Abstract
Dehydroepiandrosterone sulfate (DHEAS) is thought to be associated with life expectancy and anti-aging. Although skeletal muscle disorders are often found in diabetic people, the clinical significance of DHEAS in skeletal muscle remains unclear. Therefore, we aimed to determine whether DHEAS is associated with the development of skeletal muscle disorders in individuals with type 2 diabetes (T2D). A cross-sectional study was conducted in 361 individuals with T2D. Serum DHEAS levels, skeletal muscle mass index (SMI), handgrip strength (HS), and gait speed (GS) were measured in the participants. Pre-sarcopenia, sarcopenia, and dynapenia were defined according to the definitions of the AWGS 2019 criteria. DHEAS level was positively associated with HS but not with SMI or GS after adjustment of confounding factors. Multiple logistic regression analyses in total subjects showed that DHEAS level had an inverse association with the prevalence of dynapenia but not with the prevalence of pre-sarcopenia or sarcopenia. Furthermore, a significant association between DHEAS level and dynapenia was found in males but not in females. ROC curve analysis indicated that cutoff values of serum DHEAS for risk of dynapenia in males was 92.0 μg/dL. Therefore, in male individuals with T2D who have low serum levels of DHEAS, adequate exercise might be needed to prevent dynapenia.
1. Introduction
Populations in developed countries are aging, and significant attention has therefore been paid to geriatric syndrome. Aging-related muscle diseases, known as pre-sarcopenia, sarcopenia, and dynapenia, are included in this syndrome. Sarcopenia is defined as muscle loss and weakness, dynapenia is defined as impairment of muscle strength without reduction in muscle mass, and pre-sarcopenia is defined as reduction in muscle mass without impairment of muscle strength [1]. Previous studies have shown that these aging-related muscle diseases are more frequent in individuals with diabetes than in individuals without diabetes and that they might contribute to impaired quality of life and incidental falls [2,3]. In addition, diabatic peoples with sarcopenia have a higher risk of mortality after hospital discharge [4]. Therefore, prevention and/or prediction of skeletal muscle disorders in individuals with diabetes are pivotal clinical issues.
Dehydroepiandrosterone (DHEA) and its sulfate “DHEAS” are hormones produced by the adrenal cortex. They are mostly produced in young adulthood and their concentrations gradually decline over time [5]. Previous studies showed that a high level of DHEAS is associated with a lower risk of worsening frailty in the elderly [6] and that a lower level of DHEAS may indicate a poorer prognosis in patients with atherosclerosis [7] and cardiovascular diseases [8].
Although Yanagita et al. showed that serum cortisol-to-DHEAS ratio is strongly associated with sarcopenia in individuals with type 2 diabetes (T2D) [9], the relationships between serum DHEAS level and skeletal muscle disorders, including pre-sarcopenia, sarcopenia and dynapenia, have remained unclear. Therefore, the aim of the present study was to determine the pathophysiological role of DHEAS in skeletal muscle disorders in individuals with T2D.
2. Materials and Methods
2.1. Subjects for Cross-Sectional Study
A total of 361 Japanese subjects (202 males and 159 females) who were outpatients or inpatients with T2D and 20 years of age or older were recruited consecutively from the Department of Internal Medicine at Anan Medical Center, Anan Tokushima, Japan, between April 2020 and March 2022. All subjects underwent a standardized interview and physical examination. The diagnosis of T2D was based on the criteria proposed by the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus [10]. Body mass index was calculated as an index of obesity. Blood pressure was measured twice and averaged. Hypertensive patients were defined as those with systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg or those receiving antihypertensive agents. Patients with dyslipidemia were defined as those with low-density lipoprotein cholesterol (LDL-C) ≥ 140 mg/dL or triglycerides (TG) level ≥ 150 mg/dL or high-density lipoprotein cholesterol (HDL-C) less than 40 mg/dl or those receiving lipid-lowering agents. Current smokers were defined as subjects who had smoked within the past two years. Habitual exercisers were defined as those who engaged in aerobic exercise including walking (3 Mets or more) for 30 min or more at a time, at least three times a week for at least 1 year.
Body composition, muscle strength, and physical performance were evaluated in all the participants in this study in accordance with following described procedures.
The exclusion criteria for individuals with T2D were as follows: (1) patients with advanced cancer, (2) patients with secondary diabetes such as steroid-induced diabetes or pancreatic diabetes, (3) patients who were pregnant, (4) patients with advanced renal disease with a serum creatinine (Cr) level of >2.0 mg/dL, and (5) patients with liver cirrhosis and malnutrition (serum albumin (ALB) level of <3.0 g/dL).
