Growth Hormone Alters Circulating Levels of Glycine and Hydroxyproline in Mice
Abstract
1. Introduction
2. Materials and Methods
3. Results
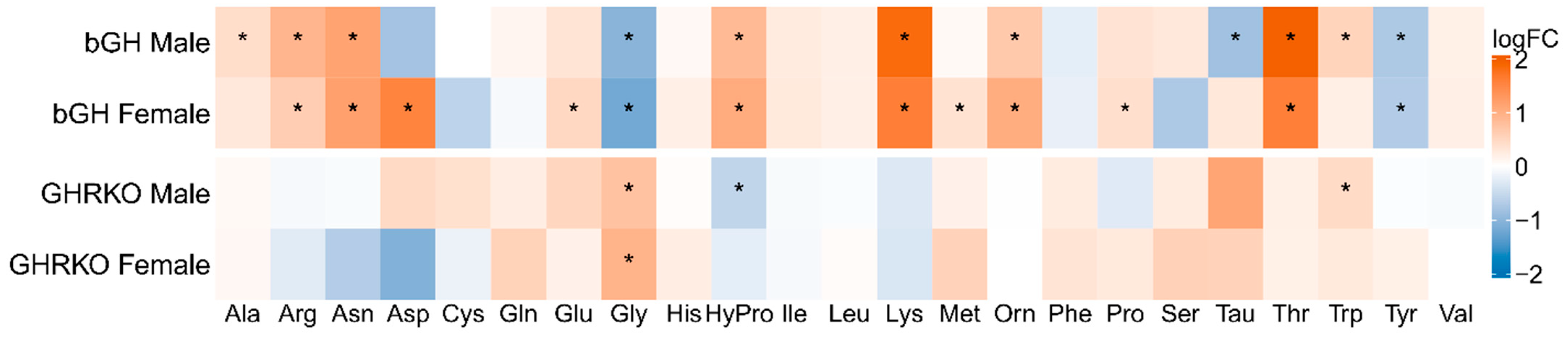
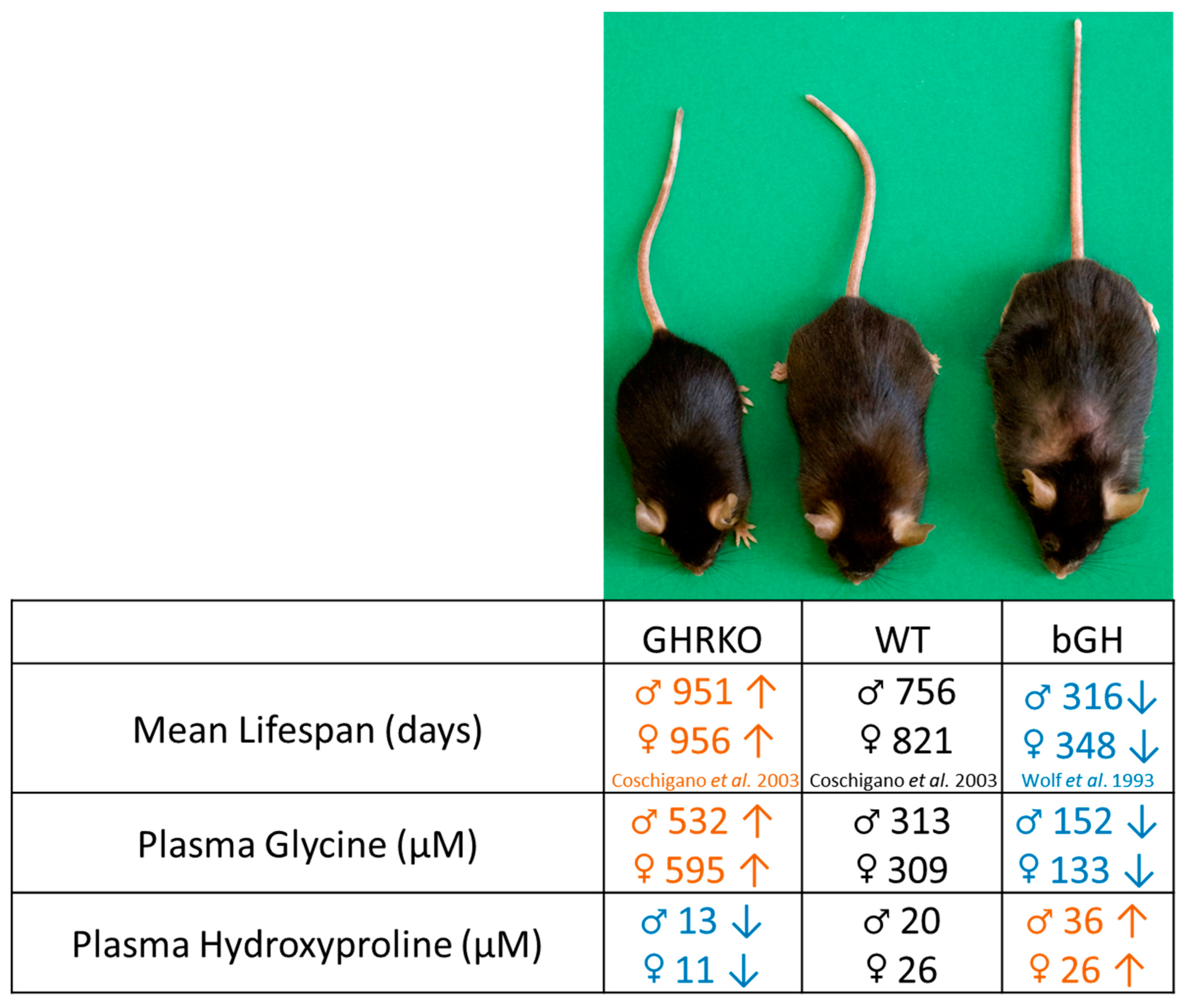
3.1. GH Action Is Positively Associated with Plasma Hydroxyproline and Negatively Associated with Plasma Glycine Levels
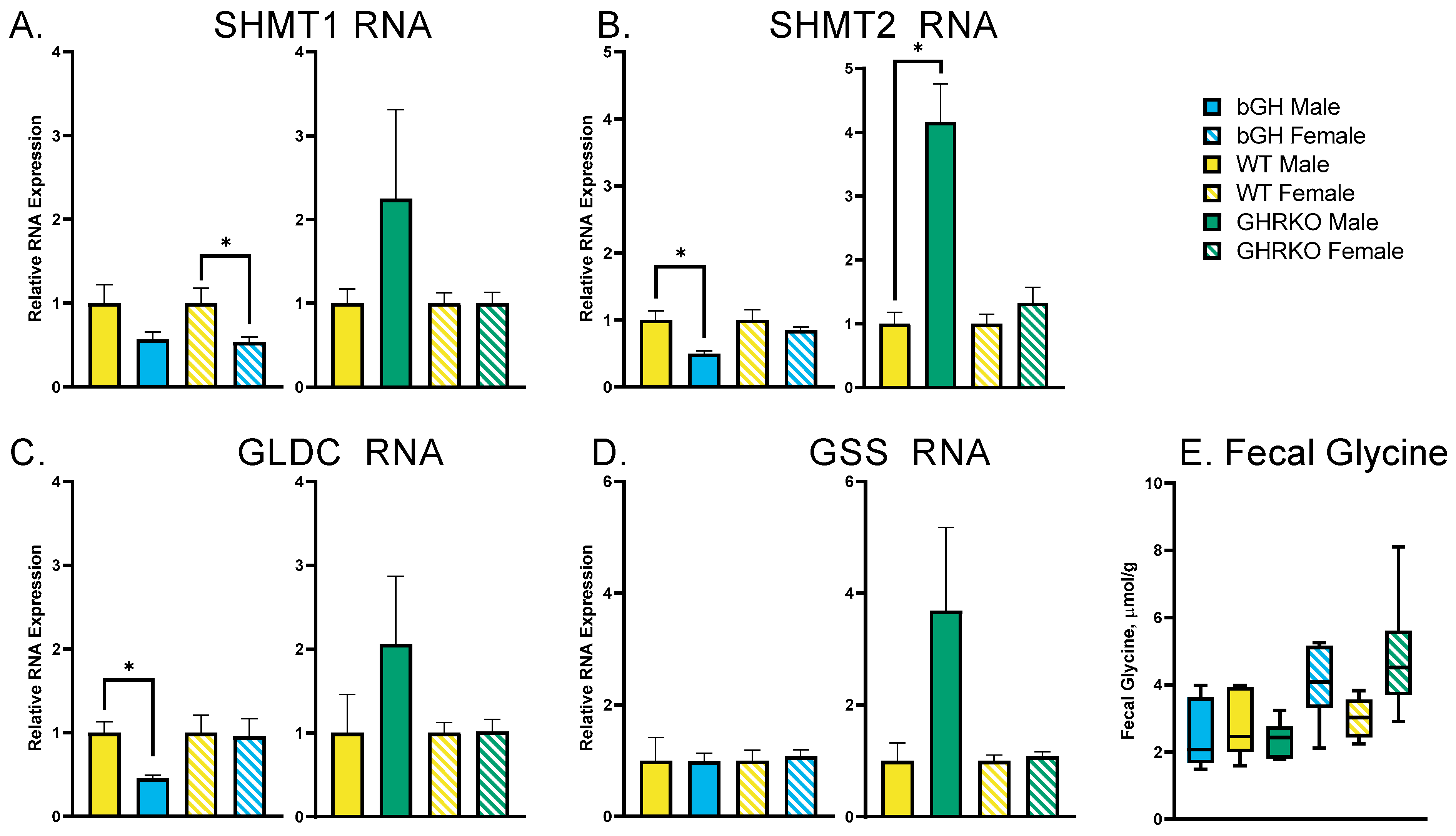
3.2. Chronic GH Alterations Have No Effect on Glycine Synthesis or Disposal
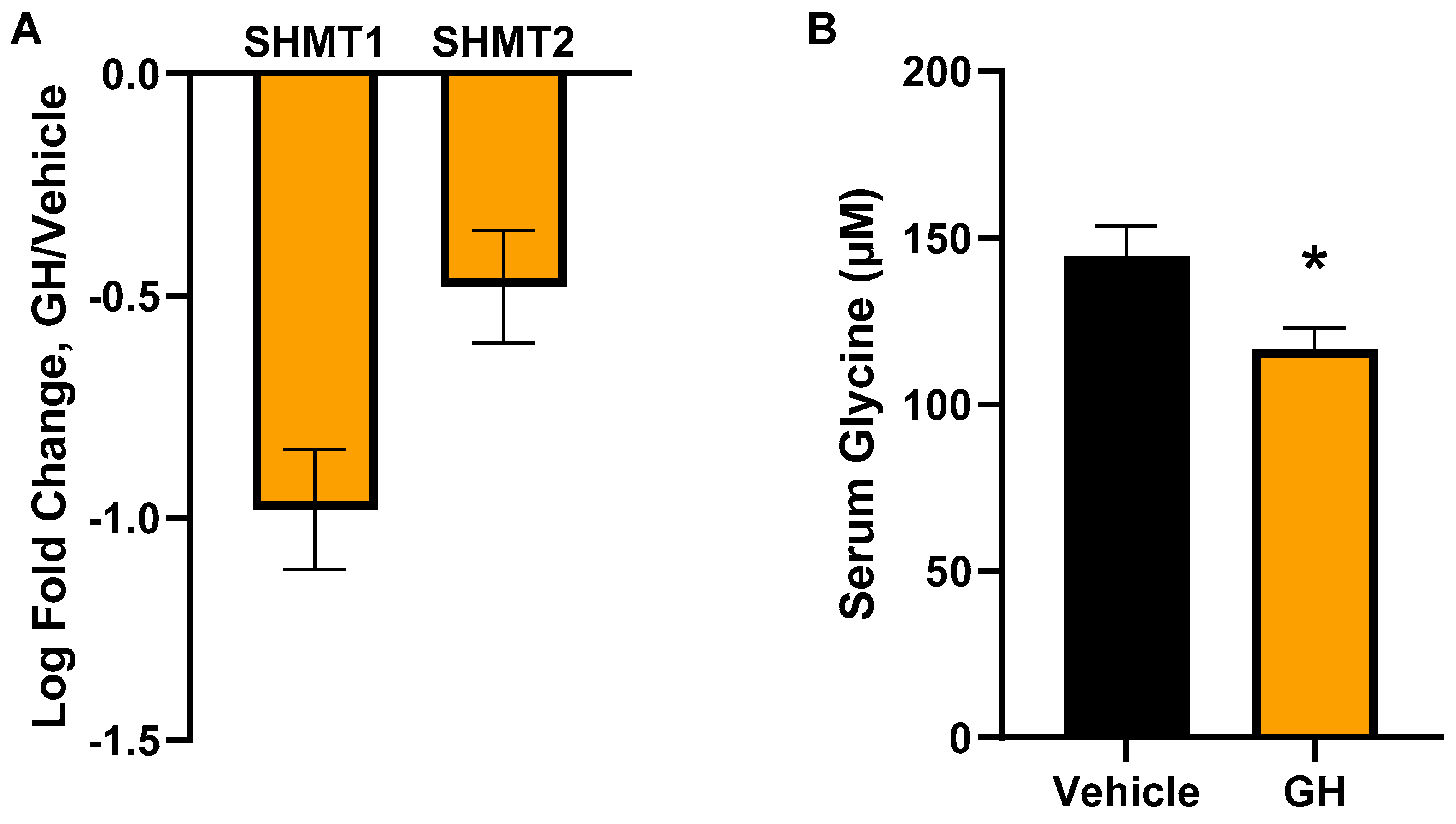
3.3. Acute GH Treatment Decreases Expression of Glycine Synthesis Genes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Houssay, B.A.; Foglia, V.G.; Smyth, F.S.; Rietti, C.T.; Houssay, A.B. The Hypophysis and Secretion of Insulin. J. Exp. Med. 1942, 75, 547–566. [Google Scholar] [CrossRef] [PubMed]
- Moller, N.; Jorgensen, J.O. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr. Rev. 2009, 30, 152–177. [Google Scholar] [CrossRef] [PubMed]
- Moller, N.; Vendelbo, M.H.; Kampmann, U.; Christensen, B.; Madsen, M.; Norrelund, H.; Jorgensen, J.O. Growth hormone and protein metabolism. Clin. Nutr. 2009, 28, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Chromiak, J.A.; Antonio, J. Use of amino acids as growth hormone-releasing agents by athletes. Nutrition 2002, 18, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Fryburg, D.A.; Barrett, E.J. Growth hormone acutely stimulates skeletal muscle but not whole-body protein synthesis in humans. Metabolism 1993, 42, 1223–1227. [Google Scholar] [CrossRef]
