1. Introduction
Metabolomics comprehensively analyzes small molecules, metabolites, in any biological system [
1], which, with the rapid development of high-throughput analytical technologies, has enabled the measurement of thousands of metabolites [
2]. However, the diversity of metabolites, their chemical complexity, and, most importantly, how different authors report them make it challenging to annotate and compare metabolite data generated by different laboratories or platforms [
3]. A data harmonization process involves integrating and standardizing data from various sources to ensure that they can be effectively compared and analyzed. The goal is to make the data coherent and compatible, allowing researchers, analysts, and decision-makers to work with a unified and consistent dataset. This involves addressing issues such as data format differences, naming conventions, data quality, and data structure variations. Data format, quality, and structure will be specific to each source used. The RefMet reference nomenclature for metabolomics [
4] was proposed to overcome the naming convention issues in metabolomics. Also, the inclusion of different kinds of identifiers will aid in the usability and comparability between different datasets’ results and can be divided into systematic and non-systematic chemical identifiers.
Systematic chemical identifiers are names assigned to chemical substances according to a set of rules established by an international body. These names are designed to be unique and unambiguous, and they provide a standardized way to represent chemical structures. Examples of systematic chemical identifiers include the International Union of Pure and Applied Chemistry (IUPAC) names, which are based on a set of rules for naming organic and inorganic compounds, and the International Chemical Identifier or InChI [
5], which encodes the chemical structure of a compound into a unique sequence of characters. InChI is designed to provide a comprehensive and unique identifier for a chemical compound, which can be used for database management, chemical search, and comparison. While InChI primarily focuses on the structural aspects of a molecule, such as the arrangement of atoms and bonds, it has some limitations [
5]. These limitations include: (i) limited representation of stereochemistry, as a more accurate depiction of cis/trans isomerism and other stereochemical features is needed; (ii) handling complex chemical scenarios, such as mixtures, polymers, complex chiral chemicals, and inorganic compounds; (iii) limited ability to represent multiple structures, such as antibodies with hundreds of amino acids, handling tautomeric forms, representation of metal bonds, and complex mixtures with varying stoichiometries, positional isomers, and variable bonding situations; (iv) limitations in representing proton moieties [
6]. Despite these limitations, InChI has become a canonical identifier for the communication of well-defined chemicals and has greatly simplified the linking and aggregation of most content in public structure-centric chemical databases. Dashti et al. introduced ALATIS [
7], an adaptation of InChI unique compound identifiers that rely on three-dimensional (3D) structures, allowing precise atom labeling in small molecules. The ALATIS naming convention has been adopted by the BMRB small molecule NMR archive [
8] and the NMReData initiative [
9], making it particularly valuable for NMR-based harmonization studies. It has also been employed to verify InChI consistency in databases like PubChem [
10], unveiling non-standard InChI strings, inconsistent atom labeling, and inaccurate cross-references. However, the specialized focus of ALATIS on 3D structures and individual molecule identification may limit its suitability for the more extensive and intricate task of harmonizing diverse metabolite datasets, which encompasses broader objectives beyond database use.
The other types of identifiers used are non-systematic chemical identifiers which, on the other hand, are not based on a standardized set of rules and may be ambiguous or inconsistent across different databases. These identifiers are often assigned by individual researchers or organizations and may include common names, trivial names, or proprietary codes or identifiers. Examples of non-systematic chemical identifiers include PubChem Compound IDs, HMDB IDs, and KEGG IDs, which are unique identifiers assigned to each compound in their respective databases. Akhondi et al. [
11] investigated the ambiguity of non-systematic chemical identifiers within and between eight widely used chemical databases. The central objective of the study was to quantify the extent of this ambiguity associated with the identifiers and to systematically assess their consistency within and between studies. The study revealed that while ambiguity within individual datasets is generally low, non-systematic identifiers shared among databases exhibit higher levels of ambiguity, leading to potential inconsistencies in associated compound structures. This underscores the complexity of validating non-systematic identifiers, often necessitating manual verification.
Given the challenges faced in metabolomics regarding the variation in metabolite nomenclature and reporting practices, addressing the issue of non-systematic chemical identifiers requires the implementation of a comprehensive strategy. Several databases have been developed to store and curate metabolite information, such as the Human Metabolome Database (HMDB) [
12], BinVestigate [
13], Marker DB [
14], COCONUT [
15], KNApSAcK [
16], and BinDiscover [
17], among others. However, each database has strengths and limitations, such as differences in data content, format, and accessibility. Merging multiple metabolite datasets implies integrating data from distinct sources, such as web-specific databases (e.g., HMDB, BinVestigate, Marker DB, COCONUT, KNApSAcK, BinDiscover, etc.), literature (application and user specific), and repositories (e.g., Metabolomics Workbench [
18], MetaboLights [
19], GNPS [
20], among others). In addition, harmonizing metabolite identifiers is essential to facilitate the integration of metabolite data from different sources. In this regard, the IUPAC InChI and standard InChI hashes (InChIKey) [
5] are systematic chemical identifiers widely used in metabolomics research due to their unique representation of molecular structures. The InChIKey is a hashed version of the InChI that is shorter and more convenient to use in databases and search engines. The InChIKey is used by several databases to identify and link chemical structures to other resources. For example, the UniChem database [
21] provides a large-scale non-redundant database of pointers between chemical structures and chemistry resources using InChIKey. The US-EPA CompTox Chemicals Dashboard [
22] also uses InChIKey to support non-targeted analysis and high-throughput toxicity testing. The HMDB [
12] uses InChIKey to encode the chemical structure of metabolites.
