1. Introduction
The development of new non-invasive and comfortable methods to diagnose various diseases is an urgent task in modern medicine. Exhaled breath [
1], exhaled breath condensate [
2], saliva [
1,
3], skin [
1,
4,
5], and urine [
1,
6] are intensively studied to develop new diagnostic approaches. Exhaled breath is especially interesting for diagnostic purposes since it can be obtained without any discomfort for patients [
7].
A few non-invasive tests have already been implemented in clinical practice: 13C-urea breath test in the diagnostics of Helicobacter pylori infection [
8], nitric oxide breath test in asthma, and allergic airway inflammation management [
9]. However, many diseases with high mortality rate are still diagnosed using complex and invasive procedures. Lung cancer remains the leading cause of death [
10], since the disease develops rapidly and asymptomatically at the initial stage and can be diagnosed only by harmful and invasive procedures such as low dose computed tomography (LDCT) and biopsy. Biopsy is an invasive procedure; LDCT scanning includes radiation exposure. As such, the development of new, accurate, simple to use and non-invasive methods for lung cancer diagnostics is highly required.
Lung cancer biomarkers can be identified using various analytical methods [
11,
12]. Among them, gas chromatography coupled with mass spectrometry (GC-MS) is extremely useful since it allows to conduct quantitative and qualitative analysis of the samples. Many scientists reported results of exhaled breath analysis using GC-MS [
13,
14,
15,
16,
17] to identify lung cancer biomarkers. Multidimensional gas chromatography seems to be particularly useful to consider such complex issues as biomarkers identification [
13].
GC-MS is a laborious method which demands extensive experience. Proton transfer reaction mass spectrometry (PTR-MS) [
18], selected ion flow tube mass spectrometry (SIFT MS) [
19], ion mobility spectrometry (IMS) [
20] are also applied to solve the task. They allow to perform fast analysis of exhaled breath without any sample preparation.
Another possible analytical scheme is obtaining the informative signal from the whole exhaled breath composition instead of searching for specific biomarkers. Electronic noses based on different sensor systems are vigorously applied to address the issue. The most popular electronic noses are Aeonose
® [
21] and Cyranose 320 [
22] which are commercially available. They were used to analyze exhaled breath for lung cancer diagnostics. There exist electronic noses based on a plethora of different sensor types such as colorimetric sensors [
23], nanomaterials [
24], quartz crystal microbalance sensors [
25,
26], and combined gas sensors [
27] for exhaled breath analysis.
It is hardly possible to diagnose lung cancer by a unique marker; no biomarker separately can diagnose the disease accurately enough to be applied in clinical practice. Mostly, the discrimination of lung cancer patients and healthy controls can be achieved applying statistical data analysis methods. As a rule, Wilcoxon rank sum test [
28,
29] or Mann–Whitney U-test [
18] are applied to identify statistically significant differences between exhaled breath samples of the investigated cohorts of people. Different machine learning algorithms, such as support vector machine [
22], k-nearest neighbor classifier [
27], and others [
13,
16,
19,
21,
30,
31], have been applied to create diagnostic models.
The results obtained by different research groups in the field of exhaled breath analysis for lung cancer diagnostics using various analytical methods are significantly different. No consistency in the set of biomarkers, statistical data analysis methods, and performance of predictive models is observed [
32]. Traditionally, the group of lung cancer patients includes patients with various exact diagnoses. The resulting products of metabolomic pathways excreted in exhaled breath can vary significantly for different kinds of malignancy. It can contribute to inconsistency of the results. On the other hand, patients with lung cancer can suffer from other diseases, which can also affect the results. To find lung cancer biomarkers, the scientists prefer to exclude the patients with other lung diseases. However, not only lung comorbidities, but other pathologies can alter VOC profile, such as diabetes, hypertension, and so on. Several researchers have presented the results of exhaled breath analysis for diagnosing other diseases: diabetes [
33], obesity [
34], heart failure [
35], and others. However, variation in VOC profile of lung cancer patients dependently from other non-pulmonary comorbidities has not been studied.
This research is devoted to the study of lung cancer variability and comorbidities. Effects of chronic heart failure, hypertension, anemia, acute cerebrovascular accident, obesity, and diabetes on VOC profile of lung cancer patients were studied. The influence of the most frequently occurring comorbidities among lung cancer patients was investigated for the first time. TD-GC-MS was used to analyze exhaled breath samples of lung cancer patients and healthy volunteers. VOC peak areas and their ratios were considered as quantitative parameters. Additionally, variation in exhaled breath composition of lung cancer patients dependently from tumor histological type, localization, TNM stage, and effect of chemotherapy was studied. The parameters, which varied dependently from treatment status and comorbidities, were not considered as putative lung cancer biomarkers. Two kinds of machine learning algorithms, namely, gradient boosted decision trees (GBDT) and artificial neural network (ANN), were applied for the creation of a diagnostic model.