2.2. Biochemical Analyses
Blood and spot urine samples were collected and used for determination of blood cell counts, plasma glucose (PG), HbA1c, and serum biochemical parameters including LDL-C, TG, HDL-C, ALB, uric acid (UA), and Cr. PG and serum levels of LDL-C, TG, HDL-C, ALB, UA, and Cr were measured by enzymatic methods using an automatic analyzing apparatus (LABOSPECT 008, Hitachi High-Tech Co., Tokyo, Japan). HbA1c was assayed by high-performance liquid chromatography using an analyzing apparatus (HLC-723 G11, Tosoh Co., Tokyo Japan). These biochemical analyzers have been widely used in Japanese hospitals. Serum level of DHEAS was determined by the chemiluminescent enzyme immunoassay (Access DHEA-S®, Beckman Coulter, Tokyo, Japan). The coefficients of variance for intra- and inter-assays in DHEAS measurements were 3.04% and 3.97%, respectively. These values mean statistically sufficient reproducibility in this examination.
2.3. Assessments of Muscle Strength and Physical Performance
Handgrip strength (HS) was evaluated as an indicator of muscle strength, and gait speed (GS) was determined to evaluate physical performance. The maximum isometric grip strength in each hand was measured in a standing position (103S TARZAN®; HATAS, Osaka, Japan). GS in each participant was measured as the time needed to walk 6 m expressed in meters per second.
2.4. Definitions of Robust, Presarcopenia, Sarcopenia and Dynapenia
Body composition was measured by using multifrequency bioelectrical impedance analysis as previously described in [11]. In brief, body composition analysis was performed using a portable direct segmental multifrequency bioimpedance analysis (DSM-BIA) device (InBody S10®, InBody Japan, Tokyo, Japan). DSM-BIA was conducted using an 8-point tactile electrode system with 30 impedance examinations taken by using 6 frequencies (1, 5, 50, 250, 500, 1000 kHz) at each of the 5 segments (bilateral thumbs, third fingers, and ankles). Patient characteristics, including information on age, sex, body weight, and height, were entered into the DSM-BIA device. Electrical currents of 1, 5, 50, 250, 500, and 1000 kHz were applied through the electrodes in the standing position. Body fat mass and skeletal muscle mass index (SMI) were calculated using formulas in the inner software based on the height, weight, and impedance examined [11].
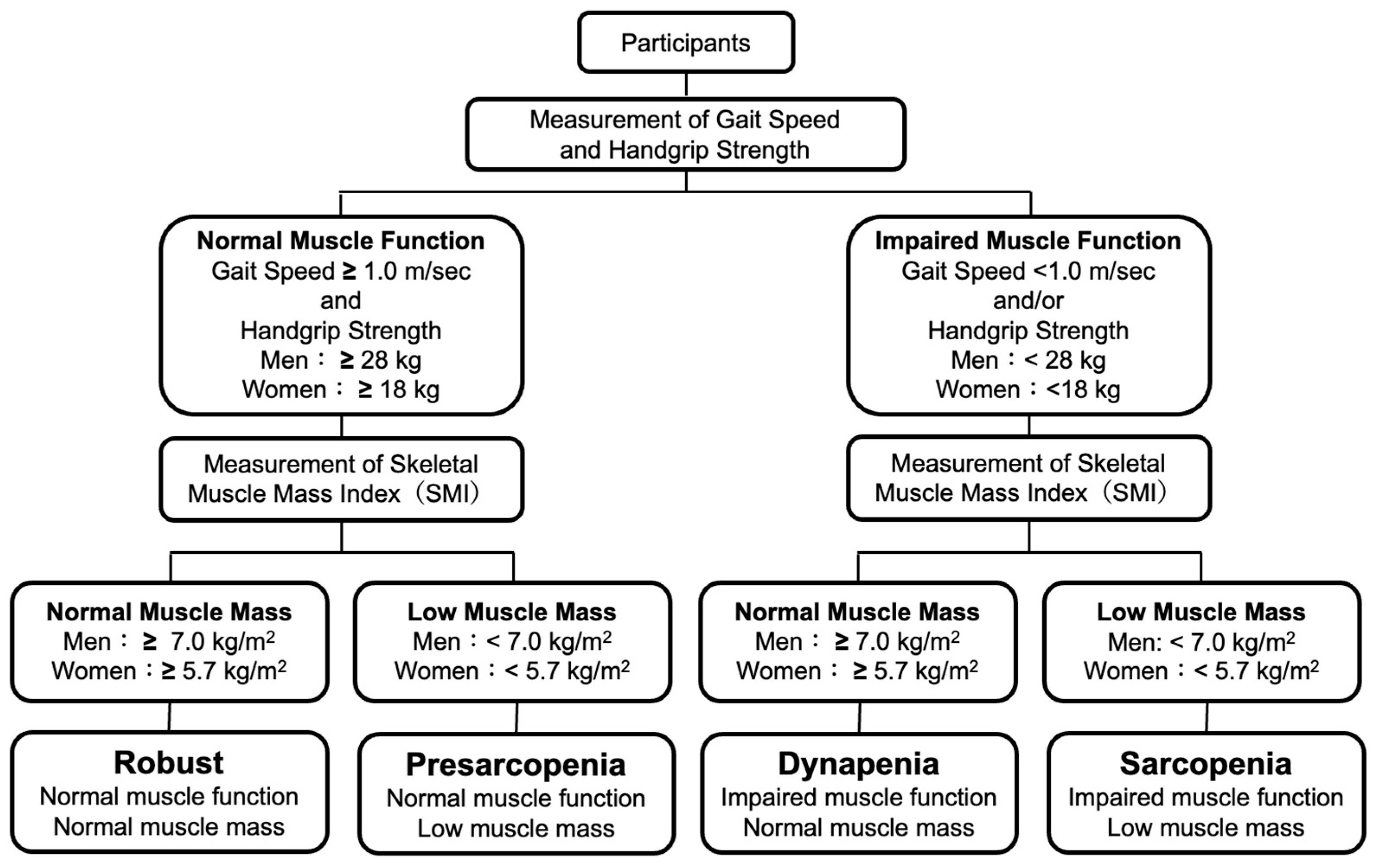
Sarcopenia was defined as a low SMI with low HS or slow GS and pre-sarcopenia was defined as a low SMI with normal HS and normal GS according to the definitions of the EWGSOP 2019 criteria [12]. Dynapenia was defined as a normal SMI with low HS or slow GS according to the definitions of the AWGS 2019 criteria [13]. Subjects without sarcopenia, pre-sarcopenia or dynapenia were defined as robust subjects. Definitions of robust, pre-sarcopenia, sarcopenia, and dynapenia in the present study are shown in Figure 1.
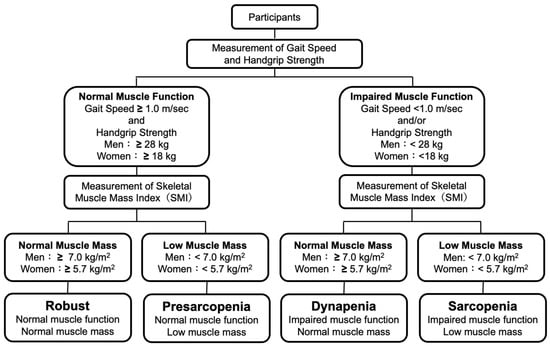
Figure 1.
Definitions of robust and skeletal muscle disorders.
2.5. Statistical Analysis
