Diagnosis, Prevalence and Significance of Obesity in a Cohort of CKD Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Biochemistry
2.3. Anthropometry
2.4. Body Composition Analysis
2.5. Central Obesity Measures
2.6. FM%/MAMC Ratio
2.7. Statistical Analysis
3. Results
3.1. Anthropometric and Clinical Features
3.2. Agreement between the Main Measures of Obesity
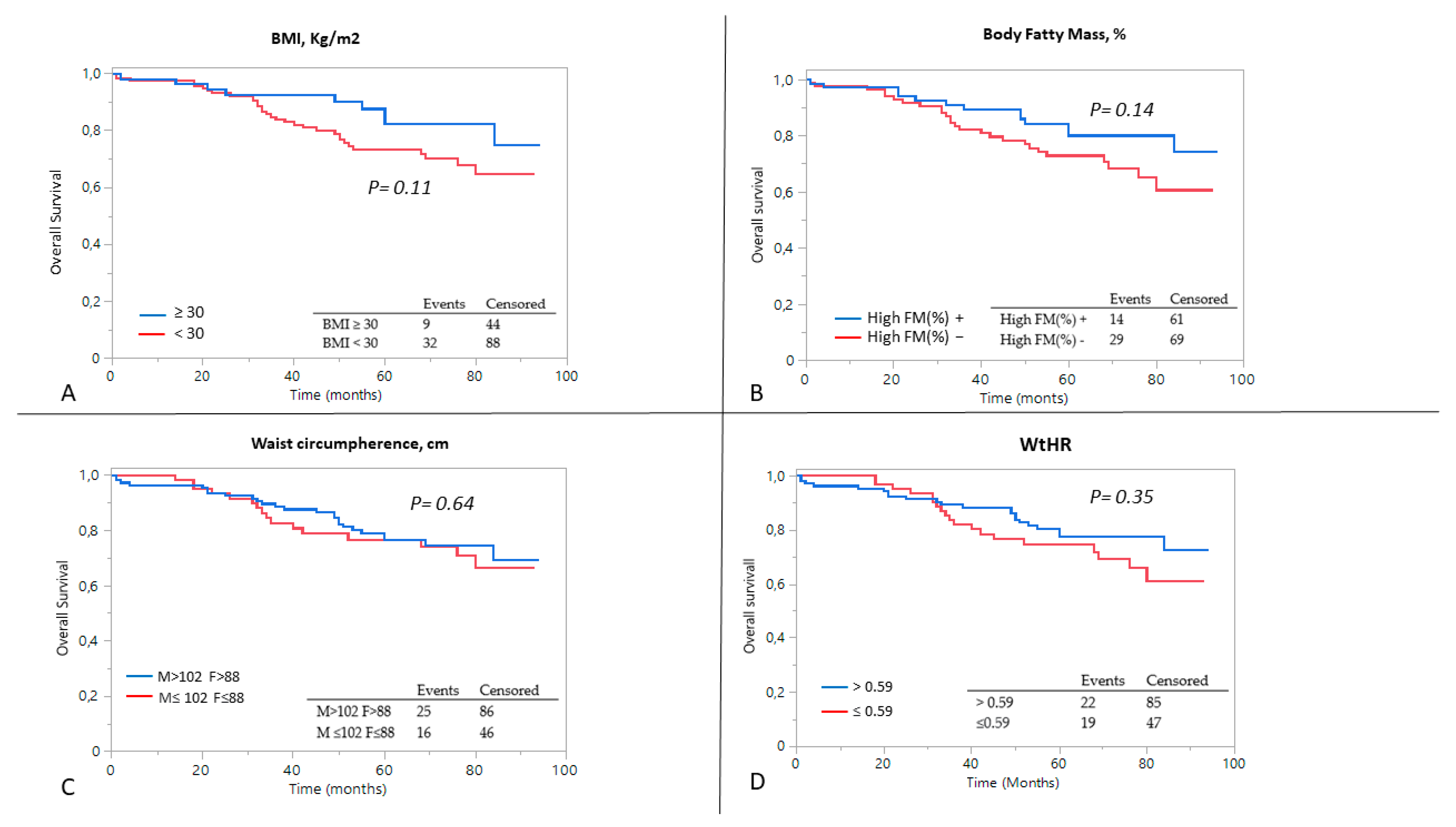
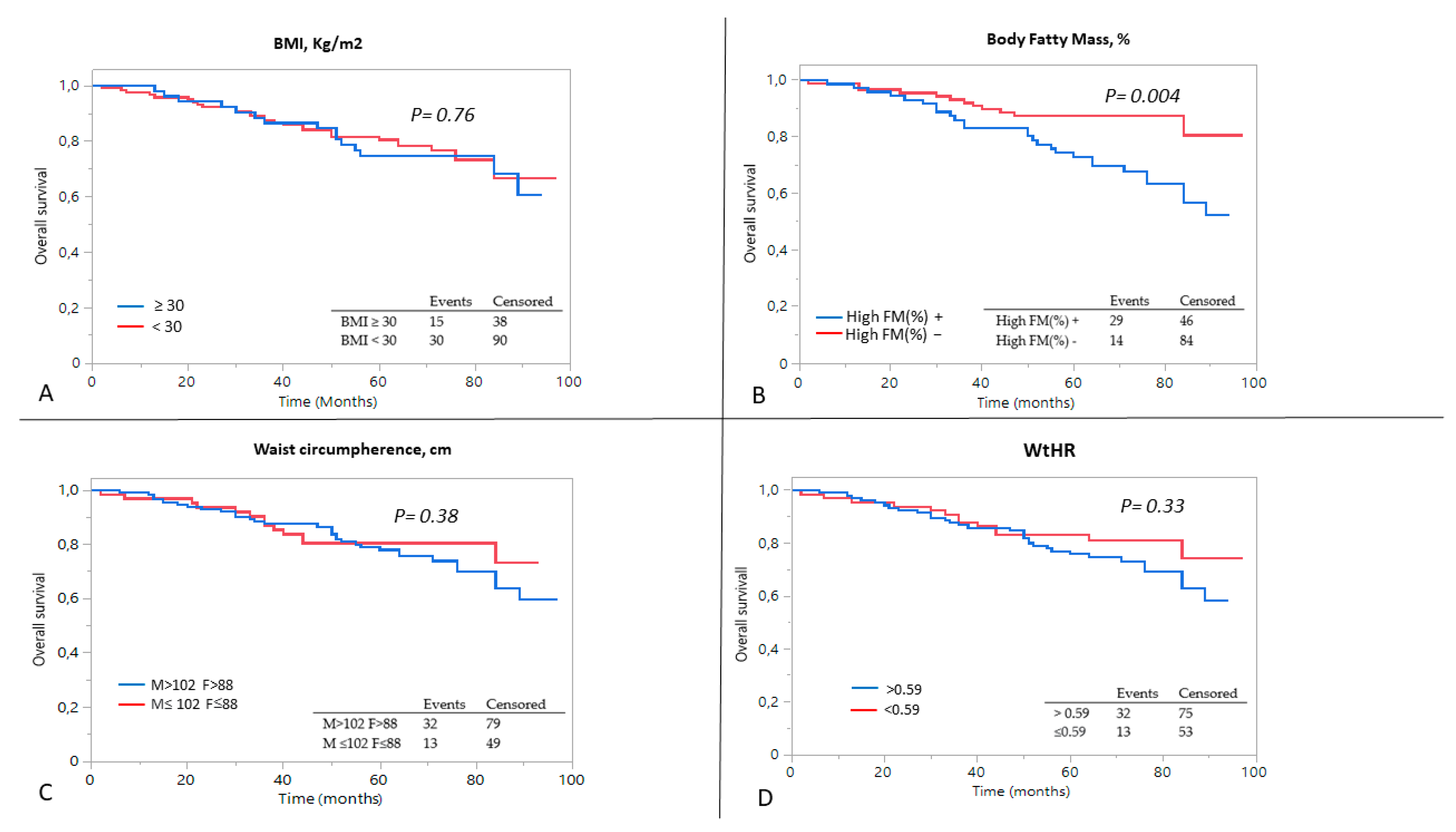
3.3. Predictors of Outcome
3.4. FM%/MAMC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moriconi, D.; Antonioli, L.; Masi, S.; Bellini, R.; Pellegrini, C.; Rebelos, E.; Taddei, S.; Nannipieri, M. Glomerular hyperfiltration in morbid obesity: Role of the inflammasome signalling. Nephrology 2022, 27, 673–680. [Google Scholar] [CrossRef]
- Câmara, N.O.; Iseki, K.; Kramer, H.; Liu, Z.H.; Sharma, K. Kidney disease and obesity: Epidemiology, mechanisms and treatment. Nat. Rev. Nephrol. 2017, 13, 181–190. [Google Scholar] [CrossRef]
- Hoogeveen, E.K.; Halbesma, N.; Rothman, K.J.; Stijnen, T.; van Dijk, S.; Dekker, F.W.; Boeschoten, E.W.; de Mutsert, R. Netherlands Cooperative Study on the Adequacy of Dialysis-2 (NECOSAD) Study Group. Obesity and mortality risk among younger dialysis patients. Clin. J. Am. Soc. Nephrol. 2012, 7, 280–288. [Google Scholar] [CrossRef]
- Hall, Y.N.; Xu, P.; Chertow, G.M. Relationship of body size and mortality among US Asians and Pacific Islanders on dialysis. Ethn. Dis. 2011, 21, 40–46. [Google Scholar] [PubMed]
- Inagaki, K.; Tawada, N.; Takanashi, M.; Akahori, T. The association between body mass index and all-cause mortality in Japanese patients with incident hemodialysis. PLoS ONE 2022, 17, e0269849. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Liu, J.S.; Hung, S.C. Obesity and risk of end-stage renal disease in patients with chronic kidney disease: A cohort study. Am. J. Clin. Nutr. 2018, 108, 1145–1153. [Google Scholar] [CrossRef]
- Port, F.K.; Ashby, V.B.; Dhingra, R.K.; Roys, E.C.; Wolfe, R.A. Dialysis dose and body mass index are strongly associated with survival in hemodialysis patients. J. Am. Soc. Nephrol. 2002, 13, 1061–1066. [Google Scholar] [CrossRef]
- Fleischmann, E.; Teal, N.; Dudley, J.; May, W.; Bower, J.D.; Salahudeen, A.K. Influence of excess weight on mortality and hospital stay in 1346 hemodialysis patients. Kidney Int. 1999, 55, 1560–1567. [Google Scholar] [CrossRef]