- Hoybye, C.; Wahlstrom, E.; Tollet-Egnell, P.; Norstedt, G. Metabolomics: A tool for the diagnosis of GH deficiency and for monitoring GH replacement? Endocr. Connect. 2014, 3, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Biagetti, B.; Herance, J.R.; Ferrer, R.; Aulinas, A.; Palomino-Schatzlein, M.; Mesa, J.; Castano, J.P.; Luque, R.M.; Simo, R. Metabolic Fingerprint of Acromegaly and its Potential Usefulness in Clinical Practice. J. Clin. Med. 2019, 8, 1549. [Google Scholar] [CrossRef]
- Berryman, D.E.; List, E.O.; Coschigano, K.T.; Behar, K.; Kim, J.K.; Kopchick, J.J. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm. IGF Res. 2004, 14, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Wolf, E.; Kahnt, E.; Ehrlein, J.; Hermanns, W.; Brem, G.; Wanke, R. Effects of long-term elevated serum levels of growth hormone on life expectancy of mice: Lessons from transgenic animal models. Mech. Ageing Dev. 1993, 68, 71–87. [Google Scholar] [CrossRef]
- Berryman, D.E.; List, E.O.; Palmer, A.J.; Chung, M.Y.; Wright-Piekarski, J.; Lubbers, E.; O’Connor, P.; Okada, S.; Kopchick, J.J. Two-year body composition analyses of long-lived GHR null mice. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 31–40. [Google Scholar] [CrossRef]
- Coschigano, K.T.; Holland, A.N.; Riders, M.E.; List, E.O.; Flyvbjerg, A.; Kopchick, J.J. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology 2003, 144, 3799–3810. [Google Scholar] [CrossRef] [PubMed]
- Pilcher, H. Money for old mice. Nature 2003. [Google Scholar] [CrossRef]
- Miller, R.A.; Harrison, D.E.; Astle, C.M.; Bogue, M.A.; Brind, J.; Fernandez, E.; Flurkey, K.; Javors, M.; Ladiges, W.; Leeuwenburgh, C.; et al. Glycine supplementation extends lifespan of male and female mice. Aging Cell 2019, 18, e12953. [Google Scholar] [CrossRef] [PubMed]