Despite all the existing tools, the practice of merging information from metabolomics studies remains scarce within the scientific community. Researchers have predominantly relied on two primary strategies to address this challenge. The first strategy involves the integration of data from a single platform over time, with the objective of ensuring consistent compound identification [
23]. The second strategy, when opting for a cross-platform approach, relies on the straightforward merging of compounds based solely on their names [
24,
25]. These two strategies represent the prevailing approaches to merging metabolomics data, but they are not without their limitations. While they have been instrumental in some cases, they may not always be sufficient to fully harness the potential insights that can be derived from metabolomics data integration. Hence, there is a need to explore additional methods and techniques that can enhance the merging of metabolomics information, ultimately advancing our understanding of complex metabolic processes.
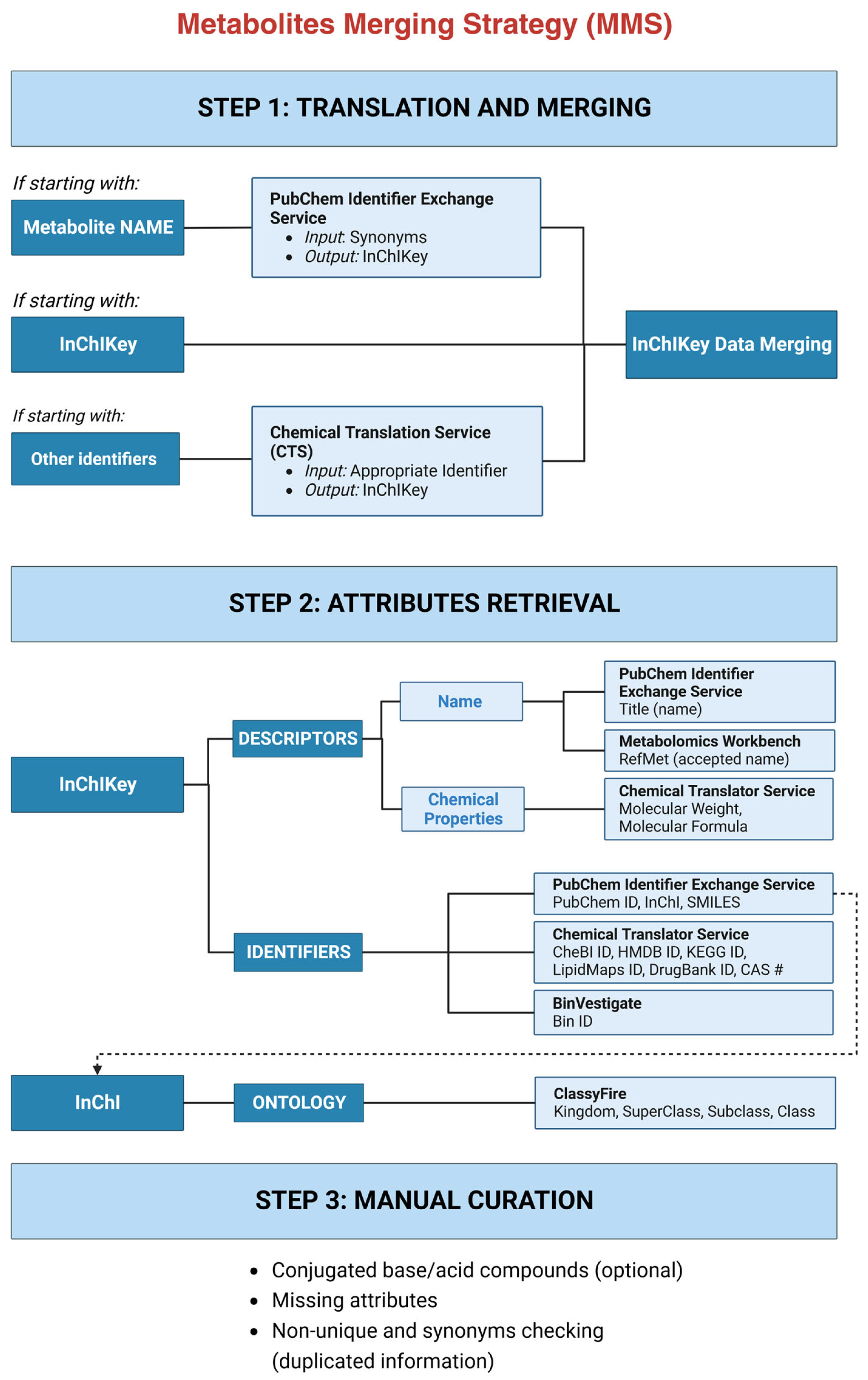
Here, we present the Metabolites Merging Strategy (MMS) that enables the cross-platform and cross-technique integration of multiple metabolite datasets. The aim of the MMS is to obtain reliable datasets of metabolites with several identifiers to enable databases’ and studies’ intercomparisons. The first step of MMS is to translate all metabolite names to systematic identifier InChIKeys and merge them. Second, attributes for each InChIKey are obtained, which include: (a) descriptors: chemical name as PubChem, RefMet accepted name and chemical properties (molecular weight, molecular formula, InChI, SMILES); (b) non-systematic identifiers: PubChem ID (CID), HMDB ID, Bin ID, KEGG ID, LipidMaps ID, DrugBank ID, CAS number, ChEBI ID); and (c) ontology. The third and last step is to perform a manual curation to check for conjugated base/acid compounds, missing attributes, and duplicated information. To usability of the MMS is exemplified by the case study of urine asthma metabolites, which included 10 sources of information (three studies from Metabolomics Workbench, the Human Metabolome DB, and six journal articles), obtaining a merged dataset with 391 metabolites. Finally, an enrichment analysis shows the importance of using the MMS, as two pathways are found significant (FDR < 0.05), in contrast to when the MMS is not followed and no significant pathways are returned.