- Ladhani, M.; Craig, J.C.; Irving, M.; Clayton, P.A.; Wong, G. Obesity and the risk of cardiovascular and all-cause mortality in chronic kidney disease: A systematic review and meta-analysis. Nephrol. Dial. Transplant. 2017, 32, 439–449. [Google Scholar] [CrossRef]
- Doshi, M.; Streja, E.; Rhee, C.M.; Park, J.; Ravel, V.A.; Soohoo, M.; Moradi, H.; Lau, W.L.; Mehrotra, R.; Kuttykrishnan, S.; et al. Examining the robustness of the obesity paradox in maintenance hemodialysis patients: A marginal structural model analysis. Nephrol. Dial. Transplant. 2016, 31, 1310–1319. [Google Scholar] [CrossRef]
- Frankenfield, D.C.; Rowe, W.A.; Cooney, R.N.; Smith, J.S.; Becker, D. Limits of body mass index to detect obesity and predict body composition. Nutrition 2001, 17, 26–30. [Google Scholar] [CrossRef]
- Kittiskulnam, P.; Chertow, G.M.; Kaysen, G.A.; Delgado, C.; Dalrymple, L.S.; Johansen, K.L. Misclassification of Obesity by Body Mass Index Among Patients Receiving Hemodialysis. Am. J. Kidney Dis. 2016, 67, 709–711. [Google Scholar] [CrossRef]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef]
- Romero-Corral, A.; Sert-Kuniyoshi, F.H.; Sierra-Johnson, J.; Orban, M.; Gami, A.; Davison, D.; Singh, P.; Pusalavidyasagar, S.; Huyber, C.; Votruba, S.; et al. Modest visceral fat gain causes endothelial dysfunction in healthy humans. J. Am. Coll. Cardiol. 2010, 56, 662–666. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Furth, S.L.; Zoccali, C.; World Kidney Day Steering Committee. Obesity and Kidney Disease: Hidden Consequences of the Epidemic. Can. J. Kidney Health Dis. 2017, 4, 2054358117698669. [Google Scholar] [CrossRef] [PubMed]
- Mor, V.; Laliberte, L.; Morris, J.N.; Wiemann, M. The Karnofsky Performance Status Scale: An examination of its reliability and validity in a research setting. Cancer 1984, 53, 2002–2007. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Kotler, D.P.; Burastero, S.; Wang, J.; Pierson, R.N., Jr. Prediction of body cell mass, fat-free mass, and total body water with bioelectrical impedance analysis: Effects of race, sex, and disease. Am. J. Clin. Nutr. 1996, 64 (Suppl. 3), 489S–497S. [Google Scholar] [CrossRef]
- Heim, N.; Snijder, M.B.; Heymans, M.W.; Deeg, D.J.; Seidell, J.C.; Visser, M. Optimal cutoff values for high-risk waist circumference in older adults based on related health outcomes. Am. J. Epidemiol. 2011, 174, 479–489. [Google Scholar] [CrossRef]
