318–1325. [Google Scholar] [CrossRef]
- Tu, P.; Chi, L.; Bian, X.; Gao, B.; Ru, H.; Lu, K. A Black Raspberry-Rich Diet Protects From Dextran Sulfate Sodium-Induced Intestinal Inflammation and Host Metabolic Perturbation in Association With Increased Aryl Hydrocarbon Receptor Ligands in the Gut Microbiota of Mice. Front. Nutr. 2022, 9, 842298. [Google Scholar] [CrossRef]
- Tu, P.; Xue, J.; Bian, X.; Chi, L.; Gao, B.; Leng, J.; Ru, H.; Knobloch, T.J.; Weghorst, C.M.; Lu, K. Dietary administration of black raspberries modulates arsenic biotransformation and reduces urinary 8-oxo-2′-deoxyguanosine in mice. Toxicol. Appl. Pharmacol. 2019, 377, 114633. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health. Part C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef] [PubMed]
- Kresty, L.A.; Morse, M.A.; Morgan, C.; Carlton, P.S.; Lu, J.; Gupta, A.; Blackwood, M.; Stoner, G.D. Chemoprevention of esophageal tumorigenesis by dietary administration of lyophilized black raspberries. Cancer Res. 2001, 61, 6112–6119. [Google Scholar] [PubMed]
- Stoner, G.D.; Wang, L.S.; Sardo, C.; Zikri, N.; Hecht, S.S.; Mallery, S.R. Cancer prevention with berries: Role of anthocyanins, in Bioactive compounds and cancer. In Bioactive Compounds and Cancer; Springer: Berlin/Heidelberg, Germany, 2010; pp. 703–723. [Google Scholar]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Kind, T.; Nakabayashi, R.; Yukihira, D.; Tanaka, W.; Cajka, T.; Saito, K.; Fiehn, O.; Arita, M. Hydrogen Rearrangement Rules: Computational MS/MS Fragmentation and Structure Elucidation Using MS-FINDER Software. Anal. Chem. 2016, 88, 7946–7958. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Milani, C.; de Giori, G.S.; Sesma, F.; van Sinderen, D.; Ventura, M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar] [CrossRef]
- Liu, K.Y.; Nakatsu, C.H.; Jones-Hall, Y.; Kozik, A.; Jiang, Q. Vitamin E alpha- and gamma-tocopherol mitigate colitis, protect intestinal barrier function and modulate the gut microbiota in mice. Free. Radic. Biol. Med. 2021, 163, 180–189. [Google Scholar] [CrossRef]
- Cai, J.; Sun, L.; Gonzalez, F.J. Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe 2022, 30, 289–300. [Google Scholar] [CrossRef]
- Claus, S.P.; Ellero, S.L.; Berger, B.; Krause, L.; Bruttin, A.; Molina, J.; Paris, A.; Want, E.J.; de Waziers, I.; Cloarec, O.; et al. Colonization-induced host-gut microbial metabolic interaction. MBio 2011, 2, e00271-10. [Google Scholar] [CrossRef]
- Heijtz, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA. 2011, 108, 3047–3052. [Google Scholar] [CrossRef]
- Gao, B.; Bian, X.; Mahbub, R.; Lu, K. Sex-Specific Effects of Organophosphate Diazinon on the Gut Microbiome and Its Metabolic Functions. Environ. Health Perspect. 2017, 125, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.; Bouscarel, B. Bile acids and signal transduction: Role in glucose homeostasis. Cell. Signal. 2008, 20, 2180–2197. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, Y.; Gao, H.; Li, D.; Jiang, R.; Ge, L.; Tong, C.; Xu, K. Associations among Dietary Omega-3 Polyunsaturated Fatty Acids, the Gut Microbiota, and Intestinal Immunity. Mediat. Inflamm. 2021, 2021, 8879227. [Google Scholar] [CrossRef] [PubMed]