4. Case Study: Urinary Asthma Metabolites
For the intercomparison of studies of urinary asthma metabolites, three studies were retrieved from Metabolomics Workbench, specific metabolites were retrieved from one database (HMDB), and six reported studies were retrieved from the literature. Following the MMS, a total of 547 metabolites have been retrieved and merged, leading to a final dataset of 391 metabolites (
Supplementary Table S2). The repository studies contributed 135 metabolites from study I (
Supplementary Table S3), 234 metabolites from study II (
Supplementary Table S4), and 183 metabolites from study III (
Supplementary Table S5). The HMDB had 13 additional metabolites associated with asthma in urine (
Supplementary Table S6). And finally, 71 metabolites were retrieved from the six selected articles (
Supplementary Table S7).
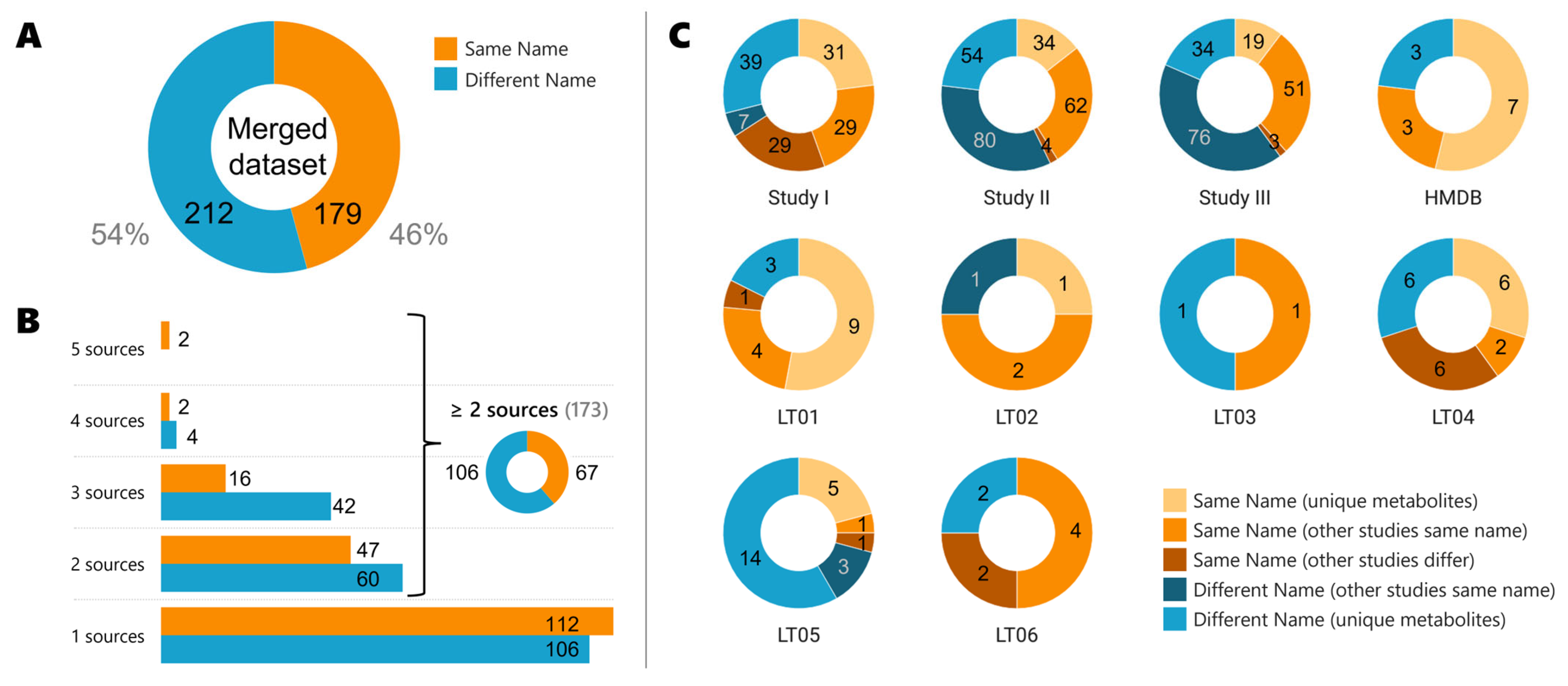
In the merged dataset, 179 metabolites (46%) are reported with the same name, while 212 (54%) are reported with different names (see
Figure 2A). As explained in the Materials and Methods section, the name was selected from PubChem name title, therefore, when we say “same name” it means the compound is reported in the repository, databases, or literature reports with the PubChem name title. Similarly, when a “different name” is stated, it means the reported name is not the PubChem “title” name. Even though we checked 10 sources of information (3 Metabolomics Workbench studies, the HMDB, and 6 literature articles (LT01–06)), only two metabolites have been found in 5 sources of information (see
Figure 2B): stearic acid and uric acid. Stearic acid (IUPAC systematic name: octadecanoic acid, abbreviated as C18:0) is a saturated long-chain fatty acid (C
18H
36O
2) present in many animal and vegetable fats and oils. In asthmatic patients, stearic acid has been found to be significantly decreased in asthmatic bronchial smooth muscle [
38]. On the other hand, uric acid (IUPAC name: 7,9-dihydro-3H-purine-2,6,8-trione, abbreviated as UA) is a xanthine. Uric acid is a product of the metabolic breakdown of purine nucleotides; in fact, it is the last product of purine metabolism in humans, and it is a normal component of urine. Serum levels of uric acid (SUA) have been associated with asthmatic patients during an acute exacerbation [
39]. SUA is one of the non-enzymatic antioxidants during an asthma exacerbation [
40]. It acts as a protective mechanism against lung damage by large amounts of oxidants.
Of the 173 metabolites that are found in at least two different sources of information (see
Figure 2B), only 67 (39%) have the same name reported. We have analyzed the individual sources of information (