Normally distributed continuous data were presented as means ± standard deviation (SD). Skewed continuous data were presented as medians and interquartile range (IQR). Categorical variables were compared by performing the χ2 test or Fisher’s exact test. For comparisons among groups, we performed the ANOVA or Kruskal–Wallis’s test for numeric variables depending on the variables’ distribution. Simple linear regression analyses to evaluate associations of levels of DHEAS with SMI, HS, and GS were performed and multiple linear regression analyses for determinants of SMI, HS, and GS were conducted. In addition, the degrees of associations between the prevalence of each skeletal muscle disorder (pre-sarcopenia, sarcopenia, and dynapenia) and clinical variables including DHEAS were determined by performing logistic regression analysis. Receiver operating characteristic (ROC) curve analysis was conducted to determine the optimal cutoff values of serum DHEAS levels in relation to the prevalences of skeletal muscle disorders. The optimal cutoff point selection of serum DHEAS levels in the context of ROC curve analysis was determined on the basis of the maximum value of the Youden index.
The analyses were performed by using GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA) and EZR (Saitama Medical Center, Jichi Medical University). The threshold for statistical significance was set at p < 0.05.
3. Results
3.1. Clinical Characteristics of Subjects Enrolled in This Study
The physical and laboratory-determined characteristics of subjects enrolled in this study are shown in Table 1. On average, the robust subjects were younger than the subjects with skeletal muscle disorders. The BMI values of subjects with pre-sarcopenia and sarcopenia were lower than those of robust subjects and subjects with dynapenia. Although there was no significant difference in basic clinical factors including SBP, serum lipids, ALB, PG, HbA1c, UA, and Cr among the four groups, serum level of DHEAS in the dynapenia group was significantly lower than that in the robust group. The percentage of subjects with dynapenia who did habitual exercise was lower than the percentage of robust subjects who did habitual exercise. The prevalence of dyslipidemia in subjects with pre-sarcopenia was lower than that in robust subjects. The durations of T2D in the three skeletal muscle disorder groups were longer than the duration of T2D in the robust group. The SMI values of subjects with pre-sarcopenia and sarcopenia were significantly lower than the SMI values of robust subjects and subjects with dynapenia. The HS values of subjects with sarcopenia and dynapenia were lower than the HS values of robust subjects and subjects with pre-sarcopenia. The GS values of subjects with sarcopenia and dynapenia were slower than the GS values of robust subjects and subjects with pre-sarcopenia. The percentages of subjects with sarcopenia and dynapenia using antiplatelets were higher than those of robust subjects and subjects with pre-sarcopenia.