- Orentreich, N.; Matias, J.R.; DeFelice, A.; Zimmerman, J.A. Low methionine ingestion by rats extends life span. J. Nutr. 1993, 123, 269–274. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, L.; Wang, Y.; Tang, H. Age-related topographical metabolic signatures for the rat gastrointestinal contents. J. Proteome Res. 2012, 11, 1397–1411. [Google Scholar] [CrossRef]
- Jensen, E.A.; Young, J.A.; Jackson, Z.; Busken, J.; List, E.O.; Carroll, R.K.; Kopchick, J.J.; Murphy, E.R.; Berryman, D.E. Growth Hormone Deficiency and Excess Alter the Gut Microbiome in Adult Male Mice. Endocrinology 2020, 161, bqaa026. [Google Scholar] [CrossRef]
- Jensen, E.A.; Young, J.A.; Jackson, Z.; Busken, J.; Kuhn, J.; Onusko, M.; Carroll, R.K.; List, E.O.; Brown, J.M.; Kopchick, J.J.; et al. Excess Growth Hormone Alters the Male Mouse Gut Microbiome in an Age-dependent Manner. Endocrinology 2022, 163, bqac074. [Google Scholar] [CrossRef]
- Tian, Y.; Rimal, B.; Gui, W.; Koo, I.; Yokoyama, S.; Perdew, G.H.; Patterson, A.D. Early Life Short-Term Exposure to Polychlorinated Biphenyl 126 in Mice Leads to Metabolic Dysfunction and Microbiota Changes in Adulthood. Int. J. Mol. Sci. 2022, 23, 8220. [Google Scholar] [CrossRef]
- Young, J.A.; Buchman, M.; Duran-Ortiz, S.; Kruse, C.; Bell, S.; Kopchick, J.J.; Berryman, D.E.; List, E.O. Transcriptome profiling of insulin sensitive tissues from GH deficient mice following GH treatment. Pituitary 2021, 24, 384–399. [Google Scholar] [CrossRef]