Figure 2C) and detailed if the compound was reported with the same name or if it differed, but also if the compound was unique to the study, and if the compound was found in the other studies analyzed with the same or a different name. This has led to five subgroups labeled as “same name (unique metabolites)”, “same name (also in other studies)”, “same name (other studies differ)”, “different names (other studies correct)”, or as “different name”. By following the MMS, 65 compounds of study I were merged instead of the 29 if the MMS was not followed (65/29). These 65 account for 58 of the “same name” compounds not unique to the study, plus 7 “different name (other studies correct)”. Similarly, 146/62 in study II, 130/51 in study III, and 28/14 if all articles are combined (LT01-06) were merged with/without following the MMS. In total, if we sum the “same name (other studies differ)” and “different name (other studies correct)” groups, this would lead to 213 compounds out of 372 that would not have been merged if the MMS was not followed.
Even though the number of compounds with the same name title as PubChem is higher in almost all sources analyzed, the number of compounds with different names between studies varies largely from one specific study to another (
Figure 2C). For example, study I has 89 metabolites that have the same name title as PubChem, while 46 metabolites had different names. Interestingly, other studies had a higher number of metabolites with different names, e.g., 134 for study II of which 80 are found correctly in other studies, or 110 for study III of which 76 are correct in other studies. The HMDB reported 77% of the metabolites to have the same names (10 of 13). For the literature studies analyzed, only the reported significant metabolites have been added for each study, and 60% of the metabolites (45 of 75) have the same name if all literature articles were combined (LT01–LT06).
5. Discussion
The Metabolites Merging Strategy (MMS) described here implies a notable advancement in the field of metabolomics. Its significance lies in bridging the knowledge gap of those unfamiliar with studies’ harmonization, thereby promoting standardization in the field. Built on the premise that the integration of metabolomics data from diverse sources can yield a more comprehensive view of the metabolome, MMS transcends the constraints of individual studies, enabling a deeper and more precise comprehension of the metabolites present in biological samples. The MMS involves three steps. First, it integrates metabolomics data from diverse sources, by standardizing the representation of metabolites with the systematic and standardized InChIKey identifier. Second, to allow the results’ intercomparisons and a better dataset curation, several attributes are retrieved. In the same line, a metabolomics standard reporting strategy has been proposed by Alseekh et al. [
41], which includes multiple information from each compound to ensure cross-study merging. The third and final step involves the dataset manual curation, to rectify disparities for conjugated base/acid compounds (optional step), missing attributes, and for synonym checking (duplicated information). Once the MMS has been followed and the final dataset generated, a final data interpretation step will be followed, where the merged information will provide a more comprehensive understanding of the metabolome, enabling researchers to draw meaningful biological insights from the integrated data.