- Can, A.S.; Yildiz, E.A.; Samur, G.; Rakicioğlu, N.; Pekcan, G.; Ozbayrakçi, S.; Palaoğlu, K.E.; Gönen, M.; Bersot, T.P. Optimal waist:height ratio cut-off point for cardiometabolic risk factors in Turkish adults. Public Health Nutr. 2010, 13, 488–495. [Google Scholar]
- Swainson, M.G.; Batterham, A.M.; Tsakirides, C.; Rutherford, Z.H.; Hind, K. Prediction of whole-body fat percentage and visceral adipose tissue mass from five anthropometric variables. PLoS ONE 2017, 12, e0177175. [Google Scholar] [CrossRef] [PubMed]
- Deurenberg, P.; Yap, M.; van Staveren, W.A. Body mass index and percent body fat: A meta analysis among different ethnic groups. Int. J. Obes. Relat. Metab. Disord. 1998, 22, 1164–1171. [Google Scholar] [CrossRef]
- Di Renzo, L.; Itani, L.; Gualtieri, P.; Pellegrini, M.; El Ghoch, M.; De Lorenzo, A. New BMI Cut-Off Points for Obesity in Middle-Aged and Older Adults in Clinical Nutrition Settings in Italy: A Cross-Sectional Study. Nutrients 2022, 14, 4848. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.W.; Lin, Y.Y.; Kao, T.W.; Lin, C.M.; Liaw, F.Y.; Wang, C.C.; Peng, T.C.; Chen, W.L. Mid-arm muscle circumference as a significant predictor of all-cause mortality in male individuals. PLoS ONE 2017, 12, e0171707. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.F.; Zahmatkesh, G.; Ahmadi, E.; Streja, E.; Rhee, C.M.; Gillen, D.L.; De Nicola, L.; Minutolo, R.; Ricardo, A.C.; Kovesdy, C.P.; et al. Association of Body Mass Index with Clinical Outcomes in Non-Dialysis-Dependent Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Cardiorenal Med. 2015, 6, 37–49. [Google Scholar] [CrossRef]
- Katzmarzyk, P.T.; Craig, C.L.; Bouchard, C. Original article underweight, overweight and obesity: Relationships with mortality in the 13-year follow-up of the Canada Fitness Survey. J. Clin. Epidemiol. 2001, 54, 916–920. [Google Scholar] [CrossRef]
- Bigaard, J.; Frederiksen, K.; Tjønneland, A.; Thomsen, B.L.; Overvad, K.; Heitmann, B.L.; Sørensen, T.I. Body fat and fat-free mass and all-cause mortality. Obes. Res. 2004, 12, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Babayev, R.; Whaley-Connell, A.; Kshirsagar, A.; Klemmer, P.; Navaneethan, S.; Chen, S.C.; Li, S.; McCullough, P.A.; Bakris, G.; Bomback, A. KEEP Investigators. Association of race and body mass index with ESRD and mortality in CKD stages 3–4: Results from the Kidney Early Evaluation Program (KEEP). Am. J. Kidney Dis. 2013, 61, 404–412. [Google Scholar] [CrossRef]