3. Results
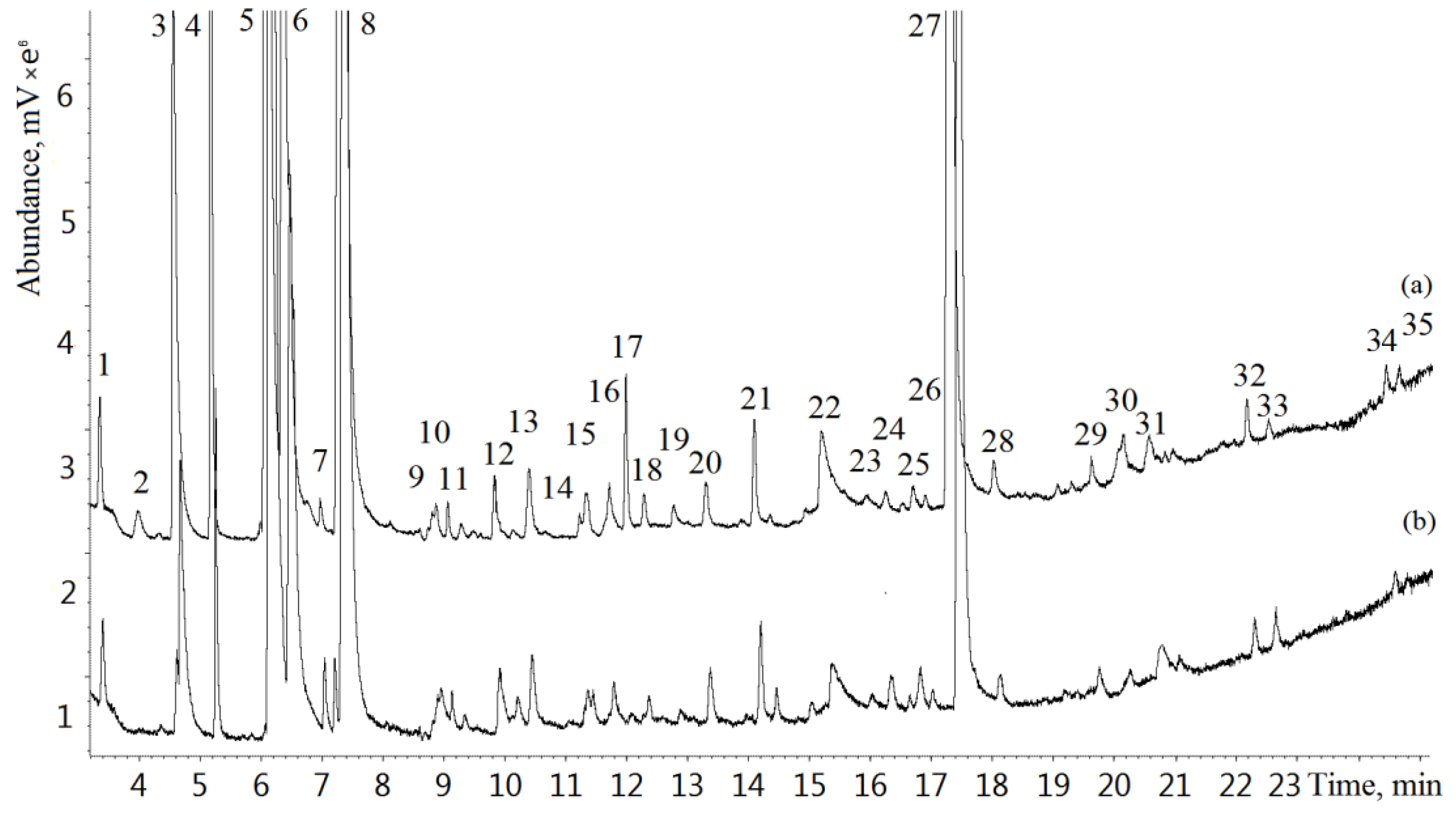
Exhaled breath samples of 112 lung cancer patients and 120 healthy volunteers were analyzed by GC-MS. Typical GC-MS chromatograms of exhaled breath samples from a lung cancer patient and a healthy volunteer are shown in
Figure 1. A total of 205 VOCs were identified in the study.
Table 3 represents the most frequently occurring compounds. The VOCs occurring in more than 50% of samples were used for statistical analysis.
Statistical analysis was conducted using VOC peak areas and their ratios. In case of ratios, to avoid division by zero, it is reasonable to use VOCs with frequency of occurring of 100% as a denominator, which was observed for acetone, isoprene, and dimethyl sulfide (
Table 3). To consider a wider list of ratios, it was rational to apply the VOCs occurring the most frequently in the samples of both groups as a denominator, which was observed for the first 10 VOCs (
Table 3). Among them, the lowest frequency was observed for acetonitrile. The frequency of occurring for rest compounds was lower and was different in the studied groups. These VOCs were applied only as a numerator.
At the initial step of the study, the correlation coefficients between VOC peak areas, their ratios and different factors were evaluated. Several ratios of 2-heptanone significantly correlated with the treatment status (before or under chemotherapy course): 2-heptanone/1-methylthiopropane, 2-heptanone/1-methylthiopropene, and 2-heptanone/dimethyl disulfide (correlation coefficients of −0.196, −0.206, −0.202, respectively).
The influence of different comorbidities on exhaled breath VOC profile of lung cancer patients was estimated using the correlation analysis. Statistically significant correlation between such comorbidities as anemia and acute cerebrovascular accident status, and VOC peak areas and their ratios was not found. Benzaldehyde/acetonitrile and benzaldehyde/2.3-butandione ratios significantly correlated with obesity status (correlation coefficients of 0.237 and 0.240). 2-Butanone (0.245) and some ratios of benzaldehyde and 2-butanone, i.e., benzaldehyde/allyl methyl sulfide (0.211), benzaldehyde/1-methylthiopropene (0.212), benzaldehyde/dimethyl sulfide (0.230), significantly correlated with diabetes status. Toluene and some ratios significantly correlated with both chronic heart failure and hypertension: toluene (−0.220 and −0.268), toluene/acetonitrile (−0.196 and −0.237), toluene/isoprene (−0.214 and −0.257), toluene/1-methylthiopropene (−0.208 and −0.257), toluene/dimethyl disulfide (−0.252 and −0.270), and 1-pentanol/dimethyl disulfide (−0.213 and −0.215). Significant correlation only with hypertension was observed for 1-pentanol (−0.205), toluene/allyl methyl sulfide (−0.236), 1-pentanol/2-butanone (−0.214), 1-pentanol/2,3-butandione (−0.218), and pentanal/acetone (−0.202).
In case of tumor localization, statistically significant correlations were found for 1-pentanol (correlation coefficient of 0.222), 1-pentanol/2,3-butandione (0.262), 1-pentanol/isoprene (0.210), 1-pentanol/acetone (0.193), dimethyl disulfide/acetonitrile (0.196), and 2-butanone/isoprene (0.191).