The MMS has been successfully implemented for urinary asthma metabolites. The merged dataset includes complete information regarding chemical properties (molecular weight and molecular formula), the systematic identifiers InChIKey, InChI, and SMILES, the non-systematic identifier PubChem ID (CID), and the ontology (for kingdom and superclass). Other non-systematic identifier attributes are partially covered: CAS number (99%), RefMet accepted name (95%), CheBI ID (88%), HMDB ID (87%), KEGG ID (83%), BinVestigate ID (42%), DrugBank ID (42%), and LipidMaps ID (21%). The variation in percentage coverage of these non-systematic identifiers can be attributed to the specific scope and content of each database. The observed differences emphasize the challenge of harmonizing data from diverse sources with distinct foci and methodologies. For example, BinVestigate is a database of GC-MS data only, reducing the number of metabolites it contains with respect to those detected by LC-MS. On the other hand, DrugBank provides information about compounds used in drug formulation, and LipidMaps is a lipid-specific database.
The distribution of metabolite names within the merged dataset bears notable implications for the comprehensive integration and harmonization achieved by the proposed MMS. The observation that 46% of the metabolites are reported with the same name across different sources highlights the presence of a substantial level of consistency in nomenclature. However, improvement is needed. If researchers start to adopt the use of systematic chemical identifiers, such as InChIKeys, along with the name of the metabolites, it will provide a standardized and unambiguous representation of molecular structures and will ease the intercomparison of studies. Such uniformity in nomenclature not only facilitates efficient data integration but also minimizes the potential for errors stemming from discrepancies in metabolite naming. However, the significant proportion of metabolites (54%) reported with different names points to the persisting challenge of diverse metabolite nomenclature practices. This heterogeneity in naming conventions is indicative of the complexities inherent in metabolite annotation, arising from factors such as historical context, variations in reporting practices, and differences in data curation methodologies across different sources. As a result, the MMS’s capability to merge data with differing metabolite names acquires heightened significance, as it bridges gaps in data integration that could otherwise hinder meaningful cross-study comparisons.
Of the 173 metabolites that are found in more than one study, 106 metabolites (61%) differ in their naming between studies. Three situations are found for metabolites with different names. First, the use of conjugated forms like palmitate instead of palmitic acid. To target these cases, we checked the first 14 characters of the InChIKey (27 characters long). They inform of the connectivity and tautomeric representation of an InChI string and, consequently, offer insight into the metabolite structure. The second part contains 10 characters that are related to all other InChI layers (isotopes, stereochemistry, etc.). The duplicated entries are combined keeping the non-conjugated ones. Second, the use of a synonym like 3,4-dihydroxycinnamic acid from LT04 instead of caffeic acid. Finally, the use of acronyms. For example, CAR 12:0 from ST001039 is referred to as lauroylcarnitine in ST001048, whose PubChem title name is in fact O-dodecanoylcarnitine. A critical aspect of the MMS is the manual curation step, which aims to ensure data accuracy and completeness. This step involves checking for conjugated compounds, addressing missing attributes, and resolving duplicated information. Manual curation remains essential due to the intricacies of metabolite data, highlighting the need for human expertise in validating and refining integrated datasets.
Without the implementation of the MMS, the merged dataset, instead of 391 entries, would have 547 entries and data analysis would lead to different results and misinterpretations. We conducted an enrichment analysis for the metabolites found at least in two sources, wherein we evaluated their overrepresentation in specific biological functions or pathways to unveil potential insights into their functional significance. If the MMS was followed (
Figure 3A), seven pathways exhibited relevance (
p < 0.05) and two pathways demonstrated significance (false discovery rate (FDR) < 0.05), specifically, tryptophan and beta-alanine metabolism pathways (
Supplementary Table S7). Conversely, when the MMS merging procedure was not employed (
Figure 3B), three pathways were relevant (
p < 0.05) but none achieved significance (
Supplementary Table S8). Tryptophan metabolic pathways have been implicated in asthma’s pathogenesis [
42], with metabolites demonstrating the potential to mitigate lung inflammation and airway hyperreactivity [
43]. On the other hand, beta-alanine metabolism exhibited higher levels in severely asthmatic patients [
44]. The MMS procedure indicated the involvement of these metabolic pathways in asthma, which otherwise would not be found relevant.
The proposed strategy effectively mitigates challenges stemming from inherent biases and variability within repositories, databases, and literature sources through a meticulously designed framework encompassing systematic data harmonization, rigorous verification, and meticulous curation processes. By leveraging InChIKeys as systematic chemical identifiers, the strategy ensures a standardized representation of metabolite structures, facilitating integration and comparability across heterogeneous sources. The translation of metabolite names and subsequent attribute consolidation, encompassing identifiers, chemical properties, and ontology, ensures comprehensive data representation. A rigorous three-step curation process further rectifies disparities, encompassing the resolution of conjugated compounds, retrieval of missing information, and identification of duplicates.
The MMS approach to data harmonization, verification, and curation enhances the accuracy and reliability of the integrated dataset, effectively addressing biases and variability intrinsic to diverse data origins and establishing a robust foundation for cross-study comparisons in metabolomics research. Moreover, the MMS’s adaptability and versatility are evident in its capacity to effectively accommodate a spectrum of data sources encountered in diverse metabolomics studies. The strategy’s applicability extends beyond the presented case study, as it can readily accommodate metabolomics data from repositories, databases, and literature articles characterized by dissimilarities in content, format, and quality. By customizing the attribute retrieval step to align with specific data source characteristics, the MMS can seamlessly handle varied metabolite identifiers and attributes, allowing the user to further use the merged dataset with several other databases or applications. This adaptability positions the MMS as a versatile tool for harmonizing and integrating metabolite data from multifaceted sources, elucidating pathways, and enabling meaningful cross-study comparisons across a spectrum of metabolomics investigations.