- Ricardo, A.C.; Madero, M.; Yang, W.; Anderson, C.; Menezes, M.; Fischer, M.J.; Turyk, M.; Daviglus, M.L.; Lash, J.P. Adherence to a healthy lifestyle and all-cause mortality in CKD. Clin. J. Am. Soc. Nephrol. 2013, 8, 602–609. [Google Scholar] [CrossRef]
- Tobias, D.K. Addressing Reverse Causation Bias in the Obesity Paradox Is Not “One Size Fits All”. Diabetes Care 2017, 40, 1000–1001. [Google Scholar] [CrossRef]
- Santomauro, F.; Lorini, C.; Pieralli, F.; Niccolai, G.; Picciolli, P.; Vezzosi, S.; Bonaccorsi, G. Waist-to-height ratio and its associations with body mass index in a sample of Tuscan children in primary school. Ital. J. Pediatr. 2017, 43, 53. [Google Scholar] [CrossRef]
- Naderi, N.; Kleine, C.E.; Park, C.; Hsiung, J.T.; Soohoo, M.; Tantisattamo, E.; Streja, E.; Kalantar-Zadeh, K.; Moradi, H. Obesity Paradox in Advanced Kidney Disease: From Bedside to the Bench. Prog. Cardiovasc. Dis. 2018, 61, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.N.; Wayne, S.J.; Waters, D.L.; Janssen, I.; Gallagher, D.; Morley, J.E. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes. Res. 2004, 12, 1995–2004. [Google Scholar] [CrossRef]
- Barreto Silva, M.I.; Picard, K.; Klein, M.R.S.T. Sarcopenia and sarcopenic obesity in chronic kidney disease: Update on prevalence, outcomes, risk factors and nutrition treatment. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Krachler, B.; Völgyi, E.; Savonen, K.; Tylavsky, F.A.; Alén, M.; Cheng, S. BMI and an anthropometry-based estimate of fat mass percentage are both valid discriminators of cardiometabolic risk: A comparison with DXA and bioimpedance. J. Obes. 2013, 2013, 862514. [Google Scholar] [CrossRef]
- Sedlmeier, A.M.; Baumeister, S.E.; Weber, A.; Fischer, B.; Thorand, B.; Ittermann, T.; Dörr, M.; Felix, S.B.; Völzke, H.; Peters, A.; et al. Relation of body fat mass and fat-free mass to total mortality: Results from 7 prospective cohort studies. Am. J. Clin. Nutr. 2021, 113, 639–646. [Google Scholar] [CrossRef]
- Androga, L.; Sharma, D.; Amodu, A.; Abramowitz, M.K. Sarcopenia, obesity, and mortality in US adults with and without chronic kidney disease. Kidney Int. Rep. 2017, 2, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.A.; Cordeiro, A.C.; Avesani, C.M.; Carrero, J.J.; Lindholm, B.; Amparo, F.C.; Amodeo, C.; Cuppari, L.; Kamimura, M.A. Sarcopenia in chronic kidney disease on conservative therapy: Prevalence and association with mortality. Nephrol. Dial. Transplant. 2015, 30, 1718–1725. [Google Scholar] [CrossRef]