Statistically significant correlations were found between TNM stage and some VOC peak areas and their ratios (
Table 4).
In case of tumor histological type ranged by malignant course, statistically significant correlations were found only for some VOC peak area ratios (
Table 5).
Further, the correlation analysis was applied to find difference between the parameters of lung cancer patients and healthy individuals. Significant correlations between the disease status and peak areas of several VOC were found, i.e., acetone (−0.163), 1-methylthiopropene (0.140), 2-pentanone (0.244), hexane (−0.287), toluene (0.249), pentanal (−0.254), and dimethyl trisulfide (0.260). Additionally, a lot of ratios were significantly different between lung cancer patients and healthy volunteers. The ratios with the highest correlation coefficients were selected for the development of diagnostic models (
Table 6). The group of healthy volunteers was significantly younger than lung cancer patients. To avoid confounding influence of age, correlation between age and all ratios selected for the creation of diagnostic models was estimated in groups of lung cancer patients and healthy volunteers separately (
Table 6). None of ratios selected for the creation of diagnostic models had statistically significant correlation with age.
Diagnostic models were created using GBDT and ANN. The input values of each model represented the same set of 12 ratios (
Table 6). To provide reliability of diagnostic models, 3-fold cross validation method was applied. Performance of models created using 3 datasets is shown in
Table 7. The highest sensitivity on the training dataset was observed in case of GBDT. However, ANN diagnostic model has the best accuracy on the test dataset.
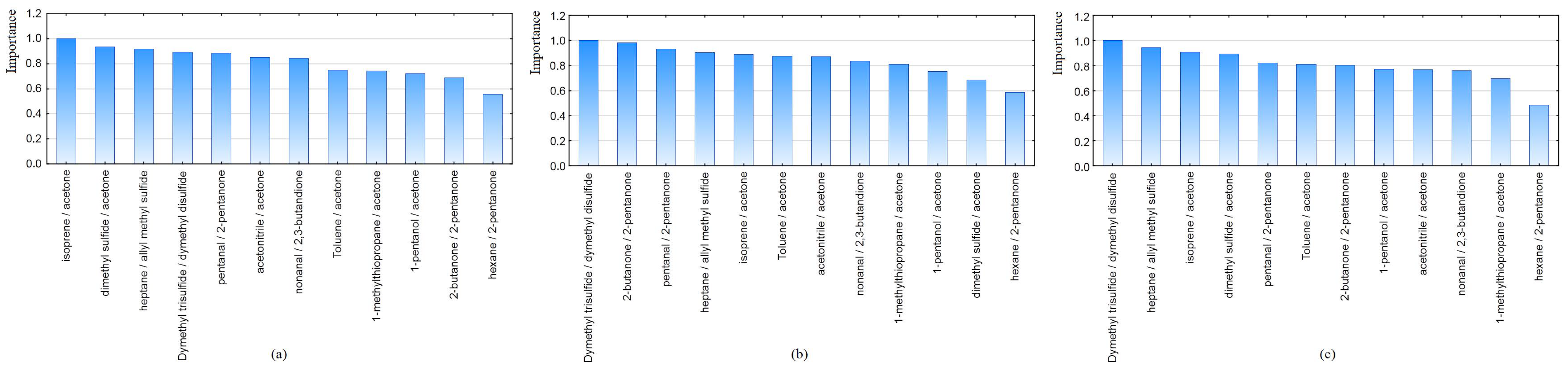
GBDT allows to estimate the importance of all variables which construct the model in relation to the most important ones. Bar plots illustrate the importance of the variables for each dataset (
Figure 2). As it can be seen, the ratio of hexane/2-pentanone contributes less to the prediction in all datasets, but ratios of dimethyl trisulfide/dimethyl disulfide and isoprene/acetone were the most significant for distinguishing the two groups.
4. Discussion
Different research groups have shown an ability of exhaled breath analysis to diagnose lung cancer [
17,
18,
19,
22,
31]. However, the results obtained by these groups were incoherent. Numerous analysis conditions, groups of volunteers, putative biomarker sets were used for the creation of diagnostic models, various learning algorithms and different performances of the models were obtained.
Inconformity of the results partially can be explained by variability of lung cancer groups in different studies. One of the aims of this study was to evaluate possible VOC profile variations in lung cancer group dependently from different factors. Considering that a part of lung cancer patients involved in the study was under the treatment, it was essential to evaluate the influence of treatment on exhaled breath composition. For this, correlations between the treatment status (before or under chemotherapy course) and VOC profile were calculated. Several ratios of 2-heptanone significantly correlated with the treatment status. The majority of other research groups have studied the effect of treatment only in case of surgery [
16,
38]. The results of exhaled breath analysis for monitoring response to treatment in lung cancer were demonstrated in work [
39]. The effect of different kinds of treatment was studied. Alterations in concentrations of dodecane, styrene, 4-methyldodecane, and α-phellandrene were observed after treatment. We did not consider these VOCs due to low frequency of occurring in samples. In our study, the majority of lung cancer patients were under the treatment. Therefore, it was essential to exclude the VOCs and ratios affected by the treatment status. It was found that a small number of ratios were affected by the treatment status. However, the issue should be considered in detail in further studies.
Another issue which can influence the results is comorbidities. The better part of scientists prefers to exclude patients with lung comorbidities when it comes to involving volunteers to the study. We also excluded patients with other lung comorbidities in the study. However, metabolic pathway of other pathologies can also lead to alterations in exhaled breath profile. In this study, for the first time, the effect of non-pulmonary comorbidities on exhaled breath profile of lung cancer patients was studied. Hypertension and diabetes affect the VOC profile the most. The parameters correlating with other pathologies should be excluded to avoid their effect on discrimination of lung cancer patients and heathy volunteers. The current study has several limitations considering comorbidities. First, we have not studied the effect of other lung comorbidities, which can influence other parameters. Second, not all possible comorbidities were considered. Third, the ratio of the patients with comorbidities was low compared with patients without them. Therefore, the list of parameters correlating with other comorbidities can be extended with increasing the cohort of participants. However, the analysis of comorbidities effect allows us to exclude parameters correlating with other diseases and eliminate their influence.