Table 1.
Clinical characteristics of subjects in total, robust, pre-sarcopenia, sarcopenia, and dynapenia group.
3.2. Associations of Serum Levels of DHEAS with SMI, HS and GS
Simple linear regression analyses showed that serum levels of DHEAS had positive associations with all skeletal muscle-associated indices, including SMI, HS, and GS, in total subjects and male subjects with T2D (Table 2). On the other hand, simple linear regression analyses showed that serum levels of DHEAS had a positive association with HS but not with SMI or GS in female subjects with T2D (Table 2).

Table 2.
Simple linear regression analysis for associations of DHEAS with SMI, HS and GS in total, male and female individuals with T2D.
3.3. Associations of Clinical Factors Including DHEAS with SMI, HS and GS
To determine independent clinical factors associated with SMI, HS, and GS in patients with T2D, we performed multiple linear regression analyses with clinical confounding factors including DHEAS. As shown in Table 3, we found that male gender, BMI, exercise, and Cr were significantly and positively correlated with SMI. In contrast, age, SBP, and LDL-C were significantly and negatively correlated with SMI. Next, we found that male gender, BMI, exercise, Cr, ALB, and DHEAS were significantly and positively correlated with HS. Conversely, age, duration of T2D, and UA were significantly and negatively correlated with HS. In regard to determinants of GS, we observed that exercise and ALB were significantly and positively correlated with GS. On the other hand, male gender, age, and BMI were significantly and negatively correlated with GS. To assess the influence of medication used in this study on associations of identified clinical factors with SMI, HS, and GS, we performed additional multiple linear regression analyses with identified clinical factors and medications used in the participants. As shown in Table S1, DHEAS remained a positive contributor for HS.

Table 3.
Multiple linear regression analysis for determinants of SMI, HS, and GS in total individuals with T2D.
3.4. Determination of Clinical Factors for Prevalences of Presarcopenia, Sarcopenia and Dynapenia
To determine clinical factors related to the prevalences of pre-sarcopenia, sarcopenia, and dynapenia in individuals with T2D, we performed multiple logistic regression analyses in each skeletal muscle disorder with reference to robust subjects. As shown in Table 4, in terms of risk factors for prevalence of the pre-sarcopenia, male gender, lower BMI, and duration of T2D were corelated with pre-sarcopenia. In the analysis for prevalence of sarcopenia, aging and lower BMI were found to be significant risk factors. As for the prevalence of dynapenia, aging and lower DHEAS were identified as significant risk factors for the prevalence of dynapenia in individuals with T2D. Lastly, we divided the subjects into male subjects and female subjects for the identification of dynapenia-associated factors, and a significant association between DHEAS and dynapenia was found in males but not in females (Table 5). The association between DHEAS and dynapenia in males remained to be significant in a multiple logistic regression analysis, including nutritional status represented by geriatric nutritional risk index: GNRI (14.89 × ALB (g/dL) + 41.7 × body weight (kg)/ideal body weight (kg)) (Table S2). The increase in the odds ratio of 0.985 at 1 μg/dL of DHEAS for prevalence of dynapenia in male individuals with T2D was considered that the increase in the odds ratio of 0.860 at 10 μg/dL of DHEAS for prevalence of dynapenia in male individuals with T2D.

Table 4.
Multiple logistic regression analysis for determinants of pre-sarcopenia, sarcopenia, and dynapenia in total individuals with T2D.

Table 5.
Multiple logistic regression analysis for determinants of dynapenia in male and female individuals with T2D.