Author Contributions
Conceptualization, R.C. and J.B.; methodology, R.C. and M.L.; software, H.V. and M.L.; validation, M.L. and R.C.; formal analysis, H.V. and R.C.; investigation, H.V., M.L. and R.C.; resources, J.G., J.B. and R.C.; data curation, H.V., M.L. and R.C.; writing—original draft preparation, H.V.; writing—review and editing, M.L. and R.C.; visualization, R.C.; supervision, R.C., J.B. and J.G.; project administration, R.C.; funding acquisition, R.C. and J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This project received funding from the European Union’s Horizon 2020 Research and Innovation Programme under the Marie Sklodowska-Curie grant agreement No 798038 and Grants PID2021-126543OB-C22 and RTI2018-098577-B-C21 funded by MCIN/AEI/10.13039/501100011033 and by “ERDF A way of making Europe”. M.L. is thankful for her graduate fellowship from the URV PMF-PIPF program (ref. 2019PMF-PIPF-37).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Datasets are available at the Zenodo repository (10.5281/zenodo.8226097).
Acknowledgments
We are thankful to Oliver Fiehn, Gert Wohlgemuth, and Diego Pedrosa for the BinVestigate REST code.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dunn, W.B.; Ellis, D.I. Metabolomics: Current Analytical Platforms and Methodologies. TrAC Trends Anal. Chem. 2005, 24, 285–294. [Google Scholar] [CrossRef]
- Patti, G.J.; Yanes, O.; Siuzdak, G. Innovation: Metabolomics: The Apogee of the Omics Trilogy. Nat. Rev. Mol. Cell Biol. 2012, 13, 263–269. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed Minimum Reporting Standards for Chemical Analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Fahy, E.; Subramaniam, S. RefMet: A Reference Nomenclature for Metabolomics. Nat. Methods 2020, 17, 1173–1174. [Google Scholar] [CrossRef]
- Heller, S.R.; McNaught, A.; Pletnev, I.; Stein, S.; Tchekhovskoi, D. InChI, the IUPAC International Chemical Identifier. J. Cheminformatics 2015, 7, 23. [Google Scholar] [CrossRef]
- Spronk, S.A.; Glick, Z.L.; Metcalf, D.P.; Sherrill, C.D.; Cheney, D.L. A Quantum Chemical Interaction Energy Dataset for Accurately Modeling Protein-Ligand Interactions. Sci. Data 2023, 10, 619. [Google Scholar] [CrossRef]
- Dashti, H.; Westler, W.M.; Markley, J.L.; Eghbalnia, H.R. Unique Identifiers for Small Molecules Enable Rigorous Labeling of Their Atoms. Sci. Data 2017, 4, 170073. [Google Scholar] [CrossRef]
- Hoch, J.C.; Baskaran, K.; Burr, H.; Chin, J.; Eghbalnia, H.R.; Fujiwara, T.; Gryk, M.R.; Iwata, T.; Kojima, C.; Kurisu, G.; et al. Biological Magnetic Resonance Data Bank. Nucleic Acids Res. 2023, 51, D368–D376. [Google Scholar] [CrossRef]
- Pupier, M.; Nuzillard, J.M.; Wist, J.; Schlörer, N.E.; Kuhn, S.; Erdelyi, M.; Steinbeck, C.; Williams, A.J.; Butts, C.; Claridge, T.D.W.; et al. NMReDATA, a Standard to Report the NMR Assignment and Parameters of Organic Compounds. Magn. Reson. Chem. 2018, 56, 703–715. [Google Scholar] [CrossRef]
- Dashti, H.; Wedell, J.R.; Westler, W.M.; Markley, J.L.; Eghbalnia, H.R. Automated Evaluation of Consistency within the PubChem Compound Database. Sci. Data 2019, 6, 190023. [Google Scholar] [CrossRef]
- Akhondi, S.A.; Muresan, S.; Williams, A.J.; Kors, J.A. Ambiguity of Non-Systematic Chemical Identifiers within and between Small-Molecule Databases. J. Cheminformatics 2015, 7, 54. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.C.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622. [Google Scholar] [CrossRef]