Exhaled breath composition can be varied dependently of tumor localization. A lung tumor localized in the central part is closer to the airways than peripheral, which can affect the alterations in VOC profile of patients differently. Further, 1-pentanol and some its ratios as well as dimethyl disulfide/acetonitrile and 2-butanone/isoprene significantly correlated with tumor localization. Differences in VOC profiles of lung cancer patients in relation to tumor localization have never been investigated by other researchers. This issue should be considered further to confirm the results.
In case of TNM stage, peak areas of 2-butanone, 1-methylthiopropene and some ratios (
Table 4) significantly correlated with TNM stage. Concentration of 2-butanone was found to be higher in advanced stages [
29], which was proved by our findings, since positive significant correlation with TNM stage was observed. However, in other studies, no differences were found in VOC profiles of patients with different stages of lung cancer [
15,
29,
40]. In this study, VOC profiles were considered based on the detailed TNM diagnosis instead of lung cancer stage (I, II, III, IV), which allows to reveal common tendencies not unclear when considering the groups separately. However, conformity with other works was observed, which proves the effect of disease stage on 2-butanone levels.
The results of different research groups concerning histological type effect are inconsistent. In a study [
15], no differences were found in exhaled breath composition of patients with different histological types. Statistically significant differences in 1-butanol and 3-hydroxy-2-butanone concentrations in samples of patients with adenocarcinoma and squamous cell carcinoma were found in a study [
29]. We have studied VOC profiles of patients with different histological types dependently from tumor malignancy (squamous cell carcinoma, adenocarcinoma, and small cell carcinoma). Statistical analysis has shown that no VOC significantly correlated with the tumor histological type. However, predominantly dimethyl trisulfide and 3-heptanone ratios (
Table 5) significantly correlated with the histological type. Considering the inconformity of the results obtained by different research groups, it is worthy to continue the study involving larger cohorts of participants.
Previously, we have optimized analysis conditions, proposed a new approach for the data analysis by using VOC ratios instead of VOC peak area values and demonstrated the efficiency of the proposed approach using different analytical methods (GC-FID and GC-MS) and different cohorts of participants [
36,
41]. In this work, we analyzed exhaled breath of a significantly larger cohort of volunteers by GC-MS. A wider range of VOC ratios was considered for the creation of diagnostic models as well. We used GBDT to estimate the contribution of ratios in predictive power of the model. Dimethyl trisulfide/dimethyl disulfide ratio contributes the most in classification of the groups. The same results were obtained earlier on a lower cohort of people [
41], which confirms reliability of the ratio as a lung cancer biomarker. ANN outperform GBDT in terms of performance on the test dataset with 82–88% sensitivity and 80–86% specificity. The performance of the previous model [
36] was higher (more than 90% for both sensitivity and specificity), which can be caused by several reasons: first, the larger cohort of people was involved in the present research and 30% of samples instead of 15% were assigned to the test dataset, which increases the reliability of the present models; second, in this research, the participants were fasted overnight before sampling, which can additionally decrease interfering effects; third, the number of smoking participants was equal; thus, the influence of smoking was eliminated. Most lung cancer patients are active smokers, which does not allow us to consider only the patients who do not smoke. However, cigarette smoking significantly influences exhaled breath composition [
42]. Thus, to consider smoking factor we had to involve a comparable number of smokers in both lung cancer patient and healthy volunteer groups. The group of healthy volunteers was significantly younger, than lung cancer patients. However, none of parameters selected for the creation of diagnostic models had statistically significant correlation with age. Further, it should be noted that the young participants (from 21 years old) were also present in the group of lung cancer patients. Unfortunately, the increasing trend in the number of young people among cancer patients should be considered.
Notably, significant correlations with the disease status were observed for ratios of toluene/acetonitrile, hexane/acetonitrile, and pentanal/isoprene. The same results were obtained in the previous research (correlation coefficients of 0.248, −0.307, −0.296, respectively). It evidences the robustness of the parameters as potential lung cancer biomarkers. Further study is required to confirm the reliance of other biomarkers found in this study.
Author Contributions
Conceptualization, V.A.P., I.S.P. and A.Z.T.; methodology A.Z.T. and E.M.G.; software, E.M.G.; validation, E.M.G., D.V.P. and E.V.D.; formal analysis, A.Z.T. and E.M.G.; investigation E.M.G.; resources A.Z.T.; data curation, E.M.G.; writing—original draft preparation E.M.G.; writing—review and editing, E.M.G., A.Z.T., D.V.P. and E.V.D.; visualization, E.M.G. and E.V.D.; supervision A.Z.T.; project administration A.Z.T.; funding acquisition A.Z.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian Science Foundation and the Kuban Science Foundation, grant number 22-13-20018 using the scientific equipment of the Center for Environmental Analysis at the Kuban State University.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by State budgetary healthcare institution “Research Institute–Regional Clinical Hospital № 1 named after Professor S.V. Ochapovsky”.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available on request due to restrictions eg privacy or ethical. The data presented in this study are available on request from the corresponding author. The data are not publicly available due to [ethical restrictions].