- Wallert, M.; Ziegler, M.; Wang, X.; Maluenda, A.; Xu, X.; Yap, M.L.; Witt, R.; Giles, C.; Kluge, S.; Hortmann, M.; et al. α-Tocopherol preserves cardiac function by reducing oxidative stress and inflammation in ischemia/reperfusion injury. Redox Biol. 2019, 26, 101292. [Google Scholar] [CrossRef]
- Demirel-Yalciner, T.; Sozen, E.; Ozaltin, E.; Sahin, A.; Ozer, N.K. Alpha-Tocopherol supplementation reduces inflammation and apoptosis in high cholesterol mediated nonalcoholic steatohepatitis. BioFactors 2021, 47, 403–413. [Google Scholar] [CrossRef]
- Lewis, E.D.; Meydani, S.N.; Wu, D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life 2019, 71, 487–494. [Google Scholar] [CrossRef]
- Abraham, A.; Kattoor, A.J.; Saldeen, T.; Mehta, J.L. Vitamin E and its anticancer effects. Crit. Rev. Food Sci. Nutr. 2019, 59, 2831–2838. [Google Scholar] [CrossRef]
- Yang, C.S.; Luo, P.; Zeng, Z.; Wang, H.; Malafa, M.; Suh, N. Vitamin E and cancer prevention: Studies with different forms of tocopherols and tocotrienols. Mol. Carcinog. 2020, 59, 365–389. [Google Scholar] [CrossRef]
- Schmidt, T.S.B.; Raes, J.; Bork, P. The Human Gut Microbiome: From Association to Modulation. Cell 2018, 172, 1198–1215. [Google Scholar] [CrossRef]
- Wolter, M.; Grant, E.T.; Boudaud, M.; Steimle, A.; Pereira, G.V.; Martens, E.C.; Desai, M.S. Leveraging diet to engineer the gut microbiome. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 885–902. [Google Scholar] [CrossRef]
- Lee, M.; Chang, E.B. Inflammatory Bowel Diseases (IBD) and the Microbiome-Searching the Crime Scene for Clues. Gastroenterology 2021, 160, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Knox, N.C.; Forbes, J.D.; Van Domselaar, G.; Bernstein, C.N. The Gut Microbiome as a Target for IBD Treatment: Are We There Yet? Curr. Treat. Options Gastroenterol. 2019, 17, 115–126. [Google Scholar] [CrossRef] [PubMed]



| Gut Bacteria | Mean Abundance (BRB+ As) | Mean Abundance (76+ As) | Up/Down | Fold Change | p-Value | q-Value |
|---|---|---|---|---|---|---|
| p_Bacteroidetes | ||||||
| c_Bacteroidia; o_Bacteroidales; f_Rikenellaceae; g_ | 0.04249 | 0.01664 | up | 2.6 | 0.003 | 0.042 |
| p_Firmicutes | ||||||
| c_Bacilli; o_Turicibacterales; f_Turicibacteraceae; g_Turicibacter | 0.01999 | 0.00503 | up | 4.0 | 0.001 | 0.025 |
| c_Clostridia; o_Clostridiales; f_; g_ | 0.17616 | 0.32849 | down | −1.9 | 0.001 | 0.025 |
| c_Clostridia; o_Clostridiales; f_Clostridiaceae; g_ | 0.06063 | 0.25752 | down | −4.2 | 0.001 | 0.025 |
| c_Clostridia; o_Clostridiales; f_Clostridiaceae; Other | 0.00006 | 0.00036 | down | −6.0 | 0.001 | 0.025 |
| c_Clostridia; o_Clostridiales; f_Lachnospiraceae; Other | 0.00012 | 0.00073 | down | −6.1 | 0.003 | 0.042 |
| c_Clostridia; o_Clostridiales; f_Ruminococcaceae; g_Anaerotruncus | 0.00004 | 0.00013 | down | −3.3 | 0.003 | 0.042 |
| c_Erysipelotrichi; o_Erysipelotrichales; f_Erysipelotrichaceae; g_Coprobacillus | 0.00003 | 0.00019 | down | −6.3 | 0.003 | 0.042 |
| p_Verrucomicrobia | ||||||
| c_Verrucomicrobiae; o_Verrucomicrobiales; f_Verrucomicrobiaceae; g_Akkermansia | 0.27359 | 0.00005 | up | 5471.8 | 0.001 | 0.025 |
| Metabolites | Formula | m/z | Mean Intensity (76+ As) | Mean Intensity (BRB+ As) | Up/Down | Fold Change | Class | HMDB ID |
|---|---|---|---|---|---|---|---|---|
| Glutarylcarnitine | C12H21NO6 | 276.1507 | 1,889,595.8 | 4,226,664.3 | up | 2.2 | Acyl carnitines | HMDB0013130 |