3.5. Determination of Cutoff Value of Serum DHEAS Levels for Prevalence of Dynapenia in Male Individuals with T2D
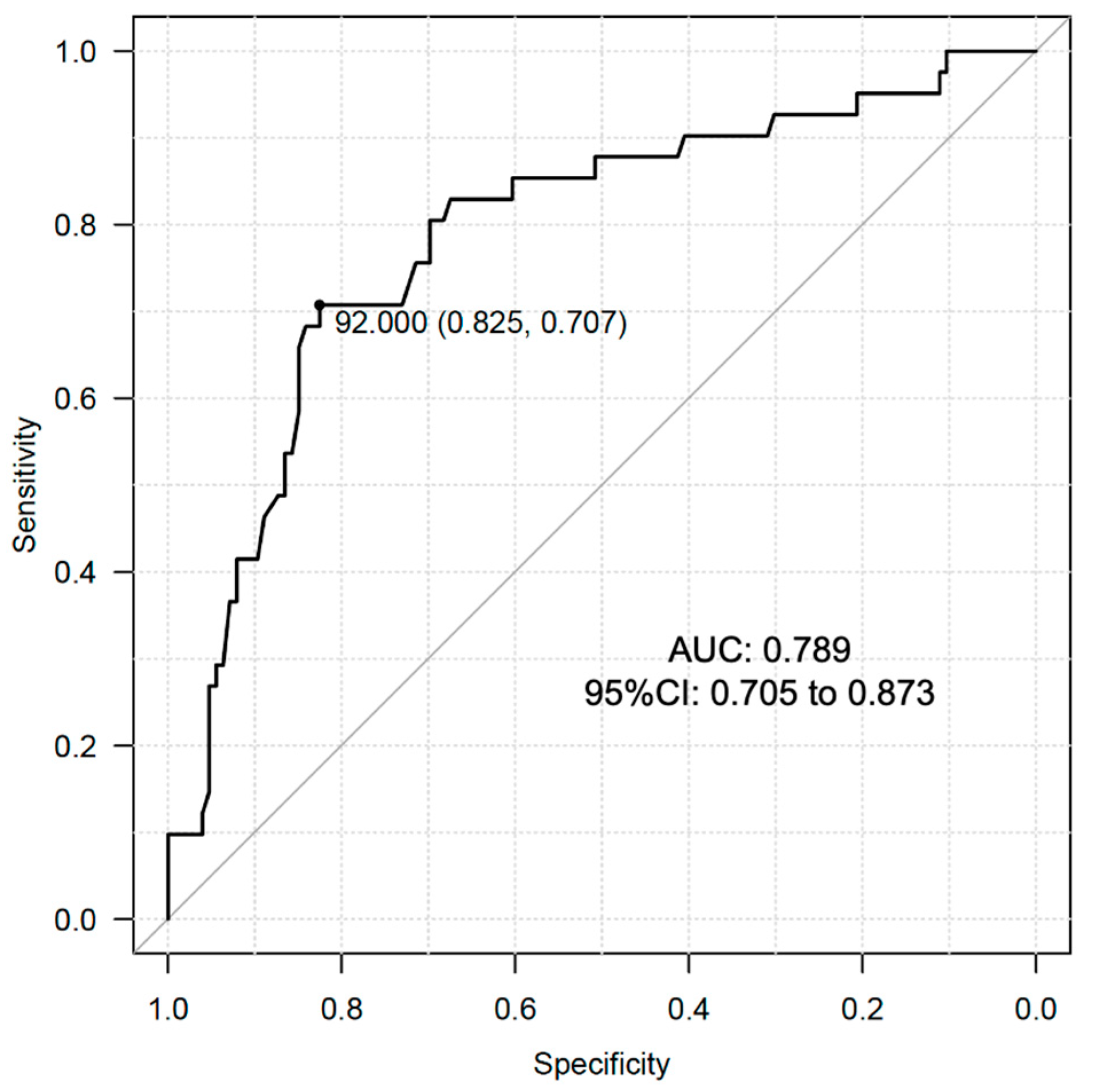
We conducted ROC curve analysis to determine the optimal cutoff value of serum DHEAS levels in relation to the prevalence of dynapenia in the male subjects of the present study. The analysis showed that the optimal cutoff value was 92.0 μg/dL with the area under the curve (AUC): 0.789 (95% CI: 0.705 to 0.873) (Figure 2). This AUC value is reasonable for indicating the significance of DHEAS in identification of dynapenia in male individuals with T2D.
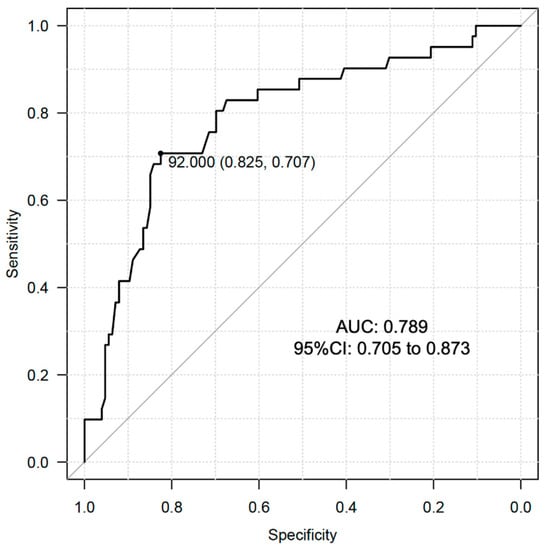
Figure 2.
ROC curve analysis for cutoff value determination of DHEAS for the prevalence of dynapenia in male subjects with T2D.
4. Discussion
In this study, we found that serum levels of DHEAS have a negative association with the prevalence of dynapenia in males with T2D but not in females with T2D.