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gouzerh, F.; Bessiere, J.-M.; Ujvari, B.; Thomas, F.; Dujon, A.M. Laurent Dormont 6 Odors and cancer: Current status and future directions. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188644. [Google Scholar] [CrossRef]
- Campanella, A.; Summa, S.D.; Tommasi, S. Exhaled breath condensate biomarkers for lung cancer. J. Breath Res. 2019, 13, 044002. [Google Scholar] [CrossRef]
- Eftekhari, A.; Hasanzadeh, M.; Sharifi, S.; Dizaj, S.M.; Khalilov, R.; Ahmadian, E. Bioassay of saliva proteins: The best alternative for conventional methods in non-invasive diagnosis of cancer. Int. J. Biol. Macromol. 2019, 124, 1246–1255. [Google Scholar] [CrossRef]
- Vishinkin, R.; Busool, R.; Mansour, E.; Fish, F.; Esmail, A.; Kumar, P.; Ghara, A.; Cancilla, J.C.; Torrecilla, J.S.; Skenders, G.; et al. Profiles of volatile biomarkers detect tuberculosis from skin. Adv. Sci. 2021, 8, e2100235. [Google Scholar] [CrossRef]
- Monedeiro, F.; Dos Reis, R.B.; Peria, F.M.; Sares, C.T.G.; De Martinis, B.S. Investigation of sweat VOC profiles in assessment of cancer biomarkers using HS-GC-MS. J. Breath Res. 2020, 14, 026009. [Google Scholar] [CrossRef]
- Bax, C.; Taverna, G.; Eusebio, L.; Sironi, S.; Grizzi, F.; Guazzoni, G.; Capelli, L. Innovative diagnostic methods for early prostate cancer detection through urine analysis: A review. Cancers 2018, 10, 123. [Google Scholar] [CrossRef]
- Kaloumenou, M.; Skotadis, E.; Lagopati, N.; Efstathopoulos, E.; Tsoukalas, D. Breath Analysis: A Promising Tool for Disease Diagnosis—The Role of Sensors. Sensors 2022, 22, 1238. [Google Scholar] [CrossRef]
- Lopes, A.I.; Vale, F.F.; Oleastro, M. Helicobacter pylori infection—Recent developments in diagnosis. World J. Gastroenterol. 2014, 20, 9299–9313. [Google Scholar]
- Duong-Quy, S. Clinical utility of the exhaled nitric oxide (NO) measurement with portable devices in the management of allergic airway inflammation and asthma. J. Asthma Allergy 2019, 12, 331–341. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Choueiry, F.; Barham, A.; Zhu, J. Analyses of lung cancer-derived volatiles in exhaled breath and in vitro models. Exp. Biol. Med. 2022, 247, 1179–1190. [Google Scholar] [CrossRef]
- Hua, Q.; Zhu, Y.; Liu, H. Detection of volatile organic compounds in exhaled breath to screen lung cancer: A systematic review. Future Oncol. 2018, 14, 1647–1662. [Google Scholar] [CrossRef] [PubMed]
- Pesesse, R.; Stefanuto, P.-H.; Schleich, F.; Louis, R.; Focant, J.-F. Multimodal chemometric approach for the analysis of human exhaled breath in lung cancer patients by TD-GC × GC-TOFMS. J. Chromatogr. B 2019, 1114–1115, 146–153. [Google Scholar] [CrossRef]
- Phillips, M.; Altorki, N.; Austin, J.H.M.; Cameron, R.B.; Cataneo, R.N.; Kloss, R.; Maxfield, R.A.; Munawar, M.I.; Pass, H.I.; Rashid, A.; et al. Detection of lung cancer using weighted digital analysis of breath biomarkers. Clin. Chim. Acta 2008, 393, 76–84. [Google Scholar] [CrossRef]
- Ligor, T.; Pater, L.; Buszewski, B. Application of an artificial neural network model for selection of potential lung cancer biomarkers. J. Breath Res. 2015, 9, 027106. [Google Scholar] [CrossRef]
- Broza, Y.Y.; Kremer, R.; Tisch, U.; Gevorkyan, A.; Shiban, A.; Best, L.A.; Haick, H. Nanomaterial-based breath test for short-term follow-up after lung tumor resection. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 15–21. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; Wang, D.; Yu, K.; Wang, L.; Zou, Y.; Zhao, C.; Zhang, X.; Wang, P.; Ying, K. The analysis of volatile organic compounds biomarkers for lung cancer in exhaled breath, tissues and cell lines. Cancer Biomark. 2012, 11, 129–137. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, Y.; Sun, C.; Liu, H.; Wang, Y.; Jiang, X. Analysis of volatile organic compounds from patients and cell lines for the validation of lung cancer biomarkers by proton-transfer-reaction mass spectrometry. Anal. Methods 2019, 11, 3188–3197. [Google Scholar] [CrossRef]
- Meng, S.; Li, Q.; Zhou, Z.; Li, H.; Liu, X.; Pan, S.; Li, M.; Wang, L.; Guo, Y.; Qiu, M.; et al. Assessment of an exhaled breath test using high-pressure photon ionization time-of-flight mass spectrometry to detect lung cancer. JAMA Netw. Open 2021, 4, e213486. [Google Scholar] [CrossRef]
- Handa, H.; Usuba, A.; Maddula, S.; Baumbach, J.I.; Mineshita, M.; Miyazawa, T. Exhaled breath analysis for lung cancer detection using ion mobility spectrometry. PLoS ONE 2014, 9, e114555. [Google Scholar] [CrossRef]
- Goor, R.; Hooren, M.; Dingemans, A.-M.; Kremer, B.; Kross, K. Training and validating a portable electronic nose for lung cancer screening. J. Thorac. Oncol. 2018, 13, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Tirzite, M.; Li, W.; He, Z.; Chen, W.; Liu, H.; Chen, K.; Pi, X. Detection of lung cancer in exhaled breath with an electronic nose using support vector machine analysis. J. Breath Res. 2017, 11, 036009. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, P.J.; Wang, X.-F.; Xu, Y.; Mekhail, T.; Beukemann, M.C.; Na, J.; Kemling, J.W.; Suslick, K.S.; Sasidhar, M. Exhaled breath analysis with a colorimetric sensor array for the identification and characterization of lung cancer. J. Thorac. Oncol. 2012, 7, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Shlomi, D.; Abud, M.; Liran, O.; Bar, J.; Gai-Mor, N.; Ilouze, M.; Onn, A.; Ben-Nun, A.; Haick, H.; Peled, N. Detection of Lung Cancer and EGFR Mutation by Electronic Nose System. J. Thorac. Oncol. 2017, 12, 1544–1551. [Google Scholar] [CrossRef]