- Sciascia, Q.L.; Pacheco, D.; McCoard, S.A. Administration of Exogenous Growth Hormone Is Associated with Changes in Plasma and Intracellular Mammary Amino Acid Profiles and Abundance of the Mammary Gland Amino Acid Transporter SLC3A2 in Mid-Lactation Dairy Cows. PLoS ONE 2015, 10, e0134323. [Google Scholar] [CrossRef]
- Ottaway, J.H. The concentration of asparagine in the tissues of rats treated with growth hormone. Biochem. J. 1972, 129, 503–505. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Bassot, A.; Bulteau, A.L.; Pirola, L.; Morio, B. Glycine Metabolism and Its Alterations in Obesity and Metabolic Diseases. Nutrients 2019, 11, 1356. [Google Scholar] [CrossRef] [PubMed]
- Kopchick, J.J.; Basu, R.; Berryman, D.E.; Jorgensen, J.O.L.; Johannsson, G.; Puri, V. Covert actions of growth hormone: Fibrosis, cardiovascular diseases and cancer. Nat. Rev. Endocrinol. 2022, 18, 558–573. [Google Scholar] [CrossRef] [PubMed]
- Simsek, B.; Karacaer, O.; Karaca, I. Urine products of bone breakdown as markers of bone resorption and clinical usefulness of urinary hydroxyproline: An overview. Chin. Med. J. 2004, 117, 291–295. [Google Scholar] [PubMed]
- Tritos, N.A.; Klibanski, A. Effects of Growth Hormone on Bone. Prog. Mol. Biol. Transl. Sci. 2016, 138, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.A.; Young, J.A.; Kuhn, J.; Onusko, M.; Busken, J.; List, E.O.; Kopchick, J.J.; Berryman, D.E. Growth hormone alters gross anatomy and morphology of the small and large intestines in age- and sex-dependent manners. Pituitary 2022, 25, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Brooks, N.E.; Hjortebjerg, R.; Henry, B.E.; List, E.O.; Kopchick, J.J.; Berryman, D.E. Fibroblast growth factor 21, fibroblast growth factor receptor 1, and beta-Klotho expression in bovine growth hormone transgenic and growth hormone receptor knockout mice. Growth Horm. IGF Res. 2016, 30–31, 22–30. [Google Scholar] [CrossRef]