Age-related declines of skeletal muscle mass and function are critical problems that have gained attention in clinical practice. Recent studies have shown that skeletal muscle strength deficits with aging are more rapid than the concomitant loss of skeletal muscle quantity [14]. Chao et al. reported that 18.49%, 3.52%, and 1.06% of 568 robust elderly subjects with a mean age of 70.1 years transited to dynapenia, pre-sarcopenia, and sarcopenia, respectively, during a 6-year follow up period [15]. Those results are consistent with our results, showing that the incidence of dynapenia was remarkably higher than the incidences of pre-sarcopenia and sarcopenia. The results suggest that elderly individuals have more problems with skeletal muscle strength rather than with skeletal muscle mass loss during the aging process.
It has been shown that dynapenia had a direct effect on increased mortality in 610 subjects aged 65 years or older [16]. In addition, a 10-year follow-up study involving 5310 older adults showed that the coexistence of anemia and dynapenia increased the mortality risk in both males and females [17]. Therefore, risk detection and prediction of dynapenia are urgent challenges for the promotion of health and longevity in elderly individuals with diabetes.
Although the detailed mechanisms of muscle strength weakness have not been fully clarified, aging-induced reactive oxygen species (ROS) has been proposed to impair muscle quality at various locations [14]. Muscle regeneration following damage diminishes with aging and it has been clarified that muscle regeneration is dependent on appropriate reinnervation [18]. Since accumulation of oxidative damage in nerves and muscles occurs with aging, abnormal activities of ROS promote defective muscle reinnervation throughout life [19]. Oxidative stress induces glycoxidation reactions, which lead to the formation of highly reactive and electrophilic compounds that results in the generation of advanced glycation end products (AGEs) [20,21]. Mori et al. showed that accumulated levels of AGEs represented by skin autofluorescence (SAF) were significantly higher in patients with sarcopenia and patients with dynapenia among individuals with T2D [22], and Kato et al. reported that SAF was an independent factor associated with low SMI among middle-aged and older Japanese men and women [23], suggesting that accumulation of AGEs promotes reduction of skeletal muscle mass and strength, leading to sarcopenia and/or dynapenia in elderly individuals.
It has been reported that DHEA treatment induced a marked decrease in the plasma concentration of pentosidine as a biomarker of AGEs in individuals with T2D [24] and it has been shown that DHEA treatment counteracted the enhanced AGE receptor activation in the heart of streptozotocin-diabetic rats and Zucker diabetic fatty rats and resulted in amelioration of diabetic cardiomyopathy [25]. Taken together, those results indicate the possibility that DHEA and/or DHEAS contribute to the reduction in oxidative stress accompanied by accumulation of AGEs in skeletal muscle, leading to prevention of the development of muscle weakness.
In a systematic review assessment of previous clinical studies regarding the association of endogenous serum DHEA(S) levels and skeletal muscle disorders [26], an early cross-sectional study showed that lower quadricep maximal muscle power and lower optimal shortening velocity were associated with levels of DHEAS in elderly females but not in elderly males [27]. A previous study showed that serum DHEAS was an independent predictor of muscle strength and mass in elderly males aged 60–79 years [28]; however, other studies did not show a significant association [29,30]. Although we reported that DHEAS has sex-dependent diverse vascular protective effects against carotid atherosclerosis in individuals with cardiovascular risk factors [7], the precise differential mechanisms of the sex-dependent pathophysiological effects of DHEAS have remained unclear and the diverse associations between DHEAS and skeletal muscle disorders in males and females are issues to be further investigated.
Limitations
The main limitation of our study is the retrospective cross-sectional nature with a relatively small sample size, which precludes conclusions regarding the temporal nature of our findings and no solid conclusions can be established. Even though we found that the levels of serum DHEAS were associated with the prevalence of dynapenia in patients with T2D, we cannot confirm a causal relationship between DHEAS and incidence of dynapenia. In addition, dietary content was not considered in our analysis. Since the protein contents in daily meals contribute to the maintenance of skeletal muscle mass, further investigation regarding dietary content is needed. Another limitation of this study is that the results cannot be extended to the general population because we enrolled only subjects with T2D. For these reasons, prospective studies are required to establish the time sequence in the relationships between serum levels of DHEAS and incidences of skeletal muscle disorders including mass and strength abnormalities in elderly individuals with or without diabetes.
5. Conclusions
In summary, the present study showed that serum levels of DHEAS were inversely associated with the prevalence of dynapenia in male individuals with T2D. Taken together, the results indicate that efficient exercise should be performed to preserve skeletal muscle strength for healthy aging in male individuals with T2D who have low serum levels of DHEAS.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/metabo13111129/s1, Table S1: Multiple linear regression analysis with identified variabales and medications used for determinants of SMI, HS and GS in individuals with T2D; Table S2: Multiple logistic regression analysis including GNRI for determinants of dynapenia in male and female individuals with T2D.