- Gasparri, R.; Santonico, M.; Valentini, C.; Sedda, G.; Borri, A.; Petrella, F.; Maisonneuve, P.; Pennazza, G.; D’Amico, A.; Natale, C.D.; et al. Volatile signature for the early diagnosis of lung cancer. J. Breath Res. 2016, 10, 016007. [Google Scholar] [CrossRef]
- Rocco, R.; Incalzi, R.A.; Pennazza, G.; Santonico, M.; Pedone, C.; Bartoli, I.R.; Vernile, C.; Mangiameli, G.; Rocca, A.L.; Luca, G.D.; et al. BIONOTE E-Nose Technology May Reduce False Positives in Lung Cancer Screening Programmes. Eur. J. Cardiothorac. Surg. 2016, 49, 1112–1117. [Google Scholar] [CrossRef]
- Liu, B.; Yu, H.; Zeng, X.; Zhang, D.; Gong, J.; Tian, L.; Qian, J.; Zhao, L.; Zhang, S.; Liu, R. Lung cancer detection via breath by electronic nose enhanced with a sparse group feature selection approach. Sens. Actuators B Chem. 2021, 339, 129896. [Google Scholar] [CrossRef]
- Song, G.; Qin, T.; Liu, H.; Xu, G.B.; Pan, Y.Y.; Xiong, F.X.; Gu, K.; Sun, G.; Chen, Z. Quantitative breath analysis of volatile organic compounds of lung cancer patients. Lung Cancer 2010, 67, 227–231. [Google Scholar] [CrossRef]
- Fu, X.; Li, M.; Knipp, R.J.; Nantz, M.H.; Bousamra, M. Noninvasive detection of lung cancer using exhaled breath. Cancer Med. 2014, 3, 174–181. [Google Scholar] [CrossRef]
- Rudnicka, J.; Walczak, M.; Kowalkowski, T.; Jezierski, T.; Buszewski, B. Determination of volatile organic compounds as potential markers of lung cancer by gas chromatography-mass spectrometry versus trained dogs. Sens. Actuator B 2014, 202, 615–621. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, Y.; Jiang, Z.; Zhou, Y.; Chen, Y.; Hu, Y.; Jiang, G.; Xie, D. Breath profile as composite biomarkers for lung cancer diagnosis. Lung Cancer 2021, 154, 206–213. [Google Scholar] [CrossRef]
- Gashimova, E.M.; Temerdashev, A.Z.; Porkhanov, V.A.; Polyakov, I.S.; Perunov, D.V. Volatile Organic Compounds in Exhaled Breath as Biomarkers of Lung Cancer: Advances and Potential Problems. J. Anal. Chem. 2022, 77, 785–810. [Google Scholar] [CrossRef]
- Kalidoss, R.; Umapathy, S.; Sivalingamb, Y. An investigation of GO-SnO2-TiO2 ternary nanocomposite for the detection of acetone in diabetes mellitus patient’s breath. Appl. Surf. Sci. 2018, 449, 667–684. [Google Scholar] [CrossRef]
- Alkhouri, N.; Eng, K.; Cikach, F.; Patel, N.; Yan, C.; Brindle, A.; Rome, E.; Hanouneh, I.; Grove, D.; Lopez, R.; et al. Breathprints of childhood obesity: Changes in volatile organic compounds in obese children compared with lean controls. Pediatr. Obes. 2015, 1, 23–29. [Google Scholar] [CrossRef]
- Marcondes-Braga, F.G.; Batista, G.L.; Bacal, F.; Gutz, I. Exhaled Breath Analysis in Heart Failure. Curr. Heart Fail. Rep. 2016, 13, 166–171. [Google Scholar] [CrossRef]
- Gashimova, E.M.; Temerdashev, A.Z.; Porkhanov, V.A.; Polyakov, I.S.; Perunov, D.V.; Azaryan, A.A.; Dmitrieva, E.V. Investigation of different approaches for exhaled breath and tumor tissue analyses to identify lung cancer biomarkers. Helyion 2020, 6, e04224. [Google Scholar] [CrossRef] [PubMed]
- Bikov, A.; Hernadi, M.; Korosi, B.Z.; Kunos, L.; Zsamboki, G.; Sutto, Z.; Tarnoki, A.D.; Tarnoki, D.L.; Losonczy, G.; Horvath, I. Expiratory flow rate, breath hold and anatomic dead space influence electronic nose ability to detect lung cancer. BMC Pulm. Med. 2014, 14, 202. [Google Scholar] [CrossRef] [PubMed]
- Bousamra, M.; Schumer, E.; Li, M.; Knipp, R.J.; Nantz, M.H.; Berkel, V.; Fu, X.A. Quantitative analysis of exhaled carbonyl compounds distinguishes benign from malignant pulmonary disease . J. Thorac. Cardiovasc. Surg. 2014, 148, 1074–1080. [Google Scholar] [CrossRef]
- Nardi-Agmon, I.; Abud-Hawa, M.; Liran, O.; Gai-Mor, N.; Ilouze, M.; Onn, A.; Bar, J.; Shlomi, D.; Haick, H.; Peled, N. Exhaled breath analysis for monitoring response to treatment in advanced lung cancer. J. Thorac. Oncol. 2016, 11, 827–837. [Google Scholar] [CrossRef]
- Phillips, M.; Altorki, N.; Austin, J.H.; Cameron, R.B.; Cataneo, R.N.; Greenberg, J.; Kloss, R.; Maxfield, R.A.; Munawar, M.I.; Pass, H.I.; et al. Prediction of lung cancer using volatile biomarkers in breath. Cancer Biomark. 2007, 3, 95–109. [Google Scholar] [CrossRef]
- Gashimova, E.M.; Osipova, A.K.; Temerdashev, A.Z.; Porkhanov, V.A.; Polyakov, I.S.; Perunov, D.V.; Dmitrieva, E.V. Exhaled breath analysis by using GC-MS and «electronic nose» for lung cancer diagnostics. Anal. Methods 2021, 13, 4793–4804. [Google Scholar] [CrossRef]
- D’Amicoa, A.; Pennazzab, G.; Santonicoa, M.; Martinellia, E.; Roscionic, C.; Galluccioc, G.; Paolessed, R.; Natalea, C.D. An investigation on electronic nose diagnosis of lung cancer. Lung Cancer 2010, 68, 170–176. [Google Scholar] [CrossRef]
Figure 1.