- Goisser, S.; Kemmler, W.; Porzel, S.; Volkert, D.; Sieber, C.C.; Bollheimer, L.C.; Freiberger, E. Sarcopenic obesity and complex interventions with nutrition and exercise in community-dwelling older persons--a narrative review. Clin. Interv. Aging 2015, 10, 1267–1282. [Google Scholar]
- Antonioli, L.; Moriconi, D.; Masi, S.; Bottazzo, D.; Pellegrini, C.; Fornai, M.; Anselmino, M.; Ferrannini, E.; Blandizzi, C.; Taddei, S.; et al. Differential Impact of Weight Loss and Glycemic Control on Inflammasome Signaling. Obesity 2020, 28, 609–615. [Google Scholar] [CrossRef]
- Akchurin, O.M.; Kaskel, F. Update on inflammation in chronic kidney disease. Blood Purif. 2015, 39, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.R. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 2006, 84, 475–482. [Google Scholar] [CrossRef]
- Xu, J.; Wan, C.S.; Ktoris, K.; Reijnierse, E.M.; Maier, A.B. Sarcopenia Is Associated with Mortality in Adults: A Systematic Review and Meta-Analysis. Gerontology 2022, 68, 361–376. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Løfblad, L.; Hov, G.G.; Åsberg, A.; Videm, V. Inflammatory markers and risk of cardiovascular mortality in relation to diabetes status in the HUNT study. Sci. Rep. 2021, 11, 15644. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; Brunori, G.; Di Iorio, B.R.; D’Alessandro, C.; Pasticci, F.; Cosola, C.; Bellizzi, V.; Bolasco, P.; Capitanini, A.; Fantuzzi, A.L.; et al. Nutritional treatment of advanced CKD: Twenty consensus statements. J. Nephrol. 2018, 31, 457–473. [Google Scholar] [CrossRef]
- Garibotto, G.; Picciotto, D.; Saio, M.; Esposito, P.; Verzola, D. Muscle protein turnover and low-protein diets in patients with chronic kidney disease. Nephrol. Dial. Transplant. 2020, 35, 741–751. [Google Scholar] [CrossRef]
- Cupisti, A.; D’Alessandro, C.; Fumagalli, G.; Vigo, V.; Meola, M.; Cianchi, C.; Egidi, M.F. Nutrition and physical activity in CKD patients. Kidney Blood Press. Res. 2014, 39, 107–113. [Google Scholar] [CrossRef]
- D’Alessandro, C.; Giannese, D.; Avino, M.; Cupisti, A. Energy Requirement for Elderly CKD Patients. Nutrients 2021, 13, 3396. [Google Scholar] [CrossRef]