Author Contributions
Conceptualization, K.-i.A.; methodology, S.Y., Y.K., H.Y. and K.-i.A.; formal analysis, T.H. (Taiki Hori), S.N. and K.-i.A.; data curation, M.H., A.T., T.H. (Tomoyo Hara) and K.K.; writing, original draft preparation, S.Y., Y.K. and H.Y.; writing, review and editing, T.H. (Takeshi Harada), T.S. and K.-i.A.; visualization, A.K.; supervision, H.M. and M.M.; project administration, T.O., T.Y. and I.E. All authors have read and agreed to the latest version of the manuscript.
Funding
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 22K08033.
Institutional Review Board Statement
This study followed the institutional guidelines and was approved by the Institutional Review Board of Anan Medical Center (approval ID R2-3), and the study was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Written informed consent was obtained from all participants involved in this study.
Data Availability Statement
The datasets generated in the present study are available from the corresponding author upon reasonable request. Data is not publicly available due to privacy.
Acknowledgments
We would like to thank S.E.S Translation and Proofreading Services for English language editing.
Conflicts of Interest
The authors declare that there is no conflicts of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Manini, T.M.; Clark, B.C. Dynapenia and aging: An update. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Feng, X.; Zhou, J.; Gong, H.; Xia, S.; Wei, Q.; Hu, X.; Tao, R.; Li, L.; Qian, F.; et al. Type 2 diabetes mellitus is associated with increased risks of sarcopenia and pre-sarcopenia in Chinese elderly. Sci. Rep. 2016, 6, 38937. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Kuroda, A.; Yoshida, S.; Yasuda, T.; Umayahara, Y.; Shimizu, S.; Ryomoto, K.; Yoshiuchi, K.; Yamamoto, T.; Matsuoka, T.A.; et al. High prevalence and clinical impact of dynapenia and sarcopenia in Japanese patients with type 1 and type 2 diabetes: Findings from the Impact of Diabetes Mellitus on Dynapenia study. J. Diabetes Investig. 2021, 12, 1050–1059. [Google Scholar] [CrossRef]
- Beretta, M.V.; Dantas Filho, F.F.; Freiberg, R.E.; Feldman, J.V.; Nery, C.; Rodrigues, T.C. Sarcopenia and Type 2 diabetes mellitus as predictors of 2-year mortality after hospital discharge in a cohort of hospitalized older adults. Diabetes Res. Clin. Pract. 2020, 159, 107969. [Google Scholar] [CrossRef]
- Lamberts, S.W.; van den Beld, A.W.; van der Lely, A.J. The endocrinology of aging. Science 1997, 278, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Swiecicka, A.; Lunt, M.; Ahern, T.; O’Neill, T.W.; Bartfai, G.; Casanueva, F.F.; Forti, G.; Giwercman, A.; Han, T.S.; Lean, M.E.J.; et al. Nonandrogenic Anabolic Hormones Predict Risk of Frailty: European Male Ageing Study Prospective Data. J. Clin. Endocrinol. Metab. 2017, 102, 2798–2806. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Aihara, K.; Azuma, H.; Uemoto, R.; Sumitomo-Ueda, Y.; Yagi, S.; Ikeda, Y.; Iwase, T.; Nishio, S.; Kawano, H.; et al. Dehydroepiandrosterone sulfate is inversely associated with sex-dependent diverse carotid atherosclerosis regardless of endothelial function. Atherosclerosis 2010, 212, 310–315. [Google Scholar] [CrossRef]
- Wu, T.T.; Chen, Y.; Zhou, Y.; Adi, D.; Zheng, Y.Y.; Liu, F.; Ma, Y.T.; Xie, X. Prognostic Value of Dehydroepiandrosterone Sulfate for Patients With Cardiovascular Disease: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2017, 6, e004896. [Google Scholar] [CrossRef]
- Yanagita, I.; Fujihara, Y.; Kitajima, Y.; Tajima, M.; Honda, M.; Kawajiri, T.; Eda, T.; Yonemura, K.; Yamaguchi, N.; Asakawa, H.; et al. A High Serum Cortisol/DHEA-S Ratio Is a Risk Factor for Sarcopenia in Elderly Diabetic Patients. J. Endocr. Soc. 2019, 3, 801–813. [Google Scholar] [CrossRef]
- American Diabetes, A. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef]
- Hori, T.; Nakamura, S.; Yamagami, H.; Yasui, S.; Hosoki, M.; Hara, T.; Mitsui, Y.; Masuda, S.; Kurahashi, K.; Yoshida, S.; et al. Phase angle and extracellular water-to-total body water ratio estimated by bioelectrical impedance analysis are associated with levels of hemoglobin and hematocrit in patients with diabetes. Heliyon 2023, 9, e14724. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef]
- Baumann, C.W.; Kwak, D.; Liu, H.M.; Thompson, L.V. Age-induced oxidative stress: How does it influence skeletal muscle quantity and quality? J. Appl. Physiol. (1985) 2016, 121, 1047–1052. [Google Scholar] [CrossRef]