Typical total ion current (TIC) chromatograms of a lung cancer patient (a) and a healthy individual (b): 1—acetaldehyde, 2—isobutene, 3—ethanol, 4—acetonitrile, 5—acetone, 6—2-propanol, 7—ethyl ester, 8—isoprene, 9—2,3-butandione, 10—2-butanone, 11—dimethyl carbonate, 12—hexane, 13—benzene, 14—2-pentanone, 15—allyl methyl sulfide, 16—1-methylthiopropane, 17—1-methylthiopropene, 18—heptane, 19—1-pentanol, 20—toluene, 21—hexanal, 22—N,N-dimethylacetamide, 23—ethylbenzene, 24—o-xylene, 25—2-heptanone, 26—heptanal, 27—phenol, 28—benzaldehyde, 29—octanal, 30—decane, 31—limonene, 32—nonanal, 33—undecane, 34—decanal, 35—dodecane.
Figure 2.
Importance bar plots of variables using GBDT, created on the datasets (a) 1, (b) 2, (c) 3.
Table 1.
Clinical characteristics of subjects.
| Cohort | Feature | Number |
|---|
| Healthy participant | Number | 120 |
| Male | 36 |
| Female | 84 |
| Age (median, range) | 21, 21–67 |
| Smokers | 17 |
| Lung cancer | Number | 112 |
| Male | 88 |
| Female | 24 |
| Age (median, range) | 63, 21–77 |
| Smokers | 22 |
| Localization of tumor |
| Central | 59 |
| Peripheral | 53 |
| Histology |
| Adenocarcinoma | 50 |
| Squamous cell carcinoma | 38 |
| Small cell carcinoma | 12 |
| Non differentiated | 12 |
| TNM (tumor, nodules, metastasis) stage |
| T1N0M1 | 1 |
| T2N0M0 | 8 |
| T2N0M1 | 5 |
| T2N1M0 | 8 |
| T2N1M1 | 1 |
| T2N2M0 | 2 |
| T2N2M1 | 6 |
| T2N3M0 | 1 |
| T2N3M1 | 2 |
| T3N0M0 | 6 |
| T3N0M1 | 2 |
| T3N1M0 | 7 |
| T3N1M1 | 1 |
| T3N2M0 | 15 |
| T3N2M1 | 4 |
| T3N3M0 | 1 |
| T4N0M0 | 5 |
| T4N0M1 | 5 |
| T4N1M0 | 8 |
| T4N1M1 | 1 |
| T4N2M0 | 10 |
| T4N2M1 | 10 |
| T4N3M0 | 2 |
| T4N3M1 | 1 |
| Comorbidity |
| Chronic heart failure | 19 |
| Hypertension | 18 |
| Anemia | 8 |
| Acute cerebrovascular accident | 5 |
| Obesity | 4 |
| Diabetes | 4 |
Table 2.
Thermal desorber and GC-MS operation modes.
| Equipment | Parameter | Value |
|---|
| Thermal desorber | Carrier gas | Helium |
| Carries gas flow rate (desorption from the sorption tube), mL/min | 30 |
| Valve temperature, °C | 150 |
| Transition line temperature, °C | 180 |
| Desorption temperature, °C | 250 |
| Initial trap temperature, °C | −10 |
| Final trap temperature, °C | 250 |
| Carrier gas flow rate (desorption from the trap), mL/min | 50 |
| Desorption time, min | 5 |
| Speed of the trap heating, °C/min | 2000 |
| GC-MS | Carrier gas | Helium |
| Injector temperature, °C | 250 |
| Split ratio | 1:10 |
| Ion source temperature, °C | 200 |
| Transfer line temperature, °C | 250 |
| Scan mode | Full scan |
| Scan range, amu | 29–250 |
| Electron impact ionization, eV | 70 |
Table 3.
Frequency of VOCs occurring in exhaled breath of healthy volunteers and lung cancer patients (%).