- Lai, Z.; Tsugawa, H.; Wohlgemuth, G.; Mehta, S.; Mueller, M.; Zheng, Y.; Ogiwara, A.; Meissen, J.; Showalter, M.; Takeuchi, K.; et al. Identifying Metabolites by Integrating Metabolome Databases with Mass Spectrometry Cheminformatics. Nat. Methods 2017, 15, 53–56. [Google Scholar] [CrossRef]
- Wishart, D.S.; Bartok, B.; Oler, E.; Liang, K.Y.H.; Budinski, Z.; Berjanskii, M.; Guo, A.; Cao, X.; Wilson, M. MarkerDB: An Online Database of Molecular Biomarkers. Nucleic Acids Res. 2021, 49, D1259–D1267. [Google Scholar] [CrossRef]
- Sorokina, M.; Merseburger, P.; Rajan, K.; Yirik, M.A.; Steinbeck, C. COCONUT Online: Collection of Open Natural Products Database. J. Cheminformatics 2021, 13, 2. [Google Scholar] [CrossRef]
- Afendi, F.M.; Okada, T.; Yamazaki, M.; Hirai-Morita, A.; Nakamura, Y.; Nakamura, K.; Ikeda, S.; Takahashi, H.; Altaf-Ul-Amin, M.; Darusman, L.K.; et al. KNApSAcK Family Databases: Integrated Metabolite–Plant Species Databases for Multifaceted Plant Research. Plant Cell Physiol. 2012, 53, e1. [Google Scholar] [CrossRef]
- Bremer, P.L.; Wohlgemuth, G.; Fiehn, O. The BinDiscover Database: A Biology-Focused Meta-Analysis Tool for 156,000 GC–TOF MS Metabolome Samples. J. Cheminformatics 2023, 15, 66. [Google Scholar] [CrossRef]
- Sud, M.; Fahy, E.; Cotter, D.; Azam, K.; Vadivelu, I.; Burant, C.; Edison, A.; Fiehn, O.; Higashi, R.; Nair, K.S.; et al. Metabolomics Workbench: An International Repository for Metabolomics Data and Metadata, Metabolite Standards, Protocols, Tutorials and Training, and Analysis Tools. Nucleic Acids Res. 2016, 44, D463. [Google Scholar] [CrossRef]
- Haug, K.; Cochrane, K.; Nainala, V.C.; Williams, M.; Chang, J.; Jayaseelan, K.V.; O’Donovan, C. MetaboLights: A Resource Evolving in Response to the Needs of Its Scientific Community. Nucleic Acids Res. 2020, 48, D440–D444. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Chambers, J.; Davies, M.; Gaulton, A.; Hersey, A.; Velankar, S.; Petryszak, R.; Hastings, J.; Bellis, L.; McGlinchey, S.; Overington, J.P. UniChem: A Unified Chemical Structure Cross-Referencing and Identifier Tracking System. J. Cheminformatics 2013, 5, 3. [Google Scholar] [CrossRef]
- Williams, A.J.; Grulke, C.M.; Edwards, J.; McEachran, A.D.; Mansouri, K.; Baker, N.C.; Patlewicz, G.; Shah, I.; Wambaugh, J.F.; Judson, R.S.; et al. The CompTox Chemistry Dashboard: A Community Data Resource for Environmental Chemistry. J. Cheminformatics 2017, 9, 61. [Google Scholar] [CrossRef]
- Mutter, S.; Worden, C.; Paxton, K.; Mäkinen, V.P. Statistical Reporting of Metabolomics Data: Experience from a High-Throughput NMR Platform and Epidemiological Applications. Metabolomics 2019, 16, 5. [Google Scholar] [CrossRef]
- Roth, H.E.; Powers, R. Meta-Analysis Reveals Both the Promises and the Challenges of Clinical Metabolomics. Cancers 2022, 14, 3992. [Google Scholar] [CrossRef]
- Goveia, J.; Pircher, A.; Conradi, L.-C.; Kalucka, J.; Lagani, V.; Dewerchin, M.; Eelen, G.; DeBerardinis, R.J.; Wilson, I.D.; Carmeliet, P. Meta-Analysis of Clinical Metabolic Profiling Studies in Cancer: Challenges and Opportunities. EMBO Mol. Med. 2016, 8, 1134–1142. [Google Scholar] [CrossRef]
- PubChem Identifier Exchange Service. Available online: https://pubchem.ncbi.nlm.nih.gov/idexchange/idexchange.cgi (accessed on 10 July 2023).
- Wohlgemuth, G.; Haldiya, P.K.; Willighagen, E.; Kind, T.; Fiehn, O. The Chemical Translation Service—A Web-Based Tool to Improve Standardization of Metabolomic Reports. Bioinformatics 2010, 26, 2647–2648. [Google Scholar] [CrossRef]
- Metabolomics Workbench: Databases: RefMet. Available online: https://www.metabolomicsworkbench.org/databases/refmet/browse.php (accessed on 31 July 2023).