| D-Urobilinogen | C33H42N4O6 | 591.3179 | 4,540,924.1 | 10,010,036.4 | up | 2.2 | Bilirubins | HMDB0004158 |
| D-Urobilin | C33H40N4O6 | 589.3026 | 7,015,796.9 | 18,248,399.2 | up | 2.6 | Bilirubins | HMDB0004160 |
| 2-(3,4-dihydroxyphenyl)-8-[1-(2,4-dihydroxyphenyl)-3-(3,4-dihydroxyphenyl)-2-hydroxypropyl]-3,4-dihydro-2H-1-benzopyran-3,5,7-triol | C30H28O11 | 565.1689 | 383,161.4 | 27,032.6 | down | −14.2 | Catechins | n.a. |
| 2,4-Toluenediamine | C7H10N2 | 123.0911 | 1,251,131.3 | 671,048.6 | down | −1.9 | Diaminotoluenes | HMDB0041799 |
| Leucyl-phenylalanine | C15H22N2O3 | 279.171 | 1,583,370.0 | 9,250,466.2 | up | 5.8 | Dipeptides | HMDB0302841 |
| Trehalose 6-phosphate | C12H23O14P | 423.0907 | 420,035.5 | 40,736.4 | down | −10.3 | Disaccharide phosphates | HMDB0001124 |
| Riboflavin | C17H20N4O6 | 377.1487 | 1,382,839.9 | 831,508.2 | down | −1.7 | Flavins | n.a. |
| trans-Ferulic acid | C10H10O4 | 195.0674 | 47,598.4 | 22,572.8 | down | −2.1 | Hydroxycinnamic acids | n.a. |
| 1H-Indole-3-carboxaldehyde | C9H7NO | 146.0587 | 586,796.9 | 197,339.2 | down | −3.0 | Indoles | n.a. |
| Alpha-Linolenic acid | C18H30O2 | 279.2302 | 241,165.2 | 445,495.3 | up | 1.8 | Lineolic acids and derivatives | HMDB0001388 |
| (R)-lipoic acid | C8H14O2S2 | 207.0452 | 49,071.2 | 9526.7 | down | −5.2 | Lipoic acids and derivatives | n.a. |
| 12a-Hydroxy-3-oxocholadienic acid | C24H34O4 | 387.2529 | 129,220.8 | 260,182.1 | up | 2.0 | Monohydroxy bile acids, alcohols and derivatives | HMDB0000385 |
| Coproporphyrin III | C36H38N4O8 | 655.2771 | 5,374,874.8 | 11,839,347.1 | up | 2.2 | Porphyrins | HMDB0000570 |
| Pyrrolidine | C4H9N | 72.0799 | 350,852.3 | 1,307,818.2 | up | 3.7 | Pyrrolidines | HMDB0031641 |
| 13′-Carboxy-alpha-tocopherol | C29H48O4 | 461.358 | 194,959.3 | 293,584.2 | up | 1.5 | Tocopherols | HMDB0012555 |
| Triethylamine | C6H15N | 102.1287 | 636,910.8 | 1,085,599.8 | up | 1.7 | Trialkylamines | HMDB0032539 |
| 4a-Carboxy-4b-methyl-5a-cholesta-8,24-dien-3b-ol | C29H46O3 | 443.35 | 396,953.2 | 655,442.3 | up | 1.7 | Triterpenoids | HMDB0062383 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, P.; Tang, Q.; Mo, Z.; Niu, H.; Hu, Y.; Wu, L.; Chen, Z.; Wang, X.; Gao, B. Dietary Administration of Black Raspberries and Arsenic Exposure: Changes in the Gut Microbiota and Its Functional Metabolites. Metabolites 2023, 13, 207. https://doi.org/10.3390/metabo13020207
Tu P, Tang Q, Mo Z, Niu H, Hu Y, Wu L, Chen Z, Wang X, Gao B. Dietary Administration of Black Raspberries and Arsenic Exposure: Changes in the Gut Microbiota and Its Functional Metabolites. Metabolites. 2023; 13(2):207. https://doi.org/10.3390/metabo13020207
Chicago/Turabian StyleTu, Pengcheng, Qiong Tang, Zhe Mo, Huixia Niu, Yang Hu, Lizhi Wu, Zhijian Chen, Xiaofeng Wang, and Bei Gao. 2023. "Dietary Administration of Black Raspberries and Arsenic Exposure: Changes in the Gut Microbiota and Its Functional Metabolites" Metabolites 13, no. 2: 207. https://doi.org/10.3390/metabo13020207
APA StyleTu, P., Tang, Q., Mo, Z., Niu, H., Hu, Y., Wu, L., Chen, Z., Wang, X., & Gao, B. (2023). Dietary Administration of Black Raspberries and Arsenic Exposure: Changes in the Gut Microbiota and Its Functional Metabolites. Metabolites, 13(2), 207. https://doi.org/10.3390/metabo13020207