| No | Retention Time, min | VOC | CAS Number | Lung Cancer Patients | Healthy Volunteers |
|---|
| 1 | 7.40 | Isoprene | 78-79-5 | 100 | 100 |
| 2 | 6.20 | Acetone | 67-64-1 | 100 | 100 |
| 3 | 6.56 | Dimethylsulfide | 75-18-3 | 100 | 100 |
| 4 | 11.99 | 1-Methylthiopropene | 10152-77-9 | 100 | 92 |
| 5 | 11.34 | Allyl methyl sulfide | 10152-76-8 | 98 | 97 |
| 6 | 11.76 | 1-Methylthiopropane | 3877-15-4 | 98 | 95 |
| 7 | 11.43 | 2-Pentanone | 107-87-9 | 97 | 99 |
| 8 | 12.20 | Dimethyl disulfide | 624-92-0 | 97 | 93 |
| 9 | 8.81 | 2.3-Butandione | 431-03-8 | 88 | 92 |
| 10 | 5.20 | Acetonitrile | 75-05-8 | 87 | 88 |
| 11 | 8.95 | 2-Butanone | 78-93-3 | 76 | 86 |
| 12 | 18.51 | Dimethyl trisulfide | 3658-80-8 | 71 | 55 |
| 13 | 18.26 | Benzaldehyde | 100-52-7 | 58 | 57 |
| 14 | 12.83 | 1-Pentanol | 71-41-0 | 56 | 59 |
| 15 | 16.72 | 2-Heptanone | 110-43-0 | 54 | 51 |
| 16 | 12.41 | Heptane | 142-82-5 | 53 | 73 |
| 17 | 22.45 | Nonanal | 124-19-6 | 53 | 56 |
| 18 | 9.86 | Hexane | 110-54-3 | 51 | 62 |
| 19 | 16.54 | 3-Heptanone | 106-35-4 | 51 | 52 |
| 20 | 19.64 | Octanal | 124-13-0 | 51 | 52 |
| 21 | 15.13 | Octane | 111-65-9 | 51 | 50 |
| 22 | 13.36 | Toluene | 108-88-3 | 51 | 32 |
| 23 | 11.39 | Pentanal | 110-62-3 | 50 | 72 |
| 24 | 14.31 | Hexanal | 66-25-1 | 49 | 49 |
| 25 | 20.16 | Decane | 124-18-5 | 49 | 48 |
| 26 | 25.04 | Dodecane | 112-40-3 | 46 | 49 |
| 27 | 22.54 | Undecane | 1120-21-4 | 45 | 49 |
| 28 | 17.25 | Propylbenzene | 103-65-1 | 45 | 13 |
| 29 | 24.97 | Decanal | 112-31-2 | 42 | 49 |
| 30 | 17.15 | Heptanal | 111-71-7 | 39 | 48 |
| 31 | 8.90 | Butanal | 123-72-8 | 37 | 48 |
| 32 | 20.37 | Nonane | 111-84-2 | 37 | 46 |
| 33 | 10.44 | Benzene | 71-43-2 | 31 | 22 |
| 34 | 7.87 | 1.3-pentadiene | 504-60-9 | 25 | 15 |
| 35 | 16.14 | Ethylbenzene | 100-41-4 | 25 | 12 |
| 36 | 10.32 | 1-Butanol | 71-36-3 | 24 | 49 |
| 37 | 7.78 | 1.4-pentadiene | 591-93-5 | 22 | 10 |
| 38 | 14.57 | Butyl acetate | 123-86-4 | 21 | 48 |
| 39 | 16.25 | o-Xylene | 95-47-6 | 20 | 13 |
| 40 | 16.76 | M + p-Xylene | 108-38-3 + 106-42-3 | 20 | 12 |
Table 4.
Correlation coefficients between VOCs (their ratios) and TNM stage.
| VOC (Ratio) | Correlation Coefficient |
|---|
|
2,3-Butandione
| 0.343 |
|
Dimethyl trisulfide
| −0.235 |
|
Octane
| 0.272 |
|
Octane/Acetone
| 0.300 |
|
Octane/Acetonitrile
| 0.319 |
|
Octane/Isoprene
| 0.312 |
|
Octane/1-methylthiopropene
| 0.356 |
|
Octane/Dimethyl disulfide
| 0.375 |
|
Dimethyl trisulfide/Acetone
| −0.272 |
|
Dimethyl trisulfide/Isoprene
| −0.237 |
|
Dimethyl trisulfide/2-butanone
| −0.259 |
|
Dimethyl trisulfide/dimethyl sulfide
| −0.242 |
|
Dimethyl trisulfide/dimethyl disulfide
| −0.237 |
|
Dimethyl trisulfide/2-pentanone
| −0.279 |
|
Benzaldehyde/acetonitrile
| 0.249 |
|
2,3-Butandione/2-pentanone
| 0.380 |
|
Acetonitrile/allyl methyl sulfide
| 0.275 |
Table 5.
Correlation coefficients between VOCs (their ratios) and histological type.
| VOC (Ratio) | Correlation Coefficient |
|---|
|
Octane/acetone
| 0.207 |
|
3-Heptanone/acetone
| 0.234 |
|
3-Heptanone/2-butanone
| 0.229 |
|
3-Heptanone/allyl methyl sulfide
| 0.229 |
|
3-Heptanone/1-methylthiopropane
| 0.235 |
|
Dimethyl trisulfide/Acetone
| 0.204 |
|
Dimethyl trisulfide/Isoprene
| 0.199 |
|
Dimethyl trisulfide/2-butanone
| 0.244 |
|
Dimethyl trisulfide/dimethyl sulfide
| 0.215 |
|
Dimethyl trisulfide/1-methylthiopropane
| 0.256 |
Table 6.
Ratios selected for the creation of diagnostic models.
| Ratio | Correlation Coefficient |
|---|
| Disease Status | Age
(Lung Cancer Patients) | Age
(Healthy Volunteers) |
|---|
|
Hexane/2-Pentanone
| −0.309 | 0.005 | −0.024 |
|
Toluene/Acetone
| 0.252 | −0.169 | −0.038 |
|
1-Pentanol/Acetone
| 0.136 | −0.089 | 0.026 |
|
Pentanal/2-Pentanone
| −0.346 | 0.059 | −0.159 |
|
Dimethyl trisulfide/Dimethyl disulfide
| 0.271 | 0.028 | 0.005 |
|
Nonanal/2.3-Butandione
| −0.153 | 0.082 | −0.166 |
|
Heptane/Allyl methyl sulfide
| −0.157 | −0.028 | 0.135 |
|
2-Butanone/2-Pentanone
| −0.320 | 0.006 | −0.121 |
|
Isoprene/Acetone
| 0.227 | −0.163 | 0.174 |
|
1-Methylthiopropane/Acetone
| −0.149 | −0.146 | 0.175 |
|
Dimethyl sulfide/Acetone
| 0.205 | −0.123 | 0.097 |
|
Acetonitrile/Acetone
| −0.269 | −0.033 | 0.149 |
Table 7.
Accuracy of diagnostic models.
| Machine Learning Algorithm | Training Dataset | Test Dataset |
|---|
| Sensitivity, % | Specificity, % | Sensitivity, % | Specificity, % |
|---|
|
Dataset
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
|
GBDT
| 92 | 94 | 96 | 82 | 85 | 92 | 88 | 78 | 77 | 77 | 68 | 81 |
|
ANN
| 89 | 88 | 87 | 85 | 85 | 75 | 88 | 85 | 82 | 86 | 80 | 81 |
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).