- Djoumbou Feunang, Y.; Eisner, R.; Knox, C.; Chepelev, L.; Hastings, J.; Owen, G.; Fahy, E.; Steinbeck, C.; Subramanian, S.; Bolton, E.; et al. ClassyFire: Automated Chemical Classification with a Comprehensive, Computable Taxonomy. J. Cheminformatics 2016, 8, 61. [Google Scholar] [CrossRef]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC-HRMS Spectra Processing, Multi-Omics Integration and Covariate Adjustment of Global Metabolomics Data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef]
- Jewison, T.; Su, Y.; Disfany, F.M.; Liang, Y.; Knox, C.; Maciejewski, A.; Poelzer, J.; Huynh, J.; Zhou, Y.; Arndt, D.; et al. SMPDB 2.0: Big Improvements to the Small Molecule Pathway Database. Nucleic Acids Res. 2014, 42, D478–D484. [Google Scholar] [CrossRef]
- Carraro, S.; Bozzetto, S.; Giordano, G.; El Mazloum, D.; Stocchero, M.; Pirillo, P.; Zanconato, S.; Baraldi, E. Wheezing Preschool Children with Early-Onset Asthma Reveal a Specific Metabolomic Profile. Pediatr. Allergy Immunol. 2018, 29, 375–382. [Google Scholar] [CrossRef]
- Chiu, C.-Y.; Lin, G.; Cheng, M.-L.; Chiang, M.-H.; Tsai, M.-H.; Su, K.-W.; Hua, M.-C.; Liao, S.-L.; Lai, S.-H.; Yao, T.-C.; et al. Longitudinal Urinary Metabolomic Profiling Reveals Metabolites for Asthma Development in Early Childhood. Pediatr. Allergy Immunol. 2018, 29, 496–503. [Google Scholar] [CrossRef]
- Chiu, C.-Y.; Cheng, M.-L.; Chiang, M.-H.; Wang, C.-J.; Tsai, M.-H.; Lin, G. Metabolomic Analysis Reveals Distinct Profiles in the Plasma and Urine Associated with IgE Reactions in Childhood Asthma. J. Clin. Med. 2020, 9, 887. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Zhou, J.; Wang, Y.; Jin, F.; Chen, X.; Yang, J.; Chen, Z. Urinary Metabolomic Profiling Reveals Biological Pathways and Predictive Signatures Associated with Childhood Asthma. J. Asthma Allergy 2020, 13, 713–724. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Liu, X.; Wang, X.; Li, J.; Lin, K.; Sun, S.; Yue, H.; Dai, Y. Untargeted Metabolomic Study of Acute Exacerbation of Pediatric Asthma via HPLC-Q-Orbitrap-MS. J. Pharm. Biomed. Anal. 2022, 215, 114737. [Google Scholar] [CrossRef]
- Tao, J.-L.; Chen, Y.-Z.; Dai, Q.-G.; Tian, M.; Wang, S.-C.; Shan, J.-J.; Ji, J.-J.; Lin, L.-L.; Li, W.-W.; Yuan, B. Urine Metabolic Profiles in Paediatric Asthma. Respirology 2019, 24, 572–581. [Google Scholar] [CrossRef]
- Esteves, P.; Blanc, L.; Celle, A.; Dupin, I.; Maurat, E.; Amoedo, N.; Cardouat, G.; Ousova, O.; Gales, L.; Bellvert, F.; et al. Crucial Role of Fatty Acid Oxidation in Asthmatic Bronchial Smooth Muscle Remodelling. Eur. Respir. J. 2021, 58, 2004252. [Google Scholar] [CrossRef]
- Papamichael, M.M.; Katsardis, C.; Sarandi, E.; Georgaki, S.; Frima, E.-S.; Varvarigou, A.; Tsoukalas, D. Application of Metabolomics in Pediatric Asthma: Prediction, Diagnosis and Personalized Treatment. Metabolites 2021, 11, 251. [Google Scholar] [CrossRef]
- Sahiner, U.M.; Birben, E.; Erzurum, S.; Sackesen, C.; Kalayci, O. Oxidative Stress in Asthma. World Allergy Organ. J. 2011, 4, 151–158. [Google Scholar] [CrossRef]
- Alseekh, S.; Aharoni, A.; Brotman, Y.; Contrepois, K.; D’Auria, J.; Ewald, J.; Ewald, J.C.; Fraser, P.D.; Giavalisco, P.; Hall, R.D.; et al. Mass Spectrometry-Based Metabolomics: A Guide for Annotation, Quantification and Best Reporting Practices. Nat. Methods 2021, 18, 747–756. [Google Scholar] [CrossRef]
- Licari, A.; Fuchs, D.; Marseglia, G.; Ciprandi, G. Tryptophan Metabolic Pathway and Neopterin in Asthmatic Children in Clinical Practice. Ital. J. Pediatr. 2019, 45, 114. [Google Scholar] [CrossRef]
- Hu, Q.; Jin, L.; Zeng, J.; Wang, J.; Zhong, S.; Fan, W.; Liao, W. Tryptophan Metabolite-Regulated Treg Responses Contribute to Attenuation of Airway Inflammation during Specific Immunotherapy in a Mouse Asthma Model. Hum. Vaccin Immunother. 2020, 16, 1891–1899. [Google Scholar] [CrossRef]
- Comhair, S.A.A.; McDunn, J.; Bennett, C.; Fettig, J.; Erzurum, S.C.; Kalhan, S.C. Metabolomic Endotype of Asthma. J. Immunol. 2015, 195, 643–650. [Google Scholar] [CrossRef]
- Ebastien Moretti, S.; Du, V.; Tran, T.; Mehl, F.; Ibberson, M.; Pagni, M. MetaNetX/MNXref: Unified Namespace for Metabolites and Biochemical Reactions in the Context of Metabolic Models. Nucleic Acids Res. 2021, 49, D570–D574. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).