- Bigman, G.; Ryan, A.S. Implications of Race and Ethnicity in Sarcopenia US National Prevalence of Sarcopenia by Muscle Mass, Strength, and Function Indices. Gerontol. Geriatr. Res. 2021, 4, 126. [Google Scholar]

| BMI ≥ 30 | 30 > BMI ≥ 25 | BMI < 25 | p-Value | |
|---|---|---|---|---|
| Subjects, n (%) | 53 (30.7) | 81 (46.8) | 39 (22.5) | |
| Age, yrs | 68 ± 9 | 71 ± 10 * | 66 ± 16 | 0.037 |
| Gender, F% | 9 (17.0) | 11 (13.6) | 7 (18.0) | 0.781 |
| Weight, kg | 92 ± 11 | 77 ± 8 | 67 ± 8 | <0.0001 |
| BMI, Kg/m2 | 32.5 ± 2.3 | 27.3 ± 1.3 | 23.2 ± 2.4 | <0.0001 |
| Diabetes, n (%) | 23 (43.4) | 15 (18.5) | 10 (25.6) | 0.007 |
| Waist circumpherence, cm | 111 ± 8 | 101 ± 8 | 90 ± 9 | <0.0001 |
| Hip circumpherence, cm | 111 ± 7 | 104 ± 5 | 98 ± 4 | <0.0001 |
| WHR | 1.01 ± 0.07 | 0.98 ± 0.08 | 0.91 ± 0.07 | 0.001 |
| WtHR | 0.66 ± 0.03 | 0.60 ± 0.04 | 0.53 ± 0.05 | <0.0001 |
| FM, % | 33.8 ± 6.7 | 29.1 ± 5.9 | 22.4 ± 6.4 | <0.0001 |
| TBW (%) | 58.3 ± 4.6 | 53.4 ± 4.3 | 50.9 ± 5.1 | <0.0001 |
| TST, mm | 13 [11–20] § | 11 [9–16] | 10 [7–14] | 0.002 |
| MAC, cm | 32.6 ± 4.0 | 29.5 ± 2.3 | 27.2 ± 2.5 | <0.0001 |
| MAMC, cm | 28 [26–30] | 25 [24–27] | 24 [22–25] | <0.0001 |
| FM%/MAMC | 1.21± 0.23 | 1.17± 0.27 | 0.98± 0.30 * | 0.0002 |
| Categorical variables related to obesity and adiposity | ||||
| Waist (>102 M or >88 F), n (%) | 52 (98.1) | 54 (66.7) | 5 (12.8) | <0.0001 |
| WtHR (>0.59), n (%) | 53 (100.0) | 52 (64.2) | 2 (5.1) | <0.0001 |
| High FM%, n (%) | 41 (77.4) | 31 (39.5) | 5 (12.8) | <0.0001 |
| Haematochemical features | ||||
| eGFR, mL/min/1.73 | 34 ± 12 | 30 ± 13 | 30 ± 12 | 0.222 |
| S Creatinine, mg/dL | 1.98 [1.57–2.76] | 2.19 [1.65–2.99] | 2.34 [2.00–2.81] | 0.213 |
| S Urea, mg/dL | 77 [57–92] | 82 [63–101] | 68 [56–99] | 0.286 |
| S Total cholesterol, mg/dL | 170 ± 37 | 177 ± 31 | 176 ± 36 | 0.632 |
| S LDL cholesterol, mg/dL | 99 ± 32 | 106 ± 31 | 106 ± 27 | 0.501 |
| S Tryglicerides, mg/dL | 121 [112–156] | 119 [111–158] | 118 [113–154] | 0.522 |
| S Glucose, mg/dL | 105 [91–113] § | 94 [84–105] | 90 [80–108] | 0.013 |
| S Albumin, g/dL | 4.20 ± 0.31 | 4.15 ± 0.36 | 4.23 ± 0.52 | 0.732 |
| S Potassium, mEq/L | 4.7 ± 0.5 | 4.6 ± 0.5 | 4.7 ± 0.4 | 0.776 |
| S Phosphorus, mg/dL | 3.3 ± 0.6 | 3.3 ± 0.6 | 3.2 ± 0.6 | 0.509 |
| Unweighted K Cohen | 95% IC | p Value | |
|---|---|---|---|