- Palmer, A.J.; Chung, M.Y.; List, E.O.; Walker, J.; Okada, S.; Kopchick, J.J.; Berryman, D.E. Age-related changes in body composition of bovine growth hormone transgenic mice. Endocrinology 2009, 150, 1353–1360. [Google Scholar] [CrossRef]
- Jara, A.; Benner, C.M.; Sim, D.; Liu, X.; List, E.O.; Householder, L.A.; Berryman, D.E.; Kopchick, J.J. Elevated systolic blood pressure in male GH transgenic mice is age dependent. Endocrinology 2014, 155, 975–986. [Google Scholar] [CrossRef]
- Ding, J.; Berryman, D.E.; Kopchick, J.J. Plasma proteomic profiles of bovine growth hormone transgenic mice as they age. Transgenic Res. 2011, 20, 1305–1320. [Google Scholar] [CrossRef]
- Doi, T.; Striker, L.J.; Quaife, C.; Conti, F.G.; Palmiter, R.; Behringer, R.; Brinster, R.; Striker, G.E. Progressive glomerulosclerosis develops in transgenic mice chronically expressing growth hormone and growth hormone releasing factor but not in those expressing insulinlike growth factor-1. Am. J. Pathol. 1988, 131, 398–403. [Google Scholar]
- Kopchick, J.J.; Bellush, L.L.; Coschigano, K.T. Transgenic models of growth hormone action. Annu. Rev. Nutr. 1999, 19, 437–461. [Google Scholar] [CrossRef]
- Piazza, V.G.; Matzkin, M.E.; Cicconi, N.S.; Muia, N.V.; Valquinta, S.; McCallum, G.J.; Micucci, G.P.; Freund, T.; Zotta, E.; Gonzalez, L.; et al. Exposure to growth hormone is associated with hepatic up-regulation of cPLA2alpha and COX. Mol. Cell. Endocrinol. 2020, 509, 110802. [Google Scholar] [CrossRef]