- Chao, Y.P.; Fang, W.H.; Chen, W.L.; Peng, T.C.; Yang, W.S.; Kao, T.W. Exploring Muscle Health Deterioration and Its Determinants Among Community-Dwelling Older Adults. Front. Nutr. 2022, 9, 817044. [Google Scholar] [CrossRef]
- Komatsu, T.R.; Borim, F.S.; Neri, A.L.; Corona, L.P. Association of dynapenia, obesity and chronic diseases with all-cause mortality of community-dwelling older adults: A path analysis. Geriatr. Gerontol. Int. 2019, 19, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Luiz, M.M.; Schneider, I.J.C.; Kuriki, H.U.; Fattori, A.; Correa, V.P.; Steptoe, A.; Alexandre, T.D.S.; de Oliveira, C. The combined effect of anemia and dynapenia on mortality risk in older adults: 10-Year evidence from the ELSA cohort study. Arch. Gerontol. Geriatr. 2022, 102, 104739. [Google Scholar] [CrossRef]
- Kobayashi, T.; Askanas, V.; Engel, W.K. Human muscle cultured in monolayer and cocultured with fetal rat spinal cord: Importance of dorsal root ganglia for achieving successful functional innervation. J. Neurosci. 1987, 7, 3131–3141. [Google Scholar] [CrossRef]
- Vasilaki, A.; Jackson, M.J. Role of reactive oxygen species in the defective regeneration seen in aging muscle. Free Radic. Biol. Med. 2013, 65, 317–323. [Google Scholar] [CrossRef]
- Vistoli, G.; De Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free Radic. Res. 2013, 47 (Suppl. S1), 3–27. [Google Scholar] [CrossRef]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative Stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in Aging and Age-Related Diseases. Oxid. Med. Cell Longev. 2019, 2019, 3085756. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Kuroda, A.; Ishizu, M.; Ohishi, M.; Takashi, Y.; Otsuka, Y.; Taniguchi, S.; Tamaki, M.; Kurahashi, K.; Yoshida, S.; et al. Association of accumulated advanced glycation end-products with a high prevalence of sarcopenia and dynapenia in patients with type 2 diabetes. J. Diabetes Investig. 2019, 10, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Kubo, A.; Sugioka, Y.; Mitsui, R.; Fukuhara, N.; Nihei, F.; Takeda, Y. Relationship between advanced glycation end-product accumulation and low skeletal muscle mass in Japanese men and women. Geriatr. Gerontol. Int. 2017, 17, 785–790. [Google Scholar] [CrossRef]
- Brignardello, E.; Runzo, C.; Aragno, M.; Catalano, M.G.; Cassader, M.; Perin, P.C.; Boccuzzi, G. Dehydroepiandrosterone administration counteracts oxidative imbalance and advanced glycation end product formation in type 2 diabetic patients. Diabetes Care 2007, 30, 2922–2927. [Google Scholar] [CrossRef]
- Aragno, M.; Mastrocola, R.; Medana, C.; Catalano, M.G.; Vercellinatto, I.; Danni, O.; Boccuzzi, G. Oxidative stress-dependent impairment of cardiac-specific transcription factors in experimental diabetes. Endocrinology 2006, 147, 5967–5974. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Cai, H.L.; Bao, J.P.; Wu, L.D. Dehydroepiandrosterone and age-related musculoskeletal diseases: Connections and therapeutic implications. Ageing Res. Rev. 2020, 62, 101132. [Google Scholar] [CrossRef] [PubMed]
- Kostka, T.; Arsac, L.M.; Patricot, M.C.; Berthouze, S.E.; Lacour, J.R.; Bonnefoy, M. Leg extensor power and dehydroepiandrosterone sulfate, insulin-like growth factor-I and testosterone in healthy active elderly people. Eur. J. Appl. Physiol. 2000, 82, 83–90. [Google Scholar] [CrossRef]
- Valenti, G.; Denti, L.; Maggio, M.; Ceda, G.; Volpato, S.; Bandinelli, S.; Ceresini, G.; Cappola, A.; Guralnik, J.M.; Ferrucci, L. Effect of DHEAS on skeletal muscle over the life span: The InCHIANTI study. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 466–472. [Google Scholar] [CrossRef]
- Baker, W.L.; Karan, S.; Kenny, A.M. Effect of dehydroepiandrosterone on muscle strength and physical function in older adults: A systematic review. J. Am. Geriatr. Soc. 2011, 59, 997–1002. [Google Scholar] [CrossRef]
- Percheron, G.; Hogrel, J.Y.; Denot-Ledunois, S.; Fayet, G.; Forette, F.; Baulieu, E.E.; Fardeau, M.; Marini, J.F.; Double-blind placebo-controlled, t. Effect of 1-year oral administration of dehydroepiandrosterone to 60- to 80-year-old individuals on muscle function and cross-sectional area: A double-blind placebo-controlled trial. Arch. Intern. Med. 2003, 163, 720–727. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).