| Waist (>102 M or >88 F) | 0.37 | 0.27–0.47 | <0.0001 |
| WtHR > 0.59 | 0.44 | 0.32–0.53 | <0.0001 |
| High FM% | 0.40 | 0.27–0.55 | <0.0001 |
| Factors | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | Coefficient | p Value | |
| Mortality | |||||
| eGFR, mL/min/1.73 | 0.96 (0.93–0.99) | 0.005 | 0.96 (0.94–0.99) | −0.04 | 0.008 |
| High% FM | 2.61 (1.31–5.17) | 0.001 | 2.08 (1.04–4.18) | −0.37 | 0.039 |
| WHR | 6.29 (1.28–51.35) | 0.029 | 3.74 (0.36–39.59) | 2.82 | 0.271 |
| FM%/MAMC | 6.97 (2.38–20.18) | 0.0005 | 3.64 (1.07–11.47) | 1.26 | 0.034 |
| Dialysis | |||||
| eGFR, mL/min/1.73 | 0.88 (0.85–0.91) | <0.0001 | 0.87 (0.84–0.90) | −0.14 | <0.0001 |
| BMI, kg/m2 | 0.92 (0.85–0.99) | 0.042 | 0.93 (0.85–1.01) | −0.08 | 0.079 |
| Waist circumference, cm | 0.97 (0.94–0.99) | 0.001 | 0.97 (0.94–1.01) | −0.03 | 0.091 |
| MAC, cm | 0.87 (0.80–0.94) | 0.0005 | 0.85 (0.77–0.93) | −0.17 | 0.0004 |
| MAMC, cm | 0.84 (0.78–0.91) | <0.0001 | 0.82 (0.75–0.89) | −0.20 | <0.0001 |
| S Phosphorus, mg/dL | 3.30 (1.89–5.79) | <0.0001 | 3.50 (1.94–6.41) | 1.25 | <0.0001 |
| Composite outcome | |||||
| eGFR, mL/min/1.73 | 0.92 (0.89–0.94) | <0.0001 | 0.92 (0.90–0.94) | −0.09 | <0.0001 |
| MAC, cm | 0.89 (0.83–0.94) | 0.0005 | 0.89 (0.84–0.95) | −0.12 | 0.001 |
| MAMC, cm | 0.88 (0.84–0.94) | 0.0001 | 0.89 (0.83–0.96) | −0.12 | 0.0007 |
| S Phosphorus, mg/dL | 2.29 (1.51–3.45) | <0.0001 | 2.30 (1.49–3.55) | 0.83 | 0.0002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moriconi, D.; D’Alessandro, C.; Giannese, D.; Panichi, V.; Cupisti, A. Diagnosis, Prevalence and Significance of Obesity in a Cohort of CKD Patients. Metabolites 2023, 13, 196. https://doi.org/10.3390/metabo13020196
Moriconi D, D’Alessandro C, Giannese D, Panichi V, Cupisti A. Diagnosis, Prevalence and Significance of Obesity in a Cohort of CKD Patients. Metabolites. 2023; 13(2):196. https://doi.org/10.3390/metabo13020196
Chicago/Turabian StyleMoriconi, Diego, Claudia D’Alessandro, Domenico Giannese, Vincenzo Panichi, and Adamasco Cupisti. 2023. "Diagnosis, Prevalence and Significance of Obesity in a Cohort of CKD Patients" Metabolites 13, no. 2: 196. https://doi.org/10.3390/metabo13020196
APA StyleMoriconi, D., D’Alessandro, C., Giannese, D., Panichi, V., & Cupisti, A. (2023). Diagnosis, Prevalence and Significance of Obesity in a Cohort of CKD Patients. Metabolites, 13(2), 196. https://doi.org/10.3390/metabo13020196