- Qian, Y.; Berryman, D.E.; Basu, R.; List, E.O.; Okada, S.; Young, J.A.; Jensen, E.A.; Bell, S.R.C.; Kulkarni, P.; Duran-Ortiz, S.; et al. Mice with gene alterations in the GH and IGF family. Pituitary 2022, 25, 1–51. [Google Scholar] [CrossRef]
- Piotrowska, K.; Borkowska, S.J.; Wiszniewska, B.; Laszczynska, M.; Sluczanowska-Glabowska, S.; Havens, A.M.; Kopchick, J.J.; Bartke, A.; Taichman, R.S.; Kucia, M.; et al. The effect of low and high plasma levels of insulin-like growth factor-1 (IGF-1) on the morphology of major organs: Studies of Laron dwarf and bovine growth hormone transgenic (bGHTg) mice. Histol. Histopathol. 2013, 28, 1325–1336. [Google Scholar] [CrossRef]
- Harper, J.M.; Salmon, A.B.; Chang, Y.; Bonkowski, M.; Bartke, A.; Miller, R.A. Stress resistance and aging: Influence of genes and nutrition. Mech. Ageing Dev. 2006, 127, 687–694. [Google Scholar] [CrossRef]
- Chen, W.Y.; White, M.E.; Wagner, T.E.; Kopchick, J.J. Functional antagonism between endogenous mouse growth hormone (GH) and a GH analog results in dwarf transgenic mice. Endocrinology 1991, 129, 1402–1408. [Google Scholar] [CrossRef]
- Nicoll, C.S.; Tarpey, J.F.; Mayer, G.L.; Russell, S.M. Similarities and Differences Among Prolactins and Growth Hormones and Their Receptors. Am. Zool. 1986, 26, 965–983. [Google Scholar] [CrossRef]
- Zhong, Z.; Wheeler, M.D.; Li, X.; Froh, M.; Schemmer, P.; Yin, M.; Bunzendaul, H.; Bradford, B.; Lemasters, J.J. L-Glycine: A novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 229–240. [Google Scholar] [CrossRef]
- Okekunle, A.P.; Li, Y.; Liu, L.; Du, S.; Wu, X.; Chen, Y.; Li, Y.; Qi, J.; Sun, C.; Feng, R. Abnormal circulating amino acid profiles in multiple metabolic disorders. Diabetes Res. Clin. Pract. 2017, 132, 45–58. [Google Scholar] [CrossRef]
- Tan, H.C.; Hsu, J.W.; Tai, E.S.; Chacko, S.; Wu, V.; Lee, C.F.; Kovalik, J.P.; Jahoor, F. De Novo Glycine Synthesis Is Reduced in Adults with Morbid Obesity and Increases Following Bariatric Surgery. Front. Endocrinol. 2022, 13, 900343. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Young, J.A.; Duran-Ortiz, S.; Bell, S.; Funk, K.; Tian, Y.; Liu, Q.; Patterson, A.D.; List, E.O.; Berryman, D.E.; Kopchick, J.J. Growth Hormone Alters Circulating Levels of Glycine and Hydroxyproline in Mice. Metabolites 2023, 13, 191. https://doi.org/10.3390/metabo13020191
Young JA, Duran-Ortiz S, Bell S, Funk K, Tian Y, Liu Q, Patterson AD, List EO, Berryman DE, Kopchick JJ. Growth Hormone Alters Circulating Levels of Glycine and Hydroxyproline in Mice. Metabolites. 2023; 13(2):191. https://doi.org/10.3390/metabo13020191
Chicago/Turabian StyleYoung, Jonathan A., Silvana Duran-Ortiz, Stephen Bell, Kevin Funk, Yuan Tian, Qing Liu, Andrew D. Patterson, Edward O. List, Darlene E. Berryman, and John J. Kopchick. 2023. "Growth Hormone Alters Circulating Levels of Glycine and Hydroxyproline in Mice" Metabolites 13, no. 2: 191. https://doi.org/10.3390/metabo13020191
APA StyleYoung, J. A., Duran-Ortiz, S., Bell, S., Funk, K., Tian, Y., Liu, Q., Patterson, A. D., List, E. O., Berryman, D. E., & Kopchick, J. J. (2023). Growth Hormone Alters Circulating Levels of Glycine and Hydroxyproline in Mice. Metabolites, 13(2), 191. https://doi.org/10.3390/metabo13020191





